Statistical Optimization of Urinary Organic Acids Analysis by a Multi-Factorial Design of Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Method
- Sample creatinine was determined according to Jaffè method;
- Urine samples were diluted with saline solution to obtain a concentration of 2 mM of creatinine;
- A total of 1 mL of the diluted samples was added with 100 μL of internal standard (1 M tropic acid), 20 μL of a solution of 35% w/v sodium hydroxide and 100 μL of 1 M hydroxylamine. Samples were incubated at 60 °C for 30 min;
- A total of 50 μL of 30% w/w hydrochloric acid and approx. 0.5 g of sodium chloride were added. Extraction was performed three times with 2 mL of ethyl acetate. The samples were vigorously vortexed for 1 min or shaken at 350 rpm on an automatic shaker for 10 min;
- The combined extracts were dried at 40 °C in a water bath, under a gentle nitrogen flux;
- A total of 50 μL of BSTFA with 1% of TMCS were added to dried extracts and they are heated at 50 °C for 40 min;
- Samples were diluted with 500 μL of n–heptane.
2.3. Urine Specimens
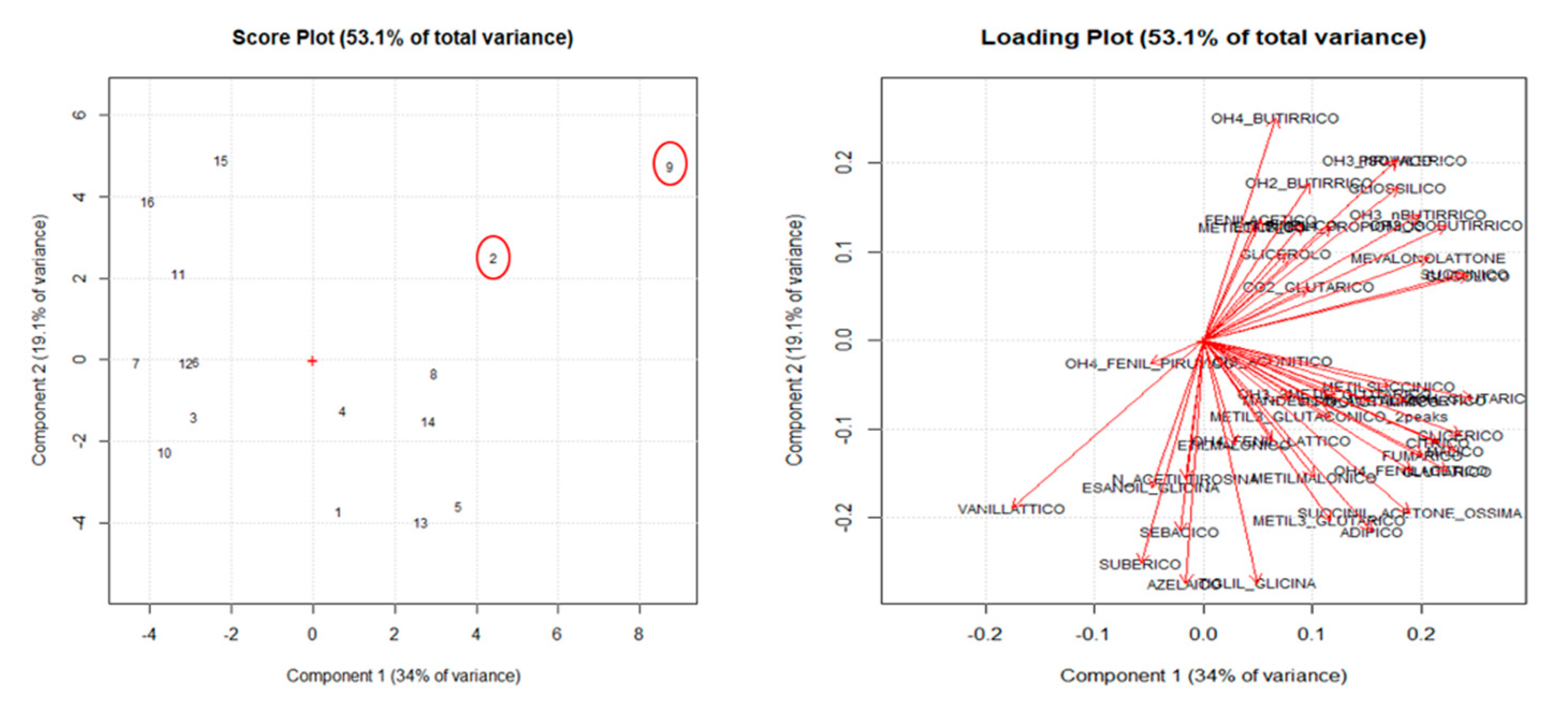
3. Chemometrics
4. Design of Experiment (DoE)
Software
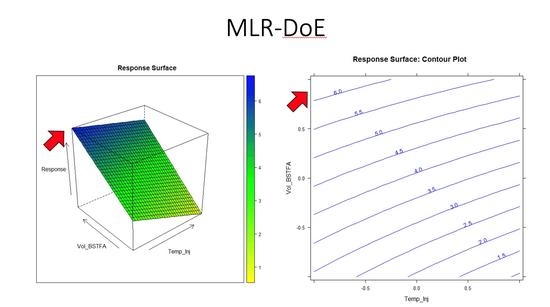
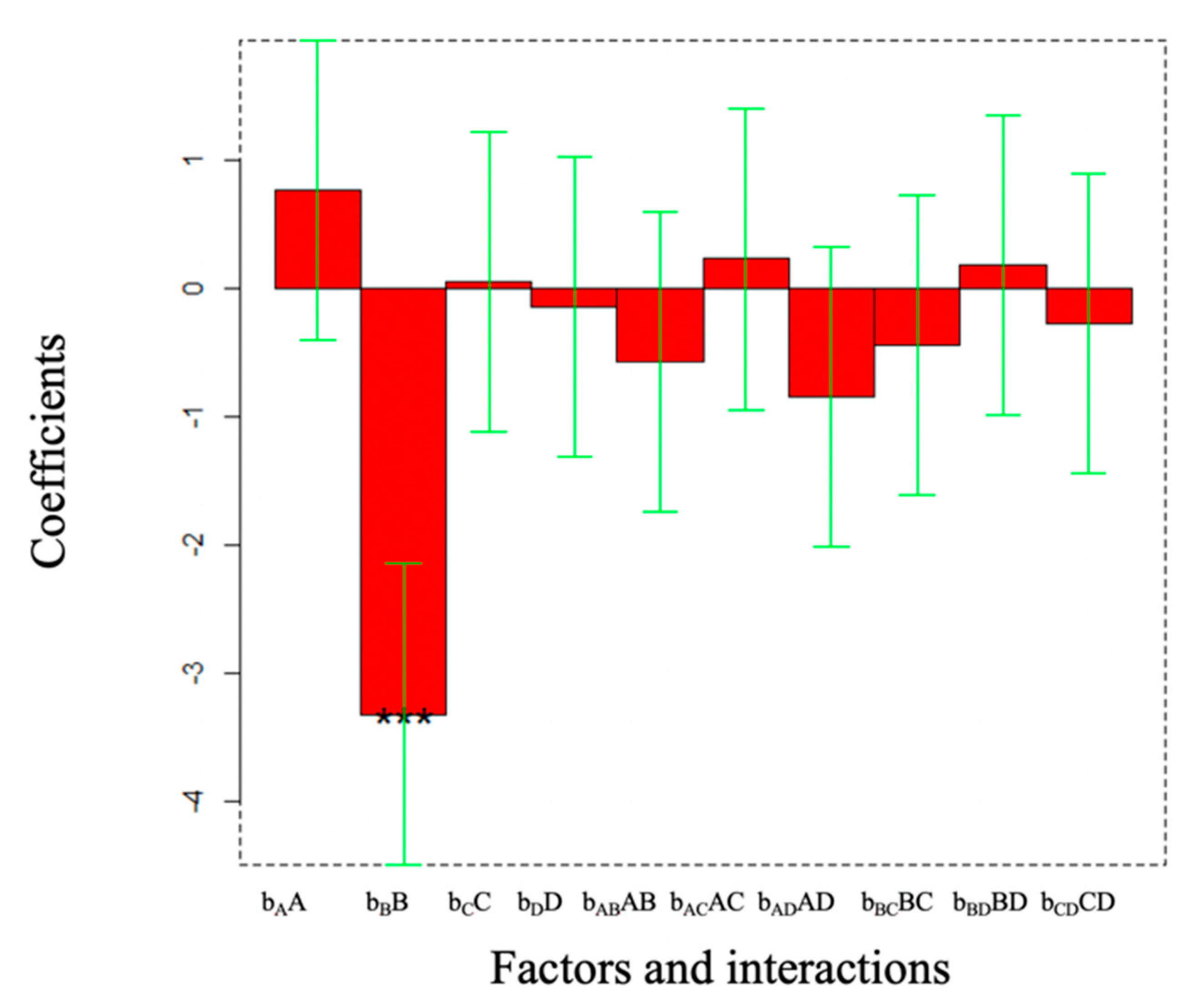
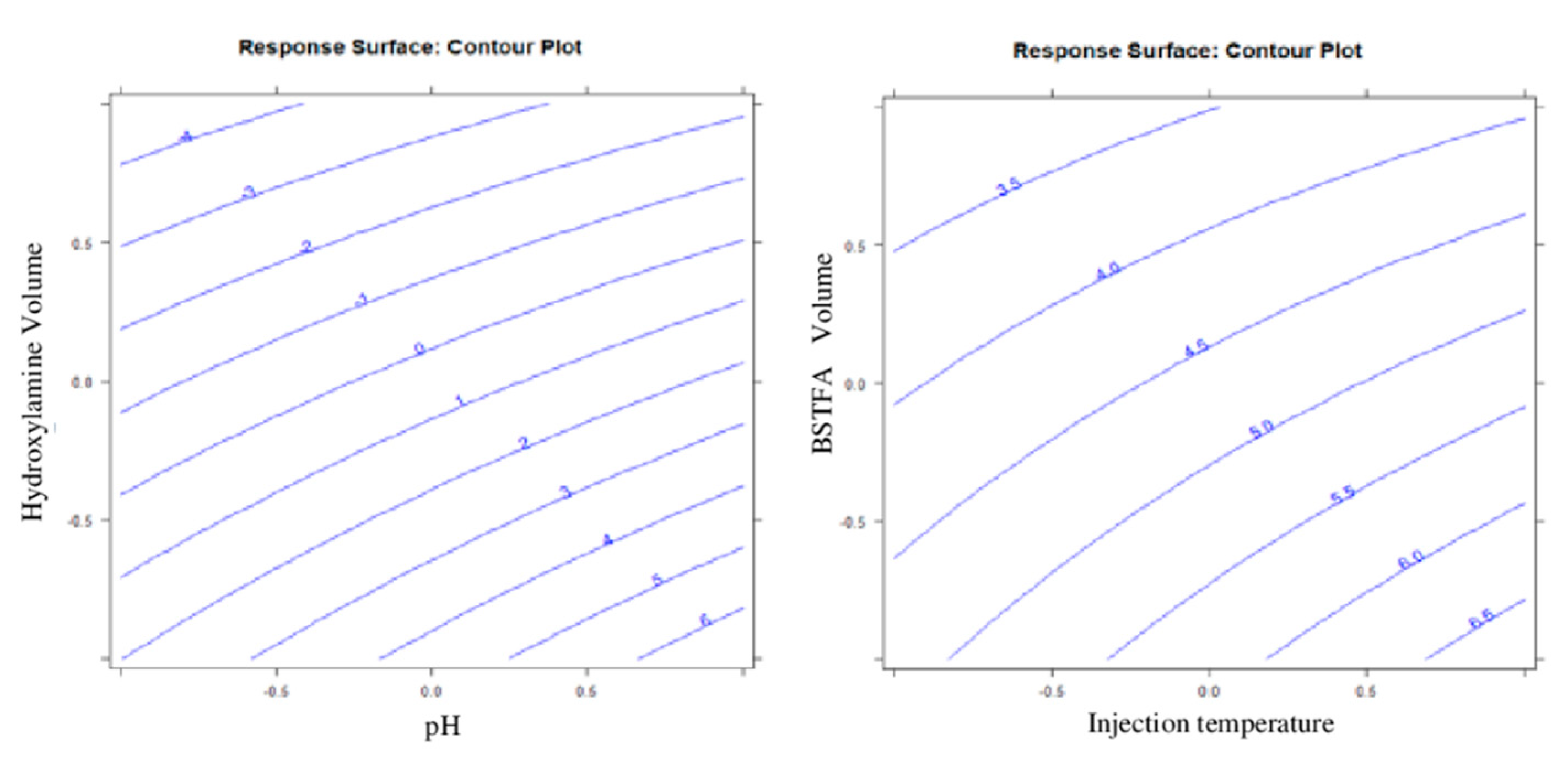
5. Results and Discussion
Design of Experiment
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Solomons, T.W.G.; Fryhle, C.B.; Snyder, S.A. Organic Chemistry, 12th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Mak, C.M.; Lee, H.-C.H.; Chan, A.Y.-W.; Lam, C.-W. Inborn errors of metabolism and expanded newborn screening, review and update. Crit. Rev. Clin. Lab. Sci. 2013, 50, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Villani, G.R.; Gallo, G.; Scolamiero, E.; Salvatore, F.; Ruoppolo, M. Classical organic acidurias: Diagnosis and pathogenesis. Clin. Exp. Med. 2017, 17, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, D.C.; Clarke, J.T. Organic acidurias and related abnormalities. Crit. Rev. Clin. Lab. Sci. 1995, 32, 377–429. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Isselbacher, K.J. The Isolation and Identification of N-Isovalerylglycine from Urine of Patients with Isovaleric Acidemia. J. Biol. Chem. 1967, 242, 2966–2972. Available online: http://www.ncbi.nlm.nih.gov/pubmed/6027258 (accessed on 30 July 2020). [PubMed]
- Christou, C.; Gika, H.G.; Raikos, N.; Theodoridis, G. GC-MS analysis of organic acids in human urine in clinical settings: A study of derivatization and other analytical parameters. J. Chromatogr. B 2014, 964, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Società Italiana Studio Malattie Metaboliche Ereditarie. Società Italiana Screenings Neonatali, Linee Guida per lo Screening Neonatale Esteso e la Conferma Diagnostica; SISMME, SISN: Genova, Italy, 2008. [Google Scholar]
- Dietzen, D.J.; Rinaldo, P.; Whitley, R.J.; Rhead, W.J.; Hannon, W.H.; Garg, U.C.; Lo, S.F.; Bennett, M.J. National academy of clinical biochemistry laboratory medicine practice guidelines: Follow-up testing for metabolic disease identified by expanded newborn screening using tandem mass spectrometry: Executive summary. Clin. Chem. 2009, 55, 1615–1626. [Google Scholar] [CrossRef]
- Tůma, P.; Samcová, E.; Štulík, K. Determination of the spectrum of low molecular mass organic acids in urine by capillary electrophoresis with contactless conductivity and ultraviolet photometric detection—An efficient tool for monitoring of inborn metabolic disorders. Anal. Chim. Acta 2011, 685, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-P.; Liao, C.-S. Comparison of ion-pair chromatography and capillary zone electrophoresis for the assay of organic acids as markers of abnormal metabolism. J. Chromatogr. A 2004, 1051, 213–219. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15532576 (accessed on 30 July 2020). [CrossRef]
- Rashed, M.S.; Ozand, P.T.; Harrison, M.E.; Watkins, P.J.F.; Evans, S.; Baillie, T.A. Electrospray Tandem Mass Spectrometry in the Diagnosis of Organic Acidemias, in Rapid Communication in Mass Spectrometry, 1st ed.; Wiley: Hoboken, NJ, USA, 1994; Volume 8, pp. 129–133. [Google Scholar] [CrossRef]
- Pan, Z.; Gu, H.; Talaty, N.; Chen, H.; Shanaiah, N.; Hainline, B.E.; Cooks, R.G.; Raftery, D. Principal component analysis of urine metabolites detected by NMR and DESI–MS in patients with inborn errors of metabolism. Anal. Bioanal. Chem. 2006, 387, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ombrone, D.; Salvatore, F.; Ruoppolo, M. Quantitative liquid chromatography coupled with tandem mass spectrometry analysis of urinary acylglycines: Application to the diagnosis of inborn errors of metabolism. Anal. Biochem. 2011, 417, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kawana, S.; Hasegawa, Y.; Yamaguchi, S. Simplified method for the chemical diagnosis of organic aciduria using GC/MS. J. Chromatogr. B 2010, 878, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Burtis, C.; Ashwood, E.; Bruns, T.D. Textbook of Clinical Chemistry and Molecular Diagnostics, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Jones, P.M.; Bennett, M.J. Urine Organic Acid Analysis for Inherited Metabolic Disease Using Gas Chromatography-Mass Spectrometry. Methods Mol. Biol. Hum. Press 2010, 603, 423–431. [Google Scholar] [CrossRef]
- Hibbert, D.B. Experimental design in chromatography: A tutorial review. J. Chromatogr. B 2012, 910, 2–13. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Draper, N.R.; Smith, H. Applied Regression Analysis (Electronic Source), Wiley Series in Probability and Statistics, 3rd ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Rinaldo, P. Organic Acids in Laboratory Guide to the Methods in Biochemical Genetics, 1st ed.; Springer: Berlin, Germany, 2008; pp. 137–169. [Google Scholar]
- Watson, M.S.; Lloyd-Puryear, M.A.; Mann, M.Y. American College of Medical Genetics (ACMG). Newborn Screening Expert Group—Main Report. Genet. Med. 2006, 117, S296–S307. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2015. Available online: https://www.r-project.org/ (accessed on 30 July 2020).
- Gruppo di Chemiometria della Divisione di Chimica Analitica della Società Chimica Italiana (SCI), Chemometric Agile Tool (CAT). Available online: http://gruppochemiometria.it/index.php/software (accessed on 30 July 2020).
| Factors | Levels: Values and Codes |
|---|---|
| A: pH of oximation | −1 = 7; +1 = 14 |
| B: VNH2OH (μL) | −1 = 100; +1 = 500 |
| C: Tinj (°C) | −1 = 250; +1 = 285 |
| D: VBSTFA (μL) | −1 = 50; +1 = 100 |
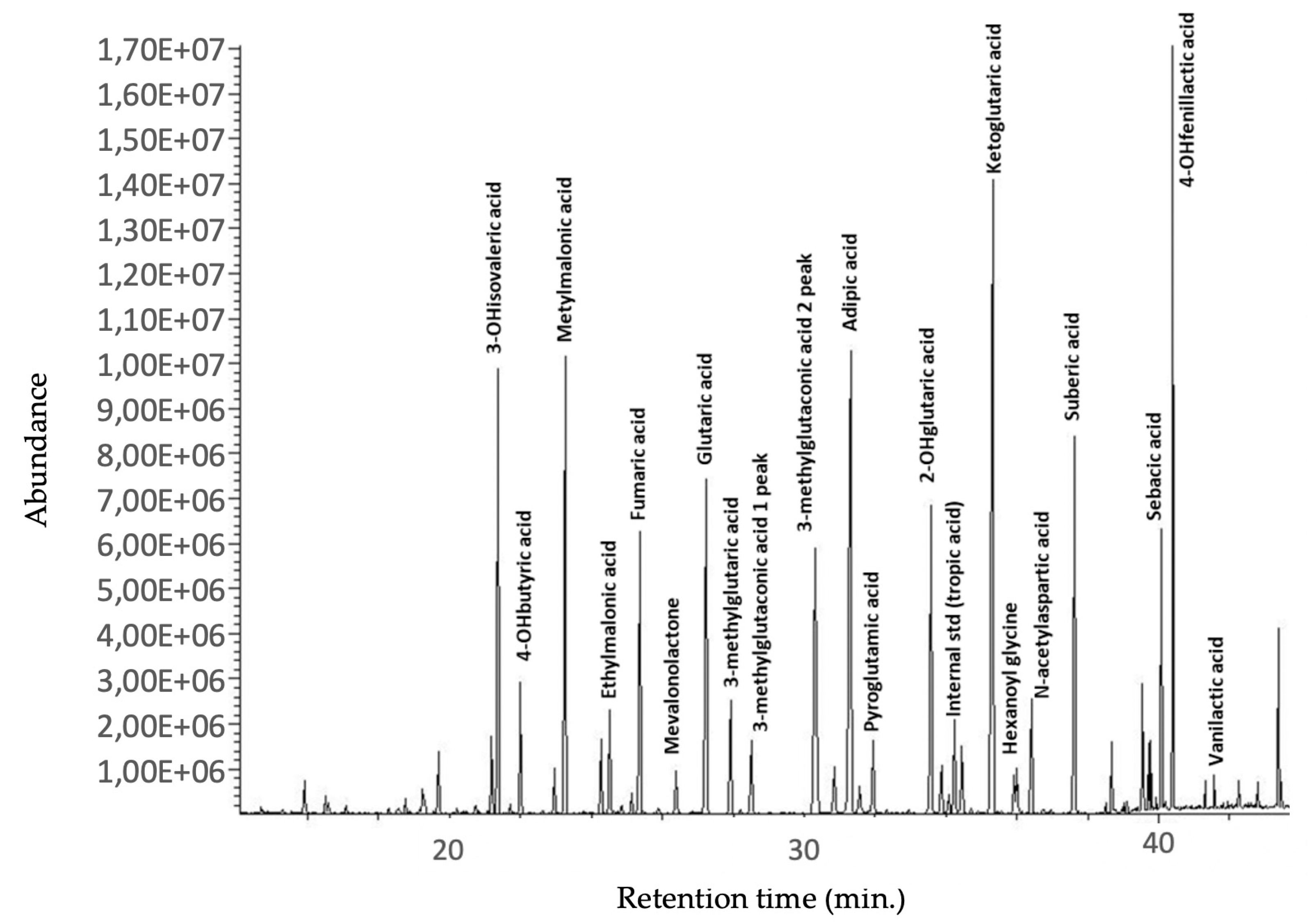
| Analytes | Retention Times (min) |
|---|---|
| 3-hydroxyisovaleric acid | 21.3 |
| 4-hydroxybutiric acid | 22.9 |
| Methylmalonic acid | 23.4 |
| Ethylmalonic acid | 24.3 |
| Fumaric acid | 25.4 |
| Mevalonolactone | 26.9 |
| Glutaric acid | 27.3 |
| 3-methylglutaric acid | 28.1 |
| 3-methylglutaconic acid (2 peaks) | 28.6–31.2 |
| Adipic acid | 31.4 |
| Pyroglutamic acid | 32.1 |
| 2-hydroxyglutaric acid | 33.7 |
| Tiglylglycine | 33.9 |
| Tropic acid (internal standard) | 34.2 |
| 3-hydroxy-3-methylglutaric acid | 34.5 |
| Ketoglutaric acid | 35.4 |
| Hexanoylglycine | 36.1 |
| N-acetylspartic acid | 36.5 |
| Suberic acid | 37.7 |
| 3-methylcitric acid (2 peaks) | 39.7–39.9 |
| Sebacic acid | 40.4 |
| Vanilactic acid | 41.2 |
| Experiment nr: | Factor A (pH) | Factor B (VNH2OH) | Factor C (Tinj) | Factor D (VBSTFA) |
|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 |
| 2 | +1 | −1 | −1 | −1 |
| 3 | −1 | +1 | −1 | −1 |
| 4 | −1 | −1 | +1 | −1 |
| 5 | −1 | −1 | −1 | +1 |
| 6 | +1 | +1 | −1 | −1 |
| 7 | −1 | +1 | +1 | −1 |
| 8 | −1 | −1 | +1 | +1 |
| 9 | +1 | −1 | +1 | −1 |
| 10 | −1 | +1 | −1 | +1 |
| 11 | +1 | +1 | +1 | −1 |
| 12 | −1 | +1 | +1 | +1 |
| 13 | +1 | −1 | −1 | +1 |
| 14 | +1 | −1 | +1 | +1 |
| 15 | +1 | +1 | −1 | +1 |
| 16 | +1 | +1 | +1 | +1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazzi, M.; Colella, S.; Alladio, E.; Puccinelli, M.P.; Mengozzi, G.; Medana, C. Statistical Optimization of Urinary Organic Acids Analysis by a Multi-Factorial Design of Experiment. Analytica 2020, 1, 14-23. https://doi.org/10.3390/analytica1010003
Pazzi M, Colella S, Alladio E, Puccinelli MP, Mengozzi G, Medana C. Statistical Optimization of Urinary Organic Acids Analysis by a Multi-Factorial Design of Experiment. Analytica. 2020; 1(1):14-23. https://doi.org/10.3390/analytica1010003
Chicago/Turabian StylePazzi, Marco, Sara Colella, Eugenio Alladio, M. Paola Puccinelli, Giulio Mengozzi, and Claudio Medana. 2020. "Statistical Optimization of Urinary Organic Acids Analysis by a Multi-Factorial Design of Experiment" Analytica 1, no. 1: 14-23. https://doi.org/10.3390/analytica1010003
APA StylePazzi, M., Colella, S., Alladio, E., Puccinelli, M. P., Mengozzi, G., & Medana, C. (2020). Statistical Optimization of Urinary Organic Acids Analysis by a Multi-Factorial Design of Experiment. Analytica, 1(1), 14-23. https://doi.org/10.3390/analytica1010003