Abstract
Background: Advanced hybrid closed-loop (AHCL) systems have improved the glycemic control in type 1 diabetes (T1D). While time in range (TIR) (70–180 mg/dL) is the standard metric, time in tight range (TITR) (70–140 mg/dL) offers a stricter assessment. Real-world comparisons using the TITR are limited. This study compared the TIR and TITR metrics between the MiniMed™ 780G and Tandem Control-IQ™ in adults with T1D. Methods: This retrospective, single-center study included 42 propensity-score-matched adults with T1D (28 MM780G users and 14 Tandem Control-IQ users), each with ≥3 months of AHCL system use. Glycemic metrics from continuous glucose monitoring (CGM) were analyzed over a 14-day period. Comparisons between groups were conducted using Mann–Whitney U tests, adjusted linear regression, and linear mixed-effects models accounting for repeated measures. Results: At three months, the MM780G users achieved significantly higher TITR (60.1% ± 12 vs. 49.5% ± 9.3; p = 0.005) and TIR (83.7% ± 7.6 vs. 72.1% ± 7.5; p < 0.001) values, along with lower glucose variability, compared to these values in the Tandem Control-IQ users. The linear regression model confirmed that the MM780G was independently associated with a higher TITR (β = 14.2; p = 0.005). Mixed-effects modeling for the TIR showed a significant interaction between timepoint and device type (β = 4.81; p = 0.006), favoring the MM780G. Conclusions: In this real-world analysis, both AHCL systems improved glycemic control, but the MiniMed 780G could be associated with a superior performance in achieving tighter glucose targets without increasing hypoglycemia. TITR may serve as a valuable complementary metric alongside TIR in evaluating AHCL systems’ effectiveness. However, these findings should be interpreted cautiously due to limitations such as the retrospective design, small sample size, potential residual confounding, and lack of standardization in the device settings. Further studies are warranted to confirm these results and assess their generalizability.
1. Introduction
Advanced hybrid closed-loop (AHCL) systems are the latest development for managing type 1 diabetes (T1D). These systems integrate continuous glucose monitoring (CGM) with an insulin pump, utilizing sophisticated algorithms to automate insulin delivery [1]. AHCL systems have revolutionized T1D management, enhancing glycemic control and reducing the risks of hypoglycemia [2,3,4]. Historically, glycemic control has been evaluated using the time in range (TIR: 70–180 mg/dL), as a TIR exceeding 70% is correlated with glycated hemoglobin (HbA1c) levels below 7% and a reduced risk of long-term complications [1,5,6]. However, following a recent study using CGM technology in non-diabetic individuals—reporting a median time in tight range (TITR, 70–140 mg/dL) of 96%—a narrower glycemic target of 70–140 mg/dL has been proposed as a new benchmark [7]. The TITR has emerged as a more precise metric for evaluating tighter glycemic control [5].
The International Consensus recommends glycemic targets including a TIR > 70%; a time below range (TBR < 70 mg/dL) < 4%, with a TBR < 54 mg/dL < 1%; and a time above range (TAR > 180 mg/dL) < 25%, with TAR > 250 mg/dL < 5% [8]. The existing evidence demonstrates that AHCL systems improve the TIR and reduce hypoglycemia and hyperglycemia episodes when compared to non-automated therapies [2,3,4]. Studies have shown that a lower TIR is associated with increased risks of microvascular complications, such as retinopathy, nephropathy, and neuropathy, and macrovascular complications [6,9,10,11]. The TITR is particularly relevant in situations where tighter glycemic control is desired, such as in pediatric populations, in diabetic neuropathy, in pregnancy (63–140 mg/dL), and generally in all diabetic patients except for the frail or the elderly [12]. The strong correlation between the TITR and TIR indicates that both metrics can effectively predict HbA1c levels, although the TITR may offer advantages in specific clinical scenarios [13].
In Spain, several AHCL systems are available through the National Health System: the MiniMed 780G (MM780G) (Northridge, CA, USA) integrated with the Guardian 4 sensor, the Tandem Control-IQ t:Slim x2 (Diabetes Care, Inc., San Diego, CA, USA) combined with Dexcom G6/G7, the Diabeloop Generation 1 system (DBLG1) (Diabeloop SA, Grenoble, France) that connects to Dexcom G6, the Roche Insight pump (Diabetes Care GmbH, Mannheim, Germany), and the Ypsopump (AG, Burgdorf, Switzerland) with the CamAPS FX system. The two most commonly used AHCL systems are the Medtronic 780G and the Tandem Control-IQ. Notably, the MiniMed 780G and the Control-IQ differ in several algorithmic and functional aspects that may influence glucose outcomes. The MiniMed 780G features automated basal adjustments and automatic correction boluses delivered every 5 min, with a customizable glucose target as low as 100 mg/dL. In contrast, the Control-IQ delivers automatic corrections up to once per hour and targets a fixed range of 112.5–160 mg/dL. These algorithmic differences may influence the systems’ ability to optimize tighter glycemic control, such as the TITR, a metric not specifically targeted by either system but potentially relevant to long-term outcomes. Understanding how these distinctions translate into real-world glycemic performance is essential.
Nevertheless, direct comparisons between AHCL systems—particularly using advanced metrics like the TITR—remain limited [4,14,15]. Therefore, the aim of the present study was to compare the glycemic control, including the TITR, between the MM780G and Tandem Control-IQ systems in real-world clinical practice among patients with T1D.
2. Materials and Methods
This was a single-center retrospective observational study. A cohort of 116 individuals with T1D was included. All patients had used their respective AHCL system for at least 3 months in active automatic mode and had uploaded CGM data to a cloud-based platform.
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [16].
The inclusion criteria were an established diagnosis of T1D with at least one year of evolution, previous therapy with continuous subcutaneous insulin infusion (CSII) or multiple-dose insulin (MDI), the use of an AHCL system for at least three months, and CGM data available for at least three months after AHCL system initiation.
Exclusion criteria were the use of an AHCL system for less than three months at the time of the data collection, incomplete or missing CGM data for the evaluation period, poor adherence to sensor use (<70% CGM data availability during the evaluation window), and a diagnosis of type 2 diabetes (T2D) or any other form of diabetes.
2.1. Data Collection
Clinical and glucometric data were collected from before and after the three months of AHCL system initiation over a 14-day period (17 September–1 October 2024) from electronic health records, including Clinical data: Sex, age, BMI, duration of diabetes, previous therapy, insulin dose/kg; Glucometric parameters: TIR, TITR, TBR, TBR < 54 mg/dL, TAR, TAR > 250 mg/dL, and HbA1c. HbA1c was measured using liquid chromatography (ADAMS A1c HA8180 V, ARKRAY®, Kyoto, Japan).
2.2. The Statistical Analysis
Outliers were examined, and the normality of the data distributions was assessed using the Kolmogorov–Smirnov test and normal probability plots. Validated extreme values were truncated at the 1st and 99th percentiles. Continuous variables are presented as the mean ± standard deviation (SD) and categorical variables as absolute frequencies and percentages.
To minimize confounding bias from the lack of randomization, propensity score matching (1:2 nearest-neighbor) was performed using logistic regression, adjusting for age, sex, and insulin dose/kg. This approach ensured the comparability of the baseline characteristics between the groups, except for sex, which was included as a covariate in the multivariable analysis due to the higher proportion of females in the Medtronic group. The final analysis included 14 Tandem Control-IQ users and 28 MM780G users, with all participants utilizing automatic mode.
First, the 14-day glucose metrics, including the time in tight range, were compared between the two treatment groups using the Mann–Whitney U test and linear regression adjusted for covariates that were not well balanced between groups.
Second, a linear mixed-effects regression model was applied using the conventional time in range (70–180 mg/dL) metric as the dependent variable. Fixed effects included the type of AHCL system, the interaction between timepoint and AHCL system type, and sex (to account for the imbalance between treatment groups), while a random intercept was specified for each subject ID.
All analyses were performed using STATA v17.0 BE-Basic Edition (StataCorp, College Station, TX, USA), with statistical significance defined as p < 0.05.
3. Results
A total of 116 individuals with T1D on AHCL system therapy were assessed, comprising 16 Tandem Control-IQ users and 100 MM780G users. After excluding individuals with missing data for key variables, 42 subjects were included in the final analysis. The mean age of this population was 39.2 ± 12.8 years, and 55% of the patients were women. Their mean duration of diabetes was 23.4 ± 12.3 years, and the mean insulin dose was 0.6 ± 0.2 IU/kg. Their mean BMI was 25.9 ± 4.1 kg/m2.
3.1. Baseline Characteristics
The patients using the Tandem Control-IQ system had a mean age of 40.3 ± 11.8 years and a mean diabetes duration of 24.5 ± 14.1 years. In comparison, the MM780G group had a mean age of 38.7 ± 13.4 years and a mean diabetes duration of 22.9 ± 11.5 years. No significant differences in the baseline demographic characteristics were observed between the two groups (Table 1).

Table 1.
Baseline demographic characteristics of the sample.
3.2. Baseline Differences According to the Pre-AHCL System Use Glucose Metrics
The patients using the Tandem Control-IQ system had a mean pre-treatment HbA1c value of 6.9 ± 0.6%, a mean TIR of 68 ± 19%, a mean TAR of 21 ± 4%, a mean TBR of 3.5 ± 4%, and a mean CV of 33.9 ± 3.3%. Those in the MM780G group had a mean pre-treatment HbA1c value of 7.0 ± 0.9%, a mean TIR of 69 ± 22%, a mean TAR of 19 ± 14%, a mean TBR of 6 ± 4%, and a mean CV of 36.7 ± 9.15%.
When comparing the pre-AHCL system use glucose metrics, no statistically significant differences were observed between the MM780G and Tandem groups (neither in HbA1c, TIR, TAR, nor TBR), except for the TAR pre > 250, which was significantly higher in the Tandem group (p = 0.04), and average glucose, which was significantly higher in the Tandem group (148 ± 13 vs. 145.3 ± 18, p = 0.013), as shown in Table 2.

Table 2.
Glucometric data before and 3 months after the use of the advanced hybrid closed-loop systems.
3.3. Post-AHCL System Use Glucometric Outcomes
At three months post-AHCL system initiation, the MM780G group achieved significantly higher TITR (60.1 ± 12% vs. 49.5 ± 9.3%, p = <0.001) and TIR (83.7 ± 7.6% vs. 72.1 ± 7.5%, p = 0.0001) values compared to those in the Tandem group. Moreover, the MM780G group showed a significantly lower CV (29.85% vs. 34.9%, p < 0.001). Also, the MM780G group showed a lower TAR > 250 mg/dL (1.9± 1.8% vs. 4.1 ± 3.7%, p = 0.17) and TAR (12.5 ± 4.7% vs. 16.1 ± 7.7%, p = 0.14). No significant differences in the TBR were observed between the two groups. A simple linear regression adjusted for sex and insulin/kg confirmed the superiority of the MM780G in terms of the TITR (difference: +11.5%, 95% CI [3.93–19.1], p = 0.004). The rest of the findings from the analysis of the glucose metrics are shown in Table 2.
Although the GMI values improved in the MM780G group, no significant between-group differences were observed post-intervention, likely due to the fact that the GMI is derived from mean glucose—which remained similar across groups—and the Tandem group, being smaller, showed increased variability, reducing the statistical power.
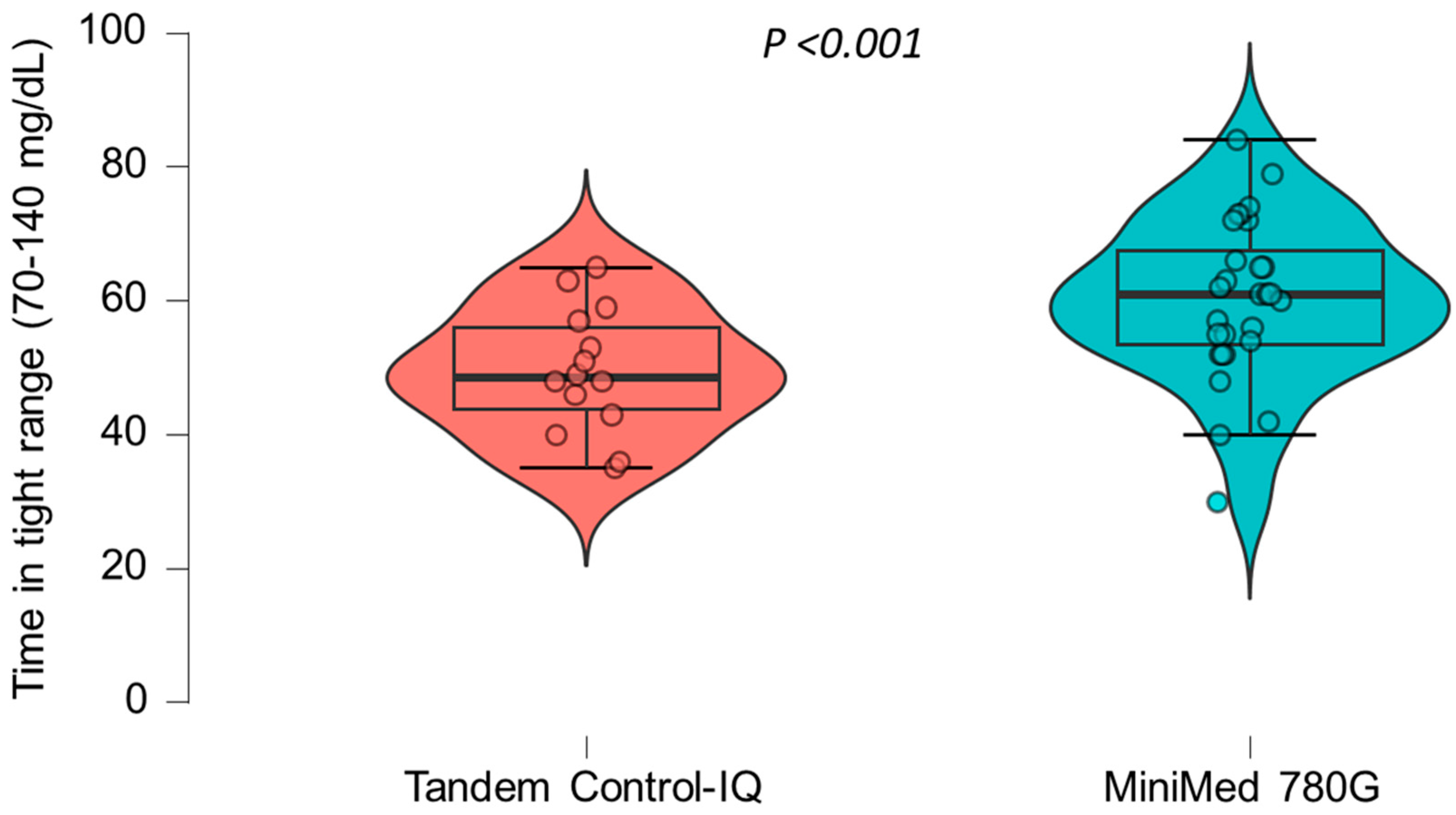
A multiple linear regression model was applied to assess the association between AHCL system type and time in tight range (TITR), adjusted for sex, age, pre-AHCL system use time in range (TIR), and pre-AHCL system use HbA1c (A1CPRE). The model was statistically significant overall (F(5, 27) = 2.79; p = 0.037) and explained approximately 34% of the variability in the TITR (R2 = 0.34). Use of the MiniMed 780G system was independently associated with a significantly higher TITR compared to that for the Tandem Control-IQ (β = 14.2, 95% CI: 4.7 to 23.7; p = 0.005). No other covariates, including sex, age, prior TIR, and prior HbA1c, were significantly associated with the TITR in this model. The crude differences in the TITR between the two treatment groups are illustrated in Figure 1.
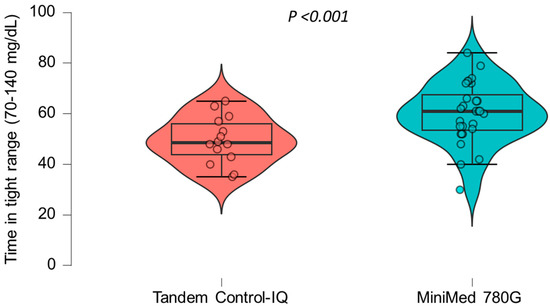
Figure 1.
The differences in the time in tight range (70–140 mg/dL) between the two treatment groups.
A statistically significant trend toward a higher time in tight range was observed in the group treated with the MiniMed 780G system compared to that in the Tandem Control-IQ group.
A linear mixed-effects model was fitted to assess the impact of automated hybrid closed-loop (AHCL) pump initiation on the TIR, using a Winsorized version of the TIR to limit the influence of extreme values. The model included fixed effects for timepoint (before vs. after AHCL system initiation), pump type (Tandem Control-IQ vs. MiniMed 780G), their interaction, and sex. A random intercept for subject ID accounted for repeated measures (Table 3).

Table 3.
The linear mixed-effects model evaluating the time in range (TIR) before and after automated hybrid closed-loop (AHCL) pump initiation.
The linear mixed-effects model with the Winsorized TIR (time in range 70–180 mg/dL) as the dependent variable was fitted. “Timepoint” refers to pre-(0) and post-(1) AHCL pump initiation. “Pump type” was coded as 0 for the Tandem Control-IQ and 1 for the MiniMed 780G. An interaction term was included to assess the differential response by pump type over time. Random intercepts were included for each subject (ID) to account for within-subject clustering.
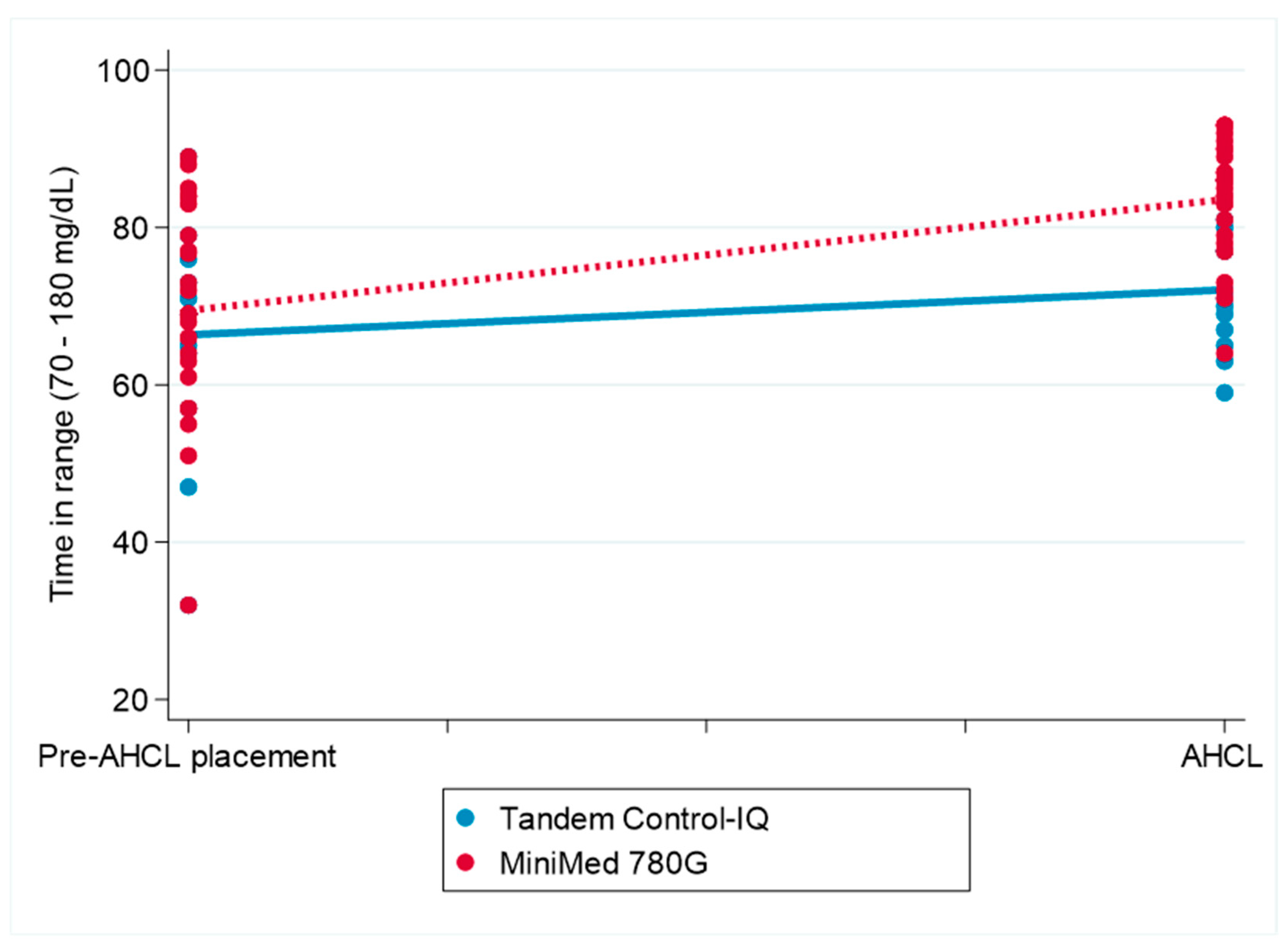
The interaction between pump type and timepoint was statistically significant (β = 4.81, 95% CI: 1.40 to 8.22; p = 0.006), indicating a greater increase in the TIR following AHCL system initiation in the participants using the MiniMed 780G system compared to that in those using the Tandem Control-IQ. No main effects were observed for timepoint, pump type, or sex individually. Figure 2 displays the change in the TIR over the follow-up period for both treatment groups.
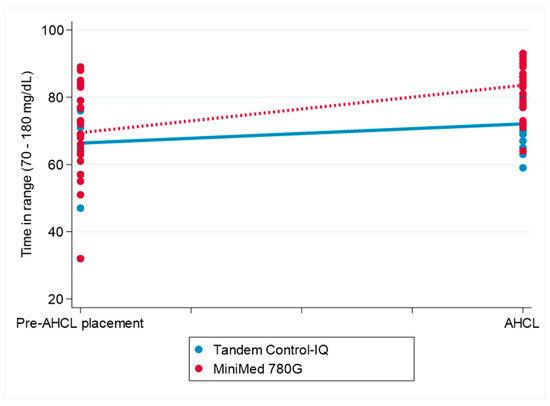
Figure 2.
The change in the conventional time in range (70–180 mg/dL) before and after the initiation of MiniMed 780G and Tandem Control-IQ use. AHCL: automated hybrid closed-loop (system) The figure illustrates the differences in conventional time in range (70–180 mg/dL) before and after AHCL system initiation in the Tandem Control-IQ and MiniMed 780G groups.
4. Discussion
In this retrospective observational study, we compared the performance of two commercially available AHCL insulin delivery systems—the MM780G and Tandem Control-IQ™—in a real-world cohort of adults with type 1 diabetes, adjusted by propensity scores. Our analysis revealed that while both systems improved glucose control, the MM780G provided a significantly higher TITR and TIR, as well as lower glucose variability, compared to these values with the Tandem Control-IQ. Crucially, these improvements were not accompanied by an increase in the time below range (TBR). However, it should be noted that neither the MM780G nor the Tandem Control-IQ™ system was specifically designed to optimize the TITR, as their algorithms do not currently support this narrower target range. This represents an important limitation of this study and should be considered when interpreting its results.
Despite the lack of randomization, the baseline clinical and glycemic characteristics were similar between groups, without statistically significant differences. This comparability reduces the risk of bias and supports the validity of the observed differences in glycemic outcomes.
Previous studies have reported mixed results regarding the superiority of one system over the other. For instance, Bassi et al. found that the Tandem Control-IQ was associated with a reduced time in hypoglycemia, while the MiniMed 780G tended to achieve higher TIR values [17], results that are consistent with our cohort. However, these studies did not assess the TITR, a key metric that we evaluated. Conversely, another prospective trial in adults with T1D reported a better nighttime TIR using the Tandem [15]. Moreover, other studies, including Beato-Vibora et al.’s multicenter prospective study, reported no significant differences between systems [18,19]. However, none of these studies included the TITR as a primary outcome measure.
TITR and TIR are known to be highly correlated, as demonstrated by Beck et al., which implies that TIR can be used to estimate TITR [20]. Our findings support this correlation across both technologies. However, other studies have demonstrated that the TIR-TITR relationship varies according to the CV and TBR [20,21,22], where higher CV or TBR values are associated with a higher TITR, raising concerns that optimizing the TITR might inadvertently increase hypoglycemia risk [20].
Despite being correlated metrics, TITR may offer complementary insights beyond TIR, especially in patients approaching normoglycemia. TIR is sensitive to changes in the CV when the mean glucose is between 150 and 160 mg/dL, which is near the generally appropriate A1C target of 7% [20,21,22]. However, at these glucose levels, a higher CV is associated with an increased TITR, giving the false impression of improved glucose control [22]. In contrast, the TITR becomes more sensitive to changes in the CV than the TIR when trying to bring glucose levels to near-normal ranges, particularly <140 mg/dL, potentially offering a more refined assessment of glycemic quality [22].
Emerging evidence also suggests that the TITR may be more strongly associated with diabetes-related complications. Several studies have linked both microvascular and macrovascular outcomes to the TITR [9,23,24]. Wang et al. found that a lower TITR was associated with an increased risk of diabetic retinopathy in patients with type 2 diabetes (T2D), even among patients with a well-controlled TIR (≥70%), suggesting that the TITR may be a more sensitive predictor of microvascular outcomes than the TIR alone [24]. TITR may be especially valuable in clinical scenarios requiring strict glycemic control, such as recent-onset T1D in pregnancy or in patients whose average glucose levels are already within the target range, where subtle glycemic fluctuations may go undetected when using the TIR [22].
This study has several limitations, despite the use of propensity score matching and the application of a linear mixed-effects regression model to assessing the changes in the TIR. First, the small and heterogeneous sample size, particularly in the Tandem Control-IQ group, was a limitation. Second, this study’s retrospective single-center analysis limits its generalizability. Third, while baseline comparability was demonstrated, residual confounding cannot be excluded. In addition, various factors that may impact glycemic outcomes—such as physical activity, dietary habits, and behavioral patterns—were not accounted for. Furthermore, the customizable settings within each AHCL system were not standardized. Notably, the MiniMed 780G includes an auto-correction bolus feature and allows for more aggressive glycemic targets (down to 100 mg/dL), which may contribute to its tighter glucose control. Lastly, no pre-AHCL system use TITR data were available for either group, limiting the ability to assess within-subject changes in tight glucose control.
In conclusion, our data suggest that while both AHCL systems significantly improve the glycemic control in individuals with T1D, the MiniMed 780G system may offer a superior performance in achieving tighter glucose control. Importantly, the improvements in the TITR and TIR with the MiniMed 780G did not come at the cost of increased hypoglycemia. Our findings support the consideration of TITR as a complementary metric alongside TIR in the evaluation of AHCL system therapy’s efficacy.
Author Contributions
Conceptualization: M.S.T.S. and V.N.-M.; Methodology: M.L.V.; Software: F.S.V.; Validation: J.A.A.M., C.S.L.G. and E.C.L. Formal Analysis: V.N.-M.; Investigation: F.S.V. and M.M.; Resources: J.J.R.L.; Data Curation: M.S.T.S.; Writing—Original Draft Preparation: M.S.T.S.; Writing—Review and Editing: S.A.; Visualization: S.G.C.; Supervision: V.N.-M.; Project Administration: M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University Hospital La Princesa (protocol code 5814, CEIm 01/25).
Informed Consent Statement
We consider that this study does not require obtaining informed consent from patients, given its observational and retrospective design, the absence of direct patient contact, and the fact that all necessary data are contained in reports documented by their regular physicians as part of routine clinical care. The proposed project is observational in nature and does not involve any diagnostic or therapeutic intervention beyond that which is part of standard clinical practice.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Lu, J.; Wang, C.; Shen, Y.; Chen, L.; Zhang, L.; Cai, J.; Lu, W.; Zhu, W.; Hu, G.; Xia, T.; et al. Time in Range in Relation to All-Cause and Cardiovascular Mortality in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care 2021, 44, 549–555. [Google Scholar] [CrossRef]
- Nathan, D.M.; Bayless, M.; Cleary, P.; Genuth, S.; Gubitosi-Klug, R.; Lachin, J.M.; Lorenzi, G.; Zinman, B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Advances and Contributions. Diabetes 2013, 62, 3976–3986. [Google Scholar] [CrossRef]
- Moreno, V.N.; Sebastian-Valles, F.; Sampedro-Nuñez, M.; Lahera Vargas, M.; Marazuela, M.; Arranz Martin, J.A.A. Patient satisfaction in three advanced hybrid closed-loop systems at 6 months of treatment in adults with type 1 diabetes mellitus: A follow-up study. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2023, 70, 548–555. [Google Scholar] [CrossRef]
- Henry, Z.; Villar Fimbel, S.; Bendelac, N.; Thivolet, C. Real world evidence of the efficacy of two hybrid closed loop systems for children and adults with type 1 diabetes with some clinical warnings. Diabetes Metab. 2022, 48, 101396. [Google Scholar] [CrossRef]
- Batellino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza Rubin, G.; Beck, R.W.; Bergenstal, R.M. Continuous glucose monitoring and metrics for clinical trials: An international consensus statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef]
- Mayeda, L.; Katz, R.; Ahmad, I.; Bansal, N.; Batacchi, Z.; Hirsch, I.B.; Robinson, N.; Trence, D.L.; Zelnick, L.; de Boer, I.H. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res. Care 2020, 8, e000991. [Google Scholar] [CrossRef]
- Shah, V.N.; DuBose, S.N.; Li, Z.; Beck, R.W.; Peters, A.L.; Weinstock, R.S.; Kruger, D.; Tansey, M.; Sparling, D.; Woerner, S.; et al. Continuous Glucose Monitoring Profiles in Healthy Nondiabetic Participants: A Multicenter Prospective Study. J. Clin. Endocrinol. Metab. 2019, 104, 4356–4364. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Meulemeester, J.; Charleer, S. The association of chronic complications with time in tight range and time in range in people with type 1 diabetes: A retrospective cross-sectional real-world study. Diabetologia 2024, 67, 1527–1535. [Google Scholar] [CrossRef]
- Beck, R.W.; Bergenstal, R.M.; Riddlesworth, T.D.; Kollman, C.; Li, Z.; Brown, A.S.; Close, K.L. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care 2019, 42, 400–405. [Google Scholar] [CrossRef]
- Guo, Q.; Zang, P.; Xu, S.; Song, W.; Zhang, Z.; Liu, C.; Guo, Z.; Chen, J.; Lu, B.; Gu, P.; et al. Time in Range, as a Novel Metric of Glycemic Control, Is Reversely Associated with Presence of Diabetic Cardiovascular Autonomic Neuropathy Independent of HbA1c in Chinese Type 2 Diabetes. J. Diabetes Res. 2020, 2020, 5817074. [Google Scholar] [CrossRef]
- Sundberg, F.; Smart, C.E.; Samuelsson, J.; Åkesson, K.; Krogvold, L. Using Time in Tight Glucose Range as a Health-Promoting Strategy in Preschoolers With Type 1 Diabetes. Diabetes Care 2025, 48, 6–14. [Google Scholar] [CrossRef]
- Burckhardt, M.-A.; Auzanneau, M.; Rosenbauer, J.; Binder, E.; Weiskorn, J.; Hess, M.; Klinkert, C.; Mirza, J.; Zehnder, L.-S.; Wenzel, S.; et al. What is the Relationship Between Time in Range, Time in Tight Range, and HbA1c in Youth and Young Adults With Type 1 Diabetes? Results From the German/Austrian/Luxembourgian/Swiss Diabetes Prospective Follow-Up Registry. J. Diabetes Sci. Technol. 2024, 16, 19322968241288870. [Google Scholar] [CrossRef]
- Folk, S.; Zappe, J.; Wyne, K.; Dungan, K.M. Comparative Effectiveness of Hybrid Closed-Loop Automated Insulin Delivery Systems Among Patients with Type 1 Diabetes. J. Diabetes Sci. Technol. 2024, 1, 19322968241234948. [Google Scholar] [CrossRef]
- Borella, N.D.; Ferramosca, A.; Castagna, G.; Ippolito, S.; Ceresoli, S.; Taverna, A.; Sonzogni, B.; Trevisan, R.; Lepore, G. Comparison of the night-time effectiveness in achieving glycemic targets in adults with type 1 diabetes of three advanced hybryd closed-loop systems. Acta Diabetol. 2025, 62, 763–771. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Bassi, M.; Teliti, M.; Lezzi, M.; Iosca, A.; Strati, M.F.; Carmisciano, L.; d’Annunzio, G.; Minuto, N.; Maggi, D. A Comparison of Two Hybrid Closed-Loop Systems in Italian Children and Adults With Type 1 Diabetes. Front. Endocrinol. 2022, 12, 802419. [Google Scholar] [CrossRef]
- Beato-Víbora, P.B.; Chico, A.; Moreno-Fernández, J.; Bellido-Castañeda, V.; Nattero-Chávez, L.; Picón-César, M.J.; Martínez-Brocca, M.A.; Giménez-Álvarez, M.; Aguilera-Hurtado, E.; Climent-Biescas, E.; et al. A Multicenter Prospective Evaluation of the Benefits of Two Advanced Hybrid Closed-Loop Systems in Glucose Control and Patient-Reported Outcomes in a Real-world Setting. Diabetes Care 2024, 47, 216–224. [Google Scholar] [CrossRef]
- Lablanche, S.; Delagenière, S.; Jalbert, M.; Sonnet, E.; Benichou, M.; Arnold, N.; Spiteri, A.; Le Berre, J.-P.; Renard, E.; Chevalier, N.; et al. 12-Month Real-Life Efficacy of the MiniMed 780G Advanced Closed-Loop System in Patients Living with Type 1 Diabetes: A French Observational, Retrospective, Multicentric Study. Diabetes Technol. Ther. 2024, 26, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.; Raghinaru, D. A Comparison of Continuous Glucose Monitoring-Measured Time-in-Range 70-180 mg/dL Versus Time-in-Tight-Range 70-140 mg/dL. Diabetes Technol. Ther. 2024, 26, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Passanisi, S.; Piona, C.; Salzano, G.; Marigliano, M.; Bombaci, B. Aiming for the Best Glycemic Control Beyond Time in Range: Time in Tight Range as a New Continuous Glucose Monitoring Metric in Children and Adolescents with Type 1 Diabetes Using Different Treatment Modalities. Diabetes Technol. Ther. 2024, 26, 161–166. [Google Scholar] [CrossRef]
- Dunn, T.C.; Ajjan, R.A.; Bergenstal, R.M.; Xu, Y. Is It Time to Move Beyond TIR to TITR? Real-World Data from Over 20,000 Users of Continuous Glucose Monitoring in Patients with Type 1 and Type 2 Diabetes. Diabetes Technol. Ther. 2024, 26, 203–210. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Lu, J.; Ni, J.; Wang, C.; Chen, L.; Lu, W.; Zhu, W.; Xia, T.; Zhou, J. Impact of time in tight range on all-cause and cardiovascular mortality in type 2 diabetes: A prospective cohort study. Diabetes Obes. Metab. 2025, 27, 2154–2162. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Bs, J.Y.; Bs, J.N.; Wang, M.; Bsn, W.L.; Bsn, W.Z.; Guo, J.; Zhou, J. Association between time in tight range and incident diabetic retinopathy in adults with type 2 diabetes. Diabetes Obes. Metab. 2025, 27, 1415–1422. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).