Abstract
Background: De novo malignancies (DNMs) after liver transplantation (LT) are a major cause of long-term mortality. However, no definitive screening protocol has been established due to their diversity. This study aimed to evaluate DNM diagnosis methods, screening protocols, and prognoses. Methods: This retrospective study included 231 adult LT recipients from April 1997 to March 2021. Disease-specific survival (DSS) was analyzed to assess the impact of screening on prognosis. Most recipients underwent serum tests every three months, annual gastrointestinal endoscopy, and chest-abdominal CT as part of routine surveillance. Results: Twenty-five DNMs were diagnosed in 22 patients, with median age of 61 years (range, 23–72), of whom 13 (59.1%) were female. The duration from transplantation to DNM diagnosis of DNM was 88 months (range, 4–195). DNM was diagnosed as follows: seven patients (31.8%) through screening (screening group) and 15 patients (68.2%) by other means (non-screening group). Curative treatment was achieved in all of the patients diagnosed by screening, whereas it was possible in only 60.0% of patients diagnosed by other means (p = 0.026). DSS in the screening group was significantly longer than that in the non-screening group (p = 0.024). Conclusions: While screening was associated with earlier-stage diagnosis and improved outcomes in some patients, the overall efficacy of the protocol requires further validation in larger studies.
1. Introduction
Liver transplantation (LT) has become the optimal treatment for end-stage decompensated cirrhosis and hepatocellular carcinoma (HCC) in carefully selected cases [1]. Over the years, improvements in surgical techniques, perioperative intensive care, and advancements in immunosuppressive therapies have significantly enhanced early post-transplant survival rates [2]. Many transplant centers now report a 1-year survival rate exceeding 90%, while 10-year survival rates are approximately 60% [3,4,5].
With improved long-term survival, various complications have emerged, including those associated with chronic immunosuppressive therapy. Additionally, prolonged survival has increased the risk of long-term complications such as cardiovascular diseases, infectious diseases, renal failure, and recurrence of pre-transplant liver disease [6,7].
Among these complications, de novo malignancies (DNMs) have emerged as a leading cause of long-term mortality following solid organ transplantation [8]. LT recipients have a 3- to 7-fold higher risk of developing DNMs compared to the general population due to prolonged immunosuppression [9,10,11]. The mechanisms include impaired immune surveillance, the reactivation of oncogenic viruses (e.g., EBV, HPV), and the promotion of tumorigenesis via cytokine-rich environments and angiogenesis [12]. Previous studies have reported that the incidence of DNM after LT varies significantly by region and patient background, with Western populations showing a high prevalence of non-melanoma skin cancers [13,14,15], whereas Asian populations are more likely to develop gastrointestinal malignancies and post-transplant lymphoproliferative disorders (PTLDs) [16,17,18]. Due to these epidemiological differences, establishing standardized screening protocols remains difficult, and their effectiveness in improving curative treatment rates and long-term survival has not been fully established. Although some studies have suggested that intensified surveillance (e.g., replacing conventional chest X-ray with chest CT) improves early detection rates [19,20], evidence regarding the prognostic benefit of such screening programs remains limited.
The aim of this study was to describe our institutional experience with post-LT surveillance and to explore the potential impact of screening-triggered diagnosis on patient prognosis.
2. Patients and Methods
2.1. Patient Selection
This retrospective study included adult patients (aged ≥ 18 years at the time of LT) who underwent liver transplantation (LT) at Keio University Hospital between April 1997 and March 2021. Patients were followed up for at least six months postoperatively to assess the development of DNMs.
Patients who underwent LT for various liver diseases, including viral hepatitis, alcohol-related liver disease, primary biliary cholangitis, and metabolic liver disorders, were included. Only those with a minimum follow-up period of six months and available medical records containing detailed clinical, radiological, and pathological data were considered eligible. This study excluded patients who had a history of pre-transplant malignancies excluded HCC, lacked complete follow-up data, or were lost to follow-up within six months after transplantation. Additionally, patients with insufficient clinical information regarding post-transplant cancer surveillance were excluded. This study was approved by the Institutional Review Board of Keio University (approval number: 20120443, approval date: 8 August 2013) and was conducted in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study.
2.2. Posttransplant Cancer Surveillance
After LT, a standardized surveillance protocol was generally adopted, which included routine outpatient examinations, physical examinations, cell blood counts and blood chemistry, including tumor markers such as alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9), were scheduled every three months. Chest CT and abdominal CT, esophagogastroduodenoscopy (EGD), and colonoscopy (CS) were also generally performed annually according to the protocol. The components and frequency of this surveillance protocol are summarized in Table 1. The surveillance protocol was consistently applied throughout the study period without major changes in frequency, modality, or interpretation criteria. Although the protocol was generally followed, some patients had incomplete adherence to specific components, such as CS or imaging coverage. Routine medical checkups were recommended for patients with inadequate adherence to annual screening.

Table 1.
Details of routine post-transplant cancer surveillance protocol.
2.3. Patient Classification
Patients diagnosed with DNMs were categorized into two groups based on how the malignancy was initially detected. The screening group included patients whose malignancy was incidentally identified during scheduled surveillance examinations (e.g., blood tests, CT, or endoscopy) and who were asymptomatic at the time of diagnosis. The non-screening group included patients whose malignancy was detected either due to symptoms or during incidental examinations unrelated to the surveillance protocol. The overall study design and patient classification process are illustrated in Figure 1. Importantly, classification was based solely on the diagnostic trigger of the malignancy—not on whether the patient had undergone surveillance or adhered to the protocol. Therefore, even if a patient had received regular screening, they were assigned to the non-screening group if the malignancy was not detected through those scheduled tests.
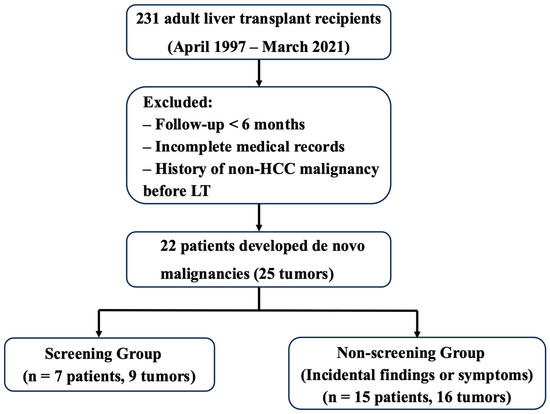
Figure 1.
Flowchart of study design and patient selection. Among 231 adult recipients who underwent liver transplantation (LT) between April 1997 and March 2021, 22 patients developed 25 de novo malignancies (DNMs). Based on the method of initial DNM detection, patients were classified into the screening group (n = 7) or the non-screening group (n = 15). One patient developed DNMs detected by both screening and non-screening methods and was classified according to the first detected malignancy.
This classification strategy was applied consistently to all cases. In patients who developed multiple DNMs, grouping and analysis were based on the detection method of the first diagnosed malignancy to minimize within-patient dependency and ensure statistical validity.
2.4. Data Collection and Endpoints
Patient data, including demographic characteristics, primary liver disease, transplant details, immunosuppressive regimen, and follow-up information, were collected from institutional medical records and databases. Screening history and cancer detection data, such as imaging techniques and tumor markers, were also reviewed.
This study included 231 adult liver transplant recipients, of whom only those who developed DNMs were included in the final analysis. The primary endpoint was disease-specific survival (DSS), defined as the time from DNM diagnosis to death due to DNM. We analyzed the mode of DNM detection (screening-based vs. non-screening-based), its association with the presence of distant metastases at diagnosis, and its impact on prognosis.
2.5. Statistical Analysis
Continuous variables were expressed as medians with corresponding ranges, while categorical variables were expressed as counts with associated percentages. Comparisons between groups were performed using the χ2 test or Student’s t-test. Patient survival was calculated from the date of DNM diagnosis to the date of death or last clinical visit. DSS was estimated using the Kaplan–Meier method and compared using a log-rank test. Statistical analyses were conducted using IBM SPSS Software for Windows, version 29.0 (IBM Corp., Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. DNM After LT
Of the total 231 patients who underwent LT, 25 cases of DNMs were diagnosed in 22 patients (9.5%). Three patients developed two distinct DNMs at different time points. For analysis, patients were grouped according to the diagnostic method of their first DNM. The DNM cohort included 9 men and 13 women, with a median age of 61 years (range, 23–72) at the time of LT. Indications for LT included alcohol-related liver disease in five cases, chronic hepatitis B infection in three cases, chronic hepatitis C infection in three cases, primary biliary cholangitis in two cases, and other conditions in nine cases. All 22 patients received livers from living donors. The median time from transplantation to cancer diagnosis was 88 months (range, 4–195).
The distribution of DNM was as follows: post-transplant lymphoproliferative diseases (PTLD) in eight cases, gastric cancers in two, laryngeal cancers in two, lung cancers in two, thyroid cancers in two, colorectal cancers in two, breast cancers in two, and one case each of renal cancer, skin cancer, prostate cancer, cholangiocarcinoma, and cancer of unknown primary. Baseline characteristics and management details for both groups are summarized in Table 2 and Table 3.

Table 2.
Clinical Characteristics of nine patients with DNM detected by screening examinations.

Table 3.
Clinical characteristics of the 16 cases with DNM detected by methods excluding screening.
3.2. Details for Detection of DNM
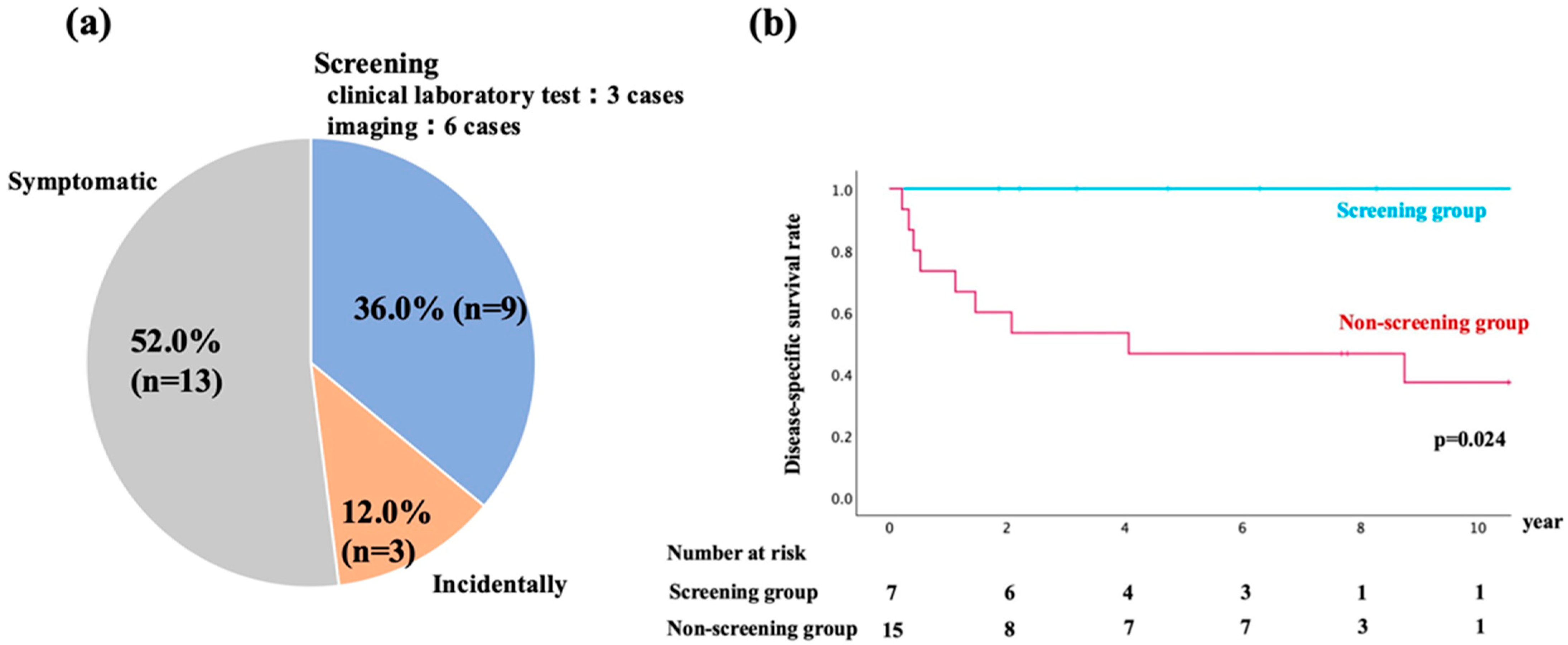
The details of DNM diagnoses are shown in Figure 2a. DNM was diagnosed in the following ways: nine cases (36.0%) through screening, 13 cases (52.0%) based on presenting symptoms, and three cases (12.0%) during incidental examinations. Among the nine cases diagnosed through screening, four cases were identified via EGD, one through fecal occult blood test, two by CT, and two through serum laboratory tests. In cases where a single patient developed multiple DNMs, each malignancy was counted separately according to its detection method.
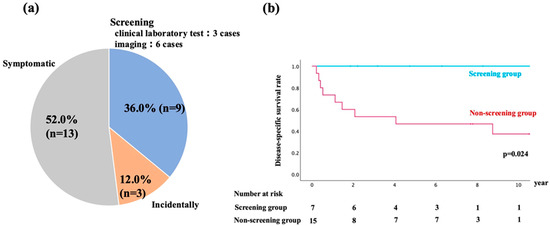
Figure 2.
(a) Distribution of diagnostic methods for de novo malignancies (DNMs) after liver transplantation. Patients were categorized into three groups based on the method of DNM detection: screening examinations (n = 9, 36.0%), symptomatic presentation (n = 13, 52.0%), and incidental findings (n = 3, 12.0%). (b) Kaplan–Meier survival curves comparing the disease-specific survival (DSS) between the screening group (n = 7) and the non-screening group (n = 15). Patients in the screening group demonstrated significantly longer DSS compared to the non-screening group (p = 0.024). The number of patients at risk in each group is shown below the graph.
Although screening had been performed in 7 of the 15 patients in the non-screening group, no DNMs were detected during these examinations. Therefore, their malignancies were ultimately diagnosed based on symptoms or incidental findings, and they were classified into the non-screening group. In contrast, all seven patients in the screening group were diagnosed with localized disease during routine surveillance, allowing curative treatment in all cases. Among the non-screening group, two patients (13.3%) were found to have distant metastases at the time of diagnosis, significantly limiting curative treatment options.
3.3. Survival Outcomes
The median DSS after DNM diagnosis was 56.8 months (range, 2.4–157.8 months).
Kaplan–Meier survival curves showed significantly higher DSS rates in the screening group than in the non-screening group (Figure 2b). The 1-, 5-, and 10-year survival probabilities were 100%, 100%, and 100% in the screening group, and 73.3%, 46.7%, and 37.3% in the non-screening group (p = 0.026). The median survival time (MST) also differed significantly between the two groups: MST was not reached in the screening group, while it was 4.1 years in the non-screening group (p = 0.024; Table 4). The proportion of patients with distant metastasis at the time of DNM diagnosis tended to be higher in the non-screening group (p = 0.167). Consequently, curative treatment was possible in only nine cases (60.0%) in the non-screening group, compared to all cases in the screening group (p = 0.026).

Table 4.
Comparison between the screening group and the non-screening group.
4. Discussion
In this study of 231 LT recipients, DNMs were identified in 22 patients (9.5%) at 25 sites, with a median interval of 88 months from LT. The most common DNM was PTLD (32.0%). Notably, patients whose DNMs detected through screening had significantly better prognoses compared to those diagnosed based on symptoms or incidental findings, although the overall detection rate was low. Although screening was associated with improved DSS and higher curative treatment rates, only 9 of 25 DNMs were detected through scheduled examinations. The majority were diagnosed due to symptoms or unrelated incidental findings.
Our results demonstrated that the incidence of DNMs (9.5%), including eight cases of PTLD, was consistent with previous reports [1,11,13,14]. However, the types of DNMs vary by region and ethnicity. Western studies report non-melanoma skin cancers as the most common DNM among solid organ transplant recipients, largely influenced by sun exposure [15,16]. In contrast, in Asian populations, the most frequent DNMs are colorectal cancer, stomach cancer, and PTLD, which is consistent with our findings [17,18]. Thus, not only do the number of DNM diseases differ by area but also by disease type, resulting in the absence of an established screening protocol.
This study uniquely focused on the triggers for DNM detection and their relationship with prognosis. Our findings showed that all patients with DNMs detected through screening could undergo curative treatment, resulting in prolonged survival. While DSS was selected as the primary endpoint in this study, it should be noted that overall survival (OS) may show less divergence between groups due to non-DNM-related deaths. Therefore, DSS better reflects the impact of early DNM detection, but the Kaplan–Meier curves should be interpreted with this in mind. Furthermore, the median interval from liver transplantation to DNM diagnosis was 88 months. This long latency raises questions about whether annual screening is justified over such an extended period, particularly in the absence of individualized risk stratification. In addition, we acknowledge that this study cannot determine the efficacy of the screening strategy due to the limited sample size and retrospective single-center design. Rather, our aim was to describe real-world outcomes under our institutional surveillance protocol. Previous studies have emphasized the importance of effective screening programs for the early detection and improved prognosis of DNM [19,20]. For example, Finkenstedt et al. demonstrated that the intensifying surveillance protocol—such as replacing chest X-ray and abdominal ultrasound with chest and abdominal CT—led to increased detection rates and earlier diagnoses of DNMs [21]. Nonetheless, further validation through prospective multicenter studies is warranted to determine the true benefit of such protocols.
Our surveillance program includes serum laboratory tests, chest/abdominal CT, EGD, and total CS. However, as shown in Table 3, two cases of PTLD and thyroid cancer were initially missed because routine CT scans did not include the cervical region. In response, we recognized the potential value of incorporating cervical CT into the surveillance protocol. Although neck CT was not routinely performed during the study period, it is currently under consideration for future practice. If implemented, cervical imaging would not be applied uniformly to all recipients but rather considered for patients with relevant risk factors such as history of thyroid disease, female sex, or prolonged immunosuppression [22].
Moreover, periodic screening was not performed in eight of fifteen patients. Total CS, in particular, was performed less frequently than EGD due to factors such as postoperative adhesions, the burden of patient preparation, and other logical challenges. In particular, the two patients who presented with hematochezia had either not undergone a recent colonoscopy or had incomplete adherence to the recommended schedule, which may have contributed to the delayed diagnosis.
In several patients, malignancies were detected incidentally during imaging studies performed for reasons unrelated to cancer screening. These included cases such as renal cancer, breast cancer, and cancers of unknown primary. Although the early detection of localized disease allowed for curative treatment in some of these cases, the overall incidence of these tumors was low, and no common risk factors were identified. Therefore, we do not advocate routine screening for these tumor types. Instead, incidental findings should be interpreted as part of the broader benefits of regular imaging surveillance after liver transplantation.
To optimize while avoiding unnecessary costs, several studies have suggested surveillance protocols tailored to individual risk factors, such as gender, smoking, and alcohol consumption [12]. In our cohort, smoking history was confirmed in several patients who developed smoking-related malignancies, such as lung and laryngeal cancers (Table 2 and Table 3). These observations support the potential utility of incorporating smoking status into risk-adapted screening protocols following liver transplantation. Moreover, our findings showed that only 9 of 25 DNMs were detected through scheduled screening, whereas the majority were diagnosed based on symptoms or incidental findings, despite the existence of a surveillance protocol. These findings suggest that while screening enabled early detection of localized disease in select patients, its overall diagnostic yield was limited, and the cost-effectiveness and invasiveness of annual imaging and endoscopy in all patients remain questionable. In addition, considering the increasing mortality from colorectal cancer in Japan, regular CS remains essential for earlier detection [14]. Moving forward, our protocols may require region-specific or patient-specific modifications and future strategies should consider both clinical effectiveness and cost-efficiency. Our findings support a transition from uniform annual screening to more tailored protocols, adjusted for individual risk factors such as age, EBV status, cancer history, and duration of immunosuppression. In this context, a more comprehensive risk-stratified strategy—based not only on lifestyle but also on clinical and immunological parameters—may help further improve outcomes while minimizing unnecessary interventions.
Although PTLD was the most common DNM (32%) in this study, only one case was detected through screening. The International Liver Transplantation Society recommends EBV–DNA monitoring during the first post-LT year for EBV–seronegative recipients [13]. Additionally, Tajima et al. identified age > 18 years, poor performance status (>2) at PTLD diagnosis, and the monomorphic subtype as independent prognostic factors, elevated serum soluble interleukin-2 receptor levels potentially indicating monomorphic PTLD [23]. These findings highlight the importance of periodic screening, targeted serum biomarkers, and thorough physical examinations for the early detection of PTLD. However, in our cohort, EBV viral load monitoring was not consistently performed, which may have contributed to delayed diagnosis in some cases. Routine monitoring, particularly in EBV–seronegative recipients, should be considered essential as part of future surveillance protocols to improve the early detection of PTLD.
This study has several limitations. First, the sample size was relatively small. Second, as a retrospective, single-center study, the generalizability of our findings may be limited. Therefore, our findings should be interpreted with caution, and further validation through larger multicenter studies is necessary to confirm the clinical utility of screening protocols after liver transplantation. Additionally, this study was conducted at a Japanese liver transplant center, where annual gastrointestinal endoscopy is routinely implemented as part of cancer surveillance. This reflects local healthcare practices and insurance systems, which differ from those in many Western countries. Therefore, the applicability of our surveillance protocol to non-Asian populations may be limited. Furthermore, the ILTS-SETH consensus guidelines, which propose alternative screening strategies more suitable for global implementation, were published in 2022—after the conclusion of our study in March 2021 [13]. These temporal and regional differences should be considered when interpreting the generalizability of our findings. Finally, although PTLD was the most frequently observed DNM in our cohort, EBV viral load monitoring using EBV–PCR was not routinely performed during the study period. This limitation may have contributed to delayed diagnosis of PTLD in some patients. As a result, our findings regarding PTLD incidence and timing should be interpreted with caution. Future surveillance protocols should incorporate routine EBV monitoring, especially in high-risk populations such as EBV–seronegative recipients.
5. Conclusions
In conclusion, the incidence of DNMs after LT was 9.5%, with PTLD being the most common malignancy. DNMs identified through screening allowed curative treatment in all cases, resulting in significantly improved prognoses. While screening was associated with better disease-specific outcomes in select patients, further large-scale prospective studies are required to validate its overall efficacy and justify the current intensity of surveillance protocols. Risk-adapted approaches may offer more practical and cost-effective alternatives.
Author Contributions
S.U., Y.H. and H.O. conceived and designed this study. S.U. and Y.H. drafted the manuscript. M.K., H.Y., Y.A., Y.H., S.H., M.T., Y.N. and Y.K. analyzed the data and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Keio University School of Medicine (protocol code: 20120443, approval date: 8 August 2013).
Informed Consent Statement
The requirement for informed consent was waived due to the retrospective nature of the study and use of anonymized data.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the staff of the Department of Anesthesiology at Keio University School of Medicine and the recipient transplantation coordinators at Keio University Hospital for their assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Z.N.; Wang, W.T.; Yan, L.N. Liver Surgery Group. De novo malignancies after liver transplantation with 14 cases at a single center. Transpl. Proc. 2015, 47, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Michael, R.C. Roadmap for Improving Patient and Graft Survival in the Next 10 Years. Liver Transpl. 2016, 22, S71–S78. [Google Scholar]
- Jimenez-Romero, C.; Manrique Municio, A.; Marques Medina, E.; Colina, F.; Domene, P.O.; Sanz, R.G.; Meneu Diaz, J.C.; Abradelo de Usera, M.; Moreno Elola, A.; Moreno Gonzalez, E. Incidence of de novo nonmelanoma skin tumors after liver transplantation for alcoholic and nonalcoholic liver diseases. Transpl. Proc. 2006, 38, 2505–2507. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Ufere, N.N.; Bucuvalas, J.C. Liver transplant survivor-ship. Liver Transpl. 2020, 26, 1030e3. [Google Scholar] [CrossRef]
- Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Noreen, S.M.; M Robinson, A.; Miller, E.; Snyder, J.J.; Israni, A.K.; et al. OPTN/SRTR2017Annual Data Report: Liver. Am. J. Transpl. 2019, 19 (Suppl. 2), e184–e283. [Google Scholar] [CrossRef]
- Jain, A.; Fiaz, O.; Sheikh, B.; Sharma, R.; Safadjou, S.; Kashyap, R.; Bryan, L.; Batzold, P.; Orloff, M. Recurrent nonhepatic and de novo malignancies after liver transplantation. Transplantation 2009, 88, 706–710. [Google Scholar] [CrossRef]
- Yoshida, E.M.; Steinbrecher, U.P.; Donovan, J.A.; Erb, S.R.; Esrason, K.T.; Medkiff, K.A.; Pruthi, J.; Fong, T.-L. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001, 7, 811–815. [Google Scholar]
- Sérée, O.; Altieri, M.; Guillaume, E.; De Mil, R.; Lobbedez, T.; Robinson, P.; Segol, P.; Salamé, E.; Abergel, A.; Boillot, O.; et al. Longterm risk of solid organ de novo malignancies after liver transplantation: A French national study on 11,226 patients. Liver Transpl. 2018, 24, 1425–1436. [Google Scholar] [CrossRef]
- Pillai, A.A. Management of de novo malignancies after liver transplantation. Transpl. Rev. 2015, 29, 38–41. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011, 306, 1891–1901. [Google Scholar] [CrossRef]
- Herrero, J.I. De novo malignancies following liver transplantation: Impact and recommendations. Liver Transpl. 2009, 15 (Suppl. 2), S90–S94. [Google Scholar] [CrossRef] [PubMed]
- Anisha, T.; Sanjiv, S.; Narendra, S.; Saha, S.; Rastogi, A.; Bhangui, P.; Saraf, N.; Srinivasan, T.; Yadav, S.K.; Gautam, D.; et al. De Novo Malignancy After Living Donor Liver Transplantation: A Large Volume Experience. J. Clin. Exp. Hepatol. 2020, 10, 448–452. [Google Scholar]
- Colmenero, J.; Tabrizian, P.; Bhangui, P.; Pinato, D.J.; Rodriguez-Per-alvarez, M.L.; Sapisochin, G.; Bhoori, S.; Pascual, S.; Senzolo, M.; Al-Adra, D.; et al. De Novo malignancy after liver trans- plantation: Risk assessment, prevention, and management-guidelines from the ILTS-SETH consensus conference. Transplantation 2022, 106, e30–e45. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Hata, K.; Tanaka, K.; Iyama, N.; Kusakabe, J.; Kageyama, S.; Ogawa, E.; Okamoto, T.; Haga, H.; Uemoto, S.; et al. Chronological alterations in de novo malignancies after living-donor liver transplantation: A cohort study of 1781 recipients using annual comparisons of standardized incidence ratios. J. Hepato-Biliary-Pancreat. Sci. 2024, 31, 455–467. [Google Scholar] [CrossRef]
- Herrero, J.I.; Lorenzo, M.; Quiroga, J.; Sangro, B.; Pardo, F.; Rotellar, F.; Alvarez-Cienfuegos, J.; Prieto, J. De novo neoplasia after liver transplantation: An analysis of risk factors and influence on survival. Liver Transpl. 2005, 11, 89. [Google Scholar] [CrossRef]
- Tanaka, T.; Michael, D.V. Decision tree analysis to stratify risk of de novo non-melanoma skin cancer following liver transplantation. J. Cancer Res. Clin. Oncol. 2018, 144, 607–615. [Google Scholar] [CrossRef]
- Kaneko, J.; Sugawara, Y.; Tamura, S.; Aoki, T.; Sakamoto, Y.; Hasegawa, K.; Yamashiki, N.; Kokudo, N. De novo malig- nancies after adult-to-adult living-donor liver transplantation with a malignancy surveillance program: Comparison with a Japanese population-based study. Transplantation 2013, 95, 1142e7. [Google Scholar] [CrossRef]
- Mizuno, S.; Hayasaki, A.; Ito, T.; Fujii, T.; Iizawa, Y.; Kato, H.; Murata, Y.; Tanemura, A.; Kuriyama, N.; Azumi, Y.; et al. De novo Malignancy Following Adult-to-Adult Living Donor Liver Transplantation Focusing on Posttransplantation Lymphoproliferative Disorder. Transpl. Proc. 2018, 50, 2699–2704. [Google Scholar] [CrossRef]
- Kobayashi, T.; Miura, K.; Ishikawa, H.; Sakata, J.; Takizawa, K.; Hirose, Y.; Toge, K.; Saito, S.; Abe, S.; Kawachi, Y.; et al. Malignancy After Living Donor Liver Transplantation. Transpl. Proc. 2024, 56, 660–666. [Google Scholar] [CrossRef]
- Masuda, Y.; Mita, A.; Ohno, Y.; Kubota, K.; Notake, T.; Shimizu, A.; Soejima, Y. De novo malignancy after adult-to-adult living donor liver trans- plantation: A single-center long-term experience. Transpl. Proc. 2023, 55, 952–955. [Google Scholar] [CrossRef]
- Finkenstedt, A.; Graziadei, I.W.; Oberaigner, W.; Hilbe, W.; Nachbaur, K.; Mark, W.; Margreiter, R.; Vogel, W. Extensive Surveillance Promotes Early Diagnosis and Improved Survival of De Novo Malignancies in Liver Transplant Recipients. Am. J. Transpl. 2009, 9, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. N. Engl. J. Med. 2016, 375, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Hata, K.; Haga, H.; Nishikori, M.; Umeda, K.; Kusakabe, J.; Miyauchi, H.; Okamoto, T.; Ogawa, E.; Sonoda, M.; et al. Post-transplant Lymphoproliferative Disorders After Liver Transplantation: A Retrospective Cohort Study Including 1954 Transplants. Liver Transpl. 2021, 27, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).