The Symptom Burden of Autonomic Neuropathy Is Associated with Decreased Quality of Life in 6961 People with Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Source
2.2. Survey Information
2.3. Statistical Analysis
3. Results
3.1. Response Rate
3.2. Clinical Characteristics
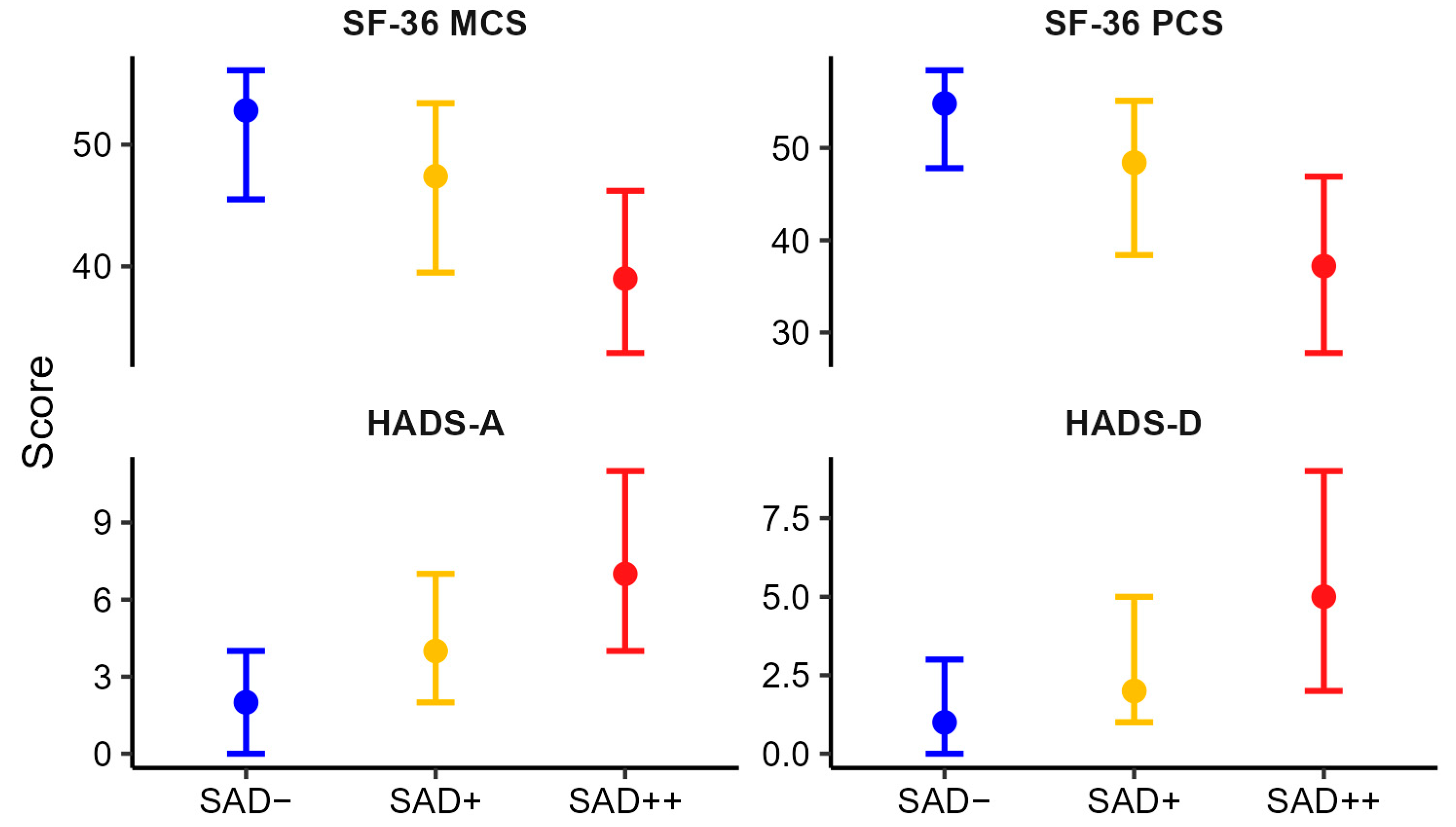
3.3. Association Between Levels of Autonomic Dysfunction and General and Psychological Well-Being
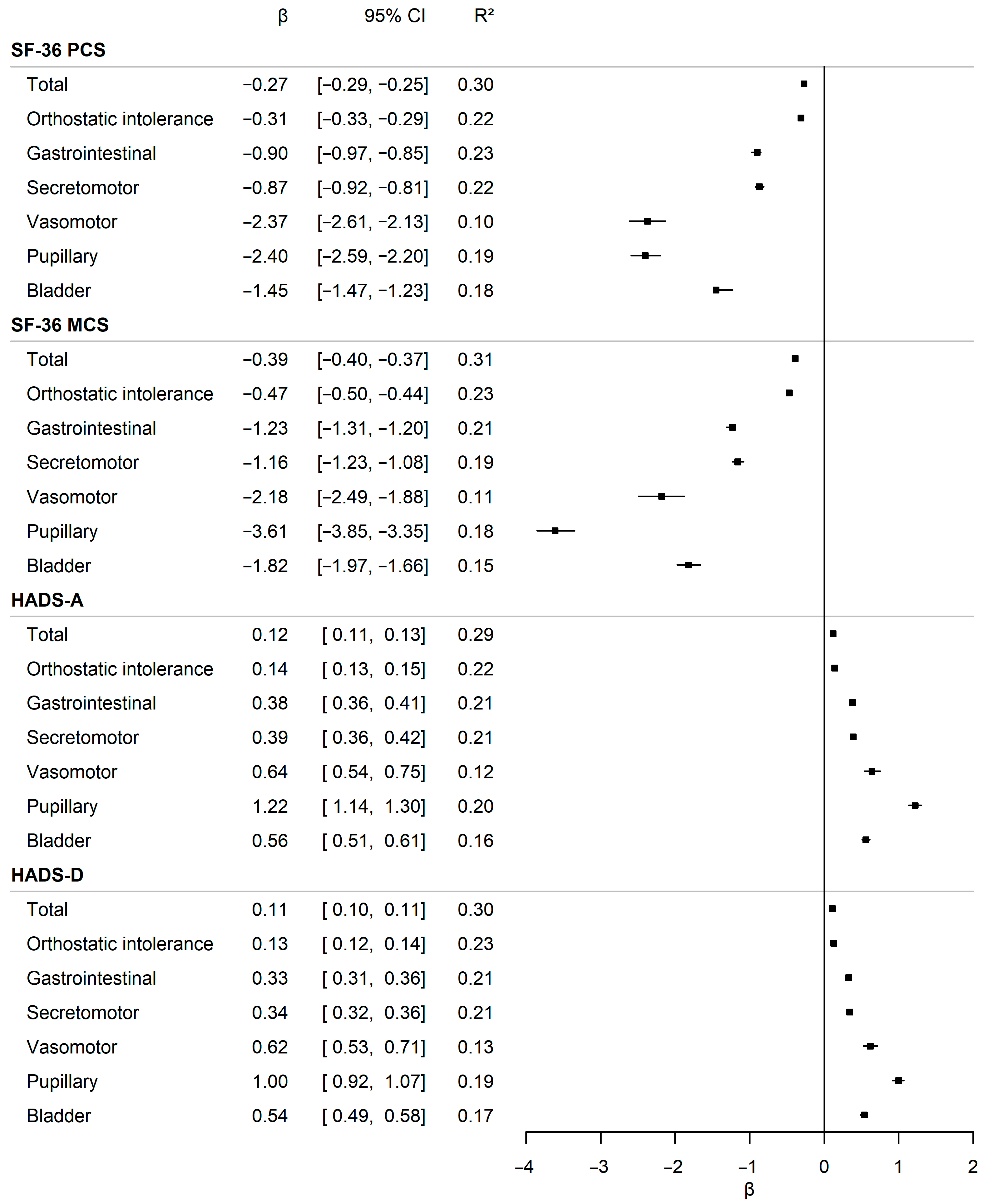
3.4. Impact of Autonomic Symptom Burden on General and Psychological Well-Being
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| QoL | Quality of life |
| COMPASS-31 | Composite Autonomic Symptom Score 31 |
| HADS | Hospital Anxiety and Depression Scale |
| SF-36 | Short Form Health Survey |
| PCS | Physical composite score |
| MSC | Mental composite score |
| SAD | Self-reported autonomic dysfunction |
References
- Bitsch Poulsen, M.; Wegeberg, A.-M.; Røikjer, J.; Nikontovic, A.; Vestergaard, P.; Brock, C. Prevalence of self-reported symptoms of diabetic autonomic dysfunction in the North Denmark Region: A population-based survey. Acta Diabetol. 2025, 62, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Cruz, J.N.; Manuel-Apolinar, L.; Arellano-Flores, M.L.; Gutierrez-Gonzalez, A.; Najera-Ahumada, A.G.; Cisneros-González, N. Health and quality of life outcomes impairment of quality of life in type 2 diabetes mellitus: A cross-sectional study. Health Qual. Life Outcomes 2018, 16, 94. [Google Scholar] [CrossRef]
- Leal, J.; Becker, F.; Feenstra, T.; Pagano, E.; Jensen, T.M.; Vistisen, D.; Witte, D.R.; Jorgensen, M.E. Health-related quality of life for normal glycaemia, prediabetes and type 2 diabetes mellitus: Cross-sectional analysis of the ADDITION-PRO study. Diabet. Med. 2022, 39, e14825. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Gao, Y.; Zhao, L.; Chen, L.; Sui, W.; Hu, J. The impact of chronic diseases on the health-related quality of life of middle-aged and older adults: The role of physical activity and degree of digitization. BMC Public Health 2024, 24, 2335. [Google Scholar] [CrossRef]
- Petersen, N.; König, H.-H.; Hajek, A. The link between falls, social isolation and loneliness: A systematic review. Arch. Gerontol. Geriatr. 2020, 88, 104020. [Google Scholar] [CrossRef]
- Lee, P.P.; Spritzer, K.; Hays, R.D. The Impact of Blurred Vision on Functioning and Well-being. Ophthalmology 1997, 104, 390–396. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Rasmussen, V.F.; Thrysøe, M.; Karlsson, P.; Nyengaard, J.R.; Kristensen, K.; Vestergaard, E.T. Impact of Neuropathy on Well-Being and Health-Related Quality of Life in Adolescents With Type 1 Diabetes. J. Diabetes Res. 2025, 2025, 6620727. [Google Scholar] [CrossRef]
- Junaković, A.; Skočić Hanžek, M.; Adamec, I.; Krbot Skorić, M.; Habek, M. A complex interplay between autonomic symptoms and symptoms of depression, anxiety, and stress. Neurol. Sci. 2023, 44, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Renno-Busch, S.; Hildesheim, H.; van Uem, J.M.T.; Sünkel, U.; Röben, B.; Brockmann, K.; Mychajliw, C.; Eschweiler, G.W.; Berg, D.; Maetzler, W. Autonomic Symptoms in Older Adults Are Common and Associated with Health-Related Quality of Life. Front. Neurol. 2021, 12, 757748. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, H.A.; Anderson, B.J. Management of diabetes: Are doctors framing the benefits from the wrong perspective? BMJ 2001, 323, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Borbjerg, M.K.; Wegeberg, A.M.; Nikontovic, A.; Mørch, C.D.; Arendt-Nielsen, L.; Ejskjaer, N.; Brock, C.; Vestergaard, P.; Røikjer, J. Understanding the Impact of Diabetic Peripheral Neuropathy and Neuropathic Pain on Quality of Life and Mental Health in 6960 People with Diabetes. Diabetes Care 2025, 48, 588–595. [Google Scholar] [CrossRef]
- Røikjer, J.; Wegeberg, A.M.; Nikontovic, A.; Brock, C.; Vestergaard, P. Prevalence of painful and painless diabetic peripheral neuropathy in the Northern Danish Region: A population-based study. Prim. Care Diabetes 2024, 18, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Danmarks Statistik [Statistics Denmark]. Befolkningstal. 2023. Available online: https://www.dst.dk/da/Statistik/emner/borgere/befolkning/befolkningstal (accessed on 12 December 2023).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Brinth, L.; Pors, K.; Mehlsen, J.; Sletten, D.M.; Terkelsen, A.J.; Singer, W. Translation and linguistic validation of the Translation and linguistic validation of the Composite Autonomic Symptom Score Composite Autonomic Symptom Score COMPASS 31 in Danish COMPASS 31 in Danish. Dan. Med. J. 2021, 69, A07210576. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated composite autonomic symptom score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. SF-36 Physical and Mental Health Summary Scales: A User’s Manual; Health Assessment Lab.: Boston, MA, USA, 1994. [Google Scholar]
- Svedbo Engström, M.; Leksell, J.; Johansson, U.B.; Borg, S.; Palaszewski, B.; Franzén, S.; Gudbjörnsdottir, S.; Eeg-Olofsson, K. Health-related quality of life and glycaemic control among adults with type 1 and type 2 diabetes—A nationwide cross-sectional study. Health Qual. Life Outcomes 2019, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Mersha, A.G.; Tollosa, D.N.; Bagade, T.; Eftekhari, P. A bidirectional relationship between diabetes mellitus and anxiety: A systematic review and meta-analysis. J. Psychosom. Res. 2022, 162, 110991. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, A.B.; Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. Prevalence of anxiety in adults with diabetes. J. Psychosom. Res. 2002, 53, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Trief, P.M.; Xing, D.; Foster, N.C.; Maahs, D.M.; Kittelsrud, J.M.; Olson, B.A.; Young, L.A.; Peters, A.L.; Bergenstal, R.M.; Miller, K.M.; et al. Depression in adults in the t1d exchange clinic registry. Diabetes Care 2014, 37, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.K.; Klein, R.; Lee, K.E.; Cruickshanks, K.J. Performance-based and self-assessed measures of visual function as related to history of falls, hip fractures, and measured gait time. Ophthalmology 1998, 105, 160–164. [Google Scholar] [CrossRef]
- Brown, J.S.; Wessells, H.; Chancellor, M.B.; Howards, S.S.; Stamm, W.E.; Stapleton, A.E.; Steers, W.D.; Van Den Eeden, S.K.; McVary, K.T. Urologic Complications of Diabetes. Diabetes Care 2005, 28, 177–185. [Google Scholar] [CrossRef]
- Dansk Endokrinologisk Selskab. Diabetisk Neuropati. 2025. Available online: https://endocrinology.dk/nbv/diabetes-melitus/diabetisk-neuropati/#elementor-toc__heading-anchor-6 (accessed on 18 June 2025).

| Type 1 Diabetes | Type 2 Diabetes | |
|---|---|---|
| n (%) | 981 (14.1) | 5980 (85.9) |
| Age, median (IQR) | 59 (48; 68) | 67 (60; 74) |
| BMI, median (IQR) | 25.7 (23.2; 29.1) | 29.4 (26.2; 33.5) |
| Sex, n (%) | ||
| Female | 432 (44.0) | 2328 (38.9) |
| Male | 545 (55.6) | 3641 (60.9) |
| Other | <5 (.) | 11 (0.2) |
| Diabetes duration, median (IQR) | 26 (13; 40) | 10 (4; 15) |
| Education, n (%) | ||
| Municipal primary/lower secondary school (10 years) | 230 (23.4) | 1978 (33.2) |
| Upper secondary school/Academy profession graduate (11–15 years) | 385 (39.2) | 2048 (34.3) |
| University graduate, BSc/MSc (16 ≥ years) | 366 (37.3) | 1939 (32.5) |
| Employment status, n (%) | ||
| Employed/self-employed/student | 554 (56.4) | 3063 (34.6) |
| Unemployed | 41 (4.2) | 192 (3.3) |
| Retirement/disability pension | 387 (39.4) | 3704 (61.2) |
| Income, n (%) | ||
| Low | 244 (24.9) | 1756 (29.4) |
| Middle | 318 (32.4) | 2044 (34.2) |
| High | 266 (27.1) | 1105 (18.5) |
| Unknown | 153 (15.6) | 1075 (18.0) |
| Questionnaire scores, median (IQR) | ||
| COMPASS-31 | 8.9 (2.9; 22.8) | 12.4 (5.3; 26.1) |
| SF-36 PCS | 52.1 (43.2; 56.4) | 49.3 (40.3; 54.8) |
| SF-36 MCS | 50.7 (40.3; 56.9) | 51.4 (41.2; 57.2) |
| HADS-A | 4 (1; 7) | 3.0 (1; 6) |
| HADS-D | 2 (1; 5) | 2 (0; 4) |
| Moderate or severe anxiety, % (95 CI) | 9.9 (8.1–11.9) | 8.9 (8.1–9.6) |
| Moderate or severe depression, % (95 CI) | 5.9 (4.5–7.6) | 5.1 (4.5–5.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsby, S.K.; Bitsch Poulsen, M.; Mark, E.B.; Røikjer, J.; Nikontovic, A.; Vestergaard, P.; Brock, C. The Symptom Burden of Autonomic Neuropathy Is Associated with Decreased Quality of Life in 6961 People with Diabetes. Diabetology 2025, 6, 128. https://doi.org/10.3390/diabetology6110128
Morsby SK, Bitsch Poulsen M, Mark EB, Røikjer J, Nikontovic A, Vestergaard P, Brock C. The Symptom Burden of Autonomic Neuropathy Is Associated with Decreased Quality of Life in 6961 People with Diabetes. Diabetology. 2025; 6(11):128. https://doi.org/10.3390/diabetology6110128
Chicago/Turabian StyleMorsby, Sigurd Kassow, Maria Bitsch Poulsen, Esben Bolvig Mark, Johan Røikjer, Amar Nikontovic, Peter Vestergaard, and Christina Brock. 2025. "The Symptom Burden of Autonomic Neuropathy Is Associated with Decreased Quality of Life in 6961 People with Diabetes" Diabetology 6, no. 11: 128. https://doi.org/10.3390/diabetology6110128
APA StyleMorsby, S. K., Bitsch Poulsen, M., Mark, E. B., Røikjer, J., Nikontovic, A., Vestergaard, P., & Brock, C. (2025). The Symptom Burden of Autonomic Neuropathy Is Associated with Decreased Quality of Life in 6961 People with Diabetes. Diabetology, 6(11), 128. https://doi.org/10.3390/diabetology6110128







