Diabetes Control and Clinical Outcomes in Chronic Obstructive Pulmonary Disease (COPD) Exacerbation
Abstract
1. Introduction
2. Methods
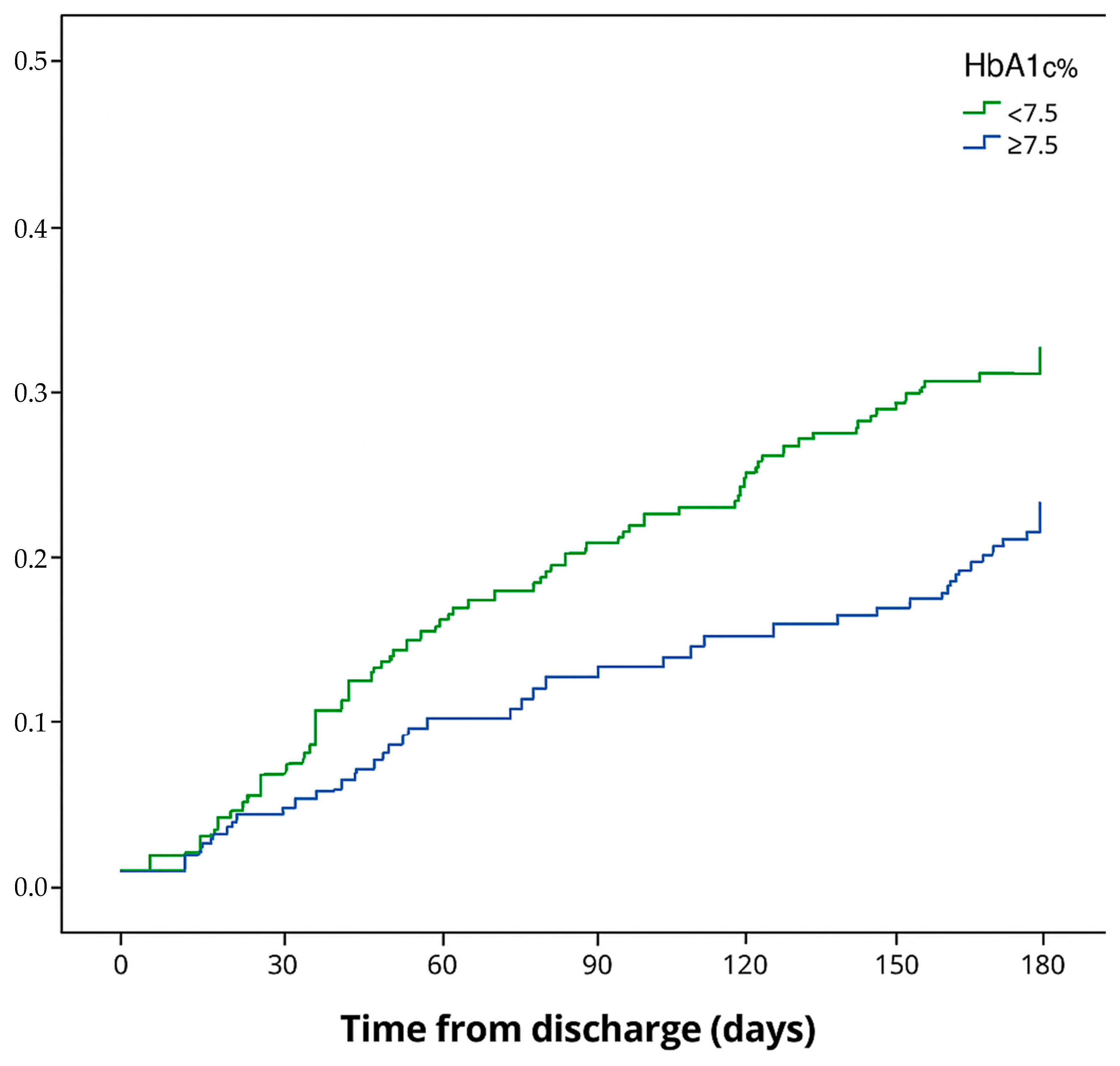
3. Results
Baseline Patient Characteristics: A Total of 426 Patients Were Included in the Study
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belligund, P.; Attaway, A.; Lopez, R.; Damania, D.; Hatipoğlu, U.; Zein, J. Diabetes associated with higher health care utilization and poor outcomes after COPD-related hospitalizations. Am. J. Manag. Care 2022, 28, e325–e332. [Google Scholar] [PubMed]
- Kiani, F.Z.; Ahmadi, A. Prevalence of different comorbidities in chronic obstructive pulmonary disease among Shahrekord PERSIAN cohort study in southwest Iran. Sci. Rep. 2021, 11, 1548. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-W.; Huang, C.-T.; Ruan, S.-Y.; Tsai, Y.-J.; Lai, F.; Yu, C.-J. Diabetes mellitus in patients with chronic obstructive pulmonary disease-The impact on mortality. PLoS ONE 2017, 12, e0175794. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.-C.; Mao, I.-C.; Lin, C.-H.; Lin, S.-H.; Hsieh, M.-C. Chronic obstructive pulmonary disease: A risk factor for type 2 diabetes: A nationwide population-based study. Eur. J. Clin. Investig. 2013, 43, 1113–1119. [Google Scholar] [CrossRef]
- Peng, Y.; Zhong, G.-C.; Wang, L.; Guan, L.; Wang, A.; Hu, K.; Shen, J. Chronic obstructive pulmonary disease, lung function and risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. BMC Pulm. Med. 2020, 20, 137. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E. Chronic obstructive pulmonary disease and glucose metabolism: A bitter sweet symphony. Cardiovasc. Diabetol. 2012, 11, 132. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Rabe, K.F. From COPD to chronic systemic inflammatory syndrome? Lancet 2007, 370, 797–799. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, H.; Chen, D.; Tse, L.A.; Kinra, S.; Li, Y. Genetic correlation and bidirectional causal association between type 2 diabetes and pulmonary function. Front. Endocrinol. 2021, 12, 777487. [Google Scholar] [CrossRef]
- Saeed, M.I.; Eklöf, J.; Achir, I.; Sivapalan, P.; Meteran, H.; Løkke, A.; Biering-Sørensen, T.; Knop, F.K.; Jensen, J.S. Use of inhaled corticosteroids and the risk of developing type 2 diabetes in patients with chronic obstructive pulmonary disease. Diabetes Obes. Metab. 2020, 22, 1348–1356. [Google Scholar] [CrossRef]
- Price, D.B.; Russell, R.; Mares, R.; Burden, A.; Skinner, D.; Mikkelsen, H.; Ding, C.; Brice, R.; Chavannes, N.H.; Kocks, J.W.H.; et al. Metabolic Effects Associated with ICS in Patients with COPD and Comorbid Type 2 Diabetes: A Historical Matched Cohort Study. PLoS ONE 2016, 11, e0162903. [Google Scholar] [CrossRef]
- van den Borst, B.; Gosker, H.R.; Zeegers, M.P.; Schols AMWJ. Pulmonary function in diabetes: A metaanalysis. Chest 2010, 138, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.L.; Aviles-Santa, L.; Cai, J.; Collard, H.R.; Kanaya, A.M.; Kaplan, R.C.; Kinney, G.L.; Mendes, E.; Smith, L.; Talavera, G.; et al. Hispanics/latinos with type 2 diabetes have functional and symptomatic pulmonary impairment mirroring kidney microangiopathy: Findings from the hispanic community health study/study of latinos (HCHS/SOL). Diabetes Care 2016, 39, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, M.; Wan, X.; Yu, H.; Wan, Y.; Hang, D.; Lu, Y.; Tao, R.; Wu, M.; Zhou, J.; et al. Associations of diabetes, prediabetes and diabetes duration with the risk of chronic obstructive pulmonary disease: A prospective UK Biobank study. Diabetes Obes. Metab. 2023, 25, 2575–2585. [Google Scholar] [CrossRef]
- Gläser, S.; Krüger, S.; Merkel, M.; Bramlage, P.; Herth, F.J.F. Chronic obstructive pulmonary disease and diabetes mellitus: A systematic review of the literature. Respiration 2015, 89, 253–264. [Google Scholar] [CrossRef]
- Castañ-Abad, M.T.; Montserrat-Capdevila, J.; Godoy, P.; Marsal, J.R.; Ortega, M.; Alsedà, M.; Barbé, F. Diabetes as a risk factor for severe exacerbation and death in patients with COPD: A prospective cohort study. Eur. J. Public Health 2020, 30, 822–827. [Google Scholar] [CrossRef]
- Lin, L.; Shi, J.; Kang, J.; Wang, Q. Analysis of prevalence and prognosis of type 2 diabetes mellitus in patients with acute exacerbation of COPD. BMC Pulm. Med. 2021, 21, 7. [Google Scholar] [CrossRef]
- Raslan, A.S.; Quint, J.K.; Cook, S. All-Cause, Cardiovascular and Respiratory Mortality in People with Type 2 Diabetes and Chronic Obstructive Pulmonary Disease (COPD) in England: A Cohort Study Using the Clinical Practice Research Datalink (CPRD). Int. J. Chron Obstruct. Pulmon Dis. 2023, 18, 1207–1218. [Google Scholar] [CrossRef]
- Roversi, S.; Fabbri, L.M.; Sin, D.D.; Hawkins, N.M.; Agustí, A. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am. J. Respir. Crit. Care Med. 2016, 194, 1319–1336. [Google Scholar] [CrossRef]
- Trinkmann, F.; Saur, J.; Borggrefe, M.; Akin, I. Cardiovascular Comorbidities in Chronic Obstructive Pulmonary Disease (COPD)-Current Considerations for Clinical Practice. J. Clin. Med. 2019, 8, 69. [Google Scholar] [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Zewari, S.; Hadi, L.; van den Elshout, F.; Dekhuijzen, R.; Heijdra, Y.; Vos, P. Obesity in COPD: Comorbidities with Practical Consequences? COPD 2018, 15, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Torres, A.; Huerta, A.; Méndez, R.; Guerrero, M.; Martinez, R.; Liapikou, A.; Soler, N.; Sethi, S.; Menéndez, R. C-Reactive Protein at Discharge, Diabetes Mellitus and ≥1 Hospitalization During Previous Year Predict Early Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. COPD 2015, 12, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Almagro, P.; Cabrera, F.J.; Diez, J.; Boixeda, R.; Ortiz, M.B.A.; Murio, C.; Soriano, J.B. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: The EPOC en Servicios de medicina interna (ESMI) study. Chest 2012, 142, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- McGhan, R.; Radcliff, T.; Fish, R.; Sutherland, E.R.; Welsh, C.; Make, B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007, 132, 1748–1755. [Google Scholar] [CrossRef]
- Baker, C.L.; Zou, K.H.; Su, J. Risk assessment of readmissions following an initial COPD-related hospitalization. Int. J. Chron. Obs. Pulmon Dis. 2013, 8, 551–559. [Google Scholar]
- Roberts, M.H.; Clerisme-Beaty, E.; Kozma, C.M.; Paris, A.; Slaton, T.; Mapel, D.W. A retrospective analysis to identify predictors of COPD-related rehospitalization. BMC Pulm. Med. 2016, 16, 68. [Google Scholar] [CrossRef]
- Koskela, H.O.; Salonen, P.H.; Romppanen, J.; Niskanen, L. A history of diabetes but not hyperglycaemia during exacerbation of obstructive lung disease has impact on long-term mortality: A prospective, observational cohort study. BMJ Open 2015, 5, e006794. [Google Scholar] [CrossRef]
- Maan, H.B.; Meo, S.A.; Al Rouq, F.; Meo, I.M.U.; Gacuan, M.E.; Alkhalifah, J.M. Effect of glycated hemoglobin (hba1c) and duration of disease on lung functions in type 2 diabetic patients. Int. J. Environ. Res. Public Health 2021, 18, 6970. [Google Scholar] [CrossRef]
- Yeh, H.C.; Punjabi, N.M.; Wang, N.Y.; Pankow, J.S.; Duncan, B.B.; Cox, C.E.; Selvin, E.; Brancati, F.L. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2008, 31, 741–746. [Google Scholar] [CrossRef]
- Paul, S.; Ali, A.; Katare, R. Molecular complexities underlying the vascular complications of diabetes mellitus—A comprehensive review. J. Diabetes Complicat. 2020, 34, 107613. [Google Scholar] [CrossRef]
- Khan, A.W.; Jandeleit-Dahm, K.A.M. Atherosclerosis in diabetes mellitus: Novel mechanisms and mechanism-based therapeutic approaches. Nat. Rev. Cardiol. 2025, 22, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.J.; Clough, G.; Byrne, C.D. Interactions between microvascular and macrovascular disease in diabetes: Pathophysiology and therapeutic implications. Diabetes Obes. Metab. 2007, 9, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Jagadapillai, R.; Rane, M.J.; Lin, X.; Roberts, A.M.; Hoyle, G.W.; Cai, L.; Gozal, E. Diabetic microvascular disease and pulmonary fibrosis: The contribution of platelets and systemic inflammation. Int. J. Mol. Sci. 2016, 17, 1853. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Pirera, E.; Pintus, C.; De Rosa, R.; Profita, M.; Musiari, G.; Siscaro, G.; Tuttolomondo, A. The role of the cumulative illness rating scale (CIRS) in estimating the impact of comorbidities on chronic obstructive pulmonary disease (COPD) outcomes: A pilot study of the MACH (multidimensional approach for COPD and high complexity) study. J. Pers. Med. 2023, 13, 1674. [Google Scholar] [CrossRef]
- Garvey, C.; Criner, G.J. Impact of comorbidities on the treatment of chronic obstructive pulmonary disease. Am. J. Med. 2018, 131, 23–29. [Google Scholar] [CrossRef]
| Total N = 426 | HbA1c < 7.5% N = 247 (58%) | HbA1c ≥ 7.5% N = 179 (42%) | p-Value | |
|---|---|---|---|---|
| Age (years) | 73.2 ± 10.2 | 74.0 ± 9.5 | 72.2 ± 11.1 | 0.06 |
| Male | 280 (65.7) | 159 (64.4) | 121 (67.6) | 0.489 |
| Hypertension | 393 (92.3) | 223 (90.3) | 170 (95.0) | 0.074 |
| Ischemic Heart Disease | 275 (64.6) | 138 (55.9) | 137 (76.5) | <0.0001 |
| Heart Failure | 239 (56.1) | 123 (49.8) | 116 (64.8) | 0.002 |
| Inhaled Corticosteroids | 244 (57.3) | 137(55.5) | 107 (59.8) | 0.375 |
| LABA/LAMA inhaler | 237 (55.6) | 136 (55.1) | 101 (56.4) | 0.78 |
| Home oxygen therapy | 182 (42.8) | 107 (43.3) | 75 (42.1) | 0.873 |
| Mechanical ventilation | 117 (27.5) | 68 (27.5) | 49 (27.4) | 0.972 |
| Days of hospital stay: mean ± std. | 7.0 ± 4.1 | 6.7 ± 3.8 | 7.3 ± 4.6 | 0.283 |
| Univariate Analysis | ||||
|---|---|---|---|---|
| 6-Month Rehospitalization/Mortality | Adjusted HR95%CI | p-Value | ||
| No | Yes | |||
| N = 296 | N = 130 | |||
| HbA1c% | ||||
| <7% | 183 (61.8) | 113 (38.2) | ||
| ≥7.5% | 64 (49.2) | 66 (50.8) | 1.60 (1.11–2.3) | 0.01 |
| Age | 72.6 ± 10.4 | 75.0 ± 9.7 | 1.02 (1.00–1.04) | 0.04 |
| Home oxygen therapy | ||||
| No | 187 (63.1) | 57 (43.9) | ||
| Yes | 109 (36.9) | 73 (56.2) | 1.98 (1.37–2.88) | 0.0003 |
| Gender | ||||
| Male | 191 (64.5) | 89 (68.5) | ||
| Female | 105 (35.5) | 41 (31.5) | 0.86 (0.58–1.28) | 0.464 |
| Inhaled Corticosteroids | ||||
| No | 159 (45.0) | 23 (31.5) | ||
| Yes | 194 (55.0) | 50 (68.5) | 1.47 (0.87–2.5) | 0.147 |
| LABA/LAMA | ||||
| No | 140 (47.3) | 49 (37.7) | ||
| Yes | 156 (52.7) | 81 (62.3) | 1.35 (0.94–1.96) | 0.109 |
| Hypertension | ||||
| No | 20 (6.8) | 13 (10.0) | ||
| Yes | 276 (93.2) | 117 (90.0) | 0.71 (0.39–1.3) | 0.271 |
| Ischemic Heart Disease | ||||
| No | 106 (35.8) | 45 (34.6) | ||
| yes | 190 (64.2) | 85 (65.4) | 1.05 (0.71–1.54) | 0.802 |
| Heart Failure | ||||
| No | 132 (44.6) | 55 (42.3) | ||
| Yes | 164 (55.4) | 75 (57.7) | 1.11 (0.77–1.61) | 0.562 |
| Multivariate analysis | ||||
| Adjusted HR | p-value | |||
| HbA1c ≥ 7.5% | 1.82 (1.24–2.67) | 0.002 | ||
| Age | 1.02 (1.00–1.04) | 0.036 | ||
| Home Oxygen Therapy | 2.22 (1.53–3.22) | <0.0001 | ||
| Univariate Analysis | 6-Month Rehospitalization | Adj HR95%CI | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| N = 353 | N = 73 | |||
| HbA1c% | ||||
| <7.5 | 214 (60.6) | 33 (45.2) | ||
| ≥7.5% | 139 (39.4) | 40 (54.8) | 1.94 (1.16–3.23) | 0.011 |
| Age | 73.6 ± 10.3 | 71.4 ± 9.4 | 0.99 (0.96–1.01) | 0.353 |
| Home oxygen therapy | ||||
| No | 216 (61.4) | 27 (37) | ||
| Yes | 136 (38.6) | 46 (63.0) | 2.23 (1.33–3.75) | 0.003 |
| Gender | ||||
| Male | 231 (65.4) | 49 (67.1) | ||
| Female | 122 (34.6) | 24 (32.9) | 0.99 (0.57–1.74) | 0.997 |
| Inhaled Corticosteroids | ||||
| No | 159 (45.0) | 23 (31.5) | ||
| Yes | 194 (55.0) | 50 (68.5) | 1.47 (0.87–2.5) | 0.147 |
| LABA/LAMA | ||||
| No | 165 (46.7) | 24 (32.9) | ||
| Yes | 188 (53.3) | 49 (67.1) | 1.58 (0.94–2.66) | 0.087 |
| Hypertension | ||||
| No | 25 (7.1) | 8 (11.0) | ||
| Yes | 328 (92.9) | 65 (89.0) | 0.66 (0.29–1.49) | 0.319 |
| Ischemic Heart Disease | ||||
| No | 124 (35.1) | 27 (37.0) | ||
| Yes | 229 (64.9) | 46 (63.0) | 0.98 (0.57–1.68) | 0.946 |
| Heart Failure | ||||
| No | 149 (42.2) | 38 (52.1) | ||
| Yes | 204 (57.8) | 35 (47.9) | 0.79 (0.48–1.31) | 0.366 |
| Multivariate analysis | ||||
| Adjusted HR | p-value | |||
| HbA1c ≥ 7.5 | 1.98 (1.19–3.27) | 0.008 | ||
| LABA/LAMA Inhaler | 1.40 (0.82–2.38) | 0.219 | ||
| Home oxygen Therapy | 1.84 (2.2–3.2) | 0.028 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassem, S.; Zaina, A.; Stein, N.; Naoum, I. Diabetes Control and Clinical Outcomes in Chronic Obstructive Pulmonary Disease (COPD) Exacerbation. Diabetology 2025, 6, 66. https://doi.org/10.3390/diabetology6070066
Kassem S, Zaina A, Stein N, Naoum I. Diabetes Control and Clinical Outcomes in Chronic Obstructive Pulmonary Disease (COPD) Exacerbation. Diabetology. 2025; 6(7):66. https://doi.org/10.3390/diabetology6070066
Chicago/Turabian StyleKassem, Sameer, Adnan Zaina, Nili Stein, and Ibrahim Naoum. 2025. "Diabetes Control and Clinical Outcomes in Chronic Obstructive Pulmonary Disease (COPD) Exacerbation" Diabetology 6, no. 7: 66. https://doi.org/10.3390/diabetology6070066
APA StyleKassem, S., Zaina, A., Stein, N., & Naoum, I. (2025). Diabetes Control and Clinical Outcomes in Chronic Obstructive Pulmonary Disease (COPD) Exacerbation. Diabetology, 6(7), 66. https://doi.org/10.3390/diabetology6070066







