Abstract
A solid phase microextraction (SPME) method coupled with liquid chromatography (LC) and fluorescence/ultraviolet-diode array detection was developed for the simultaneous determination of quercetin and trans-resveratrol. The chromatographic, detection, and SPME extraction/desorption conditions were systematically optimized. The performance of four commercial SPME fibers—polyacrylate (PA), polyethylene glycol (PEG), polydimethylsiloxane (PDMS), and polydimethylsiloxane-divinylbenzene (PDMS-DVB)—was evaluated and compared with a homemade polydopamine (PDA)-coated fiber. While all of the fibers successfully extracted the target analytes, their efficiencies varied significantly. The PA, PEG, and PDA fibers demonstrated superior performance, exhibiting wide linearity ranges (0.03–1 µg/mL (PA and PEG) and 0.06–1 µg/mL (PDA) for quercetin, 0.01–1 µg/mL for trans-resveratrol); high sensitivity (LODs of 0.01 µg/mL (PA and PEG) and 0.02 µg/mL (PDA) for quercetin, 0.003 µg/mL for trans-resveratrol); and excellent precision. Among these, the polyacrylate coating delivered the best analytical performance and was selected for further application. The optimized method was applied to analyze winemaking by-products (seeds, skins, and stalks) using SPME on ethanol-macerated extracts subjected to brief ultrasonication. Quercetin and trans-resveratrol were quantified in pomace extracts at concentrations of 104.3 ± 8.2 µg/g and 38.5 ± 4.1 µg/g, respectively. Recovery experiments confirmed the method’s accuracy, with recoveries of 99.1 ± 7.4% for quercetin and 98.5 ± 9.8% for trans-resveratrol. This study establishes a reliable, sensitive, and efficient approach for the determination of these bioactive compounds in complex matrices, with potential applications in the food and pharmaceutical industries.
1. Introduction
Polyphenols are substances with numerous useful nutritional and pharmaceutical properties [1]. Quercetin (Q) or 3,3′,4′,5,7-pentahydroxyflavone is one of the most abundant plant flavonoids in nature and has been the focus of numerous biomedical studies in the last decade for its anti-inflammatory, anticancer, protective and preventive therapeutic properties for heart health, and low systemic toxicity [2,3,4,5]. It has also recently been shown to have inhibitory activity against SARS-CoV [6]. Resveratrol (R), or 3,5,4-trihydroxystilbene, is a non-flavonoid phenol, naturally produced by various plants to protect against stressful situations, such as fungal or bacterial infections, injury, and exposure to ultraviolet rays [7,8,9]. Resveratrol may exert a protective effect on the central nervous system and is associated with a reduced risk of cardiovascular disease, diabetes, and Alzheimer’s disease and it has anti-aging effects [7,10,11,12]. In addition, it may play an important role in the inhibition, promotion, and progression of multistage carcinogenesis [7,10,11].
Q and R have been proposed, alone and in combination, as components of formulations for important therapeutic applications, such as in the treatment of colorectal cancer [13,14,15,16]. However, a serious obstacle to their pharmacological use [16] is represented by their rapid metabolism in humans. Slow-release nano-formulations have been recommended to improve their pharmacokinetics and prolong their benefits in the treatment of neoplasms [16].
Grapes, particularly their skins, and grape-derived products such as wine, are among the most significant dietary sources of R [1,8]. Similarly, Q is abundantly found in fruits and vegetables, with notable concentrations in apples, grapes, medicinal herbs, and various fruit waste materials [17,18,19,20]. These compounds are recognized as potent antioxidants, making grape and wine waste a valuable resource for extracting both Q and R.
Given the importance of these natural antioxidants in food supplements and pharmaceutical applications, numerous analytical methods have been developed for their determination in various matrices, including grapes, wine, and winemaking by-products. Among these, liquid chromatography (LC) coupled with ultraviolet (UV) detection is the most widely used technique due to its affordability and accessibility for most laboratories [20,21,22,23,24]. Additionally, fluorescence detection (FLD) and mass spectrometry (MS) have been successfully employed for the quantification of Q and R in food samples, offering higher sensitivity and selectivity [23,24,25,26]. Other techniques, such as thin-layer chromatography (TLC), gas chromatography (GC), capillary electrophoresis (CE), and electrochemical methods, have also been utilized for their determination, though to a lesser extent [23,24,27].
For the extraction of Q and R from winemaking waste, solid–liquid extraction (SLE) and supercritical fluid extraction (SFE) are commonly employed. However, these methods are often labor-intensive, costly, and require significant amounts of organic solvents, which can limit their practicality and sustainability [20,23,24]. As a result, there is a growing interest in developing more efficient and environmentally friendly extraction techniques to optimize the recovery of these valuable antioxidants.
Solid phase microextraction (SPME) is a fast and sensitive sample preparation technology that uses a fiber coated with a suitable polymer extraction phase to concentrate different analytes present in a liquid, solid, or gaseous sample [28]. The most typical uses include environmental, food, forensic and toxicological analyses for the search for contaminants, aromas, odors, and drugs, even at the trace level in aqueous or gaseous matrices [29], as well as pharmacokinetic studies, therapeutic drug monitoring, biomarker discovery, and targeted and untargeted metabolomics [30,31,32,33]. Since commercially available SPME fibers are limited, there is also a growing interest in the development and characterization of new SPME extraction sorbents. The natural organic polymer polydopamine (PDA) has recently been proposed as an extractive phase for SPME fibers because of its outstanding chemical and environmental stability, convenience for further modification, and eco-friendliness [34].
This work reports the development of a simple SPME extraction method of Q and R from aqueous matrices, which were subsequently analyzed by liquid chromatography (LC) with in series fluorescence (FD) and ultraviolet-diode array (UV-DAD) detection. The performance of one homemade polydopamine (PDA) silica fiber and of four commercial LC fibers, namely, polyacrylate (PA), polyethylene glycol (PEG), polydimethylsiloxane-divinylbenzene (PDMS-DVB), and polydimethylsiloxane (PDMS), were tested and compared. The optimized method was then successfully applied to the determination of the two polyphenols in winemaking by-products after a previous ethanol extraction step.
2. Material and Methods
2.1. Reagents and Materials
All solvents (HPLC-grade) and substances were purchased from Sigma-Aldrich (Milan, Italy). Quercetin and trans-resveratrol were USP (United States Pharmacopoeia reference standard)-grade powders. Stock solutions (1 mg/mL) were prepared in ethanol and stored in the dark at −20 °C. More dilute solutions were prepared in mobile phase just before use.
SPME-LC commercial fibers, i.e., 85 μm polyacrylate (PA), 65 μm polydimethylsiloxane–divinylbenzene (PDMS–DVB), 60 µm polyethylene glycol (PEG), and 100 μm polydimethylsiloxane (PDMS), were supplied by Merck (Sigma-Aldrich).
Homemade polydopamine (PDA)-coated fibers were prepared according to Aresta et al. [34]. Briefly, silica fibers (Supelco, Bellefonte, PA, USA) were washed 2 times in stirred Tris(hydroxymethyl)aminomethane hydrochloride (TRIS-HCl) and water buffer (25 mM, pH 8.5). Then, the PDA coating (PDA) was obtained by adding 3 mg dopamine hydrochloride in the TRIS buffer solution (10 mL; 25 mM, pH 8.5) and immersing the fiber for 2 h at room temperature under stirring. After the coating treatment, the fiber was extensively washed with the TRIS buffer, water, water/ethanol (50:50, v/v), ethanol, ethanol/acetonitrile (50:50, v/v) and water/acetonitrile/methanol (25:10:65, v/v/v) to remove dopamine. PDA fibers that did not have the appropriate morphological, compositional, and structural characteristics were discarded after careful inspection.
2.2. HPLC Apparatus
Chromatographic analyses were performed using a system composed of two Nexera LC-30AD pumps (Shimadzu, Milan, Italy), an SPME interface (Supelco), an RF-20AXS fluorescence detector (Shimadzu), an SPD-20M20A UV photodiode array detector (Shimadzu), an AccucoreTM C18 HPLC column (100 mm × 4.6 mm, 4 μm, Thermo Scientific, Milan, Italy), and an Accucore C18 guard column (Thermo Scientific). The data were acquired by LabService software, v.5.03.
2.3. Chromatographic and Detection Conditions
The mobile phase was a water/methanol/ethanol mixture (56:40:6, v/v/v) with 1.2% acetic acid, filtered daily through a 0.45 μm nylon membrane (Lab Service Analytica, Bologna, Italy). The flow rate was 0.8 mL/min. UV spectra were recorded in the wavelength range 220–500 nm. The acquisition software automatically selected and stored the maximum absorbances for each peak (i.e., 307 nm and 370 nm for R and Q, respectively), plotting Max Plot chromatograms, which report the maximum signal for each detected compound. The fluorescence detector was operated at excitation and emission wavelengths of 310 and 380 nm for R, and 370 and 540 nm for Q, respectively.
2.4. Solid Phase Micro Extraction
A manual SPME device (Supelco) was used to hold the fiber. Extractions were performed for 60 min at 18 °C (room temperature), by direct immersion of the fiber in 15 mL amber vials (Sigma-Aldrich) with PTFE hole caps (Sigma-Aldrich), containing 6.6% ethanol/10% sodium chloride solution at pH 2.3, under magnetic stirring. An SPME interface, consisting of a standard six-port HPLC injector with a special fiber desorption chamber (60 μL, volume) installed in place of the sample loop, was used to inject the sample into the column. Analyte desorption was performed in static mode by soaking the fiber in the mobile phase into the desorption chamber of the SPME interface for 20 min. After, the valve was changed to the inject position for 10 s. Then, the fiber was removed and soaked for 5 min in ethanol and subsequently rinsed with water, to ensure complete cleaning. Three replicates for each sample were performed.
2.5. Sample Collection and Pre-Treatment
The winemaking waste, consisting of seeds, skins and stalks, was supplied from a local supplier, dried, and stored at −20 °C until before use. Extraction of quercetin and trans-resveratrol was carried out through the following procedure. A 0.5 g portion of sample was weighed into a screw-capped glass tube. The sample was crushed, pressed further, and extracted with 1 mL of ethanol by ultrasonication (AU-32, Argo Lab, Carpi, Italy) for 15 min and shaking for 24 h at room temperature. After centrifugation at 5000× g, the remaining pellet was re-extracted for 24 h using 1 mL of fresh extraction solvent. The procedure was repeated 3 more times. The extracts were combined and 1 mL was sealed in a 15 mL vial filled with a 6.6% ethanol/10% sodium chloride solution at pH 2.3, immediately subjected to SPME using the polyacrylate fiber for 1 h at room temperature under magnetic stirring, and eventually desorbed into the SPME interface. Each analysis was conducted in triplicate.
3. Results and Discussion
3.1. Optimization of Chromatographic Conditions
The preliminary experiments were conducted using standard solutions and direct injection through the SPME interface (60 µL volume) to determine the optimal chromatographic conditions for analyte separation. Table 1 summarizes the efficiency factor (N), retention factor (k′), and symmetry factor (S), measured as the peak width at 1/20 of its height above baseline (w0.05h), for both analytes using the optimized mobile phase: a water/methanol/ethanol mixture (56:40:6, v/v/v) with 1.2% acetic acid. Both the N and k′ values were appropriate for the column type, and the Sw0.05h coefficient indicated excellent peak symmetry for both analytes.

Table 1.
Efficiency factor (N), retention factor (k’), and symmetry factor (S) of the peak width at 1/20 of its height above baseline (w0.05h) obtained for R and Q.
Solubility is a critical property of flavonoids, significantly influencing their behavior [4]. However, the literature on quercetin (Q) solubility is inconsistent [35]. Q can engage in intermolecular interactions with solvents, leading to variability in solubility and properties [36]. While Q is highly soluble in organic solvents and poorly soluble in water, its solubility increases with temperature [36]. However, in this study, higher temperatures negatively affected the stability of Q solutions. Aliquots of 1 mL of Q and R solutions (0.1 µg/mL) were incubated at 37 °C and 100 °C, and then kept at room temperature and analyzed at different time intervals (0, 30, 60, and 120 min). As shown in Table 2, the R peak areas remained constant, whereas the Q peak areas decreased significantly at higher temperatures.

Table 2.
Q and R peak areas obtained, keeping the relevant solutions (0.1 µg/mL) at different temperatures for various times.
Calibration curves were constructed using a UV-DAD detector in the concentration range of 0.5 ng/mL to 5 µg/mL. The linear regression equations were y = 12839·x − 199 for Q and y = 22998·x + 126 for R, where x is the injected amount (ng) and y is the peak area. Both analytes exhibited good linearity (correlation coefficients > 0.999). The limits of detection (LOD, S/N = 3) and quantification (LOQ, S/N = 10) were 1.7 ng/mL (0.1 ng) and 5.0 ng/mL (0.3 ng) for Q and 0.8 ng/mL (0.05 ng) and 2.8 ng/mL (0.17 ng) for R, respectively. The precision (% RSD) was evaluated at 0.005, 0.05, and 0.5 µg/mL, with intra-day (n = 3) and inter-day (n = 3 × 5 days) precision values below 3.0% for Q and 1.8% for R, demonstrating excellent accuracy and repeatability.
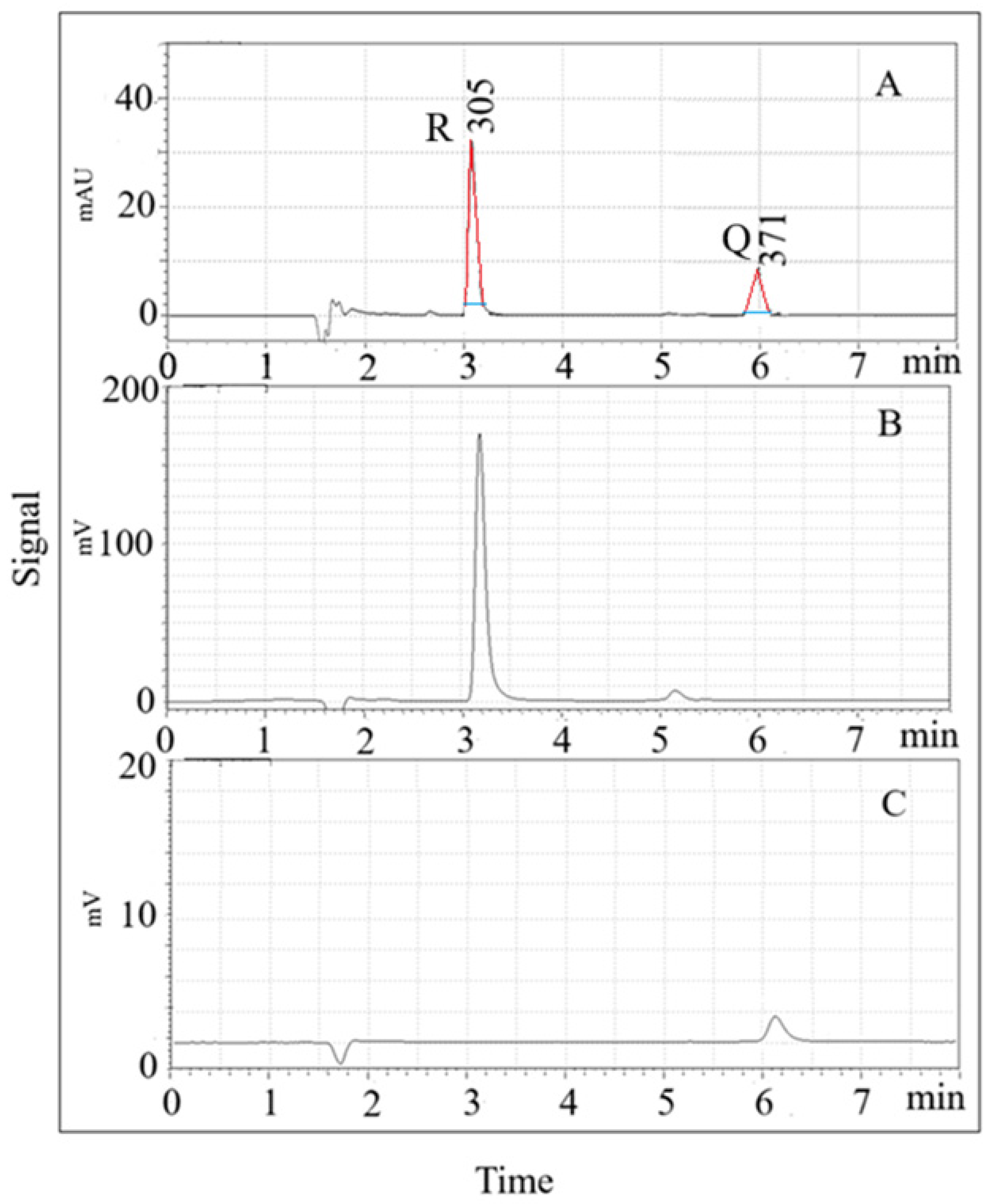
Fluorescence detection (FD) optimization was performed by evaluating excitation and emission wavelengths. For Q, the highest peak area was observed at 370 nm (excitation) and 540 nm (emission), while for R, optimal wavelengths were 310 nm (excitation) and 380 nm (emission). The calibration curves using FD in the range of 0.01–20 ng/mL showed linearity with correlation coefficients > 0.999. The LOD and LOQ values were 3.3 ng/mL and 11.1 ng/mL for Q, and 0.07 ng/mL and 0.22 ng/mL for R, respectively. The intra-day and inter-day precision values were below 3.3% for Q and 1.0% for R. Figure 1 shows typical LC-UV and LC-FD profiles for a 1 µg/mL solution of both analytes under optimized conditions.
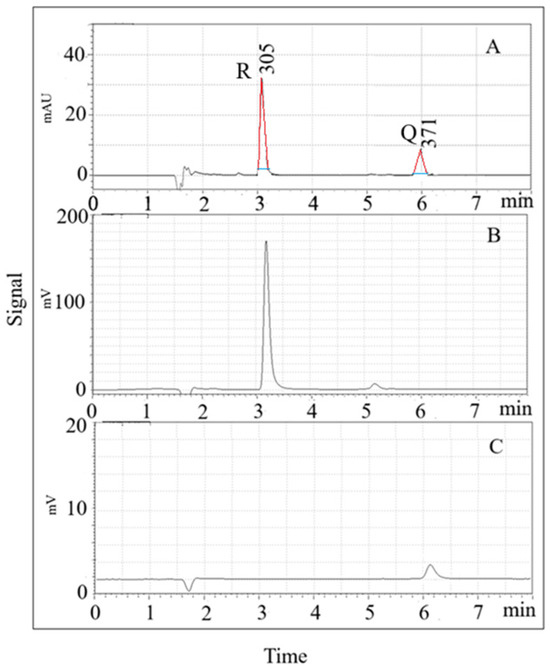
Figure 1.
SPME- LC-UV (A) and SPME-LC-FD (B,C) chromatograms for a 1 µg/mL solution of both analytes. (A) Max Plot chromatogram: resveratrol (R) = 3.1 min, and quercetin (Q) = 5.9 min; (B) excitation and emission wavelengths: 310, and 380 nm, respectively; (C) excitation and emission wavelengths: 370, and 540 nm, respectively. Red line: tangent to the side of the peak; blue line: peak width at 1/20 of its height above baseline (W0.05h).
3.2. Optimization of the SPME Procedure
The SPME procedure was optimized by evaluating salt addition, pH, and sample volume, which significantly influence analyte extraction efficiency. A constant stirring rate of 700 rpm and a polyacrylate (PA) coating were used, as PA has demonstrated good extraction efficiency for R in aqueous solutions [1]. Ethanol (6.6%) was added to enhance the extraction efficiency [37]. Standard mixtures of Q (3.0 µg/mL) and R (1.0 µg/mL) in 6.6% ethanol were used for optimization, with UV detection.
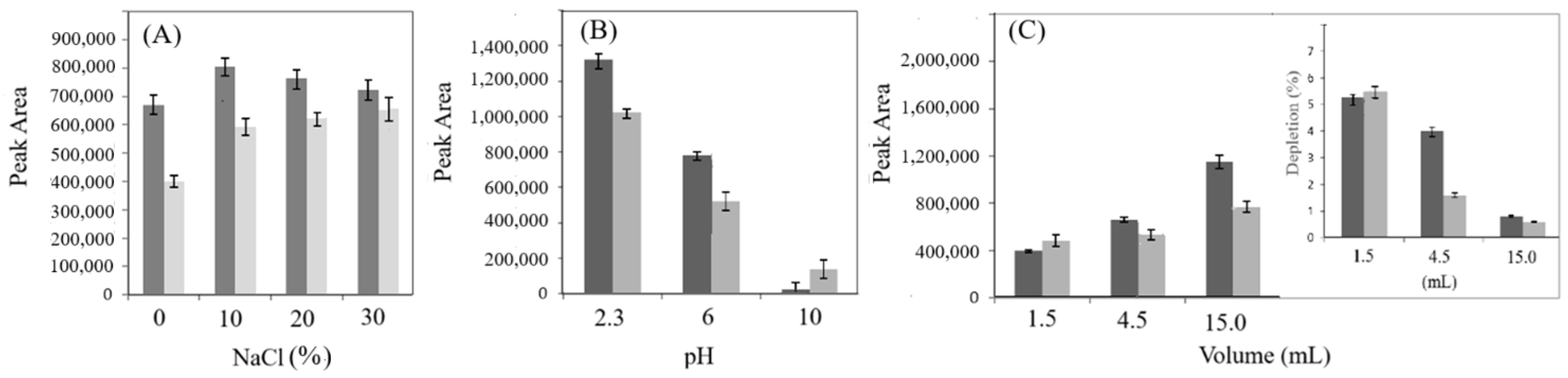
Since the extraction efficiency of some analytes can be enhanced by increasing the ionic strength of the sample solution, leveraging the salting-out effect [38], four NaCl concentrations (0%, 10%, 20%, and 30%) were subjected to SPME and analyzed. As shown in Figure 2A, the presence of NaCl generally led to a significant increase in the amount of both the analytes. The best results were obtained using 10% NaCl, which was selected for the subsequent experiments. The pH is also a crucial factor for the extraction of certain analytes [30]. Consequently, its influence on the extraction capacity was studied conducting experiments with saline solutions (10%NaCl) at different pH values (2.3, 6, and 10). As shown in Figure 2B, the extraction efficiency of both analytes decreased as the pH increased. Therefore, an acidic pH was selected for the next optimization step. In addition, the effect of sample volume was evaluated, since variations in sample volume can significantly affect quantification and accuracy [39]. As shown in the insert in Figure 2C, among the volumes examined, 15 mL is the minimum sample volume ensuring that the extracted amount remains less than 1% of the total analyte present. In contrast, smaller volumes lead to greater depletions of the analyte, reaching up to 5% with a 1.5 mL sample.
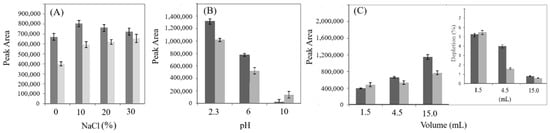
Figure 2.
Extraction efficiency of quercetin (dark grey) and trans-resveratrol (light grey). (A) Effects of salt addition (NaCl, g/100 mL); (B) effect of pH in the presence of 10% NaCl; (C) effect of the sample volume. The insert in figure (C) shows the percentage depletion. Fiber used: PA; extraction time: 60 min at room temperature; desorption mode: dynamic.
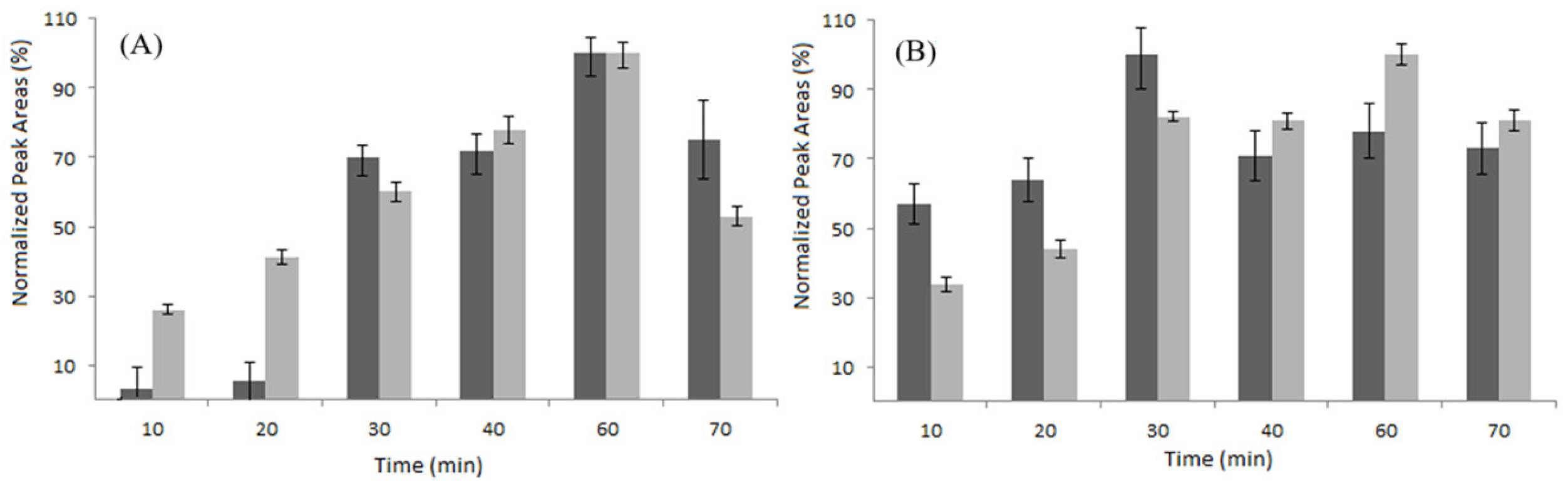
Once the extraction conditions were optimized, a comparative study on the performance of one homemade polydopamine (PDA) silica fiber and of four commercial LC fibers, i.e., polyacrylate (PA), polyethylene glycol (PEG), polydimethylsiloxane-divinylbenzene (PDMS-DVB), and polydimethylsiloxane (PDMS), was conducted. Figure 3A and Figure 3B reports the extraction time profiles obtained for quercetin and trans-resveratrol, respectively, using the PDA and PA fibers. Both of the fibers effectively extracted the target analytes, while the optimal extraction times were 60 min for Q and 30 min for R. An equilibrium time of 60 min was adopted for further experiments.
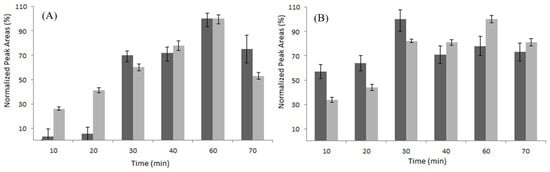
Figure 3.
Comparison of the normalized response of PDA fiber (dark grey) and PA fiber (light grey) at different times for Q (A) and R (B).
Experiments were subsequently carried out under the same extraction conditions to compare the extraction efficiency of the PDA, PA, PEG, PDMS-DVB, and PDMS fibers. For this purpose, a mixed standard solution (1 µg/mL) in 6.6% ethanol and 10% NaCl and at pH 2.3 was used.
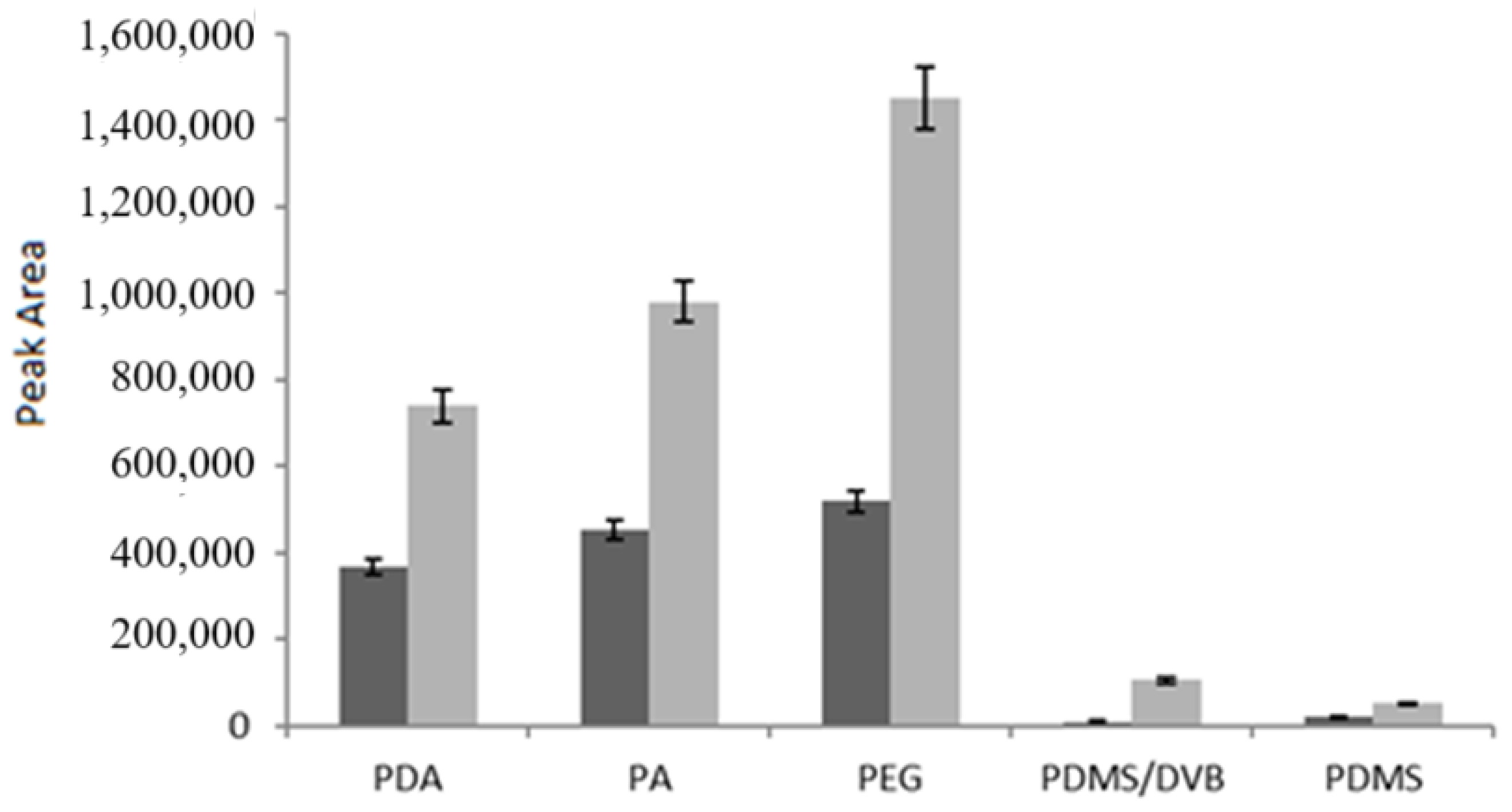
As shown in Figure 4, both analytes were successfully extracted by the PDA fiber, while the best results were observed for the PEG fiber, followed by the PA fiber; on the contrary, the PDMS-DVB and PDMS fibers were the least efficient. These findings were in good agreement with those of Kumar et al. [40], who compared the performance of PDMS-DVB and PDMS fibers with that of Carbowax/Templated Resin (CW/TPR) for determining quercetin and myricetin in grapes, vegetables, and red wine samples, with CW/TPR yielding the best results. Although CW/TPR is not currently commercially available, PEG exhibits a comparable degree of polarity. Data from Aresta et al. [1] for trans-resveratrol further support these results.
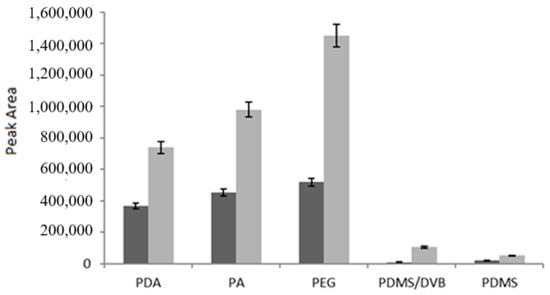
Figure 4.
Comparison of fibers under the same extraction conditions (15 mL volume, 6.6% ethanol, 10% NaCl, pH 2.3, 60 min at room temperature). Q (dark grey) and R (light grey) concentration: 1 μg/mL. Dynamic desorption mode.
The dynamic and static desorption modes [41] were evaluated. Static desorption improved the peak symmetry but resulted in carry-over, particularly for the PEG and PDA fibers, as shown in Table 3, which summarizes the SW0.05h values detected for each analyte. Dynamic desorption was selected for the subsequent experiments due to its quantitative recoveries.

Table 3.
Sw0.05h and carry-over obtained in dynamic and static desorption modes.
The SPME-HPLC method was validated for the PA, PEG, and PDA fibers using seven standard solutions (0.1 ng/mL–1 µg/mL). Linear calibration curves with high correlation coefficients and satisfactory reproducibility were obtained (Table 4). The PDA fiber performed comparably to PA and PEG for R but had higher LODs for Q. The intra-day precision (% RSD) ranged from 3.4% to 9.8% at 0.06 µg/mL. The inter-day precision was not assessed due to Q instability in aqueous solutions.

Table 4.
Linearity, sensitivity, and precision of the developed SPME-LC- UV-DAD method using the PA, PEG, and PDA fibers.
3.3. Winemaking Waste Analysis
The PA fiber was used to analyze wine pomace ethanolic extracts obtained by maceration. The SPME-LC-F-UV analysis revealed Q (104.3 ± 8.2 µg/g dry weight) and R (38.5 ± 4.1 µg/g dry weight) in the extracts, with Q present at significantly higher levels. The optimized SPME procedure demonstrated high selectivity, yielding chromatograms similar to those of standard solutions despite matrix complexity. The accuracy of the extraction procedure was evaluated via recovery experiments in which varying amounts of mixed standard solutions were added at three concentration levels (one, two, and five times the estimated levels) to wine pomace extracts. Very high recoveries were obtained, i.e., 99.1 ± 7.4% for quercetin and 98.5 ± 9.8% for trans-resveratrol. The results obtained confirmed that ethanolic wine pomace extract contains high levels of quercetin [21], being a great source of these phenolic compounds. In addition, our results show that fluorescence detection is more effective for trans-resveratrol than for quercetin. Since Q and R are considered valuable fluorescent sensors for chemical and biological detection, further investigations are underway in our laboratory to combine the SPME-LC technique with fluorescence detection to elucidate aspects related to the dispersion of these molecules in water—factors that are responsible for their solubility and bioactivity.
4. Conclusions
In this study, robust and sensitive solid phase microextraction (SPME) coupled with liquid chromatography (LC) and fluorescence/ultraviolet-diode array detection was successfully developed for the determination of quercetin and trans-resveratrol. The optimization of the chromatographic, detection, and SPME extraction/desorption conditions revealed that polyacrylate, polyethylene glycol, and a homemade polydopamine (PDA)-coated fiber exhibited the best performance, with wide linearity ranges (0.03–1 µg/mL for quercetin and 0.01–1 µg/mL for trans-resveratrol), high sensitivity (LODs of 0.01 µg/mL for quercetin and 0.003 µg/mL for trans-resveratrol), and good precision. Among these, the polyacrylate coating demonstrated a superior analytical performance and was selected for the analysis of winemaking by-products (seeds, skins, and stalks). Quercetin and trans-resveratrol were successfully quantified in pomace extracts at concentrations of 104.3 ± 8.2 µg/g and 38.5 ± 4.1 µg/g, respectively. Recovery experiments confirmed the method’s accuracy, with recoveries of 99.1 ± 7.4% for quercetin and 98.5 ± 9.8% for trans-resveratrol. This method provides a reliable and efficient approach for the analysis of these bioactive compounds in complex matrices, offering potential applications in the food and pharmaceutical industries.
Author Contributions
Conceptualization: A.M.A. and C.Z.; methodology: A.M.A., N.D.V., and C.Z.; validation: A.M.A. and G.M.; investigation: A.M.A. and G.M.; resources: A.M.A., N.D.V., and C.Z.; data curation: A.M.A.; writing—original draft preparation: A.M.A. and C.Z.; writing—review and editing: A.M.A.; supervision: C.Z.; funding acquisition: A.M.A. and C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for tender No. 341 of 15 March 2022 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code PE00000003, Concession Decree No. 1550 of 11 October 2022, adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods”.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Danilo Vona for the preparation of homemade polydopamine (PDA)-coated fibers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aresta, A.; Cotugno, P.; Massari, F.; Zambonin, C. Determination of Trans-resveratrol in Wines, Spirits, and Grape Juices Using Solid-Phase Micro Extraction Coupled to Liquid Chromatography with UV Diode-Array Detection. Food Anal. Methods 2018, 11, 426–431. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1855–1868. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Zhu, X.; Ding, G.; Ren, S.; Xi, J.; Liu, K. The bioavailability, absorption, metabolism, and regulation of glucolipid metabolism disorders by quercetin and its important glycosides: A review. Food Chem. 2024, 458, 140262. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Ajami, M.; Sotoudeheian, M.; Houshiar-Rad, A.; Esmaili, M.; Naeini, F.; Nasrabadi, F.M.; Doaei, S.; Milani-Bonab, A. Quercetin may reduce the risk of developing the symptoms of COVID-19. Avicenna J. Phytomed. 2024, 14, 189–201. [Google Scholar]
- Yu, X.; Jia, Y.; Ren, F. Multidimensional biological activities of resveratrol and its prospects and challenges in the health field. Front. Nutr. 2024, 12, 1408651. [Google Scholar] [CrossRef]
- Hanzouli, F.; Zemni, H.; Gargouri, M.; Boubakri, H.; Mliki, A.; Vincenzi, S.; Daldoul, S. Evidence of an active role of resveratrol derivatives in the tolerance of wild grapevines (Vitis vinifera ssp. sylvestris) to salinity. J. Plant Res. 2024, 137, 265–277. [Google Scholar] [CrossRef]
- Samia, D.; Hanzouli, F.; Farès, N.; Tabbene, O.; Zemni, H.; Vincenzi, S.; Mliki, A.; Gargouri, M. Enhancing biological activities and phenolic content of wild grapevine roots by severe drought stress. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2024, 158, 344–353. [Google Scholar]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Cheng, J.; Li, J.; Wu, S.X.; Xiong, R.G.; Huang, S.Y.; Cheung, P.C.; Li, H.B. Resveratrol and Its Analogues: Anti-ageing Effects and Underlying Mechanisms. Subcell. Biochem. 2024, 107, 183–203. [Google Scholar] [PubMed]
- Eity, T.A.; Bhuia, M.S.; Chowdhury, R.; Ahmmed, S.; Sheikh, S.; Akter, R.; Islam, M.T. Therapeutic Efficacy of Quercetin and Its Nanoformulation Both the Mono- or Combination Therapies in the Management of Cancer: An Update with Molecular Mechanisms. J. Trop. Med. 2024, 10, 5594462. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.; Yang, Z.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Zou, Y.; Chen, G.; Jin, Z. Co-encapsulation of quercetin and resveratrol: Comparison in different layers of zein-carboxymethyl cellulose nanoparticles. Int. J. Biol. Macromol. 2024, 278, 134827. [Google Scholar] [CrossRef] [PubMed]
- More, K.S.; Kadavakollu, S.; Nigar, S.; Gul, K.; Sehrawat, R.; Mir, N.A. Encapsulation of resveratrol in alginate microcapsules using internal gelation technique: Fabrication, characterization and release kinetics. LWT 2024, 207, 116663. [Google Scholar] [CrossRef]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Khan, S.A. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer-An Updated Review. Pharmaceutics 2024, 16, 761. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity-a review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Lojkovà, L.; Pluhackovà, H.; Benesova, K.; Kudlackova, B.; Cerkal, R. The highest yield, or greener solvents? Latest trends in quercetin extraction methods. Trends Anal. Chem. 2023, 167, 117229. [Google Scholar] [CrossRef]
- Eftekhari, M.; Alizadeh, M.; Ebrahimi, P. Evaluation of the total phenolics and quercetin content of foliage in mycorrhizal grape (Vitis vinifera L.) varieties and effect of postharvest drying on quercetin yield. Ind. Crops Prod. 2012, 38, 160–165. [Google Scholar] [CrossRef]
- Buiarelli, F.; Bernardini, F.; Di Filippo, P.; Riccardi, C.; Pomata, D.; Simonetti, G.; Risoluti, R. Extraction, Purification, and Determination by HPLC of Quercetin in Some Italian Wines. Food Anal. Methods 2018, 11, 3558–3562. [Google Scholar] [CrossRef]
- Careri, M.; Corradini, C.; Elviri, L.; Nicoletti, I.; Zagnoni, I. Direct HPLC Analysis of Quercetin and trans-Resveratrol in Red Wine, Grape, and Winemaking Byproducts. J. Agric. Food Chem. 2003, 51, 5226–5231. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.; Peeva, P. Rapid HPLC Method for Simultaneous Quantification of trans-Resveratrol and Quercetin in the Skin of Red Grapes. Food Anal. Methods 2018, 11, 514–521. [Google Scholar] [CrossRef]
- Li, W.; Yuan, H.; Liu, Y.; Wang, B.; Xu, X.; Xu, X.; Hussain, D.; Ma, L.; Chen, D. Current analytical strategies for the determination of resveratrol in foods. Food Chem. 2024, 431, 137182. [Google Scholar] [CrossRef]
- Mansour, F.R.; Abdallah, I.A.; Bedair, A.; Hamed, M. Analytical methods for the determination of quercetin and quercetin glycosides in pharmaceuticals and biological samples. Crit. Rev. Anal. Chem. 2025, 55, 187–212. [Google Scholar] [CrossRef]
- Merás, I.D.; Díaz, T.G.; Rodríguez, D.A. Determination of piceid by photochemically induced fluorescence and second-derivative: Response surface methodology for the optimization of a liquid–liquid extraction procedure for its analysis in wine samples. Talanta 2008, 74, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Moretón-Lamas, E.; Lago-Crespo, M.; Lage-Yusty, M.A.; López-Hernández, J. Comparison of methods for analysis of resveratrol in dietary vegetable supplements. Food Chem. 2017, 224, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Pillai, D.; Pandita, N. Validated high performance thin layer chromatography method for the quantification of bioactive marker compounds in Draksharishta, an ayurvedic polyherbal formulation. Rev. Bras. Farmacogn. 2016, 26, 558–563. [Google Scholar] [CrossRef][Green Version]
- Zambonin, C.; Aresta, A. Review: Recent Applications of Solid Phase Microextraction coupled to Liquid Chromatography. Separation 2021, 8, 34. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Hallmann, A.; Treder, N.; Bączek, T.; Roszkowska, A. Recent advances in the use of SPME for drug analysis in clinical, toxicological, and forensic medicine studies. Talanta 2024, 270, 125613. [Google Scholar] [CrossRef]
- Bojko, B.; Cudjoe, E.; Gómez-Ríos, G.A.; Gorynski, K.; Jiang, R.; Reyes-Garcés, N.; Risticevic, S.; Silva, É.A.S.; Togunde, O.; Vuckovic, D.; et al. SPME–Quo vadis? Anal. Chim. Acta 2012, 750, 132–151. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Zhou, L.-D.; Chen, H.; Wang, C.-Z.; Xia, Z.-N.; Yuan, C.-S. Solid-phase microextraction technology for in vitro and in vivo metabolite analysis. TrAC Trends Anal. Chem. 2016, 80, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zambonin, C.G.; Aresta, A. SPME-LC with UV detection to study delorazepam-serum albumin interactions. J. Pharm. Biomed. Anal. 2002, 29, 895–900. [Google Scholar] [CrossRef]
- Aresta, A.; Di Grumo, F.; Zambonin, C. Determination of Major Isoflavones in Soy Drinks by Solid-Phase Micro Extraction Coupled to Liquid Chromatography. Food Anal. Methods 2015, 9, 925–933. [Google Scholar] [CrossRef]
- Aresta, A.; Cicco, S.R.; Vona, D.; Farinola, G.M.; Zambonin, C. Mussel Inspired Polydopamine as Silica Fibers Coating for Solid-Phase Microextraction. Separations 2022, 9, 194. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E., Jr. On the solubility of quercetin. J. Mol. Liq. 2014, 197, 157–159. [Google Scholar] [CrossRef]
- Lanati, D.; Cascio, P.; Pollón, M.; Corona, O.; Marchi, D. Solubility of Quercetin in Wines. S. Afr. J. Enol. Vitic. 2022, 43, 146–156. [Google Scholar] [CrossRef]
- Zhang, L.; Gionfriddo, E.; Acquaro, V., Jr.; Pawliszyn, J. Direct immersion solid-phase microextraction analysis of multi-class contaminants in edible seaweeds by gas chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1031, 83–97. [Google Scholar] [CrossRef]
- Górecki, T. Effect of Sample Volume on Quantitative Analysis by Solid-phase Microextraction Part 1. Theoretical Considerations. Analyst 1997, 122, 1079–1086. [Google Scholar] [CrossRef]
- Kumar, A.; Malik, A.K.; Tewary, D.K. A new method for determination of myricetin and quercetin using solid phase microextraction–high performance liquid chromatography–ultra violet/visible system in grapes, vegetables and red wine samples. Anal. Chim. Acta 2009, 631, 177–181. [Google Scholar] [CrossRef]
- Zambonin, C.G. Coupling solid-phase microextraction to liquid chromatography. A review. Anal. Bioanal. Chem. 2003, 375, 73–80. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).