Multimodal Imaging of Metals in a Retinal Degeneration Model to Inform on Ocular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tissue Collection
2.3. Masson’s Trichrome Staining
2.4. Preparation of Calibration Arrays
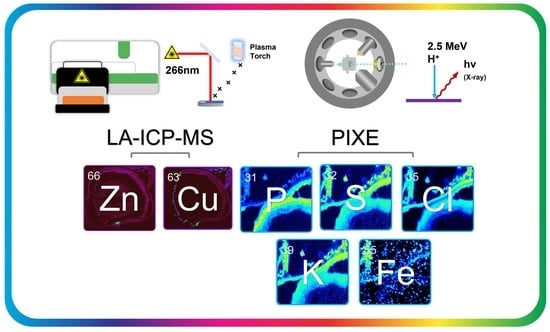
2.5. LA-ICP-MS
2.6. PIXE
3. Results
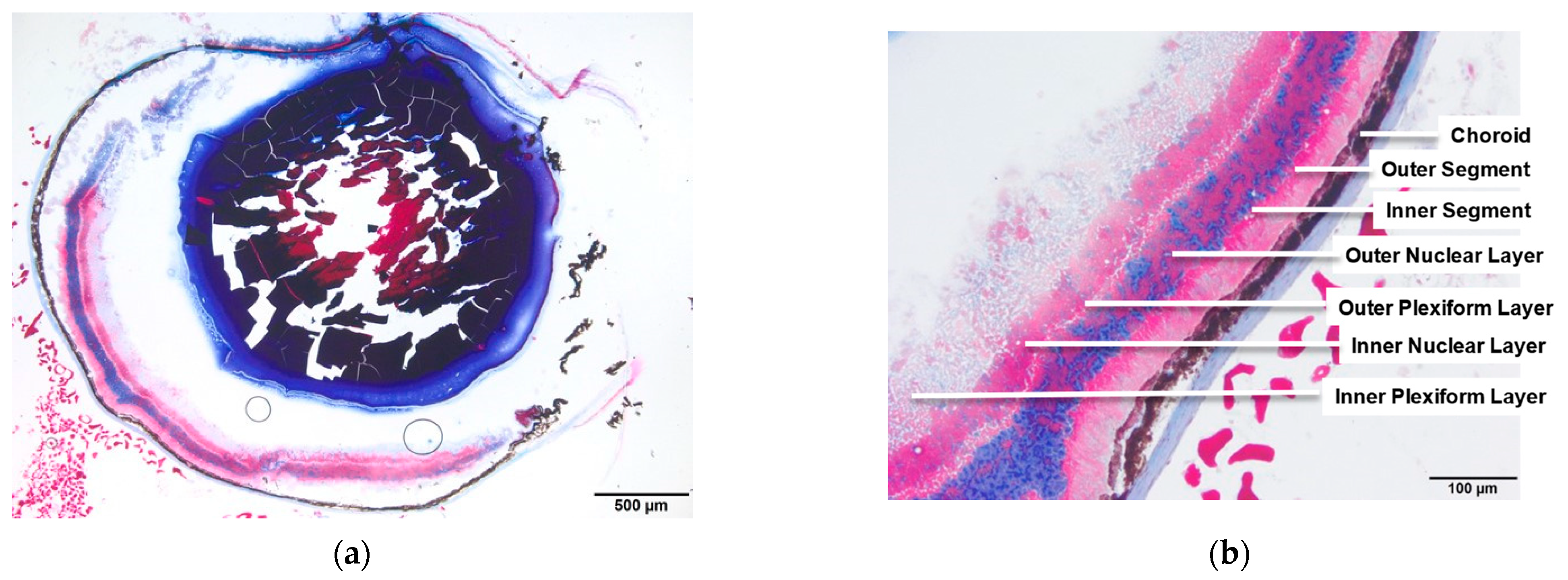
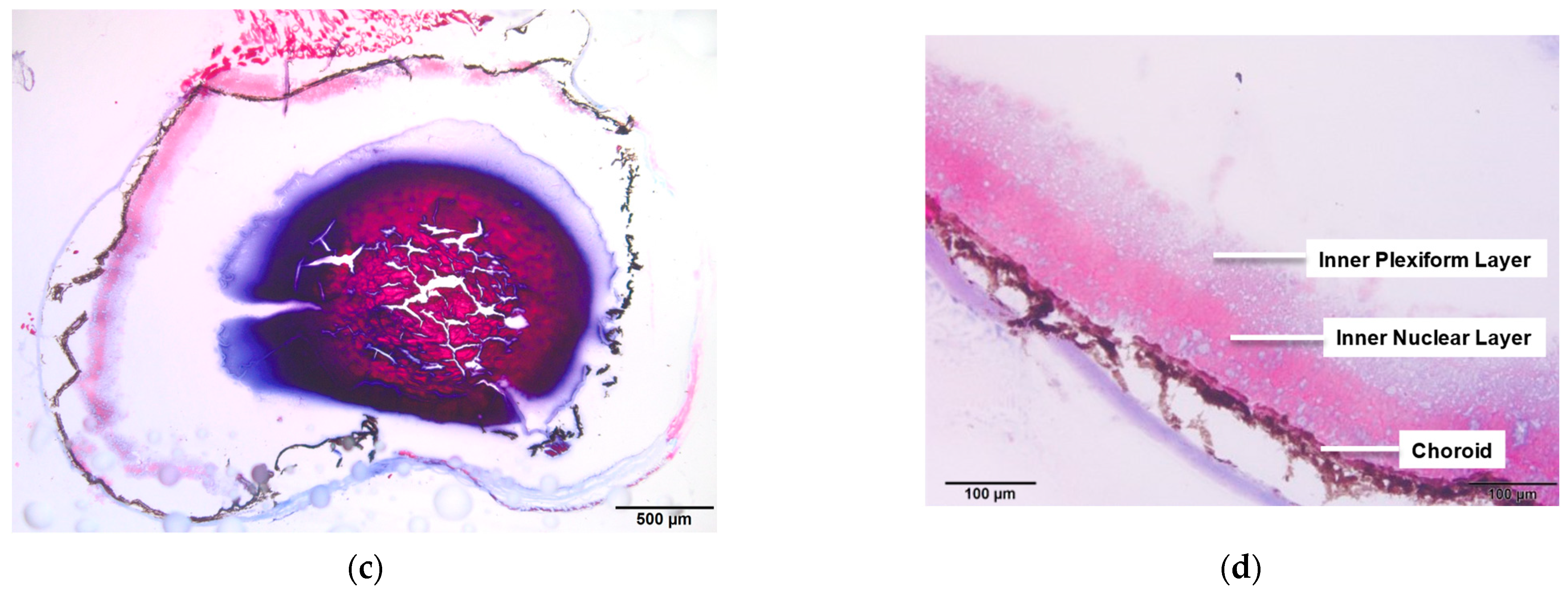
3.1. WT and Rho−/− Retinal Anatomy
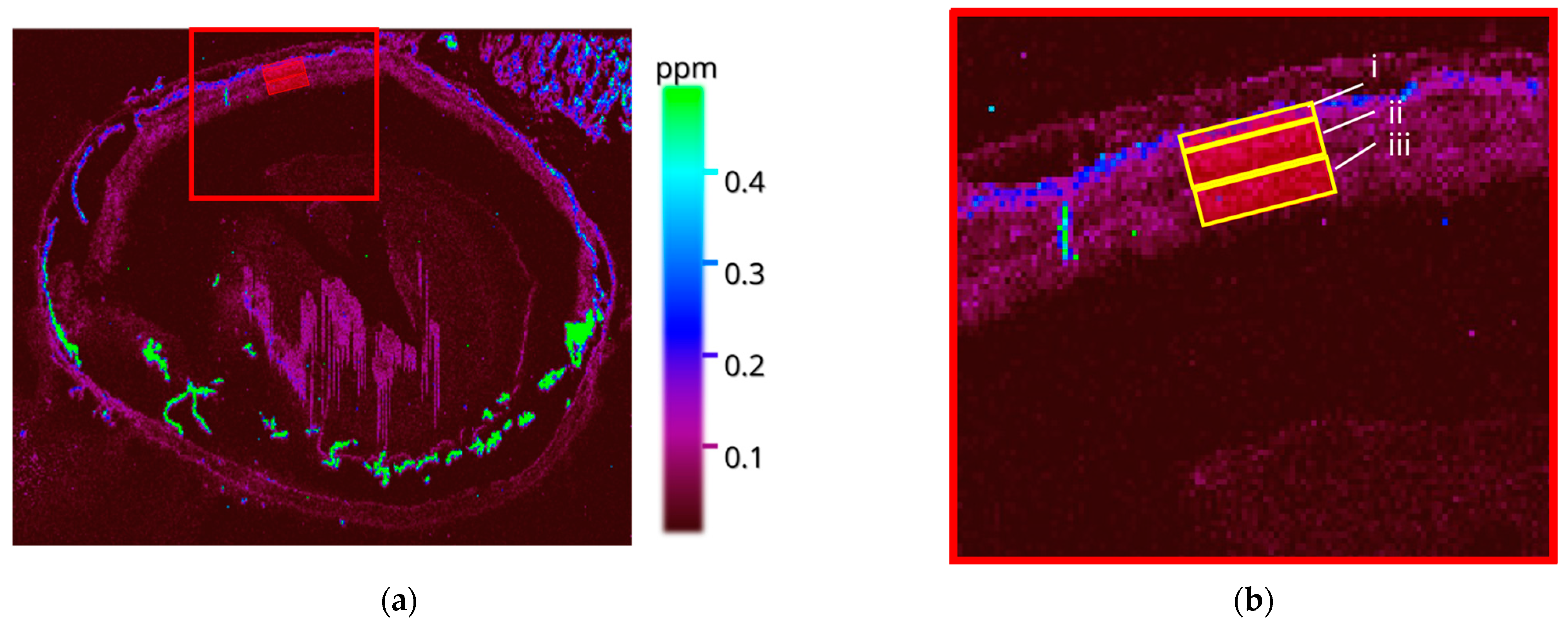
3.2. LA-ICP-MSI
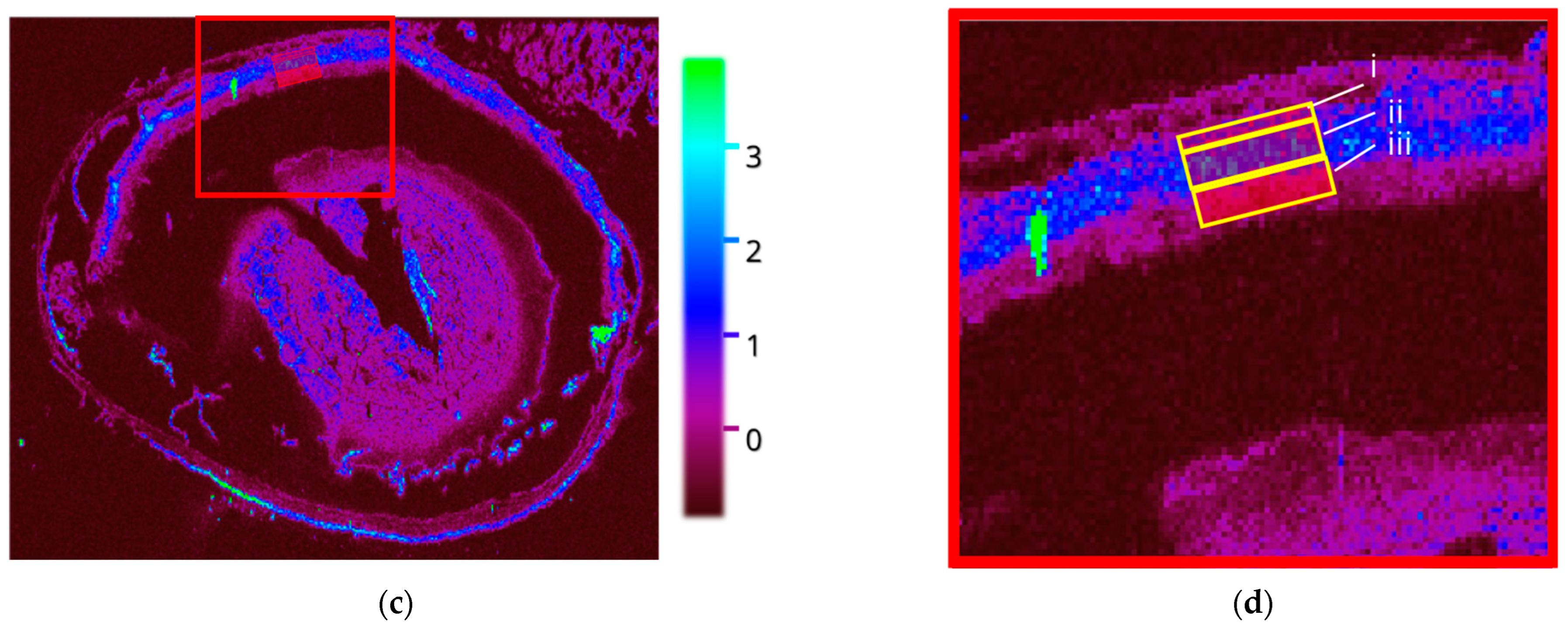
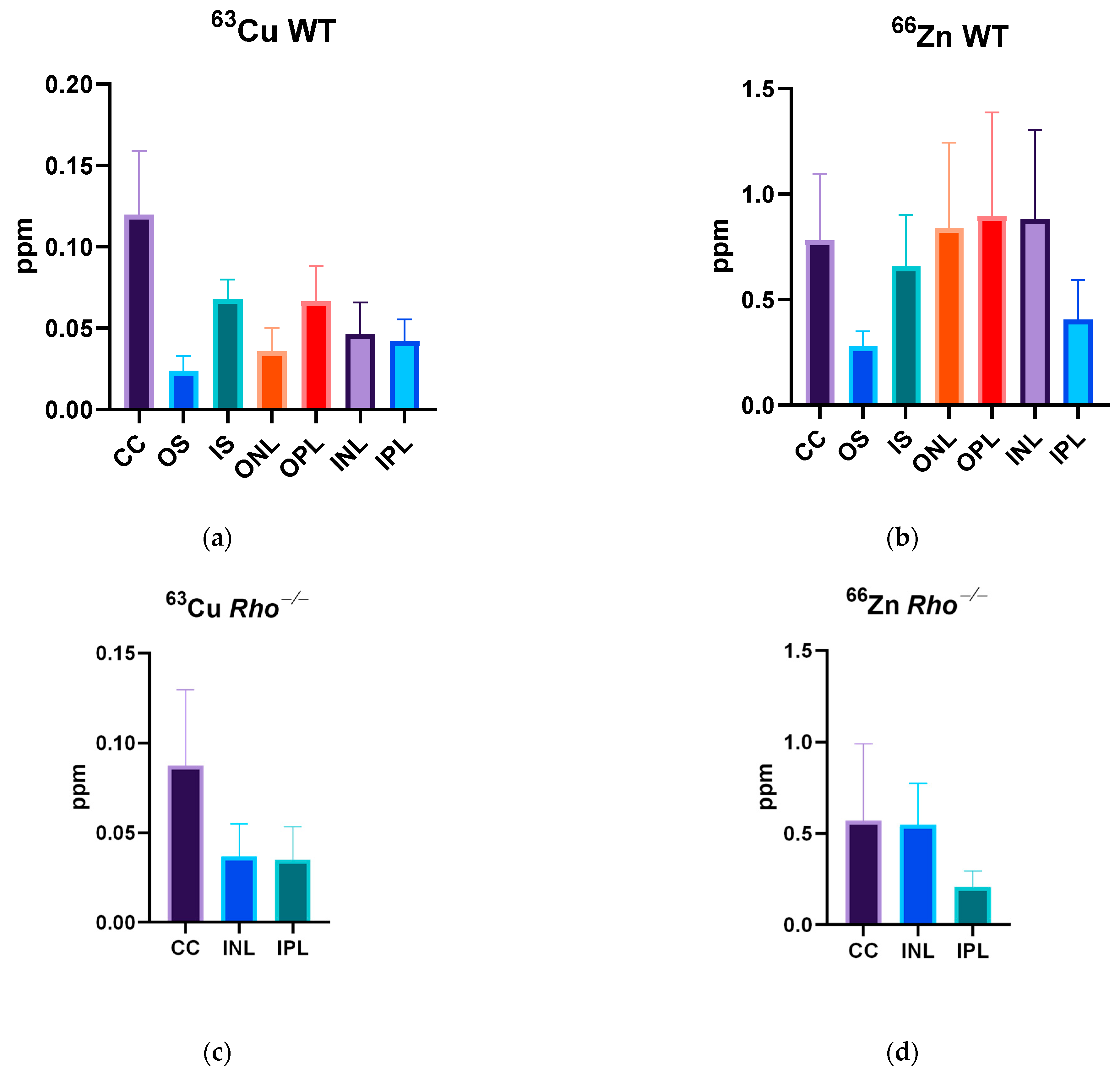
3.2.1. Wildtype Cu
3.2.2. Wildtype 66Zn
3.2.3. Rho−/− Cu
3.3. PIXE
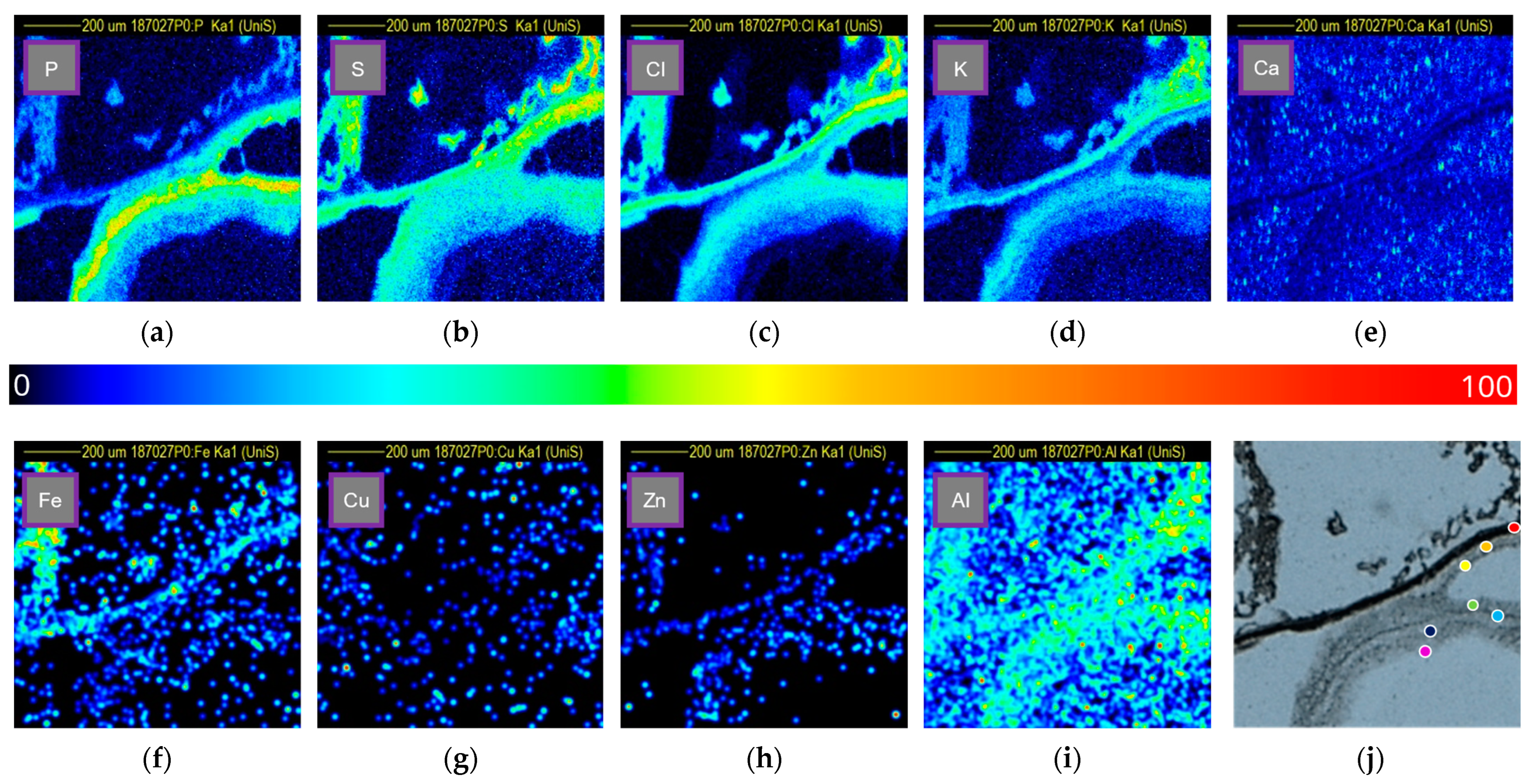
3.3.1. Phosphorus
3.3.2. Sulfur
3.3.3. Chlorine
3.3.4. Potassium
3.3.5. Calcium
3.3.6. Iron
3.3.7. Copper
3.3.8. Zinc
3.3.9. Aluminium
3.4. Quantitative PIXE
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-Related Macular Degeneration |
| amu | Atomic mass unit |
| AREDS | Age Related Eye Disease Study |
| BrM | Brusch’s Membrane |
| CC | Chorio Capillaris |
| CLIC4 | Chliride Intracellular Channel 4 |
| CMC | Carboxymethylcellulose |
| DAMPS | Damage-Associated Molecular Patterns |
| ESL | Elemental Scientific Lasers |
| IBA | Ion Beam Analysis |
| ICP | Inductively Coupled Plasma |
| INL | Inner Nuclear Layer |
| IPL | Inner Plexiform Layer |
| IS | Inner Segment |
| LA | Laser Ablation |
| MS | Mass Spectrometry |
| ONL | Outer Nuclear Layer |
| OPL | Outer Plexiform Layer |
| OS | Outer Segment |
| PET | Polyethylene Terephthalate |
| PIPS | Passivated Implanted Planar Silicon |
| PIXE | Particle-Induced X-Ray Emission |
| PRR | Pattern Recognition Receptors |
| RHO | Rhodopsin |
| ROI | Region Of Interest |
| ROS | Reactive Oxygen Species |
| RP | Retinis Pigmentosa |
| RPE | Retinal Pigment Epithelium |
| SDD | Silicon Drifted Detector |
| WT | Wildtype |
References
- Bourne, S.R.; Flaxman, H.R.; Taylor, R.J.; Casson, A.; Abdoli, E.; Abu-Gharbieh, A.A.; Alamneh, W.; Alemayehu, V.; Alipour, E.W.; Anbesu, J.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, Z. Recent Developments in the Treatment of Wet Age-related Macular Degeneration. Curr. Med. Sci. 2020, 40, 851–857. [Google Scholar] [CrossRef]
- Celkova, L.; Doyle, S.L.; Campbell, M. NLRP3 Inflammasome and Pathobiology in AMD. J. Clin. Med. 2015, 4, 172–192. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study Research Group. The effect of five-year zinc supplementation on serum zinc, serum cholesterol and hematocrit in persons randomly assigned to treatment group in the age-related eye disease study: AREDS Report No. 7. J. Nutr. 2002, 132, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The Sterile Inflammatory Response. Annu. Rev. Immunol. 2009, 28, 321–342. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Pålsgård, E.; Ugarte, M.; Rajta, I.; Grime, G. The role of zinc in the dark-adapted retina studied directly using microPIXE. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 2001, 181, 489–492. [Google Scholar] [CrossRef]
- Randomized, A. Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Newsome, D.A.; Miceli, M.V.; Tate, D.J.; Alcock, N.W.; Oliver, P.D. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration, but superoxide dismutase activity increases. J. Trace Elem. Exp. Med. 1996, 8, 193–199. [Google Scholar] [CrossRef]
- Wong, C.P.; Rinaldi, N.A.; Ho, E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol. Nutr. Food Res. 2015, 59, 991–999. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N. Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics 2014, 6, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, A.G.; Stopa, P.; Michalke, B.; Chaudhri, A.; Reulbach, U.; Huchzermeyer, C.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Zrenner, E.; Rejdak, R. Levels of Aqueous Humor Trace Elements in Patients with Non-Exsudative Age-related Macular Degeneration: A Case-control Study. PLoS ONE 2013, 8, e56734. [Google Scholar] [CrossRef]
- May, T.W.; Wiedmeyer, R.H. A table of polyatomic interferences in ICP-MS. At. Spectrosc. 1998, 19, 150–155. [Google Scholar]
- Chuang, J.Z.; Yang, N.; Nakajima, N.; Otsu, W.; Fu, C.; Yang, H.H.; Lee, M.P.; Akbar, A.F.; Badea, T.C.; Guo, Z.; et al. Retinal pigment epithelium-specific CLIC4 mutant is a mouse model of dry age-related macular degeneration. Nat. Commun. 2022, 13, 374. [Google Scholar] [CrossRef]
- Trueman, C. Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 171–181. [Google Scholar]
- Limbeck, A.; Galler, P.; Bonta, M.; Bauer, G.; Nischkauer, W.; Vanhaecke, F. Recent advances in quantitative LA-ICP-MS analysis: Challenges and solutions in the life sciences and environmental chemistry. Anal. Bioanal. Chem. 2015, 407, 6593–6617. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, zinc, and copper in retinal physiology and disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef] [PubMed]
- Aberami, S.; Nikhalashree, S.; Bharathselvi, M.; Biswas, J.; Sulochana, K.N.; Coral, K. Elemental concentrations in Choroid-RPE and retina of human eyes with age-related macular degeneration. Exp. Eye Res. 2019, 186, 107718. [Google Scholar] [CrossRef]
- Millar, J.; Ozaki, E.; Campbell, S.; Duckett, C.; Doyle, S.; Cole, L.M. Multiomic Mass Spectrometry Imaging to Advance Future Pathological Understanding of Ocular Disease. Metabolites 2022, 12, 1239. [Google Scholar] [CrossRef]
- Greenhalgh, C.J.; Karekla, E.; Miles, G.J.; Powley, I.R.; Costa, C.; De Jesus, J.; Bailey, M.J.; Pritchard, C.; MacFarlane, M.; Pringle, J.H.; et al. Exploration of Matrix Effects in Laser Ablation Inductively Coupled Plasma Mass Spectrometry Imaging of Cisplatin-Treated Tumors. Anal. Chem. 2020, 92, 9847–9855. [Google Scholar] [CrossRef]
- Jeynes, C.; Bailey, M.J.; Bright, N.J.; Christopher, M.E.; Grime, G.W.; Jones, B.N.; Palitsin, V.V.; Webb, R.P. “Total IBA”–Where are we? Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 271, 107–118. [Google Scholar] [CrossRef]
- Jaissle, G.B.; May, C.A.; Reinhard, J.; Kohler, K.; Fauser, S.; Lutjen–Drecoll, E.; Zrenner, E.; Seeliger, M.W. Evaluation of the Rhodopsin Knockout Mouse as a Model of Pure Cone Function. Investig. Ophthalmol. Vis. Sci. 2001, 42, 506–513. [Google Scholar]
- Wills, N.; Ramanujam, V.S.; Kalariya, N.; Lewis, J.; van Kuijk, F. Copper and zinc distribution in the human retina: Relationship to cadmium accumulation, age, and gender. Exp. Eye Res. 2008, 87, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Petrus, J.A.; Chew, D.M.; Leybourne, M.I.; Kamber, B.S. A new approach to laser-ablation inductively-coupled-plasma mass-spectrometry (LA-ICP-MS) using the flexible map interrogation tool ‘Monocle’. Chem. Geol. 2017, 463, 76–93. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508. [Google Scholar] [CrossRef]
- Woodhead, J.D.; Hellstrom, J.; Hergt, J.M.; Greig, A.; Maas, R. Isotopic and Elemental Imaging of Geological Materials by Laser Ablation Inductively Coupled Plasma-Mass Spectrometry. Geostand. Geoanalytical Res. 2007, 31, 331–343. [Google Scholar] [CrossRef]
- Erie, J.C.; Good, J.A.; Butz, J.A.; Pulido, J.S. Reduced Zinc and Copper in the Retinal Pigment Epithelium and Choroid in Age-related Macular Degeneration. Am. J. Ophthalmol. 2009, 147, 276–282.e1. [Google Scholar] [CrossRef]
- Boyer, N.P.; Higbee, D.; Currin, M.B.; Blakeley, L.R.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: Their origin is 11-cis-retinal. J. Biol. Chem. 2012, 287, 22276–22286. [Google Scholar] [CrossRef] [PubMed]
- Bito, L.Z.; DiBenedetto, F.E.; Stetz, D. Homeostasis of the retinal micro-environment: I. Magnesium, potassium and calcium distributions in the avian eye. Exp. Eye Res. 1982, 34, 229–237. [Google Scholar] [CrossRef]
- Mannu, G.S. Retinal phototransduction. Neurosciences 2014, 19, 275–280. [Google Scholar] [PubMed]
- Nakatani, K.; Chen, C.; Yau, K.; Koutalos, Y. Advances in Experimental Medicine and Biology; Springer USA: Boston, MA, USA, 2002; pp. 1–20. [Google Scholar]
- Tisdale, A.K.; Agrón, E.; Sunshine, S.B.; Clemons, T.E.; Ferris, F.L.; Chew, E.Y. Association of Dietary and Supplementary Calcium Intake With Age-Related Macular Degeneration: Age-Related Eye Disease Study Report 39. Arch. Ophthalmol. (1960) 2019, 137, 543–550. [Google Scholar] [CrossRef]
- Flinn, J.M.; Kakalec, P.; Tappero, R.; Jones, B.; Lengyel, I. Correlations in distribution and concentration of calcium, copper and iron with zinc in isolated extracellular deposits associated with age-related macular degeneration. Metallomics 2014, 6, 1223–1228. [Google Scholar] [CrossRef]
- Wong, R.W.; Dchimene, R.; Hahn, P.; Green, W.R.; Dunaief, J.L. Iron Toxicity as a potential factor in AMD. Retina 2007, 27, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. JAMA 2003, 290, 2525. [Google Scholar] [CrossRef] [PubMed]
- Christopher, M.E.; Warmenhoeven, J.W.; Romolo, F.S.; Donghi, M.; Webb, R.P.; Jeynes, C.; Ward, N.I.; Kirkby, K.J.; Bailey, M.J. A new quantitative method for gunshot residue analysis by ion beam analysis. Analyst 2013, 138, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.E.I.; da Silva, L.R.M.; Boufleur, L.A.; Debastiani, R.; Stefenon, C.A.; Amaral, L.; Yoneama, M.L.; Dias, J.F. Elemental characterisation of Cabernet Sauvignon wines using Particle-Induced X-ray Emission (PIXE). Food Chem. 2010, 121, 244–250. [Google Scholar] [CrossRef]
- Ash, J.D.; Grimm, C.; Hollyfield, J.G.; Anderson, R.E.; LaVail, M.M.; Bowes, C.R. Retinal Degenerative Diseases; Springer: New York, NY, USA, 2014. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millar, J.; Gibbons, L.; Costa, C.; Schneider, E.; von Gerichten, J.; Bailey, M.J.; Campbell, S.; Duckett, C.; Doyle, S.; Cole, L.M. Multimodal Imaging of Metals in a Retinal Degeneration Model to Inform on Ocular Disease. Analytica 2023, 4, 264-279. https://doi.org/10.3390/analytica4030021
Millar J, Gibbons L, Costa C, Schneider E, von Gerichten J, Bailey MJ, Campbell S, Duckett C, Doyle S, Cole LM. Multimodal Imaging of Metals in a Retinal Degeneration Model to Inform on Ocular Disease. Analytica. 2023; 4(3):264-279. https://doi.org/10.3390/analytica4030021
Chicago/Turabian StyleMillar, Joshua, Luke Gibbons, Catia Costa, Ella Schneider, Johanna von Gerichten, Melanie J. Bailey, Susan Campbell, Catherine Duckett, Sarah Doyle, and Laura M. Cole. 2023. "Multimodal Imaging of Metals in a Retinal Degeneration Model to Inform on Ocular Disease" Analytica 4, no. 3: 264-279. https://doi.org/10.3390/analytica4030021
APA StyleMillar, J., Gibbons, L., Costa, C., Schneider, E., von Gerichten, J., Bailey, M. J., Campbell, S., Duckett, C., Doyle, S., & Cole, L. M. (2023). Multimodal Imaging of Metals in a Retinal Degeneration Model to Inform on Ocular Disease. Analytica, 4(3), 264-279. https://doi.org/10.3390/analytica4030021