Pancreas Transplantation in Minorities including Patients with a Type 2 Diabetes Phenotype
Abstract
1. Introduction: Scope of Diabetes Mellitus
2. Methods
3. Status of Pancreas Transplantation (PTx)
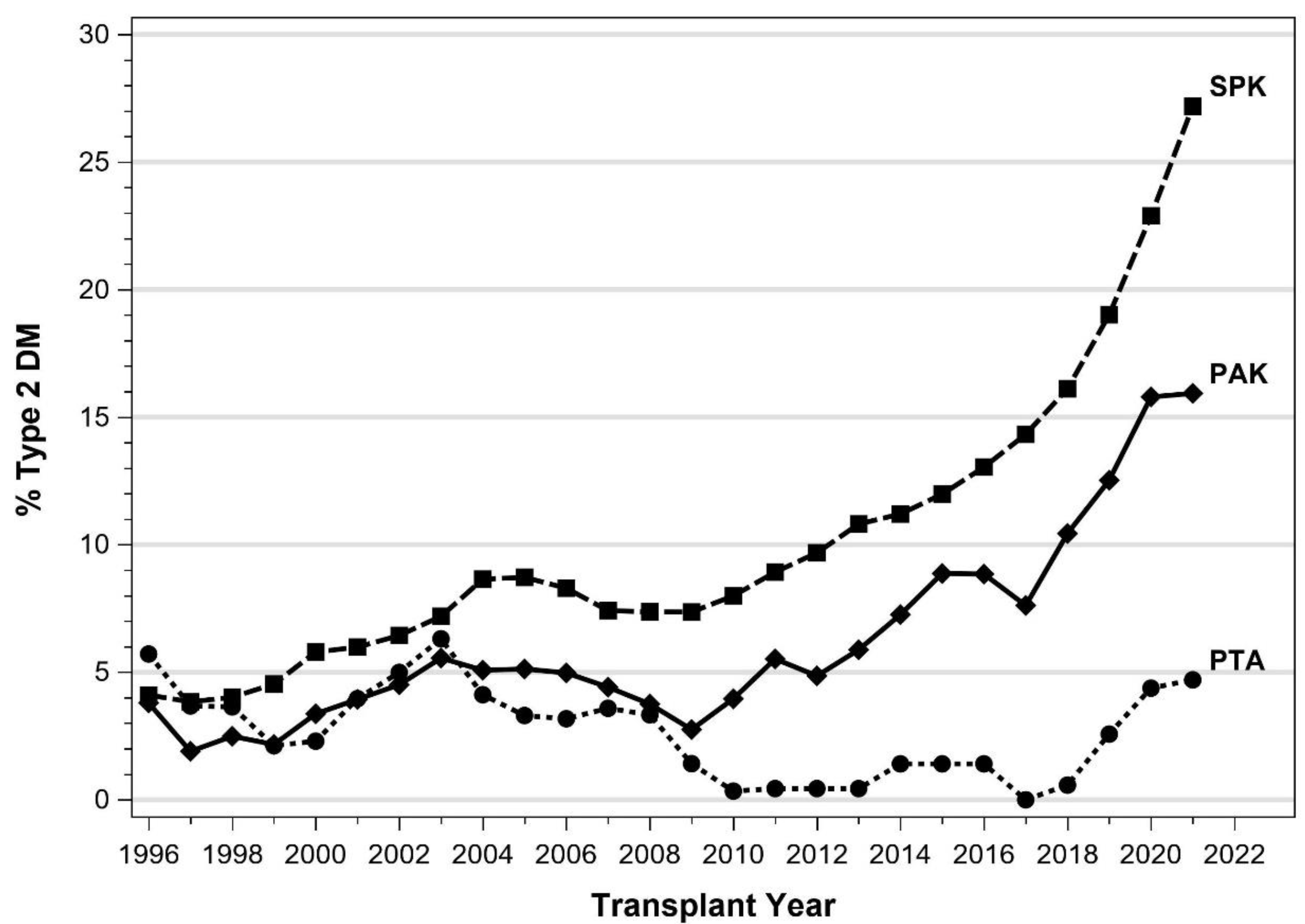
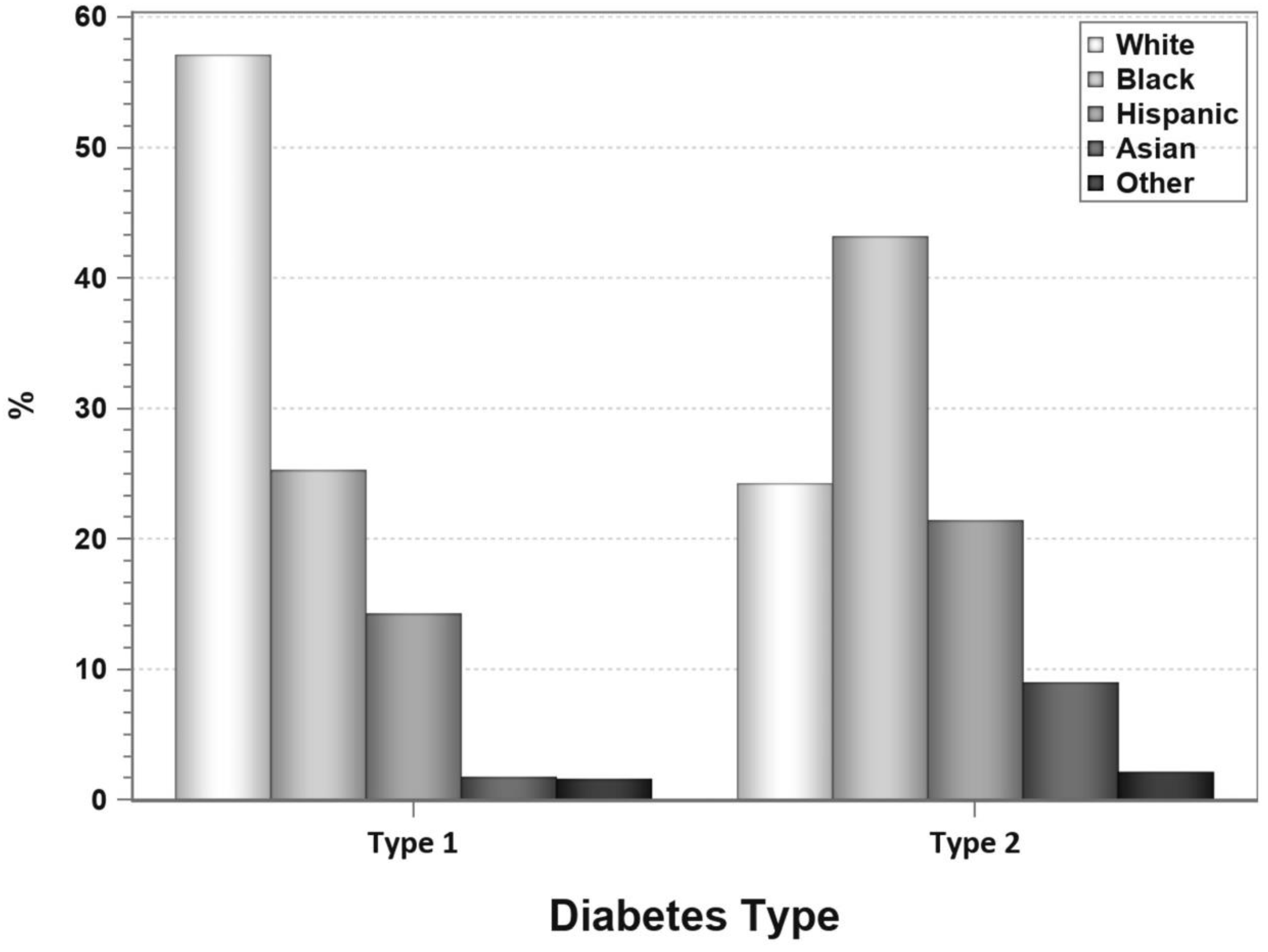
4. Pancreas Transplantation in Patients with T2DM
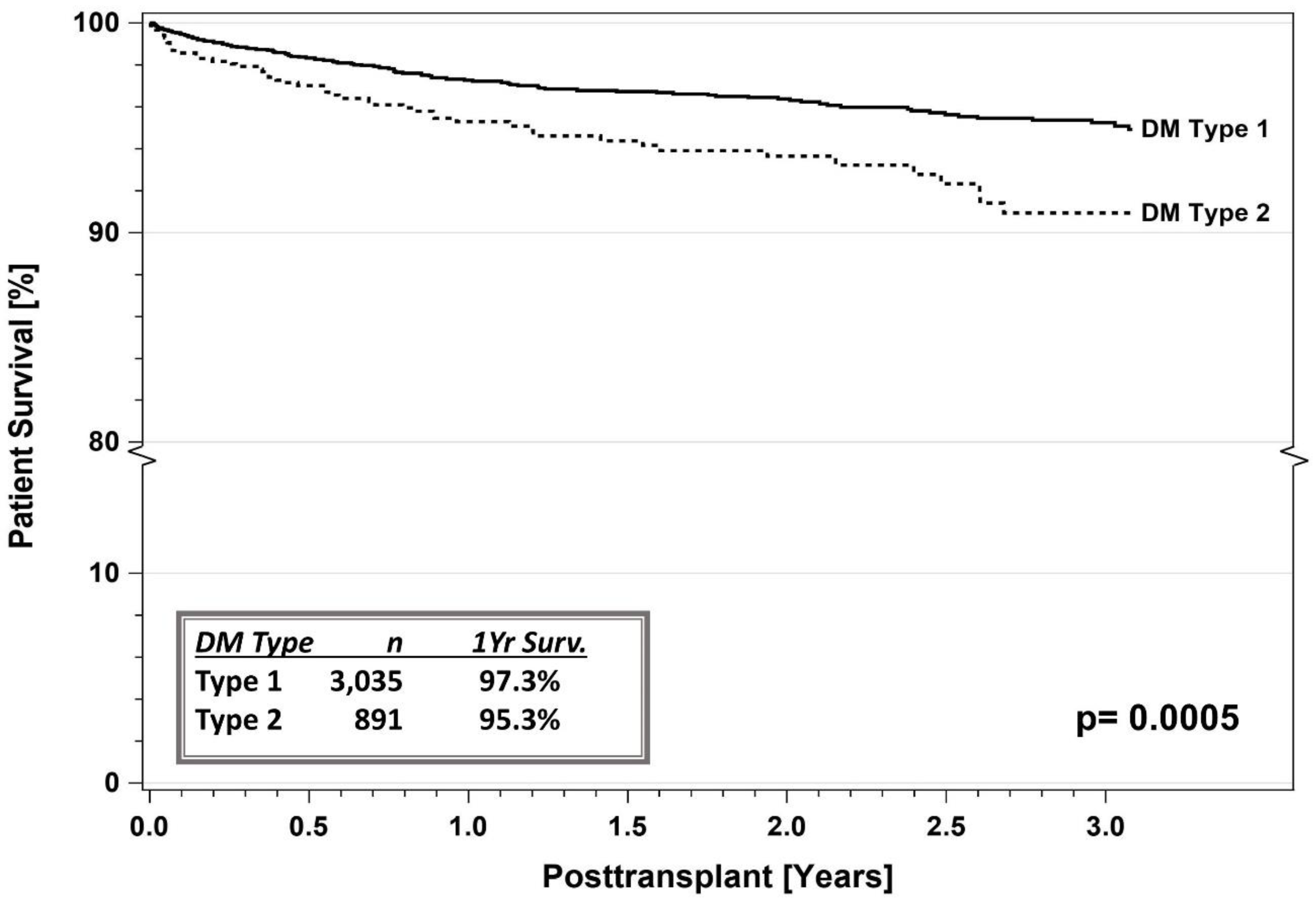
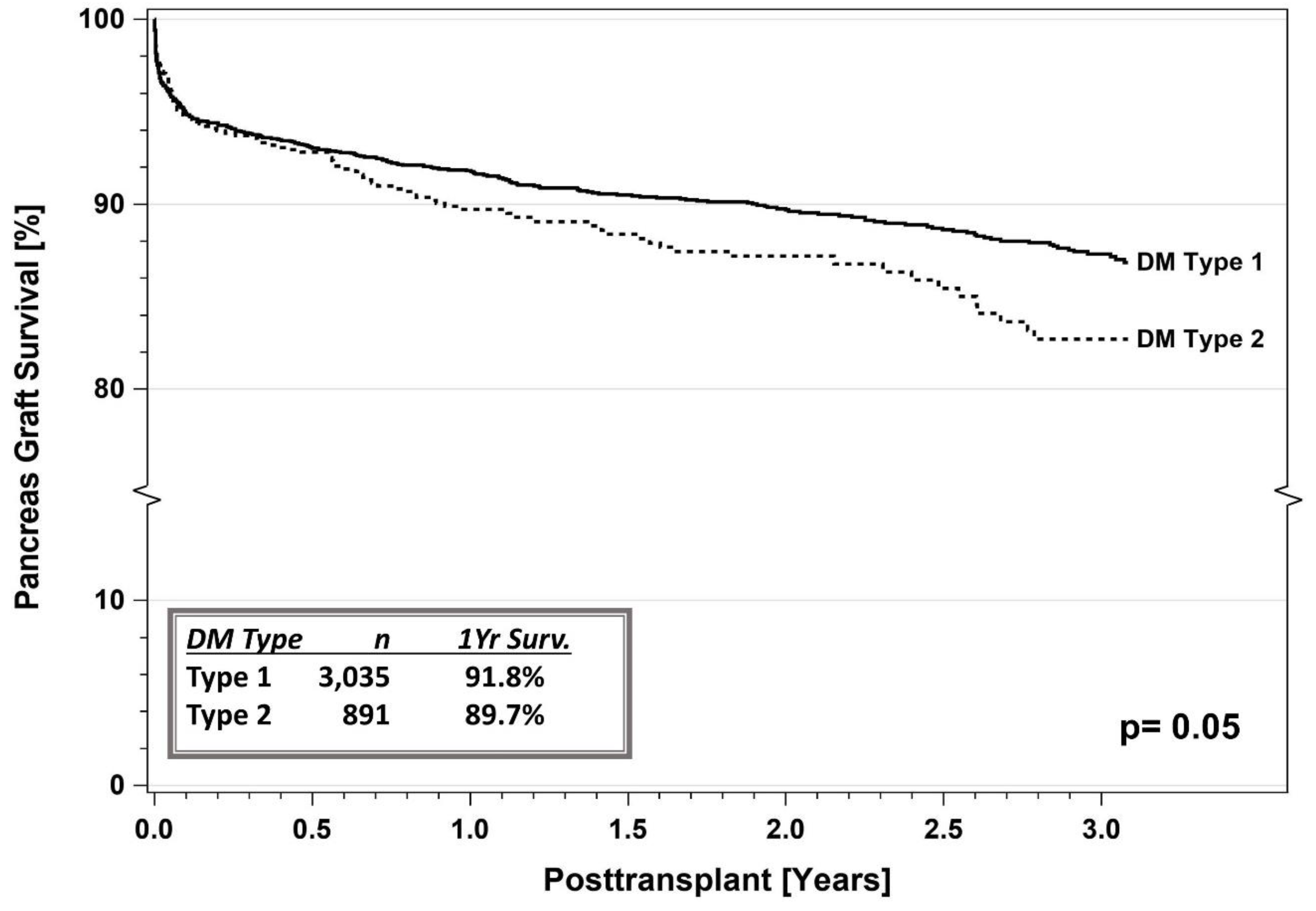
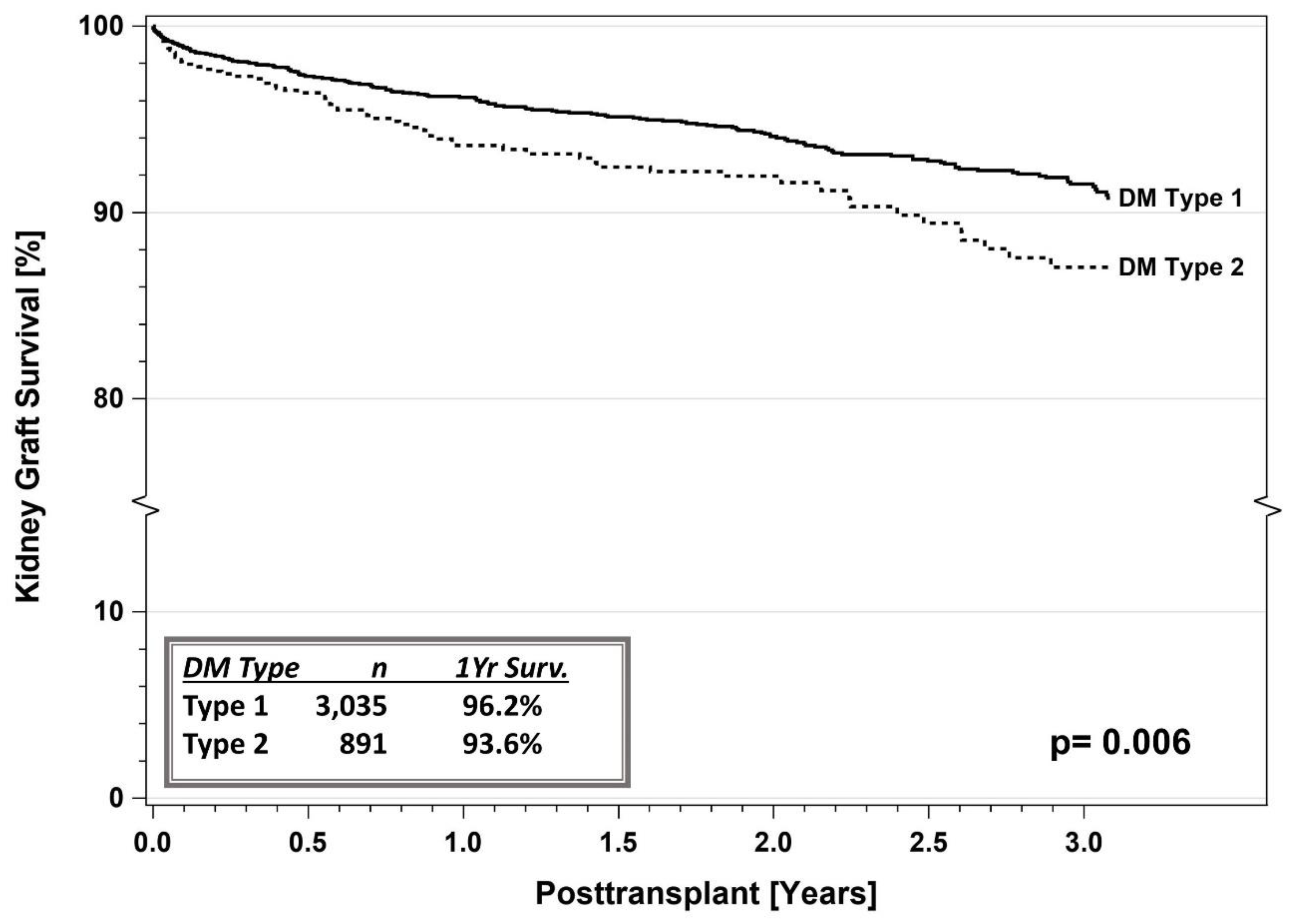
5. SPKT Outcomes in T2DM
5.1. IPTR Data
5.2. Single Center Studies
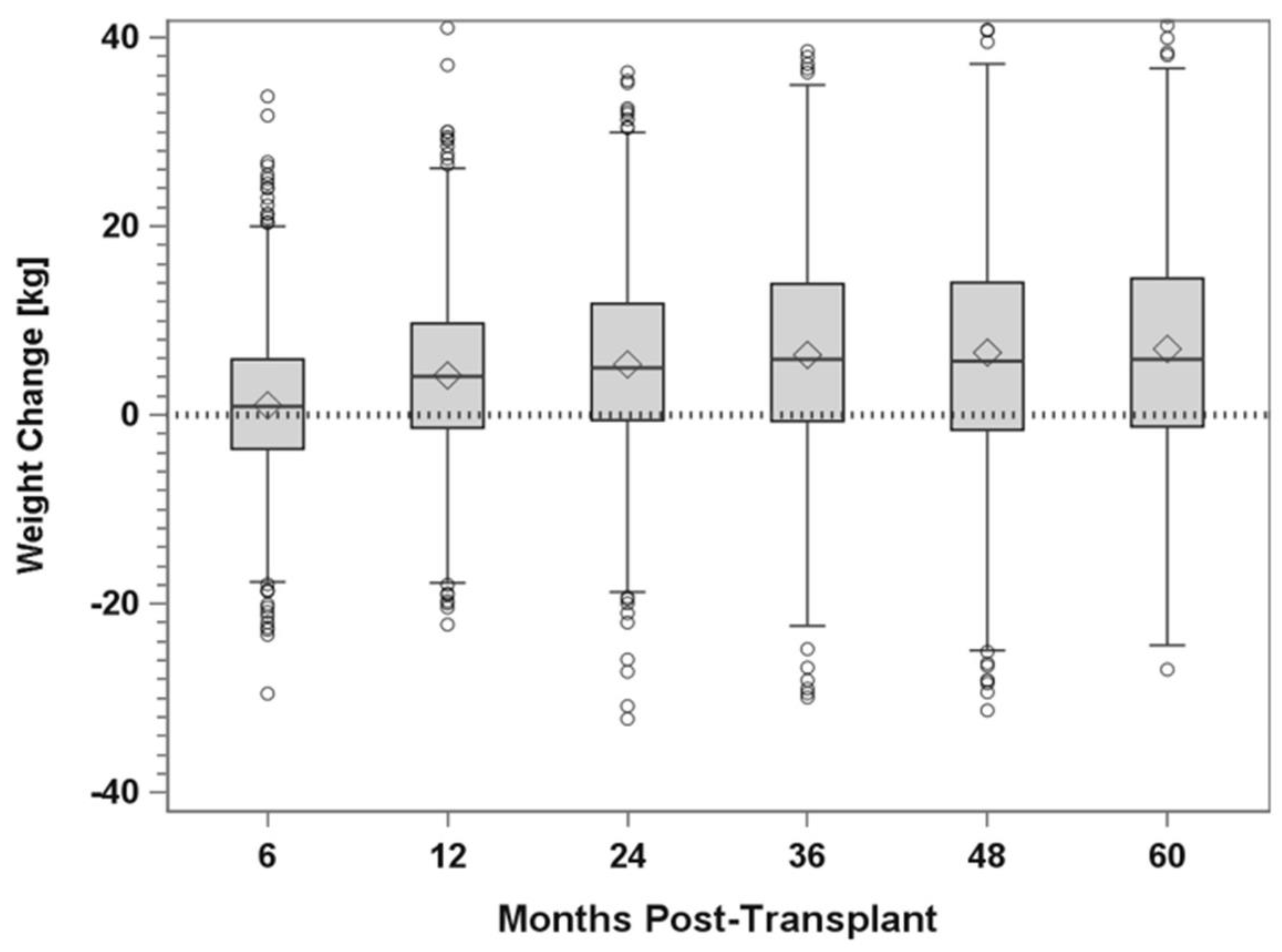
5.3. Registry Studies
6. Recipient Selection for T2DM
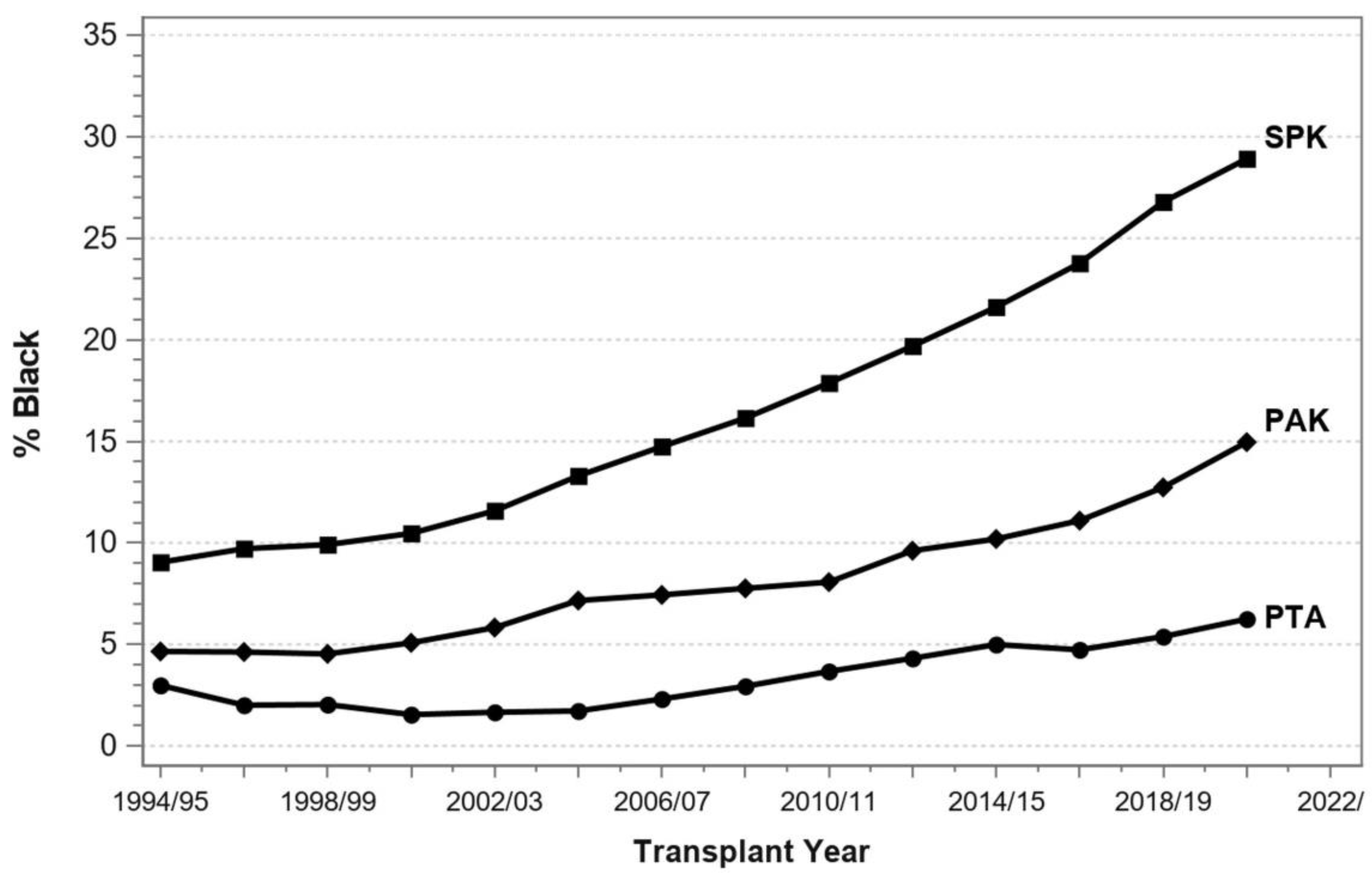
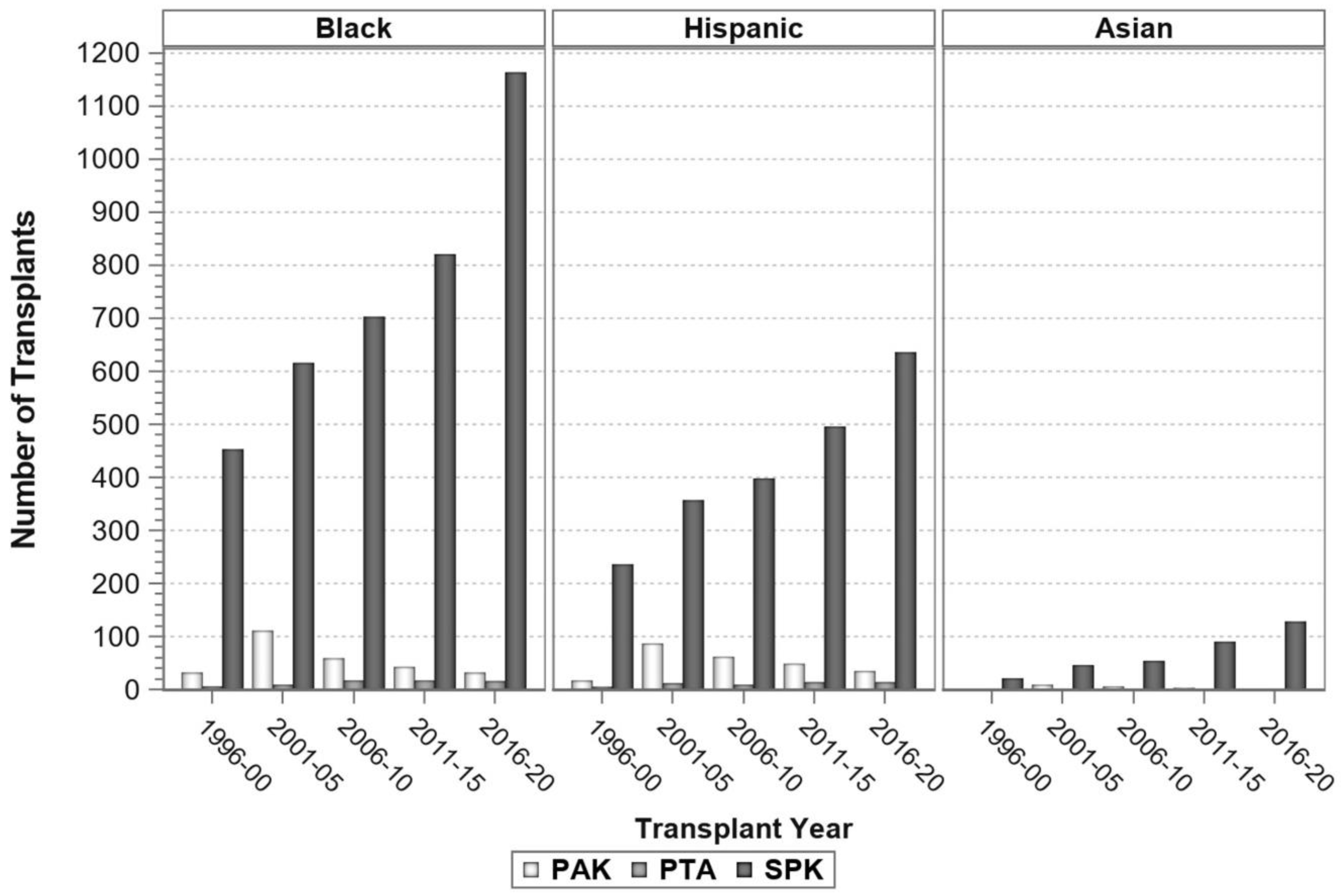
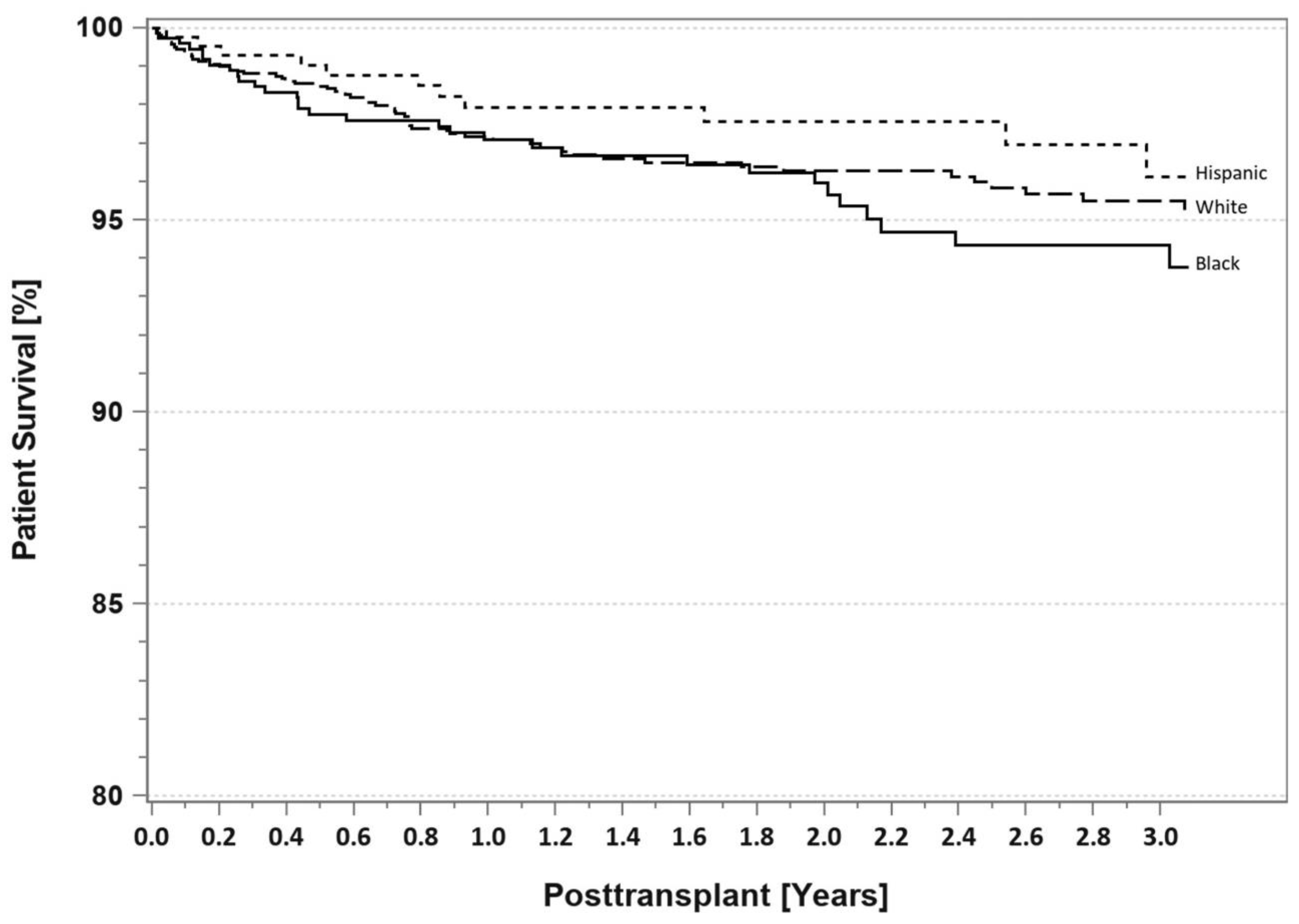
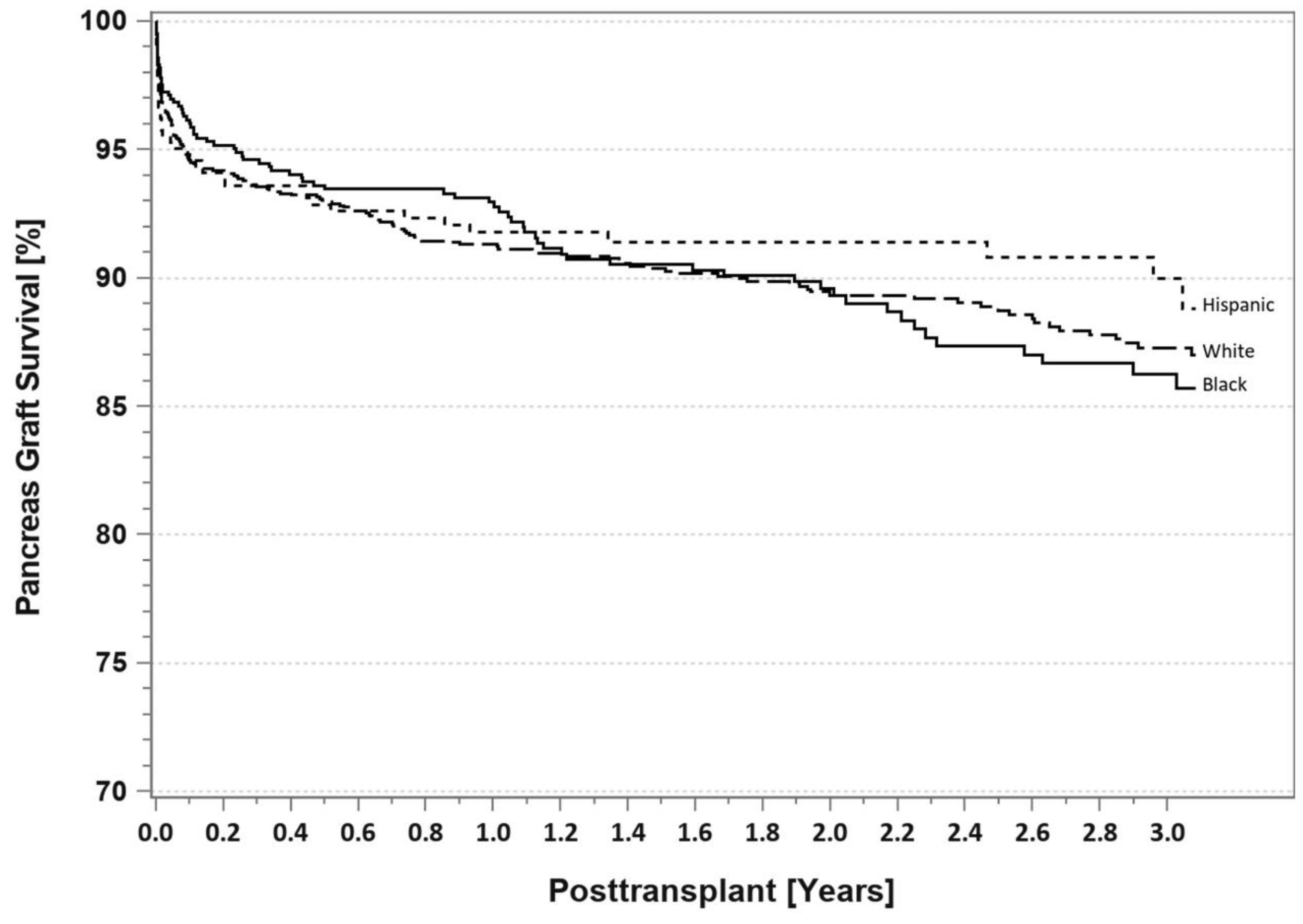
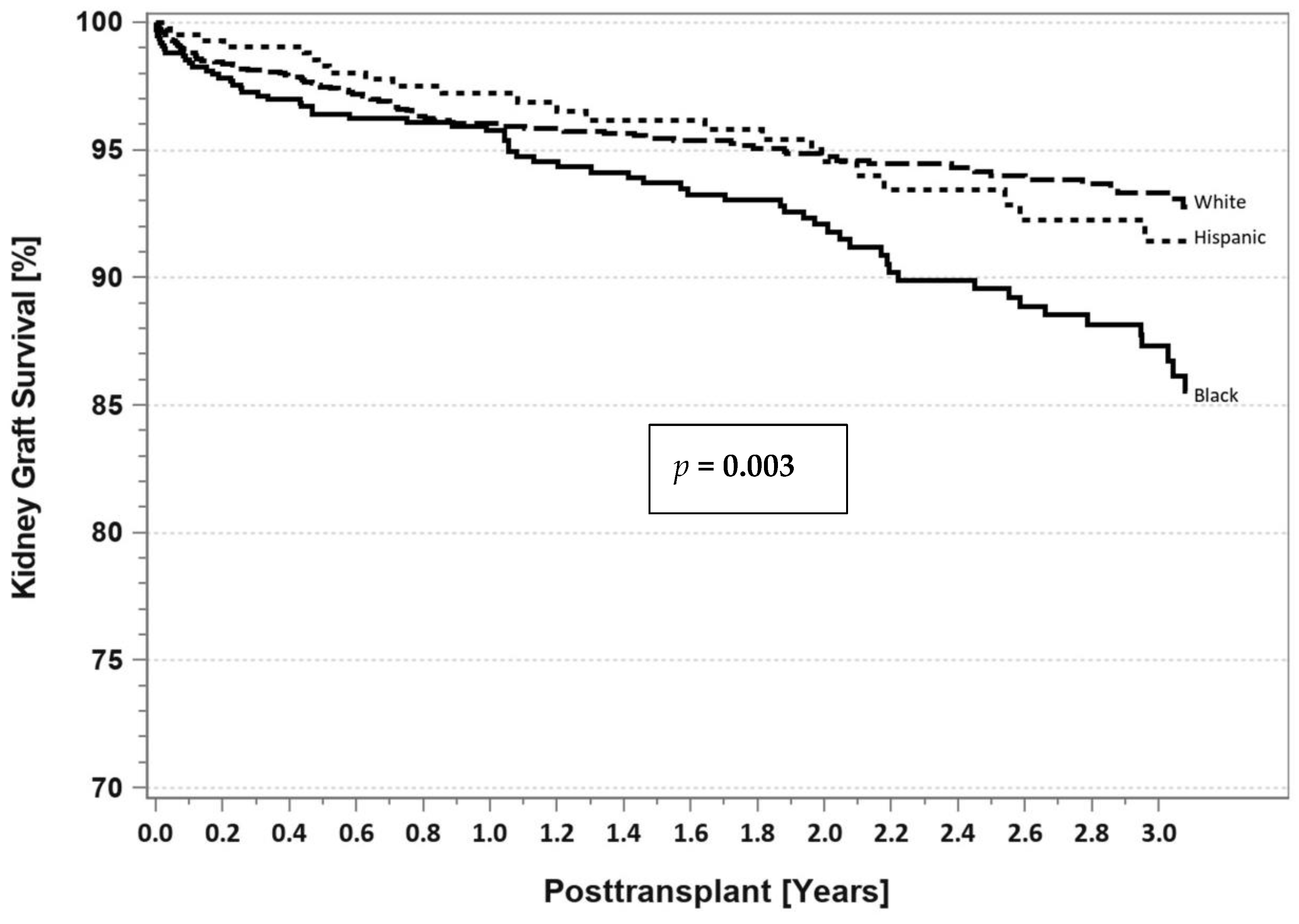
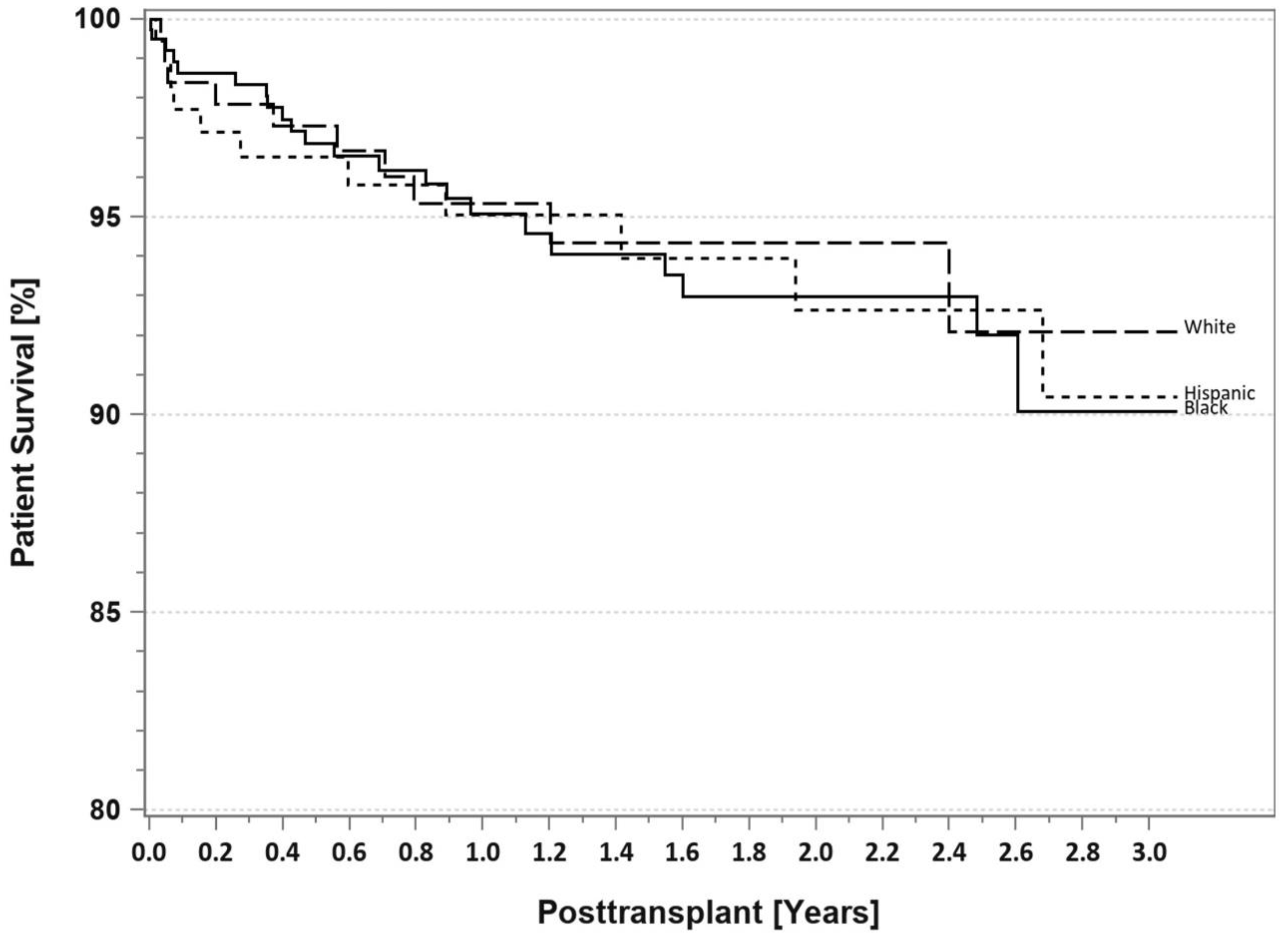
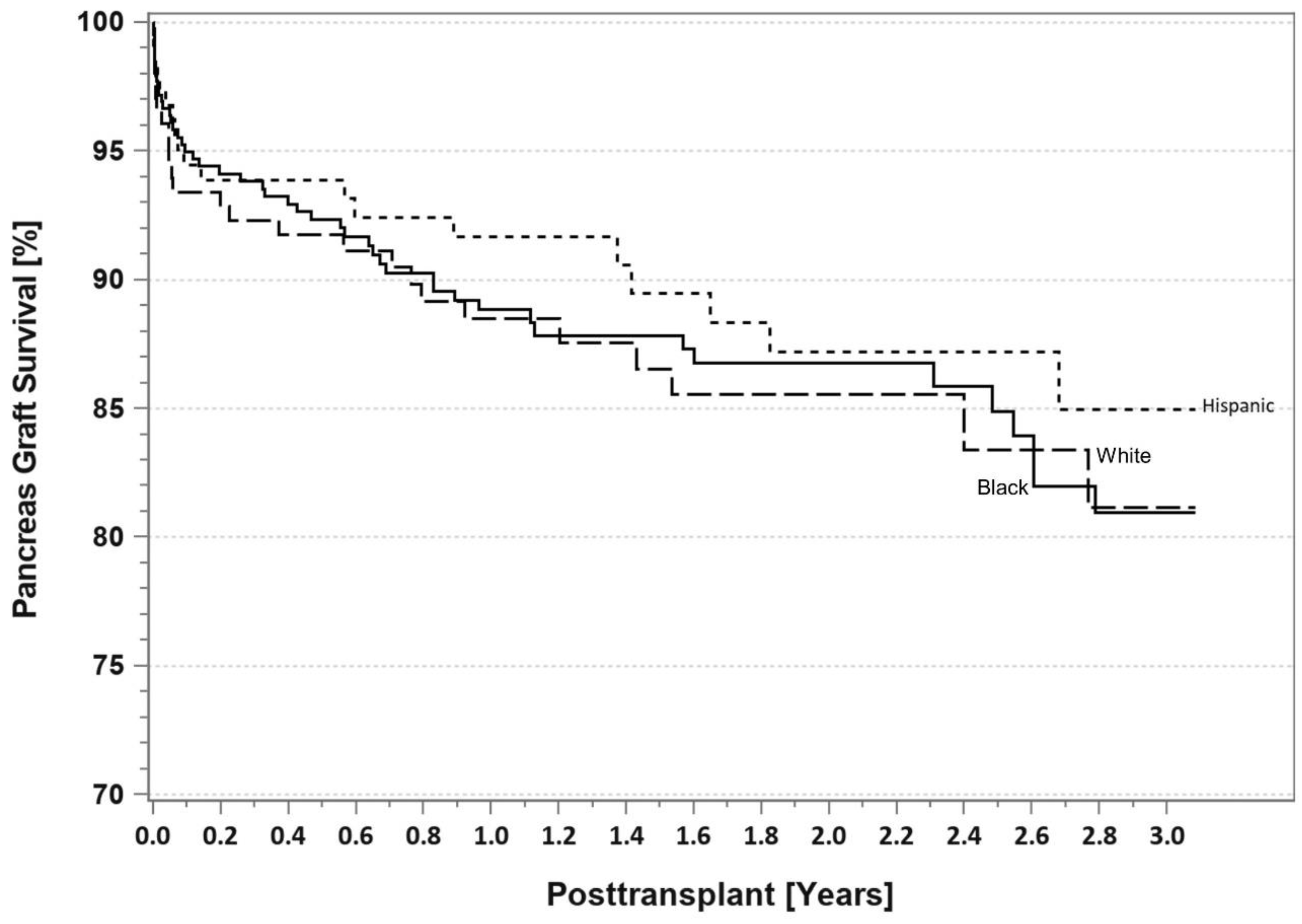
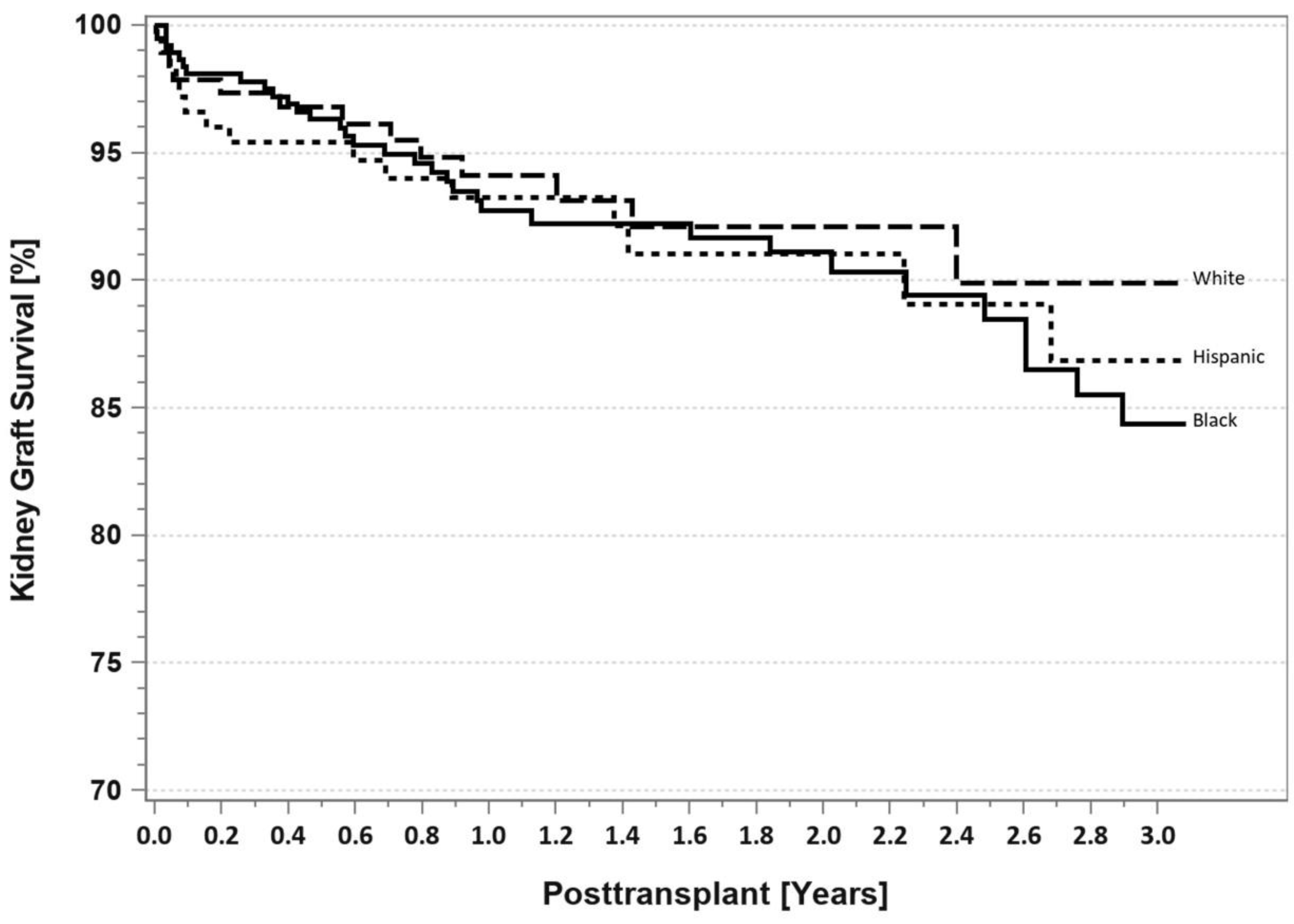
7. Pancreas Transplantation in Minorities
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | African American |
| BMI | Body mass index |
| Cp | C-peptide |
| DM | Diabetes mellitus |
| ESRD | End stage renal disease |
| HbA1c | Hemoglobin A1c |
| HLA | Human leukocyte antigen |
| IPTR | International Pancreas Transplant Registry |
| PAK | Pancreas after kidney |
| PRA | Panel reactive antibody |
| PTA | Pancreas transplant alone |
| PTx | Pancreas transplant |
| SPKT | Simultaneous pancreas-kidney transplant |
| SRTR | Scientific Registry of Transplant Recipients |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| UNOS | United Network for Organ Sharing |
| US | United States |
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef]
- IDF. 10th Edition. Available online: Diabetesatlas.org (accessed on 1 July 2022).
- Available online: http://www.cdc.gov/diabetes/data/statistics-report/index.html?CDC_AA_refVal (accessed on 1 July 2022).
- Duru, O.K.; Middleton, T.; Tewari, M.K.; Norris, K. The landscape of diabetic kidney disease in the United States. Curr. Diabetes Rep. 2018, 18, 14. [Google Scholar] [CrossRef]
- American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2020, 75 (Suppl. S1), A6–A7. [Google Scholar] [CrossRef]
- Larsen, J.L. Pancreas transplantation: Indications and consequences. Endocr. Rev. 2004, 25, 919–946. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Gruessner, R.W.G. Pancreas Transplantation for Patients with Type 1 and Type 2 Diabetes Mellitus in the USA Registry Report. Gastroenterol. Clin. N. Am. 2018, 47, 417–441. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Gruessner, R.W.G. The Current State of Pancreas Transplantation in the USA—A Registry Report. Curr. Transplant. Rep. 2018, 5, 304–314. [Google Scholar] [CrossRef]
- Updated International Pancreas Transplant Registry (IPTR) Data, Angelika Gruessner (Personal Communication). United Network for Organ Sharing (UNOS) Data. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ (accessed on 27 July 2022).
- Kandaswamy, R.; Stock, P.G.; Miller, J.; White, S.E.; Booker, S.E.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2020 Annual Data Report: Pancreas. Am. J. Transplant. 2022, 22 (Suppl. S2), 137–203. Available online: https://srtr.transplant.hrsa.gov (accessed on 1 July 2022). [CrossRef]
- Gruessner, A.C.; Sutherland, D.E. Pancreas Transplant Outcomes for United States (US) Cases Reported to the United Network for Organ Sharing (UNOS) and Non-US Cases Reported to the International Pancreas Transplant Registry (IPTR) as of October, 2000; Cecka, J.M., Terasaki, P.I., Eds.; UCLA Tissue Typing Laboratory: Los Angeles, CA, USA, 2001; pp. 45–72. [Google Scholar]
- Sener, A.; Cooper, M.; Bartlett, S.T. Is there a role for pancreas transplantation in type 2 diabetes mellitus? Transplantation 2010, 90, 121–123. [Google Scholar] [CrossRef]
- Orlando, G.; Stratta, R.J.; Light, J. Pancreas transplantation for type 2 diabetes mellitus. Curr. Opin. Organ. Transplant. 2011, 16, 110–115. [Google Scholar] [CrossRef]
- Cantarovich, D.; Murat, A.; Krempf, M.; Paineau, J.; Soulillou, J.P. Simultaneous pancreas and kidney transplantation in a type II (non-insulin-dependent) diabetic uremic patient requiring pregraft insulin therapy. Transplant. Proc. 1990, 22, 662. [Google Scholar]
- Ratner, R.E.; Gray, R.S.; Sasaki, T.; Light, J.A. Combined kidney-pancreas transplantation in patients with unrecognized NIDDM. Diabetologia 1996, 39 (Suppl. S1), 500. [Google Scholar]
- Stegall, M.; Wachs, M.; Kam, I. Successful pancreas transplantation in adult-onset diabetes mellitus (AODM). Diabetes 1997, 46 (Suppl. S1), A64. [Google Scholar]
- Sasaki, T.M.; Gray, R.S.; Ratner, R.E.; Currier, C.; Aquino, A.; Barhyte, D.Y.; Light, J.A. Successful long-term kidney-pancreas transplants in diabetic patients with high C-peptide levels. Transplantation 1998, 65, 1510–1512. [Google Scholar] [CrossRef]
- Light, J.A.; Sasaki, T.M.; Currier, C.B.; Barhyte, D.Y. Successful long term kidney-pancreas transplants regardless of C-peptide status and race. Transplantation 2001, 71, 152–154. [Google Scholar] [CrossRef]
- Pox, C.; Ritzel, R.; Büsing, M.; Meier, J.; Klempnauer, J.; Schmiegel, W.; Nauck, M. Combined pancreas and kidney transplantation in a lean type 2 diabetic patient. Effects on insulin secretion and sensitivity. Exp. Clin. Endocrinol. Diabetes 2002, 110, 420. [Google Scholar] [CrossRef]
- Friedman, A.L.; Friedman, E.A. Pancreas transplantation for type 2 diabetes at U.S. transplant centers. Diabetes Care 2002, 25, 1896. [Google Scholar] [CrossRef]
- Saudek, F.; Pruhova, S.; Boucek, P.; Lebl, J.; Adamec, M.; Ek, J.; Pedersen, O.; Hansen, T. Maturity-onset diabetes of the young with end-stage nephropathy: A new indication for simultaneous pancreas and kidney transplantation? Transplantation 2004, 77, 1298–1300. [Google Scholar] [CrossRef]
- Light, J.A.; Barhyte, D.Y. Simultaneous pancreas-kidney transplants in type I and type II diabetic patients with end-stage renal disease: Similar 10-year outcomes. Transplant. Proc. 2005, 37, 1283–1284. [Google Scholar] [CrossRef]
- Nath, D.S.; Gruessner, A.C.; Kandaswamy, R.; Gruessner, R.W.; Sutherland, D.E.; Humar, A. Outcomes of pancreas transplants for patients with type 2 diabetes mellitus. Clin. Transplant. 2005, 19, 792–797. [Google Scholar] [CrossRef]
- Singh, R.P.; Rogers, J.; Farney, A.C.; Hartmann, E.L.; Reeves-Daniel, A.; Doares, W.; Ashcraft, E.; Adams, P.L.; Stratta, R.J. Do pretransplant C-peptide levels influence outcomes in simultaneous kidney-pancreas transplantation? Transplant. Proc. 2008, 40, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, T.J. The accelerator hypothesis: A review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int. J. Obes. 2009, 33, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Alhamad, T.; Kunjai, R.; Wellen, J.; Brennan, D.C.; Wiseman, A.; Ruano, K.; Hicks, V.; Wang, M.; Schnitzler, M.A.; Chang, S.-H.; et al. Three-month pancreas graft function significantly influences survival following simultaneous pancreas-kidney transplantation in type 2 diabetes patients. Am. J. Transplant. 2020, 20, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, H.; Bodner, J.; Heilman, R.; Mulligan, D.; Moss, A.; Mekeel, K.; Mazur, M.; Hamawi, K.; Ray, R.; Beck, G.; et al. Outcomes after simultaneous pancreas and kidney transplantation and the discriminative ability of the C-peptide measurement pretransplant among type 1 and type 2 diabetes mellitus. Transplant. Proc. 2010, 42, 2650–2652. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.J.; Lawless, A.; Patel, S.J.; Gaber, A.O. Simultaneous kidney-pancreas transplantation for end-stage renal disease patients with insulin-dependent diabetes and detectable C-peptide. Transplant. Proc. 2010, 42, 4195–4196. [Google Scholar] [CrossRef]
- Light, J.; Tucker, M. Simultaneous pancreas kidney transplants in diabetic patients with end-stage renal disease: The 20-yr experience. Clin. Transplant. 2013, 27, E256–E263. [Google Scholar] [CrossRef]
- Sampaio, M.S.; Kuo, H.T.; Bunnapradist, S. Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1198–1206. [Google Scholar] [CrossRef]
- Kaufman, D.B.; Sutherland, D.E.R. Simultaneous pancreas-kidney transplants are appropriate in insulin-treated candidates with uremia regardless of diabetes type. Clin. J. Am. Soc. Nephrol. 2011, 6, 957–959. [Google Scholar] [CrossRef][Green Version]
- Wiseman, A.C.; Gralla, J. Simultaneous pancreas kidney transplant versus other kidney transplant options in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2012, 7, 656–664. [Google Scholar] [CrossRef]
- Cohen, D.J.; Ratner, L.E. Type 2 diabetes: The best transplant option is still uncertain. Clin. J. Am. Soc. Nephrol. 2012, 7, 530–532. [Google Scholar] [CrossRef]
- Scalea, J.R.; Cooper, M. Surgical strategies for type II diabetes. Transplant. Rev. 2012, 26, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Margreiter, C.; Resch, T.; Oberhuber, R.; Aigner, F.; Maier, H.; Sucher, R.; Schneeberger, S.; Ulmer, H.; Bösmüller, C.; Margreiter, R.; et al. Combined pancreas-kidney transplantation for patients with end-stage nephropathy caused by type-2 diabetes mellitus. Transplantation 2013, 95, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Bohannon, L.; Bry, W. 11 year experience with simultaneous pancreas and kidney transplantation in patients with type 1 and type 1 diabetes at a single center. Transplantation 2013, 96 (Suppl. S74), A382. [Google Scholar]
- Bry, W.; Mahanty, H.; Patel, P.; Peddi, V.R.; Hassoun, A.; Neidlinger, N.; Katznelson, S. Elevated BMI does not affect outcome in type II diabetics undergoing whole organ pancreas transplantation. Transplantation 2013, 96 (Suppl. S84), A419. [Google Scholar]
- Wiseman, A.C. Kidney transplant options for the diabetic patient. Transplant. Rev. 2013, 27, 112–116. [Google Scholar] [CrossRef]
- Ciancio, G.; Burke, G.W. Type 2 diabetes: Is pancreas transplantation an option? Curr. Diabetes Rep. 2014, 14, 542. [Google Scholar] [CrossRef]
- Weems, P.; Cooper, M. Pancreas transplantation in type II diabetes mellitus. World J. Transplant. 2014, 4, 216–221. [Google Scholar] [CrossRef]
- Mittal, S.; Gough, S.C. Pancreas transplantation: A treatment option for people with diabetes. Diabet. Med. 2014, 31, 512–521. [Google Scholar] [CrossRef]
- Stratta, R.J.; Rogers, J.; Farney, A.C.; Orlando, G.; El-Hennawy, H.; Gautreaux, M.D.; Reeves-Daniel, A.; Palanisamy, A.; Iskandar, S.S.; Bodner, J.K. Pancreas transplantation in C-peptide positive patients: Does type of diabetes really matter? J. Am. Coll. Surg. 2015, 22, 716–727. [Google Scholar] [CrossRef]
- Stratta, R.J.; Farney, A.C.; Orlando, G.; Rogers, J. Pancreas transplantation for type 2 diabetes mellitus: Who and why? Curr. Transpl. Rep. 2015, 2, 149–158. [Google Scholar] [CrossRef]
- Fourtounas, C. Transplant options for patients with type 2 diabetes and chronic kidney disease. World J. Transplant. 2014, 4, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Laftavi, M.R.; Pankewycz, O.; Gruessner, R.W.G. Simultaneous pancreas and kidney transplantation—Is it a treatment option for patients with type 2 diabetes mellitus? An analysis of the International Pancreas Transplant Registry. Curr. Diabetes Rep. 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.J.; Sayed, B.A.; Turgeon, N.A. Pancreas transplantation in unconventional recipients. Curr. Opin. Organ. Transplant. 2016, 21, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jung, C.H.; Choi, J.Y.; Kwon, H.W.; Jung, J.H.; Kim, Y.H.; Han, D.J. Long-term metabolic outcomes of functioning pancreas transplants in Type 2 diabetic recipients. Transplantation 2017, 101, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaoud, T.; Odorico, J.S.; Redfield, R.R., III. Pancreas transplantation in type 2 diabetes: Expanding the criteria. Curr. Opin. Organ. Transplant. 2018, 23, 454–460. [Google Scholar] [CrossRef]
- Gondolesi, G.E.; Aguirre, N.F.; Ramisch, D.A.; Mos, F.; Pedraza, N.; Fortunato, M.; Gutiérrez, L.; Fraguas, H.; Marrugat, R.; Rabin, G.; et al. Pancreas transplantation at a single Latin-American center: Overall results with type 1 and type 2 diabetes mellitus. Transplant. Proc. 2018, 50, 1475–1481. [Google Scholar] [CrossRef]
- Rohan, V.; Taber, D.; Palanisamy, A.; Mcgillicuddy, J.; Chavin, K.; Baliga, P.; Bratton, C. Impact of type 1 and type 2 diabetes mellitus on pancreas transplant outcomes. Exp. Clin. Transplant. 2019, 6, 796–802. [Google Scholar] [CrossRef]
- Andacoglu, O.M.; Himmler, A.; Geng, X.; Ahn, J.; Ghasemian, S.; Cooper, M.; Abrams, P. Comparison of glycemic control after pancreas transplantation for type 1 and type 2 diabetic recipients at a high volume center. Clin. Transplant. 2019, 33, e13656. [Google Scholar] [CrossRef]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef]
- Torabi, J.; Rocca, J.P.; Kestenbaum, E.; Ajaimy, M.; DeFeo, M.; Konicki, A.; Liriano-Ward, L.; Azzi, Y.; Pynadath, C.; Akalin, E.; et al. Preoperative C-peptide predicts weight gain after pancreas transplantation. Prog. Transplant. 2020, 39, 117–124. [Google Scholar] [CrossRef]
- Hau, H.M.; Jahn, N.; Brunotte, M.; Lederer, A.A.; Sucher, E.; Rasche, F.M.; Seehofer, D.; Sucher, R. Short and long-term metabolic outcomes in patients with type 1 and type 2 diabetes receiving a simultaneous pancreas kidney transplant. BMC Endocr. Disord. 2020, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.H.; Stalter, L.N.; Martinez, E.J.; Wang, J.F.; Welch, B.M.; Leverson, G.; Marka, N.; Al-Qaoud, T.; Mandelbrot, D.; Parajuli, S.; et al. Single center results of simultaneous pancreas-kidney transplantation in patients with type 2 diabetes. Am. J. Transplant. 2021, 21, 2810–2823. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cao, Y.; Wang, H.; Zhao, J.; Wang, Z.; Mo, C.; Shi, X.; Feng, G.; Song, W. Metabolic outcomes and renal function after simultaneous kidney/pancreas transplantation compared with kidney transplantation alone for type 2 diabetes mellitus. Transplant. Int. 2021, 34, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Lan, X.; Ni, K.; Li, L.; Fu, Y. Simultaneous pancreas and kidney transplantation for end-stage kidney disease patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2022, 2, 660–671. [Google Scholar] [CrossRef]
- Covic, A.M.C.; Schelling, J.R.; Constantiner, M.; Iyengar, S.K.; Sedor, J.R. Serum C-peptide concentrations poorly phenotype type 2 diabetic end-stage renal disease patients. Kidney Int. 2000, 58, 1742–1750. [Google Scholar] [CrossRef]
- Esmatjes, E.; Fernandez, C.; Rueda, S.; Nicolau, J.; Chiganer, G.; Ricart, M.J.; Junca, E.; Fernández-Cruz, L. The utility of the C-peptide in the phenotyping of patient candidates for pancreas transplantation. Clin. Transplant. 2007, 21, 358–362. [Google Scholar] [CrossRef]
- Wang, L.; Lovejoy, N.F.; Faustman, D.L. Persistence of prolonged C-peptide production in Type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care 2012, 35, 465–470. [Google Scholar]
- Dean, P.G.; Kudva, R.; Larson, T.S.; Kremers, W.K.; Stegall, M.D. Postransplant diabetes mellitus after pancreas transplantation. Am. J. Transplant. 2008, 8, 175–182. [Google Scholar]
- Egidi, M.F.; Lin, A.; Bratton, C.F.; Baliga, P.K. Prevention and management of hyperglycemia after pancreas transplantation. Curr. Opin. Organ. Transplant. 2008, 13, 72–78. [Google Scholar] [CrossRef]
- Neidlinger, N.; Singh, N.; Klein, C.; Odorico, J.; Del Rio, A.M.; Becker, Y.; Sollinger, H.; Pirsch, J. Incidence of and risk factors for posttransplant diabetes mellitus after pancreas transplantation. Am. J. Transplant. 2010, 10, 398–406. [Google Scholar] [CrossRef]
- Kandaswamy, R.; Stock, P.G.; Skeans, M.A.; Gustafson, S.K.; Sleeman, E.F.; Wainright, J.L.; Carrico, R.J.; Ghimire, V.; Snyder, J.J.; Israni, A.K.; et al. OPTN/SRTR 2011 Annual Data Report: Pancreas. Am. J. Transplant. 2013, 13 (Suppl. S1), 47–72. [Google Scholar] [CrossRef]
- Gruessner, A.C. 2011 update on pancreas transplantation: Comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. 2011, 8, 6–16. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Gruessner, R.W. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. 2016, 13, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaoud, T.; Redfield, R.; Odorico, J. The case for simultaneous pancreas and kidney (SPK) transplantation for obese T2DM patients. Rev. Diabet. Stud. 2017, 14, 103, IPITA Congress abstracts (PO1.4). [Google Scholar] [CrossRef]
- Manzanares, J.; Conget, I.; Gonzalez-Clemente, J.; Vidal, J.; Rodríguez-Villar, C.; Rojas, I.; Fernández-Fernández, F.; Casamitjana, R.; Gomis, R. Insulin treatment in diabetes mellitus type II: The usefulness of the breakfast test. Med. Clin. 1995, 104, 761–765. [Google Scholar]
- Gurram, V.; Gurung, K.; Rogers, J.; Farney, A.C.; Orlando, G.; Jay, C.; Reeves-Daniel, A.; Mena-Gutierrez, A.; Sakhovskaya, N.; Doares, W.; et al. Do pretransplant C-peptide levels influence outcomes in simultaneous pancreas-kidney transplantation? A matched case-control study. Clin. Transplant. 2021, 35, e14498. [Google Scholar] [CrossRef]
- Stratta, R.J.; Taylor, R.J.; Wahl, T.O.; Duckworth, W.C.; Gallagher, T.F.; Knight, T.F.; Fischer, J.L.; Neumann, T.V.; Miller, S.; Langnas, A.N.; et al. Recipient selection and evaluation for vascularized pancreas transplantation. Transplantation 1993, 55, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.R.; Limwattananon, C.; Matthees, B.J. Quality of life after pancreas transplantation: A review. Clin. Transplant. 1998, 12, 351–361. [Google Scholar]
- Sureshkumar, K.K.; Mubin, T.; Mikhael, N.; Kashif, M.A.; Nghiem, D.D.; Marcus, R.J. Assessment of quality of life after simultaneous pancreas-kidney transplantation. Am. J. Kidney Dis. 2002, 39, 1300–1306. [Google Scholar] [CrossRef]
- Joseph, J.T.; Baines, L.S.; Morris, M.C.; Jindal, R.M. Quality of life after kidney and pancreas transplantation: A review. Am. J. Kidney Dis. 2003, 42, 431–445. [Google Scholar] [CrossRef]
- Stratta, R.J. Pancreas transplantation: Long-term aspects and effect on quality of life. In Pancreas and Islet Transplantation; Hakim, N.S., Stratta, R.J., Gray, D., Eds.; Oxford University Press: New York, NY, USA, 2002; p. 229. [Google Scholar]
- Pera, P.I.; Vasallo, J.M.; Rabasa, A.T.; Salinas, F.O.; Pérez, L.F.C.; Brulles, M.J.R. Quality of life in simultaneous pancreas-kidney transplant recipients. Clin. Transplant. 2009, 23, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Outerelo, C.; Malheiro, J.; Fonseca, I.M.; Henriques, A.C.; Dias, L.S.; Rodrigues, A.S.; Cabrita, A.M.; Noronha, I.L. Health-related quality of life may improve after transplantation in pancreas-kidney recipients. Clin. Transplant. 2015, 29, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, M.; Cooper, M.; Abrams, P. Quality of life after pancreas transplantation: Time to look again. Curr. Opin. Organ. Transplant. 2019, 24, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Posegger, K.; Linhares, M.; Mucci, S.; Romano, T.M.; Gonzalez, A.M.; Netto, A.A.S.; Rangel, B.; Filho, G.D.J.L.; Silva-Junior, H.T.; Medina-Pestana, J. The quality of life in type 1 diabetic patients with end-stage kidney disease before and after simultaneous pancreas-kidney transplantation: A single center prospective study. Transplant. Int. 2020, 33, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, A.; Cinnirella, M.; Bayfield, J.; Watson, C.J.E.; Oniscu, G.C.; Draper, H.; Tomson, C.R.V.; Ravanan, R.; Johnson, R.J.; Forsythe, J.; et al. Changes in quality of life, health status and other patient-reported outcomes following simultaneous pancreas and kidney transplantation (SPKT): A quantitative and qualitative analysis within a UK-wide programme. Transplant. Int. 2020, 33, 1230–1243. [Google Scholar] [CrossRef]
- Nijhoff, M.; Hovens, J.G.F.M.; Huisman, S.D.; Ringers, J.; Rabelink, T.; de Fijter, H.J.; van der Boog, P.J.; de Koning, E.J. Psychological symptoms and quality of life after simultaneous kidney and pancreas transplantation. Transplant. Direct 2020, 6, e552. [Google Scholar] [CrossRef]
- Shingde, R.; Calis, V.; Craig, J.C.; Chapman, J.R.; Webster, A.C.; Pleass, H.; O’Connell, P.J.; Allen, R.; Robertson, P.; Yuen, L.; et al. Relative survival and quality of life benefits of pancreas-kidney transplantation, deceased kidney transplantation and dialysis in type 1 diabetes mellitus—A probabilistic simulation model. Transplant. Int. 2020, 33, 1393–1404. [Google Scholar] [CrossRef]
- Sutherland, D.E.R.; Gruessner, R.W.G.; Dunn, D.L.; Matas, A.J.; Humar, A.; Kandaswamy, R.; Mauer, S.M.; Kennedy, W.R.; Goetz, F.C.; Robertson, R.P.; et al. Lessons learned from more than 1000 pancreas transplants at a single institution. Ann. Surg. 2001, 233, 463–501. [Google Scholar] [CrossRef]
- Manske, C.L.; Thomas, W.; Wang, Y.; Wilson, R.F. Screening diabetic transplant candidates for coronary artery disease: Identification of a low risk subgroup. Kidney Int. 1993, 44, 617–621. [Google Scholar] [CrossRef]
- Lin, K.; Stewart, D.; Cooper, S.; Davis, C.L. Pre-transplant cardiac testing for kidney-pancreas transplant candidates and association with cardiac outcomes. Clin. Transplant. 2001, 15, 269–275. [Google Scholar] [CrossRef]
- Ma, I.W.Y.; Valantine, H.A.; Shibata, A.; Waskerwitz, J.; Dafoe, D.C.; Alfrey, E.J.; Tan, J.C.; Millan, M.; Busque, S.; Scandling, J.D. Validation of a screening protocol for identifying low-risk candidates with type 1 diabetes mellitus for kidney with or without pancreas transplantation. Clin. Transplant. 2006, 20, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Eller, K.; Kniepeiss, D.; Rosenkranz, A.R. Preoperative risk evaluation: Where is the limit for recipients of a pancreatic graft? Curr. Opin. Organ. Transplant. 2013, 18, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Masson, P.; Turner, R.M.; Lord, S.W.; Baines, L.A.; Craig, J.C.; Webster, A.C. Prognostic value of cardiac tests in potential kidney transplant recipients: A systematic review. Transplantation 2015, 99, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Knapper, J.T.; Raval, Z.; Harinstein, M.E.; Friedewald, J.J.; Skaro, A.I.; Abecassis, M.I.; Ali, Z.A.; Gheorghiade, M.; Flaherty, J.D. Assessment and management of coronary artery disease in kidney and pancreas transplant candidates. J. Cardiovasc. Med. 2019, 20, 51–58. [Google Scholar] [CrossRef]
- Mangus, R.S.; Powelson, J.; Kinsella, S.B.; Farar, D.T.; Creal, C.A.; Fridell, J.A. Pretransplant coronary artery disease associated with worse clinical outcomes in pancreas transplantation. Clin. Transplant. 2013, 27, E442–E447. [Google Scholar] [CrossRef]
- Laurence, J.M.; Barbas, A.S.; Sapisochin, G.; Marquez, M.A.; Bazerbachi, F.; Selzner, M.; Norgate, A.; McGilvray, I.D.; Schiff, J.; Ross, H.; et al. The significance of pre-operative coronary interventions on outcome after pancreas transplantation. Clin. Transplant. 2016, 30, 233–240. [Google Scholar] [CrossRef]
- Kim, J.; Schulman-Marcus, J.; Watkins, A.C.; Feldman, D.N.; Swaminathan, R.; Lee, J.B.; Muthukumar, T.; Serur, D.; Kim, L.; Hartono, C. In-hospital cardiovascular complications after pancreas transplantation in the United States from 2003 to 2012. Am. J. Cardiol. 2017, 129, 682–687. [Google Scholar] [CrossRef]
- Hathaway, D.K.; El-Gebely, S.; Cardoso, S.; Elmer, D.S.; Gaber, A.O. Autonomic cardiac dysfunction in diabetic transplant recipients succumbing to sudden cardiac death. Transplantation 1995, 59, 634–637. [Google Scholar] [CrossRef]
- Stratta, R.J. Mortality after vascularized pancreas transplantation. Surgery 1998, 124, 823–830. [Google Scholar] [CrossRef]
- Freise, C.E.; Stock, P.G.; Melzer, J.S. Increased morbidity and mortality of simultaneous pancreas-renal transplantation in patients over 49 years of age. Transplant. Proc. 1998, 30, 292. [Google Scholar] [CrossRef]
- Ablorsu, E.; Ghazanfar, A.; Mehra, S.; Campbell, B.; Riad, H.; Pararajasingam, R.; Parrott, N.; Picton, M.; Augustine, T.; Tavakoli, A. Outcome of pancreas transplantation in recipients older than 50 years: A single-centre experience. Transplantation 2008, 86, 1511–1514. [Google Scholar] [CrossRef]
- Schenker, P.; Vonend, O.; Krüger, B.; Klein, T.; Michalski, S.; Wunsch, A.; Krämer, B.K.; Viebahn, R. Long-term results of pancreas transplantation in patients older than 50 years. Transplant. Int. 2011, 24, 136–142. [Google Scholar] [CrossRef]
- Afaneh, C.; Rich, B.S.; Aull, M.J.; Hartono, C.; Leeser, D.B.; Kapur, S. Pancreas transplantation: Does age increase morbidity? J. Transplant. 2011, 2011, 596801. [Google Scholar] [CrossRef]
- Shah, A.P.; Mangus, R.S.; Powelson, J.A.; Samy, K.P.; Taber, T.E.; Goble, M.L.; Fridell, J.A. Impact of recipient age on whole organ pancreas transplantation. Clin. Transplant. 2013, 27, E49–E55. [Google Scholar] [CrossRef] [PubMed]
- Siskind, E.; Maloney, C.; Akerman, M.; Alex, A.; Ashburn, S.; Barlow, M.; Siskind, T.; Bhaskaran, M.; Ali, N.; Basu, A.; et al. An analysis of pancreas transplantation outcomes based on age groupings—An update of the UNOS database. Clin. Transplant. 2014, 28, 990–994. [Google Scholar] [CrossRef]
- Laurence, J.M.; Marquez, M.A.; Seal, J.B.; Sapisochin, G.; Bazerbachi, F.; Selzner, M.; Norgate, A.; Greig, P.D.; McGilvray, I.; Schiff, J.; et al. The effect of recipient age on outcome after pancreas transplantation. Transplantation 2015, 99, e13–e14. [Google Scholar] [CrossRef]
- Scalea, J.R.; Redfield, R.R., III; Arpali, E.; Leverson, G.; Sollinger, H.W.; Kaufman, D.B.; Odorico, J.S. Pancreas transplantation in older patients is safe, but patient selection is paramount. Transplant. Int. 2016, 29, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Schenker, P. The elderly pancreas transplant recipient. Transplant. Int. 2016, 29, 807–809. [Google Scholar] [CrossRef]
- Mittal, S.; Smilevska, R.; Franklin, R.; Hammer, C.; Knight, S.; Vrakas, G.; Reddy, S.; Gilbert, J.; Quiroga, I.; Sharples, E.; et al. An analysis of the association between older recipient age and outcomes after whole-organ pancreas transplantation—A single-centre retrospective study. Transplant. Int. 2020, 33, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Alejo, J.; Rogers, J.; Farney, A.C.; Orlando, G.; Jay, C.; Reeves-Daniel, A.; Mena-Gutierrez, A.; Sakhovskaya, N.; Doares, W.; et al. Recipient age and outcomes following simultaneous pancreas-kidney transplantation in the new millennium: Single-center experience and review of the literature. Clin. Transplant. 2021, 38, e14302. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner Gl Henry, M.L.; Elkhammas, E.; Wilson, G.A.; Tso, P.; Davies, E.; Qiu, W.; Ferguson, R.M. Obesity as a risk factor after combined pancreas/kidney transplantation. Transplantation 1995, 60, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Chavin, K.D.; Baliga, P.K.; Lin, A.; Emovon, O.; Afzal, F.; Ashcraft, E.E.; Baillie, G.M.; Taber, D.J.; Rajagopalan, P.R. Influence of mild obesity on outcome of simultaneous pancreas and kidney transplantation. J. Gastrointest. Surg. 2003, 7, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Hanish, S.I.; Petersen, R.P.; Collins, B.H.; Tuttle-Newhall, J.; Marroquin, C.E.; Kuo, P.C.; Butterly, D.W.; Smith, S.R.; Desai, D.M. Obesity predicts increased overall complications following pancreas transplantation. Transplant. Proc. 2005, 37, 3564–3566. [Google Scholar] [CrossRef]
- Sampaio, M.S.; Reddy, P.N.; Kuo, H.T.; Poommipanit, N.; Cho, Y.W.; Shah, T.; Bunnapradist, S. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010, 89, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Afaneh, C.; Rich, B.; Aull, M.J.; Hartono, C.; Kapur, S.; Leeser, D.B. Pancreas transplantation considering the spectrum of body mass indices. Clin. Transplant. 2011, 25, E520–E529. [Google Scholar] [CrossRef] [PubMed]
- Fridell, J.A.; Mangus, R.S.; Tabor, T.E.; Goble, M.L.; Milgrom, M.L.; Good, J.; Vetor, R.; Powelson, J.A. Growth of a nation part II: Impact of recipient obesity on whole-organ pancreas transplantation. Clin. Transplant. 2011, 25, E366–E374. [Google Scholar] [CrossRef]
- Laurence, J.M.; Marquez, M.A.; Bazerbachi, F.; Seal, J.B.; Selzner, M.; Norgate, A.; McGilvray, I.D.; Schiff, J.; Cattral, M.S. Optimizing pancreas transplantation outcomes in obese recipients. Transplantation 2015, 99, 1282–1287. [Google Scholar] [CrossRef]
- Bedat, B.; Niclauss, N.; Jannot, A.S.; Andres, A.; Toso, C.; Morel, P.; Berney, T. Impact of recipient body mass index on short-term and long-term survival of pancreatic grafts. Transplantation 2015, 99, 94–99. [Google Scholar] [CrossRef]
- Porubsky, M.; Powelson, J.A.; Selzer, D.J.; Mujtaba, M.A.; Taber, T.; Carnes, K.L.; Fridell, J.A. Pancreas transplantation after bariatric surgery. Clin. Transplant. 2012, 26, E1–E6. [Google Scholar]
- Bonatti, H.; Schmitt, T.; Northup, J.; Schirmer, B.; Swenson, B.; Pruett, T.; Sawyer, R.; Brayman, K. Laparoscopic gastric banding in a kidney-pancreas transplant recipient with new onset type II diabetes mellitus associated with morbid obesity. Clin. Transplant. 2008, 22, 829–832. [Google Scholar]
- Zelones, J.; Biswas, O.; Mehran, A. Laparoscopic sleeve gastrectomy after simultaneous pancreas-kidney transplant. Am. Surg. 2012, 78, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Elli, E.F.; Gonzalez-Heredia, R.; Sanchez-Johnsen, L.; Patel, N.; Garcia-Roca, R.; Oberholzer, J. Sleeve gastrectomy surgery in obese post-organ transplantation. Surg. Obes. Relat. Dis. 2016, 12, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Boggi, U.; Signori, S.; Vistoli, F.; Amorese, G.; Consani, G.; De Lio, N.; Perrone, V.; Croce, C.; Marchetti, P.; Cantarovich, D.; et al. Current perspectives on laparoscopic robot-assisted pancreas and pancreas-kidney transplantation. Rev. Diabet. Stud. 2011, 8, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Spaggiari, M.; Tzvetanov, I.; Oberholzer, J. Robotic pancreas transplantation in a type 1 diabetic patient with morbid obesity: A case report. Medicine 2017, 96, e5847. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, L.A.; Oberholzer, J. Robotic pancreas transplantation: The state of the art. Curr. Opin. Organ. Transplant. 2018, 23, 423–427. [Google Scholar] [CrossRef]
- Isaacs, R.; Lobo, P.I.; Nock, S.L.; Hanson, J.A.; Ojo, A.O.; Pruett, T.L. Racial disparities in access to simultaneous pancreas-kidney transplantation. Am. J. Kidney Dis. 2000, 36, 526–533. [Google Scholar] [CrossRef]
- Young, C.Y.; Gaston, R.S. Renal transplantation in Black Americans. N. Engl. J. Med. 2000, 343, 1545–1552. [Google Scholar] [CrossRef]
- Young, C.Y.; Kew, C. Health disparities in transplantation: Focus on complexity and challenge of renal transplantation in African Americans. Med. Clin. N. Am. 2005, 89, 1003–1031. [Google Scholar] [CrossRef]
- Melancon, J.K.; Kucirka, L.M.; Boulware, L.E.; Powe, N.R.; Locke, J.E.; Montgomery, R.A.; Segev, D.L. Impact of Medicare coverage on disparities in access to simultaneous pancreas and kidney transplantation. Am. J. Transplant. 2009, 9, 2785–2791. [Google Scholar] [CrossRef][Green Version]
- Crook, E.D.; Patel, S.R. Diabetic nephropathy in African-American patients. Curr. Diabetes Rep. 2004, 4, 455–461. [Google Scholar] [CrossRef]
- Kirk, J.K.; D’Agostino, R.B., Jr.; Bell, R.A.; Passmore, L.V.; Bonds, D.E.; Karter, A.J.; Narayan, K.M. Disparities in HbA1c levels between African-American and non-Hispanic White adults with diabetes: A meta-analysis. Diabetes Care 2006, 29, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Douzdjian, V.; Bhaskar, S.S.; Baliga, P.K.; Gugliuzza, K.; Rajagopalan, P.R. Effect of race on outcome after kidney and kidney-pancreas transplantation in type 1 diabetic patients. Diabetes Care 1997, 20, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Douzdjian, V.; Thacker, L.R.; Blanton, J.W. Effect of race on outcome following kidney-pancreas transplantation in type 1 diabetics: The South-Eastern Organ Procurement Foundation experience. Clin. Transplant. 1997, 11, 470–475. [Google Scholar]
- Burke, G.W.; Ciancio, G.; Colona, J.; Roth, D.; Miller, J. African-Americans with type 1 insulin-dependent diabetes mellitus and end stage renal disease: Results after simultaneous pancreas kidney transplantation. Transplant. Proc. 1997, 29, 3715–3716. [Google Scholar] [CrossRef]
- Lo, A.; Stratta, R.J.; Egidi, M.F.; Shokouh-Amiri, M.H.; Grewal, H.P.; Kizilisik, A.T.; Alloway, R.R.; Gaber, L.W.; Gaber, A.O. Outcome of simultaneous kidney-pancreas transplantation in African-American recipients: A case control study. Transplant. Proc. 2001, 33, 1675–1677. [Google Scholar] [CrossRef]
- Rogers, J.; Baliga, P.K.; Chavin, K.D.; Lin, A.; Emovon, O.; Afzal, F.; Baillie, G.M.; Ashcraft, E.E.; Rajagopalan, P.R. Effect of ethnicity on outcome of simultaneous pancreas and kidney transplantation. Am. J. Transplant. 2003, 3, 1278–1288. [Google Scholar] [CrossRef]
- Rogers, J.; Stratta, R.J.; Alloway, R.R.; Lo, A.; Hodge, E. African-American ethnicity is no longer a risk factor for early adverse outcomes in simultaneous kidney-pancreas transplantation with contemporary immunosuppression. Transplant. Proc. 2004, 36, 1055–1057. [Google Scholar] [CrossRef]
- Rogers, J.; Stratta, R.J.; Lo, A.; Alloway, R.R. Inferior late functional and metabolic outcomes in African American simultaneous kidney-pancreas recipients. Transplant. Proc. 2005, 37, 3552–3554. [Google Scholar] [CrossRef]
- Zhang, R.; Florman, S.; Devidoss, S.; Zarifian, A.; Yau, C.L.; Paramesh, A.; Killackey, M.; Alper, B.; Fonseca, V.; Slakey, D. A comparison of long-term survivals of simultaneous pancreas-kidney transplant between African American and Caucasian recipients with basiliximab induction therapy. Am. J. Transplant. 2007, 7, 1815–1821. [Google Scholar] [CrossRef]
- Zhang, R.; Florman, S.; Paramesh, A.; Islam, T.; Zarifian, A.; Simon, E.; Hamm, L.L.; Slakey, D. Pancreas transplantation in African American patients undergoing basiliximab induction. Am. J. Med. Sci. 2009, 337, 307–311. [Google Scholar] [CrossRef]
- Luan, F.L.; Kommareddi, M.; Cibrik, D.M.; Samaniego, M.; Ojo, A.O. Influence of recipient race on the outcome of simultaneous pancreas and kidney transplantation. Am. J. Transplant. 2010, 10, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T.; Liu, R.; Oliver, M.; DeLeonibus, A.; Mei, J.; White, D.; Siskind, E.; Ortiz, J. Simultaneous pancreas and kidney transplantation is associated with inferior long-term outcomes in African Americans. Pancreas 2018, 47, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Young, C.J.; MacLennan, P.A.; Mannon, E.C.; Reed, R.D.; Shelton, B.A.; Hanaway, M.J.; Agarwal, G.; Gaston, R.S.; Julian, B.A.; Kew, C.E.; et al. Redefining the influence of ethnicity on simultaneous kidney-pancreas transplantation outcomes. Ann. Surg. 2020, 271, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, H.M.; Taber, D.J.; Nadig, S.; Patel, N.; Lin, A.; Baliga, P.K.; Rohan, V.S. The impact of race on metabolic, graft, and patient outcomes after pancreas transplantation. Am. J. Surg. 2022, 223, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, J.; Liu, J.; Wang, L. Race does not predict pancreas graft failure after pancreas transplantation in the modern era. Clin. Transplant. 2022, 36, e14576. [Google Scholar] [CrossRef]
- McElroy, L.; Fridell, J. Impact of race on pancreas transplant outcomes in the current era: It is not all black and white. Clin. Transplant. 2022, 36, e14615. [Google Scholar] [CrossRef]
- Rogers, J.; Jay, C.J.; Farney, A.C.; Orlando, G.; Jacobs, M.L.; Harriman, D.; Gurram, V.; Sharda, B.; Gurung, K.; Reeves-Daniel, A.; et al. Simultaneous pancreas-kidney transplantation in Caucasian versus African-American patients: Does recipient race influence outcomes. Clin. Transplant. 2022, 36, e14599. [Google Scholar] [CrossRef]
- Feyssa, E.; Jones-Burton, C.; Ellison, G.; Philosophe, B.; Howell, C. Racial-ethnic disparity in kidney transplantation outcomes: Influence of donor and recipient characteristics. J. Natl. Med. Assoc. 2009, 101, 111–115. [Google Scholar] [CrossRef]
- Ciancio, G.; Burke, G.W.; Gomez, C.; Olson, L.; Esquenazi, V.; Miller, J. Simultaneous pancreas-kidney transplantation in Hispanic recipients with type 1 diabetes mellitus and end-stage renal disease. Transplant. Proc. 1997, 29, 3717. [Google Scholar] [CrossRef]
- Perosa, M.; Boggi, U.; Cantarovich, D.; Robertson, P. Pancreas transplantation outside the USA: An update. Curr. Opin. Organ. Transplant. 2011, 16, 135–141. [Google Scholar] [CrossRef]
- Ishibashi, M.; Ito, T.; Sugitani, A.; Furukawa, H.; Sekiguchi, S.; Gotoh, M.; Teraoka, S.; Sato, Y.; Matsuno, N.; Kenmochi, S.; et al. Present status of pancreas transplantation in Japan: Donation predominantly from marginal donors and modified surgical technique—Report of Japan pancreas transplantation registry. Transplant. Proc. 2008, 40, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Perosa, M.; Crescentini, F.; Noujaim, H.; Mota, L.T.; Branez, J.R.; Ianhez, L.E.; Ferreira, G.; de Oliveira, R.A.; Genzini, T. Over 500 pancreas transplants by a single team in Sao Paulo, Brazil. Clin. Transplant. 2011, 25, E422–E429. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jung, J.H.; Shin, S.; Kim, Y.H.; Han, D.J. Association between the pancreas transplantation and survival of patients with diabetes: A single center experience. PLoS ONE 2017, 12, e0186827. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Gruessner, R.W.G. Long-term outcome after pancreas transplantation: A registry analysis. Curr. Opin. Organ. Transplant. 2016, 21, 377–385. [Google Scholar] [CrossRef]
- Odorico, J.S.; Heisey, D.M.; Voss, B.J.; Steiner, D.; Knechtle, S.; D’Alessandro, A.; Hoffmann, R.; Sollinger, H. Donor factors affecting outcome after pancreas transplantation. Transplant. Proc. 1998, 30, 276–277. [Google Scholar] [CrossRef]
- Boggi, U.; Del Chiaro, M.; Signori, S.; Vistoli, F.; Amorese, G.; Croce, C.; Morelli, L.; Bartolo, T.V.; Pietrabissa, A.; Barsotti, M.; et al. Pancreas transplants from donors aged 45 years or older. Transplant. Proc. 2005, 37, 1265–1267. [Google Scholar] [CrossRef]
- Salvalaggio, P.R.; Schnitzler, M.A.; Abbott, K.C.; Brennan, D.C.; Irish, W.; Takemoto, S.K.; Axelrod, D.; Santos, L.S.; Kocak, B.; Willoughby, L.; et al. Patient and graft survival implications of simultaneous pancreas kidney transplantation from old donors. Am. J. Transplant. 2007, 7, 1561–1571. [Google Scholar] [CrossRef]
- Schenker, P.; Wunsch, A.; Ertas, N.; Schaeffer, M.; Rump, L.; Viebahn, R.; Vonend, O. Long-term results after simultaneous pancreas-kidney transplantation using donors aged 45 years or older. Transplant. Proc. 2008, 40, 923–926. [Google Scholar] [CrossRef]
- Fridell, J.A.; Rogers, J.; Stratta, R.J. The Pancreas Allograft Donor: Current Status, Controversies, and Challenges for the Future. Clin. Transplant. 2010, 24, 433–449. [Google Scholar] [CrossRef]
- Axelrod, D.A.; Sung, R.S.; Meyer, K.H.; Wolfe, R.A.; Kaufman, D.B. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am. J. Transplant. 2010, 10, 837–845. [Google Scholar] [CrossRef]
- Finger, E.B.; Radosevich, D.M.; Dunn, T.B.; Chinnakotla, S.; Sutherland, D.E.; Matas, A.J.; Pruett, T.L.; Kandaswamy, R. A composite risk model for predicting technical failure in pancreas transplantation. Am. J. Transplant. 2013, 13, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Kayler, L.K.; Wen, X.; Zachariah, M.; Casey, M.; Schold, J.; Magliocca, J. Outcomes and survival analysis of old-to-old simultaneous pancreas and kidney transplantation. Transplant. Int. 2013, 26, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Bonilla, A.J.; Campos-Hernandez, J.P.; Carrasco-Valiente, J.; Márquez-López, F.; Ruiz-García, J.; Sánchez-Gónzalez, A.; Salamanca-Bustos, J.; Regueiro-López, J.; Navarro-Cabello, M.; Requena-Tapia, M. Influence of donor and recipient ages in survival of simultaneous pancreas-kidney transplantation. Transplant. Proc. 2016, 48, 3033–3036. [Google Scholar] [CrossRef] [PubMed]
- Proneth, A.; Schnitzbauer, A.A.; Schenker, P.; Wunsch, A.; Rauchfuss, F.; Arbogast, H.; Manekeller, S.; Nadalin, S.; Heise, M.; Ströhlein, M.A.; et al. Extended pancreas donor program—The EXPAND study: A prospective multicenter trial testing the use of pancreas donors older than 50 years. Transplantation 2018, 102, 1330–1337. [Google Scholar] [CrossRef]
- Mensink, J.W.; de Vries, K.M.; Huurman, V.A.L.; Pol, R.A.; Alwayn, I.P.; Braat, A.E. Risk analysis of extended pancreas donor selection criteria. Pancreatology 2019, 19, 994–999. [Google Scholar] [CrossRef]
- Ito, T.; Kenmochi, T.; Aida, N.; Kurihara, K.; Asaoka, T.; Ito, T. Are the outcomes of Japanese pancreas transplantation utilizing extended-criteria donors acceptable? A propensity score matching analysis for donors < 50 or ≥50 years old. Transplant. Int. 2020, 33, 1046–1060. [Google Scholar] [CrossRef]
- Gruessner, A.; Laftavi, M.; Pankewycz, O.; Gruessner, R. Pancreas transplants in the elderly patient: Should it be done? Am. J. Transplant. 2018, 18 (Suppl. S4), 391. [Google Scholar]
- Meier, R.P.H.; Noguchi, H.; Kelly, Y.M.; Sarwal, M.; Conti, G.; Ward, C.; Halleluyan, R.; Tavakol, M.; Stock, P.G.; Freise, C.E. Impact of sarcopenia on simultaneous pancreas and kidney transplantation outcomes: A retrospective observational cohort study. Transplant. Direct 2020, 6, e610. [Google Scholar] [CrossRef]
- Lentine, K.L.; Alhamad, T.; Cheungpasitporn, W.; Tan, J.C.; Chang, S.H.; Cooper, M.; Dadhania, D.M.; Axelrod, D.A.; Schnitzler, M.A.; Ouseph, R.; et al. Impact of functional status on outcomes of simultaneous pancreas-kidney transplantation: Risks and opportunities for patient benefit. Transplant. Direct 2020, 6, e599. [Google Scholar] [CrossRef]
- Fukuda, Y.; Asaoka, T.; Eguchi, H.; Sasaki, K.; Iwagami, Y.; Yamada, D.; Noda, T.; Kawamoto, K.; Gotoh, K.; Kobayashi, S.; et al. Clinical impact of preoperative sarcopenia on the postoperative outcomes after pancreas transplantation. World J. Surg. 2018, 42, 3364–3371. [Google Scholar] [CrossRef]
- Landi, F.; Onder, G.; Bernabei, R. Sarcopenia and diabetes: Two sides of the same coin. J. Am. Med. Dir. Assoc. 2013, 14, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Gruessner, A.; Agopian, V.G.; Khalpey, Z.; Riaz, I.B.; Kaplan, B.; Halazun, K.J.; Busuttil, R.W.; Gruessner, R.W.G. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015, 150, 252–259. [Google Scholar] [CrossRef]
- Parajuli, S.; Mandelbrot, D.A. One more time, emphasizing the advantage of simultaneous pancreas and kidney transplantation for patients with type 1 diabetes and end-stage renal disease. Transplant. Int. 2020, 33, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.B. Life after pancreas transplantation: Reversal of diabetic lesions. Curr. Opin. Organ. Transplant. 2014, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- van Dellen, D.; Worthington, J.; Mitu-Pretorian, O.M.; Ghazanfar, A.; Forgacs, B.; Pararajasingam, R.; Campbell, B.; Parrott, N.R.; Augustine, T.; Tavakoli, A. Mortality in diabetes: Pancreas transplantation is associated with significant survival benefit. Nephrol. Dial. Transplant. 2013, 28, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.N.; Brazy, P.C.; Becker, Y.T.; Odorico, J.S.; Pintar, T.J.; Collins, B.H.; Pirsch, J.D.; Leverson, G.E.; Heisey, D.M.; Sollinger, H.W. Simultaneous pancreas-kidney transplantation reduces excess mortality in type 1 diabetic patients with end-stage renal disease. Kidney Int. 2000, 57, 2129–2135. [Google Scholar] [CrossRef]
| General Inclusion Criteria: |
|---|
| - Age below 65–70 years (physiologic age more important than chronologic age) - DM managed with insulin (however, with newer anti-diabetic agents, the need for insulin may not necessarily stay a prerequisite for PTx candidacy in the near future) - Presence of “complicated” DM (either “type 1 or 2”, regardless of C-peptide levels): complications may include symptomatic or progressive retinopathy, peripheral or autonomic neuropathy, nephropathy, microangiopathy, glucose hyperlability (episodes of diabetic ketoacidosis or unawareness of hypoglycemia), or significant psychological or physical problems associated with insulin therapy - Medical stability (predicted ability to tolerate the operative procedure, possible associated complications, and chronic immunosuppression); no recurrent Emergency Department visits - Absence of smoking tobacco, multiple amputations, severe cardiac or vascular disease - HbA1c level < 10–12% (measure of compliance) - BMI < 35 kg/m2 (body habitus and weight/adipose distribution are important determinants) - Health literacy and psychosocial suitability (as defined by being able to comprehend and manage the aftercare, presence of acceptable caregiver support, and having adequate resources in place) |
| Specific criteria for PTx in T2DM: |
| - Age < 55–60 years - BMI < 30–35 kg/m2 (avoidance of central and excessive intra-abdominal obesity) - Random Cp level ≤ 10–15 ng/mL (limited data available with PTx in patients with higher levels) - Total daily insulin dose < 100 μ/day and 1 μ/kg/day (limited data available in patients with higher doses) - Insulin-requiring for minimum of 3–5 years (absolute need for insulin may change in the near future) - Presence of “complicated” diabetes including severe glycemic excursions - No recent history of dietary and medication noncompliance - Adequate financial, caregiver, and psychosocial support |
| General Exclusion Criteria: |
| - Age > 70 years (physiology eclipses chronology) - Not on insulin with minimal glycemic excursions and no evidence for diabetic complications - BMI > 35 kg/m2 (mainly related to technical considerations) - Inadequate cardiac function such as: Significant noncorrectable coronary artery disease detected by coronary angiography; low ejection fraction (<30–40%) or cardiomyopathy; recent myocardial infarction; pulmonary hypertension (>45–50 mm Hg); recent history of stroke with residual or evolving findings - Significant dysfunction in other (non-renal) organ system (lung, liver, central nervous system) unless patient being considered for multi-organ transplant other than SPKT - Severe peripheral vascular (aorto-iliac) disease, presence of aorto-iliac bypass graft - Untreated or ongoing substance abuse (tobacco, alcohol, drug), psychiatric illness, or noncompliance - Active or inadequately treated infection (including positive Hepatitis B surface antigen serology) or malignancy (other than localized basal cell or squamous cell skin cancer) - Inadequate financial resources and social support (lives alone, relies on public transportation) - Poor overall functional and performance status (severe malnutrition or deconditioning, sarcopenia, frailty, dementia, wheelchair-bound, need for chronic oxygen therapy, recurrent falls) - Any chronic illness that cannot be treated with medication or any systemic illness that would severely compromise recovery or limit life expectancy - Peritoneal dialysis with multiple episodes of peritonitis or sclerosing encapsulating peritonitis; multiple previous laparotomies with severe adhesions; previous intra-abdominal/pelvic irradiation - Severe uncontrolled gastroparesis or orthostasis, history of uncontrolled thrombophilia - Presence of a chronic bladder drainage catheter, ostomy, or feeding tube - Unable to provide informed consent |
| Basic Testing: |
|---|
| Hematology: Complete blood count with differential, platelet count; coagulation studies, thrombophilia panel Chemistry: Standard chemistry profile including serum amylase, lipase, HbA1c, C-peptide levels Special chemistry: Iron and lipid panels; parathyroid hormone, C-peptide and HbA1c levels Urine tests: Drug screen, urinalysis with culture, urine protein/creatinine |
| Health Care Maintenance: |
| Hemoccult x3 or Cologuard®; Prostate specific antigen level in males above age 45 years; serum pregnancy test in women of childbearing potential; Colonoscopy in all patients above age 45 years (repeat every 5 years if history of polyps or positive family history, otherwise repeat every 10 years); Dermatologic exam if history of skin cancer; Mammography in women above age 40 years; Pelvic exam with Pap smear (in absence of total hysterectomy) |
| Imaging: |
| Chest radiograph, abdominal ultrasound or noncontrast abdominal and pelvic computerized tomographic scan |
| Cardiac and Vascular Studies: |
| 12-lead electrocardiogram, transthoracic echocardiography, cardiac stress testing, coronary angiography and Cardiology consultation (when indicated), carotid Doppler exam, iliac duplex arterial and venous studies (when indicated) |
| Additional Consults: |
| History and physical examination by transplant provider (Nephrologist, surgeon, other provider) Consultation by diabetes specialist (when indicated) Social work and financial assessment Evaluation by Transplant Dietitian, Coordinator, and Pharmacist Cardiology consultation Clearance be Dentistry (as needed) Assessment by Pulmonary, Urology, Infectious Disease, Neurology, Dermatology, Ophthalmology, Podiatry/Orthopedic, Psychiatry or Gastro-Intestinal when indicated Performance, Fit, or Functional testing |
| Infectious Disease, Immunologic, and Serologic Testing: |
| Blood type ABO, tissue type HLA, anti-HLA antibody testing with calculated panel reactive antibody level; viral studies (HIV, hepatitis C and B, cytomegalovirus, Epstein–Barr virus, BK virus, COVID-19, varicella-zoster), VDRL/FTA, Quantiferon Gold for TB (when indicated), Stronglyoides (when indicated) |
| Optional Testing: |
| Quality of life questionnaire (SF-36), pulmonary function studies (when indicated–smoking history); anti-GAD65, anti-insulin, anti-islet antibodies, glucose stimulation testing, gastric emptying study, voiding cystourethogram with post-void residual, urodynamics |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratta, R.J.; Gruessner, A. Pancreas Transplantation in Minorities including Patients with a Type 2 Diabetes Phenotype. Uro 2022, 2, 213-244. https://doi.org/10.3390/uro2040026
Stratta RJ, Gruessner A. Pancreas Transplantation in Minorities including Patients with a Type 2 Diabetes Phenotype. Uro. 2022; 2(4):213-244. https://doi.org/10.3390/uro2040026
Chicago/Turabian StyleStratta, Robert J., and Angelika Gruessner. 2022. "Pancreas Transplantation in Minorities including Patients with a Type 2 Diabetes Phenotype" Uro 2, no. 4: 213-244. https://doi.org/10.3390/uro2040026
APA StyleStratta, R. J., & Gruessner, A. (2022). Pancreas Transplantation in Minorities including Patients with a Type 2 Diabetes Phenotype. Uro, 2(4), 213-244. https://doi.org/10.3390/uro2040026





