Abstract
Canine olfaction is a highly developed sense and is utilized for the benefit of detection applications, ranging from medical diagnostics to homeland security and defense prevention strategies. Instrumental validation of odor delivery methods is key to standardize canine olfaction research to establish baseline data for explosive detection applications. Solid-phase microextraction gas chromatography (SPME/GC-MS) was used to validate the odor delivery of an olfactometer. Three explosive classes were used in this study: composition C-4 (C-4), trinitrotoluene (TNT), and ammonium nitrate (AN). Dynamic airflow sampling yielded the successful detection of previously reported target volatile organic compounds (VOCs): 2,3-dimethyl-2,3-dinitrobutane (DMNB) in C-4 and 2-ethylhexan-1-ol (2E1H) in ammonium nitrate and TNT across odor dilutions of 80%, 50%, 25%, 12%, and 3%. C-4 highlighted the most reliable detection from the olfactometer device, depicting a response decrease as a function of dilution factor of its key odor volatile DMNB across the entire range tested. TNT only portrayed 2-ethylhexan-1-ol as a detected odor volatile with a detection response as a function of dilution from 80% down to 12%. Comparatively, ammonium nitrate also depicted 2-ethylhexan-1-ol as an odor volatile in the dynamic airflow sampling but with detection only within the upper scale of the dilution range (80% and 50%). The results suggest the importance of monitoring odor delivery across different dilution ranges to provide quality control for explosive odor detection using dynamic airflow systems.
1. Introduction
Canines have a highly developed olfactory sense and memory associated with odor [1,2,3]. Canines can be trained in odor detection with lower detection limits compared to analytical instrumentation [4]. Canine olfaction allows for the detection and identification of explosives, illicit drugs, currencies, human scent, wildlife conservation efforts, infectious diseases, and many other applications [5,6,7]. Research efforts are ongoing to determine what odors dissipate from specific targets (possibly, odorants the canines are using to identify the source), and to determine a threshold level with the associated target odor [8]. Measuring human and animal odor detection thresholds has been useful for a variety of applications such as environmental [9], agricultural [10,11,12], and human health [13]. While odor threshold testing is not novel, there is limited understanding of explosive odor delivery using airflow dynamic systems.
An odor detection threshold is the minimum concentration of an odorant that can be detected [14]. In the case of canine detection, dogs are required to detect trace quantities of odorants, many of these in the parts per billion (ppb) or parts per trillion (ppt) range [15]. Amyl acetate is a commonly studied odorant due to its desired physiochemical properties and safe handling. Amyl acetate has a range of reported odor thresholds in dogs: 50 ppb [16] and 6.15–0.21 ppt [17]. Canine threshold detection limits are variable for specific odors across laboratories (e.g., [18]). Some of this variability may be due in part to differences in odor presentation paired with the intrinsic physical properties of each odorant molecule being tested. These variations within the calculation of odor concentrations can be due to the numerous techniques employed for odor analysis. These techniques can include dynamic olfactometry [18], electronic noses [19], and gas chromatography-mass spectrometry [20], which are recognized analytical methods to evaluate odor detection and quantitation, odor monitoring, and chemical characterization [21].
Accurate vapor generation and efficient delivery are key parameters when embarking on odor threshold types of studies. In terms of olfactory threshold experimental designs, there are basic container setups with different dilutions of odor presented to the canine for behavioral response [16,17,22,23]. These are designed to be simple and operationally relevant set-ups which can easily be performed with detector dog teams to measure their sensitivity to target odorants. However, these types of studies must carefully take into consideration environmental contamination, odorant substrate selection, and odorant dissipation into the surroundings or onto the container device. Recently, a modified trace vapor generator was created to overcome some of these odor threshold challenges [24]. In the laboratory, odor thresholds are commonly measured utilizing olfactometers. These devices are ideal for evaluating complex odor mixtures as they deliver the target odor via controlled airstream systems [25,26,27,28,29,30]. Olfactometer devices can also be exploited for dilution because they have the capability of easily manipulating airflows [28]. Previous work in our laboratory has evaluated canine thresholds to four different explosives classes using an olfactometer [31]. The air dilution range allowed for the canines to be tested under different environmental conditions and showed the feasibility of using this type of odor delivery system for effective canine behavior testing. In terms of quality of odor delivery, previous studies have used analytical instrumentation to assess the output of various olfactometers [32,33]. Work in our laboratory recently evaluated double-based smokeless powder delivery evaluating odorant amounts, longevity and residual contamination [33].
While studies have utilized olfactometers for odor dilution research, there is still a dearth of knowledge about how varying explosive materials are delivered with these systems at distinctive dilution ranges. The aim of this research is to provide an analytical validation for an in-house olfactometer as the explosive odor delivery system using three distinctive explosive bulk materials using solid-phase microextraction/gas chromatography-mass spectrometry analysis (SPME-GC-MS) as the headspace odor sampling technique.
2. Materials and Methods
The explosive materials utilized in this study included the following: Composition C-4 (C-4), ammonium nitrate (AN) prill (CAS: 6484-52-2), and trinitrotoluene (TNT) (CAS: 119-96-7), all purchased from Omni Distribution, Inc. (Marion, AR, USA). Calibration chemical standards used included 2,3-dimethyl, 2-3-dinitrobutane (DMNB) (CA#: 3964-18-9) from Sigma-Aldrich (St. Louis, MO, USA) and 2-ethylhexan-1-ol (CAS: 104-76-7) from Thermo Fisher Scientific (Waltham, MA, USA). Analytical grade solvents used for calibration included acetone (CAS: 67-64-1), methylene chloride (CAS: 75-09-2), and acetonitrile (CAS: 75-05-8) from Sigma-Aldrich (St. Louis, MO, USA). Use of all energetic materials was approved by the Texas Tech University Institutional Laboratory Safety Committee on Energetic Materials (Protocol ILSC#2110E1).
The construction, electronic components, and dimensions for the automated olfactometer are described in previous work [34]. Briefly, the activation of a solenoid valve passed clean air from the selected valve to the jar connected via a tube (“1/8” OD polytetrafluoroethylene) that pierced a polytetrafluorethylene/silicone septa. A second tube inserted into the septa carried the headspace through a stainless-steel check valve (to prevent reverse flow contamination) and to a polytetrafluorethylene manifold where it mixed with a clean dilution line. The experimenter could adjust rotameters to control the flow rates from the odor line and dilution line, producing dilutions at 80%, 50%, 25%, 12%, and 3%. These dilutions were produced with the flow settings detailed in Table 1.

Table 1.
Olfactometer dilution ratios.
Solid-Phase Microextraction (SPME) Sampling Procedure
Target odorants for AN prill, C-4, and TNT collected from the odor port of the olfatometer were detected via SPME-GC/MS. Olfactometer odor dilutions were determined by the ratio of line one and line two settings calculated in Equation (1):
where LDilution represents the dilution flow path line (cc min−1), and LOdor represents the odor line (cc min−1). All samples were analyzed with an SPME fiber inserted into the odor port to extract for 1 min. SPME fiber assembly was wrapped in parafilm to facilitate transfer from the odor port to the injected fiber and prevent odorant loss to the surrounding environment (Figure 1). The fiber was retracted, removed, and the blank valve was activated for 1 min to purge the line of residual odor. Extraction replicates (n = 6) were analyzed to obtain average peak area responses for target volatiles and evaluated for concentrations of volatiles by external standard calibration. Replicates per dilution (n = 18) were conducted for a series of 3 days for intra- and interday analyses. All chromatographic separations utilized an Agilent 7890A GC Series GC system, equipped with front and rear split/splitless injection ports and fitted with an HP5 30 m, 0.25 mm i.d., 0.25 mm capillary column. Helium was the carrier gas at a flow rate of 1 mL min−1 at an average velocity of 37 cm s−1. The mass spectrometer (MS) utilized was an Agilent 5975C inert XL MSD with a triple-axis detector operated in electron ionization (EI) full-scan mode from 45 to 550 amu with a 3-min solvent delay. Target VOCs were identified using the National Institute of Standards and Technology (NIST) (2017) mass spectral reference library and verified with external standard chemical calibration. The criteria for identified compounds were samples with detected peak areas greater than a match quality of 80%. Blank background subtraction was performed to exclude compounds as products of the column or sampling process.

Figure 1.
Olfactometer six-channel manifold and sampling. (a) Olfactometer six-channel solenoid valve manifold (inside olfactometer). (b) Solid-phase microextraction (SPME) fiber attached to odor port and secured with parafilm to entrap exiting volatiles from olfactometer.
Data Analysis
All generated data were analyzed using ChemStation software version 10.1.49 (Agilent Technologies, Santa Clara, CA, USA). Target VOC concentrations from intra- and interday sampling were analyzed with Analysis of Variance (ANOVA) and Tukey’s multiple comparison test (normally distributed) or by the Kruskal–Wallis test with Dunn’s multiple comparison test (if not normally) distributed. Standard error was calculated and reported across all dilution levels. Target VOC concentration dispersion for the dataset was analyzed with the coefficient of variation (%CV). The %CV serves as a ratio of the standard deviation to the mean for the dataset. All statistical analyses were analyzed via JMP Pro 16.0.0, SAS Institute Inc., Cary, NC, USA, 2021.
3. Results
Headspace vapor odor profiles were evaluated using optimal fiber chemistry previously identified for each explosive class [35]. For each dilution and explosive sample, a total of six (n = 6) replicates were conducted per sampling day. The results are summarized by each explosive class below.
3.1. Composition C-4
The optimal extraction fiber for this explosive was a polydimethylsiloxane (PDMS) SPME fiber. Interday mean concentrations (n = 6) of DMNB for 80%, 50%, 25%, 12%, and 3% dilutions were 42, 32, 5, 4, and 3 mg L−1,, respectively (Figure 2). The coefficient of variation (%CV) at each dilution percentage was 32%, 4.5%, 25%, 18%, and 10%, respectively. Significant differences for average concentration were reported between days at the 12% dilution. Ambient laboratory conditions were not statistically different among consecutive sampling days. Dilutions (80%, 50%, 25%, and 3%) were not affected by ambient sampling conditions due to the relative temperature and humidity each sampling day.
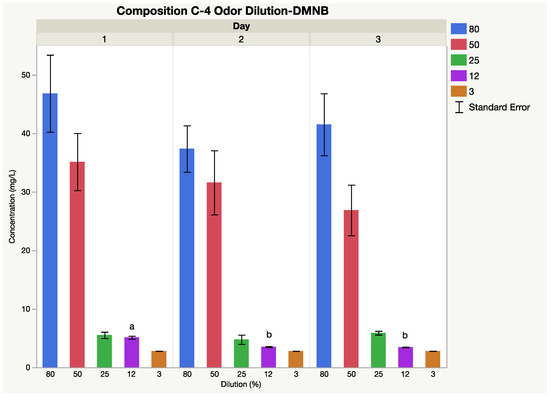
Figure 2.
Instrumental delivery validation results for composition C-4 odor dilution; Interday mean concentrations (n = 6) of DMNB for 80%, 50%, 25%, 12%, and 3% dilutions.
3.2. TNT
The optimal extraction fiber for this explosive was a polydimethylsiloxane/divinylbenzene (PDMS/DVB) SPME fiber. Interday mean concentrations of 2-ethylhexan-1-ol for 80%, 50%, 25%, 12%, and 3% dilutions were 7, 6, 4, 3, and 0 mg L−1, respectively (Figure 3). The %CV at each dilution percentage was 11%, 16%, 2.7%, 38%, and 0%, respectively. Non-matched letters indicate significant differences between average concentrations on separate sampling days. The average concentration differences are attributed to the variance among ambient laboratory conditions between sampling days. Lower dilutions (50%, 25%, 12%, and 3%) were not affected by ambient sampling conditions due to the relative temperature and humidity each sampling day.
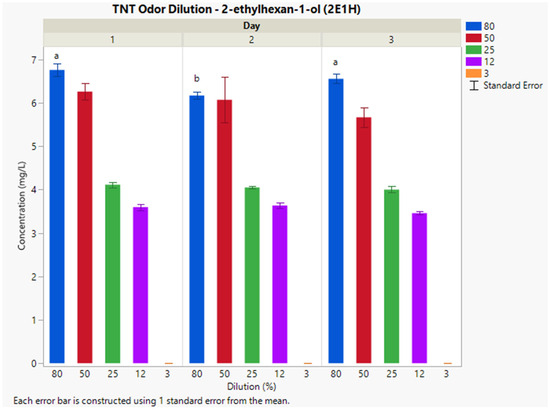
Figure 3.
Instrumental delivery validation results for TNT odor dilution; Interday concentrations (n = 6) of 2E1H for 80%, 50%, 25%, 12%, and 3% dilutions.
3.3. Ammonium Nitrate Prill
The optimal extraction fiber for this explosive was a divinylbenzene/carboxen/polydimethylsiloxane/divinylbenzene (DVB/CAR/PDMS) SPME fiber. Interday concentrations of 2E1H for the 80% and 50%, dilutions were 5 and 4, mg L−1, respectively (Figure 4). 2E1H was not detected across the intraday sampling periods at the 25%, 12%, and 3% dilutions. The %CV at each dilution factor was 19% and 4.7%, respectively. Non-matched letters indicate significant differences between average concentrations on separate sampling days. A letter-matching test was performed for each dilution percentage and indicates significance among each dilution level independently. The average concentration differences are attributed to variance among ambient laboratory conditions (temperature and relative humidity) between sampling days. The variance among temperature and relative humidity is attributed to seasonal fluctuations during sampling days. A loss of target VOC concentration was observed at 25%, 12%, and 3% dilution across all three consecutive sampling days.
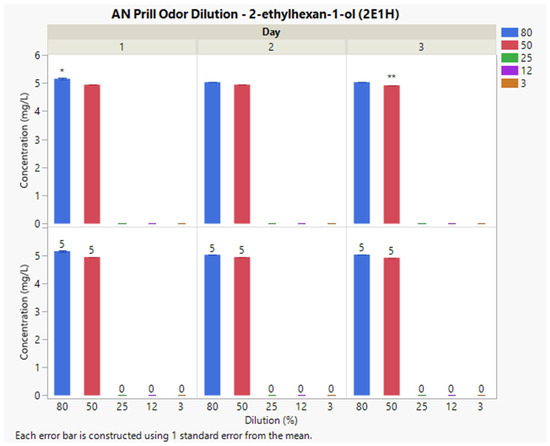
Figure 4.
Instrumental delivery validation results for AN Prill odor dilution; Interday concentrations of 2E1H for 80%, 50%, 25%, 12%, and 3% dilutions (top); * statistically significant difference at 80% dilution; ** statistically significant difference at 50% dilution. Relative concentrations are reported for numerical comparison (bottom).
4. Discussion
The main objective of this study was to instrumentally validate an olfactometer device using a range of explosive classes at different odor dilutions. Given the increased use of olfactometers in odor delivery for canine detection research, it is crucial to monitor and verify odor quality and quantity via an analytical instrumental approach. Optimization of the sampling procedure included the use of parafilm to prevent the loss of target VOC for each dilution. Method optimization concluded the use of parafilm to partially seal the odor port and SPME fiber junction to contain the maximum extraction of VOCs from the odor port of the olfactometer; however, this may have had an impact on the final flow rates, which may have impacted concentration.
Overall, peak area counts decreased as a function of increasing dilution line factors, which is an indicator of the decreased odor availability in higher calculated odor dilutions of bulk material (Figure 5). For the highly concentrated odor (low dilution factors of 80% and 50%), vapor generation is considerably higher with less demand on the vapor source (less airflow, less disturbance on odorant concentration) in comparison to high dilution (25%, 10%, and 3%) that is rate limiting, with the larger concentration of airflow affecting odorant vapor saturation and emission.
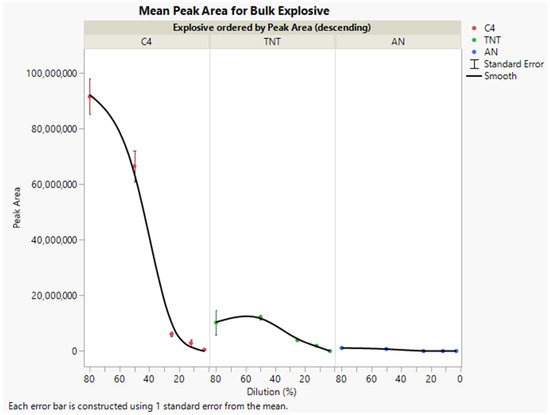
Figure 5.
Mean peak area counts for explosive bulk material by olfactometer dilution (n = 6); mean peak area for bulk explosives at 80%, 50%, 25%, 12%, and 3% dilutions.
4.1. Composition C-4
No significant differences for VOC mean concentrations were observed between intraday sampling of 2,3-dimethyl-2,3-dinitrobutane (DMNB) for all odor dilutions. The DMNB average peak area decreased as the odor dilution factor increased, which was indicative of the odor availability decreasing as a function of dilution factor. This suggests the an olfactometer can be used for the odor delivery of varying concentrations. The stability of DMNB concentrations in C-4 odor dilutions is attributed to its use as an explosive taggant, predominantly in the United States [36]. Confirmation of the presence of DMNB in diluted odor concentrations validates the use for the improved detection of plastic explosives [37]. The reported headspace VOCs for composition C-4 include cyclohexanol, cyclohexanone, 2-ethylhexan-1-ol, 2,3-dimethyl-2,3-dinitrobutane, and pentadecane [38]. The loss of cyclohexanol, cyclohexanone, 2-ethylhexan-1-ol, and pentadecane in headspace analysis correlates to the extraction time of the olfactometer (1 min) and the overall abundance in the headspace of the bulk explosive material. The detection of DMNB instrumentally validates the use of a taggant for high explosives and detection via olfactometry. Our results shed a different perspective on primary odor volatiles within an olfactometer system using C-4 as compared to previous literature which depicted cyclohexanone as the main odor chemical in their olfactometer [32]. This intrinsic variation in identified chemicals within odor delivery systems can be reflective of the limitations of each analytical technique utilized as well as the variation in the device manufacturing.
4.2. TNT
Significant differences were observed for mean concentrations between intraday sampling of 2-ethylhexan-1-ol at 80% odor dilution. Odor dilutions at 50% and 25% did not show significant differences across days. The odor availability of 2-ethylhexan-1-ol was minimally influenced by the ambient relative humidity conditions [39] between intraday sampling: Day 1 (41%), Day 2 (20%), and Day 3 (52%), respectively. 2-ethylhexan-1-ol is a common plasticizer in explosives [40]. The confirmation of 2-ethylhexan-1-ol is an indication of potential explosive detection but would require additional VOCs to positively confirm the detection of a TNT explosive. TNT direct headspace analysis has previously identified VOCs such as 1,3-dinitrobenzene, 2,6-dinitrotoluene, or TNT [41]. The loss of TNT specific volatiles via olfactometry requires further development; however, it likely results from the large difference in sampling times afforded to direct headspace analysis compared to the 1 min airflow sampling time used here. The short dynamic airflow sampling time could have prevented lower vapor pressure compounds from emitting.
The detection of 2-ethylhexan-1-ol in TNT may arise from contamination by plastic containers using plasticizers or from long-term storage along with explosives that off-gas 2-ethyl-1-hexanol. The predominant odorant for TNT is 2,4-DNT, but the sole detection of 2-ethylhexan-1-ol does not negate detection within the bulk material, as this is a limitation to SPME fiber affinity to select odorants. Previous research has identified other major impurities found in TNT bulk material sources; however, 2-ethylhexan-1-ol was not identified based on the containment and storage of samples but has been identified in an undefined source of TNT [40,42]. SPME fiber selection was based on a previous study for bulk material headspace analysis, and the consideration of SPME fiber affinity for this collection method should be evaluated in future studies.
4.3. Ammonium Nitrate Prill
Significant differences were observed for mean concentrations between intraday sampling of 2-ethylhexan-1-ol at 80% and 50% odor dilution. Detection of 2-ethylhexan-1-ol was not found at 25%, 12%, or 3% odor dilution. Ammonium nitrate prill requires an increased surface area for the detection of volatiles [43]. Odor availability is influenced by surface area for the prill form due to its porous nature. To increase detection at 25%, 12%, and 3% odor dilution, optimization of the sample vials in the olfactometer and duration of exposure are to be evaluated. The results of this study corroborate previous literature establishing 2-ethylhexan-1-ol as a volatile constituent for the calcium ammonium nitrate (CAN) variety [44]. While ammonium nitrate odor profiles vary as a function of form, purity, and environment, the ability of the olfactometer to deliver only one of its previously detected odor compounds demonstrates the challenge of odor delivery via airflow systems. The rapid sampling time with the SPME fiber represents a limitation compared to other studies that allow AN headspace sampling for up to 4 h [44]. Hence, there is a need to provide an instrumental snapshot of dynamic odor collection compared to traditional static headspace extraction techniques. This comparison of odor collection methods is key given the need for the canine detection of evolving homemade explosive threats using ammonium nitrate in their manufacture.
The results have several implications for continued dynamic odor analysis research. It is important to note that when utilizing airflow dynamic systems, there is a rapid exchange between odor and air within the device. This parameter results in an odor port purge time. Initial experiments were conducted with a 30 s purge of the odor port. This rapid timeframe could have affected the target VOCs from reaching an equilibrium (odor saturation) within the system (static sampling), therefore the limiting total abundance of VOCs. Further evaluation can target an increase to the purge time with a blank vial purging clean air to compensate for the depletion of VOCs in the headspace. Evaluation of the contributing factors in static (vapor concentration at equilibrium) and dynamic (vapor concentrations as a rate-dependent function) are important to consider as variables that affect experimental outcomes. Dynamic sampling provides continuous monitoring and real-time data on vapor quality variations, making this a suitable mode for studying temporal trends and immediate responses to changing conditions. Static vapor sampling offers spatial representation of snapshot assessments and long-term monitoring at specific sampling points [45].
Dynamic systems allow for observations of transient responses of samples or systems to changes in vapor concentrations. This provides insight to dynamic processes and time-dependent behavior. Dynamic sampling minimizes the risk of sample modifications due to adsorption and/or chemical reactions between volatiles found within the sampled gas or storage conditions [46]. However, a limitation of controlling dynamic vapor concentrations precisely is from environmental factors, such as temperature, humidity, and airflow quality, introducing a source of variability and uncertainty [47]. Static airflow systems are controlled at equilibrium and are advantageous to investigate odorant physical properties under steady-state conditions, and they do not accurately represent real-world environmental variability [48].
The initial data demonstrated a decrease in peak area throughout consecutive sampling. Replicate sampling depleted the headspace, which increases the coefficient of variance among intraday samples. An optimized purge time needs further investigation to allow for the analysis of replicate sampling without any loss of analyte peak area. A potential source of the decrease in odor abundance in high dilution (25%, 10%, and 3%) is attributed to the adsorption of target analytes in the olfactometer tubing. This adsorption is likely more efficiently avoided at lower airflow dilution concentrations compared to high airflow working ranges. Thus, system adsorption of the target analyte via SPME fiber might describe the concentrations encountered.
Flow dilution olfactometers have limitations involving the potential for intertrial and interchannel contamination, which may occur due to the adsorption of odorants onto surfaces located downstream of odorant reservoirs (such as tubing, manifolds, mixing chambers, and final delivery ports) as well as from pressure fluctuations during valve activation that could lead to a backflow of odorant vapor into upstream manifolds [27,49].
Future studies could include fiber chemistry optimization for the dynamic airflow sampling of explosives compared to the direct headspace of the bulk material to confirm appropriate SPME fiber chemistry. It has been previously shown that the collection method (bulk vs. airflow) does have an impact on SPME fiber selection for optimal target odorant collection [33]. As olfactometers improve detection capabilities for biological organisms, further studies are needed to validate the reliability of these instruments using analytical chemistry approaches concurrently with canine behavioral assays. It is important to bear in mind that the analytical chemistry detection of target odor markers for an array of explosives is key to link animal perception with chemical characterization results. Furthermore, the study emphasizes the need to evaluate explosives individually, as each odor volatile represents differences in physical properties which lead to differences in delivery patterns, emission, and ultimate detection. Previous studies in our laboratory have evaluated similar air dilution olfactometer studies for methyl benzoate which did not depict substantial differences in measured concentration at the olfactometer output online across the tested environmental conditions, thus providing different results than the target explosive classes studied here [50]. This variation in odorant behavior utilizing similar odor delivery systems indicates the need for individual odorant evaluation to effectively monitor accurate odor delivery and resulting emission patterns. Alternative air dilutions systems [50] and chromatography instrumentation [51] can be applied in tandem to provide further identification and characterization to odor profiles to give a more detailed evaluation of olfactometry studies.
5. Conclusions
This study evaluates the indirect dynamic headspace sampling of explosives for the validation of olfactometer explosive vapor delivery using key odor markers for presence confirmation and provides foundational knowledge of explosive material odor behavior across a specific dilution range.
These targeted explosive samples included the following: composition C-4 (C-4), trinitrotoluene (TNT), and ammonium nitrate prill. Dynamic airflow sampling yielded the successful detection of target VOCs: 2,3-dimethyl-2,3-dinitrobutane and 2-ethylhexan-1-ol across odor dilutions of 80%, 50%, and 25%. There was a decrease in peak area for all detected VOCs as the odor dilution factor decreased. It is important to note the non-linear behavior of odorant concentration as a function of dilution, thus suggesting dynamic airflow rate-limiting factors encountered during this type of odor sampling. Dynamic airflow sampling with the evaluated olfactometer yielded the successful detection of DMNB taggant in the C-4 energetic material, thus corroborating the odor quality and capability of the device for this odorant. While both TNT and AN prill did not yield previously reported volatile odor markers for these explosive class categories, the results depict dynamic odor delivery challenges across a range of odorants of importance. With the continuous rise of national security threats of explosive devices, it is imperative to understand odor thresholds across a range of energetic materials for optimal biological detection. Hence, the study provides a first step in evaluating an airflow delivery system across distinctive dilutions of an array of explosive classes. It is critical to monitor odor delivery for each target vapor source, as the intrinsic physical properties of the material impact the capability of the device in ultimate odor delivery quality. As can be expected, the results corroborate that the introduction of dynamic airflow reduces the concentration of target VOC concentrations compared to static sampling of the vapor source. The study further establishes the need for analytical validation on individual odorants to monitor effective odor delivery. Given the wide applicability of olfactometer use in olfactory research, the study provides a path forward for the standardization of device validation capabilities to offer a bridge between behavioral assays and instrumental odorant detection.
Author Contributions
Conceptualization, P.A.P.-T. and N.J.H.; methodology, D.E.H., P.A.P.-T. and N.J.H.; software, N.J.H.; validation, P.A.P.-T., D.E.H. and A.C.; formal analysis, D.E.H. and P.A.P.-T.; investigation, D.E.H. and A.C.; resources, P.A.P.-T. and N.J.H.; data curation, P.A.P.-T. and N.J.H.; writing—original draft preparation, D.E.H.; writing—review and editing, P.A.P.-T., N.J.H., D.E.H. and S.A.K., L.S.F.; visualization, P.A.P.-T. and D.E.H.; supervision, P.A.P.-T., N.J.H. and J.E.C.-C.; project administration, P.A.P.-T. and N.J.H.; funding acquisition, P.A.P.-T. and N.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was made possible through funding provided by the DoD Army Research Office under Contract No. W911NF2120124. The views expressed in this manuscript are those of the author and do not necessarily reflect the official policy or position of the Department of Defense, nor the U.S. Government.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jendrny, P.; Twele, F.; Meller, S.; Osterhaus, A.; Schalke, E.; Volk, H.A. Canine olfactory detection and its relevance to medical detection. BMC Infect Dis. 2021, 21, 838. [Google Scholar] [CrossRef] [PubMed]
- Barrios, A.W.; Sánchez-Quinteiro, P.; Salazar, I. Dog and mouse: Toward a balanced view of the mammalian olfactory system. Front. Neuroanat. 2014, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, F.; Albertini, M. Olfactory detection of cancer by trained sniffer dogs: A systematic review of the literature. J. Vet. Behav. 2017, 19, 105–117. [Google Scholar] [CrossRef]
- Kokocinska-Kusiak, A.; Woszczylo, M.; Zybala, M.; Maciocha, J.; Barlowska, K.; Dzieciol, M. Canine Olfaction: Physiology, Behavior, and Possibilities for Practical Applications. Animals 2021, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.; Stafford, K.; Fordham, R. The use of scent-detection dogs. Ir. Vet. J. 2006, 59, 97. [Google Scholar]
- Jezierski, T.; Adamkiewicz, E.; Walczak, M.; Sobczyńska, M.; Gorecka-Bruzda, A.; Ensminger, J.; Papet, E. Efficacy of drug detection by fully-trained police dogs varies by breed, training level, type of drug and search environment. Forensic Sci. Int. 2014, 237, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.J.; Furton, K.G. Biological Detection of Explosives. In Counterterrorist Detection Techniques of Explosives; Elsevier: Amsterdam, The Netherlands, 2007; pp. 395–431. [Google Scholar] [CrossRef]
- Cerreta, M.M.; Furton, K.G. An assessment of detection canine alerts using flowers that release methyl benzoate, the cocaine odorant, and an evaluation of their behavior in terms of the VOCs produced. Forensic. Sci. Int. 2015, 251, 107–114. [Google Scholar] [CrossRef]
- Diaz, A.; Fabrellas, C.; Ventura, F. Determination of Odor Threshold Concentrations of Chlorobrominated Anisoles in Water. J. Agric. Food Chem. 2005, 53, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of Additional Volatile Components of Tomato. J. Agric. Food Chem. 1971, 19, 524–529. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of Volatiles to Rice Aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Flath, R.A.; Mon, T.R.; Teranishi, R.; Guentert, M. Volatile Constituents of Apricot (Prunus armeniaca). J. Agric. Food Chem. 1990, 38, 471–477. [Google Scholar] [CrossRef]
- Ruth, J.H. Odor Thresholds and Irritation Levels of Several Chemical Substances: A Review. Am. Ind. Hyg. Assoc. J. 1986, 47, A-142–A-151. [Google Scholar] [CrossRef] [PubMed]
- Krantz, D.H. Threshold theories of signal detection. Psychol. Rev. 1969, 76, 308. [Google Scholar] [CrossRef] [PubMed]
- DeChant, M.T.; Hall, N.J. Training with varying odor concentrations: Implications for odor detection thresholds in canines. Anim. Cogn. 2021, 24, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Krestel, D.; Passe, D.; Smith, J.; Jonsson, L. Behavioral determination of olfactory thresholds to amyl acetate in dogs. Neurosci. Biobehav. Rev. 1984, 8, 169–174. [Google Scholar] [CrossRef]
- Walker, D.B.; Walker, J.C.; Cavnar, P.J.; Taylor, J.L.; Pickel, D.H.; Hall, S.B.; Suarez, J.C. Naturalistic quantification of canine olfactory sensitivity. Appl. Anim. Behav. Sci. 2006, 97, 241–254. [Google Scholar] [CrossRef]
- Sironi, S.; Capelli, L.; Céntola, P.; Del Rosso, R.; Pierucci, S. Odour impact assessment by means of dynamic olfactometry, dispersion modelling and social participation. Atmos. Environ. 2010, 44, 354–360. [Google Scholar] [CrossRef]
- Brooks, S.W.; Moore, D.R.; Marzouk, E.B.; Glenn, F.R.; Hallock, R.M. Canine olfaction and electronic nose detection of volatile organic compounds in the detection of cancer: A review. Cancer Investig. 2015, 33, 411–419. [Google Scholar] [CrossRef]
- DeGreeff, L.E.; Furton, K.G. Collection and identification of human remains volatiles by non-contact, dynamic airflow sampling and SPME-GC/MS using various sorbent materials. Anal. Bioanal. Chem. 2011, 401, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Giungato, P.; Di Gilio, A.; Palmisani, J.; Marzocca, A.; Mazzone, A.; Brattoli, M.; Giua, R.; de Gennaro, G. Synergistic approaches for odor active compounds monitoring and identification: State of the art, integration, limits and potentialities of analytical and sensorial techniques. TrAC Trends Anal. Chem. 2018, 107, 116–129. [Google Scholar] [CrossRef]
- Concha, A.R.; Guest, C.M.; Harris, R.; Pike, T.W.; Feugier, A.; Zulch, H.; Mills, D.S. Canine Olfactory Thresholds to Amyl Acetate in a Biomedical Detection Scenario. Front. Vet. Sci. 2018, 5, 345. [Google Scholar] [CrossRef] [PubMed]
- Abel, R.J.; Lunder, J.L.; Harynuk, J.J. A novel protocol for producing low-abundance targets to characterize the sensitivity limits of ignitable liquid detection canines. Forensic. Chem. 2020, 18, 100230. [Google Scholar] [CrossRef]
- DeGreeff, L.; Katilie, C.J.; Johnson, R.F.; Vaughan, S. Quantitative vapor delivery for improved canine threshold testing. Anal. Bioanal. Chem. 2021, 413, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Myers, L.J.; Waggoner, L.P.; Williams, M. Determination of canine olfactory thesholds using operant laboratory methods. In Proceedings of the Substance Detection Systems, 28 March 1994; SPIE: Bellingham, WA, USA, 1994; Volume 2092, pp. 238–243. [Google Scholar]
- Hall, N.J.; Collada, A.; Smith, D.W.; Wynne, C.D.L. Performance of domestic dogs on an olfactory discrimination of a homologous series of alcohols. Appl. Anim. Behav. Sci. 2016, 178, 1–6. [Google Scholar] [CrossRef]
- Burton, S.D.; Wipfel, M.; Guo, M.; Eiting, T.P.; Wachowiak, M. A Novel Olfactometer for Efficient and Flexible Odorant Delivery. Chem. Senses 2019, 44, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Tichy, H.; Zeiner, R.; Traunmuller, P.; Martzok, A.; Hellwig, M. Developing and testing of an air dilution flow olfactometer with known rates of concentration change. J. Neurosci. Methods 2020, 341, 108794. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.J.; Wynne, C.D.L. Odor mixture training enhances dogs’ olfactory detection of Home-Made Explosive precursors. Heliyon 2018, 4, e00947. [Google Scholar] [CrossRef] [PubMed]
- Gotow, N.; Hoshi, A.; Kobayakawa, T. Expanded olfactometer for measuring reaction time to a target odor during background odor presentation. Heliyon 2019, 5, e01254. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.A.; Fernandez, L.S.; Huff, D.E.; Prada-Tiedemann, P.A.; Hall, N.J. Canine detection of explosives under adverse environmental conditions with and without acclimation training. PLoS ONE 2024, 19, e0297538. [Google Scholar] [CrossRef] [PubMed]
- Hallowell, S.F.; Davies, J.P.; Gresham, G.L. Qualitative/semiquantitative chemical characterization of the Auburn olfactometer. In Proceedings of Cargo Inspection Technologies, 6 October 1994; SPIE: Bellingham, WA, USA, 1994; Volume 2276, pp. 437–448. [Google Scholar]
- Gallegos, S.F.; Aviles-Rosa, E.O.; Hall, N.J.; Prada-Tiedemann, P.A. Headspace sampling of smokeless powder odor in a dynamic airflow context. Forensic Chem. 2022, 27, 100402. [Google Scholar] [CrossRef]
- Aviles-Rosa, E.O.; Gallegos, S.F.; Prada-Tiedemann, P.A.; Hall, N.J. An Automated Canine Line-Up for Detection Dog Research. Front. Vet. Sci. 2021, 8, 775381. [Google Scholar] [CrossRef] [PubMed]
- Huff, D.E. Characterization of Odor Behavior and Canine Detection Limits in Extreme Environmental Conditions. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 2022. [Google Scholar]
- Lai, H.; Leung, A.; Magee, M.; Almirall, J.R. Identification of volatile chemical signatures from plastic explosives by SPME-GC/MS and detection by ion mobility spectrometry. Anal. Bioanal. Chem. 2010, 396, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Kranz, W.; Kitts, K.; Strange, N.; Cummins, J.; Lotspeich, E.; Goodpaster, J. On the smell of Composition C-4. Forensic Sci. Int. 2014, 236, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.L.; Huertas, A.M.; Prada, P.A.; Furton, K.G. A non-contact passive approach for the effective collection of target explosive volatiles for canine training aid development. J. Forensic Sci. Criminol. 2016, 4, 205–212. [Google Scholar]
- Simon, A.G.; DeGreeff, L.E.; Frank, K.; Peranich, K.; Holness, H.; Furton, K.G. A Method for Controlled Odor Delivery in Olfactory Field-Testing. Chem. Senses 2019, 44, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.J.; Almirall, J.R.; Furton, K.G. Identification of dominant odor chemicals emanating from explosives for use in developing optimal training aid combinations and mimics for canine detection. Talanta 2005, 67, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Psillakis, E.; Naxakis, G.; Kalogerakis, N. Detection of TNT-contamination in spiked-soil samples using SPME and GC/MS. Glob. NEST J. 2000, 2, 227–236. [Google Scholar]
- George, V.; Jenkins, T.F.; Phelan, J.M.; Leggett, D.C.; Oxley, J.C.; Webb, S.W.; Miyares, P.H.; Cragin, J.H.; Smith, J.L.; Berry, T.E. Progress on determining the vapor signature of a buried landmine. In Detection and Remediation Technologies for Mines and Minelike Targets V; SPIE: Bellingham, WA, USA, 2000; Volume 4038, pp. 590–601. [Google Scholar]
- Katilie, C.J.; Simon, A.G.; DeGreeff, L.E. Quantitative analysis of vaporous ammonia by online derivatization with gas chromatography-mass spectrometry with applications to ammonium nitrate-based explosives. Talanta 2019, 193, 87–92. [Google Scholar] [CrossRef] [PubMed]
- DeGreeff, L.E.; Peranich, K. Headspace analysis of ammonium nitrate variants and the effects of differing vapor profiles on canine detection. Forensic Chem. 2021, 25, 100342. [Google Scholar] [CrossRef]
- Smeets, M.A.; Bulsing, P.J.; van Rooden, S.; Steinmann, R.; de Ru, J.A.; Ogink, N.W.; van Thriel, C.; Dalton, P.H. Odor and irritation thresholds for ammonia: A comparison between static and dynamic olfactometry. Chem. Senses 2007, 32, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Sironi, S.; Del Rosso, R. Odor sampling: Techniques and strategies for the estimation of odor emission rates from different source types. Sensors 2013, 13, 938–955. [Google Scholar] [CrossRef] [PubMed]
- Brattoli, M.; de Gennaro, G.; de Pinto, V.; Loiotile, A.D.; Lovascio, S.; Penza, M. Odour detection methods: Olfactometry and chemical sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef] [PubMed]
- MacCrehan, W.; Moore, S.; Schantz, M. Evaluating headspace component vapor-time profiles by solid-phase microextraction with external sampling of an internal standard. Anal. Chem. 2011, 83, 8560–8565. [Google Scholar] [CrossRef] [PubMed]
- DeGreeff, L.E.; Peranich, K. Canine olfactory detection of trained explosive and narcotic odors in mixtures using a mixed odor delivery device. Forensic Sci. Int. 2021, 329, 111059. [Google Scholar] [CrossRef] [PubMed]
- Brustkern, M.; Thompson, R.; Lawhon, S.; Good, K.; Bunker, P.; Prada-Tiedemann, P.; Hall, N.J. Effect of rapid changes in environmental conditions on canine detection of methyl benzoate. Appl. Anim. Behav. Sci. 2023, 264, 105924. [Google Scholar] [CrossRef]
- Villière, A.; Arvisenet, G.; Lethuaut, L.; Prost, C.; Sérot, T. Selection of a representative extraction method for the analysis of odourant volatile composition of French cider by GC–MS–O and GC× GC–TOF-MS. Food Chem. 2012, 131, 1561–1568. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).