A Narrative Review of Current Advances and Future Changes Regarding Bladder Cancer Treatment
Abstract
1. Introduction
1.1. Major Risk Factors for Developing Bladder Cancer
1.1.1. Sex
1.1.2. Age
1.1.3. Smoking Tobacco Products
1.1.4. Obesity
1.1.5. Pathogens
1.1.6. Genetics
1.1.7. Occupational Diseases
1.1.8. Genetics–Environment Interaction
1.1.9. Eating Habits and Physical Activity
1.1.10. The Role of the Microbiome
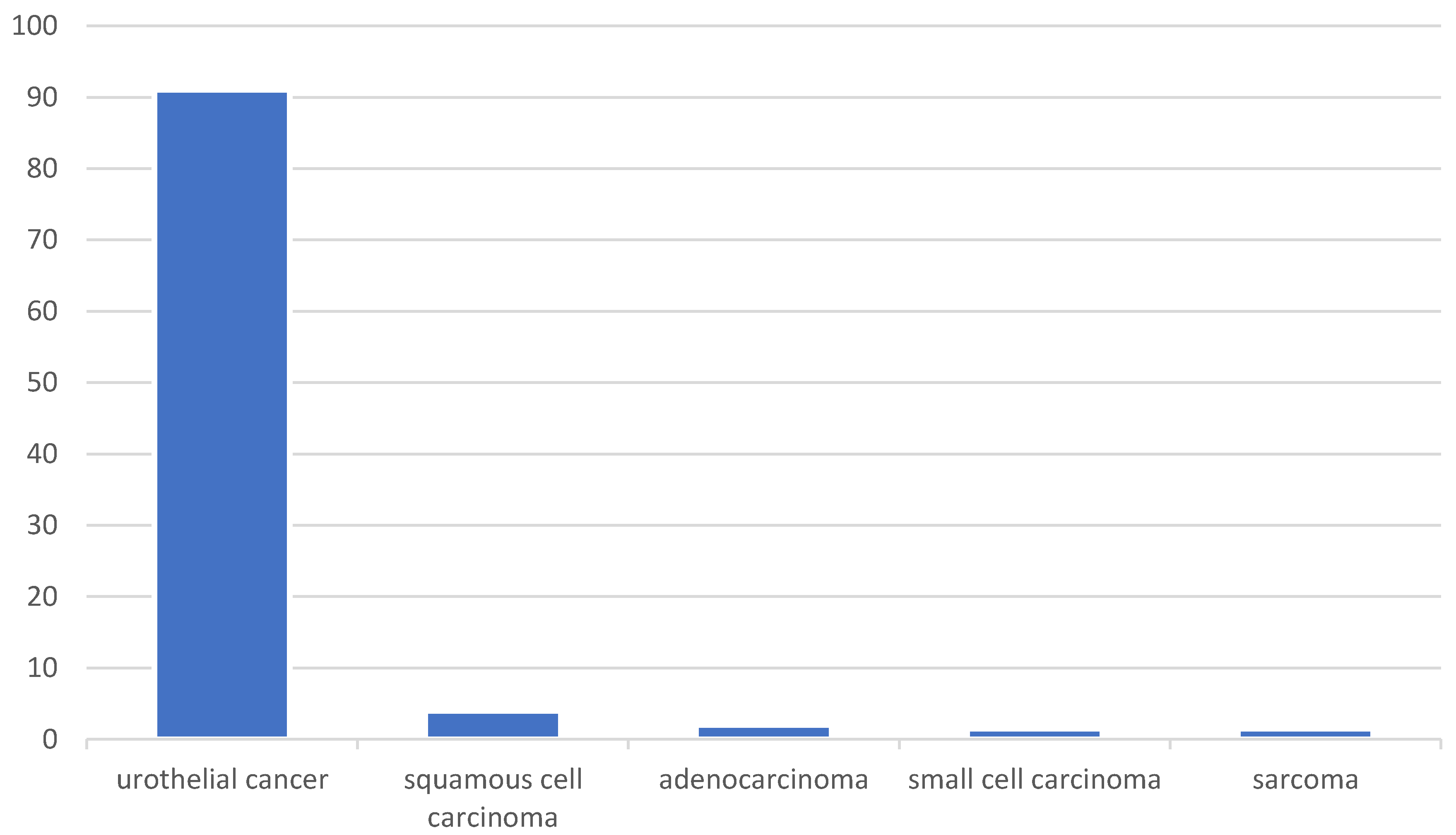
1.2. Types and Subtypes of Bladder Cancer
2. Materials and Methods
3. Results
3.1. Current Treatment Standards
3.1.1. Localized Immunotherapeutic and Chemotherapeutic Approaches
3.1.2. Surgical Approach
3.1.3. Chemotherapeutic Approach
3.1.4. Radiotherapy Approach
3.1.5. Trimodal Therapy
3.1.6. Mitomycin C (MMC) Approach
3.2. The Distinction Between Non–Muscle-Invasive and Muscle-Invasive Bladder Cancer Treatments
3.3. Potential Side Effects of Traditional Treatments
3.4. Types of Innovative Therapies and Latest Research in BC Treatment
3.4.1. Immunotherapy
New Prognostic or Predictive Biomarkers and Immune Checkpoint Inhibitors (ICIs)
New Bladder Cancer-Specific Antigens
Personalization of Immune Therapy- New Immunological Strategies
CAR-T Therapy
3.4.2. Innovations in Targeted Therapies
Innovations in Targeted Therapies
Personalization of Therapy Based on Tumor Molecular Profile
Inhibitors of Specific Signaling Pathways
Development of Technologies Related to Targeted Therapy
3.4.3. Antibody–Drug Conjugates (ADCs)
ADC Mechanism of Action
Examples of ADC Use in Bladder Cancer
3.4.4. Gene Therapies
Examples of Innovative Gene Therapies
Potential Limitations and Side Effects of Gene Therapies
3.4.5. Photodynamic Therapy
3.5. Nanomedicine
3.5.1. Nanomedicine in Photodynamic Therapy
3.5.2. Nanomedicine in Gene Therapies
3.5.3. Nanomedicine in Immunotherapy
3.5.4. Nanomedicine in Targeted Therapy
3.6. Parameters or Indices That May Impact Prognosis and Patient Eligibility for Various Treatment Options
3.7. Limitations, Clinical Challenges, and Future Perspectives Regarding Innovative Therapies for BC Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alouini, S. Risk Factors Associated with Urothelial Bladder Cancer. Int. J. Environ. Res. Public Health 2024, 21, 954. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. World Fact Sheet. GLOBOCAN. 2022. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 6 February 2023).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 6 February 2023).
- Teoh, J.Y.; Huang, J.; Ko, W.Y.; Lok, V.; Choi, P.; Ng, C.F.; Sengupta, S.; Mostafid, H.; Kamat, A.M.; Black, P.C.; et al. Global Trends of Bladder Cancer Incidence and Mortality, and Their Associations with Tobacco Use and Gross Domestic Product Per Capita. Eur. Urol. 2020, 78, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Koutros, S.; Figueroa, J.D.; Prokunina-Olsson, L.; Rothman, N. Bladder Cancer. In Schottenfeld and Fraumeni Cancer Epidemiology and Prevention, 4th ed.; Oxford University Press: New York, NY, USA, 2017; pp. 977–996. [Google Scholar]
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.W.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; Palou Redorta, J.; et al. EAU Guidelines on Non-Muscle-Invasive Urothelial Carcinoma of the Bladder: Update 2013. Eur. Urol. 2013, 64, 639–653. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hinotsu, S.; Akaza, H.; Miki, T.; Fujimoto, H.; Shinohara, N.; Kikuchi, E.; Mizutani, Y.; Koga, H.; Okajima, E.; Okuyama, A.; et al. Bladder Cancer Develops 6 Years Earlier in Current Smokers: Analysis of Bladder Cancer Registry Data Collected by the Cancer Registration Committee of the Japanese Urological Association. Int. J. Urol. 2009, 16, 64–69. [Google Scholar] [CrossRef]
- Linn, J.F.; Sesterhenn, I.; Mostofi, F.K.; Schoenberg, M. The Molecular Characteristics of Bladder Cancer in Young Patients. J. Urol. 1998, 159, 1493–1496. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between Smoking and Risk of Bladder Cancer among Men and Women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. The Insulin and Insulin-Like Growth Factor Receptor Family in Neoplasia: An Update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.W.; Zhao, L.G.; Yang, Y.; Ma, X.; Wang, Y.Y.; Xiang, Y.B. Obesity and Risk of Bladder Cancer: A Dose-Response Meta-Analysis of 15 Cohort Studies. PLoS ONE 2015, 10, e0119313. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Hämmerl, L.; Ferlay, J.; Kantelhardt, E.J. Cancer in Africa 2018: The Role of Infections. Int. J. Cancer 2020, 146, 2089–2103. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Sheweita, S.A.; O’Connor, P.J. Relationship between Schistosomiasis and Bladder Cancer. Clin. Microbiol. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef]
- Rambau, P.F.; Chalya, P.L.; Jackson, K. Schistosomiasis and Urinary Bladder Cancer in North Western Tanzania: A Retrospective Review of 185 Patients. Infect. Agents Cancer 2013, 8, 19. [Google Scholar] [CrossRef]
- Nassour, A.-J.; Jain, A.; Hui, N.; Siopis, G.; Symons, J.; Woo, H. Relative Risk of Bladder and Kidney Cancer in Lynch Syndrome: Systematic Review and Meta-Analysis. Cancers 2023, 15, 506. [Google Scholar] [CrossRef]
- Złowocka-Perłowska, E.; Tołoczko-Grabarek, A.; Narod, S.A.; Lubiński, J. Germline BRCA1 and BRCA2 Mutations and the Risk of Bladder or Kidney Cancer in Poland. Hered. Cancer Clin. Pract. 2022, 20, 13. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Ye, Y.; Siddiq, A.; Garcia-Closas, M.; Chatterjee, N.; Prokunina-Olsson, L.; Cortessis, V.K.; Kooperberg, C.; Cussenot, O.; Benhamou, S.; et al. Genome-Wide Association Study Identifies Multiple Loci Associated with Bladder Cancer Risk. Hum. Mol. Genet. 2014, 23, 1387–1398. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, K.; Hayashi, Y.; Hatano, K.; Kawashima, A.; McConkey, D.J.; Nonomura, N. Mutational Landscape and Environmental Effects in Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 6072. [Google Scholar] [CrossRef]
- Lozano, F.; Raventos, C.X.; Carrion, A.; Trilla, E.; Morote, J. Current Status of Genetic Urinary Biomarkers for Surveillance of Non-Muscle Invasive Bladder Cancer: A Systematic Review. BMC Urol. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.; Milne, R.L.; Calle, M.L.; Rothman, N.; López de Maturana, E.; Herranz, J.; Kogevinas, M.; Chanock, S.J.; Tardón, A.; Márquez, M.; et al. Genetic Variation in the TP53 Pathway and Bladder Cancer Risk: A Comprehensive Analysis. PLoS ONE 2014, 9, e89952. [Google Scholar] [CrossRef] [PubMed]
- Złowocka-Perłowska, E.; Dębniak, T.; Słojewski, M.; Lemiński, A.; Soczawa, M.; van de Wetering, T.; Trubicka, J.; Kluźniak, W.; Wokołorczyk, D.; Cybulski, C.; et al. Recurrent PALB2 Mutations and the Risk of Cancers of Bladder or Kidney in Polish Population. Hered. Cancer Clin. Pract. 2021, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur. Urol. 2016, 70, 458–466. [Google Scholar] [CrossRef]
- Kiriluk, K.J.; Prasad, S.M.; Patel, A.R.; Steinberg, G.D.; Smith, N.D. Bladder Cancer Risk from Occupational and Environmental Exposures. Urol. Oncol. 2012, 30, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.E.; Baris, D.R.; Figueroa, J.D.; Garcia-Closas, M.; Karagas, M.R.; Schwenn, M.R.; Johnson, A.T.; Lubin, J.H.; Hein, D.W.; Dagnall, C.L.; et al. GSTM1 Null and NAT2 Slow Acetylation Genotypes, Smoking Intensity and Bladder Cancer Risk: Results from the New England Bladder Cancer Study and NAT2 Meta-Analysis. Carcinogenesis 2011, 32, 182–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harling, M.; Schablon, A.; Schedlbauer, G.; Dulon, M.; Nienhaus, A. Bladder Cancer among Hairdressers: A Meta-Analysis. Occup. Environ. Med. 2010, 67, 351–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koutros, S.; Silverman, D.T.; Baris, D.; Zahm, S.H.; Morton, L.M.; Colt, J.S.; Hein, D.W.; Moore, L.E.; Johnson, A.; Schwenn, M.; et al. Hair Dye Use and Risk of Bladder Cancer in the New England Bladder Cancer Study. Int. J. Cancer 2011, 129, 2894–2904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alberg, A.J.; Hébert, J.R. Cigarette Smoking and Bladder Cancer: A New Twist in an Old Saga? J. Natl. Cancer Inst. 2009, 101, 1525–1526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, D.A.; Taylor, J.A.; Paulson, D.F.; Robertson, C.N.; Mohler, J.L.; Lucier, G.W. Genetic Risk and Carcinogen Exposure: A Common Inherited Defect of the Carcinogen-Metabolism Gene Glutathione S-Transferase M1 (GSTM1) That Increases Susceptibility to Bladder Cancer. J. Natl. Cancer Inst. 1993, 85, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Lesseur, C.; Gilbert-Diamond, D.; Andrew, A.S.; Ekstrom, R.M.; Li, Z.; Kelsey, K.T.; Marsit, C.J.; Karagas, M.R. A Case-Control Study of Polymorphisms in Xenobiotic and Arsenic Metabolism Genes and Arsenic-Related Bladder Cancer in New Hampshire. Toxicol. Lett. 2012, 210, 100–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hung, R.J.; Boffetta, P.; Brennan, P.; Malaveille, C.; Gelatti, U.; Placidi, D.; Carta, A.; Hautefeuille, A.; Porru, S. Genetic Polymorphisms of MPO, COMT, MnSOD, NQO1, Interactions with Environmental Exposures and Bladder Cancer Risk. Carcinogenesis 2004, 25, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene-Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, B.; Yan, Y.; Ye, X.; Fang, H.; Xu, H.; Liu, Y.; Li, S.; Zhao, Y. Intake of Fruit and Vegetables and Risk of Bladder Cancer: A Dose-Response Meta-Analysis of Observational Studies. Cancer Causes Control 2014, 25, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.M.; Nuñez, S.; Smith, A.H. Diet and Bladder Cancer: A Meta-Analysis of Six Dietary Variables. Am. J. Epidemiol. 2000, 151, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xie, B.; Mao, Q.; Kong, D.; Lin, Y.; Zheng, X. Tea Consumption and Risk of Bladder Cancer: A Meta-Analysis. World J. Surg. Oncol. 2012, 10, 172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, Q.Q.; Dai, Y.; Lin, Y.W.; Qin, J.; Xie, L.P.; Zheng, X.Y. Milk Consumption and Bladder Cancer Risk: A Meta-Analysis of Published Epidemiological Studies. Nutr. Cancer 2011, 63, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; An, S.; Hou, L.; Chen, P.; Lei, C.; Tan, W. Red and Processed Meat Intake and Risk of Bladder Cancer: A Meta-Analysis. Int. J. Clin. Exp. Med. 2014, 7, 2100–2110. [Google Scholar] [PubMed] [PubMed Central]
- Tang, J.E.; Wang, R.J.; Zhong, H.; Yu, B.; Chen, Y. Vitamin A and Risk of Bladder Cancer: A Meta-Analysis of Epidemiological Studies. World J. Surg. Oncol. 2014, 12, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.Y.; Wang, X.L.; Yu, Z.J. Vitamin C and E Intake and Risk of Bladder Cancer: A Meta-Analysis of Observational Studies. Int. J. Clin. Exp. Med. 2014, 7, 4154–4164. [Google Scholar] [PubMed] [PubMed Central]
- Liao, Y.; Huang, J.L.; Qiu, M.X.; Ma, Z.W. Impact of Serum Vitamin D Level on Risk of Bladder Cancer: A Systematic Review and Meta-Analysis. Tumour Biol. 2015, 36, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Cantor, K.P.; Silverman, D.T.; Malats, N. Selenium and Bladder Cancer Risk: A Meta-Analysis. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 2407–2415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myung, S.K.; Kim, Y.; Ju, W.; Choi, H.J.; Bae, W.K. Effects of Antioxidant Supplements on Cancer Prevention: Meta-Analysis of Randomized Controlled Trials. Ann. Oncol. 2010, 21, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Keimling, M.; Behrens, G.; Schmid, D.; Jochem, C.; Leitzmann, M.F. The Association between Physical Activity and Bladder Cancer: Systematic Review and Meta-Analysis. Br. J. Cancer 2014, 110, 1862–1870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogers, C.J.; Colbert, L.H.; Greiner, J.W.; Perkins, S.N.; Hursting, S.D. Physical Activity and Cancer Prevention: Pathways and Targets for Intervention. Sports Med. 2008, 38, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Al-Zalabani, A.H.; Stewart, K.F.; Wesselius, A.; Schols, A.M.; Zeegers, M.P. Modifiable Risk Factors for the Prevention of Bladder Cancer: A Systematic Review of Meta-Analyses. Eur. J. Epidemiol. 2016, 31, 811–851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heidar, N.A.; Bhat, T.A.; Shabir, U.; Hussein, A.A. The Urinary Microbiome and Bladder Cancer. Life 2023, 13, 812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussein, A.A.; Elsayed, A.S.; Durrani, M.; Jing, Z.; Iqbal, U.; Gomez, E.C.; Singh, P.K.; Liu, S.; Smith, G.; Tang, L.; et al. Investigating the Association between the Urinary Microbiome and Bladder Cancer: An Exploratory Study. Urol. Oncol. 2021, 39, 370.e9–370.e19. [Google Scholar] [CrossRef] [PubMed]
- El-Mosalamy, H.; Salman, T.M.; Ashmawey, A.M.; Osama, N. Role of Chronic E. coli Infection in the Process of Bladder Cancer—An Experimental Study. Infect. Agent Cancer 2012, 7, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curtiss, N.; Balachandran, A.; Krska, L.; Peppiatt-Wildman, C.; Wildman, S.; Duckett, J. Age, menopausal status and the bladder microbiome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Soon-Sutton, T.L.; Aeddula, N.R. Bladder Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jiang, S.; Redelman-Sidi, G. BCG in Bladder Cancer Immunotherapy. Cancers 2022, 14, 3073. [Google Scholar] [CrossRef] [PubMed]
- Colbert, L.; Jia, Y.; Sharma, A.; Hu, J.; Xu, Z.; Suzman, D.L.; Das, A.; Bross, P.; Kluetz, P.G.; Fashoyin-Aje, L.A.; et al. FDA Approval Summary: Nadofaragene Firadenovec-vncg for Bacillus Calmette–Guérin–Unresponsive Non–Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2025, 31, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Gul, M.H.; Wardak, A.B.; Raja, H.A.A.; Hussaini, H. Nogapendekin alfa inbakicept-PMLN: First approval milestone for BCG-unresponsive noninvasive bladder cancer: Editorial. Ann. Med. Surg. 2024, 86, 6386–6388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abou Chakra, M.; McElree, I.M.; Packiam, V.T.; Mott, S.L.; O’Donnell, M.A. Early experience with sequential intravesical gemcitabine and docetaxel for micropapillary variant non-muscle invasive bladder cancer. Urol. Oncol. 2024, 42, 289.e13–289.e21. [Google Scholar] [CrossRef] [PubMed]
- Meghani, K.; Cooley, L.F.; Choy, B.; Kocherginsky, M.; Swaminathan, S.; Munir, S.S.; Svatek, R.S.; Kuzel, T.; Meeks, J.J. First-in-human Intravesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Eur. Urol. 2022, 82, 602–610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, L.H.C.; Patel, M.I. Transurethral Resection of Bladder Tumour (TURBT). Transl. Androl. Urol. 2020, 9, 3056–3072. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Z.; Yao, H.; Mao, Q.; Chu, Y.; Cui, Y.; Wu, J. Robot-Assisted Radical Cystectomy vs Open Radical Cystectomy in Patients with Bladder Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. World J. Surg. Oncol. 2023, 21, 240. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, D.Y.; Liu, Z.H. Clinical Efficacy and Quality of Life Assessment of Partial Cystectomy and Plasmakinetic Transurethral Resection of Tumor in Bladder Cancer Patients. Cancer Manag. Res. 2022, 14, 389–398. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar] [CrossRef]
- McFerrin, C.; Davaro, F.; May, A.; Raza, S.; Siddiqui, S.; Hamilton, Z. Trends in Utilization of Neoadjuvant and Adjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Investig. Clin. Urol. 2020, 61, 565–572. [Google Scholar] [CrossRef]
- Pfister, C.; Gravis, G.; Fléchon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulié, M.; Allory, Y.; et al. VESPER Trial Investigators. Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Nonmetastatic Muscle-Invasive Bladder Cancer: Results of the GETUG-AFU V05 VESPER Trial. J. Clin. Oncol. 2022, 40, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Catto, J.W.F.; Galsky, M.D.; Al-Ahmadie, H.; Meeks, J.J.; Nishiyama, H.; Vu, T.Q.; Antonuzzo, L.; Wiechno, P.; Atduev, V.; et al. NIAGARA Investigators. Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N. Engl. J. Med. 2024, 14, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 3, 2102–2114, Erratum in N. Engl. J. Med. 2021, 26, 864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Apolo, A.B.; Ballman, K.V.; Sonpavde, G.; Berg, S.; Kim, W.Y.; Parikh, R.; Teo, M.Y.; Sweis, R.F.; Geynisman, D.M.; Grivas, P.; et al. Adjuvant Pembrolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2025, 392, 45–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swinton, M.; Mariam, N.B.G.; Tan, J.L.; Murphy, K.; Elumalai, T.; Soni, M.; Ferrera, A.; Richardson, C.; Walshaw, R.; Mistry, H.; et al. Bladder-Sparing Treatment with Radical Dose Radiotherapy Is an Effective Alternative to Radical Cystectomy in Patients with Clinically Node-Positive Nonmetastatic Bladder Cancer. J. Clin. Oncol. 2023, 41, 4406–4415. [Google Scholar] [CrossRef]
- Mathes, J.; Rausch, S.; Todenhöfer, T.; Stenzl, A. Trimodal therapy for muscle-invasive bladder cancer. Expert Rev. Anticancer. Ther. 2018, 18, 1219–1229. [Google Scholar] [CrossRef]
- Zargar, H.; Aning, J.; Ischia, J.; So, A.; Black, P. Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. Nat. Rev. Urol. 2014, 11, 220–230. [Google Scholar] [CrossRef]
- Suzman, D.L.; Agrawal, S.; Ning, Y.M.; Maher, V.E.; Fernandes, L.L.; Karuri, S.; Tang, S.; Sridhara, R.; Schroeder, J.; Goldberg, K.B.; et al. FDA Approval Summary: Atezolizumab or Pembrolizumab for the Treatment of Patients with Advanced Urothelial Carcinoma Ineligible for Cisplatin-Containing Chemotherapy. Oncologist 2019, 24, 563–569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raja, R.; Kuziora, M.; Brohawn, P.Z.; Higgs, B.W.; Gupta, A.; Dennis, P.A.; Ranade, K. Early Reduction in ctDNA Predicts Survival in Patients with Lung and Bladder Cancer Treated with Durvalumab. Clin. Cancer Res. 2018, 24, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Zajac, M.; Boothman, A.M.; Ben, Y.; Gupta, A.; Jin, X.; Mistry, A.; Sabalos, C.; Nielsen, A.; Manriquez, G.; Barker, C.; et al. Analytical Validation and Clinical Utility of an Immunohistochemical Programmed Death Ligand-1 Diagnostic Assay and Combined Tumor and Immune Cell Scoring Algorithm for Durvalumab in Urothelial Carcinoma. Arch. Pathol. Lab. Med. 2019, 143, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.; Bandini, M.; Gibb, E.A.; Ross, J.S.; Raggi, D.; Marandino, L.; Costa de Padua, T.; Crupi, E.; Colombo, R.; Colecchia, M.; et al. Neoadjuvant Pembrolizumab and Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Cancer: 3-Year Median Follow-Up Update of PURE-01 Trial. Clin. Cancer Res. 2022, 28, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Fléchon, A.; Morales-Barrera, R.; Powles, T.; Alva, A.; Özgüroğlu, M.; Csöszi, T.; Loriot, Y.; Rodriguez-Vida, A.; Géczi, L.; Cheng, S.Y.; et al. Association of Tumor Mutational Burden and PD-L1 with the Efficacy of Pembrolizumab with or without Chemotherapy versus Chemotherapy in Advanced Urothelial Carcinoma. Clin. Cancer Res. 2024, 30, 5353–5364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, H.; Zhou, Q.; Wang, Z.; Zhang, H.; Liu, Z.; Huang, Q.; Wang, J.; Chang, Y.; Bai, Q.; Xia, Y.; et al. Stromal LAG-3+ Cells Infiltration Defines Poor Prognosis Subtype Muscle-Invasive Bladder Cancer with Immunoevasive Contexture. J. Immunother. Cancer 2020, 8, e000651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sridhar, S.S.; Powles, T.; Climent Durán, M.A.; Park, S.H.; Massari, F.; Thiery-Vuillemin, A.; Valderrama, B.P.; Ullén, A.; Tsuchiya, N.; Aragon-Ching, J.B.; et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Analysis from JAVELIN Bladder 100 by Duration of First-Line Chemotherapy and Interval Before Maintenance. Eur. Urol. 2024, 85, 154–163. [Google Scholar] [CrossRef]
- Ding, M.; Lin, J.; Qin, C.; Fu, Y.; Du, Y.; Qiu, X.; Wei, P.; Xu, T. Novel CAR-T Cells Specifically Targeting SIA-CIgG Demonstrate Effective Antitumor Efficacy in Bladder Cancer. Adv. Sci. 2024, 11, e2400156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffman-Censits, J.H.; Lombardo, K.A.; Parimi, V.; Kamanda, S.; Choi, W.; Hahn, N.M.; McConkey, D.J.; McGuire, B.M.; Bivalacqua, T.J.; Kates, M.; et al. Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 619–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandran, E.B.A.; Iannantuono, G.M.; Atiq, S.O.; Akbulut, D.; Sinaii, N.; Simon, N.I.; Banday, A.R.; Boudjadi, S.; Gurram, S.; Nassar, A.H.; et al. Mismatch Repair Deficiency and Microsatellite Instability in Urothelial Carcinoma: A Systematic Review and Meta-Analysis. BMJ Oncol. 2024, 3, e000335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, D.; Xu, S.; Chang, T.; Ma, S.; Wang, K.; Sun, G.; Chen, S.; Xu, Y.; Zhang, H. Predicting Prognosis and Distinguishing Cold and Hot Tumors in Bladder Urothelial Carcinoma Based on Necroptosis-Associated lncRNAs. Front. Immunol. 2022, 13, 916800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giudice, G.C.; Sonpavde, G.P. Vaccine Approaches to Treat Urothelial Cancer. Hum. Vaccines Immunother. 2024, 20, 2379086. [Google Scholar] [CrossRef]
- Shen, J.; Dai, D.; Zhao, W.; Liu, T.; Zhao, Y.; Xu, Y.; Qi, Y.; Hong, J.; Shi, X.; Yang, Z.; et al. A Novel Co-Receptor with Mutated TIGIT to Enhance PSCA CAR-T Therapy for Bladder Cancer. J. Clin. Oncol. 2024, 42, e14574. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Lu, S.; Ding, F.; Wang, X.; Zhu, C.; Wang, Y.; Wang, K. Molecular Understanding and Clinical Outcomes of CAR T Cell Therapy in the Treatment of Urological Tumors. Cell Death Dis. 2024, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Su, F.; Kalyana-Sundaram, S.; Khazanov, N.; Ateeq, B.; Cao, X.; Lonigro, R.J.; Vats, P.; Wang, R.; Lin, S.F.; et al. Identification of Targetable FGFR Gene Fusions in Diverse Cancers. Cancer Discov. 2013, 3, 636–647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker, B.C.; Annala, M.J.; Cogdell, D.E.; Granberg, K.J.; Sun, Y.; Ji, P.; Li, X.; Gumin, J.; Zheng, H.; Hu, L.; et al. The Tumorigenic FGFR3-TACC3 Gene Fusion Escapes miR-99a Regulation in Glioblastoma. J. Clin. Investig. 2013, 123, 855–865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minner, S.; Sauter, G. Tumoren des ableitenden Harntrakts. Aktuelle und alte Probleme [Tumors of the urinary system. Current and old problems]. Pathologe 2009, 30 (Suppl. S2), 179–184. [Google Scholar] [CrossRef]
- Perera, T.P.S.; Jovcheva, E.; Mevellec, L.; Vialard, J.; De Lange, D.; Verhulst, T.; Paulussen, C.; Van De Ven, K.; King, P.; Freyne, E.; et al. Discovery and Pharmacological Characterization of JNJ-42756493 (Erdafitinib), a Functionally Selective Small-Molecule FGFR Family Inhibitor. Mol. Cancer Ther. 2017, 16, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aggen, D.H.; Drake, C.G. Biomarkers for Immunotherapy in Bladder Cancer: A Moving Target. J. Immunother. Cancer 2017, 5, 94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, N.H.; Chan, S.H.; Tzai, T.S.; Ho, C.L.; Liu, H.S. Expression Profiles of ErbB Family Receptors and Prognosis in Primary Transitional Cell Carcinoma of the Urinary Bladder. Clin. Cancer Res. 2001, 7, 1957–1962. [Google Scholar] [PubMed]
- Baselga, J.; Swain, S.M. Novel Anticancer Targets: Revisiting ERBB2 and Discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.S.; O’donnell, P.; Yu, E.Y.; Grivas, P.; Donnell, P.O. Systematic Review: Targeting HER2 in Bladder Cancer. Bladder Cancer 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Zvereva, M.; Pisarev, E.; Hosen, I.; Kisil, O.; Matskeplishvili, S.; Kubareva, E.; Kamalov, D.; Tivtikyan, A.; Manel, A.; Vian, E.; et al. Activating Telomerase TERT Promoter Mutations and Their Application for the Detection of Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 6034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagata, M.; Muto, S.; Horie, S. Molecular Biomarkers in Bladder Cancer: Novel Potential Indicators of Prognosis and Treatment Outcomes. Dis. Markers 2016, 2016, 8205836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shariat, S.F.; Tokunaga, H.; Zhou, J.; Kim, J.; Ayala, G.E.; Benedict, W.F.; Lerner, S.P. p53, p21, pRB, and p16 Expression Predict Clinical Outcome in Cystectomy with Bladder Cancer. J. Clin. Oncol. 2004, 22, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Lin, A.; Cao, M.; Xu, A.; Luo, P.; Zhang, J. Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit from Immune Checkpoint Inhibition. Cancer Control 2020, 27, 1073274820976665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahangar, M.; Mahjoubi, F.; Mowla, S.J. Bladder Cancer Biomarkers: Current Approaches and Future Directions. Front. Oncol. 2024, 14, 1453278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kopparapu, P.K.; Boorjian, S.A.; Robinson, B.D.; Downes, M.; Gudas, L.J.; Mongan, N.P.; Persson, J.L. Expression of VEGF and Its Receptors VEGFR1/VEGFR2 Is Associated with Invasiveness of Bladder Cancer. Anticancer Res. 2013, 33, 2381–2390. [Google Scholar] [PubMed]
- Mohammed, A.A.; El-Tanni, H.; El-Khatib, H.M.; Mirza, A.A.; Mirza, A.A.; Alturaifi, T.H. Urinary Bladder Cancer: Biomarkers and Target Therapy, New Era for More Attention. Oncol. Rev. 2016, 10, 320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Q.; Liu, Y.; Xie, H.; Zhong, Y.; Liao, X.; Zhan, H.; Zhou, Q.; Ding, M.; Yang, K.; Li, A.; et al. Lentivirus-Mediated shRNA Targeting MUTYH Inhibits Malignant Phenotypes of Bladder Cancer SW780 Cells. OncoTargets Ther. 2018, 11, 6101–6109. [Google Scholar] [CrossRef]
- Mitra, A.P. Molecular Substratification of Bladder Cancer: Moving Towards Individualized Patient Management. Ther. Adv. Urol. 2016, 8, 215–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daneshmand, S.; Zaucha, R.; Vasdev, N.; Gartrell, B.A.; Lotan, Y.; Hussain, S.A.; Lee, E.K.; Procopio, G.; Galanternik, F.; Pignot, G.; et al. Marker Lesion Study of Oral Erdafitinib in Patients with Intermediate-Risk Non-Muscle–Invasive Bladder Cancer with FGFR3/2 Alterations in THOR-2: Updated Cohort 3 Results. Urol. Oncol. 2024, 42, S58. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Petrylak, D.P.; Bellmunt, J.; Nishiyama, H.; Necchi, A.; Gurney, H.; Lee, J.L.; van der Heijden, M.S.; Rosenbaum, E.; Penel, N.; et al. FORT-1: Phase II/III Study of Rogaratinib Versus Chemotherapy in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Selected Based on FGFR1/3 mRNA Expression. J. Clin. Oncol. 2023, 41, 629–639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Necchi, A.; Pouessel, D.; Leibowitz, R.; Gupta, S.; Fléchon, A.; García-Donas, J.; Bilen, M.A.; Debruyne, P.R.; Milowsky, M.I.; Friedlander, T.; et al. Pemigatinib for Metastatic or Surgically Unresectable Urothelial Carcinoma with FGF/FGFR Genomic Alterations: Final Results from FIGHT-201. Ann. Oncol. 2024, 35, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Huan, J.; Grivas, P.; Birch, J.; Hansel, D.E. Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy. Cancers 2022, 14, 1555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McPherson, V.; Reardon, B.; Bhayankara, A.; Scott, S.N.; Boyd, M.E.; Garcia-Grossman, I.R.; Regazzi, A.M.; McCoy, A.S.; Kim, P.H.; Al-Ahmadie, H.; et al. A Phase 2 Trial of Buparlisib in Patients with Platinum-Resistant Metastatic Urothelial Carcinoma. Cancer 2020, 126, 4532–4544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenberg, J.E.; Ballman, K.A.; Halabi, S.; Atherton, P.J.; Mortazavi, A.; Sweeney, C.; Stadler, W.M.; Teply, B.A.; Picus, J.; Tagawa, S.T.; et al. Randomized Phase III Trial of Gemcitabine and Cisplatin with Bevacizumab or Placebo in Patients with Advanced Urothelial Carcinoma: Results of CALGB 90601 (Alliance). J. Clin. Oncol. 2021, 39, 2486–2496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galffy, G.; Lugowska, I.; Poddubskaya, E.V.; Cho, B.C.; Ahn, M.J.; Han, J.Y.; Su, W.C.; Hauke, R.J.; Dyar, S.H.; Lee, D.H.; et al. A Phase II Open-Label Trial of Avelumab Plus Axitinib in Previously Treated Non-Small-Cell Lung Cancer or Treatment-Naïve, Cisplatin-Ineligible Urothelial Cancer. ESMO Open 2023, 8, 101173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, K.; Yang, X.; Tai, H.; Zhong, X.; Luo, T.; Zheng, H. HER2-Targeted Therapies in Cancer: A Systematic Review. Biomark. Res. 2024, 12, 16. [Google Scholar] [CrossRef]
- Tang, D.; Yan, Y.; Li, Y.; Li, Y.; Tian, J.; Yang, L.; Ding, H.; Bashir, G.; Zhou, H.; Ding, Q.; et al. Targeting DAD1 Gene with CRISPR-Cas9 System Transmucosally Delivered by Fluorinated Polylysine Nanoparticles for Bladder Cancer Intravesical Gene Therapy. Theranostics 2024, 14, 203–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Sun, X.; Liu, G.; Li, H.; Yu, M.; Zhu, Y. Integration of Multi-Omics and Clinical Treatment Data Reveals Bladder Cancer Therapeutic Vulnerability Gene Combinations and Prognostic Risks. Front. Immunol. 2024, 14, 1301157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, H.; Liu, Z.; Weng, S.; Dang, Q.; Ge, X.; Zhang, Y.; Ren, Y.; Xing, Z.; Chen, S.; Zhou, Y.; et al. Artificial Intelligence-Driven Consensus Gene Signatures for Improving Bladder Cancer Clinical Outcomes Identified by Multi-Center Integration Analysis. Mol. Oncol. 2022, 16, 4023–4042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.S.; Seo, H.K. Emerging Treatments for Bacillus Calmette-Guérin-Unresponsive Non-Muscle-Invasive Bladder Cancer. Investig. Clin. Urol. 2021, 62, 361–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagayama, A.; Ellisen, L.W.; Chabner, B.; Bardia, A. Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments. Target Oncol. 2017, 12, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Parslow, A.C.; Parakh, S.; Lee, F.T.; Gan, H.K.; Scott, A.M. Antibody-Drug Conjugates for Cancer Therapy. Biomedicines 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.H.; Chang, I.H. A Novel Strategy for Treatment of Bladder Cancer: Antibody-Drug Conjugates. Investig. Clin. Urol. 2022, 63, 373–384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samanta, D.; Almo, S.C. Nectin Family of Cell-Adhesion Molecules: Structural and Molecular Aspects of Function and Specificity. Cell Mol. Life Sci. 2015, 72, 645–658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nadal, R.; Clara, J.A.; Valderrama, B.P.; Bellmunt, J. Current Therapy for Metastatic Urothelial Carcinoma. Hematol. Oncol. Clin. North Am. 2021, 35, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Weinstock, C.; Zhang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 922–927. [Google Scholar] [CrossRef]
- Brave, M.H.; Maguire, W.F.; Weinstock, C.; Zhang, H.; Gao, X.; Li, F.; Yu, J.; Fu, W.; Zhao, H.; Pierce, W.F.; et al. FDA Approval Summary: Enfortumab Vedotin Plus Pembrolizumab for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2024, 30, 4815–4821. [Google Scholar] [CrossRef]
- Sahota, S.; Vahdat, L.T. Sacituzumab Govitecan: An Antibody-Drug Conjugate. Expert Opin. Biol. Ther. 2017, 17, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Sharkey, R.M. Antibody-Drug Conjugates Targeting TROP-2 and Incorporating SN-38: A Case Study of Anti-TROP-2 Sacituzumab Govitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benjamin, D.J.; Kalebasty, A.R.; Mar, N. Implications and Lessons from the Withdrawal of Sacituzumab Govitecan for Treating Advanced Urothelial Carcinoma. Eur. Urol. Oncol. 2025, 8, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Tagawa, S.; Vulsteke, C.; Gross-Goupil, M.; Park, S.; Necchi, A.; De Santis, M.; Duran, I.; Morales-Barrera, R.; Guo, J.; et al. Sacituzumab govitecan in advanced urothelial carcinoma: TROPiCS-04, a phase III randomized trial. Ann. Oncol. 2025, 36, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Srinivasalu, V.K.; Robbrecht, D. Advancements in First-Line Treatment of Metastatic Bladder Cancer: EV-302 and Checkmate-901 Insights and Future Directions. Cancers 2024, 16, 2398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleischmann, A.; Rotzer, D.; Seiler, R.; Studer, U.E.; Thalmann, G.N. Her2 Amplification Is Significantly More Frequent in Lymph Node Metastases from Urothelial Bladder Cancer than in the Primary Tumours. Eur. Urol. 2011, 60, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayashi, T.; Seiler, R.; Oo, H.Z.; Jäger, W.; Moskalev, I.; Awrey, S.; Dejima, T.; Todenhöfer, T.; Li, N.; Fazli, L.; et al. Targeting HER2 with T-DM1, an Antibody Cytotoxic Drug Conjugate, Is Effective in HER2 Overexpressing Bladder Cancer. J. Urol. 2015, 194, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, C.; Willuda, J.; Kubetzko, S.; Lauffer, I.; Tschudi, D.; Waibel, R.; Plückthun, A.; Stahel, R.A.; Zangemeister-Wittke, U. A Recombinant Immunotoxin Derived from a Humanized Epithelial Cell Adhesion Molecule-Specific Single-Chain Antibody Fragment Has Potent and Selective Antitumor Activity. Clin. Cancer Res. 2003, 9, 2837–2848. [Google Scholar] [PubMed]
- Fong, D.; Seeber, A.; Terracciano, L.; Kasal, A.; Mazzoleni, G.; Lehne, F.; Gastl, G.; Spizzo, G. Expression of EpCAM(MF) and EpCAM(MT) Variants in Human Carcinomas. J. Clin. Pathol. 2014, 67, 408–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kowalski, M.; Entwistle, J.; Cizeau, J.; Niforos, D.; Loewen, S.; Chapman, W.; MacDonald, G.C. A Phase I Study of an Intravesically Administered Immunotoxin Targeting EpCAM for the Treatment of Nonmuscle-Invasive Bladder Cancer in BCG-Refractory and BCG-Intolerant Patients. Drug Des. Devel. Ther. 2010, 4, 313–320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kowalski, M.; Guindon, J.; Brazas, L.; Moore, C.; Entwistle, J.; Cizeau, J.; Jewett, M.A.; MacDonald, G.C. A Phase II Study of Oportuzumab Monatox: An Immunotoxin Therapy for Patients with Noninvasive Urothelial Carcinoma In Situ Previously Treated with Bacillus Calmette-Guérin. J. Urol. 2012, 188, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An Antibody-Drug Conjugate That Targets Tissue Factor Exhibits Potent Therapeutic Activity Against a Broad Range of Solid Tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Patry, G.; Hovington, H.; Larue, H.; Harel, F.; Fradet, Y.; Lacombe, L. Tissue Factor Expression Correlates with Disease-Specific Survival in Patients with Node-Negative Muscle-Invasive Bladder Cancer. Int. J. Cancer 2008, 122, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.P.; Arkenau, H.T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab Vedotin in Patients with Advanced or Metastatic Solid Tumours (InnovaTV 201): A First-in-Human, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef]
- Narayan, V.M.; Meeks, J.J.; Jakobsen, J.S.; Shore, N.D.; Sant, G.R.; Konety, B.R. Mechanism of Action of Nadofaragene Firadenovec-VNCG. Front. Oncol. 2024, 14, 1359725. [Google Scholar] [CrossRef]
- Konety, B.R.; Shore, N.D.; Sant, G.R. Clinical Use of Nadofaragene Firadenovec-VNCG. Ther. Adv. Urol. 2024, 16, 17562872241280005. [Google Scholar] [CrossRef]
- Zhou, Q.; Fang, L.; Tang, Y.; Wang, Q.; Tang, X.; Zhu, L.; Peng, N.; Wang, B.; Song, W.; Fu, H. Exosome-Mediated Delivery of Artificial Circular RNAs for Gene Therapy of Bladder Cancer. J. Cancer 2024, 15, 1770–1778. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in Gene Therapy: Current Status and Clinical Applications. J. Control Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- McNall, S.; Hooper, K.; Sullivan, T.; Rieger-Christ, K.; Clements, M. Treatment Modalities for Non-Muscle Invasive Bladder Cancer: An Updated Review. Cancers 2024, 16, 1843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goulet, M.-L.; Dauphinee, S.M.; Veilleux, D.; Louis, K.S.; Lazure, D.; Stevenson, S.; Bilimoria, D.; Zamzameer, F.; Chen, X.; Sublemontier, S.; et al. Abstract PR006: EG-70 (Detalimogene Voraplasmid), a Novel, Non-Viral Intravesical Gene Therapy for BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer: Preclinical Characterization and Translation into the Clinic. Clin. Cancer Res. 2024, 30, PR006. [Google Scholar] [CrossRef]
- Hannouneh, Z.A.; Hijazi, A.; Alsaleem, A.A.; Hami, S.; Kheyrbek, N.; Tanous, F.; Khaddour, K.; Abbas, A.; Alshehabi, Z. Novel Immunotherapeutic Options for BCG-Unresponsive High-Risk Non-Muscle-Invasive Bladder Cancer. Cancer Med. 2023, 12, 21944–21968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kochergin, M.; Fahmy, O.; Asimakopoulos, A.; Theil, G.; Zietz, K.; Bialek, J.; Tiberi, E.; Gakis, G. Photodynamic Therapy: Current Trends and Potential Future Role in the Treatment of Bladder Cancer. Int. J. Mol. Sci. 2024, 25, 960. [Google Scholar] [CrossRef] [PubMed]
- Kurabayashi, A.; Fukuhara, H.; Furihata, K.; Iwashita, W.; Furihata, M.; Inoue, K. Photodynamic Diagnosis and Therapy in Non-Muscle-Invasive Bladder Cancer. Cancers 2024, 16, 2299. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Wang, W.; Geng, H.; Wang, Y.; Gao, B. Current Advances in the Application of Nanomedicine in Bladder Cancer. Biomed. Pharmacother. 2023, 157, 114062. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Al-Hayek, S.; Huang, H.; Zhu, Z.; Zhu, W.; Guo, H. Photodynamic Effect of 5-Aminolevulinic Acid-Loaded Nanoparticles on Bladder Cancer Cells: A Preliminary Investigation. Scand. J. Urol. 2013, 47, 145–151. [Google Scholar] [CrossRef]
- Lin, T.Y.; Li, Y.; Liu, Q.; Chen, J.L.; Zhang, H.; Lac, D.; Zhang, H.; Ferrara, K.W.; Wachsmann-Hogiu, S.; Li, T.; et al. Novel Theranostic Nanoporphyrins for Photodynamic Diagnosis and Trimodal Therapy for Bladder Cancer. Biomaterials 2016, 104, 339–351. [Google Scholar] [CrossRef]
- Winnicka, A.; Brzeszczyńska, J.; Saluk, J.; Wigner-Jeziorska, P. Nanomedicine in Bladder Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 10388. [Google Scholar] [CrossRef]
- He, L.; Wang, L.; Yu, X.; Tang, Y.; Jiang, Z.; Yang, G.; Liu, Z.; Li, W. Full-Course NIR-II Imaging-Navigated Fractionated Photodynamic Therapy of Bladder Tumours with X-Ray-Activated Nanotransducers. Nat. Commun. 2024, 15, 8240. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Karimi-Maleh, H.; Taheriazam, A.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Makvandi, P.; Nazarzadeh Zare, E.; Sharifi, E.; et al. (Nano)platforms in Bladder Cancer Therapy: Challenges and Opportunities. Bioeng. Transl. Med. 2022, 8, e10353. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-Crosslinkable Chitosan-Hyaluronic Acid Dialdehyde Nanoparticles for CD44-Targeted siRNA Delivery to Treat Bladder Cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef]
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of Dual miRNA Through CD44-Targeted Mesoporous Silica Nanoparticles for Enhanced and Effective Triple-Negative Breast Cancer Therapy. Biomater. Sci. 2020, 8, 2939–2954. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, X.; Pang, G.; Deng, J.; Xie, Q.; Zhang, Z. Significance of KDM6A Mutation in Bladder Cancer Immune Escape. BMC Cancer 2021, 21, 635. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cui, J.; Zhao, Y.; Liu, X.; Chen, L.; Xia, Y.; Wang, Y.; Chen, S.; Sun, S.; Shi, B.; et al. KDM6A-ARHGDIB Axis Blocks Metastasis of Bladder Cancer by Inhibiting Rac1. Mol. Cancer 2021, 20, 77. [Google Scholar] [CrossRef]
- Kong, N.; Zhang, R.; Wu, G.; Sui, X.; Wang, J.; Kim, N.Y.; Blake, S.; De, D.; Xie, T.; Cao, Y.; et al. Intravesical Delivery of KDM6A-mRNA via Mucoadhesive Nanoparticles Inhibits the Metastasis of Bladder Cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2112696119. [Google Scholar] [CrossRef]
- Terán-Navarro, H.; Zeoli, A.; Salines-Cuevas, D.; Marradi, M.; Montoya, N.; Gonzalez-Lopez, E.; Ocejo-Vinyals, J.G.; Dominguez-Esteban, M.; Gutierrez-Baños, J.L.; Campos-Juanatey, F.; et al. Gold Glyconanoparticles Combined with 91-99 Peptide of the Bacterial Toxin, Listeriolysin O, Are Efficient Immunotherapies in Experimental Bladder Tumors. Cancers 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, Z.; Wang, J.; Li, L.; Wang, F.; Zhu, Z.; Wang, X. Nanomedicine for Combination Urologic Cancer Immunotherapy. Pharmaceutics 2023, 15, 546. [Google Scholar] [CrossRef]
- Lv, M.; Shang, S.; Liu, K.; Wang, Y.; Xu, P.; Song, H.; Zhang, J.; Sun, Z.; Yan, Y.; Zhu, Z.; et al. Revitalizing Bacillus Calmette-Guérin Immunotherapy for Bladder Cancer: Nanotechnology and Bioengineering Approaches. Pharmaceutics 2024, 16, 1067. [Google Scholar] [CrossRef]
- Zhou, Q.; Ding, W.; Qian, Z.; Zhu, Q.; Sun, C.; Yu, Q.; Tai, Z.; Xu, K. Immunotherapy Strategy Targeting Programmed Cell Death Ligand 1 and CD73 with Macrophage-Derived Mimetic Nanovesicles to Treat Bladder Cancer. Mol. Pharm. 2021, 18, 4015–4028. [Google Scholar] [CrossRef]
- Liu, F.; Guo, C.; Li, X.; Li, Y.; Xu, S.; James, T.D.; Wang, L. A Versatile Nano-Transformer for Efficient Localization-Specific Imaging and Synergistic Therapy of Bladder Cancer. Nano Today 2024, 54, 102116. [Google Scholar] [CrossRef]
- Simó, C.; Serra-Casablancas, M.; Hortelao, A.C.; Di Carlo, V.; Guallar-Garrido, S.; Plaza-García, S.; Rabanal, R.M.; Ramos-Cabrer, P.; Yagüe, B.; Aguado, L.; et al. Urease-Powered Nanobots for Radionuclide Bladder Cancer Therapy. Nat. Nanotechnol. 2024, 19, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, T.; Zhou, B.; Wei, J.; Fang, Y.; Lu, J.; Guo, L.; Chen, W.; Liu, Z.P.; Luo, J. Mg(II)-Catechin Nanoparticles Delivering siRNA Targeting EIF5A2 Inhibit Bladder Cancer Cell Growth In Vitro and In Vivo. Biomaterials 2016, 81, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Arista-Romero, M.; Cascante, A.; Fornaguera, C.; Borrós, S. Role of Survivin in Bladder Cancer: Issues to Be Overcome When Designing an Efficient Dual Nano-Therapy. Pharmaceutics 2021, 13, 1959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, J.; Cheang, T.; Tang, B.; Xia, H.; Xing, Z.; Chen, Z.; Fang, Y.; Chen, W.; Xu, A.; Wang, S.; et al. The Inhibition of Human Bladder Cancer Growth by Calcium Carbonate/CaIP6 Nanocomposite Particles Delivering AIB1 siRNA. Biomaterials 2013, 34, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Sari Motlagh, R.; Ghoreifi, A.; Yanagisawa, T.; Kawada, T.; Kikic, Z.; Gill, I.; Daneshmand, S.; Djaladat, H.; Shariat, S.F. Survival of Patients with Chronic Kidney Disease Treated with Radical Cystectomy and Risk Factors of Glomerular Filtration Rate Loss Following Radical Cystectomy: Two Systematic Reviews and Meta-analyses of Interplay Between Radical Cystectomy and Renal Function. Eur. Urol. Focus 2023, 10, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Panunzio, A.; Gozzo, A.; Mazzucato, G.; Ornaghi, P.I.; Di Filippo, G.; Soldano, A.; De Maria, N.; Cianflone, F.; Orlando, R.; Boldini, M.; et al. Impairment in Activities of Daily Living Assessed by the Barthel Index Predicts Adverse Oncological Outcomes After Radical Cystectomy for Bladder Cancer. Clin. Genitourin. Cancer 2023, 21, e495–e501.e2. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, R.; Tillu, N.; Cumarasamy, S.; Alerasool, P.; Rich, J.M.; Kaufmann, B.; Elkun, Y.; Attalla, K.; Mehrazin, R.; Wiklund, P.; et al. Longitudinal Tumor-informed Circulating Tumor DNA Status Predicts Disease Upstaging and Poor Prognosis for Patients Undergoing Radical Cystectomy. Eur. Urol. Oncol. 2024, 7, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Marino, F.; Rossi, F.; Bizzarri, F.P.; Ragonese, M.; Dibitetto, F.; Filomena, G.B.; Marafon, D.P.; Ciccarese, C.; Iacovelli, R.; et al. Is Systemic Immune-Inflammation Index a Real Non-Invasive Biomarker to Predict Oncological Outcomes in Patients Eligible for Radical Cystectomy? Medicina 2023, 59, 2063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nardelli, C.; Aveta, A.; Pandolfo, S.D.; Tripodi, L.; Russo, F.; Imbimbo, C.; Castaldo, G.; Pastore, L. Microbiome Profiling in Bladder Cancer Patients Using the First-morning Urine Sample. Eur. Urol. Open Sci. 2023, 59, 18–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Mutation Name | Brief Description | Reference |
|---|---|---|
| Lynch Syndrome | Lynch syndrome, especially with EPCAM/MSH2 mutations, increases bladder cancer risk by 7.5 times (RR: 7.48; 95% CI: 3.70–15.13; p < 0.01). These tumors are typically high-grade but non-invasive. Early, targeted screening is recommended, particularly for patients under 65 with a personal or family history of Lynch-related cancers. | [20] |
| Mutation in the BRCA1 and BRCA2 genes | BRCA mutations, primarily linked to breast and ovarian cancer, may also influence bladder cancer risk, particularly in European populations. In Poland, the BRCA2 C5972T variant was identified in about 5% of bladder cancer cases. Although no strong association was confirmed, its higher frequency suggests a possible role that warrants further investigation and may support broader genetic testing in selected patient groups. | [21] |
| Genome-wide analysis (GWAS) | Genome-wide association studies (GWASs) have identified genetic risk loci such as GSTM1, UGT1A, rs10936599, and rs907611, which may increase bladder cancer susceptibility, particularly in individuals with a family history. These findings suggest shared genetic and environmental influences. Further validation and functional studies are needed to clarify their role and support improved risk prediction and early detection strategies. | [22] |
| Mutations in the FGFR3 and TERT genes | FGFR3 and TERT mutations are common in bladder cancer, particularly in less aggressive forms, and are associated with early tumor development and progression. FGFR3 may also serve as a therapeutic target. Incorporating TERT and FGFR3 testing into clinical practice could enhance early detection, prognostication, and support personalized treatment approaches. | [23,24] |
| TP53 and TP63 mutations | TP53 and TP63 gene variants, such as rs1042522 and rs710521, have been linked to increased risk of bladder cancer, especially aggressive forms like MIBC. While common TP53 variants like Arg72Pro show limited association in white Europeans, SNPs in TP63 and SERPINB5 may be relevant. Larger genome-wide studies are needed to identify reliable genetic markers beyond current candidate gene findings. | [25] |
| Mutations in DNA repair pathway genes, including PALB2 | In the Polish population, rare PALB2 mutations (509_510delGA, 172_175delTTGT) have been identified in some bladder cancer cases. Although uncommon, they may contribute to risk in individuals with a family history, possibly through impaired DNA repair. Further studies are needed to determine whether PALB2 testing could help identify high-risk patients. | [26] |
| Type of Method | Clinical Outcomes | Side Effects | Reference |
|---|---|---|---|
| Surgical approach (Current Standard) | The clinical outcomes of the described bladder cancer treatments indicate high effectiveness of TURBT, particularly when the detrusor muscle is present in the sample, reducing the risk of recurrence and improving five-year survival. ReTURBT in T1 tumors enhances staging accuracy and treatment outcomes. Radical cystectomy (RC) and robot-assisted radical cystectomy (RARC) show comparable oncological results, with RARC offering reduced blood loss and better surgical field visualization, despite a longer operative time. Partial cystectomy (PC) preserves bladder function and is effective in selected patients, but carries a higher risk of recurrence and requires regular follow-up. | In TURBT, the main concern is incomplete tumor removal, which increases the risk of recurrence and disease progression. In the case of radical cystectomy (RC) and robot-assisted radical cystectomy (RARC), complications within 30 and 90 days are similar, although RC is associated with greater blood loss and a higher need for transfusions, while RARC offers better hemostasis and surgical field visualization but requires longer operative time. Partial cystectomy (PC) is more invasive than TURBT, involving abdominal incision, which prolongs surgery and recovery time and increases the risk of infections. Additionally, PC carries a higher likelihood of cancer recurrence, necessitating long-term follow-up. | [60,61,62] |
| Chemotherapeutic approach (Current Standard) | In neoadjuvant chemotherapy, the standard treatment regimen includes gemcitabine and cisplatin, administered every three weeks in four cycles, with the goal of maximizing the antitumor effect before surgery. Studies have shown that the use of neoadjuvant chemotherapy before cystectomy increases overall survival and event-free survival. Adjuvant chemotherapy, on the other hand, is used in patients at high risk of cancer recurrence, with the aim of destroying any residual cancer cells that may have remained after surgery. Analyses show that the 5-year overall survival in the AC group is 42.6%, which is significantly higher than the 37.8% observed in patients without chemotherapy, but lower than the 48.3% in patients who received neoadjuvant chemotherapy. | Both as neoadjuvant (preoperative) and adjuvant (postoperative) therapy, causes side effects typical of systemic therapies, such as weakness, nausea, damage to the kidneys, hearing, and immunity. | [63,64] |
| Radiotherapy approach (Current Standard) | In patients with clinically positive lymph nodes (cN+ M0), radiotherapy can be used in a radical form (RadRT) as an alternative that allows for bladder preservation. Studies indicate that patients undergoing RadRT achieve similar outcomes in terms of overall survival (OS) and progression-free survival (PFS) compared to those who undergo cystectomy. The effectiveness of radiotherapy can be further enhanced by using radiosensitizers such as gemcitabine or mitomycin, which strengthen the tumor’s response to treatment. | Side effects of radiotherapy for bladder cancer may include fatigue, which manifests as persistent physical and mental exhaustion that is not relieved by rest. Hematuria, or blood in the urine, can also occur, ranging from mild to more severe cases requiring medical intervention. Additionally, long-term damage to the bladder and surrounding tissues is possible, leading to complications such as reduced bladder capacity, urinary dysfunction, and, in some cases, fibrosis of adjacent organs, which can impact overall quality of life. | [69] |
| Immunotherapeutic approach (Current Standard) | Bacillus Calmette–Guérin (BCG) is the standard treatment for patients with non-muscle-invasive bladder cancer (NMIBC) at intermediate and high risk. It is also the preferred approach for tumors containing carcinoma in situ (CIS). Through intravesical administration, BCG triggers a complex immune response involving dendritic cells, macrophages, natural killer (NK) cells, and T lymphocytes, effectively aiding in tumor eradication in many patients. | BCG immunotherapy commonly causes irritative symptoms like increased urinary frequency and hematuria. In rare instances, a mycobacterial infection may develop, necessitating specialized medical intervention. | [55] |
| Trimodal therapy (TMT) (Current Standard) | Trimodal therapy (TMT) for bladder cancer, which combines maximal tumor debulking via transurethral resection of bladder tumor (TURBT) with chemoradiotherapy, has demonstrated promising clinical outcomes. According to prospective RTOG studies, the five-year overall survival (OS) rate is 57%, while the disease-specific survival rate reaches 71%. A significant advantage of TMT is the preservation of bladder function, with 79% of survivors reporting satisfaction with their urinary function, significantly improving their quality of life. Additionally, 75% of patients rated their bladder function as normal after TMT, based on urodynamic tests and questionnaires, highlighting the functional benefits of this approach. These outcomes underscore TMT as a viable alternative to radical cystectomy, particularly for patients seeking bladder preservation. | Despite its benefits, TMT is associated with potential side effects, primarily related to chemoradiotherapy. Common adverse effects include bladder irritation, urinary frequency, dysuria, and fatigue, which are typical of cisplatin-based chemoradiotherapy. Additionally, radiation therapy can lead to long-term bladder dysfunction in some patients, such as reduced bladder capacity or increased urinary urgency. Strict monitoring through cystoscopy and urine cytology is essential to detect recurrence early, as disease progression may necessitate radical cystectomy. While TMT offers significant advantages in bladder preservation and quality of life, careful patient selection and management of side effects are crucial to optimizing outcomes. | [71] |
| New prognostic and predictive biomarkers (New Approach) | A reduction in VAF in ctDNA after 6 weeks of anti-PD-L1 therapy was associated with improved PFS and OS. In the CD-ON-MEDI4736-1108 trial, durvalumab demonstrated durable clinical responses, particularly in patients with high PD-L1 expression. In the PURE-01 study, pembrolizumab as neoadjuvant therapy before RC in patients with MIBC provided better EFS in individuals with high PD-L1 CPS (89.8% vs. 59.7%; p = 0.0013). In the KEYNOTE-361 trial, high TMB (≥175 mutations/exome) and PD-L1 CPS (≥10) correlated with improved PFS, OS, and ORR, especially in patients with both parameters at high levels. | Side effects included an acceptable toxicity profile for atezolizumab and pembrolizumab, cytokine release syndrome (CRS) and neurotoxicity (ICANS) in CAR-T therapy, and grade ≥ 3 adverse events in 43–62% of patients treated with avelumab. | [72,73] |
| New Bladder Cancer-Specific Antigens (New Approach) | Studies have shown that SIA-CIgG is a promising bladder cancer-specific antigen with minimal expression in healthy tissues. CAR-T cells targeting SIA-CIgG effectively destroy cancer cells, and their cytotoxicity depends on the antigen expression level. Compared to HER2-targeted CAR-T cells, SIA-CIgG CAR-T cells demonstrated milder tumor cell lysis and better functional durability, while the combination with vorinostat significantly increased therapeutic effectiveness. Nectin-4, another antigen widely expressed in urothelial cancer, was found in 87% of non-muscle-invasive and 58% of muscle-invasive bladder cancer cases, with the highest expression observed in non-muscle-invasive papillary carcinomas (97%) and in situ carcinomas (87.5%). Low or absent expression was noted in small cell carcinomas (0%) and sarcomatoid carcinomas (10%). In these cases, alternative molecular targets like Trop2 or HER2/ERBB2 were often present. The results confirm the importance of Nectin-4 as a key therapeutic target, though alternative options should be considered in cases of low expression. | The analyzed articles did not mention any significant side effects, which may suggest a potentially safe therapeutic profile. | [77,78] |
| Personalization of Immune Therapy (New Approach) | The tumor mutational profile, including dMMR and MSI-H, provides important prognostic and predictive information in bladder cancer immunotherapy. Studies have shown that dMMR occurs in 2.3% of BC patients and 8.95% of UTUC patients, while MSI-H is present in 2.11% and 8.36%, respectively. The prevalence of MSI-H is higher in localized tumors than in metastatic ones. In metastatic urothelial cancer, patients with dMMR/MSI-H treated with ICIs achieved a 64.7% response rate, compared to 11.1% in those treated with chemotherapy, suggesting their role in predicting immunotherapy effectiveness and cisplatin resistance. Further research identified necroptosis-related lncRNAs as prognostic biomarkers, with prediction accuracy for 1-, 3-, and 5-year survival at AUC 0.707, 0.679, and 0.675, respectively. The analysis divided tumors into “hot”, with high immune activity and better response to immunotherapy, and “cold”, requiring alternative strategies. These findings highlight the importance of dMMR, MSI-H, and lncRNAs in personalizing bladder cancer treatment. | In the context of personalized bladder cancer immunotherapy, no significant information regarding side effects has been reported. The focus is primarily on the role of biomarkers such as dMMR, MSI-H, and lncRNA in predicting treatment efficacy and tailoring therapy to the tumor microenvironment. | [79,80] |
| CAR-T Therapy (New Approach) | CAR-T therapy in bladder cancer has shown promising clinical outcomes, particularly when targeting specific antigens like Nectin-4, SIA-CIgG, and PSCA. These therapies demonstrate enhanced efficacy in destroying cancer cells and reducing tumor burden, as evidenced by decreased bioluminescent tumor signals in preclinical models. The use of modified TIGIT co-receptors and CD28 activation signals further improves CAR-T cell functionality, leading to increased cytokine production (e.g., IFN-γ) and cytotoxic efficiency. Additionally, CAR-T cells’ ability to create immunological memory offers long-term protection against disease progression, potentially reducing relapse rates. | The main serious adverse events include cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). These side effects pose significant safety challenges for the therapy and require further research to develop strategies to minimize these complications. | [80,81] |
| Use of immune checkpoint inhibitors (ICIs) (New Approach) | Immune checkpoint inhibitors (ICIs) have significantly improved outcomes in the treatment of muscle-invasive bladder cancer (MIBC) and advanced urothelial cancer (UC). Targeting receptors like PD-1, PD-L1, and CTLA-4 has enhanced T lymphocyte activation, leading to better antitumor responses. The phase 3 JAVELIN Bladder 100 study demonstrated that avelumab maintenance therapy, combined with best supportive care (BSC), significantly improved overall survival (OS) and progression-free survival (PFS) in patients with advanced UC who did not progress after platinum-based chemotherapy (HR for OS: 0.63–0.79). Additionally, LAG-3 has emerged as a promising target, with high expression associated with poorer survival outcomes and reduced efficacy of adjuvant chemotherapy. LAG-3+ expression correlates with immunosuppressive tumor microenvironments and CD8+ T cell dysfunction, making it a potential biomarker for targeted therapies and immunotherapy. | While ICIs like avelumab are generally well tolerated, they are associated with adverse events. In the JAVELIN Bladder 100 study, grade ≥ 3 adverse events occurred in 43–62% of patients, highlighting the need for careful monitoring and management of side effects. Common side effects of ICIs include immune-related adverse events (irAEs) such as fatigue, skin rash, colitis, hepatitis, and endocrinopathies. The immunosuppressive nature of the tumor microenvironment, including factors like LAG-3, may also contribute to resistance and reduced efficacy of ICIs, underscoring the importance of further research to optimize therapeutic strategies and minimize complications. | [82,83] |
| Targeted therapies (New Approach) | Targeted therapies have significantly advanced the treatment of bladder cancer, particularly through the inhibition of key molecular pathways such as FGFR3, HER2, and PI3K/Akt/mTOR. Erdafitinib, an FGFR kinase inhibitor, demonstrated a complete response (CR) rate of 83.3% and a partial response (PR) rate of 11.1% in patients with intermediate-risk non-muscle-invasive bladder cancer (NMIBC) harboring FGFR3/2 alterations, with a median duration of response (DOR) of 12.8 months. Rogaratinib, another FGFR1-4 inhibitor, achieved an objective response rate (ORR) of 20.7% in advanced urothelial cancer (UC), with comparable efficacy to chemotherapy. Pemigatinib, a selective FGFR1-3 inhibitor, showed an ORR of 17.8–23.3% in UC patients with FGFR3 mutations, particularly in those with S249C, R248C, and G370C mutations. mTOR inhibitors like everolimus and temsirolimus have shown moderate efficacy, especially in patients with TSC1 or PIK3CA mutations, though their use is limited by significant toxicity. Bevacizumab, an anti-VEGF therapy, did not improve overall survival in combination with chemotherapy but slightly prolonged progression-free survival (PFS) by 1.3 months. The JAVELIN Medley VEGF trial combining avelumab (PD-L1 inhibitor) and axitinib (VEGFR inhibitor) in cisplatin-ineligible patients achieved an ORR of 10% and a median overall survival (OS) of 21.2 months, though with notable toxicity. HER2-targeted therapies, including tyrosine kinase inhibitors and antibody–drug conjugates (ADCs), have shown promise in HER2-overexpressing bladder cancer, though resistance remains a challenge. CRISPR/Cas9 technology, targeting genes like DAD1, has demonstrated preclinical efficacy in inducing cancer cell apoptosis, offering a potential breakthrough for NMIBC treatment. Multi-omics and AI-driven approaches are further enhancing personalized therapy by identifying predictive biomarkers and optimizing treatment strategies. | Targeted therapies, while effective, are often associated with significant side effects. Erdafitinib commonly causes hyperphosphatemia (100%), diarrhea (83.3%), and dry skin (50%). Rogaratinib is linked to diarrhea, hyperphosphatemia, and fatigue, with grade 3 or higher adverse events occurring in 43% of patients. Pemigatinib frequently results in diarrhea (44.6%), alopecia, stomatitis, and hyperphosphatemia (42.7%), with rare severe events like stomatitis (8.8%) and anemia (8.1%). mTOR inhibitors, such as everolimus and temsirolimus, are associated with insulin resistance, pneumonia, hyperlipidemia, and severe adverse events in 53% of patients. Bevacizumab can cause hypertension and proteinuria, while the combination of avelumab and axitinib led to serious adverse events in 50% of patients, including hypertension, fatigue, and two therapy-related deaths. These side effects highlight the need for careful patient monitoring and the development of strategies to mitigate toxicity while maintaining therapeutic efficacy. | [86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114] |
| Antibody–drug conjugates (ADCs) (New Approach) | Antibody–drug conjugates (ADCs) have emerged as a transformative therapeutic option in bladder cancer, particularly for advanced or refractory cases. Enfortumab vedotin (EV), targeting Nectin-4, has shown remarkable efficacy in advanced urothelial cancer (mUC). In the EV-201 study, EV achieved a 44% response rate with a median duration of response (DOR) of 7.6 months. The combination of EV with pembrolizumab (Pembro) in the EV-302/KEYNOTE-A39 trial demonstrated even more impressive results, with a median progression-free survival (PFS) of 12.5 months and a median overall survival (OS) of 31.5 months, significantly outperforming traditional chemotherapy (PFS: 6.3 months; OS: 16.1 months). Sacituzumab govitecan (SG), targeting TROP2, achieved an objective response rate (ORR) of 27% and a median OS of 10.5 months in the TROPHY-U-01 study. Trastuzumab emtansine (T-DM1), targeting HER2, has shown promising preclinical efficacy in HER2-positive urothelial cancer (UC), with ongoing phase II trials evaluating its clinical potential. Vicinium (Oportuzumab monatox), targeting EpCAM, demonstrated a complete response rate of 39–41% at 3 months in BCG-refractory non-muscle-invasive bladder cancer (NMIBC), with a median duration of response of 9.4 months in the phase III VISTA study. Tisotumab vedotin (TV), targeting tissue factor (TF), showed a 26.7% objective response rate in bladder cancer patients in a phase I/II trial, highlighting its potential in UC treatment. These ADCs offer significant clinical benefits, particularly in patients with limited treatment options. | Despite their efficacy, ADCs are associated with notable side effects. Enfortumab vedotin (EV) commonly causes fatigue, peripheral neuropathy, and rash, with 73% of patients experiencing high-grade adverse events. Sacituzumab govitecan (SG) is linked to neutropenia, leukopenia, and anemia, though its toxicity profile is generally manageable. Trastuzumab emtansine (T-DM1) is associated with thrombocytopenia, elevated liver enzymes, and neuropathy, particularly in HER2-positive cancers. Vicinium primarily causes mild bladder irritation, with no severe toxicity reported in clinical trials. Tisotumab vedotin (TV) has been associated with infusion-related reactions and serious adverse events in some patients, though these were manageable in clinical studies. These side effects underscore the need for careful patient monitoring and management to balance therapeutic efficacy with tolerability. | [115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139] |
| Gene therapies (New Approach) | Gene therapies have shown significant promise in the treatment of bladder cancer, particularly for patients with high-risk non-muscle-invasive bladder cancer (NMIBC) who are unresponsive to standard therapies like Bacillus Calmette–Guérin (BCG). Nadofaragene firadenovec-vncg (Adstiladrin), an adenoviral vector-based gene therapy delivering interferon alpha-2b (IFNα-2b), achieved a complete response rate of over 50% at three months in phase III trials, with some patients maintaining efficacy for over a year. This therapy offers a bladder-preserving alternative to radical cystectomy, significantly improving patients’ quality of life. CG0070, an oncolytic adenovirus, demonstrated an overall response rate of 76% in the BOND003 phase III trial, with 74% of patients maintaining a response for at least six months. When combined with pembrolizumab in the CORE001 trial, the response rate increased to 85%, highlighting its potential as an effective and well tolerated option for BCG-unresponsive NMIBC. EG-70, a nonviral gene therapy, achieved a complete response rate of 73% in the phase 1/2 LEGEND trial, with evidence of remodeling the tumor microenvironment to promote antitumor immunity. BC-819, a plasmid-based therapy targeting the H19 gene, showed promising results in a phase I/II study, with 22% of patients achieving tumor marker ablation and 55% of patients on maintenance therapy experiencing disease-free survival exceeding 35 weeks. These therapies represent innovative approaches to bladder cancer treatment, offering durable responses and bladder preservation. | While gene therapies are generally well tolerated, they are not without side effects. Nadofaragene firadenovec-vncg primarily causes local and mild adverse events, such as bladder irritation, with a low risk of systemic toxicity. CG0070 is associated with mild adverse events, including bladder spasms (20%) and urinary frequency (16%), making it a safe option for most patients. EG-70 has shown minimal systemic toxicity, with most side effects being localized to the bladder, though long-term safety data are still being collected. BC-819 has not reported serious treatment-related adverse events in initial studies, but further research is needed to confirm its safety profile. Despite these encouraging results, challenges remain, such as the toxicity of polyethyleneimine (PEI) due to its high cationic charge and limited biodegradability, which may limit its clinical application. Additionally, the long-term efficacy and safety of therapies like CG0070, EG-70, and BC-819 require further investigation to fully establish their role in bladder cancer treatment. Overall, while gene therapies offer significant potential, ongoing research is essential to optimize their safety and efficacy. | [141,142,143,144,145,146] |
| Photodynamic therapy (New Approach) | Photodynamic therapy (PDT) has shown promising results in the treatment of non-muscle-invasive bladder cancer (NMIBC), particularly for patients resistant to BCG therapy. In a phase 1b study using the photosensitizer TLD-1433, two out of three patients treated with the therapeutic dose (0.70 mg/cm2) achieved complete remission (CR) without disease recurrence for 18 months. The remaining patients experienced disease recurrence but without progression. PDT with 5-aminolevulinic acid (5-ALA) has also demonstrated efficacy in controlling carcinoma in situ (CIS) and other flat lesions, offering a minimally invasive alternative to aggressive treatments like chemotherapy or BCG therapy. The use of green laser light (penetration depth of 1.5 mm) in PDT has helped avoid complications related to damage to the detrusor muscle, which was a concern with earlier therapies using red light. Overall, PDT shows potential as a bladder-preserving treatment, reducing recurrence rates and improving local control in NMIBC. | PDT is generally well tolerated, with adverse effects primarily limited to mild to moderate lower urinary tract symptoms, such as bladder spasms, urinary frequency, and urge incontinence, which typically resolve within 90–180 days. No serious adverse events (grade ≥ 3) or photosensitivity were reported in clinical studies. However, challenges remain, such as the risk of thermal burns at high light power (e.g., 2.5 W) and the potential for muscle damage with longer wavelengths (e.g., 693 nm). The limited residence time of photosensitizers (1–2 h) after intravesical administration can also affect their diffusion into tumor cells, potentially reducing efficacy. Further optimization of therapy parameters and the development of more selective photosensitizers are needed to minimize side effects and improve outcomes. | [147,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godlewski, D.; Czech, S.; Szpara, J.; Bartusik-Aebisher, D.; Aebisher, D. A Narrative Review of Current Advances and Future Changes Regarding Bladder Cancer Treatment. Uro 2025, 5, 11. https://doi.org/10.3390/uro5020011
Godlewski D, Czech S, Szpara J, Bartusik-Aebisher D, Aebisher D. A Narrative Review of Current Advances and Future Changes Regarding Bladder Cancer Treatment. Uro. 2025; 5(2):11. https://doi.org/10.3390/uro5020011
Chicago/Turabian StyleGodlewski, Dominik, Sara Czech, Jakub Szpara, Dorota Bartusik-Aebisher, and David Aebisher. 2025. "A Narrative Review of Current Advances and Future Changes Regarding Bladder Cancer Treatment" Uro 5, no. 2: 11. https://doi.org/10.3390/uro5020011
APA StyleGodlewski, D., Czech, S., Szpara, J., Bartusik-Aebisher, D., & Aebisher, D. (2025). A Narrative Review of Current Advances and Future Changes Regarding Bladder Cancer Treatment. Uro, 5(2), 11. https://doi.org/10.3390/uro5020011








