Abstract
Fundamental research, exploration, extraction, and metallurgical studies of rare earth elements (REEs) require the use of analytical techniques. Recently, emerging developments of analytical instrumentation for REEs have taken place, with some of them having shrunk in size, becoming handheld devices. The Flame and Graphite Furnace AAS, ICP-OES, and MP-AES are standard laboratory techniques used for the analysis of REEs. ICP-MS, ICP-MS/MS, ICP-TOF-MS, HR-ICP-MS, MH-ICP-MS, and MC-ICP-MS are popular techniques for REE analysis thanks to their ultrahigh sensitivity, minimal interference effects, and broad applicability. The INAA, XRF, LIBS, and LA-based ICP-MS techniques are widely employed for the direct analysis of solid samples. The TIMS, SIMS, and SHRIMP are common techniques used for dating isotopic REE deposits. The portable XRF, LIBS, and Raman spectrometer devices can perform on-the-spot in situ analysis, which may help make speedy decisions in the exploration study of REEs. Currently, hyperspectral remote sensing platforms, such as handheld, drone, and satellite-based devices, are preferred for the exploration of REEs due to their cost-effectiveness, which enables the coverage of large areas in a limited amount of time. The use of microanalytical sensors installed on remotely operated vehicles has been successfully applied in analyzing rich REE-bearing deposits in the deep sea. In general, this review provides in-depth information on all essential aspects, from analytical instruments to cutting-edge developments in the analysis of REE-bearing resources.
1. Introduction
Rare earth elements (REEs) constitute a group of 17 metals, including the 15 lanthanides from La to Lu, as well as Sc and Y [1]. Among REEs, Pm is the only element that does not occur naturally in the Earth’s crust. The lanthanide Pm is a scarce element in nature and mostly occurs synthetically as a by-product of uranium fission [2]. Both Sc and Y technically are not part of the lanthanide series. However, these elements are included in the REE group owing to their similar physicochemical properties. In addition, Sc and Y tend to occur in the same mineral deposits as the lanthanide metals [3,4]. Based on the atomic weight and ionic radius, REEs are typically split into two groups: (i) the cerium group of light REEs, consisting of La, Ce, Pr, Nd, Pm, Sm, and Eu, and (ii) the yttrium group of heavy REEs, which consists of Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu plus Y [5,6]. In many studies, Sc is not included in either the light or heavy REE groups, probably due to its distinct geochemical characteristics [7,8,9,10]. Figure 1 presents a list of REEs and their classification.
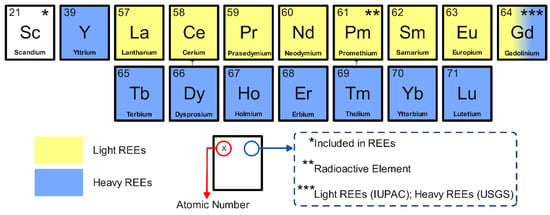
Figure 1.
Classification of REEs. Sc is not a part of either light or heavy REEs. Pm is a radioactive element. Gd is included in the light REEs according to IUPAC, whereas it is classified as a heavy REE according to the USGS.
REEs possess unique electronic, magnetic, and optical properties, which are of great importance in a wide range of high-tech and environmentally friendly technologies [11]. Furthermore, REEs are considered crucial materials for high-efficiency modern apparatuses in terms of energy consumption, enabling handheld tools with high accuracy, speed, durability, and thermal stability [12]. Therefore, the utilization of REEs has skyrocketed in the modern era for applications in various devices, including smartphones, computers, televisions, electric vehicles, wind turbines, advanced military systems, superconductors, and technology-based industries, as well as healthcare [13,14,15]. Indeed, the high demand for these elements has been constantly increasing year by year [16], which has encouraged researchers to conduct exploration, extraction, and metallurgical studies to discover more effective approaches to mining and refining rare earth metals.
In fact, except for Pm, REEs are fairly abundant in the Earth’s crust; however, their widespread dispersion presents a challenge in economic extraction. Moreover, a highly coherent characteristic of REEs is also among the factors that make these elements difficult to extract and purify either as a group or individually [17,18]. REEs are studied as a group on account of their similar characteristics and their frequent occurrence together in geological formations [19,20]. Regardless, REEs exhibit variations in their physical, electronic, and magnetic properties [21]. Thus, it is also significant to comprehensively study REEs as a single element. Recently, developments in analytical techniques for determining REEs in various geological materials have widely progressed, as reported in many investigations [22,23,24]. The scattered information available may limit readers’ understanding of it in a centralized and comprehensive manner. Hence, in the present work, we attempt to summarize emerging techniques in the exploration, extraction, and metallurgical studies of REEs (Figure 2). The study covers the fundamentals and cutting-edge developments in the analysis of REE-bearing resources. Additionally, the geological occurrence and distribution of REEs, as well as a comparison of analytical techniques and existing challenges, are incorporated into the text.
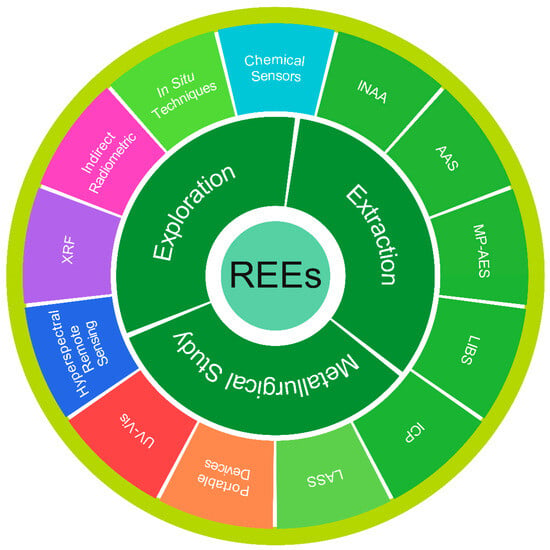
Figure 2.
Scope and objectives of the work: Emerging analytical techniques for REEs.
2. Geological Occurrence and Distribution of REEs
In general, rare earth deposits form through both primary and secondary processes. The primary geological processes include hydrothermal, magmatic, and metamorphic, while the secondary processes involve weathering and sedimentary carriage [25,26,27]. The primary rare earth deposits, originating from primary processes, are typically linked to carbonatites and alkaline-peralkaline igneous rocks. Secondary deposits, on the other hand, are formed from primary deposits that undergo erosion and weathering, such as placer and ion-adsorption deposits [28,29]. The rare earth deposits are distributed across 34 countries, with China (~42.3%) and Brazil (~16.9%) as the first and second reserves, respectively [30]. Table 1 presents the global distribution of rare earth resources.

Table 1.
Rare earth resources and distribution.
Up to the present time, more than 250 minerals in various classes (e.g., carbonates, halides, oxides, phosphates, silicates) have been identified as containing REEs [31], and some of which are presented in Table 2. Commonly, the light REEs are found in either carbonates or phosphates, whereas the heavy REEs are concentrated in oxides [32,33,34]. Bastnäsite and monazite are the two most common minerals discovered in rare earth deposits. Accordingly, the production of REEs is mostly provided by these minerals [35,36]. Among the REE minerals, bastnäsite deposits are considered to be the largest percentage of the world’s economic rare earth resources, which are found in China and the USA. This mineral contains approximately 70% rare earth oxides, excluding uranium and/or thorium. The primary sources of bastnäsite include contact metamorphic zones, pegmatites, vein-type deposits, carbonate-silicate rocks associated with alkaline intrusions, fluorite-bearing and quartz veins, and breccia fillings within Permian sandstone [37,38,39].

Table 2.
Primary rare earth minerals.
Monazite deposits are the second-largest segment occurring in Australia, Brazil, China, India, Malaysia, South Africa, Sri Lanka, Thailand, and the USA [38]. Monazite commonly occurs as an accessory mineral in beach sands, granite, gneiss, complex ores, and other igneous and metamorphic rocks [40]. Due to its high specific gravity, monazite is associated with heavy minerals and exhibits strong resistance to chemical weathering [41]. This mineral typically contains 10–40% La2O3, 4–12% ThO2, 20–30% Ce2O3, along with significant amounts of Pr, Nd, and Sm, making it a major source of Ce and light REEs [42]. Similarly to bastnaesite, monazite also contains approximately 70% rare earth oxides, with uranium and thorium also present [43]. Xenotime is an yttrium phosphate mineral that contains ~67% of rare earth oxides and constitutes a source of heavy REEs [44]. Allanite generally occurs in igneous rocks as an accessory mineral, such as diorites, granites, and syenites, as well as associated pegmatites. This mineral is rare because its concentration is not sufficiently enriched to form REE ores [45]. Euxenite is an oxide mineral typically occurring in granite pegmatites, where it is associated with feldspars, ferrocolumbite, ferrotantalite, monazite, and quartz [46]. Other resources that are less studied include apatite, cheralite, eudialyte, loparite, phosphorites, rare-earth-bearing ion-absorption clays, secondary monazite, and spent uranium solutions, among others.
3. Development of Analytical Techniques for REE Study
Before analyzing REE-containing rocks or minerals, the preparation of samples is crucial to obtain the best results. For instance, in the ICP-MS analysis, it is necessary to prepare the samples effectively through complete digestion. To ensure reliable data analysis, high-purity acids are used to eliminate interfering agents, such as oxides and hydroxides [47]. Sample preparation methods for studying REEs in geological samples include acid dissolution, fusion dissolution, microwave, and infrared radiation methods [48,49,50]. Acid dissolution methods are reasonably effective for decomposing geological materials. The mixtures of hydrofluoric, hydrochloric, nitric, and perchloric acids can be used for sample preparation to completely dissolve refractory mineral phases [51]. In some cases, acid digestion methods result in low recoveries of REEs. Alkali fluxes, such as lithium borate, are an alternative method for producing homogeneous synthetic glass disks suitable for XRF and LA-ICP-MS measurements. Accordingly, Leitzke et al. [52] utilized high-purity sodium tetraborate mixed with rock samples at high temperature. Schramm [53] used REE rock sample/lithium tetraborate flux with a 1:1 ratio in a platinum crucible. The XRF analysis was performed on the beads obtained after fusion at 1000 °C in a muffle furnace. A great analytical performance was obtained with detection precision for Sm, Gd, Eu, Y, and Th of less than 1%. The dissolution of REE ores is challenging, as it often yields low recovery rates, since associated minerals are highly resistant to conventional sample digestion methods [54]. For microwave methods, Kasar et al. [55] digested geochemical reference materials and soils containing REEs, Th, and U using an effective microwave method to assess the best quality control for REE analysis. Microwave digestion methods provide a fast and complete dissolution of REE-bearing samples. Helmeczi et al. [49] applied short-wavelength infrared radiation to enhance the acid digestion of refractory REE ores, resulting in more efficient digestion within a shorter timeframe.
Generally, REEs are present in small amounts in most rocks; therefore, selecting an appropriate technique that meets standards for reliable quantification is also an important step in the analysis, in addition to sample pretreatment [56]. The ICP-MS has led to the progression of various ICP-MS types that perform rapid elemental and isotopic analysis, with high sensitivity and minimal interference effects [57]. The integration of microelectronics and computer technology into XRF or LIBS enables these analytical techniques to shrink in size, making them portable devices that allow operators to apply them directly in the field to collect data quickly. These integrated techniques not only simplify the analysis of REEs in a wide variety of industrial materials but also enable rapid analysis [58,59]. The ICP-MS/MS and HR-ICP-MS offer an effective separation of spectral interferences in the analysis of REEs with a low detection limit at the pg/g levels [60]. Internal standardization diminishes the effects of shifts, as they are typically nonlinear, mass-dependent, and frequently change when analyzing a large mass range. Often, offline data reduction procedures assist in shift correction [61]. Figure 3 demonstrates an outline of the analytical techniques commonly employed for the analysis of REEs, which include elemental, isotopic, and mineralogy-related research studies.
Figure 3.
Schematic of analytical techniques used in REE exploration, extraction, and metallurgical studies.
In the REE study, analytical techniques ranging from the simplest, such as UV-Vis spectroscopy, to the more complex, including the SHRIM instrument, are employed. Recent advancements in such analytical techniques and their applicability in the analysis of REEs in various samples, including those during exploration, extraction, and metallurgical processes, are the focus of this review.
3.1. Ultraviolet-Visible (UV-Vis) Spectroscopy
UV-Vis spectroscopy is among the oldest techniques commonly employed to detect low levels of several metals, including REEs [62]. However, this technique is limited to the colored solution. If the desired substance is not self-colored, complexation is required to achieve a higher molar absorptivity, which enables sensitive determination. A selective ligand that binds to the metal is commonly used in this regard. In the measurements, the color intensity of the measured solution is directly proportional to the concentration of the analyzed element. By comparing it with the standard samples, an estimate can be made [63,64]. Several spectroscopic techniques were reported for the analysis of REE-containing samples. For example, Pu et al. [65] reported an ultrasensitive, selective, and accurate method based on spectrophotometry for determining the sum of REEs. Prior to analysis, REEs were preconcentrated online using flow-injection analysis with an aminophosphonic-carboxylic acid resin. The adsorbed analytes were then desorbed with hydrochloric acid and reacted with chloroacetate buffer containing Arsenazo III. Employing a spectrophotometer, the proposed method performed a linear dynamic range up to 1 µg/mL for La3+ with a detection limit of 4 ng/mL. Convincingly, the proposed method was applied to determine the sum of the REEs in real geological and aluminum alloy samples. Due to the similar properties of REEs, determining these elements individually can be challenging. Saputra et al. [66] proposed a fast simultaneous analysis method for the quantitative determination of Sm, Eu, Gd, Tb, and Dy in monazite samples by combining UV-Vis and multivariate analysis. Complexation was not required in this case since multivariate calibration was applied to analyze the data (Figure 4). The method demonstrated excellent performance towards REEs, yielding results consistent with those obtained using ICP-OES. Wyantuti et al. [67] conducted a comparative study on the determination of liquid–liquid extraction of Sm using voltammetric and UV-Vis measurements. In this investigation, the UV-Vis analysis method was reported to show better performance with a lower detection limit than the DPV method (LOD = 0.7 vs. 1.24 mg/L).
Figure 4.
Schematic diagram of REE determination by multivariate analysis-combined UV-Vis spectroscopy. Modified from [66].
Although UV-Vis spectrophotometry is a well-established technique for REE determination, it mostly suffers from spectral interference from other components. Furthermore, the methods are neither very sensitive nor simple. Moreover, with the availability of multielement analytical techniques, UV-Vis spectroscopy appears to be less popular today, especially in REE exploration, extraction, and metallurgical studies.
3.2. X-Ray Fluorescence Spectrometry (XRF)
XRF is a common analytical technique used to analyze the elemental composition of geological materials, including rocks, sediments, soils, and ores. Based on the excitation, dispersion, and detection methods, XRF is classified into energy-dispersive (ED-XRF) and wavelength-dispersive (WD-XRF) types. Table 3 presents the comparison of them. Both types of XRF are popular in REE studies [68,69]. Generally, XRF measurements involve bombarding the sample using high-energy X-rays, which causes the ionization of atoms in the sample or the release of electrons. The inner shell electron holes are filled by outer-shell electrons, accompanied by the energy release in the form of a photon. The radiation emitted indicates the difference in energy between the two shells involved, which in turn reflects the sample’s characteristics. Meanwhile, the concentration of the samples is proportional to the intensity. When the standard of a particular element is used, the measurement of the same element in a sample is possible [70]. XRF is a non-destructive, multielement, and reliable technique that has analytical capabilities at the µg/g level. Nevertheless, this technique suffers from high LOD, which makes it mostly unsuitable for the analysis of many geological materials [22]. Therefore, certain procedures are required, such as preconcentration and/or separation of REEs for accurate determination.

Table 3.
Characteristic comparison between ED-XRF and WD-XRF.
Several examples of the XRF technique’s application in REE analysis are discussed. Zhang et al. [71] proposed a simple method coupling solid phase extraction for the simultaneous detection of trace REEs in water samples employing XRF. Juras et al. [72] identified REEs in various types of geological samples from ultramafic rocks to rhyolites. An ion-exchange procedure was employed in this case to separate REEs from other constituents. De Vito et al. [73] used a complexing agent o-[3,6-disulfo-2-hydroxy-1-naphthylazo]-benzenearsonic acid to preconcentrate REEs via a chemo-filtration process. A polyamide membrane-retaining chemo filtrate leads to the removal of the interelement effect when measured using XRF. The preconcentration procedure enabled the detection of very low contents of REEs. Wu et al. [74] used XRF to analyze REEs in alloys, concentrates, compounds, functional materials, minerals, ores, raw metals, rocks, and soils. Before measurement, the samples were pretreated using a fast separation and preconcentration procedure, which included ion-exchange and precipitation. Losev et al. [75] preconcentrated REEs in fossil raw materials using aminophosphonic group-chemically modified silicas. Afterward, ICP-OES measurements were carried out to quantitatively analyze CRM, coal ash, and REE-bearing lignite and coal. The WD-XRF analytical technique was designed to analyze L-lines of REEs in the range of 4.5 to 7 keV, with a sensitivity at the µg/g level. De Pauw et al. [76] used this technique to determine REEs in geological materials (cometary, asteroidal, or interstellar materials). The method was reported to successfully detect REEs in the deep-earth diamond inclusions, performing a LOD of 0.5 µg/g for characteristic L-lines, 10 times lower than that of regular K-lines. Adeti et al. [22] confirmed the lack of a tube-based XRF technique in REE-bearing sample analysis, which is mainly from the interference disturbances between relatively low L-series line intensities of REEs and K-series X-ray emission from transition metals. XRF and ICP-MS techniques were employed to analyze carbonatite tailings, which showed a recovery of ~9% REEs [77]. An ED-XRF spectrometer can measure multiple elements in various geological samples, including those with concentrations as low as 0.03% [78].
In general, the XRF technique is insufficient to determine low concentrations of REEs at levels below µg/g. Nevertheless, the analysis is possible if the separation and/or preconcentration are carried out prior to further analysis.
3.3. Instrumental Neutron Activation Analysis (INAA)
INAA represents a highly sensitive and broadly applicable technique for precise multielement analysis. This technique can accurately determine a wide range of REE concentration levels from major to trace elements in various geological samples [79]. Typically, the measurement is performed by subjecting polythene bags containing a small number of samples with standards to a neutron flux within a nuclear reactor. During irradiation, stable nuclei capture neutrons, then transform into radioactive nuclei and subsequently decay with particle emission (Figure 5). The nuclide is identified through the gamma-ray energy, where the intensity is proportional to its concentration. The concentration of a particular nuclide is determined by comparing it with the standard. In the quantitative analyses, scintillation and semiconductor-based detectors are most commonly employed. Since nuclear reactions are almost unaffected by a material’s physicochemical structure during irradiation, the matrix composition has only a small influence on the induced activity [80]. INAA is very popular when nuclear reactors for sample irradiation are available. Although the nondestructive INAA technique detects REEs at the ng/g levels in some rock types, achieving accurate measurements is difficult when highly radioactive matrix radionuclides generate a significant background in the gamma-ray spectrum. In some cases, following sample irradiation, the matrix is separated using methods such as coprecipitation, ion-exchange, and solvent extraction, and the measurements are subsequently carried out through radiochemical NAA [81]. However, many studies employed the INAA technique to determine REEs in various earth materials.
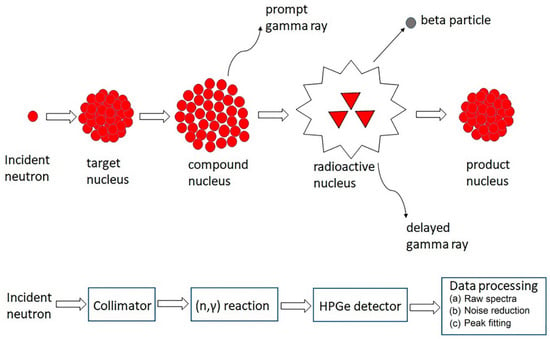
Figure 5.
NAA principles [82]. © 2023 Mathew, Kshirsagar, Abidin, Griffin, Kanarachos, James, Alamaniotis, Fitzpatrick.
Depending on the background intensity and half-life, various elements are determined using INAA and ED-XRF techniques. Ravisankar et al. [79] employed the INAA technique to determine REEs in beach rocks using the thermal comparator K0. Along with SRM 1646a estuarine sediment, the samples were irradiated with thermal neutron flux using the Kalpakkam mini reactor. As many as 15 different samples were analyzed using HR gamma spectrometry in this investigation. Kumar and Elias [83] employed the INAA technique to investigate the distribution and concentration of REEs (Sm, Eu, Yb, Lu, and Tb) in surface sediments of mangrove. Along with standard reference materials SL-1 and blank, the samples were irradiated prior to measurement. Ahmed et al. [84] determined gold-mining site-collected soil samples containing REEs using INAA to study the concentrations of REEs. The detection accuracy was evaluated toward REE data using SRM-NIST 2586. Kin et al. [85] reported a comparative study on REE determination using INAA and ICP-MS. Although INAA could not precisely determine several REEs (Pr, Gd, Dy, Ho, Er, Tm) due to their short half-lives, INAA was found to be a more powerful technique with high detection accuracy and precision. Alternatively, they can be determined using radiochemical NAA techniques. El-Taher et al. [86] investigated the capabilities of INAA, RNAA, WD-XRF, ED-XRF, and total reflection XRF in analyzing REEs in various samples, including rocks, minerals, sediments, and soils. Among the techniques, INAA appears to be an appealing method for determining REEs, as it provides reliable data within a reasonable timescale. The detection accuracy depends on the types of materials and the concentrations of elements of interest. Adeti et al. [22] employed Ag-anode X-ray tube XRF, Am-241 excitation-based XRF, INAA, and regular XRF nuclear techniques to analyze Sc, La, Ce, Nd, Sm, Eu, Tb, and Lu in volcanic rocks and the IAEA-Soil-7 CRM. Except for the Ag-anode X-ray tube XRF, other techniques demonstrated comparable analytical results, with the Am-241 excitation-based XRF technique providing the best results. This technique is faster, with a shorter analysis time, compared to others, as it requires minimal sample preparation.
Although INAA is a highly sensitive technique for multielement analysis, it possesses limitations. This technique requires matrix-matching references for calibration to enable the measurement of X-rays emitted from samples subjected to self-attenuation under the same counting conditions. The INAA is a sluggish and non-self-governing technique, which requires longer cooling times for specific elements. Instead, some nuclides cannot be determined accurately due to their very short lifetimes.
3.4. Indirect Radiometric-Based Measurement
An exploration study of REEs was reported to use radioactivity measurement [87]. The contents of radioactive U, Th, and K elements were observed to correlate directly with mineralization of REEs. Therefore, radiation measurement is a valuable exploration technique for REE study, presenting an outlook for the industry. A handheld γ-ray spectrometer is used to directly analyze drill core samples for the radioactive elements U, Th, and K. At the same time, the individual REEs were determined with ICP-MS. Interestingly, the radioactivity in the study area was observed to be entirely influenced by U, with the sum of REEs showing an acceptable correlation with both radioactivity and U. Indeed, this represents an indirect determination of the sum of REEs using a γ-ray spectrometer device. In a separate study, a high-purity germanium energy spectrometer was employed to measure the radionuclide levels of 238U, 232Th, 226Ra, and 40K in the surrounding soil samples [88]. Radiometry has been applied to the study of beaches, alluvial placers, and the east coast of India for geochemical and radionuclide exploration. The total REE concentrations in Paradeep Beach sediments were found to be approximately 90 and 20 times higher than average crustal abundance levels. Coastal sediments at specific sites along the east coast of India exhibit significant enrichment in radionuclide and REE concentrations [89].
3.5. Atomic Absorption Spectrometry (AAS)
AAS is classified into two types: flame AAS (F-AAS) and graphite furnace AAS (GF-AAS). The F-AAS quantifies an analyte by measuring the light absorbed by its atoms at specific wavelengths in the flame. The analyte concentration is estimated by comparing it with the standard samples [90]. Figure 6 demonstrates a schematic diagram of the AAS instrumentation. The F-AAS technique is typically used for single-element analysis and is most useful for very high analyte concentrations, especially in the context of REE analysis. This issue is due to the low sensitivity of the instruments. Besides that, the interference effects from elements like Si, Na, Fe, Al, HF, and phosphates, as well as other REEs, still occur even after applying a high-temperature acetylene-nitrous oxide flame. However, the F-ASS technique is widely used by many industries because of its low-cost analysis. The individual determination of REEs is successfully carried out using F-AAS by adding excess potassium, which suppresses ionization interferences. The more advanced GF-AAS technique provides superior sensitivity by introducing a small volume of sample solution into a graphite tube for analysis. This technique is suitable for analyzing metal samples at ultra-trace levels of ng/mL [91].
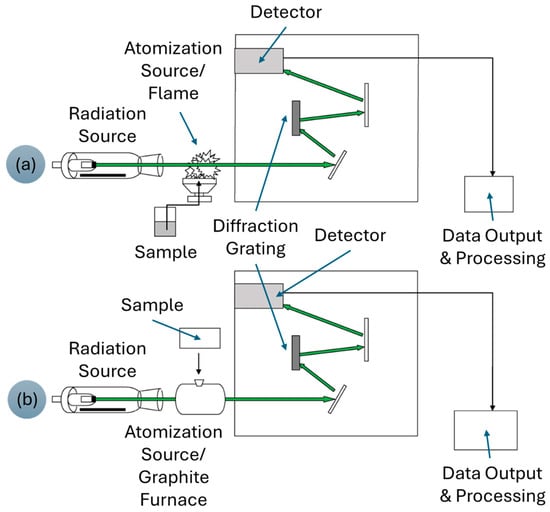
Figure 6.
Schematic diagram of (a) F-AAS and (b) GF-AAS. Adapted from [92] © 2016 Ring, O’Mullane, O’Riordan, and Furey.
Currently, powerful, sophisticated techniques, such as ICP-MS, have shifted the dominance of ASS techniques in REE studies, particularly at low concentration levels.
3.6. Microwave Plasma Atomic Emission Spectrometry (MP-AES)
Introduced in 2011, MP-AES serves as an alternative technique to F-AAS and ICP-OES [93]. The MP-AES technique utilizes a novel plasma torch design that utilizes nitrogen gas to generate high-temperature microwave plasma. This technique is particularly attractive for analyzing industrial effluent, water, sediment, soil, rock, and ore samples. The process begins with the conversion of the sample solution into an aerosol, which is then introduced into the central plasma channel for atomization. At this stage, most excited species remain in the atomic state, since the excitation temperature of the microwave plasma is insufficient for further electron release. The characteristic lines of the target elements are isolated and subsequently analyzed through a detector System [94,95]. Helmeczi et al. [49] used MP-AES to determine REEs after the dissolution of ores using a rapid digestion procedure. The results were reported in good agreement with those obtained using ICP-MS, indicating that MP-AES is a valuable technique for analyzing REEs in geological materials. The advantage of this technique is a favorable low LOD and a low analysis cost.
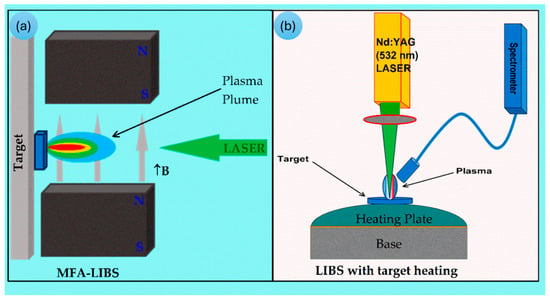
Figure 7.
Schematic representation of experimental arrangements for (a) magnetic field-assisted and (b) thermal LIBS [96]. © 2024 Fayyaz, Baig, Waqas, and Liaqat.
3.7. Laser-Induced Breakdown Spectroscopy (LIBS)
The versatile LIBS is a rapidly advancing analytical technique for characterizing a wide range of materials, enabling real-time analysis of elements, molecules, and isotopes [97]. Typically, LIBS is setup either with a magnetic field or target heating (Figure 7). The use of LIBS in the measurements minimizes sample damage. The LIBS technique is widely used for geochemical studies, industrial production processes monitoring, and remote material analysis, even in harsh environments. generating a microplasma composed of electronically excited atoms and ions. Excimer and Nd:YAG lasers are the two most common lasers used to produce very short plasma pulses, typically 5–20 ns in duration. Excimer lasers are a form of UV laser [98]. To obtain the qualitative or quantitative data on the elements in the samples, the characteristic spectral lines emitted are analyzed. LIBS is an in situ analysis technique that requires little or even no sample preparation [99]. Since LIBS suffers from a high LOD and spectral interferences, it is not easy to determine the REEs, especially when relatively low REE contents are present in complex matrices. Despite this, the LIBS technique has been widely employed for quantifying elements in various geological materials. Various sample types can be directly analyzed by LIBS, as it requires minimal or no sample preparation. In liquid sample analysis, LIBS suffers from inherent limitations, including splashing, surface ripples, intensity quenching, and a shorter plasma lifetime [100].
Abedin et al. [101] employed LIBS to identify monazite sands and found La, Ce, Pr, Nd, Y, Yb, Gd, Dy, Er, and other elements (Zr, Cr, Ti, Mg, Mn, Nb, Al). Bhatt et al. [102] determined the concentrations of La, Ce, Nd, Y, Pr, Sm, Eu, Gd, and Dy in geological samples using LIBS, demonstrating good agreement with the ICP-MS analysis results. LIBS was used to quantify phosphor-doped La and Nd, and then the performance was confirmed using CRM [103]. The main limitations of LIBS analysis are matrix effects and measurement uncertainties. To address the issues, Jie et al. [104] used a data selection method to improve the precision in LIBS measurements. The LIBS technique was applied for detecting REEs and other elements, such as Si and P, in beach sands [101]. LIBS can be utilized for the automatic recognition of coal and rock with high accuracy when combined with an artificial neural network [105]. However, LIBS is not a particularly sensitive technique for determining REEs. Detecting trace REEs is more difficult because weak signals often go undetected due to strong interference from other elements. Gaft et al. [98] effectively detected REEs through atomic and ionic emission lines. Simultaneous elemental and molecular plasma-induced luminescence-combined LIBS exhibits ultrasensitive analysis towards Eu, Sm, Dy, Gd, and Pr. This combination could accurately determine REEs with substantially longer acquisition times. In the LIBS analysis, the interpretation of complex REE spectra is mainly hindered by spectral interferences and weak emission lines at low REE concentrations. Since the emission intensity is independent of the laser wavelength, Afgan et al. [99] utilized IR, visible, and UV radiation to achieve higher intensities, even with low REE levels in monazite. Bhatt et al. [106] utilized LIBS for the study of chemical imaging and distribution analysis of REEs in coal. The results were then compared with those obtained using LA-ICP-MS. In this case, LIBS performed a relatively rapid analysis with a less complicated sample preparation procedure compared to LA-ICP-MS.
3.8. Inductively Coupled Plasma (ICP)
3.8.1. ICP-Optical Emission Spectrometry (ICP-OES)
ICP-OES is a popular multielement analytical technique for determining various metals. This technique is not only applicable to REE analysis, but also to other elements in geological materials [107]. In the ICP-OES analysis, the liquid samples are first introduced into the System to convert them into an aerosol. Afterward, the as-produced aerosols are transported to the high-temperature ICP, undergoing subsequent desolvation, vaporization, atomization, and ionization. The electrons absorb energy from the plasma, resulting in a transition from one energy level to another. When the electrons return to the ground states, they emit light of a wavelength specific to each element. The concentrations of the measured elements are estimated by comparing the measured emission intensities to the intensities of the standard. In terms of performance, the ICP-OES possesses a broad linearity range with medium sensitivity. Moreover, this technique can analyze up to 60 elements in various samples [108,109]. Makombe et al. [110] employed ICP-OES to determine REEs in sediments, with sample preparation involving four-acid digestion (HCl, HF, HNO3, and HClO4) followed by lithium metaborate fusion. The analytical results were then compared with the ICP-MS results, showing good agreement. As well, the results obtained are within the reference material’s confidence limits. Compared to quadrupole-ICP-MS, ICP-OES exhibits relatively low sensitivity, likely due to non-spectral and spectral interferences. Indeed, it makes ICP-OES susceptible to detecting low REE contents in most rock samples. However, this issue can be addressed through separation and preconcentration methods, which reduce the limit of detection and minimize interferences [111]. The ICP-OES analysis of REEs is challenging due to their complex spectra, which involve hundreds of possible orbital transitions and associated emission wavelengths. Near-overlap and overlap spectra make the trace-level analysis of REEs nearly impossible. Nonetheless, it can be addressed by using alternate wavelengths to help waive possible interferences. Additionally, some interference-reducing methods, such as separation, solvent extraction, ion-exchange, and precipitation, enable the removal of specific elements that cause spectral interferences in the rock matrices. Pradhan and Ambade [112] proposed a solvent extraction method for preconcentrating REEs from geological samples (rock, soil grabs, stream sediments, beneficiation products, minerals, and core samples) before ICP-OES analysis. These pretreated samples were then analyzed using ICP-OES to estimate the content of REEs, with detection precision (RSD) in the range of 1–10% depending on the lanthanide concentrations. Nóbrega et al. [108] utilized the ‘radial view mode’ and ‘dual view mode’ ICP-OES to determine all REEs (including Sc and Y) in the samples. A minimal matrix effect was observed, as geological and agricultural samples were treated using a microwave digestion method prior to measurement.
3.8.2. ICP-Mass Spectrometry (ICP-MS)
Currently, ICP-MS techniques hold a valuable position in modern laboratories due to their ability to perform multielement analysis, their limited interferences, high detection sensitivity, precision, and accuracy, as well as the instrument’s simplicity. Numerous instrumental advances have been implemented in various forms of ICP-MS instruments to enhance analytical performance, particularly in overcoming interference and improving the detection accuracy of elemental and isotopic determinations [113,114]. Four decades ago, the integration of a quadrupole mass spectrometer with an ICP presented a respected addition to the existing elemental and isotopic analysis techniques [115]. The solution samples are passed through a nebulizer and spray chamber, where they are converted into an aerosol. After that, the aerosol is introduced to the ICP, where it undergoes heating at ~9000 K. The process as a whole encompasses desolvation, vaporization, dissociation, atomization, and ionization. Next, singly charged positive ions pass through a differentially pumped interface in the quadrupole mass spectrometer. The quadrupole mass spectrometer, depending on the applied RF/DC potential, transmits only ions with a specific mass-to-charge (m/z) ratio. Any neutral species present in the plasma gas is removed by being pushed out by the vacuum pump. After mass isolation, the ions pass through the detector System. It typically includes an electron multiplier, which generates signals using a pulse-counting approach. Additional sample introduction systems, such as laser ablation and chromatography, can be coupled with ICP-MS to enable direct solid analysis and improve speciation capabilities [51].
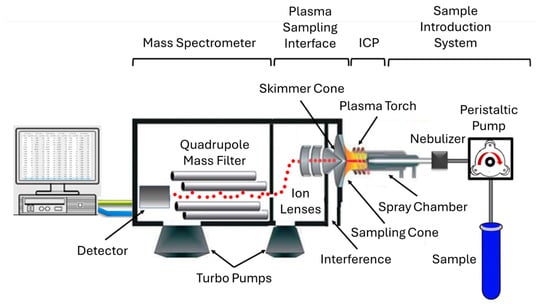
Figure 8.
Schematic illustration showing the basic components of ICP-MS [116]. © 2022 Mazarakioti, Zotos, Thomatou, Kontogeorgos, Patakas, and Ladavos.
F-AAS and ICP-OES require the separation of REEs from the rock matrices to minimize the interferences. This separation can be carried out using coprecipitation, or solid-phase or solvent extraction methods [117]. Otherwise, the ICP-MS technique can analyze REEs, where the samples are nebulized without requiring separation and preconcentration steps. ICP-MS has been established as a powerful analytical tool, particularly for the analysis of geological materials. Figure 8 shows a typical instrumentation of ICP-MS. Even compared to ICP-MS, INAA is a relatively slow technique for analyzing geological REE samples, likely due to the requirements for sample irradiation and cooling. In terms of performance, quadrupole ICP-MS offers very low detection limits in the ng/mL level [118,119]. Regardless of its advantages in REE analysis, the ICP-MS suffers from matrix interferences that hamper the detection accuracy [120]. To address this interference issue, methods like internal standard, matrix-matching calibration, ion-exchange, solvent extraction, and collision cell can be applied instead of standard methods (standard addition, isotope dilution, and integrated sample introduction and aerosol dilution systems). Moreover, the optimization of ion lenses plays a decisive role in ensuring accurate determination of REEs, as it directly influences ion transmission efficiency and signal stability in ICP-MS analysis. When coupled with a collision cell, ion lens tuning enables the significant reduction in interferences, thereby enhancing the reliability and precision of REE measurements, even in complex sample matrices [121].
Often, exploration samples comprise a highly refractory phase, which is effectively digested by alkali fusion. ICP-MS can then analyze such samples with a very low LOD and fast analysis without sample preconcentration [122]. Lin et al. [123] employed ICP-MS to study coal-bearing REEs. The investigation revealed that approximately 25% of the REE concentration was associated with the organic matter, where the heavy REEs were relatively enhanced. Regardless, Buyko et al. [124] determined REEs in lignite and volcanic fumarole sediment by ICP-MS after sample preconcentration using polyamines and carboxyarsenazo-modified biosilica layer-by-layer. Baghaliannejad et al. [125] performed the selective extraction of REEs from uranium materials using ICP-MS analysis and ICP-OES. Before analysis, the samples were separated from the matrix using tri(2-ethylhexyl) phosphate via solvent extraction. Xu et al. [126] employed the ICP-MS technique to analyze ore samples for high-throughput comprehensive REE fractionation. Petrova et al. [127] separated and preconcentrated impurities in RE materials for ICP-AES and ICP-MS analysis. Nguyen et al. [128] proposed a simple separation method coupled with HPLC-ICP-MS for determining ultra-trace REE impurities in pure europium and ytterbium oxides (REE2O3). The formation of 153Eu16O+ and 174YbH+ directly overlaps with monoisotopic 169Tm+, and the most abundant isotope of Lu (175Lu+). The proposed method meets the standard for the routine analysis of trace-level REE impurities in high-purity Eu and Yb oxides. ICP-MS was reported in a study for the determination of Sc, Y, and other REEs in Ni laterites [129]. Except in barite samples, barium is normally under 2 mg/g in several types of geological materials. The detection of REEs in geological materials is hindered by spectroscopic interferences derived from Ba, Ba oxides, and hydroxides in barite samples. Accordingly, separation techniques like ion-exchange chromatography are occasionally required to remove Ba impurities from REEs. Liu et al. [130] developed an ion-exchange-based separation method for use in barium removal. The pretreated samples, which are barium-free, were then analyzed using ICP-MS to precisely determine the REE contents.
Determining REEs in surface, groundwater, rainwater, and ice is challenging by the reason of the inadequate sensitivity of analytical techniques. In addition, matrix interference, potential contamination, and the lack of water CRMs are also among the limitations. Wysocka [131] addressed such problems by offering analytical strategies to obtain the most reliable REE measurements in natural water samples. Mathematical evaluations, multivariate analysis, and internal standards can be applied to accurately detect REEs with a precision of less than 15%. The ICP-MS technique was used to investigate the economic aspects of the REEs in coal by-products [132]. In a different study, ICP-MS was used to detect REEs and trace elements (e.g., U and Th) to understand the characteristics of the parent sediments and to establish elemental fingerprints in phosphate rocks [133]. ICP-MS was used to analyze fly and bottom ash containing REEs, which in turn can be a promising source of REEs. ICP-MS was employed to detect REEs in lake bottom sediments, surface waters, and ore processing areas to assess anthropogenic inputs [134]. Wencai et al. [45] employed ICP-MS to detect REEs in marine sediments with 115In and 185Re as internal standards to minimize signal drift and matrix effects. Two pretreatment methods for ICP-MS analysis of REEs were compared in this regard, namely, closed acid dissolution and alkali sodium peroxide fusion.
Accurately detecting REEs in seawater is challenging due to the combination of high salt concentrations and inherently low REE concentrations. This environment will cause matrix interference, signal shift, and frequent clogging of the sample recognition system. Li et al. [135] integrated an automatic separation System (ELSPE-2 Precon) with ICP-MS to eliminate the high-salt matrix in seawater samples, providing accurate measurements of REEs. Wysocka et al. [136] employed a similar method to determine REEs in natural waters from wells and mineral water bottles, achieving a high recovery rate of approximately 98%. Li et al. [137] employed ICP-MS to determine REEs from seawater after sample pretreatment. A mini column packed with calcium carbonate-containing crab shell particles was used to simultaneously separate the matrix agents and preconcentrate REEs. The REE contents in seawater were found to be in the range of 0025–0.172 ng/mL, with a low LOD ranging from 0.6 to 8.8 pg/mL. The recoveries of REEs were obtained in the range of 95.3 to 104.4%, indicating the efficiency of the current extraction procedures.
3.8.3. ICP-Tandem MS (ICP-MS/MS)
Although the ionization efficiency is nearly 100%, the classical quadrupole ICP-MS cannot easily determine REEs, likely because of the very close physicochemical and spectral characteristics associated with inter-elemental spectral interferences. For instance, polyatomic oxide and hydroxide species, such as 137Ba16O+ and 136Ba16O1H+, cause spectral interference toward 153Eu+ and 139La16O+ toward 155Gd. The spectral interference issue can be addressed using approaches such as blank subtraction, temperature-controlled sample introduction Systems, interference correction, sample pretreatments, internal standards, standard addition, isotope dilution, mixed plasma, matrix-matched calibration, cool plasma technology, HR-ICP-MS, and collision/reaction cell techniques [138]. Recent studies have shown that tandem ICP-MS (ICP-MS/MS), featuring collision capabilities and two quadrupole analyzers, provides optimal REE analysis with detection limits at the pg/mL level. An NH3 cell gas was observed to react with polyatomic ions that interfere with REE measurements, thereby providing great benefit in reducing interferences. Nevertheless, NH3 gas reacts with some REEs, which obviously leads to a reduction in detection sensitivity. Zhu et al. [139] carried out a comparative study on the reaction gases for removing interference in the determination of REEs in river water reference materials. Using ICP-MS/MS, N2O gas was observed to perform more accurately compared to O2 gas when applied in the analysis. The use of N2O reaction gas helps minimize Ba-related interferences, yielding a lower LOD. Santoro et al. [140] utilized N2O2 fusion coupled with ICP-MS/MS to rapidly analyze REE levels in quartz-rich materials. In this investigation, four acquisition modes of (i) no cell gas, (ii) He, (iii) H2, and (iv) indirect mass-shift mode using O2 gas were applied to the most abundant isotopes. The analytical performance in terms of suitability and detection accuracy was evaluated by analyzing the geological CRM and QLO-1 (USGS). Additionally, it was compared with the results from INAA, showing good agreement.
Lancaster et al. [141] studied the application of N2O as a reaction gas for determining more than 70 elements, including REEs. The ICP-MS/MS method was applicable for fast sample screening of quartz-rich geological areas to determine REE concentrations during an exploration study. Tandem ICP-MS or ICP-MS/MS is a powerful technique comparable to high-resolution ICP-MS (HR-ICP-MS). Zhu [142] employed ICP-MS/MS to detect REEs in seawater after sample pretreatment, which involved the coprecipitation with magnesium hydroxide. The mass-shift mode was employed by passing each isotope through the first quadrupole, where it reacted with oxygen to form a metal monoxide. The resulting monoxide was subsequently transmitted and measured by the detector. The U-Th-Pb in situ LA-ICP-MS/MS with ESI NWR193UC was used to date monazite and bastnaesite [143]. The results showed that bastnaesite and monazite, respectively, are ~602 and ~589 Ma in age, indicating that the crystallization of bastnaesite was speedily followed by monazite alteration. This study established the applicability of the ICP-MS/MS technique in conjunction with LA sampling or in situ dating studies.
3.8.4. ICP-TOF-MS
ICP time-of-flight MS (ICP-TOF-MS) advances the analysis of geological material-bearing REEs to a new level [144,145,146]. Primarily, ions drawn from the ICP source are guided by ion optics and steered toward a Repeller region. In this stage, all ions are electrostatically accelerated to a uniform kinetic energy within a field-free flight tube and reflected toward a drift region. After carrying an equal distance in the reverse direction, these ions reach the detector. This results in a faster focusing speed and an enhanced overall flight trajectory, which significantly improves resolution. Since all ions are accelerated to the same kinetic energy, those with varying masses travel at different velocities through the flight tube (Figure 9). ICP-TOF-MS enables the rapid acquisition of approximately 30 K scans per second, offers high ion transmission, and allows for quasi-simultaneous analysis of all masses within an ion packet extracted from the source. This leads to improved performance, particularly in terms of detection limit, compared to quadrupole ICP-MS analysis. Dick et al. [147] used ICP-TOF-MS to detect REEs in Antarctic ice, achieving recovery rates of approximately 103% with a detection precision of around 3.4%. This investigation showed that ICP-TOF-MS meets the demands of restricted sample mass by demonstrating good agreement results with ICP-MS and HR-ICP-MS analyses. LA-ICP-TOF-MS was applied to determine REEs from zircon crystals. Also, this instrument was employed to determine U-Pb ages at sampling depths ranging from 0.59 to 0.66 µm [148]. Peng et al. [149] employed rapid, high-resolution LA-ICP-TOF-MS mapping to visualize critical metals by determining trace elements, including REEs. The advantage of this process lies in its time-saving nature, due to high-speed stage movement and microscale lateral resolution. The simultaneous analysis capability of LA-TOF-MS enables the rapid multi-element detection of transient signals, such as laser ablation imaging in a low-dispersion ablation cell. Additionally, it is well-suited for 2D and 3D material imaging and is expected to supersede LA-ICP-MS for these applications in the near future [150].
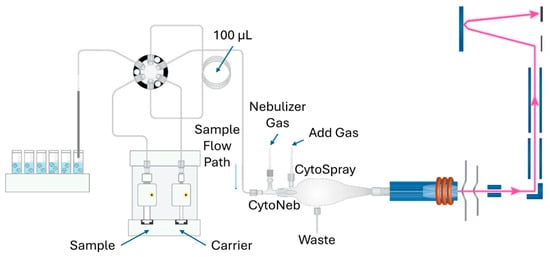
Figure 9.
Schematic illustration of the detection System of the ICP-TOF-MS setup. Adapted from [151] © 2022 Manard, Bradley, Quarles, Hendriks, Dunlap, Hexel, Sullivan, Andrews.
3.8.5. Magnetic Sector/High-Resolution ICP-MS (HR-ICP-MS)
The HR-ICP-MS technique basically integrates an ICP source with a magnetic sector analyzer that has double-focusing capability. This instrument is typically used for the analysis of trace metals and/or isotope ratios [152,153]. The HR-ICP-MS technique utilizes electromagnetic fields to separate trace analytes within a complex system. The ions are produced from the plasma and then accelerated into the ion optic region to a few kilovolts (kV) prior to entering the mass analyzer. The magnetic field directs ions with varying trajectories toward a common focal point beyond the entrance slit. Then, the electrostatic analyzer focuses the ions onto the exit slit, in which a detector is positioned. High mass-resolution HR-ICP-MS can identify almost any targeted analyte ions from interfering molecules and/or atomic isobars [154]. Another advantage of this method over traditional quadrupole ICP-MS is the higher transmission, supported by low background levels. Typical oxide and hydroxide interferences can be minimized by adjusting the instrument’s resolution settings.
To achieve optimum analysis performance, the use of an ultrasensitive HR-ICP-MS technique should be under a clean environment. This technique offers ultrasensitivity by convincingly resolving spectroscopic interferences. Balaram et al. [155] proposed a quantification procedure for REEs in seawater using HR-ICP-MS. Prior to analysis, the samples were preconcentrated using a bis-2-ethylhexyl phosphoric acid complexing compound. Gao et al. [156] employed HR-ICP-MS to determine REEs in coals and silty mudstones. REEs were observed primarily derived from felsic and intermediate rocks. The HR-ICP-MS technique was employed to determine the preconcentrated samples, showing recoveries in the range of 85–100% and a detection precision (RSD) of 3–5%. The preconcentrated samples were prepared by separating impurities from the matrix using HPLC [157]. The analysis of REEs in seawater is challenging, even when an ultrasensitive HR-ICP-MS technique is employed, likely due to the high concentration of total dissolved salts. For this sample, Soto-Jiménez et al. [158] performed filtration and acidification of the samples, matrix separation using a SeaFAST-SP3™ System for separation and preconcentration, offline injection of eluted samples, and HR-ICP-MS analysis. To validate the analysis results, estuarine water CRM, SLEW-3, was utilized in this regard.
3.8.6. MH-ICP-MS
MH-ICP-MS is a fully ICP-MS system designed in a compact Mattauch-Herzog geometry. This instrument integrates a spatially resolving ion detector and a permanent magnet. In the Mattauch-Herzog geometry, ions across all masses are focused along lines that align with the boundaries of the second magnetic field. The resolved ion beam is recorded on a high-spatial resolution semiconductor ion detector, which can simultaneously detect multiple elements/isotopes. The MH-ICP-MS instrument, equipped with a single 4.8K-pixel element detector, can detect isotopes across the full inorganic mass range, from 6Li to 238U, including REE-containing liquid samples with volumes of 1 to 4 mL [159,160]. This instrument is designed to analyze the elemental distribution and composition, particularly in geological samples.
3.8.7. Multi-Collector ICP-MS (MC-ICP-MS)
MC-ICP-MS consists of an ICP source, a Neir-Johnson double-focusing mass spectrometer, and a detector array [161]. In the MC-ICP-MS analysis, the samples are introduced to a high-temperature ICP, which results in the evaporation, dissociation, atomization, and ionization of the elements. The positively charged ions formed in the ICP-MS system are then transported from the plasma to the high-vacuum MS. Subsequently, the ions are accelerated and passed through an electrostatic analyzer for focusing. Ions exiting the electrostatic analyzer enter a magnetic field, which distributes the ions based on their m/z ratio. Careful alignment of the detector with the individual isotope beams is necessary when analyzing multiple elements [162]. Among REEs, Nd, with several isotopes, is highly incompatible and refractory. The variation in Nd isotopes is likely due to both mass-dependent and mass-independent effects, which are used to study various geological processes. Their precise determination is a great challenge due to severe interference effects. when combined with advanced sample preparation and separation techniques, such as a two-column ion-exchange procedure, MC-ICP-MS enables highly precise determination of isotope ratios. Moreover, the MC-ICP-MS is of great use for determining elemental concentrations [163]. Kent et al. [164] employed isotope dilution-MC-ICP-MS to accurately determine REEs in geochemical reference materials. The rock powders and synthetic silicate glasses were analyzed to support the use of CRMs for in situ trace element detection techniques. A comparative study on REE concentration determination was also conducted using ID-MC-ICP-MS and ID-TIMS. The ID method does not apply to monoisotopic elements. In other words, it requires at least two isotopes. Replicate analyses of rock powder and glass materials are in good agreement with analytical uncertainties (typically ~1%). Lee and Ko [56] detected the concentration of REEs in geological materials, including natural water CRM and SLRS-6, with high detection accuracy and precision, after group separation using 2-hydroxyisobutyric acid. In this study, REEs are divided into three groups: light REEs (La, Ce, Pr, Nd), middle REEs (Sm, Eu, Gd, Tb), and heavy REEs (Dy, Ho, Er, Tm, Yb, Lu). Pourmand et al. [165] developed an extraction chromatography method for the fast analysis of lanthanides, Sc, and Y. After minimizing impurities in the flux fusion, the MC-ICP-MS technique was employed to analyze all REEs in CI-chondrites, applying a desolvating nebulizer and a standard bracketing method. Baker et al. [166] proposed a high-precision analysis method for REEs using a high-performance ID-MC-ICP-MS. The fast and reproducible analysis was performed using ID-MC-ICP-MS on smaller sample amounts compared to ID-ICP-MS and ID-TIMS. Bastnäsite is the end member of a large group of carbonate-fluoride minerals that represent the major economic light REE deposits. Li et al. [167] conducted in situ major and trace elemental and Tb-Pb and Sm-Nd isotopic analysis for bastnäsite. LA-MC-ICP-MS was employed for determining age using the U-Th-Pb geochronology method and Sr-Nd isotopic composition. Sm-Nd geochronometers and LA-MC-ICP-MS were utilized to comprehend the timing of primary crystallization of REE deposits.
3.8.8. Thermal Ionization MS (TIMS)
In the TIMS measurements, the analytes interact with a heated surface to generate ions. The singly charged ions are then separated based on the m/z ratios and detected using a Faraday cup detector. For the simultaneous detection of multiple isotopes, the multi-connector TIMS is equipped with some detectors. Meanwhile, for isotope dilution analysis, the REEs are chemically separated into light and heavy REEs to minimize the inter-elemental interferences. At least two isotopes are necessary for this analysis, making it inapplicable to monoisotopic elements such as Pr, Ho, and Tm. TIMS is used primarily for dating applications in addition to element and isotope determination [168]. Ramesh et al. [169] employed TIMS to investigate the distribution of REEs in surficial sediments. Also, the behavior of REEs during weathering and transport in a secondary sedimentary environment was studied. The TIMS is a valuable technique for studying the ages of geological events using the decay systems of the radioactive REE isotopes La-138, Sm-147, and Lu-176, from the first step of planetary formation to more recent events, such as Deccan volcanism. TIMS using 234U/235U/238U isotopes was also reported to be employed to comprehend the occurrences of the REE deposits [170].
3.8.9. Sensitive High-Resolution Ion Micro Probe (SHRIMP)
SHRIMP is an ultrasensitive, high-resolution apparatus mainly applied for analyzing geological materials, particularly in situ U-Pb dating of minerals such as apatite and zircon [171]. Essentially, SHRIMP is an MS technique based on the secondary ion MS principle. The samples are bombarded to produce the second ionization yield using negatively charged oxygen molecules generated from the ion beam. This negatively charged O2 is used due to its effectiveness as the primary species in this regard. The collisions occurred between the surface and the primary ions, physically eroding, or ‘sputtering’, the sample, which in turn leads to the ejection of particles from the surface. Afterwards, the released secondary ions are focused and then directed towards the magnetic analyzer. In this stage, the multi-collector assembly simultaneously detects different ions/isotopes of interest [172]. Generally, SHRIMP dating methods have been broadly applied to some REE deposits. The deposits of REEs, including carbonite, can be accurately dated using the decay systems of radioactive REE isotopes, such as La-138, Sm-147, and Lu-176. This applies not only to determining the age of REE deposits, but to comprehending events ranging from the initial stages of planetary formation to more recent geodynamic processes as well. Thus, for such studies, it is necessary to analyze the abundances of all REEs at varying concentrations in different geomaterials, in addition to their precise isotope ratios [173]. Campbell et al. [174] studied carbonatite-related magnetism and mineralization events of REEs by dating zircon from the Bayan Obo Fe-Nb-REE deposits. The study showed that the zircon cores from Bayan Obo belong to a sequence of magmatic and intrusive carbonate lithologies. Bhunia et al. [175] employed SHRIMP to date one of the well-studied carbonatite-hosted REE deposits. SHRIMP enables the acquisition of high-resolution data on REE abundances in minerals such as zircon and apatite [176]. SHRIMP-RG was utilized to study the REE distribution in coal, which is crucial for designing cost-effective extraction methods for REEs in coal and its byproducts [177]. The SHRIMP U-Pb dating of zircons from Koga syenites, spatially associated with carbonatite deposits, was used to date carbonatite-alkaline complexes, providing important constraints on the timing of alkaline magmatism. Such in-depth studies of REE deposits have generally provided a comprehensive picture of the origins of these world-class mineral deposits [178].
3.9. In Situ Analytical Techniques
The mineralogical properties of REE resources are of great importance to investigate, as the leaching process relies on both the mineral composition and the morphological distribution of elements. Such studies help determine the optimal leaching/extraction process for taking out REEs from ore samples. Characterization techniques like XRD, SEM-EDS, and LA-ICP-MS are of great use for the analysis of REE-containing minerals. In exploration studies, portable XRD and Raman spectroscopy are used to analyze the mineralogy of geological formations on-site, aiding in the understanding of the deposit and guiding subsequent actions. Several significant analytical instruments used in mineralogy studies are discussed in this section.
3.9.1. X-Ray Diffractometry (XRD)
The investigation of indicator minerals in earth materials is highly significant in REE exploration studies. XRD is among the most effective techniques for analyzing minerals in different geological materials [179]. When a sample is exposed to monochromatic X-rays, constructive interference and diffraction occur if the conditions meet Bragg’s law. This law connects the wavelength of electromagnetic radiation with the diffraction angle and the lattice spacing of a crystal. The diffracted X-rays are subsequently detected, processed, and calculated. All possible diffraction grating directions should be reached by scanning the sample over an angular range of 2θ, owing to the powder material’s random orientation. Since each mineral has a distinct set of d-spacings, converting diffraction peaks into d-spacings allows for accurate mineral identification. This is achieved by matching the results with standard reference patterns. XRD has been successfully used to identify various minerals within the clay fraction of the REE-rich weathered anorthosite complex [180]. Villanova-de-Benavent et al. [181] performed powder and monocrystal EMP, SEM-EDS, and XRD analyses to elucidate the differences between REE phosphates and carbonates in the karst bauxite deposits. It was observed that phosphates are enriched in Y and heavy REEs of Gd and Dy, while carbonates are composed of light REEs of La, Ce, Nd, and Sm and heavy REEs of Gd. The analytical techniques of XRF, XRD, and ICP-MS were employed to characterize the lignite deposits from the Barmer Basin, with a focus on their mineralogical and elemental distribution. XRD measurements can identify the presence of minerals such as andalusite, anhydrite, anatase, hematite, nepheline, and spinel. Minor minerals were also identified, which include albite, calcite, cristobalite, hauyne, kaolinite, mayenite, nosean, periclase, pyrite, quartz, and siderite. XRF identified the most abundant elements, while ICP-MS determined REEs and trace elements. This study showed that the dominant mineral concentrations, elemental composition, trace elements, and REEs are associated with lignite deposits [182].
3.9.2. Electron Probe Micro Analyzer (EPMA)
In EPMA analysis, a solid sample is bombarded using a focused electron beam. This leads to an interaction between electrons and the sample, resulting in the release of heat, electrons, and X-rays. This process likely results from inelastic collisions between incoming electrons and the inner-shell electrons of the sample atoms. Various detector types are installed around the chamber to capture X-rays and electrons that are emitted from the samples. Light microscopes permit direct optical observation of samples. By using ED-XRF, the chemical composition of the samples is studied with X-rays. EPMA cannot only be used to detect high contents of REEs in rocks, but also be utilized to study the distribution patterns of REEs in minerals. EPMA is a nondestructive instrument for measuring samples [183]. The integration of EMA with EDS enables the accurate determination of the chemical composition of small solid samples by referencing them against a standard. EPMA, ICP-MS, and SEM were utilized to investigate the presence and distribution of REE in fly ash. SEM and EPMA clearly demonstrated the widespread presence of REE mineral particles, revealing that coal fly ash contained 630.51 μg/g of the total REEs. Furthermore, wet milling combined with acid leaching was found to enhance REE recovery during extraction [184]. Such a multidisciplinary approach often aids in developing cost-effective and environmentally sustainable methods for extracting REEs from coal fly ash, which has emerged as a promising alternative REE source [27]. Sano et al. [185] used EPMA to detect REEs in oceanic basalt glasses. The results were then compared with those obtained from the ICP-MS analysis, showing an overall accuracy of approximately ≤20% at one standard deviation.
3.9.3. Secondary Ion Mass Spectrometry (SIMS)/Ion Microprobe
SIMS, or known as an ion microprobe, is a highly effective method for performing in situ REE analyses in geological samples. Primarily, this technique uses a primary ion beam of O− or Cs+ to bombard polished rock samples or thin sections. The secondary ions generated by the sputtering process are then extracted into the MS for analysis. SIMS provides ultra-sensitive detection of elements ranging from hydrogen to uranium, as well as REEs, offering high spatial resolution and very low LOD at ng/g levels [186]. HR-SIMS, with its large diameter and double-focusing capabilities, enables measurements of over 5000 R. Additionally, it can estimate isotopic and elemental abundances in minerals with diameters ranging from 10 to 30 µm and a depth resolution from 1 to 5 µm. The large-diameter and double-focusing HR-SIMS technique can also be used as an ion microscope to provide imaging maps of elemental distributions. Zinner and Crozaz [187] employed SIMS for quantifying REEs in phosphates. A linear dynamic range of REEs was obtained in a wide range, with LODs of >50 and ~200 ng/g for light and heavy REEs, respectively. SIMS is applicable for the analysis of REE contents in individual mineral grains, including zircon and monazite. Shi et al. [188] employed a high-lateral-resolution nanoSIMS with a mass-resolving power of 9400 R to determine REEs in silicate glasses and zircon minerals. Bastnäsite is a main economic mineral of REEs, considered a promising geochronological means for determining the timing of mineralization using U-Pb dating methods. Ling et al. [189] employed HR-SIMS to study REE mineralization in the Himalayan Mianning-Dechang deposits by dating bastnaesites. Sahijpal et al. [190] proposed an analytical procedure for analyzing REEs in two primitive carbonaceous chondrites, showing uncertainties in the range from 10 to 15%. REE abundances were given to provide insights into understanding even the microscopic features of the early Solar System.
3.9.4. Scanning Electron Microprobe-Energy Dispersive X-Ray Spectroscopy (SEM-EDS)
SEM is a powerful imaging technique that employs a finely focused, scanned electron beam to produce high-resolution images of a sample’s surface. The EDS detector measures the samples through characteristic X-rays emitted by the corresponding elements in the top few µm. Therefore, SEM-EDS combines SEM and ED-XRF for characterizing materials, including minerals [191]. Pan et al. [192] employed SEM-EDS and XRD to investigate that aluminosilicate, apatite, monazite, and scheelite are the main phases of REE carriers. These studies provide insights that support the development of cost-effective leaching strategies for extracting REEs from ores. Palozzi et al. [132] analyzed fine monazite grains in coal fly ash using SEM-EDS and an integrated mineral analyzer, which are commonly bound to Al/Si-rich phases. This work contributed to the development of more efficient extraction with aqua regia. Sarker et al. [77] used XRD and SEM-EDX to search for the potential of carbonatite and to identify the principal mineral phases relevant to REE recovery. In this study, researchers discovered that monazite and florencite are the primary minerals containing REEs. SEM-EDX-based liberation shows that REE-bearing minerals are locked within large particles of over 100 µm and associated with goethite. These mineral grains could be liberated by reducing the particle size to 50 µm. To completely liberate most minerals containing REEs, the finding suggested grinding the materials to 63 µm prior to subsequent analysis. Li et al. [193] identified high contents of REEs in the acid drainage from a coal mine. Using SEM-EDS, they identified bastnaesite and monazite in the claystone. These minerals were mostly absorbed onto abundant kaolinite. Comprehensive characterization of the mineralogical and geochemical variability in REE deposits is essential for addressing mineral processing challenges and for improving purification strategies. SEM-EDS and XRD were employed to study the mineralogy of the Bear Lodge REE deposits. The corresponding minerals were grouped into fluor-carbonates (bastnäsite, parisite, and synchysite), phosphates (monazite, xenotime, florencite, rhabdophane, and churchite), cerianite, and ancylite. Moreover, the REE distribution was discovered to be uneven across the deposits [194].
3.9.5. Laser Ablation ICP-MS (LA-ICP-MS)
The direct determination of elemental concentrations and isotope ratios from a solid sample has emerged as an interesting area of application in geoscience studies. Gray [195] used LA-ICP-MS to demonstrate the feasibility of direct solid analysis. The samples were ablated by a pulsed laser prior to analysis with ICP-MS. LA-ICP-MS has been reported to offer several advantages, including the ability to minimize oxide and hydroxide interferences, ease of sample preparation, rapid analysis, and cost-effectiveness, particularly in the analysis of REEs. Nonetheless, the formation of polyatomic, oxide, and hydroxide ions in the plasma during analysis leads to interference. Fractionation effects, which result in nonstoichiometric behavior, also impact isotope ratio measurements. The LA-ICP-MS technique is among the most powerful laser ablation techniques for directly analyzing minerals and their inclusions, typically using spot sizes of 20–50 μm to measure major, minor, and even trace elements. Liu et al. [196] utilized LA-ICP-MS with regression analysis of time-resolved signals to directly determine major and trace elements (including REEs) in micrometer-scale ilmenite lamellae in titanomagnetite, showing good detection accuracy and precision. Guo et al. [197] employed LA-ICP-MS for in situ determination of REE contents in scheelite minerals from scheelite-quartz veins. Scheelite was observed to contain elevated contents of REEs with total REEs plus Y ranging from 3.339 to 6.321 mg/g. This information is crucial for understanding the crystallization processes of minerals from relatively reduced hydrothermal fluids during their formation. Instead of a nanosecond laser, a femtosecond laser in LA-ICP-MS could reduce issues of fractionation and matrix effects [162]. Mohanty et al. [89] utilized LA-ICP-MS to analyze detrital zircon grains from the riverbanks and beach placers, identifying the most abundant trace elements of Hf and Y. This type of geochemical study is highly significant in understanding the resource potential of a particular region. Jiu et al. [198] employed the LA-ICP-MS imaging technique to study the distribution of REEs in coal. The technique, coupled with strong organic materials, is very challenging. La and Ce elements are concentrated in related materials, which include monazite, bastnaesite, and lanthanides in coal. The LA-ICP-MS analysis of major, minor, and trace elements was used to map barren sandstone from the Proterozoic Athabasca Basin, in order to identify signs of hydrothermal REE mineralization. Quantitative evaluation of elemental correlations, together with SEM-EDS analysis, reveals that the enrichment of U and REEs is linked to aluminum phosphate sulfate phases rather than to apatite or monazite minerals [199]. For chemical analysis, LA-ICP-MS is a valuable technique for directly analyzing particular materials, such as barite, corundum minerals, and tourmaline. Oostingh [200] used the LA-ICP-MS technique to determine REEs in barites. To achieve this, a fusion method using a 1:10 ratio of barite and Na2CO3 was developed. The resultant fused material was dissolved in 6N HCl. Ion-exchange chromatography was employed to remove Ba ions using 2(ethylhexyl) orthophosphoric acid as the binding fluid. Nonetheless, a challenge was faced in LA sampling, in which PrO interferes with Gd and Ce affects the fusion flux. To address the interference issues, LA-ICP-MS/MS offers an effective alternative in this context. Liu et al. [201] presented a concise discussion on the LA-ICP-MS for microgeochemistry and bulk analysis of intact soil and rock samples. Challenges, including sample preparation, matrix-related interferences, sensitivity drift correction, calibration procedures, and the use of matrix-matched CRMs, were comprehensively considered. Maruyama et al. [202] used LA-ICP-MS to measure elements from major to trace levels in volcanic glass shards employing a femtosecond laser. LA-ICP-MS was considered a viable technique for characterizing rhyolitic and basaltic glasses.
3.9.6. LA-ICP-MS/MS
The in situ LA-ICP-MS/MS Lu-Hf dating technique has been proven effective for Paleozoic-Precambrian apatite, garnet, and xenotime. Although high-purity NH3 performed an effective reaction, the NH3-He mixture was utilized in this study as matrix-matched reference materials. Mass-shift mode was applied to detect Lu. The accuracy of single-point ages was better than 1.5%, and for xenotime was comparable to in situ U-Pb analysis [203]. Ham-Meert et al. [204] demonstrated that the LA-ICP-MS/MS technique, combined with the CH3F/He reaction gas in a mass-shift approach, can resolve spectral overlaps. This technique was used to evaluate the REE patterns and their effects on the isotopic composition of Sr. Indeed, this approach offers a practical alternative in situations where non-destructive analysis is necessary.
3.9.7. Laser Ablation Split Stream (LASS)
In LASS instrumentation, the ablated aerosol generated during sampling is divided and simultaneously directed to two spectrometers, typically an MC-ICP-MS and an additional ICP-MS system. This instrument configuration permits the simultaneous determination of elemental and isotopic ratios from a single sampling event [162]. Qian and Zhang [205] used SF-ICP-MS and MC-ICP-MS for the simultaneous measurement of REEs and the study of Nd isotopic compositions, respectively, in apatite crystals. The detection accuracy and precision of this study were evaluated using an apatite standard by both techniques. Kylander-Clark et al. [206] presented a laser ablation split-flow technology that couples a Nu AttoM SC-ICP-MS and a Nu Plasma HR MC-ICP-MS, for high-speed, high-spatial-resolution, and simultaneous isotopic and elemental analysis, enabling petrochronology at a new level. This new technique is of great use in helping to understand rock evolution, and it also explains more complex geological events.
3.10. Handheld Analytical Techniques
Portable instruments generally provide rapid, reliable, and economical analysis of geological samples, often with little to no sample preparation required. These platforms are superior, particularly in the on-site measurements during the extraction and transportation of ores. Various kinds of portable digital devices are utilized in the REE study, including portable XRFs, LIBS, and GPS, as well as computers that provide access to real-time imagery, maps, and geochemistry on-site.
3.10.1. Portable XRF
Portable XRF is a versatile technology suitable for various geological, mineralogical, and exploration studies, as well as mining and element extraction applications (Figure 10). This technology is very useful to find deposits of REEs that contain especially La, Ce, Pr, and Nd of <∑REEs 300 in the mining sites. The industry cut-off grade for certain REE deposits is typically set at ∑REEs of 300 µg/g. Previous studies have shown that achieving this threshold may require Y concentrations in the range of 20–30 µg/g, resulting in an overall ∑REE content of approximately 300 µg/g. Rock samples can be analyzed directly on-site or formed into a pressed pellet using sample preparation equipment. For inherently heterogeneous rock samples, it is crucial to perform measurements at multiple locations to obtain a representative analysis result. Phosphate deposits represent a significant REE source [27]. Simandl et al. [207] employed portable XRF to evaluate REE-enriched sedimentary phosphate deposits, obtaining the concentrations of Nd, Ce, La, Pr, and Y with satisfactory detection precision and accuracy. By ensuring proper sample preparation and calibration, portable XRF can effectively assess phosphate deposits enriched with REEs in the field, supporting large-scale exploration activities. Improved detection of low-concentration REEs with portable XRF can be achieved using a commercially available advanced 55 kV X-ray tube, which enables efficient analysis of light REEs (La, Ce, Pr, Nd, Sm) and selected heavy REEs (Eu, Gd). Standard instruments operating at the typical industry voltage of 50 kV are presently used to detect Y. Yang et al. [208] conducted a case study on anthracite-associated clays by determining Y content using portable XRF. Interestingly, Y can be used as an indicator element for estimating the total concentration of REEs. Portable XRF offers a cost-efficient analysis for conducting REE exploration studies. Fajber and Simandl [209] used a handheld XRF analyzer to achieve satisfactory quantitative and semi-quantitative estimation of Ce, La, Nd, Pr, and Y in the evaluation of REE-enriched sedimentary phosphate deposits. Since portable XRF enables the fast and reliable screening of samples during exploration, a subset of samples can be considered for laboratory analysis using more precise methods, such as ICP-MS, to verify the results. Sukadana et al. [210] employed micro-XRF to characterize minerals containing REEs. The study aimed to investigate the influence of carbonatite magma on the hydrothermal mineralization process. Figueiredo et al. [211] used an ED-XRF portable spectrometer for field semiquantitative screening of REEs in CRT phosphors and NdFeB magnet residues. They claimed that this handheld device can be implemented in a speedy and accurate manner for REE concentrations in e-waste recycling plants.
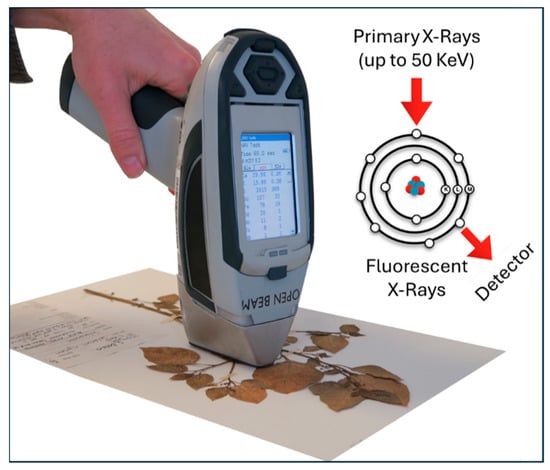
Figure 10.
Photograph of a handheld XRF analyzer. Adapted from [212] © 2019 van Der Ent, Echevarria, Pollard, and Erskine.
3.10.2. Portable LIBS
In the analysis using a handheld LIBS device, samples are typically prepared by forming consolidated pellets using a hydraulic press. Gibaga et al. [213] employed portable LIBS to detect REEs in ion-absorption clays and then compared the results with those obtained from ICP-MS analysis. The results obtained by both techniques were found to be in good agreement, indicating that portable LIBS could be a viable alternative for the real-time detection of REEs in ion adsorption clays. Indeed, portable LIBS is well-suited for on-site analysis, making it a cost-effective analysis technique. Bellie et al. [214] proposed a portable LIBS system for the rapid identification of elements at a low cost. This handheld device provides smartphone access to the emission spectrum and enables online monitoring via the cloud.
3.10.3. Portable XRD
Hydrometallurgical procedures are applied to improve extraction yields and achieve variable recovery rates, especially when processing REE ores. However, the understanding of the microscale processes (e.g., within fly ash) that govern REE extraction remains limited. To address this issue, the portable XRD was employed to characterize the fly ash at various stages of the process. Interestingly, the morphology and elemental composition of the ash matrix were identified to play a crucial role in REE accessibility. The portable XRD can provide valuable insights into the geological System by in situ identification of minerals [215].
3.10.4. Portable Raman Spectrometer
Raman spectroscopy provides data about the molecular form in which the compound is discovered. Fundamentally, the laser interacts with molecular vibrations, phonons, or other surface excitations of the sample, producing a shift in the energy of the scattered photons. This energy shift provides information in the form of a spectrum that characterizes the chemical composition of the samples. The laser Raman technique is an ideal instrument for use in terrestrial fields and extreme environments due to its ability to in situ, optically identify mineralogy. The deep-ocean Raman in situ spectrometer (or known as DORISS) is the first stage in utilizing the Raman technique in the deep ocean [216]. Moroz et al. [217] employed Raman microspectroscopy with 532 nm visible wavelength excitation to identify rock from the Tomtor Nb-REE deposits. This investigation confirmed that the presence of biogenic cyanobacterial mats has a significant impact on the formation of the unique Tomtor Nb-REE deposits.
3.10.5. Portable FT-IR Spectrometry
IR spectroscopy is similar to Raman in terms of information generated; however, they are complementary to each other. In the analysis, the FT-IR spectrometer provides absorption and intensity data from a continuous scan of the surface in the NIR range. The scan process takes approximately one minute to complete, and it is then returned to the control System [218]. The FT-IR technique is a popular online analyzer in industrial processes in the industries, including bauxite/alumina, coal, and phosphate. The benefit of this technique is that no sampling is required. Ye and Bai [219] employed microscopy, FT-IR, Raman microprobe, and UV-Vis to comprehend the spectral features, mineralogical structure and composition, fluid inclusions, and REE geochemistry. It was also observed that the FT-IR technique is occasionally used to verify the data obtained by the analysis of single-crystal X-ray diffraction [220].
3.11. Hyperspectral Remote Sensing Techniques
Hyperspectral remote sensing is an analytical technique based on the sharp and distinctive absorption features in the visible and near-infrared (VNIR) and shortwave infrared (SWIR) electromagnetic spectral ranges [216]. A real photograph of a portable SWIR (400–2500 nm) used in geological studies is shown in Figure 11. This technique has great potential for identifying REE minerals due to its high spatial resolution. The wavelength positions of specific absorption bands associated with REE3+ are sensitive to dissimilar crystal structures and are not merely related to variations in the specific REE content. Nd was identified as the dominant light REE, exhibiting characteristic absorption features in the VNIR region of the electromagnetic spectrum, even at concentrations as low as ~1 mg/g [221]. Besides satellite deployment, the system can also be installed on drones or operated as a handheld spectrometer for ground-based applications. This advanced remote sensing method has emerged as an innovative approach for studying REEs in geological samples, providing a non-destructive, cost-effective, and efficient alternative. Boesche et al. [222] utilized multitemporal hyperspectral imagery from two sensors, one covering the visible to near-infrared wavelength range (350–1000 nm) and the other covering the shortwave infrared range (1000–2500 nm), to identify neodymium-rich surface materials during hyperspectral mapping of REE-containing igneous carbonate rocks. The European Space Agency launched a multispectral instrument-equipped Sentinel-2 that is capable of detecting Nd-bearing rare earth minerals and showcasing the potential of multispectral instrument data for this purpose. Remote sensing data collected in the visible to shortwave infrared (VNIR-SWIR) range revealed distinct absorption features of neodymium (Nd), serving as a crucial indicator for REEs during the investigation of the Esfordi phosphate deposit, which contains rich REEs [223]. Booysen et al. [224] employed hyperspectral data collected by sensors installed on lightweight unmanned aerial vehicles (UAVs) to identify REEs, particularly Nd, with a low LOD of <200 µg/g in the carbonatite complexes, presenting a fast and effective method for REE deposit exploration. This type of research encourages the adoption of cost-effective drone-based hyperspectral imaging for exploration purposes. The main advantage of these methods is their ability to survey extensive areas quickly, with the added benefit that their results can be validated using laboratory techniques such as XRD and ICP-MS. Brazil possesses some of the world’s largest U-phosphate deposits, where high U and REE concentrations are found in close association with phosphorus, enabling the simultaneous extraction of all three resources. In these regions, NIR spectroscopy was applied to investigate the REE content within the soils of the U-phosphate deposits. REE concentrations were then verified with ICP-OES. These results demonstrate the potential of NIR spectroscopy as an instrument for mapping REE levels in topsoil [225]. Wang et al. [226] employed Vis-NIR reflectance spectroscopy to determine the concentrations of La, Nd, Pr, Sm, and total light REEs in soil. Turner et al. [227] investigated RE-bearing silicate minerals using short-wave IR reflectance spectroscopy within the VSWIR region (500–2500 nm). Several specific absorptions related to REEs, such as those of Er3+ and Yb3+ near 978 nm and Nd3+ near 746, 803, and 875 nm, were utilized to identify various REEs. The findings were subsequently confirmed through SEM and EPMA measurements.

Figure 11.
Field handheld SWIR spectrometer. From [228] © 2023 Balaram.
3.12. Chemical Sensors
Chemical sensors consist of an analytical technique that works on the interaction between recognition elements and targets of interest to generate signals proportional to the analytes. The type of signals depends on the transducers employed. Chemical sensors are easily miniaturized, leading to the development of on-site analysis [229,230,231]. Featherston et al. [232] developed a sensitized luminescence platform to probe Lanmodulin’s lanthanide. Using this sensor, Tb was successfully quantified from acid mine drainage in the presence of REEs and other elements. This technology leads to the development of a fast, cost-effective device for the specific detection of individual REEs. Cruickshank et al. [233] fabricated an electrochemical sensor using 2-pyridinol-1-oxide on an AuE for the rapid sensing of Eu in environmental samples. The developed sensor offers simple, effective, ultrasensitive, precise, and reliable analysis without the need for sample pre-treatment. Dehabadi et al. [234] developed a simple, cost-effective, ion-selective potentiometric sensor for detecting Sc3+ ions in acidic solutions. The proposed sensors demonstrated good selectivity towards Sc in the presence of REEs and divalent metal cations. Indeed, the sensors have considerable potential for use in analyzing complex multicomponent mixtures. Rare earth ions are mainly present on the surface of clay ores as impurities. These substances are commonly investigated using traditional methods, which involve complex and long procedures. Although alternative methods have been proposed, as the use of organic ligand-containing hydrogels, this material is harmful to the environment. To address this issue, Zhang et al. [235] proposed a simple and environmentally friendly detection platform for rare earth element ions. The developed sensor was based on a visual fluorescence method using a ligand-free hydrogel. The developed sensor performed anti-interference effects against other ions, which is of great use for preliminary field exploration. Crawford et al. [236] developed a REE sensor by modifying a fiber optic tip with a zinc adeninate MOF using a sol–gel method. The sensor could measure the emission of targets Sm, Eu, Tb, and Dy at ppb levels. In a recent study, a fluorescence-based chemical sensor was used to support the separation of REEs [237]. The sensor could specifically detect REEs in the presence of interfering molecules in the solution matrix. Obviously, this demonstrates that the proposed sensor is a viable and appealing platform for use in industrial streams.
3.13. Miscellaneous
Fluorite is among the most common minerals, which contains a relatively abundant amount of REEs. This mineral was successfully analyzed using XRF, FT-IR, and a UV-Vis spectrometer for its mineralogical information. Interestingly, the coloration of the fluorite samples was attributed to the presence of REEs [238]. In a different study, Hirose et al. [239] used glow discharge MS to determine La, Pr, Nd, Gd, and Tb in metallic REE matrices. The great performance was demonstrated in the REE analysis, where the detection limit was achieved at ng/g levels and the detection precision was ≤5% for 100 ppm. Xiong et al. [240] employed a linear ion trap MS-coupled microwave plasma torch (MPT-MS) for the analysis of REEs in aqueous solution samples. Different LODs were observed for different REEs in the range from 0.04 to 0.574 ng/mL. Using the same MPT-MS technique, in some of their works, Yuan et al. [241] determined several trace elements, including REEs, in water samples. To obtain a more accurate analysis, the individual separation of REEs is of great importance. Fayyaz et al. [242] employed the LA-TOF-MS technique to analyze REE-containing geological ore samples. Good agreement results were obtained for this analysis as compared to the calibration-free LIBS and ED-XRF techniques. Near-infrared spectroscopy serves as an effective technique for the rapid detection and monitoring of REEs (Sc, La, Pr, Sm, Th) in soil and bed sediment samples. Nevertheless, the standard analytical techniques, such as ICP-OES or ICP-MS, are required to confirm the exact concentrations of the elements being analyzed [243].
A fast analysis of bastnäsite, monazite, and xenotime facilitates efficient exploration of REE-containing ores. Imashuku [244] applied cathodoluminescence imaging to detect bastnäsite within ores and subsequently used an SEM-EDX to roughly estimate its content. The individual isolation of REEs is a challenging task due to their similar physicochemical characteristics. Zeolites (ferrierite, faujasite, Linde type L) possess ion-exchange ability that is useful for the separation of REEs. Duplouy [245] investigated ferrierite, Faujasite, and Linde type L through XRD, ED-XRF, and ATR-FTIR analyses. The uptake capacity toward REEs was observed to decrease as the atomic number increases, following the order La > Nd > Dy in this study. To obtain REEs, the REE-loaded zeolite could be leached off at pH 1.51 with HNO3. This study not only helps to understand the ion exchange behavior but also aids in designing suitable separation processes. Borst et al. [246] employed X-ray absorption spectroscopy to study the distribution and local bonding environment, using Y and Nd as representative proxies for heavy and light REEs in regolith-hosted ion-adsorbed clay deposits. This insight is essential to devising the best method for extracting REEs from ores. Obhodaš et al. [247] carried out in situ Lu-176 radioactivity and Gd measurements using a nuclear technique and a neutron sensor-based device, respectively. The total REEs in deep-sea sediments can be estimated based solely on the Lu and Gd contents, as they exhibit a strong correlation with each other. Notably, these techniques can be integrated into remotely operated vessels to enable in situ elemental analysis during deep-sea exploration for REE deposits. To clarify the binding environments and associations of REEs with mineral phases in coal and its combustion residues, obtaining speciation information is essential. Stuckman et al. [248] used synchrotron microscopy and spectroscopy to collect such information. Jochum et al. [249] quantitatively determined REEs in geological materials using isotope dilution spark source MS. The analytical performance showed the LOD ranging from 0.001 to 0.05 µg/g and detection accuracy and precision of <5%. Convincingly, this technique is applicable to multielement analysis with very small sample quantities.
Quick and continuous feedback on the contents of specific elements is crucial for maintaining appropriate process control during a metallurgical process. The main limitation is that analytical methods such as XRF, ICP-OES, and ICP-MS are unable to provide real-time feedback. To address this issue, Zhao et al. [250] designed an analytical device that can detect changes in sample composition as it migrates through a pipe. The device operates on in situ, ultrasensitive detection of La by tracking the 1435.8 keV peak through online germanium gamma spectrometry, achieving a minimum detectable concentration between 4.66 and 4.99 µg/g. The determination of lanthanum using the characteristic gamma-ray is applicable since La-138 is an isotope with a stable abundance of about 0.089% that emits characteristic gamma-rays during the decay activity. Coal mine overburden is reportedly still underexplored as a potential source of REEs. Attry et al. [9] addressed this issue by exploring it using the analytical techniques FE-SEM, TEM, and XPS to obtain the information regarding geochemical behaviors and commercial viability of REEs and Y.
4. Comparison of Analytical Techniques in REE Study
The isotope dilution method represents the most precise approach for quantifying metals using the MS technique. The isotope dilution MC-ICP-MS was successfully used to determine all REEs in the USGS BCR-2 and BCR-1 rock standards without the need for separation of individual elements. The results showed deviations of ≤4% of the expected values (4% for Gd). This suggests that the method can result in high-quality REE patterns comparable to those obtained using standard methods [251]. Separating REEs collectively from the rock matrix is sometimes favored, as it can yield more accurate results compared to analyzing them individually. Adeti et al. [22] carried out a comparative evaluation of multiple analytical techniques, including ICP-MS, INAA, tube-based XRF, and 241Am excitation-based XRF, in analyzing volcanic rock specimens. Meanwhile, Folkedahl et al. [252] carried out a round-robin interlaboratory investigation on geological materials. This study involved 13 laboratories that analyze REEs in coal, fly ash, mine waste, and shale. Various sample preparation procedures were employed for the analysis, which utilized INAA, ICP-OES, and ICP-MS techniques. Although the variability of the results was relatively high (RSD > 13%), three labs were reported to exhibit excellent repeatability and reproducibility. Ardini et al. [253] employed ICP-MS, HR-ICP-MS, and ICP-AES techniques to determine REEs in geological materials. By using a cooled spray chamber in a quadrupole ICP-MS, a significant decline of approximately 50% in the formation of oxide ions was observed, which minimizes polyatomic interferences. A comparable or even better performance in terms of detection limit for REEs was observed for HR-ICP-MS. Accurate analysis results were found for the majority of REEs using a pneumatic/ultrasonic nebulization System-equipped ICP-AES. Additionally, this platform was utilized after carefully selecting emission lines and compensating for non-spectral interference through internal standardization. Nonetheless, the ICP-AES could not measure Pr, Tb, Ho, Tm, and Lu, probably owing to insufficient sensitivity and interference disturbance. Zuma et al. [254] employed ICP-MS and ICP-OES to analyze REEs in geological and fossil samples, including rocks, ores, minerals, crude oil, and coal. Prior to measurement, the samples were pretreated with acid and heat digestion or extraction. Brouziotis et al. [255] carried out a comparative study on the analysis of REE contents in abandoned mining sites of bauxite using ICP-MS and TXRF. The significant amounts of REEs were observed through the corresponding techniques, where among the REEs, Ce is the most abundant. Conclusively, ICP-MS performed better than TXRF in this study. The ICP-MS is favored for the detection of REEs in geological samples due to its capability for multielement analysis with minimal interference effects, low detection limits, and rapid analysis. However, the development of more efficient, simple, and eco-friendly methods is of great importance for the study of REEs in various samples.
Table 4 presents representative data summarizing all the analytical information, including suitable application scenarios, analytical techniques, analytical performances, and sample information (type and handling).

Table 4.
The analytical data for REE analysis employing emerging analytical techniques.
5. Conclusions and Challenges
Analytical techniques play a significant part in all aspects of REE study, from exploration to metallurgical studies. Analytical methods are employed to examine ores, concentrates, and final products, providing insights into their elemental, isotopic, and mineralogical compositions, as well as minimal interference, multi-element and isotopic capabilities, and exceptional accuracy and precision in detection. Among the available analytical techniques, solution nebulization or laser ablation ICP-MS/MS represents the most cost-effective approach. Although less sensitive, the XRF technique remains popular for analyzing samples with high REE concentrations due to its ease of use. The portable XRF is a valuable technique that offers reasonable sensitivity for Y, which correlates with the total REEs. Currently, the handheld analytical techniques are available, for instance, portable XRF and LIBS. Indeed, these instruments significantly enhance REE exploration by providing a more efficient, rapid, and economical approach. Hyperspectral sensing techniques, such as those used in handheld, drone, and satellite applications, are highly cost-effective due to their ability to cover a large area in a short time. Microanalytical sensor devices installed in an unmanned vehicle are successfully applied for detecting deposits rich in REEs in the deep ocean. At last, an in situ nuclear γ-spectroscopy technique is employed to continuously monitor variations in sample composition during the industrial extraction of REEs, enabling real-time data collection as the material flows through the pipeline. Additionally, SHRIMP, TIMS, and MC-ICP-MS serve as important tools for the precise dating of REE ore bodies. They are also of great importance in understanding the primary crystallization timing of REE deposits. EPMA, SEM-EDS, Raman, and XRD techniques are significant tools for comprehending the mineralogical properties of various resources. Such a study is substantial for determining the best leaching/extraction process during REE extraction from ores, as the leaching stages are dependent on both the mineralogical composition and the morphological distribution.
REE exploration faces various challenges due to their complex geological occurrence and low natural concentrations, as well as environmental concerns. Unlike other metal deposits, REEs are rarely found in economically viable concentrations, often being dispersed in a wide range of mineral matrices that are not only difficult to identify but also to extract. The lack of advanced geophysical and geochemical tools tailored specifically for REEs further complicates their detection and mapping. Furthermore, REE-bearing minerals are often associated with radioactive elements such as thorium and uranium, presenting environmental and regulatory challenges during exploration. These factors collectively hamper the efficient and sustainable exploration efforts of REEs globally. In addition, geopolitical control over large REE reserves also poses strategic and economic challenges for other countries seeking to secure their own REE supply chains.
Author Contributions
H.A.S.: conceptualization, methodology, visualization, writing—original draft; D.A.: visualization, writing—review and editing; B.T.I.A.: writing—review and editing; A.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No data were used for the research described in the article.
Acknowledgments
The authors thank the referees for their comments and useful suggestions.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Lambert, C.E. Lanthanide Series of Metals. In Encyclopedia of Toxicology; Academic Press: Oxford, UK, 2005; pp. 691–694. [Google Scholar]
- Nurdeni; Saputra, H.A.; Atje Setiawan, A.; Budi Nurani, R.; Husein Hernandi, B.; Hedi. Quantitative Structure-Property Relationship of the Rare-Earth Elements-Dibutyl Dithiophosphate Derivative Complexes Using Principal Component Analysis. Fine Chem. Eng. 2024, 5, 333–344. [Google Scholar] [CrossRef]
- Horovitz, C.T. Chemical and physical properties of scandium and yttrium. In Biochemistry of Scandium and Yttrium, Part 1: Physical and Chemical Fundamentals; Springer: Berlin/Heidelberg, Germany, 1999; Volume 13A, pp. 29–74. [Google Scholar] [CrossRef]
- Drobniak, A.; Mastalerz, M. Rare Earth Elements: A brief overview. Indiana J. Earth Sci. 2022, 4. [Google Scholar] [CrossRef]
- Balaram, V. Sources and applications of rare earth elements. In Environmental Technologies to Treat Rare Earth Element Pollution: Principles and Engineering; IWA Publishing: London, UK, 2022; pp. 75–114. [Google Scholar]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Dijkstra, A.H.; Bakker, W.H.; Deon, F.; Marcatelli, C.; Plokker, M.P.; Hintzen, H.T. Identification of rare earth elements in synthetic and natural monazite and xenotime by visible-to-shortwave infrared reflectance spectroscopy. Phys. Chem. Miner. 2024, 51, 16. [Google Scholar] [CrossRef]
- Attry, B.; Subramanyam, K.S.V.; Tiwari, V.M.; Saikia, B.K. Exploring rare earth elements in coalmine overburden: Nanoscale insights from FESEM, TEM and XPS analysis. RSC Adv. 2025, 15, 19218–19235. [Google Scholar] [CrossRef]
- Klimpel, F.; Bau, M. Decoupling of scandium and rare earth elements in organic (nano)particle-rich boreal rivers draining the Fennoscandian Shield. Sci. Rep. 2023, 13, 10357. [Google Scholar] [CrossRef]
- Liang, B.; Gu, J.; Zeng, X.; Yuan, W.; Rao, M.; Xiao, B.; Hu, H. A Review of the Occurrence and Recovery of Rare Earth Elements from Electronic Waste. Molecules 2024, 29, 4624. [Google Scholar] [CrossRef] [PubMed]
- Palle Paul Mejame, M.; King, D.; Banhalmi-zakar, Z.; He, Y. Circular economy: A sustainable management strategy for rare earth elements consumption in Australia. Curr. Res. Environ. Sustain. 2022, 4, 100157. [Google Scholar] [CrossRef]
- Duchna, M.; Cieślik, I. Rare Earth Elements in New Advanced Engineering Applications. In Rare Earth Elements—Emerging Advances, Technology Utilization, and Resource Procurement; IntechOpen: London, UK, 2023. [Google Scholar]
- Preinfalk, C.; Morteani, G. The Industrial Applications of Rare Earth Elements. In Lanthanides, Tantalum and Niobium; Springer: Berlin/Heidelberg, Germany, 1989; pp. 359–370. [Google Scholar]
- Giese, E.C. Rare Earth Elements: Therapeutic and diagnostic applications in modern medicine. Clin. Med. Rep. 2018, 2, 1–2. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements, resources, applications, extraction technologies, chemical characterization, and global trade—A comprehensive review. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2025; pp. 193–233. [Google Scholar]
- Gkika, D.A.; Chalaris, M.; Kyzas, G.Z. Review of Methods for Obtaining Rare Earth Elements from Recycling and Their Impact on the Environment and Human Health. Processes 2024, 12, 1235. [Google Scholar] [CrossRef]
- Leal Filho, W.L.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Dushyantha, N.; Ilankoon, I.M.S.K.; Ratnayake, N.P.; Premasiri, H.M.R.; Dharmaratne, P.G.R.; Abeysinghe, A.M.K.B.; Rohitha, L.P.S.; Chandrajith, R.; Ratnayake, A.S.; Dissanayake, D.M.D.O.K.; et al. Recovery Potential of Rare Earth Elements (REEs) from the Gem Mining Waste of Sri Lanka: A Case Study for Mine Waste Management. Minerals 2022, 12, 1411. [Google Scholar] [CrossRef]
- Dushyantha, N.; Ratnayake, N.; Premasiri, R.; Batapola, N.; Panagoda, H.; Jayawardena, C.; Chandrajith, R.; Ilankoon, I.M.S.K.; Rohitha, S.; Ratnayake, A.S.; et al. Geochemical exploration for prospecting new rare earth elements (REEs) sources: REE potential in lake sediments around Eppawala Phosphate Deposit, Sri Lanka. J. Asian Earth Sci. 2023, 243, 105515. [Google Scholar] [CrossRef]
- Judge, T.A.; Dadzie, R.A.; Skinner, W.; Abaka-Wood, G.B. Characterisation and magnetic separation of complex low grade saprolite ore for rare earth elements minerals recovery. Miner. Eng. 2025, 231, 109437. [Google Scholar] [CrossRef]
- Adeti, P.J.; Amoako, G.; Tandoh, J.B.; Gyampo, O.; Ahiamadjie, H.; Amable, A.S.K.; Kansaana, C.; Annan, R.A.T.; Bamford, A. Rare-earth element comparative analysis in chosen geological samples using nuclear-related analytical techniques. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2023, 540, 122–128. [Google Scholar] [CrossRef]
- Jolis, E.M.; Szentpéteri, K.; O’Brien, H.; Menzies, A.H.; Nikkola, P.; Heikkilä, P.; Butcher, A.R.; Heino, P. Modern scanning- and micro-analytical spectroscopy—Technologies and workflow to characterise critical raw materials: A case study of the Sokli Phosphate and Rare Earth Elements deposit, Finland. Ore Geol. Rev. 2025, 185, 106683. [Google Scholar] [CrossRef]
- VanBommel, S.J.; Sharma, S.; Kizovski, T.V.; Heirwegh, C.M.; Christian, J.R.; Knight, A.L.; Ganly, B.; Allwood, A.C.; Hurowitz, J.A.; Tice, M.M.; et al. Rare earth element assessment in Jezero crater using the Planetary Instrument for X-ray Lithochemistry on the Mars 2020 rover Perseverance: A case study of cerium. Icarus 2025, 425, 116355. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Element Deposits: Sources, and Exploration Strategies. J. Geol. Soc. India 2022, 98, 1210–1216. [Google Scholar] [CrossRef]
- Al-Ani, T.; Molnár, F.; Lintinen, P.; Leinonen, S. Geology and Mineralogy of Rare Earth Elements Deposits and Occurrences in Finland. Minerals 2018, 8, 116355. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Dostal, J. Rare Earth Element Deposits of Alkaline Igneous Rocks. Resources 2017, 6, 34. [Google Scholar] [CrossRef]
- Patel, K.S.; Sharma, S.; Maity, J.P.; Martín-Ramos, P.; Fiket, Ž.; Bhattacharya, P.; Zhu, Y. Occurrence of uranium, thorium and rare earth elements in the environment: A review. Front. Environ. Sci. 2023, 10, 1058053. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries 2018; U.S. Geological Survey: Reston, VA, USA, 2018. [Google Scholar] [CrossRef]
- Sager, M.; Wiche, O. Rare Earth Elements (REE): Origins, Dispersion, and Environmental Implications—A Comprehensive Review. Environments 2024, 11, 24. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Santosh, M.; Hu, J.; Wei, H.; Yang, J.; Liu, S.; Zhang, G.; Yang, D.; Li, S. A review of retrieving pristine rare earth element signatures from carbonates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 586, 110765. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Mongelli, G. Geology, geochemistry, and genesis of REY minerals of the late Cretaceous karst bauxite deposits, Zagros Simply Folded Belt, SW Iran: Constraints on the ore-forming process. J. Geochem. Explor. 2022, 240, 107030. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare Earth Elements: Overview of Mining, Mineralogy, Uses, Sustainability and Environmental Impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Voncken, J.H.L. SpringerBriefs in Earth Sciences. In The Rare Earth Elements; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Chen, W.; Honghui, H.; Bai, T.; Jiang, S. Geochemistry of Monazite within Carbonatite Related REE Deposits. Resources 2017, 6, 51. [Google Scholar] [CrossRef]
- Thompson, W.; Lombard, A.; Santiago, E.; Singh, A. Mineralogical Studies in Assisting Beneficiation of Rare Earth Element Minerals from Carbonatite Deposits. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2011; pp. 665–672. [Google Scholar]
- Gupta, C.K.; Krishnamurthy, N. Extractive metallurgy of rare earths. Int. Mater. Rev. 2013, 37, 197–248. [Google Scholar] [CrossRef]
- Cesbron, F.P. Mineralogy of the Rare-Earth Elements. In Lanthanides, Tantalum and Niobium; Springer: Berlin/Heidelberg, Germany, 1989; pp. 3–26. [Google Scholar]
- Rasoulnia, P.; Barthen, R.; Lakaniemi, A.-M. A critical review of bioleaching of rare earth elements: The mechanisms and effect of process parameters. Crit. Rev. Environ. Sci. Technol. 2020, 51, 378–427. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, W. Review of allanite: Properties, occurrence and mineral processing technologies. Green Smart Min. Eng. 2024, 1, 40–52. [Google Scholar] [CrossRef]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective. In Non-Renewable Resource Issues; Springer: Berlin/Heidelberg, Germany, 2012; pp. 131–155. [Google Scholar]
- Balaram, V.; Subramanyam, K.S.V. Sample preparation for geochemical analysis: Strategies and significance. Adv. Sample Prep. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Dinali, G.S.; Ramos, S.J.; de Carvalho, T.S.; Carvalho, G.S.; de Oliveira, C.; Siqueira, J.O.; Guilherme, L.R.G. Dissolution techniques for determination of rare earth elements in phosphate products: Acid digestion or alkaline fusion? J. Geochem. Explor. 2019, 197, 114–121. [Google Scholar] [CrossRef]
- Helmeczi, E.; Wang, Y.; Brindle, I.D. A novel methodology for rapid digestion of rare earth element ores and determination by microwave plasma-atomic emission spectrometry and dynamic reaction cell-inductively coupled plasma-mass spectrometry. Talanta 2016, 160, 521–527. [Google Scholar] [CrossRef]
- Cora Jofre, F.; Savio, M. Infrared radiation: A sustainable and promising sample preparation alternative for inorganic analysis. TrAC Trends Anal. Chem. 2024, 170, 117469. [Google Scholar] [CrossRef]
- Balaram, V. Strategies to overcome interferences in elemental and isotopic geochemical analysis by quadrupole inductively coupled plasma mass spectrometry: A critical evaluation of the recent developments. Rapid Commun. Mass Spectrom. 2021, 35, e9065. [Google Scholar] [CrossRef]
- Leitzke, F.P.; Wegner, A.C.; Porcher, C.C.; Vieira, N.I.M.; Berndt, J.; Klemme, S.; Conceição, R.V. Whole-rock trace element analyses via LA-ICP-MS in glasses produced by sodium borate flux fusion. Braz. J. Geol. 2021, 51, e20200057. [Google Scholar] [CrossRef]
- Schramm, R. Use of X-ray Fluorescence Analysis for the Determination of Rare Earth Elements. Phys. Sci. Rev. 2016, 1, 20160061. [Google Scholar] [CrossRef]
- Hanchar, J.M.; Finch, R.J.; Hoskin, P.W.O.; Watson, E.B.; Cherniak, D.J.; Mariano, A.N. Rare earth elements in synthetic zircon: Part 1. Synthesis, and rare earth element and phosphorus doping. Am. Mineral. 2001, 86, 667–680. [Google Scholar] [CrossRef]
- Kasar, S.; Murugan, R.; Arae, H.; Aono, T.; Sahoo, S.K. A Microwave Digestion Technique for the Analysis of Rare Earth Elements, Thorium and Uranium in Geochemical Certified Reference Materials and Soils by Inductively Coupled Plasma Mass Spectrometry. Molecules 2020, 25, 5178. [Google Scholar] [CrossRef]
- Lee, S.-G.; Ko, K.-S. Development of an analytical method for accurate and precise determination of rare earth element concentrations in geological materials using an MC-ICP-MS and group separation. Front. Chem. 2023, 10, 906160. [Google Scholar] [CrossRef]
- Wilschefski, S.; Baxter, M. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Jatteau, M.; Cauzid, J.; Fabre, C.; Voudouris, P.; Soulamidis, G.; Tarantola, A. Portable Analyses of Strategic Metal-Rich Minerals Using pXRF and pLIBS: Methodology and Database Development. Data 2025, 10, 12. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectrochim. Acta Part B At. Spectrosc. 2021, 175, 106013. [Google Scholar] [CrossRef]
- Zhu, Y.; Nakano, K.; Shikamori, Y.; Itoh, A. Direct determination of rare earth elements in natural water samples by inductively coupled plasma tandem quadrupole mass spectrometry with oxygen as the reaction gas for separating spectral interferences. Spectrochim. Acta Part B At. Spectrosc. 2021, 179, 106100. [Google Scholar] [CrossRef]
- Fernández, A.S.; Gago, A.G.; Naveda, F.A.; Calleja, J.G.; Zawadzka, A.; Czarnocki, Z.; Barrallo, J.C.M.; Menéndez, R.M.S.; Rodríguez-González, P.; Alonso, J.I.G. Evaluation of different internal standardization approaches for the quantification of melatonin in cell culture samples by multiple heart-cutting two dimensional liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2022, 1663, 462752. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Gysi, A.P. UV-Vis spectrophotometric determination of rare earth elements (REE) speciation at near-neutral to alkaline pH. Part I: M-cresol purple properties from 25–75 °C and Er hydrolysis. Dalton Trans. 2024, 53, 13129–13141. [Google Scholar] [CrossRef]
- Dadi, M.; Yasir, M. Spectroscopy and Spectrophotometry: Principles and Applications for Colorimetric and Related Other Analysis. In Colorimetry; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Alaboodi, A.S.; Kadhim, S.A.; Hussein, A.S. Ultraviolet-Visible Spectroscopy, Importance, Principle, Structure and Most Important Applications: A Study Review. Int. J. Nov. Res. Phys. Chem. Math. 2025, 12, 53–60. [Google Scholar] [CrossRef]
- Pu, Q.; Liu, P.; Hu, Z.; Su, Z. Spectrophotometric Determination of the Sum of Rare Earth Elements by Flow-Injection on-Line Preconcentration with a Novel Aminophosphonic–Carboxylic Acid Resin. Anal. Lett. 2002, 35, 1401–1414. [Google Scholar] [CrossRef]
- Saputra, H.A.; Anggraeni, A.; Mutalib, A.; Bahti, H.H. Development of a Fast Simultaneous Analysis Method for Determination of Middle Rare-Earth Elements in Monazite Samples. J. Kim. Sains Dan Apl. 2021, 24, 177–184. [Google Scholar] [CrossRef]
- Wyantuti, S.; Nurwulanda, S.; Mardiah, N.; Anggraeni, A.; Pratomo, U.; Fauzia, R.P.; Bahti, H.H. Comparative Study of Voltammetric Analysis with UV-Vis Spectrophotometry in Determining the Results of Liquid-Liquid Extraction of Samarium (III). J. Kim. Val. 2024, 10, 229–236. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; de Almeida, E. X-ray fluorescence spectrometry for environmental analysis: Basic principles, instrumentation, applications and recent trends. Chemosphere 2022, 303, 135006. [Google Scholar] [CrossRef]
- Neikov, O.D.; Lotsko, D.V.; Gopienko, V.G. Powder characterization and testing. Handb. Non-Ferr. Met. Powders 2009, 2, 7–44. [Google Scholar] [CrossRef]
- Jaworowski, R.J.; Cosgrove, J.F.; Bracco, D.J.; Walters, R.M. Determination of trace rare earths by X-ray excited optical fluorescence. Spectrochim. Acta Part B At. Spectrosc. 1968, 23, 751–763. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, J.; Tang, Q.; Zhang, J.; Jiang, X.; Hou, X. Phosphonic Acid-Functionalized MIL-53(Al) As an Efficient Sorbent for Trace Rare Earth Elements Preconcentration, Storage and Their Determination by X-ray Fluorescence Spectrometry. Anal. Chem. 2023, 95, 14169–14174. [Google Scholar] [CrossRef] [PubMed]
- Juras, S.J.; Hickson, C.J.; Horsky, S.J.; Godwin, C.I.; Mathews, W.H. A practical method for the analysis of rare-earth elements in geological samples by graphite furnace atomic absorption and X-ray fluorescence. Chem. Geol. 1987, 64, 143–148. [Google Scholar] [CrossRef]
- De Vito, I.E.; Olsina, R.A.; Masi, A.N. Enrichment method for trace amounts of rare earth elements using chemofiltration and XRF determination. Fresenius’ J. Anal. Chem. 2000, 368, 392–396. [Google Scholar] [CrossRef]
- Wu, W.; Xu, T.; Hao, Q.; Wang, Q.; Zhang, S.; Zhao, C. Applications of X-ray fluorescence analysis of rare earths in China. J. Rare Earths 2010, 28, 30–36. [Google Scholar] [CrossRef]
- Losev, V.N.; Buyko, O.V.; Borodina, E.V.; Zhizhaev, A.M.; Samoilo, A.S. Preconcentration and ICP-OES determination of rare earth elements using silicas chemically modified with aminophosphonic groups in fossil raw materials. Int. J. Environ. Anal. Chem. 2023, 104, 7523–7539. [Google Scholar] [CrossRef]
- De Pauw, E.; Tack, P.; Lindner, M.; Ashauer, A.; Garrevoet, J.; Vekemans, B.; Falkenberg, G.; Brenker, F.E.; Vincze, L. Highly Sensitive Nondestructive Rare Earth Element Detection by Means of Wavelength-Dispersive X-ray Fluorescence Spectroscopy Enabled by an Energy Dispersive pn-Charge-Coupled-Device Detector. Anal. Chem. 2019, 92, 1106–1113. [Google Scholar] [CrossRef]
- Sarker, S.K.; Bruckard, W.; Haque, N.; Roychand, R.; Bhuiyan, M.; Pramanik, B.K. Characterization of a carbonatite-derived mining tailing for the assessment of rare earth potential. Process Saf. Environ. Prot. 2023, 173, 154–162. [Google Scholar] [CrossRef]
- Yao, M.; Wang, D.; Zhao, M. Element Analysis Based on Energy-Dispersive X-Ray Fluorescence. Adv. Mater. Sci. Eng. 2015, 2015, 290593. [Google Scholar] [CrossRef]
- Ravisankar, R.; Manikandan, E.; Dheenathayalu, M.; Rao, B.; Seshadreesan, N.P.; Nair, K.G.M. Determination and distribution of rare earth elements in beach rock samples using instrumental neutron activation analysis (INAA). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 251, 496–500. [Google Scholar] [CrossRef]
- Glascock, M.D. Instrumental Neutron Activation Analysis and Its Application to Cultural Heritage Materials. In Handbook of Cultural Heritage Analysis; Springer: Berlin/Heidelberg, Germany, 2022; pp. 69–94. [Google Scholar]
- Alghem Hamidatou, L. Overview of Neutron Activation Analysis. In Advanced Technologies and Applications of Neutron Activation Analysis; IntechOpen: London, UK, 2019. [Google Scholar]
- Mathew, J.; Kshirsagar, R.; Abidin, D.Z.; Griffin, J.; Kanarachos, S.; James, J.; Alamaniotis, M.; Fitzpatrick, M.E. A comparison of machine learning methods to classify radioactive elements using prompt-gamma-ray neutron activation data. Sci. Rep. 2023, 13, 9948. [Google Scholar] [CrossRef]
- Krishnan, K.; Saion, E. Distributions of rare earth element (REE) in mangrove surface sedimentby nuclear technique. INTI J. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Bounouira, H.; Abbo, M.A.; Amsil, H.; Didi, A.; Aarab, I. Utilizing the k0-IAEA program to determine rare earth elements in soil samples from gold-mining areas in Sudan. J. Radioanal. Nucl. Chem. 2023, 332, 1707–1721. [Google Scholar] [CrossRef]
- Kin, F.D.; Prudêncio, M.I.; Gouveia, M.Â.; Magnusson, E. Determination of Rare Earth Elements in Geological Reference Materials: A Comparative Study by INAA and ICP-MS. Geostand. Newsl. 2007, 23, 47–58. [Google Scholar] [CrossRef]
- El-Taher, A. Nuclear Analytical Techniques for Detection of Rare Earth Elements. J. Radiat. Nucl. Appl. 2018, 3, 53–64. [Google Scholar] [CrossRef]
- Ghannadpour, S.S.; Hezarkhani, A. Prospecting rare earth elements (REEs) using radiation measurement: Case study of Baghak mine, Central Sangan iron ore mine, NE of Iran. Environ. Earth Sci. 2022, 81, 363. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, W.; Liu, J.; Liang, X.; Yuan, W.; Ouyang, Q.e.; Liu, S.; Gok, C.; Wang, J.; Song, G. Preliminary Screening of Soils Natural Radioactivity and Metal(loid) Content in a Decommissioned Rare Earth Elements Processing Plant, Guangdong, China. Int. J. Environ. Res. Public Health 2022, 19, 14566. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Khan, R.; Tamim, U.; Adak, S.; Bhunia, G.S.; Sengupta, D. Geochemical and Radionuclide studies of sediments as tracers for enrichment of beach and alluvial placers along the eastern coast of India. Reg. Stud. Mar. Sci. 2023, 63, 103003. [Google Scholar] [CrossRef]
- Walsh, A. The application of atomic absorption spectra to chemical analysis. Spectrochim. Acta 1955, 7, 108–117. [Google Scholar] [CrossRef]
- L’vov, B.V. A continuum source vs. line source on the way toward absolute graphite furnace atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1637–1646. [Google Scholar] [CrossRef]
- Ring, G.; O’Mullane, J.; O’Riordan, A.; Furey, A. Trace metal determination as it relates to metallosis of orthopaedic implants: Evolution and current status. Clin. Biochem. 2016, 49, 617–635. [Google Scholar] [CrossRef]
- Balaram, V. Microwave plasma atomic emission spectrometry (MP-AES) and its applications—A critical review. Microchem. J. 2020, 159, 105483. [Google Scholar] [CrossRef]
- Kamala, C.T.; Balaram, V.; Dharmendra, V.; Satyanarayanan, M.; Subramanyam, K.S.V.; Krishnaiah, A. Application of Microwave Plasma Atomic Emission Spectrometry (MP-AES) for environmental monitoring of industrially contaminated sites in Hyderabad City. Environ. Monit. Assess. 2014, 186, 7097–7113. [Google Scholar] [CrossRef]
- Balaram, V.; Vummiti, D.; Roy, P.; Taylor, C.; Kar, P.; Raju, A.K.; Abburi, K. Determination of precious metals in rocks and ores by microwave plasma-atomic emission spectrometry for geochemical prospecting studies. Curr. Sci. 2013, 104, 1207–1215. [Google Scholar]
- Fayyaz, A.; Baig, M.A.; Waqas, M.; Liaqat, U. Analytical Techniques for Detecting Rare Earth Elements in Geological Ores: Laser-Induced Breakdown Spectroscopy (LIBS), MFA-LIBS, Thermal LIBS, Laser Ablation Time-of-Flight Mass Spectrometry, Energy-Dispersive X-ray Spectroscopy, Energy-Dispersive X-ray Fluorescence Spectrometer, and Inductively Coupled Plasma Optical Emission Spectroscopy. Minerals 2024, 14, 1004. [Google Scholar] [CrossRef]
- Pedarnig, J.D.; Trautner, S.; Grünberger, S.; Giannakaris, N.; Eschlböck-Fuchs, S.; Hofstadler, J. Review of Element Analysis of Industrial Materials by In-Line Laser—Induced Breakdown Spectroscopy (LIBS). Appl. Sci. 2021, 11, 9274. [Google Scholar] [CrossRef]
- Gaft, M.; Raichlin, Y.; Pelascini, F.; Panzer, G.; Motto Ros, V. Imaging rare-earth elements in minerals by laser-induced plasma spectroscopy: Molecular emission and plasma-induced luminescence. Spectrochim. Acta Part B At. Spectrosc. 2019, 151, 12–19. [Google Scholar] [CrossRef]
- Afgan, M.S.; Hou, Z.; Song, W.; Liu, J.; Song, Y.; Gu, W.; Wang, Z. On the Spectral Identification and Wavelength Dependence of Rare-Earth Ore Emission by Laser-Induced Breakdown Spectroscopy. Chemosensors 2022, 10, 350. [Google Scholar] [CrossRef]
- Khan, Z.H.; Ullah, M.H.; Rahman, B.; Talukder, A.I.; Wahadoszamen, M.; Abedin, K.M.; Haider, A.F.M.Y.; Galić, N. Laser-Induced Breakdown Spectroscopy (LIBS) for Trace Element Detection: A Review. J. Spectrosc. 2022, 2022, 3887038. [Google Scholar] [CrossRef]
- Abedin, K.M.; Haider, A.F.M.Y.; Rony, M.A.; Khan, Z.H. Identification of multiple rare earths and associated elements in raw monazite sands by laser-induced breakdown spectroscopy. Opt. Laser Technol. 2011, 43, 45–49. [Google Scholar] [CrossRef]
- Bhatt, C.R.; Jain, J.C.; Goueguel, C.L.; McIntyre, D.L.; Singh, J.P. Determination of Rare Earth Elements in Geological Samples Using Laser-Induced Breakdown Spectroscopy (LIBS). Appl. Spectrosc. 2017, 72, 114–121. [Google Scholar] [CrossRef]
- Unnikrishnan, V.K.; Nayak, R.; Devangad, P.; Tamboli, M.M.; Santhosh, C.; Kumar, G.A.; Sardar, D.K. Calibration based laser-induced breakdown spectroscopy (LIBS) for quantitative analysis of doped rare earth elements in phosphors. Mater. Lett. 2013, 107, 322–324. [Google Scholar] [CrossRef]
- Long, J.; Song, W.; Hou, Z.; Wang, Z. A data selection method for matrix effects and uncertainty reduction for laser-induced breakdown spectroscopy. Plasma Sci. Technol. 2023, 25, 075501. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, J.; Jiang, J.; Zhou, Z.; Ye, S. Automatic Coal-Rock Recognition by Laser-Induced Breakdown Spectroscopy Combined with an Artificial Neural Network. Spectroscopy 2023, 38, 25–32. [Google Scholar] [CrossRef]
- Bhatt, C.R.; Jain, J.C.; Bol’shakov, A.A.; McIntyre, D.L. Chemistry imaging and distribution analysis of rare earth elements in coal using LIBS and LA-ICP-MS instruments. Int. J. Coal Geol. 2025, 301, 104710. [Google Scholar] [CrossRef]
- Olesik, J. ICP-OES capabilities, developments, limitations, and any potential challengers? Spectroscopy 2020, 35, 18–21. [Google Scholar]
- Nóbrega, J.; Schiavo, D.; Amaral, C.; Barros, J.; Mogueira, A.R.; Virgilio, A.; Machado, R. Determination of rare earth elements in geological and agricultural samples by ICP-OES. Spectroscopy 2017, 32, 32–36. [Google Scholar]
- Jaron, I.; Kudowska, B.; Bulska, E. Determination of rare earth elements in geological samples by ICP-OES. At. Spectrosc. 2000, 21, 105–110. [Google Scholar]
- Makombe, M.; Horst, C.v.d.; Silwana, B.; Iwuoha, E.; Somerset, V. Optimisation of Parameters for Spectroscopic Analysis of Rare Earth Elements in Sediment Samples. In Rare Earth Element; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Gorbatenko, A.A.; Revina, E.I. A review of instrumental methods for determination of rare earth elements. Inorg. Mater. 2015, 51, 1375–1388. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Ambade, B. Extractive separation of rare earth elements and their determination by inductively coupled plasma optical emission spectrometry in geological samples. J. Anal. At. Spectrom. 2020, 35, 1395–1404. [Google Scholar] [CrossRef]
- Beauchemin, D. Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2008, 80, 4455–4486. [Google Scholar] [CrossRef]
- Thomas, R. 40 Years Old and Still Solving Problems: Evolution of the ICP-MS Application Landscape. Spectroscopy 2023, 38, 8–13. [Google Scholar] [CrossRef]
- Houk, R.S.; Fassel, V.A.; Flesch, G.D.; Svec, H.J.; Gray, A.L.; Taylor, C.E. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Anal. Chem. 2002, 52, 2283–2289. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Zotos, A.; Thomatou, A.-A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), a Useful Tool in Authenticity of Agricultural Products’ and Foods’ Origin. Foods 2022, 11, 3705. [Google Scholar] [CrossRef]
- Nakamura, A.; Kubota, R.; Ohta, A. Multi-element analysis of geological samples using ICP-MS equipped with integrated sample introduction and aerosol dilution systems. Bull. Geol. Surv. Jpn. 2023, 74, 71–85. [Google Scholar] [CrossRef]
- Bulska, E.; Wagner, B. Quantitative aspects of inductively coupled plasma mass spectrometry. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150369. [Google Scholar] [CrossRef]
- Montaser, A. Inductively Coupled Plasma Mass Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- McMahon, P.G. ICP-MS–ICP-MS instrumentation, ICP-MS analysis, strengths and limitations. Technol. Netw. 2021. Available online: https://www.technologynetworks.com/analysis/webinars/changing-the-landscape-of-screening-ai-powered-asms-ligand-identification-404407 (accessed on 10 August 2025).
- Iglesias, M.n.; Gilon, N.; Poussel, E.; Mermet, J.-M. Evaluation of an ICP-collision/reaction cell-MS system for the sensitive determination of spectrally interfered and non-interfered elements using the same gas conditions. J. Anal. At. Spectrom. 2002, 17, 1240–1247. [Google Scholar] [CrossRef]
- Mnculwane, H.T. Rare Earth Elements Determination by Inductively Coupled Plasma Mass Spectrometry after Alkaline Fusion Preparation. Analytica 2022, 3, 135–143. [Google Scholar] [CrossRef]
- Lin, R.; Bank, T.L.; Roth, E.A.; Granite, E.J.; Soong, Y. Organic and inorganic associations of rare earth elements in central Appalachian coal. Int. J. Coal Geol. 2017, 179, 295–301. [Google Scholar] [CrossRef]
- Buyko, O.V.; Metelitsa, S.I.; Losev, V.N.; Panasenko, A.E.; Shimanskii, A.F. Biosilica layer-by-layer modified with polyamines and carboxyarsenazo for REE preconcentration prior to ICP-MS determination in lignites and volcanic fumarole sediment. Anal. Methods 2020, 12, 3813–3822. [Google Scholar] [CrossRef]
- Baghaliannejad, R.; Aghahoseini, M.; Amini, M.K. Determination of rare earth elements in uranium materials by ICP-MS and ICP-OES after matrix separation by solvent extraction with TEHP. Talanta 2021, 222, 121509. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Xia, F.; Zhu, T.; Wu, D.; Chingin, K.; Chen, H. High throughput online sequential extraction of natural rare earth elements and determination by mass spectrometry. Sci. China Chem. 2021, 64, 642–649. [Google Scholar] [CrossRef]
- Petrova, K.V.; Es’kina, V.V.; Baranovskaya, V.B.; Doronina, M.S.; Korotkova, N.A.; Arkhipenko, A.A. Separation and Preconcentration of Impurities in Rare-Earth-Based Materials for Spectrometric Methods. Russ. J. Non-Ferr. Met. 2022, 63, 510–525. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Ramzan, M.; Kifle, D.; Wibetoe, G. A simple separation system for elimination of molecular interferences for purity determination of europium and ytterbium oxides by HPLC-ICP-MS. J. Anal. At. Spectrom. 2020, 35, 2594–2599. [Google Scholar] [CrossRef]
- Tupaz, C.A.J.; Gregorio, C.G.C.; Arcilla, C. Determination of Scandium (Sc), Yttrium (Y), and Rare-Earth Elements (REEs) in Mafic and Ultramafic Rock Powder by a Modified and Validated Digestion Protocol and Inductively Coupled Plasma—Mass Spectrometry (ICP-MS). Anal. Lett. 2022, 56, 932–943. [Google Scholar] [CrossRef]
- Liu, W.; An, Y.; Qu, Q.; Li, P.; Zhang, L.; Li, C.; Wei, S.; Zhou, H.; Chen, J. An efficient method for the separation of REEs from Ba for the accurate determination of REE content in Ba-rich samples by ICP-MS. J. Anal. At. Spectrom. 2023, 38, 449–456. [Google Scholar] [CrossRef]
- Wysocka, I. Determination of rare earth elements concentrations in natural waters—A review of ICP-MS measurement approaches. Talanta 2021, 221, 121636. [Google Scholar] [CrossRef] [PubMed]
- Palozzi, J.; Bailey, J.G.; Tran, Q.A.; Stanger, R. A characterization of rare earth elements in coal ash generated during the utilization of Australian coals. Int. J. Coal Prep. Util. 2023, 43, 2106–2135. [Google Scholar] [CrossRef]
- El-Taher, A.; Ashry, A.; Ene, A.; Almeshari, M.; Zakaly, H.M. Determination of phosphate rock mines signatures using XRF and ICP-MS elemental analysis techniques: Radionuclides, oxides, rare earth, and trace elements. Rom. Rep. Phys. 2023, 75, 701. [Google Scholar]
- Krasavtseva, E.; Sandimirov, S.; Elizarova, I.; Makarov, D. Assessment of Trace and Rare Earth Elements Pollution in Water Bodies in the Area of Rare Metal Enterprise Influence: A Case Study—Kola Subarctic. Water 2022, 14, 3406. [Google Scholar] [CrossRef]
- Li, H.; Tong, R.; Guo, W.; Xu, Q.; Tao, D.; Lai, Y.; Jin, L.; Hu, S. Development of a fully automatic separation system coupled with online ICP-MS for measuring rare earth elements in seawater. RSC Adv. 2022, 12, 24003–24013. [Google Scholar] [CrossRef]
- Agnieszka Wysocka, I.; Kaczor-Kurzawa, D.; Porowski, A. Development and validation of seaFAST-ICP-QMS method for determination of rare earth elements total concentrations in natural mineral waters. Food Chem. 2022, 388, 133008. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Huang, K.; Wang, Z. Multielemental Determination of Rare Earth Elements in Seawater by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) After Matrix Separation and Pre-concentration With Crab Shell Particles. Front. Environ. Sci. 2021, 9, 781996. [Google Scholar] [CrossRef]
- Balaram, V. Inductively Coupled Plasma-Tandem Mass Spectrometry (ICP-MS/MS) and Its Applications. J. ISAS 2022, 1, 1–26. [Google Scholar] [CrossRef]
- Zhu, Y. Determination of Rare Earth Elements by Inductively Coupled Plasma–Tandem Quadrupole Mass Spectrometry with Nitrous Oxide as the Reaction Gas. Front. Chem. 2022, 10, 912938. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Thoss, V.; Ribeiro Guevara, S.; Urgast, D.; Raab, A.; Mastrolitti, S.; Feldmann, J. Assessing rare earth elements in quartz rich geological samples. Appl. Radiat. Isot. 2016, 107, 323–329. [Google Scholar] [CrossRef]
- Lancaster, S.T.; Prohaska, T.; Irrgeher, J. Characterisation of gas cell reactions for 70+ elements using N2O for ICP tandem mass spectrometry measurements. J. Anal. At. Spectrom. 2023, 38, 1135–1145. [Google Scholar] [CrossRef]
- Zhu, Y. Determination of rare earth elements in seawater samples by inductively coupled plasma tandem quadrupole mass spectrometry after coprecipitation with magnesium hydroxide. Talanta 2020, 209, 120536. [Google Scholar] [CrossRef]
- Ntiharirizwa, S.; Boulvais, P.; Poujol, M.; Branquet, Y.; Morelli, C.; Ntungwanayo, J.; Midende, G. Geology and U-Th-Pb Dating of the Gakara REE Deposit, Burundi. Minerals 2018, 8, 394. [Google Scholar] [CrossRef]
- Myers, D.P.; Li, G.; Yang, P.; Hieftje, G.M. An inductively coupled plasma-time-of-flight mass spectrometer for elemental analysis. Part I: Optimization and characteristics. J. Am. Soc. Mass Spectrom. 1994, 5, 1008–1016. [Google Scholar] [CrossRef]
- Mahoney, P.P.; Ray, S.J.; Hieftje, G.M.; Li, G. Continuum background reduction in orthogonal-acceleration time-of-flight mass spectrometry with continuous ion sources. J. Am. Soc. Mass Spectrom. 1997, 8, 125–131. [Google Scholar] [CrossRef][Green Version]
- Balaram, V.; Satyanarayanan, M.; Murthy, P.K.; Mohapatra, C.; Prasad, K.L. Chemical Characterization of Cobalt Crust from Afanasy-Nikitin Seamount in the Eastern Indian Ocean by Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Mapan 2013, 28, 63–77. [Google Scholar] [CrossRef]
- Dick, D.; Wegner, A.; Gabrielli, P.; Ruth, U.; Barbante, C.; Kriews, M. Rare earth elements determined in Antarctic ice by inductively coupled plasma—Time of flight, quadrupole and sector field-mass spectrometry: An inter-comparison study. Anal. Chim. Acta 2008, 621, 140–147. [Google Scholar] [CrossRef]
- Nakazato, M.; Asanuma, H.; Niki, S.; Iwano, H.; Hirata, T. Depth-Profiling Determinations of Rare Earth Element Abundances and U-Pb Ages from Zircon Crystals Using Sensitivity-Enhanced Inductively Coupled Plasma-Time of Flight-Mass Spectrometry. Geostand. Geoanal. Res. 2022, 46, 603–620. [Google Scholar] [CrossRef]
- Peng, J.; Li, D.; Hollings, P.; Fu, Y.; Sun, X. Visualization of critical metals in marine nodules by rapid and high-resolution LA-ICP-TOFMS mapping. Ore Geol. Rev. 2023, 154, 105342. [Google Scholar] [CrossRef]
- Chew, D.; Drost, K.; Marsh, J.H.; Petrus, J.A. LA-ICP-MS imaging in the geosciences and its applications to geochronology. Chem. Geol. 2021, 559, 119917. [Google Scholar] [CrossRef]
- Manard, B.T.; Bradley, V.C.; Quarles, C.D.; Hendriks, L.; Dunlap, D.R.; Hexel, C.R.; Sullivan, P.; Andrews, H.B. Towards Automated and High-Throughput Quantitative Sizing and Isotopic Analysis of Nanoparticles via Single Particle-ICP-TOF-MS. Nanomaterials 2023, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, N.; Hall, E.F.H.; Sanderson, N.E. Communication. Inductively coupled plasma as an ion source for high-resolution mass spectrometry. J. Anal. At. Spectrom. 1989, 4, 801–803. [Google Scholar] [CrossRef]
- Satyanarayanan, M.; Balaram, V.; Sawant, S.S.; Subramanyam, K.S.V.; Vamsi Krishna, G.; Dasaram, B.; Manikyamba, C. Rapid Determination of REEs, PGEs, and Other Trace Elements in Geological and Environmental Materials by High Resolution Inductively Coupled Plasma Mass Spectrometry. At. Spectrosc. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Thomas, R. A beginner’s guide to ICP-MS-Part VII: Mass separation devices-Double-focusing magnetic-sector technology. Spectroscopy 2001, 16, 22–27. [Google Scholar]
- Balaram, V.; Roy, P.; Subramanyam, K.S.V.; Durai, L.; Mohan, M.R.; Satyanarayanan, M.; Vani, K. REE geochemistry of seawater from Afanasy-Nikitin seamount in the eastern equatorial Indian Ocean by high resolution inductively coupled plasma mass spectrometry. Indian J. Geo-Mar. Sci. 2015, 44, 339–347. [Google Scholar]
- Gao, J.; Lv, D.; Tom van Loon, A.J.; Hower, J.C.; Raji, M.; Yang, Y.; Ren, Z.; Wang, Y.; Zhang, Z. Reconstruction of provenance and tectonic setting of the Middle Jurrasic Yan’an Formation (Ordos Basin, North China) by analysis of major, trace and rare earth elements in the coals. Ore Geol. Rev. 2022, 151, 105218. [Google Scholar] [CrossRef]
- Pedreira, W.R.; Sarkis, J.E.S.; Rodrigues, C.; Tomiyoshi, I.A.; da Silva Queiroz, C.A.; Abrão, A. Determination of trace amounts of rare earth elements in highly pure praseodymium oxide by double focusing inductively coupled plasma mass spectrometry and high-performance liquid chromatography. J. Alloys Compd. 2001, 323–324, 49–52. [Google Scholar] [CrossRef]
- Soto-Jiménez, M.F.; Martinez-Salcido, A.I.; Morton-Bermea, O.; Ochoa-Izaguirre, M.J. Lanthanoid analysis in seawater by seaFAST-SP3™ system in off-line mode and magnetic sector high-resolution inductively coupled plasma source mass spectrometer. MethodsX 2022, 9, 101625. [Google Scholar] [CrossRef]
- Bäuchle, M.; Lüdecke, T.; Rabieh, S.; Calnek, K.; Bromage, T.G. Quantification of 71 detected elements from Li to U for aqueous samples by simultaneous-inductively coupled plasma-mass spectrometry. RSC Adv. 2018, 8, 37008–37020. [Google Scholar] [CrossRef]
- Louis Brown, J.H.B.; Augustyn, A.; Gaur, A.; Lotha, G.; Rodriguez, E.; Setia, V.; Young, G. The Editors of Encyclopaedia Britannica. In Electrostatic Field Analysis in Mass Spectrometry in General Principles. 2025. Available online: https://www.britannica.com/science/mass-spectrometry/Hydrogen-carbon-nitrogen-oxygen-and-sulfur-in-nature (accessed on 10 August 2025).
- Walder, A.J.; Freedman, P.A. Communication. Isotopic ratio measurement using a double focusing magnetic sector mass analyser with an inductively coupled plasma as an ion source. J. Anal. At. Spectrom. 1992, 7, 571–575. [Google Scholar] [CrossRef]
- Balaram, V.; Rahaman, W.; Roy, P. Recent advances in MC-ICP-MS applications in Earth and environmental sciences: Challenges and solutions. Geosyst. Geoenviron. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Bai, J.-H.; Liu, F.; Zhang, Z.-F.; Ma, J.-L.; Zhang, L.; Liu, Y.-F.; Zhong, S.-X.; Wei, G.-J. Simultaneous measurement stable and radiogenic Nd isotopic compositions by MC-ICP-MS with a single-step chromatographic extraction technique. J. Anal. At. Spectrom. 2021, 36, 2695–2703. [Google Scholar] [CrossRef]
- Kent, A.J.R.; Jacobsen, B.; Peate, D.W.; Waight, T.E.; Baker, J.A. Isotope Dilution MC-ICP-MS Rare Earth Element Analysis of Geochemical Reference Materials NIST SRM 610, NIST SRM 612, NIST SRM 614, BHVO-2G, BHVO-2, BCR-2G, JB-2, WS-E, W-2, AGV-1 and AGV-2. Geostand. Geoanal. Res. 2007, 28, 417–429. [Google Scholar] [CrossRef]
- Pourmand, A.; Dauphas, N.; Ireland, T.J. A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chem. Geol. 2012, 291, 38–54. [Google Scholar] [CrossRef]
- Baker, J.; Waight, T.; Ulfbeck, D. Rapid and highly reproducible analysis of rare earth elements by multiple collector inductively coupled plasma mass spectrometry. Geochim. Cosmochim. Acta 2002, 66, 3635–3646. [Google Scholar] [CrossRef]
- Li, X.-C.; Yang, K.-F.; Spandler, C.; Fan, H.-R.; Zhou, M.-F.; Hao, J.-L.; Yang, Y.-H. The effect of fluid-aided modification on the Sm-Nd and Th-Pb geochronology of monazite and bastnäsite: Implication for resolving complex isotopic age data in REE ore systems. Geochim. Cosmochim. Acta 2021, 300, 1–24. [Google Scholar] [CrossRef]
- Guerra-Sommer, M.; Cazzulo-Klepzig, M.; Menegat, R.; Formoso, M.L.L.; Basei, M.Â.S.; Barboza, E.G.; Simas, M.W. Geochronological data from the Faxinal coal succession, southern Paraná Basin, Brazil: A preliminary approach combining radiometric U-Pb dating and palynostratigraphy. J. S. Am. Earth Sci. 2008, 25, 246–256. [Google Scholar] [CrossRef]
- Ramesh, R.; Ramanathan, A.; Ramesh, S.; Purvaja, R.; Subramanian, V. Distribution of rare earth elements and heavy metals in the surficial sediments of the Himalayan river system. Geochem. J. 2000, 34, 295–319. [Google Scholar] [CrossRef]
- Natarajan, T.; Inoue, K.; Sahoo, S.K. Rare Earth Elements Geochemistry and 234U/238U, 235U/238U Isotope Ratios of the Kanyakumari Beach Placer Deposits: Occurrence and Provenance. Minerals 2023, 13, 886. [Google Scholar] [CrossRef]
- Compston, W.; Pidgeon, R.T. Jack Hills, evidence of more very old detrital zircons in Western Australia. Nature 1986, 321, 766–769. [Google Scholar] [CrossRef]
- Balaram, V. Current and emerging analytical techniques for geochemical and geochronological studies. Geol. J. 2020, 56, 2300–2359. [Google Scholar] [CrossRef]
- Sindern, S. Analysis of rare earth elements in rock and mineral samples by ICP-MS and LA-ICP-MS. Phys. Sci. Rev. 2017, 2, 20160066. [Google Scholar] [CrossRef]
- Campbell, L.S.; Compston, W.; Sircombe, K.N.; Wilkinson, C.C. Zircon from the East Orebody of the Bayan Obo Fe–Nb–REE deposit, China, and SHRIMP ages for carbonatite-related magmatism and REE mineralization events. Contrib. Mineral. Petrol. 2014, 168, 1041. [Google Scholar] [CrossRef]
- Bhunia, S.; Chalapathi Rao, N.V.; Belyatsky, B.; Talukdar, D.; Pandey, R.; Lehmann, B. SHRIMP U-Pb zircon geochronology of the carbonatite-hosted REE deposit of Kamthai, Late Cretaceous polychronous Sarnu Dandali alkaline complex, NW India: Links to plume-related metallogeny and CO2 outgassing at the K-Pg boundary. Gondwana Res. 2022, 112, 116–125. [Google Scholar] [CrossRef]
- Sano, Y.; Terada, K.; Fukuoka, T. High mass resolution ion microprobe analysis of rare earth elements in silicate glass, apatite and zircon: Lack of matrix dependency. Chem. Geol. 2002, 184, 217–230. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Hong, J.; Khan, T.; Li, W.; Khalil, Y.S.; Narejo, A.A.; Rashid, M.U.; Zeb, M.J. SHRIMP U–Pb ages, mineralogy, and geochemistry of carbonatite–alkaline complexes of the Sillai Patti and Koga areas, NW Pakistan: Implications for petrogenesis and REE mineralization. Ore Geol. Rev. 2021, 139, 104547. [Google Scholar] [CrossRef]
- Balaram, V.; Sawant, S.S. Indicator Minerals, Pathfinder Elements, and Portable Analytical Instruments in Mineral Exploration Studies. Minerals 2022, 12, 394. [Google Scholar] [CrossRef]
- Jo, J.; Shin, D. Geochemical characteristics of REE-enriched weathered anorthosite complex in Hadong district, South Korea. Geochem. J. 2023, 57, 13–27. [Google Scholar] [CrossRef]
- Villanova-de-Benavent, C.; Proenza, J.A.; Torró, L.; Aiglsperger, T.; Domènech, C.; Domínguez-Carretero, D.; Llovet, X.; Suñer, P.; Ramírez, A.; Rodríguez, J. REE ultra-rich karst bauxite deposits in the Pedernales Peninsula, Dominican Republic: Mineralogy of REE phosphates and carbonates. Ore Geol. Rev. 2023, 157, 105422. [Google Scholar] [CrossRef]
- Kumar, O.P.; Gopinathan, P.; Naik, A.S.; Subramani, T.; Singh, P.K.; Sharma, A.; Maity, S.; Saha, S. Characterization of lignite deposits of Barmer Basin, Rajasthan: Insights from mineralogical and elemental analysis. Environ. Geochem. Health 2023, 45, 6471–6493. [Google Scholar] [CrossRef]
- Reed, S.J.B.; Buckley, A. Rare-earth element determination in minerals by electron-probe microanalysis: Application of spectrum synthesis. Mineral. Mag. 2018, 62, 1–8. [Google Scholar] [CrossRef]
- Wu, L.; Ma, L.; Huang, G.; Li, J.; Xu, H. Distribution and Speciation of Rare Earth Elements in Coal Fly Ash from the Qianxi Power Plant, Guizhou Province, Southwest China. Minerals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Sano, Y.; Terada, K.; Hidaka, H.; Nishio, Y.; Amakawa, H.; Nozaki, Y. Ion-Microprobe Analysis of Rare Earth Elements in Oceanic Basalt Glass. Anal. Sci. 1999, 15, 743–748. [Google Scholar] [CrossRef]
- MacRae, N.D. Quantitative analysis of REEs by SIMS. Am. Mineral. 1987, 72, 1263–1268. [Google Scholar]
- Zinner, E.; Crozaz, G. A method for the quantitative measurement of rare earth elements in the ion microprobe. Int. J. Mass Spectrom. Ion Process. 1986, 69, 17–38. [Google Scholar] [CrossRef]
- Shi, L.; Sano, Y.; Takahata, N.; Koike, M.; Morita, T.; Koyama, Y.; Kagoshima, T.; Li, Y.; Xu, S.; Liu, C. NanoSIMS Analysis of Rare Earth Elements in Silicate Glass and Zircon: Implications for Partition Coefficients. Front. Chem. 2022, 10, 844953. [Google Scholar] [CrossRef]
- Ling, X.X.; Li, Q.L.; Liu, Y.; Yang, Y.H.; Liu, Y.; Tang, G.Q.; Li, X.H. In situ SIMS Th–Pb dating of bastnaesite: Constraint on the mineralization time of the Himalayan Mianning–Dechang rare earth element deposits. J. Anal. At. Spectrom. 2016, 31, 1680–1687. [Google Scholar] [CrossRef]
- Sahijpal, S.; Marhas, K.K.; Goswami, J.N. Determination of rare earth and refractory trace element abundances in early solar system objects by ion microprobe. J. Earth Syst. Sci. 2003, 112, 485–498. [Google Scholar] [CrossRef][Green Version]
- Singh, S.P.; Balaram, V.; Satyanarayanan, M.; Sarma, D.S.; Subramanyam, K.S.V.; Anjaiah, K.V.; Kharia, A.; Tiwari, V.M.; Pichamuthu, D.V.; Pichamuthu, D.V.; et al. Platinum Group Minerals from the Madawara Ultramafic-mafic Complex, Bundelkhand Massif, Central India: A Preliminary Note, XXV IUGG General Assembly, Melbourne. “Earth on the Edge: Science for a Sustainable Planet”, Note on the Evolution of India’s Mineral Policy and its Impact on the Mineral Industry, A Vision for the Mineral Sector in Karnataka, Recent Research in Ediacaran Fauna. J. Geol. Soc. India 2011, 78, 281–294. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Wen, Z.; Nie, T.; Zhang, N.; Zhou, C. The mechanism study on the integrated process of NaOH treatment and citric acid leaching for rare earth elements recovery from coal fly ash. J. Environ. Chem. Eng. 2023, 11, 109921. [Google Scholar] [CrossRef]
- Li, X.; Qiao, W.; Chen, D.; Wu, P.; Xie, Y.; Chen, X. Anomalous concentrations of rare earth elements in acid mine drainage and implications for rare earth resources from late Permian coal seams in northern Guizhou. Sci. Total Environ. 2023, 879, 163051. [Google Scholar] [CrossRef] [PubMed]
- Van Rythoven, A.D.; Pfaff, K.; Clark, J.G. Use of QEMSCAN® to characterize oxidized REE ore from the Bear Lodge carbonatite, Wyoming, USA. Ore Energy Resour. Geol. 2020, 2–3, 100005. [Google Scholar] [CrossRef]
- Gray, A.L. Solid sample introduction by laser ablation for inductively coupled plasma source mass spectrometry. Analyst 1985, 110, 551–556. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Jiang, S.-Y.; Chen, W.; Wang, C.Y.; Su, H.-M.; Cao, Y.; Zhang, H.-X.; Li, W.-T. Precise determination of major and trace elements in micrometer-scale ilmenite lamellae in titanomagnetite using LA-ICP-MS technique: Application of regression analysis to time-resolved signals. RSC Adv. 2023, 13, 13303–13313. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Xu, X.; Song, Z.; Dong, X.; Tian, J.; Yang, Y.; She, H.; Xiang, A.; Kang, Y. Sm-Nd dating and REE Composition of scheelite for the Honghuaerji scheelite deposit, Inner Mongolia, Northeast China. Lithos 2016, 261, 307–321. [Google Scholar] [CrossRef]
- Jiu, B.; Huang, W.; Spiro, B.; Hao, R.; Mu, N.; Wen, L.; Hao, H. Distribution of Li, Ga, Nb, and REEs in coal as determined by LA-ICP-MS imaging: A case study from Jungar coalfield, Ordos Basin, China. Int. J. Coal Geol. 2023, 267, 104184. [Google Scholar] [CrossRef]
- Chi, G.; Potter, E.; Petts, D.; Jackson, S.; Chu, H. LA-ICP-MS Mapping of Barren Sandstone from the Proterozoic Athabasca Basin (Canada)—Footprint of U- and REE-Rich Basinal Fluids. Minerals 2022, 12, 733. [Google Scholar] [CrossRef]
- Oostingh, K.F. Analysis of Rare Earth Element Concentrations in Barite (BaSO4). Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2012. [Google Scholar]
- Liu, Y.; Hu, Z.; Li, M.; Gao, S. Applications of LA-ICP-MS in the elemental analyses of geological samples. Chin. Sci. Bull. 2013, 58, 3863–3878. [Google Scholar] [CrossRef]
- Maruyama, S.; Hattori, K.; Hirata, T.; Suzuki, T.; Danhara, T. Simultaneous determination of 58 major and trace elements in volcanic glass shards from the INTAV sample mount using femtosecond laser ablation-inductively coupled plasma-mass spectrometry. Geochem. J. 2016, 50, 403–422. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Yang, Y.; Niu, J.; Lan, Z.; Zhang, L.; Huang, C.; Xie, L.; Xu, L.; Yang, J.; et al. In situLu–Hf geochronology with LA-ICP-MS/MS analysis. J. Anal. At. Spectrom. 2023, 38, 1285–1300. [Google Scholar] [CrossRef]
- Van Ham-Meert, A.; Bolea-Fernandez, E.; Belza, J.; Bevan, D.; Jochum, K.P.; Neuray, B.; Stoll, B.; Vanhaecke, F.; Van Wersch, L. Comparison of Minimally Invasive Inductively Coupled Plasma–Mass Spectrometry Approaches for Strontium Isotopic Analysis of Medieval Stained Glass with Elevated Rubidium and Rare-Earth Element Concentrations. ACS Omega 2021, 6, 18110–18122. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.-P.; Zhang, L. Simultaneous in situ determination of rare earth element concentrations and Nd isotope ratio in apatite by laser ablation ICP-MS. Geochem. J. 2019, 53, 319–328. [Google Scholar] [CrossRef]
- Kylander-Clark, A.R.C.; Hacker, B.R.; Cottle, J.M. Laser-ablation split-stream ICP petrochronology. Chem. Geol. 2013, 345, 99–112. [Google Scholar] [CrossRef]
- Simandl, G.J.; Fajber, R.; Paradis, S. Portable X-ray fluorescence in the assessment of rare earth element-enriched sedimentary phosphate deposits. Geochem. Explor. Environ. Anal. 2014, 14, 161–169. [Google Scholar] [CrossRef]
- Yang, X.; Kozar, D.; Gorski, D.; Marchese, A.; Pagnotti, J.; Sutterlin, R.; Rezaee, M.; Klima, M.S.; Pisupati, S.V. Using yttrium as an indicator to estimate total rare earth element concentration: A case study of anthracite-associated clays from northeastern Pennsylvania. Int. J. Coal Sci. Technol. 2020, 7, 652–661. [Google Scholar] [CrossRef]
- Fajber, R.; Simandl, G.J. Evaluation of rare earth element-enriched sedimentary phosphate deposits using portable X-ray fluorescence (XRF) instruments. Geol. Fieldwork 2011, 2012, 199–210. [Google Scholar]
- Sukadana, I.G.; Warmada, I.W.; Pratiwi, F.; Harijoko, A.; Adimedha, T.B.; Yogatama, A.W. Elemental Mapping for Characterizing of Thorium and Rare Earth Elements (REE) Bearing Minerals Using µXRF. At. Indones. 2022, 48, 87–98. [Google Scholar] [CrossRef]
- Figueiredo, F.M.J.; Fátima Araújo, M.; Marçalo, J.; Leal, J.P.; Sardinha, J.P. Determination of rare earth elements in CRT phosphors and NdFeB magnets residues using an ED-XRF portable spectrometer to assist field semi-quantitative screening. Microchem. J. 2024, 198, 110136. [Google Scholar] [CrossRef]
- van der Ent, A.; Echevarria, G.; Pollard, A.J.; Erskine, P.D. X-Ray Fluorescence Ionomics of Herbarium Collections. Sci. Rep. 2019, 9, 4746. [Google Scholar] [CrossRef]
- Gibaga, C.R.; Montano, M.; Samaniego, J.; Tanciongco, A.; Quierrez, R.N. Comparative Study on Determination of Selected Rare Earth Elements (REEs) in Ion Adsorption Clays Using Handheld LIBS and ICP-MS. Philipp. J. Sci. 2022, 151, 1595–1600. [Google Scholar] [CrossRef]
- Bellie, V.; Gokulraju, R.; Rajasekar, C.; Vinoth, S.; Mohankumar, V.; Gunapriya, B. Laser induced Breakdown Spectroscopy for new product development in mining industry. Mater. Today Proc. 2021, 45, 8157–8161. [Google Scholar] [CrossRef]
- Gerardo, S.; Davletshin, A.R.; Loewy, S.L.; Song, W. From Ashes to Riches: Microscale Phenomena Controlling Rare Earths Recovery from Coal Fly Ash. Environ. Sci. Technol. 2022, 56, 16200–16208. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.G.; Malby, G.; Pasteris, J.D.; White, S.N.; Peltzer, E.T.; Wopenka, B.; Freeman, J.; Brown, M.O. Development of a laser Raman spectrometer for deep-ocean science. Deep Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 739–753. [Google Scholar] [CrossRef]
- Moroz, T.N.; Edwards, H.G.M.; Zhmodik, S.M. Detection of carbonate, phosphate minerals and cyanobacteria in rock from the Tomtor deposit, Russia, by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119372. [Google Scholar] [CrossRef] [PubMed]
- Solutions, P.A. Portable FTIR Analysis: Revolutionising Industries with the Agilent 4300 Handheld FTIR. 2025. Available online: https://www.portableas.com/news/portable-ftir-analysis-revolutionising-industries-with-the-agilent-4300-handheld-ftir/ (accessed on 10 August 2025).
- Ye, X.; Bai, F. Spectral Characteristics, Rare Earth Elements, and Ore-Forming Fluid Constrains on the Origin of Fluorite Deposit in Nanlishu, Jilin Province, China. Minerals 2022, 12, 1195. [Google Scholar] [CrossRef]
- Davletshina, N.y.; Ermakova, E.; Dolgova, D.; Davletshin, R.; Ivshin, K.; Fedonin, A.; Stoikov, I.; Cherkasov, R. Structure and FT-IR spectroscopic analyses of complexes phosphorylated betaines with rare earth metal ions. Inorganica Chim. Acta 2023, 545, 121245. [Google Scholar] [CrossRef]
- Qasim, M.; Khan, S.D. Detection and Relative Quantification of Neodymium in Sillai Patti Carbonatite Using Decision Tree Classification of the Hyperspectral Data. Sensors 2022, 22, 7537. [Google Scholar] [CrossRef]
- Boesche, N.; Rogass, C.; Lubitz, C.; Brell, M.; Herrmann, S.; Mielke, C.; Tonn, S.; Appelt, O.; Altenberger, U.; Kaufmann, H. Hyperspectral REE (Rare Earth Element) Mapping of Outcrops—Applications for Neodymium Detection. Remote Sens. 2015, 7, 5160–5186. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Tangestani, M.H. Potential of Sentinel-2 MSI data in targeting rare earth element (Nd3+) bearing minerals in Esfordi phosphate deposit, Iran. Egypt. J. Remote Sens. Space Sci. 2022, 25, 697–710. [Google Scholar] [CrossRef]
- Booysen, R.; Jackisch, R.; Lorenz, S.; Zimmermann, R.; Kirsch, M.; Nex, P.A.M.; Gloaguen, R. Detection of REEs with lightweight UAV-based hyperspectral imaging. Sci. Rep. 2020, 10, 17450. [Google Scholar] [CrossRef]
- Maia, A.J.; da Silva, Y.J.A.B.; do Nascimento, C.W.A.; Veras, G.; Escobar, M.E.O.; Cunha, C.S.M.; da Silva, Y.J.A.B.; Nascimento, R.C.; de Souza Pereira, L.H. Near-infrared spectroscopy for the prediction of rare earth elements in soils from the largest uranium-phosphate deposit in Brazil using PLS, iPLS, and iSPA-PLS models. Environ. Monit. Assess. 2020, 192, 675. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Pan, X. Potential of visible and near-infrared reflectance spectroscopy for the determination of rare earth elements in soil. Geoderma 2017, 306, 120–126. [Google Scholar] [CrossRef]
- Turner, D.J.; Rivard, B.; Groat, L.A. Visible and short-wave infrared reflectance spectroscopy of selected REE-bearing silicate minerals. Am. Mineral. 2018, 103, 927–943. [Google Scholar] [CrossRef]
- Balaram, V. Advances in Analytical Techniques and Applications in Exploration, Mining, Extraction, and Metallurgical Studies of Rare Earth Elements. Minerals 2023, 13, 1031. [Google Scholar] [CrossRef]
- Saputra, H.A. Electrochemical sensors: Basic principles, engineering, and state of the art. Monatshefte Für Chem.-Chem. Mon. 2023, 154, 1083–1100. [Google Scholar] [CrossRef]
- Paderni, D.; Giorgi, L.; Fusi, V.; Formica, M.; Ambrosi, G.; Micheloni, M. Chemical sensors for rare earth metal ions. Coord. Chem. Rev. 2021, 429, 213639. [Google Scholar] [CrossRef]
- Rocha, D.L.; Maringolo, V.; Araújo, A.N.; Amorim, C.M.P.G.; Montenegro, M.d.C.B.S.M. An overview of Structured Biosensors for Metal Ions Determination. Chemosensors 2021, 9, 324. [Google Scholar] [CrossRef]
- Featherston, E.R.; Issertell, E.J.; Cotruvo, J.A. Probing Lanmodulin’s Lanthanide Recognition via Sensitized Luminescence Yields a Platform for Quantification of Terbium in Acid Mine Drainage. J. Am. Chem. Soc. 2021, 143, 14287–14299. [Google Scholar] [CrossRef]
- Cruickshank, L.; Officer, S.; Pollard, P.; Prabhu, R.; Stutter, M.; Fernandez, C. Rare Elements Electrochemistry: The Development of a Novel Electrochemical Sensor for the Rapid Detection of Europium in Environmental Samples Using Gold Electrode Modified with 2-pyridinol-1-oxide. Anal. Sci. 2015, 31, 623–627. [Google Scholar] [CrossRef]
- Dehabadi, M.; Legin, E.; Legin, A.; Yaghmaei, S.; Nechaev, A.; Babain, V.; Kirsanov, D. Developing potentiometric sensors for scandium. Sens. Actuators B Chem. 2021, 348, 130699. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Wu, Y.; Gong, C.; Feng, Z.; Wang, Z.; Huang, Y.; Zheng, Z. A ligand-free hydrogel as a visual fluorescence sensor for detection of rare-earth ions. Opt. Commun. 2024, 570, 130885. [Google Scholar] [CrossRef]
- Crawford, S.E.; Burgess, W.A.; Kim, K.-J.; Baltrus, J.P.; Diemler, N.A. Zinc adeninate metal–organic framework-coated optical fibers for enhanced luminescence-based detection of rare earth elements. RSC Appl. Interfaces 2024, 1, 689–698. [Google Scholar] [CrossRef]
- Tse, P.; Espley, A.F.; Rakos, J.M.; Wang, Q.; Subban, C.V.; Bryan, S.A.; Lines, A.M. Developing Fluorescence-Based Sensors to Support Rare Earth Element Separation. ACS Sens. 2025, 10, 4974–4982. [Google Scholar] [CrossRef]
- Gidado Shehu, I.M.B. Mineralogical and Structural Analyses of Natural Fluorite from Yantuwaru Mining Site, Nigeria. UMYU Sci. 2023, 2, 53–61. [Google Scholar] [CrossRef]
- Hirose, F.; Itoh, S.; Okochi, H. Determination of Rare-earth Elements in Metallic La, Pr, Nd, Gd and Tb by Glow Discharge Mass Spectrometry. Tetsu-to-Hagane 1991, 77, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Jiang, T.; Qi, W.; Zuo, J.; Yang, M.; Fei, Q.; Xiao, S.; Yu, A.; Zhu, Z.; Chen, H. Some Rare Earth Elements Analysis by Microwave Plasma Torch Coupled with the Linear Ion Trap Mass Spectrometry. Int. J. Anal. Chem. 2015, 2015, 156509. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, X.; Cao, Y.; Yan, N.; Peng, L.; Lai, X.; Tao, H.; Li, L.; Jiang, T.; Zhu, Z. Microwave plasma torch mass spectrometry for some rare earth elements. Arab. J. Chem. 2022, 15, 104379. [Google Scholar] [CrossRef]
- Fayyaz, A.; Ali, R.; Waqas, M.; Liaqat, U.; Ahmad, R.; Umar, Z.A.; Baig, M.A. Analysis of Rare Earth Ores Using Laser-Induced Breakdown Spectroscopy and Laser Ablation Time-of-Flight Mass Spectrometry. Minerals 2023, 13, 787. [Google Scholar] [CrossRef]
- Jamil Maia, A.; Cabral Nascimento, R.; Jacques Agra Bezerra da Silva, Y.; Williams Araújo do Nascimento, C.; de Sousa Mendes, W.; Germano Veras Neto, J.; Coelho de Araújo Filho, J.; Tiecher, T.; Jacques Agra Bezerra da Silva, Y. Near-infrared spectroscopy for prediction of potentially toxic elements in soil and sediments from a semiarid and coastal humid tropical transitional river basin. Microchem. J. 2022, 179, 107544. [Google Scholar] [CrossRef]
- Imashuku, S. Rapid determination of the approximate content of bastnäsite in ores using cathodoluminescence imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122055. [Google Scholar] [CrossRef] [PubMed]
- Duplouy, L. Preliminary Investigation of Rare Earth Elements Ion Exchange on Zeolites. Master’s Thesis, University of Helsinki Open Repository, Helsinki, Finland, 2016. [Google Scholar]
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-de-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Xu, C.; et al. Adsorption of rare earth elements in regolith-hosted clay deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef]
- Obhođaš, J.; Sudac, D.; Meric, I.; Pettersen, H.E.S.; Uroić, M.; Nađ, K.; Valković, V. In-situ measurements of rare earth elements in deep sea sediments using nuclear methods. Sci. Rep. 2018, 8, 4925. [Google Scholar] [CrossRef]
- Stuckman, M.Y.; Lopano, C.L.; Granite, E.J. Distribution and speciation of rare earth elements in coal combustion by-products via synchrotron microscopy and spectroscopy. Int. J. Coal Geol. 2018, 195, 125–138. [Google Scholar] [CrossRef]
- Jochum, K.P.; Seufert, H.M.; Midinet-Best, S.; Rettmann, E.; Schönberger, K.; Zimmer, M. Multi-element analysis by isotope dilution-spark source mass spectrometry (ID-SSMS). Fresenius Z. Anal. Chem. 1988, 331, 104–110. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, Y.; Ge, L.; Wang, Y.; Li, T.; Zhang, Q.; Wu, H.; Li, X.; Liu, Y. Direct analysis of lanthanum in extraction process by in-situ gamma spectrometry. J. Radioanal. Nucl. Chem. 2022, 331, 3807–3817. [Google Scholar] [CrossRef]
- Shen, S.; Krogstad, E.; Conte, E.; Brown, C. Rapid unseparated rare earth element analyses by isotope dilution multicollector inductively coupled plasma mass spectrometry (ID-MC-ICP-MS). Int. J. Mass Spectrom. 2022, 471, 116726. [Google Scholar] [CrossRef]
- Folkedahl, B.; Nyberg, C.; Biswas, S.; Zhang, X. Round-Robin Interlaboratory Study on Rare-Earth Elements in U.S.-Based Geologic Materials. Minerals 2023, 13, 944. [Google Scholar] [CrossRef]
- Ardini, F.; Soggia, F.; Rugi, F.; Udisti, R.; Grotti, M. Comparison of inductively coupled plasma spectrometry techniques for the direct determination of rare earth elements in digests from geological samples. Anal. Chim. Acta 2010, 678, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zuma, M.C.; Lakkakula, J.; Mketo, N. Recent trends in sample preparation methods and plasma-based spectrometric techniques for the determination of rare earth elements in geological and fossil fuel samples. Appl. Spectrosc. Rev. 2020, 57, 353–377. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Heise, S.; Saviano, L.; Zhang, K.; Giarra, A.; Bau, M.; Tommasi, F.; Guida, M.; Libralato, G.; Trifuoggi, M. Levels of rare earth elements on three abandoned mining sites of bauxite in southern Italy: A comparison between TXRF and ICP-MS. Talanta 2024, 275, 126093. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).