Comparative Analysis of Cardiovascular Outcomes in Type 2 Diabetes Patients Engaging in Aerobic, Resistance, and Combined Training: A Systematic Review
Abstract
1. Introduction
1.1. Global Impact of Type 2 Diabetes and Cardiovascular Disease
1.2. Role of Exercise in Cardiovascular Health for T2D
1.3. Study Objectives and Contributions
2. Methodology
2.1. Research Design
2.2. Rationale for the Systematic Review
2.3. Research Questions and Hypotheses
- Main Question:
- Secondary Questions:
- How do exercise intensity, frequency, and duration impact cardiovascular risk factors?
- Is combined training more synergistic or superior to single-modality training?
- H1: Aerobic activity leads to more pronounced blood pressure drops and cholesterol profile enhancements compared to strength training [6].
- H2: Strength training improves performance, indirectly lowers inflammation, improves cardiovascular health, and increases muscle mass and glucose metabolism [10].
- H3: Individuals can achieve the greatest cardiovascular benefits by improving muscle strength and cardiorespiratory fitness through resistance and aerobic exercise. This may lead to a greater reduction in cardiovascular risks [9].
2.4. Inclusion and Exclusion Criteria
- Inclusion Criteria:
- Population: Adults 18 years and older diagnosed with T2D.
- Interventions: Exercise interventions must be aerobic, resistance training, or both (combined) and at least 8 weeks long. Such a period is the minimum for detecting relevant cardiovascular and metabolic effects adaptations.
- Outcomes: Eligible studies should report cardiovascular outcomes, including blood pressure, lipid profiles (LDL, HDL, TG), HRV, and inflammatory biomarkers (e.g., C-reactive protein (CRP) and interleukin-6 (IL-6)).
- Study Design: Randomized controlled trials (RCTs), cohort studies, and longitudinal designs that isolate exercise intervention effects on cardiovascular outcomes.
- Note on Multi-Component Interventions:
- Exclusion Criteria:
- Non-T2D Populations: Studies focused on and/or only included non-T2D populations or pharmacological intervention without distinction of exercise effect [5].
- Pharmacological Interventions: This review will exclude any studies that combine exercise interventions with pharmacological medications (e.g., insulin or metformin) in which exercise is not examined separately, as it focuses solely on the effects of exercise on cardiovascular outcomes [6].
- Duration of Intervention: Interventions lasting fewer than eight weeks were excluded, as this duration is generally insufficient to produce meaningful cardiovascular or metabolic changes.
- Language and Publication Type: Only peer-reviewed, English-language publications were included to enhance methodological rigor and ensure consistency in quality assessment. While this approach reduces heterogeneity in study design and reporting, we acknowledge that the exclusion of non-English studies and gray literature may introduce language and publication bias.
Consideration of Medication Use
2.5. Data Sources and Search Strategy
- “Type 2 Diabetes” AND “Cardiovascular Outcomes”;
- “Aerobic Exercise” OR “Resistance Training” OR “Combined Training”;
- “Blood Pressure” AND “Lipid Profiles” AND “Heart Rate Variability”;
- “Inflammation” AND “C-reactive Protein” AND “IL-6”.
2.6. Study Selection Process
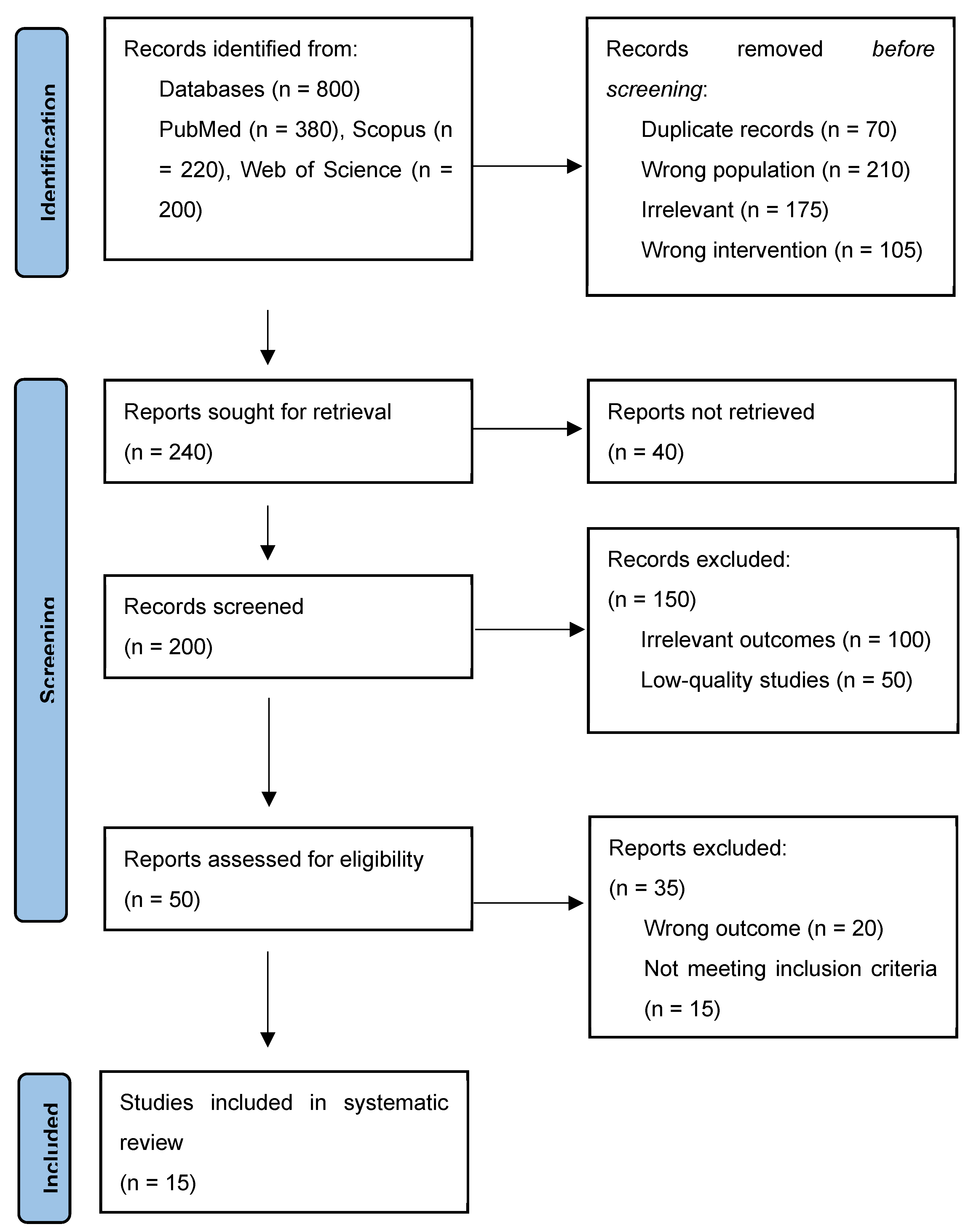
- Title and Abstract Screening: The titles and abstracts of 800 studies were initially reviewed to eliminate duplicates and irrelevant studies. Studies unrelated to cardiovascular outcomes or not involving exercise interventions were removed at this stage.
- Full-Text Review: 200 articles were reviewed in detail to assess their adherence to inclusion criteria. Only studies that met the specified intervention, population, and outcome requirements were included.
- Eligible Articles: For the final analysis, 15 studies with around 1794 subjects each were included. The above-mentioned studies included a wide range of exercise protocols and targeted several cardiovascular disease-mediated endpoints related to the management of T2D (see Figure 1).
2.7. Data Extraction and Management
- Study Information: The authors, year of publication, and journal information are provided as input.
- Demographic characteristics: Details regarding the age, gender, and duration of T2D among participants were extracted.
- Intervention characteristics: The type of exercise intervention, including intensity, frequency, and duration, were recorded.
- Cardiovascular Outcomes: The cardiovascular outcomes examined (e.g., blood pressure, cholesterol levels, and HRV) were recorded.
2.8. Quality Assessment
- Randomization: Whether participants were randomly assigned to different exercise groups [15].
- Blinding: Whether the outcome assessors were blinded to group assignments.
- Incomplete Data: How the study managed missing or incomplete data, such as participant dropouts.
- Selective Reporting: Whether the study reported all expected outcomes or only favorable results.
2.9. Data Synthesis
| No. | Study (Author, Year) | First Author | Total Participants | Average Age | Gender Distribution | Intervention Type | Frequency (per Week) | Duration (Weeks) | Intensity | Primary Outcomes | Secondary Outcomes | Statistical Measures (Mean, SD, Significance) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cassidy et al., 2019 [17] | Cassidy, Sophia—Houghton, David | 22.0 | 60 ± 2 years | 17 males | HIIT (3 sessions per week) vs. control with standard care | 3 | 12.0 | High-intensity | Glycemic Control (HbA1c): Significant improvement in the HIIT group (7.13% to 6.87%, p = 0.03); HRV: No significant changes; Blood Pressure Variability: Some variations but not statistically significant | Negative correlation between HbA1c and BRS | HbA1c improvement: Mean change, p = 0.03 |
| 2 | Byrkjeland et al., 2017 [18] | Byrkjelan, Rune | 137.0 | 63.1 ± 7.9 years | Predominantly Caucasian | Exercise Program: 12-month combined aerobic and resistance training (150 min/week); Control Group: Standard follow-up | 3 (150 min total) | 52.0 | Moderate | Glycemic Control (HbA1c): Significant decrease in HbA1c in the exercise group, correlated with reductions in endothelial biomarkers (E-selectin, ICAM-1, VCAM-1) | Endothelial Function: No direct improvement, but reduced endothelial activation correlated with HbA1c improvements | HbA1c and endothelial markers: Correlation observed, exact values not specified |
| 3 | Karjalainen et al., 2015 [19] | Karjalainen, Jaana | 1.046 | Not specified | Not specified | LTPA and Home-Based Exercise Training: 2-year controlled trial including endurance and strength training | Varied (No LTPA, irregular LTPA, LTPA > 3 times weekly) | 104.0 | Varied (endurance and strength) | Cardiovascular Events: Minor improvement in exercise capacity (0.2 ± 0.8 MET for T2D); Blood Pressure: Reduced systolic and diastolic pressure in CAD patients with T2D; Glycemic Control (HbA1c): Slight, non-significant reduction | Composite End Points: LTPA reduced CV morbidity and mortality; controlled exercise training showed no significant effect on CV risk factors | Exercise capacity: Mean ± SD; HbA1c reduction: Not significant |
| 4 | Verma et al., 2018 [20] | Verma, Shalini | 60.0 | Not specified | Not specified | Low-intensity, low-volume; Low-intensity, high-volume; High-intensity, high-volume | Varied: 3–5 sessions per week | 12.0 | Varied: Low to High Intensity | Primary outcomes include maximal oxygen consumption (VO2max), oxidative stress markers (SOD, CAT, GPX, NO), and cardiac autonomic function (HRR, HRV) | Secondary outcomes include body composition, lipid profiles, glycemic control (HbA1c, fasting glucose) | ANOVA (split-plot), Bonferroni post hoc, significance level set at p < 0.05 |
| 5 | Rech et al., 2019 [21] | Rech, Anderson | 39.0 | 68 ± 6.5 years (AC); 70.5 ± 7.4 years (RT) | Not specified | RT Group: 12-week, 3×/week resistance training with functional and traditional exercises; AC Group: Low-intensity stretching exercises once per week | RT: 3; AC: 1 | 12.0 | RT: Resistance exercises; AC: Low-intensity stretching | Inflammatory Markers: TNF-α and IL-1β decreased significantly in both groups; No changes in CRP, IL-6, or IL-10 | Endothelial Function: No significant improvements in FMD or BAD; Lipid Profile: No significant changes in triglycerides, HDL, LDL, or total cholesterol; Glycemic Control: No significant improvement in HbA1c and fasting glycemia | Inflammatory markers: Significant decrease in TNF-α and IL-1β; Lipid profile and glycemic control: No significant changes |
| 6 | Ghardashi et al., 2020 [22] | Ghardashi, Afousi | 74.0 | 45–60 years | Not specified | HIIT: Six intervals of 4 min at 85–90% HRmax, with 3 min at 45–50% HRmax, 3×/week for 12 weeks; Control group with no intervention | 3 | 12.0 | HIIT: High-intensity (85–90% HRmax) | Carotid Intima-Media Thickness (cIMT): Significant reduction in HIIT group (from 0.83 ± 0.17 to 0.71 ± 0.14 mm); Serum Markers (Dkk-1, Sclerostin): Both decreased significantly, correlating with cIMT reduction | Cardiorespiratory Fitness (VO2 Peak): Increased by 6.2 mL/kg/min in HIIT, with inverse correlation with cIMT, Dkk-1, and sclerostin levels | cIMT, VO2 Peak, Dkk-1, Sclerostin: Mean ± SD; Correlations with cIMT noted |
| 7 | Seyedizadeh et al., 2020 [23] | Seyedizadeh, Seyedeh Hoda | 24.0 | 45–65 years | Female only | Combined Training: Resistance and aerobic exercises, 3×/week for 8 weeks; Resistance Training: 2–3 sets, 8–12 reps with progressive load; Aerobic Training: Interval running at 50–65% HRR, progressively increased in duration and intensity | 3 | 8.0 | Resistance: Progressive load; Aerobic: 50–65% HRR | Serum Kinesin-1: No significant change in either group; Aerobic Endurance: Slight decline in both groups, greater decrease in control | Lower Body Strength: Significant improvement in experimental group compared to decline in control group | Aerobic endurance and lower body strength: Relative changes observed; significance noted in strength improvements |
| 8 | Li et al., 2022 [24] | Li, Jun | 37.0 | 32–47 years | Not specified | HIIT: 10 rounds of 1 min power cycling at 80–95% VO2max, 1 min rest at 25–30% VO2max, 5×/week; MICT: Continuous cycling at 50–70% VO2max for 30 min, 5×/week; Control: Standard counseling on exercise and diet | 5 | 12.0 | HIIT: High-intensity (80–95% VO2max); MICT: Moderate-intensity (50–70% VO2max) | Body Composition: Weight reduction significant in MICT (−3.52 kg); BMI reduction in MICT (from 26.75 ± 4.20 to 25.45 ± 3.51 kg/m²); Cardiorespiratory Fitness: VO2max increased in HIIT (0.53 L/min) and MICT (0.31 L/min) | Glycemic Control: FBG and HbA1c reduced in HIIT and MICT; Fasting Insulin: Reduced in HIIT (−2.39 pmol/L) and MICT (−0.99 pmol/L) | Weight, BMI, VO2max, FBG, HbA1c, and FI: Mean ± SD, significant reductions in MICT and HIIT |
| 9 | Otten et al., 2019 [25] | Otten, Julia | 22.0 | 30–70 years | Not specified | Both groups followed a Paleolithic diet for 12 weeks; PD-EX group engaged in 3 h of supervised exercise/week, combining aerobic and resistance training | 3 h of exercise for PD-EX | 12.0 | Aerobic and resistance training for PD-EX | Cardiac and Metabolic Measures: MTG reduced by 45% in PD-EX; Improvements in LV mass-to-volume ratio and end-diastolic volume in PD-EX; Significant increase in VO2max in PD-EX | Weight Loss and HbA1c: Significant reductions in both groups, with more pronounced changes in PD-EX | MTG, LV remodeling, VO2max, weight loss, and HbA1c: Mean ± SD; significant changes noted in PD-EX group |
| 10 | Naylor et al., 2016 [26] | Naylor, Louise | 13.0 | 13–21 years | 10 female, 3 male | Exercise Protocol: Personalized gym-based sessions, 3×/week for 12 weeks; Combined aerobic (65–85% HRmax) and resistance (55–70% MVC) training; Control Group: Usual activity levels without additional training | 3 | 12.0 | Aerobic: 65–85% HRmax; Resistance: 55–70% MVC | Vascular Health: FMD increased in the exercise group (+2.2%, p < 0.05); Microvascular function improved in exercise group; Body Composition: Fat mass decreased by 1.10 kg in exercise group; Lean mass increased by 1.35 kg in exercise group | Strength: Total strength increased significantly in exercise group; Glycemic Control: No significant changes in insulin sensitivity | FMD, fat mass, lean mass, and strength: Mean ± SD; significance noted for FMD and strength increases |
| 11 | MacDonald et al., 2020 [27] | MacDonald, Christopher | 98.0 | 54.4 ± 9.0 years | Not specified | U-TURN group divided into tertiles by accumulated exercise volume (12 months); Lower Tertile: 178 min/week; Intermediate Tertile: 296 min/week; Upper Tertile: 380 min/week; Standard Care: Lifestyle advice without structured exercise | Lower: 178; Intermediate: 296; Upper: 380 | 52.0 | Varied by tertile | Medication Discontinuation: 48% (lower), 60% (intermediate), 70% (upper) discontinued meds; 16% in standard care group | Glycemic Control: HbA1c reduction in intermediate and upper tertiles; Fasting glucose and insulin improved in both tertiles; Body Composition: Reduction in body mass in intermediate and upper tertiles; VO2max increased significantly in upper tertile only | Medication discontinuation and glycemic control measures: Percentages noted; significant reductions in body mass and increases in VO2max |
| 12 | Amaravadi et al., 2024 [28] | Amaravadi, Sampath Kumar | 160.0 | 30–65 years | Not specified | Structured exercise regimen, including aerobic, resistance, and combined exercises, 3–5 times weekly for 12 weeks; Conducted at a diabetic clinic with ongoing monitoring and adherence checks | 3–5 | 12.0 | Aerobic and resistance exercises | Insulin Resistance (HOMA-IR): 30.06% improvement in exercise group vs. 14.9% increase in control; Fasting Insulin: 24.34% reduction in exercise group vs. 8.11% increase in control | Glycemic Control: FBS decreased by 14.24% and PPBS by 12.66% in exercise group; HbA1c reduced by 0.55 points; Quality of Life: Significant improvements in WHOQOL-BREF across all domains; Functional Capacity: 27.43% improvement in Six-Minute Walk Test in exercise group | Insulin resistance, fasting insulin, glycemic control, and quality of life measures: Mean ± SD; significance noted for improvements |
| 13 | Kluding et al., 2015 [29] | Kluding, Patricia | 18.0 | 58.1 years (SD = 5) | 13 females, 5 males | 16-week supervised aerobic exercise program, 3×/week; Sessions: 30–50 min, progressing from 50% to 70% VO2R | 3 | 16.0 | 50% to 70% VO2R | Adverse Events: 57 non-serious events reported, mainly in initial weeks (joint pain, hypoglycemia, hyperglycemia); Cardiovascular Fitness (VO2peak): Increased by 1.1 mL/kg/min (p < 0.05) | Body Composition: 1% reduction in total body fat (p < 0.01) and 1780 g fat mass reduction; Peripheral Blood Flow: Improved by 2.27%; Fatigue: Significant reduction in general and physical fatigue scores | VO2peak, body fat, and fatigue scores: Mean ± SD; significance noted for improvements |
| 14 | Taylor et al., 2014 [30] | Taylor, J. David | 21.0 | MOD: 52.2 years; HIGH: 54.4 years | Not specified | MOD Group: Resistance at 75% of 8-RM, aerobic at 30–45% HRR; HIGH Group: Resistance at 100% of 8-RM, aerobic at 50–65% HRR; Both groups trained for 3 months, resistance exercises twice weekly, aerobic three times weekly | Resistance: 2; Aerobic: 3 | 12.0 | MOD: 75% 8-RM, 30–45% HRR; HIGH: 100% 8-RM, 50–65% HRR | Physical Fitness: Muscle strength improved in both groups, no significant difference; Exercise capacity and physical function improved in both groups without significant differences | Glycemic Control: Blood glucose decreased immediately and 1 h after exercise; MOD: 204.5 to 172.0 mg/dL; HIGH: 140.0 to 118.5 mg/dL | Muscle strength, exercise capacity, and blood glucose measures: Mean ± SD; significance noted in blood glucose reductions |
| 15 | Cassidy et al., 2016 [31] | Cassidy, Sophia—Thoma Christian | 23.0 | Around 60 years | Not specified | HIIT: 12-week cycling-based high-intensity interval training, 3x/week, intervals at 80–90% HRmax; Control: Standard care without structured exercise | 3 | 12.0 | 80–90% heart rate max | Cardiac Function: LV Wall Mass increased in HIIT (104 ± 17 g to 116 ± 20 g); Stroke volume increased from 76 ± 16 mL to 87 ± 19 mL; Early diastolic filling rate improved in HIIT; Liver Fat: 39% relative reduction in liver fat, correlated with improvements in HbA1c | Glycemic Control: HbA1c reduced from 7.1% to 6.8% in HIIT; No improvement in control group; Other glycemic markers: No significant changes in fasting glucose or insulin sensitivity | Cardiac and liver fat measures: Mean ± SD; significance noted for LV mass and stroke volume changes |
2.10. Ethical Considerations
| Study (Author, Year) | Randomization | Blinding | Incomplete Data | Selective Reporting | Overall Risk |
|---|---|---|---|---|---|
| Cassidy et al., 2019 [17] | Low | High | Low | Low | Moderate |
| Byrkjeland et al., 2017 [18] | Low | High | Low | Low | Low |
| Karjalainen et al., 2015 [19] | Low | High | Low | Low | Moderate |
| Verma et al., 2018 [20] | Low | High | Low | Low | High |
| Rech et al., 2019 [21] | Low | High | Low | Low | Moderate |
| Ghardashi et al., 2020 [22] | Low | Unclear | Low | Low | Moderate |
| Seyedizadeh et al., 2020 [23] | Low | Unclear | Low | Low | Moderate |
| Li et al., 2022 [24] | Low | Unclear | Low | Low | Moderate |
| Otten et al., 2019 [25] | Low | Unclear | Low | Low | Low |
| Naylor et al., 2016 [26] | Low | High | Low | Low | Moderate |
| MacDonald et al., 2020 [27] | Low | High | High | Low | Low |
| Amaravadi et al., 2024 [28] | Low | High | Low | Low | Low |
| Kluding et al., 2015 [29] | Unclear | Unclear | Low | Low | Moderate |
| Taylor et al., 2014 [30] | Low | High | Unclear | Low | Moderate |
| Cassidy et al., 2016 [31] | Low | High | Low | Low | Low |
| Study (Author, Year) | Ethical Approval | Informed Consent |
|---|---|---|
| Cassidy et al., 2019 [17] | Yes | Yes |
| Byrkjeland et al., 2017 [18] | Yes | Yes |
| Karjalainen et al., 2015 [19] | Yes | Yes |
| Verma et al., 2018 [20] | Yes | Yes |
| Rech et al., 2019 [21] | Yes | Yes |
| Ghardashi et al., 2020 [22] | Yes | Yes |
| Seyedizadeh et al., 2020 [23] | Yes | Yes |
| Li et al., 2022 [24] | Yes | Yes |
| Otten et al., 2019 [25] | Yes | Yes |
| Naylor et al., 2016 [26] | Yes | Yes |
| MacDonald et al., 2020 [27] | Yes | Yes |
| Amaravadi et al., 2024 [28] | Yes | Yes |
| Kluding et al., 2015 [29] | Yes | Yes |
| Taylor et al., 2014 [30] | Yes | Yes |
| Cassidy et al., 2016 [31] | Yes | Yes |
3. Results
3.1. Search Strategy and Study Selection
3.2. Study Characteristics and Intervention Protocols
- Resistance Training: Training protocols were designed to improve muscle strength and insulin sensitivity. They typically involve 2–4 sets of 8–12 repetitions performed 2–3 times weekly. Exercises target large muscle groups to enhance vascular health and glucose metabolism [20,21,23,24,25,26,27,28,31].
- Combined Training: Protocols integrate aerobic and resistance components within the same intervention. These programs vary, with some studies combining both modalities in a single session and others alternating between them. Combined training was particularly effective in simultaneously addressing multiple cardiovascular and metabolic risk factors [18,24,25,27].
3.3. Cardiovascular Outcomes by Exercise Modality
3.3.1. Aerobic Training
- Blood Pressure: Aerobic exercise reduced blood pressure, with an average decrease of 6 mmHg systolic and 3 mmHg diastolic. Systolic pressure reduction ranged from 4 to 8 mmHg, while diastolic decreases ranged from 2 to 5 mmHg [19,26]. These results can be explained by improved endothelial function and reduced arterial stiffness.
- Lipid Profiles: LDL cholesterol decreased by 8%, while HDL cholesterol increased by 5%. Study sub-analyses also showed a 6% reduction in TG [30]. Aerobic exercise enhances lipid metabolism in T2D patients by increasing lipid oxidation and lipoprotein lipase activity, facilitating transport and hydrolysis of lipids.
- Inflammatory Markers: CRP and IL-6 dropped 5–7%, indicating that aerobic exercises have anti-inflammatory effects. This may result from improved insulin sensitivity, reduced adiposity, and better lipid profiles, which decrease chronic low-grade inflammation tied to T2D-associated cardiovascular disease [22].
3.3.2. Resistance Training
- Blood Pressure: Resistance training reduced systolic and diastolic blood pressure by 5 mmHg and 3 mmHg [21], respectively. While these reductions were slightly smaller than those achieved with aerobic training, they were clinically meaningful.
- Lipid Profiles: Resistance training increased HDL cholesterol by 6%, with minor decreases in TG levels. These changes highlight the potential of resistance exercise to improve lipid metabolism [28].
- Muscle Mass and Insulin Sensitivity: Participants showed a 2 kg rise in lean muscle mass, which improved glucose uptake by muscle tissue. The improved insulin sensitivity lowers hyperglycemia and cardiovascular risk by reducing oxidative stress and inflammation [25].
- Inflammatory Markers: An average study of resistance training found that five studies showed a 5% fall in CRP levels, with changes in IL-6 being variable. This suggests a modest anti-inflammatory effect to resistance training and raises the hope that it could reduce atherosclerosis development in T2D [21].
3.3.3. Combined Training
- Blood Pressure: Combining training methods yielded the most significant reductions—9 mmHg systolic and 6 mmHg diastolic—showing the benefits of merging aerobic and resistance training exercises [18].
- Lipid Profiles: Combined training reduced LDL cholesterol by an average of 10% and increased HDL cholesterol by 8%. These changes far exceed those from aerobic or strength training alone, suggesting a synergistic effect. Combined exercise may influence lipid metabolism by altering enzymes and increasing mitochondrial function mass [24].
- HRV: Improvements (15%) surpassed those seen with aerobic or resistance training, emphasizing better autonomic function regulation [26].
- Inflammatory Markers: Combined training had the largest effect on inflammation markers: CRP and IL-6 decreased by around 10%. This result underscores the potential of combined training to mitigate chronic inflammation associated with atherosclerosis and other cardiovascular complications in T2D [27].
Overall Comparison of Modalities
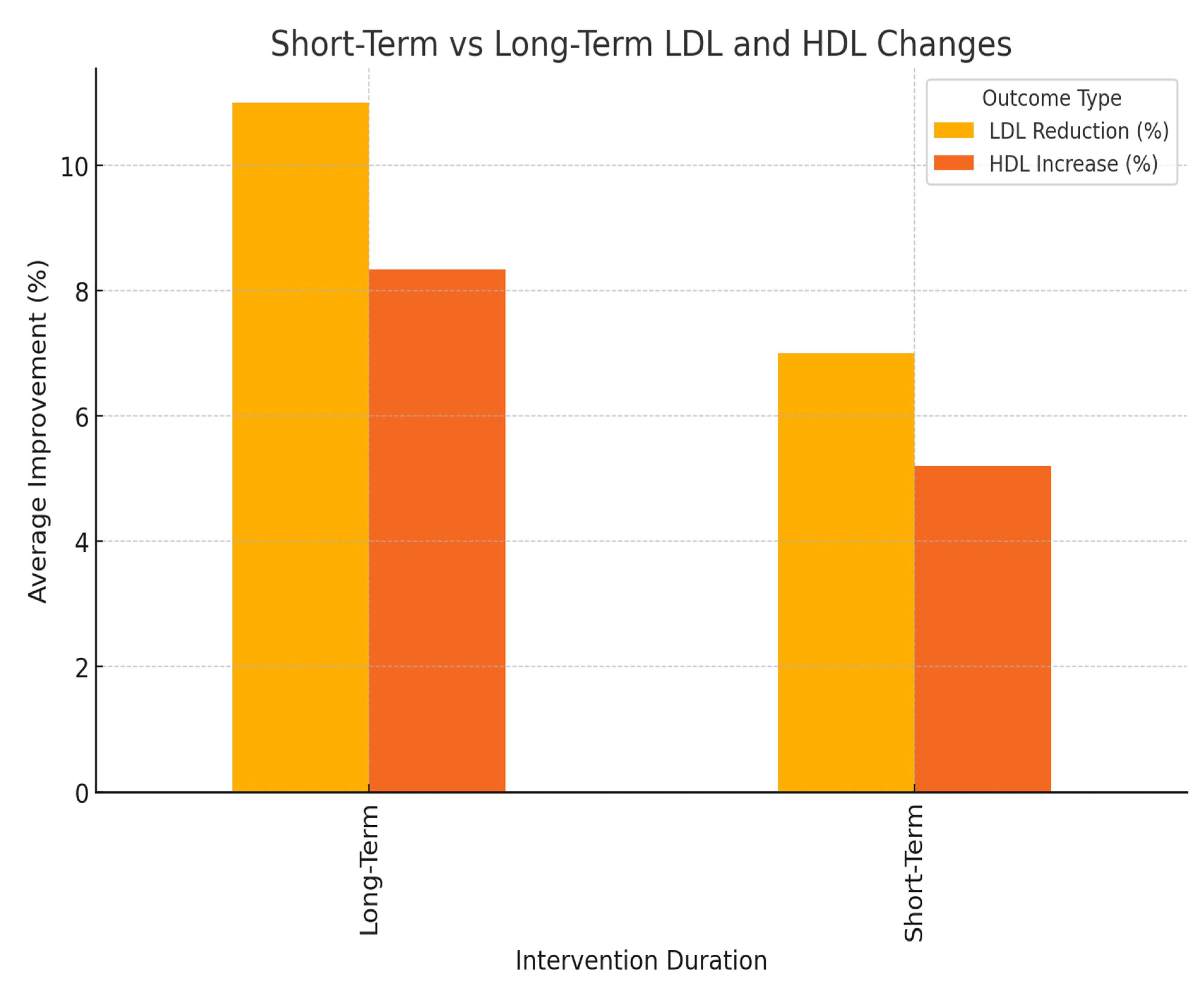
3.4. Short-Term vs. Long-Term Effects
- Lipid Profiles: Longer interventions led to greater reductions in LDL levels and higher increases in HDL cholesterol levels.
4. Summary of Findings
5. Discussion
5.1. Overview of Finding
5.2. Aerobic Training and Cardiovascular Outcomes
5.3. Resistance Training and Cardiovascular Benefits
5.4. Combined Training as a Superior Modality
5.5. Practical Considerations and Real-World Applicability
5.6. Clinical Implications and Recommendations
5.7. Behavioral Strategies and Sustainability
5.8. Future Directions
6. Conclusions
Funding
Conflicts of Interest
References
- Cantley, J.; Ashcroft, F.M. Q&A: Insulin secretion and type 2 diabetes: Why do β-cells fail? BMC Biol. 2015, 13, 33. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157. [Google Scholar] [CrossRef] [PubMed]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Available online: https://www.eurekaselect.com/article/97859 (accessed on 22 April 2025).
- Chakraborty, S.; Verma, A.; Garg, R.; Singh, J.; Verma, H. Cardiometabolic Risk Factors Associated With Type 2 Diabetes Mellitus: A Mechanistic Insight. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231220780. [Google Scholar] [CrossRef]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabolism 2014, 63, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147. [Google Scholar] [CrossRef]
- Dugan, J.A. Exercise recommendations for patients with type 2 diabetes. J. Am. Acad. Physician Assist. 2016, 29, 13–18. [Google Scholar] [CrossRef]
- Yavari, A.; Najafipoor, F.; Aliasgharzadeh, A.; Niafar, M.; Mobasseri, M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biol. Sport 2012, 29, 135–143. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C.; White, R.D. Physical Activity/Exercise and Type 2 Diabetes. Diabetes Care 2006, 29, 1433–1438. [Google Scholar] [CrossRef]
- Church, T.S.; Blair, S.N.; Cocreham, S.; Johannsen, N.; Johnson, W.; Kramer, K.; Mikus, C.R.; Myers, V.; Nauta, M.; Rodarte, R.Q.; et al. Effects of Aerobic and Resistance Training on Hemoglobin A1c Levels in Patients With Type 2 Diabetes: A Randomized Controlled Trial. JAMA 2010, 304, 2253. [Google Scholar] [CrossRef]
- Moore, M.N.; Climie, R.E.; Otahal, P.; Schultz, M.G. Exercise blood pressure and cardiovascular disease risk: A systematic review and meta-analysis of cross-sectional studies. J. Hypertens. 2021, 39, 2395–2402. [Google Scholar] [CrossRef]
- Zanuso, S.; Jimenez, A.; Pugliese, G.; Corigliano, G.; Balducci, S. Exercise for the management of type 2 diabetes: A review of the evidence. Acta Diabetol. 2010, 47, 15–22. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; López-López, J.A.; Becker, B.J.; Davies, S.R.; Dawson, S.; Grimshaw, J.M.; McGuinness, L.A.; Moore, T.H.M.; Rehfuess, E.A.; Thomas, J.; et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob. Health 2019, 4, e000858. [Google Scholar] [CrossRef]
- Xiao, M.-X.; Lu, C.-H.; Ta, N.; Wei, H.-C.; Haryadi, B.; Wu, H.-T. Machine learning prediction of future peripheral neuropathy in type 2 diabetics with percussion entropy and body mass indices. Biocybern. Biomed. Eng. 2021, 41, 1140–1149. [Google Scholar] [CrossRef]
- Cassidy, S.; Houghton, D.; Zalewski, P.; Seferovic, J.P.; Hallsworth, K.; MacGowan, G.A.; Trenell, M.I.; Jakovljevic, D.G. Unsupervised high-intensity interval training improves glycaemic control but not cardiovascular autonomic function in type 2 diabetes patients: A randomised controlled trial. Diab. Vasc. Dis. Res. 2019, 16, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Byrkjeland, R.; Njerve, I.U.; Arnesen, H.; Seljeflot, I.; Solheim, S. Reduced endothelial activation after exercise is associated with improved HbA1c in patients with type 2 diabetes and coronary artery disease. Diab. Vasc. Dis. Res. 2017, 14, 94–103. [Google Scholar] [CrossRef]
- Karjalainen, J.J.; Kiviniemi, A.M.; Hautala, A.J.; Piira, O.-P.; Lepojärvi, E.S.; Perkiömäki, J.S.; Junttila, M.J.; Huikuri, H.V.; Tulppo, M.P. Effects of Physical Activity and Exercise Training on Cardiovascular Risk in Coronary Artery Disease Patients with and Without Type 2 Diabetes. Diabetes Care 2015, 38, 706–715. [Google Scholar] [CrossRef]
- Verma, S.; Moiz, J.A.; Anwer, S.; Alghadir, A.H.; Hussain, M.E. A dose-response study of aerobic training for oxygen uptake, oxidative stress and cardiac autonomic function in type 2 diabetes mellitus: Study protocol for a randomized controlled trial. Trials 2018, 19, 289. [Google Scholar] [CrossRef]
- Rech, A.; Botton, C.E.; Lopez, P.; Quincozes-Santos, A.; Umpierre, D.; Pinto, R.S. Effects of short-term resistance training on endothelial function and inflammation markers in elderly patients with type 2 diabetes: A randomized controlled trial. Exp. Gerontol. 2019, 118, 19–25. [Google Scholar] [CrossRef]
- Ghardashi-Afousi, A.; Davoodi, M.; Hesamabadi, B.K.; Asvadi-Fard, M.; Bigi, M.A.B.; Izadi, M.R.; Gaeini, A.A. Improved carotid intima-media thickness-induced high-intensity interval training associated with decreased serum levels of Dkk-1 and sclerostin in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107469. [Google Scholar] [CrossRef]
- Seyedizadeh, S.H.; Cheragh-Birjandi, S.; Hamedi Nia, M.R. The Effects of Combined Exercise Training (Resistance-Aerobic) on Serum Kinesin and Physical Function in Type 2 Diabetes Patients with Diabetic Peripheral Neuropathy (Randomized Controlled Trials). J. Diabetes Res. 2020, 2020, 6978128. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W.; Ma, H. A Comparative Study of Health Efficacy Indicators in Subjects with T2DM Applying Power Cycling to 12 Weeks of Low-Volume High-Intensity Interval Training and Moderate-Intensity Continuous Training. J. Diabetes Res. 2022, 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.; Andersson, J.; Ståhl, J.; Stomby, A.; Saleh, A.; Waling, M.; Ryberg, M.; Hauksson, J.; Svensson, M.; Johansson, B.; et al. Exercise Training Adds Cardiometabolic Benefits of a Paleolithic Diet in Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2019, 8, e010634. [Google Scholar] [CrossRef] [PubMed]
- Naylor, L.H.; Davis, E.A.; Kalic, R.J.; Paramalingam, N.; Abraham, M.B.; Jones, T.W.; Green, D.J. Exercise training improves vascular function in adolescents with type 2 diabetes. Physiol. Rep. 2016, 4, e12713. [Google Scholar] [CrossRef]
- MacDonald, C.S.; Johansen, M.Y.; Nielsen, S.M.; Christensen, R.; Hansen, K.B.; Langberg, H.; Vaag, A.A.; Karstoft, K.; Lieberman, D.E.; Pedersen, B.K.; et al. Dose-Response Effects of Exercise on Glucose-Lowering Medications for Type 2 Diabetes: A Secondary Analysis of a Randomized Clinical Trial. Mayo Clin. Proc. 2020, 95, 488–503. [Google Scholar] [CrossRef]
- Amaravadi, S.K.; Maiya, G.A.; Vaishali, K.; Shastry, B.A. Effectiveness of structured exercise program on insulin resistance and quality of life in type 2 diabetes mellitus–A randomized controlled trial. PLoS ONE 2024, 19, e0302831. [Google Scholar] [CrossRef]
- Kluding, P.M.; Pasnoor, M.; Singh, R.; D’Silva, L.J.; Yoo, M.; Billinger, S.A.; LeMaster, J.W.; Dimachkie, M.M.; Herbelin, L.; Wright, D.E. Safety of Aerobic Exercise in People With Diabetic Peripheral Neuropathy: Single-Group Clinical Trial. Phys. Ther. 2015, 95, 223–234. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fletcher, J.P.; Mathis, R.A.; Cade, W.T. Effects of Moderate- Versus High-Intensity Exercise Training on Physical Fitness and Physical Function in People With Type 2 Diabetes: A Randomized Clinical Trial. Phys. Ther. 2014, 94, 1720–1730. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Hallsworth, K.; Parikh, J.; Hollingsworth, K.G.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2016, 59, 56–66. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Ekechukwu, N.I.; Anwara, S.U.; Mgbeojedo, U.G.; Chijioke, O.U.; Onwukwe, O.S.; Ezugwu, U.A.; Ekechukwu, E.N.D.; Okoronkwo, I.L. Effects of Structured Aerobic Exercise on Selected Clinical Profiles of Patients with Type 2 Diabetes Mellitus: A Systematic Review with Meta-Analysis. Int. J. Med. Health Dev. 2021, 26, 17–30. [Google Scholar] [CrossRef]
- Hwang, M.-H.; Kim, S. Type 2 Diabetes: Endothelial dysfunction and Exercise. J. Exerc. Nutr. Biochem. 2014, 18, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Chen, S.; Durstine, J.L. The Effects of Exercise Training on the Traditional Lipid Profile and Beyond. Curr. Sports Med. Rep. 2014, 13, 253–259. [Google Scholar] [CrossRef]
- De Nardi, A.T.; Tolves, T.; Lenzi, T.L.; Signori, L.U.; Silva, A.M.V.D. High-intensity interval training versus continuous training on physiological and metabolic variables in prediabetes and type 2 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2018, 137, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, G.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Antonopoulos, C.; Tachmatzidis, D.; Didangelos, T.; Lambadiari, V.; Kadoglou, N.P.E. The anti-inflammatory effects of aerobic exercise training in patients with type 2 diabetes: A systematic review and meta-analysis. Cytokine 2023, 164, 156157. [Google Scholar] [CrossRef]
- Westcott, W.L. Resistance Training is Medicine: Effects of Strength Training on Health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Kim, C. Resistance Training for Glycemic Control, Muscular Strength, and Lean Body Mass in Old Type 2 Diabetic Patients: A Meta-Analysis. Diabetes Ther. 2017, 8, 459–473. [Google Scholar] [CrossRef]
- Qadir, R.; Sculthorpe, N.F.; Todd, T.; Brown, E.C. Effectiveness of Resistance Training and Associated Program Characteristics in Patients at Risk for Type 2 Diabetes: A Systematic Review and Meta-analysis. Sports Med.-Open 2021, 7, 38. [Google Scholar] [CrossRef]
- Schroeder, E.C.; Franke, W.D.; Sharp, R.L.; Lee, D. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: A randomized controlled trial. PLoS ONE 2019, 14, e0210292. [Google Scholar] [CrossRef]
- Magalhães, J.P.; Santos, D.A.; Correia, I.R.; Hetherington-Rauth, M.; Ribeiro, R.; Raposo, J.F.; Matos, A.; Bicho, M.D.; Sardinha, L.B. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: A secondary analysis from a 1-year randomized controlled trial. Cardiovasc. Diabetol. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009, 3, CD002759. [Google Scholar] [CrossRef] [PubMed]
- Tavoian, D.; Russ, D.W.; Consitt, L.A.; Clark, B.C. Perspective: Pragmatic Exercise Recommendations for Older Adults: The Case for Emphasizing Resistance Training. Front. Physiol. 2020, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Jimenez, A. Differential Effects of Aerobic Exercise, Resistance Training and Combined Exercise Modalities on Cholesterol and the Lipid Profile: Review, Synthesis and Recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, b1665. [Google Scholar] [CrossRef]
- Revello, K.; Fields, W. An Educational Intervention to Increase Nurse Adherence in Eliciting Patient Daily Goals. Rehabil. Nurs. 2015, 40, 320–326. [Google Scholar] [CrossRef]
- Luhanga, E.T.; Hippocrate, A.A.E.; Suwa, H.; Arakawa, Y.; Yasumoto, K. Identifying and Evaluating User Requirements for Smartphone Group Fitness Applications. IEEE Access 2018, 6, 3256–3269. [Google Scholar] [CrossRef]
- Marrero, D.G. Time to Get Moving: Helping Patients With Diabetes Adopt Exercise as Part of a Healthy Lifestyle. Clin. Diabetes 2005, 23, 154–159. [Google Scholar] [CrossRef][Green Version]
- Shan, R.; Sarkar, S.; Martin, S.S. Digital health technology and mobile devices for the management of diabetes mellitus: State of the art. Diabetologia 2019, 62, 877–887. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Donato, K.; Kaftalli, J.; Herbst, K.L.; Stuppia, L.; Fioretti, F.; Nodari, S.; Perrone, M.; Chiurazzi, P.; et al. Polymorphisms, diet and nutrigenomics. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E125–E141. [Google Scholar] [CrossRef]
- Perrone, M.A.; Donatucci, B.; Salvati, A.; Gualtieri, P.; De Lorenzo, A.; Romeo, F.; Bernardini, S. Inflammation, oxidative stress and gene expression: The postprandial approach in professional soccer players to reduce the risk of muscle injuries and early atherosclerosis. Med. Sport 2019, 72, 234–243. [Google Scholar] [CrossRef]

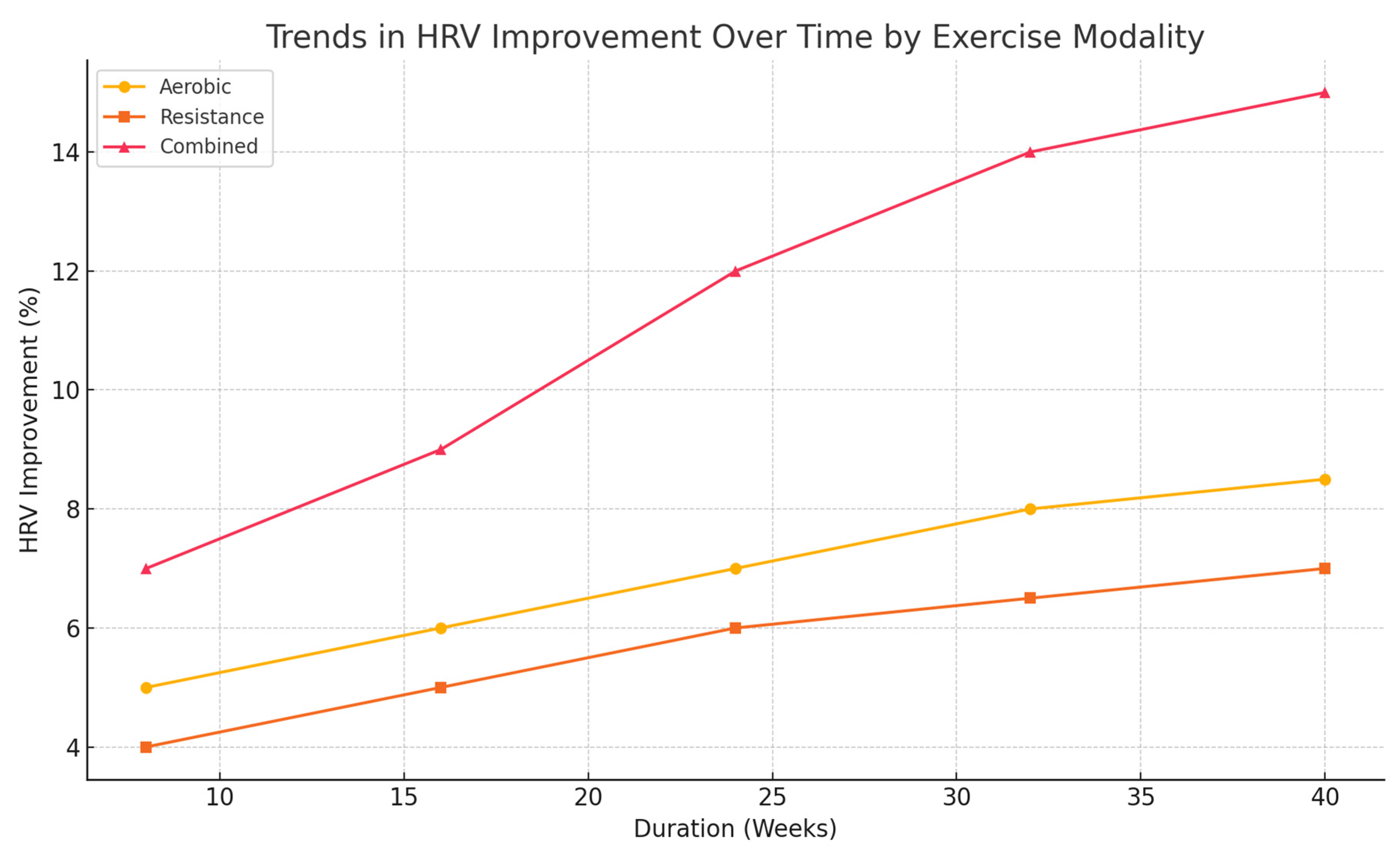
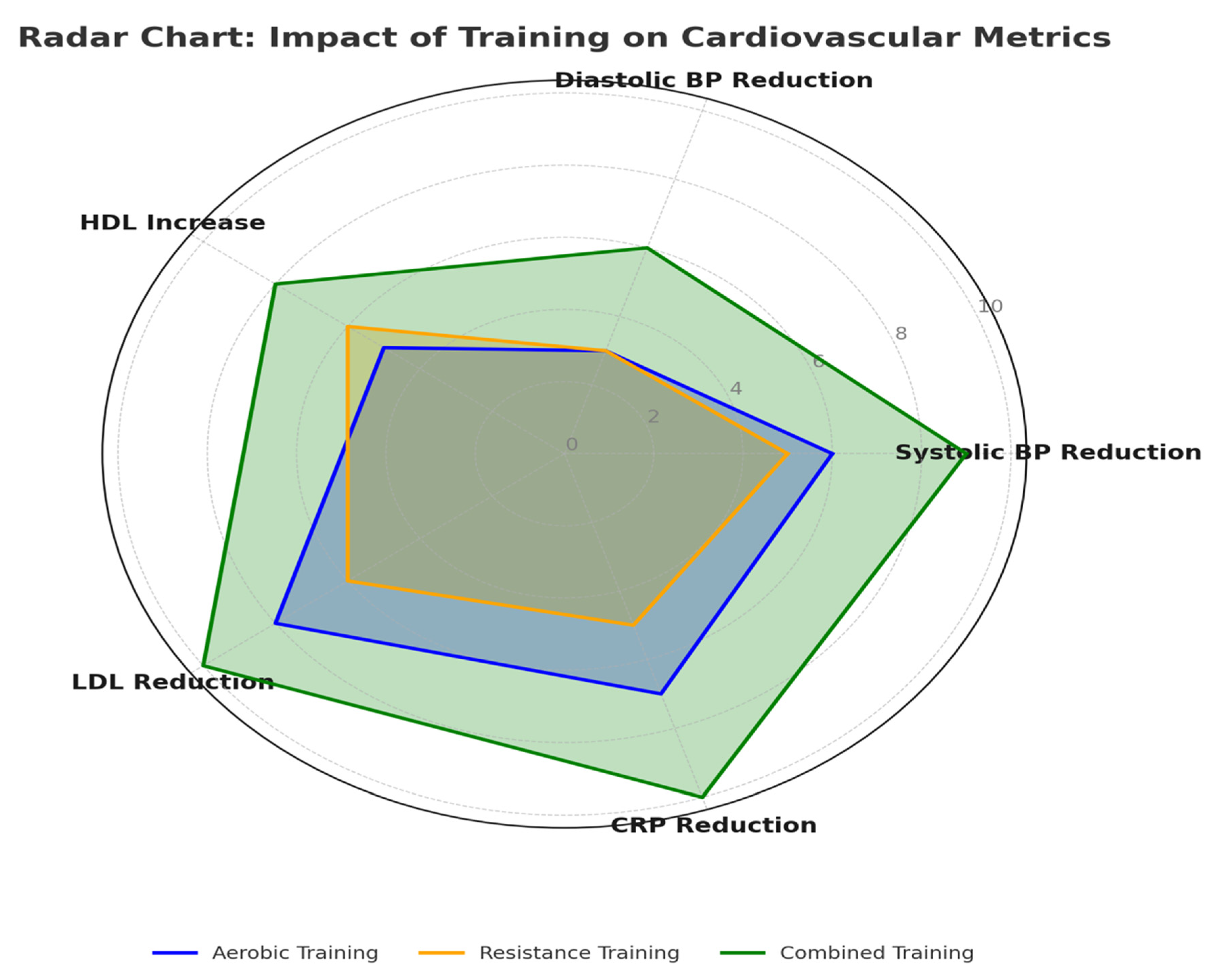
| Exercise Modality | Systolic BP Reduction (mmHg) | Diastolic BP Reduction (mmHg) | LDL Reduction (%) | HDL Increase (%) | TG Reduction (%) | HRV Improvement (%) | CRP Reduction (%) | IL-6 Reduction (%) |
|---|---|---|---|---|---|---|---|---|
| Aerobic | 6 | 3 | 8 | 5 | 6 | 8 | 7 | 6 |
| Resistance | 5 | 3 | 6 | 6 | 5 | 6 | 5 | 4 |
| Combined | 9 | 6 | 10 | 8 | 10 | 15 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi Zadeh, S.A.; Caminiti, G.; Aracri, M.; Pieri, M.; Mitterhofer, A.P.; De Lorenzo, A.; Bernardini, S.; Farsetti, P.; Volterrani, M.; Barone, R.; et al. Comparative Analysis of Cardiovascular Outcomes in Type 2 Diabetes Patients Engaging in Aerobic, Resistance, and Combined Training: A Systematic Review. Diabetology 2025, 6, 38. https://doi.org/10.3390/diabetology6050038
Mousavi Zadeh SA, Caminiti G, Aracri M, Pieri M, Mitterhofer AP, De Lorenzo A, Bernardini S, Farsetti P, Volterrani M, Barone R, et al. Comparative Analysis of Cardiovascular Outcomes in Type 2 Diabetes Patients Engaging in Aerobic, Resistance, and Combined Training: A Systematic Review. Diabetology. 2025; 6(5):38. https://doi.org/10.3390/diabetology6050038
Chicago/Turabian StyleMousavi Zadeh, Sayed Alireza, Giuseppe Caminiti, Maurizio Aracri, Massimo Pieri, Anna Paola Mitterhofer, Antonino De Lorenzo, Sergio Bernardini, Pasquale Farsetti, Maurizio Volterrani, Rosario Barone, and et al. 2025. "Comparative Analysis of Cardiovascular Outcomes in Type 2 Diabetes Patients Engaging in Aerobic, Resistance, and Combined Training: A Systematic Review" Diabetology 6, no. 5: 38. https://doi.org/10.3390/diabetology6050038
APA StyleMousavi Zadeh, S. A., Caminiti, G., Aracri, M., Pieri, M., Mitterhofer, A. P., De Lorenzo, A., Bernardini, S., Farsetti, P., Volterrani, M., Barone, R., Iellamo, F., & Perrone, M. A. (2025). Comparative Analysis of Cardiovascular Outcomes in Type 2 Diabetes Patients Engaging in Aerobic, Resistance, and Combined Training: A Systematic Review. Diabetology, 6(5), 38. https://doi.org/10.3390/diabetology6050038














