Factors Influencing the Prescription of First-Line Treatment for Type 2 Diabetes Mellitus: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection Process
2.4. Data Extraction
2.5. Risk of Bias and Data Analysis
3. Results
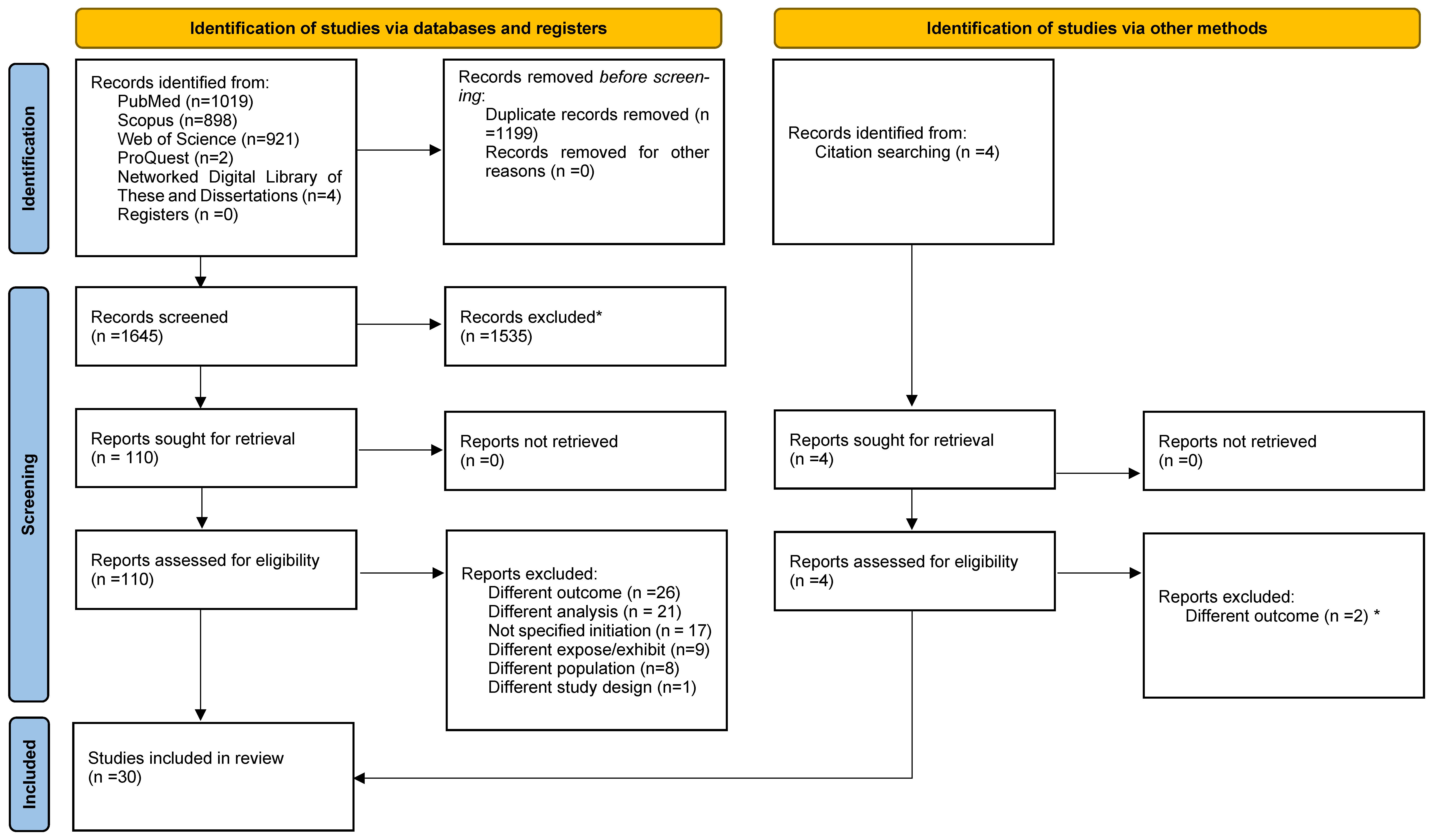
3.1. Study Selection
3.2. Study Characteristics
3.3. Data Extracted and Analysed
3.3.1. Physician-Related Factors
3.3.2. Healthcare System-Related Factors
3.3.3. Patient-Related Factors: Sociodemographic
3.3.4. Patient-Related Factors: Lifestyle and Metabolic
3.3.5. Patient-Related Factors: Cardiovascular
3.3.6. Patient-Related Factors: Renal
3.3.7. Patient-Related Factors: Other Clinical Factors
3.3.8. Disease-Related Factors
3.4. Quality Assessment
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ADA | American Diabetes Association |
| ADs | Antidiabetic drugs |
| AHA | Other oral antihyperglycemic agents |
| ARBs | Angiotensin receptor blockers |
| BMI | Body mass index |
| Cat. | Categorical |
| CKD | Chronic kidney disease |
| Cont. | Continuous |
| COPD | Chronic obstructive pulmonary disease |
| CT | Combination therapy |
| DCSI | Diabetes complication severity index |
| DPP4i | Dipeptidyl peptidase-4 inhibitors |
| EASD | European Association for the Study of Diabetes |
| GLP1-RA | Glucagon-like peptidase-1 receptor agonists |
| Glip | Glipizide |
| GPs | General practitioners |
| HbA1c | Glycated haemoglobin |
| HDL | High-density lipoprotein |
| IHD | Ischemic heart disease |
| JBI | Joanna Briggs Institute |
| LDL | Low-density lipoprotein |
| Metf. | Metformin |
| MT | Monotherapy |
| Non-Metf | Non-metformin |
| PECO-S | Population, Exposure, Comparator, Outcomes, Study type |
| Pio | Pioglitazone |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SGLT2i | Sodium-glucose transporter-2 inhibitors |
| SU | Sulfonylureas |
| TZDs | Thiazolidinediones |
| W/CVB | Drugs without cardiovascular benefits |
| WCVB | Drugs with cardiovascular benefits |
| WHO | World Health Organization |
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Engler, C.; Leo, M.; Pfeifer, B.; Juchum, M.; Chen-Koenig, D.; Poelzl, K.; Schoenherr, H.; Vill, D.; Oberdanner, J.; Eisendle, E.; et al. Long-term trends in the prescription of antidiabetic drugs: Real-world evidence from the Diabetes Registry Tyrol 2012-2018. BMJ Open Diabetes Res. Care 2020, 8, e001279. [Google Scholar] [CrossRef]
- Heintjes, E.M.; Overbeek, J.A.; Hall, G.C.; Prieto-Alhambra, D.; Lapi, F.; Hammar, N.; Bezemer, I.D. Factors Associated with Type 2 Diabetes Mellitus Treatment Choice Across Four European Countries. Clin. Ther. 2017, 39, 2296–2310.e14. [Google Scholar] [CrossRef]
- Nicolucci, A.; Charbonnel, B.; Gomes, M.B.; Khunti, K.; Kosiborod, M.; Shestakova, M.V.; Shimomura, I.; Watada, H.; Chen, H.; Cid-Ruzafa, J.; et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second-line therapy: Results from the global DISCOVER study programme. Diabetes Obes. Metab. 2019, 21, 2474–2485. [Google Scholar] [CrossRef]
- Cahn, A.; Cefalu, W.T. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care 2016, 39, S137–S145. [Google Scholar] [CrossRef]
- Corallo, A.N.; Croxford, R.; Goodman, D.C.; Bryan, E.L.; Srivastava, D.; Stukel, T.A. A systematic review of medical practice variation in OECD countries. Health Policy 2014, 114, 5–14. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Reames, B.N.; McCulloch, P.; Carr, A.J.; Campbell, W.B.; Wennberg, J.E. Understanding of regional variation in the use of surgery. Lancet 2013, 382, 1121–1129. [Google Scholar] [CrossRef]
- Davari, M.; Khorasani, E.; Tigabu, B.M. Factors Influencing Prescribing Decisions of Physicians: A Review. Ethiop. J. Health Sci. 2018, 28, 795–804. [Google Scholar] [CrossRef]
- Rodrigues, A.T.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Raets, L.; Ingelbrecht, A.; Benhalima, K. Management of type 2 diabetes in pregnancy: A narrative review. Front. Endocrinol. 2023, 14, 1193271. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S254–S266. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis; Epub ahead of print; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Brouwer, E.S.; West, S.L.; Kluckman, M.; Wallace, D.; Masica, A.L.; Ewen, E.; Kudyakov, R.; Cheng, D.; Bowen, J.; Fleming, N.S. Initial and subsequent therapy for newly diagnosed type 2 diabetes patients treated in primary care using data from a vendor-based electronic health record. Pharmacoepidemiol. Drug Saf. 2012, 21, 920–928. [Google Scholar] [CrossRef]
- Zhang, Q.; Rajagopalan, S.; Marrett, E.; Davies, M.J.; Radican, L.; Engel, S.S. Time to treatment initiation with oral antihyperglycaemic therapy in US patients with newly diagnosed type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 149–154. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Alexander, C.M.; Davies, M.J.; Zhao, C.; Mavros, P. Factors associated with initiation of antihyperglycaemic medication in UK patients with newly diagnosed type 2 diabetes. BMC Endocr. Disord. 2012, 12, 1. [Google Scholar] [CrossRef]
- Raebel, M.A.; Xu, S.; Goodrich, G.K.; Schroeder, E.B.; Schmittdiel, J.A.; Segal, J.B.; O’Connor, P.J.; Nichols, G.A.; Lawrence, J.M.; Kirchner, H.L.; et al. Initial Antihyperglycemic Drug Therapy Among 241 327 Adults With Newly Identified Diabetes From 2005 Through 2010: A Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM) Study. Ann. Pharmacother. 2013, 47, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Geier, A.S.; Wellmann, I.; Wellmann, J.; Kajüter, H.; Heidinger, O.; Hempel, G.; Hense, H.W. Patterns and determinants of new first-line antihyperglycaemic drug use in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 106, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.K. Examining Drug Utilisation Patterns and Optimal Treatment Pathways of Antidiabetic Medications; The University of Manchester: Manchester, UK, 2014; Available online: https://pure.manchester.ac.uk/ws/portalfiles/portal/54566935/FULL_TEXT.PDF (accessed on 25 August 2023).
- Li, Y. Factors Associated with Initiation of Glucose-Lowering Agents Among Medicare Beneficiaries with Newly Diagnosed Type 2 Diabetes, 2007–2017; Epub ahead of print; University of Pittsburgh: Pittsburgh, PA, USA, 2019. [Google Scholar] [CrossRef]
- Carrillo Balam, G.G. Glucose-Lowering Medication Initiation in People with Newly Diagnosed Type 2 Diabetes in Scotland: A Mixed-Methods Study; The University of Edinburgh: Edinburgh, UK, 2020. [Google Scholar] [CrossRef]
- Ouchi, D.; Giner-Soriano, M.; Vilaplana-Carnerero, C.; Monfa, R.; Torres, F.; Morros, R. Longitudinal treatment patterns in patients recently diagnosed with type 2 diabetes mellitus in Catalonia. Diabetes Res. Clin. Pract. 2023, 202, 110777. [Google Scholar] [CrossRef] [PubMed]
- Winkelmayer, W.C.; Stedman, M.R.; Pogantsch, M.; Wieninger, P.; Bucsics, A.; Asslaber, M.; Bauer, R.; Burkhardt, T.; Schautzer, A.; Brookhart, M.A. Guideline-conformity of initiation with oral hypoglycemic treatment for patients with newly therapy-dependent type 2 diabetes mellitus in Austria. Pharmacoepidemiol. Drug Saf. 2011, 20, 57–65. [Google Scholar] [CrossRef]
- Desai, N.R.; Shrank, W.H.; Fischer, M.A.; Avorn, J.; Liberman, J.N.; Schneeweiss, S.; Pakes, J.; Brennan, T.A.; Choudhry, N.K. Patterns of medication initiation in newly diagnosed diabetes mellitus: Quality and cost implications. Am. J. Med. 2012, 125, e1–e302. [Google Scholar] [CrossRef]
- Grimes, R.T.; Bennett, K.; Tilson, L.; Usher, C.; Smith, S.M.; Henman, M.C. Initial therapy, persistence and regimen change in a cohort of newly treated type 2 diabetes patients. Br. J. Clin. Pharmacol. 2014, 79, 1000–1009. [Google Scholar] [CrossRef]
- Abdelmoneim, A.S.; Eurich, D.T.; Gamble, J.M.; Simpson, S.H. Use patterns of antidiabetic regimens by patients with type2 diabetes. Can. J. Diabetes 2013, 37, 394–400. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Eguale, T.; Tamblyn, R. Guidelines adherence in the treatment of patients with newly diagnosed type 2 diabetes: A historical cohort comparing the use of metformin in Quebec pre and post-Canadian Diabetes Association guidelines. BMC Health Serv. Res. 2013, 13, 442. [Google Scholar] [CrossRef]
- Mitchell, B.D.; Eby, E.L.; Lage, M.J. Glycemic control and the first use of oral antidiabetic agents among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2013, 29, 1587–1597. [Google Scholar] [CrossRef]
- Vashisht, R.; Jung, K.; Shah, N. Learning Effective Treatment Pathways for Type-2 Diabetes from a clinical data warehouse. AMIA Annu. Symp. Proc. 2016, 2016, 2036–2042. [Google Scholar]
- Fujihara, K.; Igarashi, R.; Matsunaga, S.; Matsubayashi, Y.; Yamada, T.; Yokoyama, H.; Tanaka, S.; Shimano, H.; Maegawa, H.; Yamazaki, K.; et al. Comparison of baseline characteristics and clinical course in Japanese patients with type 2 diabetes among whom different types of oral hypoglycemic agents were chosen by diabetes specialists as initial monotherapy (JDDM 42). Medicine 2017, 96, e6122. [Google Scholar] [CrossRef]
- Tanabe, M.; Motonaga, R.; Terawaki, Y.; Nomiyama, T.; Yanase, T. Prescription of oral hypoglycemic agents for patients with type 2 diabetes mellitus: A retrospective cohort study using a Japanese hospital database. J. Diabetes Investig. 2017, 8, 227–234. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, S.T.; Chang, C.H.; Chuang, L.M.; Lai, M.S. Prescription trends and the selection of initial oral antidiabetic agents for patients with newly diagnosed type 2 diabetes: A nationwide study. Public Health 2017, 152, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Murayama, H.; Odawara, M.; Bauer, M. Treatment patterns of drug-naive patients with type 2 diabetes mellitus: A retrospective cohort study using a Japanese hospital database. Diabetol. Metab. Syndr. 2019, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Rodrigues, A.P.; Nunes, B. Initial Therapeutic Choices for Type 2 Diabetes in the Portuguese Sentinel Practice Network. Acta Med. Port. 2019, 32, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Juste, A.M.; Menditto, E.; Orlando, V.; Monetti, V.M.; Miguel, A.G.; Rubio, F.G.; Aza-Pascual-Salcedo, M.M.; Cahir, C.; Torres, A.P.; Riccardi, G. Treatment patterns of diabetes in Italy: A population-based study. Front. Pharmacol. 2019, 10, 870. [Google Scholar] [CrossRef]
- Moreno-Juste, A.; Poblador-Plou, B.; Aza-Pascual-Salcedo, M.; González-Rubio, F.; Malo, S.; López, J.L.; Pico-Soler, V.; Labrador, E.G.; Mucherino, S.; Orlando, V.; et al. Initial therapy, regimen change, and persistence in a spanish cohort of newly treated type 2 diabetes patients: A retrospective, observational study using real-world data. Int. J. Environ. Res. Public Health 2020, 17, 3742. [Google Scholar] [CrossRef]
- Yabe, D.; Higashiyama, H.; Kadowaki, T.; Origasa, H.; Shimomura, I.; Watada, H.; Tobe, K.; Iglay, K.; Tokita, S.; Seino, Y. Real-world Observational Study on Patient Outcomes in Diabetes (RESPOND): Study design and baseline characteristics of patients with type 2 diabetes newly initiating oral antidiabetic drug monotherapy in Japan. BMJ Open Diabetes Res. Care 2020, 8, e001361. [Google Scholar] [CrossRef]
- Wood, S.J.; Magliano, D.J.; Bell, J.S.; Shaw, J.E.; Keen, C.S.; Ilomäki, J. Pharmacological treatment initiation for type 2 diabetes in Australia: Are the guidelines being followed? Diabet. Med. 2020, 37, 1367–1373. [Google Scholar] [CrossRef]
- Campbell, D.J.T.; Campbell, D.B.; Ogundeji, Y.; Au, F.; Beall, R.; Ronksley, P.E.; Quinn, A.E.; Manns, B.J.; Hemmelgarn, B.R.; Tonelli, M.; et al. First-line pharmacotherapy for incident type 2 diabetes: Prescription patterns, adherence and associated costs. Diabet. Med. 2021, 38, e14622. [Google Scholar] [CrossRef]
- Shin, H.J.; Schneeweiss, S.; Glynn, R.J.; Patorno, E. Trends in First-Line Glucose-Lowering Drug Use in Adults With Type 2 Diabetes in Light of Emerging Evidence for SGLT-2i and GLP-1RA. Diabetes Care 2021, 44, 1774–1782. [Google Scholar] [CrossRef]
- Bonora, E.; Cataudella, S.; Marchesini, G.; Miccoli, R.; Vaccaro, O.; Fadini, G.P.; Martini, N.; Rossi, E. Initial treatment of diabetes in Italy. A nationwide population-based study from of the ARNO Diabetes Observatory. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2661–2668. [Google Scholar] [CrossRef]
- Barth, S.D.; Kostev, K.; Krensel, M.; Mathey, E.; Rathmann, W. Do Glucagonlike Peptide-1 Receptor Agonist and Sodium-glucose Co-transporter 2 Inhibitor Prescriptions in Germany Reflect Recommendations for Type 2 Diabetes with Cardiovascular Disease of the ADA/EASD Consensus Report? Exp. Clin. Endocrinol. Diabetes 2022, 131, 153–161. [Google Scholar] [CrossRef]
- Bouchi, R.; Sugiyama, T.; Goto, A.; Imai, K.; Ihana-Sugiyama, N.; Ohsugi, M.; Yamauchi, T.; Kadowaki, T.; Ueki, K. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J. Diabetes Investig. 2022, 13, 280–291. [Google Scholar] [CrossRef]
- Hulley, S.B.; Cummings, S.R.; Browner, W.S.; Grady, D.G.; Newman, T.B. Designing Clinical Research; Epub ahead of print; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Centers for Medicare & Medicaid Services. What’s Medicare? Available online: https://www.medicare.gov/ (accessed on 7 December 2024).
- Morieri, M.L.; Longato, E.; Di Camillo, B.; Sparacino, G.; Avogaro, A.; Fadini, G.P. Management of type 2 diabetes with a treat-to-benefit approach improved long-term cardiovascular outcomes under routine care. Cardiovasc. Diabetol. 2022, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee; Cheng, A.Y.Y. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. diabetes 2018, 42 (Suppl. 1), S1–S325. [Google Scholar] [CrossRef]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. J. Diabetes Investig. 2020, 11, 1020–1076. [Google Scholar] [CrossRef] [PubMed]
- Mardetko, N.; Nabergoj Makovec, U.; Locatelli, I.; Janez, A.; Kos, M. Uptake of new antidiabetic medicines in 11 European countries. BMC Endocr. Disord. 2021, 21, 127. [Google Scholar] [CrossRef]
- Funakoshi, M.; Nishioka, D.; Haruguchi, S.; Yonemura, S.; Takebe, T.; Nonaka, M.; Iwashita, S. Diabetes control in public assistance recipients and free/low-cost medical care program beneficiaries in Japan: A retrospective cross-sectional study. BMJ Public Health 2024, 2, e000686. [Google Scholar] [CrossRef]
- Glennie, J.L.; Kovacs Burns, K.; Oh, P. Bringing patient centricity to diabetes medication access in Canada. Clinicoecon. Outcomes Res. 2016, 8, 599–611. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Bashier, A.; Bin Hussain, A.; Abdelgadir, E.; Alawadi, F.; Sabbour, H.; Chilton, R. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol. Metab. Syndr. 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Allyhiani, M.; Kurdi, A.; Abdulaziz, A.; Faqeh, S.; Alhajjaji, A.; Alansari, S.; Althaqafi, A.; Alzaman, N.; Ali, M. Prescribing patterns of antidiabetics in type 2 diabetes and factors affecting them. Saudi Pharm. J. 2022, 30, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Halm, E.A.; Atlas, S.J.; Borowsky, L.H.; Benzer, T.I.; Metlay, J.P.; Chang, Y.C.; Singer, D.E. Understanding physician adherence with a pneumonia practice guideline: Effects of patient, system, and physician factors. Arch. Intern. Med. 2000, 160, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Grustam, A.; Jovic Vranes, A.; Soldatovic, I.; Stojicic, P.; Jovanovic Andersen, Z. Factors Associated with Utilization of Primary and Specialist Healthcare Services by Elderly Cardiovascular Patients in the Republic of Serbia: A Cross-Sectional Study from the National Health Survey 2013. Int. J. Environ. Res. Public Health 2020, 17, 2602. [Google Scholar] [CrossRef]
- Qi, M.; Santos, H.; Pinheiro, P.; McGuinness, D.L.; Bennett, K.P. Demographic and socioeconomic determinants of access to care: A subgroup disparity analysis using new equity-focused measurements. PLoS ONE 2023, 18, e0290692. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, Q.; Tang, J.; Fan, C.P.S.; Li, Z.; Apecechea, M.; Hegar, R.; Shankar, R.; Kurtyka, K.M.; Engel, S.S. Why physicians do not initiate dual therapy as recommended by AACE guidelines: A survey of clinicians in the United States. Diabetes Res. Clin. Pract. 2015, 108, 456–465. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012, 35, 731–737. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Confederat, L.; Constantin, S.; Lupaşcu, F.; Pânzariu, A.; Hăncianu, M.; Profire, L. Hypoglycemia induced by antidiabetic sulfonylureas. Rev. Med. Chir. Soc. Med. Nat. Iasi 2015, 119, 579–584. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Type 2 Diabetes in Adults: Management. NICE Guideline [NG28]; NICE: London, UK, 2015; Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 10 September 2024).
- Direção-Geral da Saúde. Abordagem Terapêutica Farmacológica na Diabetes Mellitus Tipo 2 no Adulto. Norma DGS No.052/2011 Updated 27 April 2015; pp. 1–28. Available online: https://www.mgfamiliar.net/wp-content/uploads/NOC_Diabetes_2015-3.pdf (accessed on 12 September 2024).
- Riddle, M.C. Modern Sulfonylureas: Dangerous or Wrongly Accused? Diabetes Care 2017, 40, 629–631. [Google Scholar] [CrossRef]
- Hirst, J.A.; Farmer, A.J.; Dyar, A.; Lung, T.W.C.; Stevens, R.J. Estimating the effect of sulfonylurea on HbA1c in diabetes: A systematic review and meta-analysis. Diabetologia 2013, 56, 973–984. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009, 52, 17–30. [Google Scholar] [CrossRef]
- Giorda, C.B.; Orsi, E.; De Cosmo, S.; Bossi, A.C.; Guerzoni, C.; Cercone, S.; Gilio, B.; Cavalot, F. Prescription of Sulphonylureas among Patients with Type 2 Diabetes Mellitus in Italy: Results from the Retrospective, Observational Multicentre Cross-Sectional SUSCIPE (Sulphonyl_UreaS_Correct_Internal_Prescription_Evaluation) Study. Diabetes Ther. 2020, 11, 2105–2119. [Google Scholar] [CrossRef] [PubMed]
- Thulé, P.M.; Umpierrez, G. Sulfonylureas: A new look at old therapy. Curr. Diab. Rep. 2014, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Lalau, J.-D.; Kajbaf, F.; Bennis, Y.; Hurtel-Lemaire, A.-S.; Belpaire, F.; De Broe, M.E. Metformin Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease Stages 3A, 3B, or 4. Diabetes Care 2018, 41, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Beigi, F.; Moghissi, E.; Tiktin, M.; Hirsch, I.B.; Inzucchi, S.E.; Genuth, S. Individualizing glycemic targets in type 2 diabetes mellitus: Implications of recent clinical trials. Ann. Intern. Med. 2011, 154, 554–559. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Hazra, N.C.; Rudisill, C.; Gulliford, M.C. Determinants of health care costs in the senior elderly: Age, comorbidity, impairment, or proximity to death? Eur. J. Health Econ. 2018, 19, 831–842. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; de Oliveira Rezende, A.T.; Delpino, F.M.; Mendonça, C.R.; Noll, M.; Nunes, B.P.; de Oliviera, C.; Silveira, E.A. Association between multimorbidity and hospitalization in older adults: Systematic review and meta-analysis. Age Ageing 2022, 51, afac155. [Google Scholar] [CrossRef]
- Mahmoud, F.; Mullen, A.; Sainsbury, C.; Rushworth, G.F.; Yasin, H.; Abutheraa, N.; Mueller, T.; Kurdi, A. Meta-analysis of factors associated with antidiabetic drug prescribing for type 2 diabetes mellitus. Eur. J. Clin. Investig. 2023, 53, e13997. [Google Scholar] [CrossRef]
| Category | Inclusion Criteria |
| Study population | Studies including ≥80% of adults (≥18 years) with T2DM naïve to antidiabetic treatment or those with relevant subgroup analyses. |
| Expose/Exhibit | Studies assessing factors influencing prescribing decisions (e.g., physician-, patient-, and system-related, pharmaceutical influence, cost). |
| Outcomes | Outcomes related to the initiation of metformin or oral combination therapy (two or more drugs initiated simultaneously). |
| Publication type | Observational analytical studies. |
| Category | Exclusion criteria |
| Study population | Studies on pregnant or breastfeeding women. |
| Study and Country | Participants’ Characteristics | Period to Identify Sample | Follow-Up (Months) | Source of Data | Setting | Antidiabetic Drug Studied | |||
|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Male (%) | Mean Age (Years) | Prevalence Initiation (%) | ||||||
| Retrospective cohort studies | |||||||||
| Brouwer et al. (2012) [19], US | 1972 | 48 | >21 | January 1998 to March 2009 | Vary * | Vendor-based electronic health records (from BHCS and CCHS) | Primary care | Metf vs. SU (ref.) Metf vs. TZDs (ref.) Metf vs. CT (ref.) SU vs. CT (ref.) | 66.63 (M) 10.60 (S) 5.22 (T) 13.74 (CT) |
| Zhang et al. (2012) [20], US | 10,743 | 45 | 61 | January 2003 to December 2005 | 24 | General Electronic Healthcare’s Clinical Data Services electronic medical record database | Multicentre | Metf | 64 (M) (<65years) 1 49 (M) (≥65years) 1 |
| Sinclair et al. (2012) [21], UK | 9158 | 54 | 62 | January 2003 to December 2005 | 24 | International Medical Statistics (IMS) MediPlus database | Information from general practitioners. | Metf | 76 (M) 1 |
| Raebel et al. (2013) [22], US | 241,327 | 53 | 59 | January 2005 to December 2010 | 6 | Surveillance Prevention and Management of diabetes mellitus (SUPREME-DM) | Multicentre | SU vs. Metf (ref.) | 19.17 (S) 1 65.53 (M) 1 |
| Geier et al. (2014) [23], Germany | 27,138 | 49 | 63 | June 2003 to December 2009 | Vary * | German Disease Management Programme for T2DM (DMP-DM2), funding by health insurance | Multicentre | Metf vs. SU (ref.) | 33 (M) 2 7 (S) 2 |
| Wright (2014) [24], UK | 44,838 | 57 | 61 | January 2005 to December 2009 | Vary * | Clinical Practice Research Datalink | Primary care | SU vs. Metf (ref.) | 10.4 (S) 87.8 (M) |
| Li (2019) [25], US | 231,408 | 38 | 72 | January 2007 to December 2017 | 12 | Health insurance database (Medicare) | Multicentre | Metf Metf vs. SU (ref.) | 68.4 (M) 1 14.8 (S) 1 |
| Carrillo Balam (2020) [26], Scotland | 154,660 | 56 | 61 | January 2004 to December 2012 | 24 | Scottish care information—diabetes | Multicentre | Metf | 82.3 (M) |
| Ouchi et al. (2023) [27], Spain | 86,854 | 58 | 59 | January 2015 to December 2020 | Vary * | Electronic medical records from SIDIAP | Primary care | CT vs. MT | 78.3 (MT) 21.7 (CT) |
| Study and Country | Participants’ Characteristics | Period to Identify Sample | Source of Data | Setting | Antidiabetic Drug Studied | |||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Male (%) | Mean Age (Years) | Prevalence Initiation (%) | |||||
| Cross-sectional studies | ||||||||
| Winkelmayer et al. (2011) [28], Austria | 39,077 | 50 | 63 | January 2006 to June 2008 | Insurance claims data (public and non-for-profit health insurance company) | Multicentre | Metf vs. AHA (ref.) | 71.7 (M) |
| Desai et al. (2012) [29], US | 254,973 | 53 | 58 | January 2006 to December 2008 | Prescription claims data from CVS Caremark | Multicentre | Metf Metf vs. MT (ref.) | 51 (M) |
| Grimes et al. (2014) [30], Ireland | 20,947 | 58 | >40 | January 2008 to December 2009 | National pharmacy claims databases in Ireland 1 | Multicentre | Metf vs. MT | 76 (M) |
| Abdelmoneim et al. (2013) [31], Canada | 39,276 | NR | ≥66 | January 1998 to December 2010 | Alberta Blue Cross provincial insurance programme | Multicentre | Metf vs. SU (ref.) | 84.2 (M, 2010) 4.5 (S, 2010) |
| Wang et al. (2013) [32], Canada | 1279 | 49 | ≥18 | January 2003 to December 2011 | Electronic health record (MOXXI: Medical Office of the XXIst Century) | Primary care | SU vs. Metf * (ref.) TZDs vs. Metf * (ref.) Metf * vs. AHA (ref) | 92 (M *) |
| Mitchell et al. (2013) [33], US | 4627 | 48 | 53 | January 2006 to June 2010 | The i3 Invision Data Mart database (OptumInsight, Eden Prairie, MN, US) | Multicentre | CT, Metf | 93.24 (MT) |
| Vashisht et al. (2016) [34], US | 6121 | 51 | NR | NR | Electronic medical records from Stanford Clinical Data Warehouse | Hospital | Glipizide vs. Metf (ref.) Pioglitazone vs. Metf (ref.) | NR |
| Fujihara et al. (2017) [35], Japan | 2666 | 64 | 61 | December 2009 to March 2015 | The Japan Diabetes Clinical Data Management Study Group (JDDM) | Outpatient clinics (clinical diabetologists) | Metf vs. SU (ref.) | 35.7 (M) 11.4 (S) |
| Tanabe et al. (2017) [36], Japan | 7108 | 63 | NR | April 2008 to April 2013 | Electronic information systems constructed by Medical Data Vision (MDV) | Multicentre | SU vs. Metf | 18.4 (S) 26.5 (M) |
| Liu et al. (2017) [37], Taiwan | 28,640 | 53 | 57 | January 2006 to December 2010 | Taiwan National Insurance Research Database | Multicentre | AHA vs. Metf * (ref.) | 43.8 (AHA, 2006) 26.2 (AHA, 2010) |
| Morita et al. (2019) [38], Japan | 224,761 | 61 | 66 | October 2012 to September 2016 | Medical Data Vision database, a Diagnosis Procedure Combination database | Outpatient | DPP4i vs. Metf (ref.) | 26.2 (D) 7.1(M) |
| Pinto et al. (2019) [39], Portugal | 415 | 55 | NR | January 2014 to December 2015 | Portuguese Sentinel Practice Network | Multicentre | Metf, CT. | 85.5 (M) 6.5 (CT) |
| Juste et al. (2019) [40], Italy | 14,679 | 55 | 64 | January 2016 to December 2016 | Campania Regional Database for Medication Consumption | Primary care | MT vs. CT | 86.9(MT) 13.1(CT) |
| Moreno-Juste et al. [41] (2020), Spain | 4247 | 58 | 65 | October 2013 to September 2014 | Electronic health records and pharmacy billing records from health system (EpiChron Cohort) | Multicentre | Metf MT vs. CT | 80.5 (M) 88.7(MT) 11.3 (CT) |
| Yabe et al. (2020) [42], Japan | 1485 | 62 | 60 | June 2016 to May 2019 | Real-world observational study on patient outcomes in diabetes (RESPOND) | Multicentre | Metf | 16 (M) |
| Wood et al. (2020) [43], Australia | 47,860 | 53 | 61 | July 2013 to February 2018 | Random sample from Australia’s Pharmaceutical Benefits Scheme | Multicentre | SU vs. Metf (ref.) Non-Metf vs. Metf (ref.) CT vs. Metf (ref.) | 85.8 (M) 4.6 (S) 1.9 (Non–M) 7.7 (CT) |
| Campbell et al.(2021) [44], Canada | 17,932 | 55 | 56 | April 2012 to March 2017 | Multiple administrative health datasets from Alberta, Canada | Multicentre | AHA 2 vs. Metf (ref.) | 89 (M) |
| Shin et al. (2021) [45], US | 264,542 (Clinf.) 285,213 (Med.) | 55 (Clinf.) 46 (Med.) | 59 (Clinf.) 73 (Med.) | April 2013 to December 2019 (Clinf.) April 2013 to December 2017 (Med.) | Health insurance databases (Optum Clinformatics (Clinf.) and Medicare fee-for-service (Med.)) | Multicentre | Metf Drugs without cardiovascular benefits vs. Metf (ref.) Drugs With cardiovascular benefits benefits vs. Metf (ref.) | Last data available: 83.1 (M, Clinf.) 80.6 (M, Med.) |
| Bonora et al. (2021) [46], Italy | 65,932 | 51 | NR | January 2018 to December 2018 | Administrative data from National Health System (ARNO Diabetes Observatory database) | Multicentre | Metf, CT | 71.9 (M) 3.8 (CT) |
| Barth et al. (2022) [47], Germany | 16,006 | 57 | 61 | January 2015 to December 2020 | Health care database (Disease Analyzer database (IQVIA)) | Multicentre | Metf | 77 (M) |
| Bouchi et al. (2022) [48], Japan | 1,136,723 | 58 | >20 | October 2014 to March 2018 | National Database of health Insurance Claims and Specific Health Chek-ups in Japan | Outpatient clinic | Metf vs. MT (ref.) | 15.9 (M) |
| Physician-Related Factors | Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| Metf. | W/CVB | WCVB | AHA | CT | |||
| Age (cat.) | Metf. | +++ [28] ++ [37] | |||||
| Years of experience (cont.) | Metf. | - - [32] | |||||
| Sex | Metf. | ++ [37] LR - - - [28] - - [32,37] ML | |||||
| Medicine evidence questionnaire (cat.) | Metf. | - - [32] | |||||
| Physician Speciality | Metf. | ++ [45] | ++ [45] | +++ [28] ++ [37,44] | |||
| _________ | + [42] - [39] | + [39] | |||||
| Healthcare System- Related Factors | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metf. | SU | DPP4i | W/CVB | WCVB | MT | AHA | |||
| Guidelines (updates) | Metf. | - - [32] | ++ [32] | ||||||
| TZDs | ++ [32] | ||||||||
| Time (cont.) | Metf. | ++ [31] | ++ [29] | ||||||
| ______ | + [25,29,45] 1 - [45] 2 | ||||||||
| Time (cat.) | Metf. | +++ [22] ++ [23,43] - - [19] | ++ [43,48] | +++ [28] ++ [37] | |||||
| TZDs | ++ [19] | ||||||||
| CT | ++ [43] - - [19] | - - [19] | |||||||
| HbA1c tests (cat.) | Metf. | ++ [45] | ++ [45] | ||||||
| Office visits (cont.) | Metf. | +++ [31] | + [38] | - - [45] | ++ [45] ALX - - [45] 2(TB) | ||||
| Hospitalisation (cont.) | Metf. | + [31] - - [31] | |||||||
| Hospitalisation (cat.) | Metf. | ++ [25] | ++[45] | ++ [45] ALX - -[45] 1(TB),2(TB) | |||||
| Length of hospital stay (cont.) | Metf. | ++ [45] 2(TB,TC) - - [45] ALX | ++ [45] 2(TA) - - [45] ALX | ||||||
| Length of hospital stay (cat.) | Metf. | +++ [28] | |||||||
| Emergency visits (cont.) | Metf. | + [31] - - [31] | |||||||
| Emergency visits (cat.) | Metf. | - - [45] | ++ [45] ALX - - [45] 1(TA) | ||||||
| Preventive healthcare service | Metf. | ++ [45] | ++ [45] 2(TC) - - [45] ALX | ||||||
| Healthcare settings | Metf. | ++ [48] | ++ [37] LR - - [37] ML | ||||||
| Healthcare ownership | Metf. | ++ [37] | |||||||
| Location urbanisation | Metf. | ++ [37] | |||||||
| Hospital beds (cat.) | Metf. | ++ [48] | |||||||
| Health insurance | Metf. | ++ [29] | +++ [28] | ||||||
| Co-payment (cat.) | Metf. | +++ [28] | |||||||
| Costs drugs (cat.) | Metf. | ++ [25] | |||||||
| Brand/generic ratio | Metf. | ++ [45] | ++ [45] | ||||||
| Patient-Related Factors: Sociodemographic | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metf. | SU | W/CVB | WCVB | MT | AHA | CT | |||
| Age (cont.) | Metf. | +++ [31] ++ [23,25,35] + [22,36] | ++ [45] | ++ [45] | ++ [44] | ||||
| DPP4i | + [38] | ||||||||
| CT | + [40,41] | ||||||||
| Age (cat.) | Metf. | +++ [22] ++ [19,24,43] | ++ [29,30,43] | +++ [28] ++ [32,37] | |||||
| TZDs | ++ [19] | ||||||||
| CT | ++ [19,43] | - - [19] | |||||||
| ______ | + [20,21,26] | ||||||||
| Age (cat.) and health insurance | Metf. | ++ [48] | |||||||
| Sex | Metf. | +++ [22] ++ [25] - - - [31] - - [19,23,35,43] | ++ [45] ALX - - [45] 2(TC) | ++ [45] ALX - - [45] 1(TA,TB) | ++ [29,30,48] - - [43] | +++ [28] ++ [37] - - [32,44] | |||
| TZDs | - - [19] | ||||||||
| DPP4i | + [38] | ||||||||
| CT | ++ [19,43] | - - [19] | + [40] - [41] | ||||||
| ____ | - [46] | + [46] 3 - [46] 3 | |||||||
| Race/Ethnicity | Metf. | +++ [22] ++ [19,25] | ++ [45] 1(TA,TB) | ++ [45] 1(TA,TB) | |||||
| TZDs | - - [19] | ||||||||
| CT | - - [19] | - - [19] | |||||||
| Socioeconomic status | Metf. | ++ [25] + [22] | ++ [29] | ++ [37] LR - - [37] ML | |||||
| CT | - [41] | ||||||||
| Doctor or has a doctor in family | Metf. | ++ [37] LR - - [37] ML | |||||||
| Geographic region | Metf. | ++ [25] | ++ [45] | ++ [45] | |||||
| CT | + [40,41] | ||||||||
| Immigrant status | Metf. | + [41] | |||||||
| Lifestyle and metabolic | |||||||||
| BMI (cont.) | Metf. | ++ [23,35] + [22] | |||||||
| DPP4i | + [38] | ||||||||
| BMI (cat.) | Metf. | ++ [24] | |||||||
| Obesity or overweight | Metf. | ++ [45] | ++ [45] | ||||||
| Smoker (cat.) | Metf. | ++ [45] 2(TA,TB) - - [45] ALX | ++ [45] ALX - - [45] 1(TB),2(TC) | ||||||
| Ex-smoker (cat.) | Metf. | + [22] - - [22] | |||||||
| Current smoker (cat.) | Metf. | - - - [22] - - [23] | |||||||
| Substance abuse (cat.) | Metf. | ++ [45] 2(TA) - - [45] ALX | ++ [45] ALX - - [45] 1(TA,TB) | ||||||
| Liver disease | Metf. | +++ [31] ++ [24] | |||||||
| DPP4i | - [38] | ||||||||
| Hyperlipidaemia | Metf. | - [22] | ++ [45] ALX - - [45] 1(TA),2(TA) | ++ [45] | |||||
| Dyslipidaemia | Metf. | ++ [43] | ++ [43] | ++ [43] | |||||
| HDL (cont.) | Metf. | - [22] | |||||||
| LDL (cont.) | Metf. | + [22] | |||||||
| Lipid-lowering meds | Metf. | +++ [31] - - [23] | ++ [48] | ||||||
| Statin use | Metf. | ++ [45] | ++ [45] | ||||||
| Patient-Related Factors: Cardiovascular | Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metf. | SU | DPP4i | W/CVB | WCVB | MT | AHA | CT | |||
| Hypertension | Metf. | +++ [31] ++ [35] + [22] - - [43] | ++ [45] | ++ [45] | - - [43] | - - [43] | ||||
| Coagulopathy | Metf. | + [31] - - [31] | ||||||||
| Cardiovascular disease | Metf. | ++ [25] + [22] | ++ [45] | ++ [45] | - - [32] | |||||
| ___ | - [47] | |||||||||
| IHD | Metf. | + [31] - - [31] | ++ [48] | |||||||
| IHD/angina | Metf. | - - [43] | - - [43] | - - [43] | ||||||
| IHD/hypertension | Metf. | - - [43] | - - [43] | - - [43] | ||||||
| IHD/Stroke | Metf. | + [38] | ||||||||
| Coronary atherosclerosis of native coronary artery | Pio (TZDs) | ++ [34] | ||||||||
| Heart failure | Metf. | +++ [31] ++ [43] + [22] | - - [43] | ++ [43] | ||||||
| Valvular disease | Metf. | +++ [31] | ||||||||
| Arrhythmia | Metf. | + [31] - - [31] | ||||||||
| Atrial fibrillation | Metf. | ++ [43] | - - [43] | - - [43] | ||||||
| Peripheral vascular disease | Metf. | + [31] - - [31] | ||||||||
| Cerebrovascular disease | Metf. | ++ [43] + [31] - - [31] | - - [43] | ++ [43] | ||||||
| Micro/macrovascular complications | CT | + [40] | ||||||||
| Microvascular complications | Metf. | ++ [45] | ++ [45] ALX - - [45] 2(TA) | |||||||
| Diabetic retinopathy | Metf. | - - - [31] - - [24] | + [38] | |||||||
| Diabetic neuropathy | Metf. | - - - [31] - - [24] | - [38] | |||||||
| Cardiovascular meds | Metf. | ++ [24] 3 - - [24] 4 | ||||||||
| Antihypertensive meds | Metf. | - - [23] | ++ [48] | |||||||
| ACE inhibitors or ARBs | Metf. | ++ [45] | ++ [45] | |||||||
| Beta-blockers | Metf. | ++ [45] 1(TB) - - [45] ALX | - - [45] | |||||||
| Calcium channel blockers | Metf. | ++ [45] 1(TA)2(TC) - - [45] ALX | ++ [45] 2(TA,TC) - - [45] ALX | |||||||
| Loop diuretics | Metf. | ++ [45] | ++ [45] ALX - - [45] 2(TA) | |||||||
| Thiazide diuretics | Metf. | ++ [45] | ++ [45] ALX - - [45] 1(TB) | |||||||
| Anticoagulants meds | Metf. | ++ [45] 2(TC) - - [45] ALX | ++ [45] ALX - - [45] 1(TA)2(TC) | |||||||
| Antiplatelet meds | Metf. | ++ [45] | ++ [45] ALX - - [45] 1(TB)2(TB) | |||||||
| Renal | ||||||||||
| Serum creatinine (cont.) | Metf. | + [22] | ||||||||
| Serum creatinine (cat.) | Metf. | +++ [22] ++ [19] | - - [19] | |||||||
| TZDs | ++ [19] | |||||||||
| CT | ++ [19] | |||||||||
| Renal disease | Metf. | + [38] | ++ [32] | |||||||
| Chronic kidney disease | Metf. | +++ [22] ++ [24,25] | ++ [45] | ++ [45] | - - [48] | |||||
| CT | + [41] | |||||||||
| Renal failure | Metf. | + [22] | ||||||||
| Glip (SU) | ++ [34] | |||||||||
| Diabetic nephropathy | Metf. | +++ [31] | - [38] | |||||||
| Glip (SU) | ++ [34] | |||||||||
| Patient-Related Factors: Other Clinical | Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metf. | SU | DPP4i | W/CVB | WCVB | MT | AHA | CT | |||
| Esophageal varices, without bleeding, in disease classified elsewhere | Glip (SU) | ++ [34] | ||||||||
| Fluid and electrolyte disorder | Metf. | + [31] - - [31] | ||||||||
| Paracetamol | Pio (TZDs) | ++ [34] | ||||||||
| COPD | Metf. | + [31] - - [31] | ++ [45] 1(TA)2(TA) - - [45] ALX | ++ [45] 1(TC) - - [45] ALX | ||||||
| Pulmonary collapse | Pio (TZDs) | ++ [34] | ||||||||
| Dementia | Metf. | ++ [24] | ||||||||
| Depression | Metf. | ++ [43] + [22,31] - - [31] | - - [43] | ++ [43] | ||||||
| Neuropsychiatric meds | CT | + [40] | ||||||||
| Antipsychotic meds | Metf. | - - [24] 3,4 | ||||||||
| Cancer | Metf. | +++ [31] | ||||||||
| Lymphoma | Metf. | + [31] - - [31] | ||||||||
| Hypothyroidism | Metf. | - - - [31] | ||||||||
| Rheumatoid arthritis | Metf. | + [31] - - [31] | ||||||||
| Immune modulators/suppressants | Metf. | ++ [24] 3 - - [24] 4 | ||||||||
| Oral corticosteroids | Metf. | ++ [24] 3 - - [24] 4 | ||||||||
| Tacrolimus | Glip (SU) | ++ [34] | ||||||||
| Cefepime | Glip (SU) | ++ [34] | ||||||||
| Medication use (cont.) | Metf. | - - [25] | ++ [29] | |||||||
| CT | + [41] | |||||||||
| Medication use (cat.) | Metf. | +++ [28] | ||||||||
| CT | + [40] | |||||||||
| Comorbidities (cont.) | Metf. | ++ [25] | ||||||||
| CT | + [41] | |||||||||
| Comorbidities (cat.) | Metf. | ++ [43] | ++ [43] | ++ [44] | ++ [43] | |||||
| Quan Score (cont.) | Metf. | + [22] | ||||||||
| Rx-Risk comorbidity index (cont.) | CT | + [40] | ||||||||
| Disease-Related Factors | Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metf. | SU | TZDs | DPP4i | W/CVB | WCVB | MT | AHA | CT | |||
| HbA1c (cont.) | Metf. | ++ [23,35] + [22] - [36] | + [38] | ++ [44] | |||||||
| HbA1c (cat.) | Metf. | +++ [22] ++ [19,24] | - - [19] | ++ [19] | |||||||
| CT | ++[19] | + [27] | |||||||||
| ______ | + [33] | + [33] | |||||||||
| Fasting glucose (cont.) | Metf. | + [22] | |||||||||
| Random glucose (cont.) | Metf. | + [22] | |||||||||
| Glucose | Pio (TZDs) | ++ [34] | |||||||||
| Diabetes duration (cont.) | Metf. | ++ [23,35] | |||||||||
| Time to initiation (cont.) | Metf. | ++ [23] | |||||||||
| CT | + [27] | ||||||||||
| Number of antidiabetics at initiation (cat.) | Metf | - - [32] | |||||||||
| Diabetes without complications | Pio (TZDs | ++ [34] | |||||||||
| Hypoglycaemic events (cat.) | Metf. | ++ [45] ALX - - [45] 1(TB)2(TB) | ++ [45]ALX - - [45] 1(TA,TB) | ||||||||
| DCSI (cat.) | Metf. | ++ [37] LR - - [37] ML | |||||||||
| Topics Assessed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective Cohort Studies | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | D10 | D11 |
| Brouwer et al. [19] | Unclear | Unclear | Unclear | No | No | Unclear | Unclear | Yes | Unclear | N/A | Unclear |
| Zhang et al. [20] | Unclear | Unclear | Unclear | No | No | Yes | Yes | Yes | Unclear | N/A | No |
| Sinclair et al. [21] | Unclear | Unclear | Unclear | No | No | Yes | Yes | Yes | Unclear | N/A | No |
| Raebel et al. [22] | Unclear | Unclear | Unclear | No | No | Yes | Unclear | Yes | Unclear | N/A | Unclear |
| Geier et al. [23] | Unclear | Unclear | Unclear | No | No | Yes | Unclear | Yes | Unclear | N/A | Unclear |
| Wright [24] | Unclear | Unclear | Unclear | No | No | Yes | Yes | Yes | Unclear | N/A | Unclear |
| Li [25] | Unclear | Unclear | Unclear | No | No | Yes | Unclear | Yes | Unclear | N/A | Unclear |
| Carrillo Balam [26] | Unclear | Unclear | Unclear | No | No | Yes | Yes | Yes | Unclear | N/A | No |
| Ouchi et al. [27] | Unclear | Unclear | Unclear | No | No | Unclear | Unclear | Yes | Unclear | N/A | Unclear |
| Topics Assessed | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cross-Sectional Studies | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 |
| Winkelmayer et al. [28] | Yes | Yes | Unclear | Yes | No | No | Unclear | Unclear |
| Desai et al. [29] | Yes | No | N/A | Yes | No | No | Unclear | Unclear |
| Grimes et al. [30] | Yes | No | N/A | Yes | No | No | Unclear | Unclear |
| Abdelmoneim et al. [31] | Yes | Yes | Unclear | Yes | No | No | Unclear | Unclear |
| Wang et al. (2013) [32] | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Unclear |
| Mitchell et al. [33] | No | Yes | Unclear | Unclear | No | No | Unclear | No |
| Vashisht et al. [34] | No | No | Unclear | Yes | No | No | Yes | Unclear |
| Fujihara et al. [35] | No | No | Unclear | Unclear | No | No | Yes | Unclear |
| Tanabe et al. [36] | No | No | Unclear | Unclear | No | No | Yes | No |
| Liu et al. [37] | Yes | Yes | Unclear | Yes | No | No | Unclear | Unclear |
| Morita et al. [38] | No | Yes | Unclear | Yes | No | No | Yes | No |
| Pinto et al. [39] | Yes | No | N/A | Yes | No | No | Yes | No |
| Juste et al. [40] | Yes | Yes | Unclear | Yes | No | No | Unclear | No |
| Moreno-Juste et al. [41] | Yes | Yes | Unclear | Yes | No | No | Unclear | No |
| Yabe et al. [42] | No | Yes | Unclear | Unclear | No | Yes | Yes | No |
| Wood et al. [43] | Yes | Yes | Unclear | Yes | No | No | Unclear | Unclear |
| Campbell et al. [44] | No | Yes | Unclear | Yes | Yes | Unclear | Unclear | Unclear |
| Shin et al. (2021) [45] | Yes | Yes | Unclear | Yes | No | No | Unclear | Unclear |
| Bonora et al. [46] | No | No | N/A | No | No | No | Yes | No |
| Barth et al. [47] | Yes | No | Unclear | Yes | No | Yes | Yes | No |
| Bouchi et al. [48] | Yes | Yes | Unclear | Yes | Unclear | Unclear | Yes | Unclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Moreira, H.; Moreira, F.; Jesus, Â.; Monteiro-Soares, M.; Santos, P. Factors Influencing the Prescription of First-Line Treatment for Type 2 Diabetes Mellitus: A Systematic Review. Diabetology 2025, 6, 114. https://doi.org/10.3390/diabetology6100114
Silva-Moreira H, Moreira F, Jesus Â, Monteiro-Soares M, Santos P. Factors Influencing the Prescription of First-Line Treatment for Type 2 Diabetes Mellitus: A Systematic Review. Diabetology. 2025; 6(10):114. https://doi.org/10.3390/diabetology6100114
Chicago/Turabian StyleSilva-Moreira, Helena, Fernando Moreira, Ângelo Jesus, Matilde Monteiro-Soares, and Paulo Santos. 2025. "Factors Influencing the Prescription of First-Line Treatment for Type 2 Diabetes Mellitus: A Systematic Review" Diabetology 6, no. 10: 114. https://doi.org/10.3390/diabetology6100114
APA StyleSilva-Moreira, H., Moreira, F., Jesus, Â., Monteiro-Soares, M., & Santos, P. (2025). Factors Influencing the Prescription of First-Line Treatment for Type 2 Diabetes Mellitus: A Systematic Review. Diabetology, 6(10), 114. https://doi.org/10.3390/diabetology6100114









