Albumin: Bountiful Arrow in the Quiver of Liver and Its Significance in Physiology
Abstract
1. Introduction
2. Mutations and Post-Translational Modifications of Albumin and Their Physiological Consequences
3. Association of Albumin with Cellular Physiology and Adverse Effects
4. Role of Albumin Levels in Various Pathologies
4.1. Role in Hepatic Diseases
4.2. Effects of Albumin in Neurological Diseases
4.3. Effects of Albumin in the Immune System
4.4. Effects of Albumin in Sepsis
4.5. Effects of Albumin in CVD
4.6. Effects of Albumin in Blood Coagulation
4.7. Effects of Albumin in Pancreatitis
4.8. Effects of Albumin in Colitis
5. Albumin’s Drug Interactions and Physiological Consequences
6. Albumin in Therapeutics
6.1. Infusion Therapy
6.2. Albumin as a Drug Itself, and Also as a Drug Delivery Agent
6.3. Aid in Chelation Therapy
6.4. Use of Albumin in Various Forms in Research and Cell Culture
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HGF | Hepatocyte growth factor |
| HNF | Hepatocyte nuclear factor |
| RBP | Retinol-binding protein |
| DBP | Vitamin D-binding protein |
| C/EBP | CCAAT/enhancer-binding protein |
| AGE | Advanced glycation end-products |
| ER | Endoplasmic reticulum |
| NH4Cl | Ammonium chloride |
| NAFLD | Non-alcoholic fatty liver disease |
| MASLD | Metabolism dysfunction-associated steatotic liver disease |
| CCl4 | Carbon tetrachloride |
| PGE2 | Prostaglandin E2 |
| IL1β | Interleukin-1-beta |
| CD | Cluster of differentiation |
| BDL | Bile duct ligation |
| LPS | Lipopolysaccharide |
| iNOS | Inducible nitric oxide synthase |
| CVD | Cardiovascular disease |
| PTMs | Post-translational modifications |
| CRPs | C-reactive proteins |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| TNFα | Tumor necrosis factor |
| DSS | Dextran sulfate sodium |
| EDTA | Ethylenediaminetetraacetic acid |
| MuSCs | Muscle satellite/stem cells |
| ROS | Reactive oxygen species |
| PAMPs | Pathogen-associated molecular patterns |
| DAMPs | Damage-associated molecular patterns |
| PI3K | Phosphoinositide 3-kinase |
| PD | Parkinson’s disease |
| GPCR | G-protein-coupled receptor |
| PRECIOSA | PREdiction of the effects of long-term human albumin in patients with decompensated cirrhosis and ascites |
| HRS | Hepatorenal syndrome |
| SBP | Spontaneous bacterial peritonitis |
| ANSWER | Human albumin for the treatment of ascites in patients with hepatic cirrhosis |
| MPP | 1-Methyl-4-phenylpyridinium |
| ARISS | Albumin replacement therapy in septic shock |
| FA | Fatty acid |
| LPC | Lysophosphatidylcholine |
| PMMA | Poly methyl methacrylate |
| BSA | Bovine serum albumin |
References
- Hardon, E.M.; Frain, M.; Paonessa, G.; Cortese, R. Two distinct factors interact with the promoter regions of several liver-specific genes. EMBO J. 1988, 7, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, K.D.; Sharma, S.S.; Roy, N. Hepatocyte nuclear factor-1alpha mediated upregulation of albumin expression in focal ischemic rat brain. Neurol. Res. 2012, 34, 25–31. [Google Scholar] [CrossRef]
- Huang, K.W.; Reebye, V.; Czysz, K.; Ciriello, S.; Dorman, S.; Reccia, I.; Lai, H.S.; Peng, L.; Kostomitsopoulos, N.; Nicholls, J.; et al. Liver Activation of Hepatocellular Nuclear Factor-4α by Small Activating RNA Rescues Dyslipidemia and Improves Metabolic Profile. Mol. Ther. Nucleic Acids 2020, 19, 361–370. [Google Scholar] [CrossRef]
- Feng, R.; Kan, K.; Sticht, C.; Li, Y.; Wang, S.; Liu, H.; Shao, C.; Munker, S.; Niess, H.; Wang, S.; et al. A hierarchical regulatory network ensures stable albumin transcription under various pathophysiological conditions. Hepatology 2022, 76, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.H.; Ma, F.J.; Gopinadhan, S.; Sakban, R.B.; Wang, N.-d. C/EBPα knock-in hepatocytes exhibit increased albumin secretion and urea production. Cell Tissue Res. 2007, 330, 427–435. [Google Scholar] [CrossRef]
- Masaki, T.; Matsuura, T.; Ohkawa, K.; Miyamura, T.; Okazaki, I.; Watanabe, T.; Suzuki, T. All-trans retinoic acid down-regulates human albumin gene expression through the induction of C/EBPbeta-LIP. Biochem. J. 2006, 397, 345–353. [Google Scholar] [CrossRef]
- Kubicka, S.; Kühnel, F.; Zender, L.; Rudolph, K.L.; Plümpe, J.; Manns, M.; Trautwein, C. p53 represses CAAT enhancer-binding protein (C/EBP)-dependent transcription of the albumin gene. A molecular mechanism involved in viral liver infection with implications for hepatocarcinogenesis. J. Biol. Chem. 1999, 274, 32137–32144. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, G.C.; Morgan, E.H. Albumin and transferrin synthesis during development in the rat. Biochem. J. 1974, 144, 215–224. [Google Scholar] [CrossRef]
- Misumi, Y.; Oda, K.; Fujiwara, T.; Takami, N.; Tashiro, K.; Ikehara, Y. Functional expression of furin demonstrating its intracellular localization and endoprotease activity for processing of proalbumin and complement pro-C3. J. Biol. Chem. 1991, 266, 16954–16959. [Google Scholar] [CrossRef]
- Huang, Y.; Shinzawa, H.; Togashi, H.; Takahashi, T.; Kuzumaki, T.; Otsu, K.; Ishikawa, K. Interleukin-6 down-regulates expressions of the aldolase B and albumin genes through a pathway involving the activation of tyrosine kinase. Arch. Biochem. Biophys. 1995, 320, 203–209. [Google Scholar] [CrossRef]
- Brenner, D.A.; Buck, M.; Feitelberg, S.P.; Chojkier, M. Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia. J. Clin. Investig. 1990, 85, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.A.; Oratz, M.; Mongelli, J.; Schreiber, S.S. Effects of a short-term fast on albumin synthesis studied In Vivo, in the perfused liver, and on amino acid incorporation by hepatic microsomes. J. Clin. Investig. 1968, 47, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Kelman, L.; Saunders, S.J.; Wicht, S.; Frith, L.; Corrigall, A.; Kirsch, R.E.; Terblanche, J. The effects of amino acids on albumin synthesis by the isolated perfused rat liver. Biochem. J. 1972, 129, 805–809. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Mongelli, J.; Fishman, L.; Schreiber, S.S. Amino acid regulation of albumin synthesis. J. Nutr. 1969, 98, 395–403. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Alcohol, amino acids, and albumin synthesis. Gastroenterology 1974, 67, 1200–1213. [Google Scholar] [CrossRef]
- Oratz, M.; Rothschild, M.A.; Schreiber, S.S.; Burks, A.; Mongelli, J.; Matarese, B. The role of the urea cycle and polyamines in albumin synthesis. Hepatology 1983, 3, 567–571. [Google Scholar] [CrossRef]
- Oratz, M.; Rothschild, M.A.; Schreiber, S.S. Alcohol, amino acids, and albumin synthesis. II. Alcohol inhibition of albumin synthesis reversed by arginine and spermine. Gastroenterology 1976, 71, 123–127. [Google Scholar] [CrossRef]
- Johnson, T.R.; Rudin, S.D.; Blossey, B.K.; Ilan, J.; Ilan, J. Newly synthesized RNA: Simultaneous measurement in intact cells of transcription rates and RNA stability of insulin-like growth factor I, actin, and albumin in growth hormone-stimulated hepatocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 5287–5291. [Google Scholar] [CrossRef] [PubMed]
- Pusch, W. Osmotic pressure of dextran T10 solutions. Desalination 1988, 68, 69–73. [Google Scholar] [CrossRef]
- Yamauchi, A.; Fukuhara, Y.; Yamamoto, S.; Yano, F.; Takenaka, M.; Imai, E.; Noguchi, T.; Tanaka, T.; Kamada, T.; Ueda, N. Oncotic pressure regulates gene transcriptions of albumin and apolipoprotein B in cultured rat hepatoma cells. Am. J. Physiol. 1992, 263, C397–C404. [Google Scholar] [CrossRef]
- Van Venrooij, W.J.W.; Henshaw, E.C.; Hirsch, C.A. Effects of deprival of glucose or individual amino acids on polyribosome distribution and rate of protein synthesis in cultured mammalian cells. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1972, 259, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Oratz, M.; Rothschild, M.A.; Schreiber, S.S. Alcohol, amino acids, and albumin synthesis: III. Effects of ethanol, acetaldehyde, and 4-methylpyrazole. Gastroenterology 1978, 74, 672–676. [Google Scholar] [CrossRef]
- Hradec, J.; Stiborová, M.; Dusek, Z.; Franĕk, F. Biosynthesis of rabbit serum albumin in a heterologous fractionated subcellular system. Eur. J. Biochem. 1983, 131, 277–281. [Google Scholar] [CrossRef]
- Tse, T.P.; Taylor, J.M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J. Biol. Chem. 1977, 252, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Judah, J.D.; Quinn, P.S. Calcium ion-dependent vesicle fusion in the conversion of proalbumin to albumin. Nature 1978, 271, 384–385. [Google Scholar] [CrossRef]
- Brennan, S.O.; Peach, R.J.; Boswell, D.R. Novel human proalbumin variant with intact dibasic sequence facilitates identification of its converting enzyme. Biochim. Biophys. Acta 1989, 993, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Misumi, Y.; Sohda, M.; Takami, N.; Sakaki, Y.; Ikehara, Y. Selective processing of proalbumin determined by site-specific mutagenesis. Biochem. Biophys. Res. Commun. 1991, 175, 690–696. [Google Scholar] [CrossRef]
- Oda, K.; Ikehara, Y. Weakly basic amines inhibit the proteolytic conversion of proalbumin to serum albumin in cultured rat hepatocytes. Eur. J. Biochem. 1985, 152, 605–609. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Bravo, J. High affinity binding, endocytosis, and degradation of conformationally modified albumins. Potential role of gp30 and gp18 as novel scavenger receptors. J. Biol. Chem. 1993, 268, 7562–7570. [Google Scholar] [CrossRef]
- Strobel, J.L.; Cady, S.G.; Borg, T.K.; Terracio, L.; Baynes, J.W.; Thorpe, S.R. Identification of fibroblasts as a major site of albumin catabolism in peripheral tissues. J. Biol. Chem. 1986, 261, 7989–7994. [Google Scholar] [CrossRef]
- Slattery, C.; Lee, A.; Zhang, Y.; Kelly, D.J.; Thorn, P.; Nikolic-Paterson, D.J.; Tesch, G.H.; Poronnik, P. In vivo visualization of albumin degradation in the proximal tubule. Kidney Int. 2008, 74, 1480–1486. [Google Scholar] [CrossRef]
- Carson, J.M.; Okamura, K.; Wakashin, H.; McFann, K.; Dobrinskikh, E.; Kopp, J.B.; Blaine, J. Podocytes degrade endocytosed albumin primarily in lysosomes. PLoS ONE 2014, 9, e99771. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, R.; Kulka, R.G. Degradation of microinjected methylated and unmethylated proteins in hepatoma tissue culture cells. J. Biol. Chem. 1983, 258, 9597–9600. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.P.; Croze, E.M.; Morré, D.J.; Schreiber, G. Albumin secreted by rat liver bypasses Golgi apparatus cisternae. Biochim. Biophys. Acta 1981, 678, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.K.; Rosenthal, T.C. Prealbumin: A marker for nutritional evaluation. Am. Fam. Physician 2002, 65, 1575–1578. [Google Scholar]
- Schnitzer, J.E.; Oh, P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J. Biol. Chem. 1994, 269, 6072–6082. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Albumin metabolism. Gastroenterology 1973, 64, 324–337. [Google Scholar] [CrossRef]
- Mellor, T.; Harris, N.; Goldkind, L. Severe Abdominal Pain and Intestinal Edema Due to Hypoalbuminemia: 1077. Am. J. Gastroenterol. 2015, 110, S468. [Google Scholar] [CrossRef]
- Ledgerwood, E.C.; George, P.M.; Bathurst, I.C.; Brennan, S.O. The predicted proteinase furin is not the hepatic proalbumin convertase. Biochim. Biophys. Acta 1992, 1159, 9–12. [Google Scholar] [CrossRef]
- Ledgerwood, E.C.; Brennan, S.O.; George, P.M. Endoproteases other than furin have a role in hepatic proprotein processing. Biochem. Mol. Biol. Int. 1997, 42, 1131–1142. [Google Scholar] [CrossRef]
- Brennan, S.O.; Owen, M.C.; Boswell, D.R.; Lewis, J.H.; Carrell, R.W. Circulating proalbumin associated with a variant proteinase inhibitor. Biochim. Biophys. Acta 1984, 802, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Abdo, Y.; Rousseaux, J.; Dautrevaux, M. Proalbumin Lille, a new variant of human serum albumin. FEBS Lett. 1981, 131, 286–288. [Google Scholar] [CrossRef]
- Takahashi, N.; Takahashi, Y.; Putnam, F.W. Structural changes and metal binding by proalbumins and other amino-terminal genetic variants of human serum albumin. Proc. Natl. Acad. Sci. USA 1987, 84, 7403–7407. [Google Scholar] [CrossRef]
- Kragh-Hansen, U.; Brennan, S.O.; Minchiotti, L.; Galliano, M. Modified high-affinity binding of Ni2+, Ca2+ and Zn2+ to natural mutants of human serum albumin and proalbumin. Biochem. J. 1994, 301, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; He, X.M.; Munson, S.H.; Twigg, P.D.; Gernert, K.M.; Broom, M.B.; Miller, T.Y. Three-dimensional structure of human serum albumin. Science 1989, 244, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Wu, N.; Liu, T.; Tian, M.; Liu, C.; Ma, S.; Cao, H.; Bian, H.; Wang, L.; Feng, Y.; Qi, J. Albumin, an interesting and functionally diverse protein, varies from ‘native’ to ‘effective’ (Review). Mol. Med. Rep. 2024, 29, 24. [Google Scholar] [CrossRef]
- Martin, S.C.; Ekman, P. In vitro phosphorylation of serum albumin by two protein kinases: A potential pitfall in protein phosphorylation reactions. Anal. Biochem. 1986, 154, 395–399. [Google Scholar] [CrossRef]
- Rodrigues Oliveira, A.; Chevalier, C.; Wargny, M.; Pakulska, V.; Caradeuc, C.; Cloteau, C.; Letertre, M.P.M.; Giraud, N.; Bertho, G.; Bigot-Corbel, E.; et al. Methylglyoxal-Induced Glycation of Plasma Albumin: From Biomarker Discovery to Clinical Use for Prediction of New-Onset Diabetes in Individuals with Prediabetes. Clin. Chem. 2025, 71, 688–699. [Google Scholar] [CrossRef]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef]
- Shiraki, T.; Miura, Y.; Sawada, T.; Okada, T.; Sakuraoka, Y.; Muto, T.; Kubota, K. Glycated albumin suppresses glucose-induced insulin secretion by impairing glucose metabolism in rat pancreatic β-cells. Nutr. Metab. 2011, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Yamasaki, K.; Maruyama, T.; Kragh-Hansen, U.; Otagiri, M. Effect of oxidative stress on the structure and function of human serum albumin. Pharm. Res. 2001, 18, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Kubota, K.; Yamada, N.; Tagami, U.; Takehana, K.; Sonaka, I.; Suzuki, E.; Hirayama, K. Identification and characterization of oxidized human serum albumin. FEBS J. 2006, 273, 3346–3357. [Google Scholar] [CrossRef] [PubMed]
- Sakurama, K.; Nishi, K.; Chuang, V.T.G.; Hashimoto, M.; Yamasaki, K.; Otagiri, M. Effects of Oxidation of Human Serum Albumin on the Binding of Aripiprazole. Biol. Pharm. Bull. 2020, 43, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Burczynski, F.J.; Wang, G.Q.; Hnatowich, M. Effect of nitric oxide on albumin-palmitate binding. Biochem. Pharmacol. 1995, 49, 91–96. [Google Scholar] [CrossRef]
- Chubarov, A.; Spitsyna, A.; Krumkacheva, O. Reversible Dimerization of Human Serum Albumin. Molecules 2020, 26, 108. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G. N-Acetyl-Cysteine Regenerates Albumin Cys34 by a Thiol-Disulfide Breaking Mechanism: An Explanation of Its Extracellular Antioxidant Activity. Antioxidants 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Sakata, M.; Kawaguchi, T.; Taniguchi, E.; Nakayama, A.; Ishizaki, S.; Sonaka, I.; Nakamura, T.; Itou, M.; Oriishi, T.; Abe, M.; et al. Oxidized albumin is associated with water retention and severity of disease in patients with chronic liver diseases. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2010, 5, e247–e253. [Google Scholar] [CrossRef]
- Bonifazi, M.; Meessen, J.; Pérez, A.; Vasques, F.; Busana, M.; Vassalli, F.; Novelli, D.; Bernasconi, R.; Signori, C.; Masson, S.; et al. Albumin Oxidation Status in Sepsis Patients Treated With Albumin or Crystalloids. Front. Physiol. 2021, 12, 682877. [Google Scholar] [CrossRef]
- Fujii, R.; Ueyama, J.; Aoi, A.; Ichino, N.; Osakabe, K.; Sugimoto, K.; Suzuki, K.; Hamajima, N.; Wakai, K.; Kondo, T. Oxidized human serum albumin as a possible correlation factor for atherosclerosis in a rural Japanese population: The results of the Yakumo Study. Environ. Health Prev. Med. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, H.; Höfler, M.; Beckmann, R.; Hufnagl, P.; Vanyek, E.; Bielek, E.; Wojta, J.; Fabry, A.; Lockie, S.; Binder, B.R. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J. Cell Sci. 1996, 109, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, H.; Siddiqui, S.; Kelly, E.; Medbury, H. The anti-apoptotic activity of albumin for endothelium is inhibited by advanced glycation end products restricting intramolecular movement. Cell. Mol. Biol. Lett. 2009, 14, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.A.; Castelli, I.; Pargger, H.; Drop, L.J. Nitric oxide dose-response study in the isolated perfused rat kidney after inhibition of endothelium-derived relaxing factor synthesis: The role of serum albumin. J. Pharmacol. Exp. Ther. 1995, 273, 855–862. [Google Scholar] [CrossRef]
- Keaney, J.F.; Simon, D.I.; Stamler, J.S.; Jaraki, O.; Scharfstein, J.; Vita, J.A.; Loscalzo, J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J. Clin. Investig. 1993, 91, 1582–1589. [Google Scholar] [CrossRef]
- Xu, J.; Elshazly, A.M.; Gewirtz, D.A. The Cytoprotective, Cytotoxic and Nonprotective Functional Forms of Autophagy Induced by Microtubule Poisons in Tumor Cells-Implications for Autophagy Modulation as a Therapeutic Strategy. Biomedicines 2022, 10, 1632. [Google Scholar] [CrossRef]
- Baral, A.; Park, P.-H. Leptin Induces Apoptotic and Pyroptotic Cell Death via NLRP3 Inflammasome Activation in Rat Hepatocytes. Int. J. Mol. Sci. 2021, 22, 12589. [Google Scholar] [CrossRef]
- Chang, C.P.; Lei, H.Y. Autophagy induction in T cell-independent acute hepatitis induced by concanavalin A in SCID/NOD mice. Int. J. Immunopathol. Pharmacol. 2008, 21, 817–826. [Google Scholar] [CrossRef]
- Yang, M.-C.; Chang, C.-P.; Lei, H.-Y. Endothelial cells are damaged by autophagic induction before hepatocytes in Con A-induced acute hepatitis. Int. Immunol. 2010, 22, 661–670. [Google Scholar] [CrossRef]
- Masini, M.; Bugliani, M.; Lupi, R.; del Guerra, S.; Boggi, U.; Filipponi, F.; Marselli, L.; Masiello, P.; Marchetti, P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 2009, 52, 1083–1086. [Google Scholar] [CrossRef]
- Chang, C.-P.; Yang, M.-C.; Lei, H.-Y. Concanavalin A/IFN-Gamma Triggers Autophagy-Related Necrotic Hepatocyte Death through IRGM1-Mediated Lysosomal Membrane Disruption. PLoS ONE 2011, 6, e28323. [Google Scholar] [CrossRef]
- Munasinghe, P.E.; Riu, F.; Dixit, P.; Edamatsu, M.; Saxena, P.; Hamer, N.S.; Galvin, I.F.; Bunton, R.W.; Lequeux, S.; Jones, G.; et al. Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. Int. J. Cardiol. 2016, 202, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, W.; Huang, D.; Sun, X. Possible intermediary role of autophagy in serum albumin decrease-associated cardiovascular events among patients with coronary heart disease. Int. J. Cardiol. 2018, 250, 64. [Google Scholar] [CrossRef]
- Onodera, J.; Ohsumi, Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J. Biol. Chem. 2005, 280, 31582–31586. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Davis, T.; Loos, B.; Sishi, B.; Huisamen, B.; Strijdom, H.; Engelbrecht, A.-M. Autophagy is essential for the maintenance of amino acids and ATP levels during acute amino acid starvation in MDAMB231 cells. Cell Biochem. Funct. 2018, 36, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Bok, E.; Kim, J.; Park, G.H.; Choi, D.Y. Dopaminergic neuroprotective effects of inosine in MPTP-induced parkinsonian mice via brain-derived neurotrophic factor upregulation. Neuropharmacology 2023, 238, 109652. [Google Scholar] [CrossRef]
- Kil, Y.-S.; Baral, A.; Jeong, B.-S.; Laatikainen, P.; Liu, Y.; Han, A.-R.; Hong, M.-J.; Kim, J.-B.; Choi, H.; Park, P.-H.; et al. Combining NMR and MS to Describe Pyrrole-2-Carbaldehydes in Wheat Bran of Radiation. J. Agric. Food Chem. 2022, 70, 13002–13014. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Çiğ, B.; Yazğan, Y.; Schwaerzer, G.K.; Theilig, F.; Pecze, L. Albumin evokes Ca2+-induced cell oxidative stress and apoptosis through TRPM2 channel in renal collecting duct cells reduced by curcumin. Sci. Rep. 2019, 9, 12403. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Proics, E.; de Bieville, C.H.; Rousseau, D.; Bonnafous, S.; Patouraux, S.; Adam, G.; Lavallard, V.J.; Rovere, C.; Le Thuc, O.; et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015, 6, e1879. [Google Scholar] [CrossRef]
- Baral, A. Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses. Stresses 2024, 4, 481–504. [Google Scholar] [CrossRef]
- Galehdar, Z.; Swan, P.; Fuerth, B.; Callaghan, S.M.; Park, D.S.; Cregan, S.P. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J. Neurosci. 2010, 30, 16938–16948. [Google Scholar] [CrossRef] [PubMed]
- Prola, A.; Pires Da Silva, J.; Guilbert, A.; Lecru, L.; Piquereau, J.; Ribeiro, M.; Mateo, P.; Gressette, M.; Fortin, D.; Boursier, C.; et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017, 24, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 2010, 10, 156–165. [Google Scholar] [CrossRef]
- Nolin, A.C.; Mulhern, R.M.; Panchenko, M.V.; Pisarek-Horowitz, A.; Wang, Z.; Shirihai, O.; Borkan, S.C.; Havasi, A. Proteinuria causes dysfunctional autophagy in the proximal tubule. Am. J. Physiol. Ren. Physiol. 2016, 311, F1271–F1279. [Google Scholar] [CrossRef] [PubMed]
- Delitsikou, V.; Jarad, G.; Rajaram, R.D.; Ino, F.; Rutkowski, J.M.; Chen, C.D.; Santos, C.X.C.; Scherer, P.E.; Abraham, C.R.; Shah, A.M.; et al. Klotho regulation by albuminuria is dependent on ATF3 and endoplasmic reticulum stress. FASEB J. 2020, 34, 2087–2104. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chang, J.W.; Yang, W.S.; Kim, S.B.; Park, S.K.; Park, J.S.; Lee, S.K. Albumin-induced epithelial-mesenchymal transition and ER stress are regulated through a common ROS-c-Src kinase-mTOR pathway: Effect of imatinib mesylate. Am. J. Physiol. Ren. Physiol. 2011, 300, F1214–F1222. [Google Scholar] [CrossRef]
- Gonçalves, G.L.; Costa-Pessoa, J.M.; Thieme, K.; Lins, B.B.; Oliveira-Souza, M. Intracellular albumin overload elicits endoplasmic reticulum stress and PKC-delta/p38 MAPK pathway activation to induce podocyte apoptosis. Sci. Rep. 2018, 8, 18012. [Google Scholar] [CrossRef]
- Baral, A.; Park, P.H. Interleukin-1β Signaling Contributes to Cell Cycle Arrest and Apoptotic Cell Death by Leptin via Modulation of AKT and p38MAPK in Hepatocytes. Biomol. Ther. 2024, 32, 611–626. [Google Scholar] [CrossRef]
- Baral, A. Mechanisms of Inflammasome Activation and Involvement in Liver Disease. J. Mol. Pathol. 2024, 5, 171–186. [Google Scholar] [CrossRef]
- Chang, A.; Ko, K.; Clark, M.R. The emerging role of the inflammasome in kidney diseases. Curr. Opin. Nephrol. Hypertens. 2014, 23, 204–210. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yasinta, M.; Hu, C.; Zhao, M.; Ding, G.; Bai, M.; Yang, L.; Ni, J.; Wang, R.; Jia, Z.; et al. Mitochondrial dysfunction confers albumin-induced NLRP3 inflammasome activation and renal tubular injury. Am. J. Physiol. Ren. Physiol. 2015, 308, F857–F866. [Google Scholar] [CrossRef] [PubMed]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, F.; Liang, J.; Deng, X.; Zhang, Y.; Ding, G.; Zhang, A.; Jia, Z.; Huang, S. Activation of COX-2/mPGES-1/PGE2 Cascade via NLRP3 Inflammasome Contributes to Albumin-Induced Proximal Tubule Cell Injury. Cell. Physiol. Biochem. 2017, 42, 797–807. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ding, G.; Zhao, M.; Bai, M.; Yang, L.; Ni, J.; Wang, R.; Jia, Z.; Huang, S.; Zhang, A. NLRP3 inflammasome mediates albumin-induced renal tubular injury through impaired mitochondrial function. J. Biol. Chem. 2014, 289, 25101–25111. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Li, L.; Ma, T.; Shi, M.; Yang, Y.; Fan, Q. A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1297–1309. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Z.; Zhang, C.; Shi, Y.; Han, W.; Song, S.; Mu, L.; Du, C.; Shi, Y. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism 2021, 118, 154748. [Google Scholar] [CrossRef]
- Østergaard, J.A.; Jha, J.C.; Sharma, A.; Dai, A.; Choi, J.S.Y.; de Haan, J.B.; Cooper, M.E.; Jandeleit-Dahm, K. Adverse renal effects of NLRP3 inflammasome inhibition by MCC950 in an interventional model of diabetic kidney disease. Clin. Sci. 2022, 136, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, I.; Chiarla, C.; Giuliante, F.; Vellone, M.; Ardito, F.; Nuzzo, G. The relationship between albumin, other plasma proteins and variables, and age in the acute phase response after liver resection in man. Amino Acids 2006, 31, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, R.; Zeni, N.; Piano, S. Intravenous albumin in cirrhosis: Updated clinical uses and novel perspectives. Ann. Hepatol. 2023, 28, 101150. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Zimmon, D.; Schreiber, S.S.; Weiner, I.; Van Caneghem, A. Albumin synthesis in cirrhotic subjects with ascites studied with carbonate-14C. J. Clin. Investig. 1969, 48, 344–350. [Google Scholar] [CrossRef]
- Steyl, C.; Van Zyl-Smit, R. Mechanisms of oedema formation: The minor role of hypoalbuminaemia. S. Afr. Med. J. 2009, 99, 57–59. [Google Scholar] [PubMed]
- Wei, N.; Liu, C.; Zhu, H.; Wang, C.; Zhou, Y.; Xiao, Z.; Du, L.; Song, Y. Hypoalbuminemia contributes to ascites formation via sodium and water retention: Evidence from clinical date and albumin deficient mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2024, 1870, 167275. [Google Scholar] [CrossRef] [PubMed]
- Falasca, L.; Favale, A.; Gualandi, G.; Maietta, G.; Conti Devirgiliis, L. Retinoic acid treatment induces apoptosis or expression of a more differentiated phenotype on different fractions of cultured fetal rat hepatocytes. Hepatology 1998, 28, 727–737. [Google Scholar] [CrossRef]
- Das, S.; Maras, J.S.; Hussain, M.S.; Sharma, S.; David, P.; Sukriti, S.; Shasthry, S.M.; Maiwall, R.; Trehanpati, N.; Singh, T.P.; et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017, 65, 631–646. [Google Scholar] [CrossRef]
- Fernández, J.; Clària, J.; Amorós, A.; Aguilar, F.; Castro, M.; Casulleras, M.; Acevedo, J.; Duran-Güell, M.; Nuñez, L.; Costa, M.; et al. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology 2019, 157, 149–162. [Google Scholar] [CrossRef]
- Duran-Güell, M.; Flores-Costa, R.; Casulleras, M.; López-Vicario, C.; Titos, E.; Díaz, A.; Alcaraz-Quiles, J.; Horrillo, R.; Costa, M.; Fernández, J.; et al. Albumin protects the liver from tumor necrosis factor α-induced immunopathology. FASEB J. 2021, 35, e21365. [Google Scholar] [CrossRef]
- Weinbach, E.C.; Garbus, J. Restoration by Albumin of Oxidative Phosphorylation and Related Reactions. J. Biol. Chem. 1966, 241, 169–175. [Google Scholar] [CrossRef]
- Clària, J.; Aguilar, F.; Lozano, J.J.; Jiménez-Gracia, L.; Nieto, J.C.; Romero-Grimaldo, B.; Marcos-Fa, X.; Giarracco, E.; Weiss, E.; Trebicka, J.; et al. Albumin reprograms the B cell transcriptional landscape and improves neutrophil antimicrobial function in patients with decompensated cirrhosis. JHEP Rep. 2024, 6, 101184. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Ju, A.; Zhang, S.; An, Q.; Xu, S.; Liu, J.; Yu, L.; Fu, Y.; Luo, Y. Albumosomes formed by cytoplasmic pre-folding albumin maintain mitochondrial homeostasis and inhibit nonalcoholic fatty liver disease. Signal Transduct. Target. Ther. 2023, 8, 229. [Google Scholar] [CrossRef]
- Panduro, A.; Shalaby, F.; Weiner, F.R.; Biempica, L.; Zern, M.A.; Shafritz, D.A. Transcriptional switch from albumin to alpha-fetoprotein and changes in transcription of other genes during carbon tetrachloride induced liver regeneration. Biochemistry 1986, 25, 1414–1420. [Google Scholar] [CrossRef]
- Qi, Z.; Qi, X. Alterations in the "Gut-Liver Axis" on Rats with Immunological Hepatic Fibrosis. J. Immunol. Res. 2023, 2023, 5577850. [Google Scholar] [CrossRef] [PubMed]
- Biere, A.L.; Ostaszewski, B.; Stimson, E.R.; Hyman, B.T.; Maggio, J.E.; Selkoe, D.J. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J. Biol. Chem. 1996, 271, 32916–32922. [Google Scholar] [CrossRef] [PubMed]
- Bode, D.C.; Stanyon, H.F.; Hirani, T.; Baker, M.D.; Nield, J.; Viles, J.H. Serum Albumin’s Protective Inhibition of Amyloid-β Fiber Formation Is Suppressed by Cholesterol, Fatty Acids and Warfarin. J. Mol. Biol. 2018, 430, 919–934. [Google Scholar] [CrossRef]
- Yang, M.X.; Wang, Z.R.; Zhang, Y.L.; Zhang, Z.N.; Li, Y.L.; Wang, R.; Su, Q.; Guo, J.H. Albumin antagonizes Alzheimer’s disease-related Tau pathology and enhances cognitive performance by inhibiting aberrant Tau aggregation. Exp. Neurol. 2025, 386, 115155. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, H.; Wang, L.; Xu, L.; Zhang, X.; Yu, L.; Liu, Q.; Li, Y.; Zhao, N.; Zhao, N.; et al. Human albumin attenuates excessive innate immunity via inhibition of microglial Mincle/Syk signaling in subarachnoid hemorrhage. Brain Behav. Immun. 2017, 60, 346–360. [Google Scholar] [CrossRef]
- Boada, M.; López, O.L.; Olazarán, J.; Núñez, L.; Pfeffer, M.; Paricio, M.; Lorites, J.; Piñol-Ripoll, G.; Gámez, J.E.; Anaya, F.; et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: Primary results of the AMBAR Study. Alzheimer’s Dement. 2020, 16, 1412–1425. [Google Scholar] [CrossRef]
- Martin, R.H.; Yeatts, S.D.; Hill, M.D.; Moy, C.S.; Ginsberg, M.D.; Palesch, Y.Y. ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2. Stroke 2016, 47, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Manole, M.D.; Kochanek, P.M.; Foley, L.M.; Hitchens, T.K.; Bayır, H.; Alexander, H.; Garman, R.; Ma, L.; Hsia, C.J.C.; Ho, C.; et al. Polynitroxyl Albumin and Albumin Therapy after Pediatric Asphyxial Cardiac Arrest: Effects on Cerebral Blood Flow and Neurologic Outcome. J. Cereb. Blood Flow Metab. 2011, 32, 560–569. [Google Scholar] [CrossRef]
- Kim, Y.R.; van Meer, M.P.A.; Mandeville, J.B.; Tejima, E.; Dai, G.; Topalkara, K.; Qui, J.; Dijkhuizen, R.M.; Moskowitz, M.A.; Lo, E.H.; et al. fMRI of Delayed Albumin Treatment during Stroke Recovery in Rats: Implication for Fast Neuronal Habituation in Recovering Brains. J. Cereb. Blood Flow Metab. 2007, 27, 142–153. [Google Scholar] [CrossRef]
- Hofmann, M.; McCormack, E.; Mujić, M.; Rossberg, M.; Bernd, A.; Bereiter-Hahn, J.; Gjertsen, B.T.; Wiig, H.; Kippenberger, S. Increased plasma colloid osmotic pressure facilitates the uptake of therapeutic macromolecules in a xenograft tumor model. Neoplasia 2009, 11, 812–822. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Wang, J.; Fang, F.; Cheng, G.; Jiang, Y.; Xiao, H.; Wan, Q. Impact of serum uric acid, albumin and their interaction on Parkinson’s disease. Neurol. Sci. 2017, 38, 331–336. [Google Scholar] [CrossRef]
- Pisani, V.; Stefani, A.; Pierantozzi, M.; Natoli, S.; Stanzione, P.; Franciotta, D.; Pisani, A. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J. Neuroinflamm. 2012, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Horrillo, R.; Ortiz, A.M.; Pérez, A.; Mestre, A.; Ruiz, A.; Boada, M.; Grancha, S. Increased Albumin Oxidation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2018, 63, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, A.C.V.; Razzak, M.A.; Cho, J.H.; Kim, J.Y.; Choi, S.S. Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-like Symptoms in C. elegans. Nanomaterials 2022, 12, 758. [Google Scholar] [CrossRef]
- Xu, K.; Huang, P.; Wu, Y.; Liu, T.; Shao, N.; Zhao, L.; Hu, X.; Chang, J.; Peng, Y.; Qu, S. Engineered Selenium/Human Serum Albumin Nanoparticles for Efficient Targeted Treatment of Parkinson’s Disease via Oral Gavage. ACS Nano 2023, 17, 19961–19980. [Google Scholar] [CrossRef]
- Khanal, S.; Shin, E.J.; Yoo, C.J.; Kim, J.; Choi, D.Y. Inosine exerts dopaminergic neuroprotective effects via mitigation of NLRP3 inflammasome activation. Neuropharmacology 2025, 266, 110278. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaikhan, E.; Bayarsaikhan, D.; Lee, J.; Son, M.; Oh, S.; Moon, J.; Park, H.J.; Roshini, A.; Kim, S.U.; Song, B.J.; et al. Microglial AGE-albumin is critical for neuronal death in Parkinson’s disease: A possible implication for theranostics. Int. J. Nanomed. 2015, 10, 281–292. [Google Scholar] [CrossRef]
- Choe, W.H.; Baik, S.K. Prostaglandin E2 -mediated immunosuppression and the role of albumin as its modulator. Hepatology 2015, 61, 1080–1082. [Google Scholar] [CrossRef]
- Yoo, S.-K.; Chowell, D.; Valero, C.; Morris, L.G.T.; Chan, T.A. Pre-treatment serum albumin and mutational burden as biomarkers of response to immune checkpoint blockade. NPJ Precis. Oncol. 2022, 6, 23. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, S.T.; Kim, T.J.; Jun, J.S.; Moon, J.; Jung, K.H.; Park, K.I.; Chu, K.; Lee, S.K. High albumin level is a predictor of favorable response to immunotherapy in autoimmune encephalitis. Sci. Rep. 2018, 8, 1012. [Google Scholar] [CrossRef]
- Casulleras, M.; Flores-Costa, R.; Duran-Güell, M.; Alcaraz-Quiles, J.; Sanz, S.; Titos, E.; López-Vicario, C.; Fernández, J.; Horrillo, R.; Costa, M.; et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci. Transl. Med. 2020, 12, eaax5135. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, R.; Andreola, F.; Mehta, G.; Poulton, K.; Oria, M.; Jover, M.; Soeda, J.; Macnaughtan, J.; De Chiara, F.; Habtesion, A.; et al. Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. J. Hepatol. 2015, 62, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Fleck, A.; Hawker, F.; Wallace, P.I.; Raines, G.; Trotter, J.; Ledingham, I.M.; Calman, K.C. Increased Vascular Permeability: A Major Cause of Hypoalbuminaemia in Disease and Injury. Lancet 1985, 325, 781–784. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Gabarre, P.; Desnos, C.; Morin, A.; Missri, L.; Urbina, T.; Bonny, V.; Turpin, M.; Baudel, J.L.; Berard, L.; Montil, M.; et al. Albumin versus saline infusion for sepsis-related peripheral tissue hypoperfusion: A proof-of-concept prospective study. Crit. Care 2024, 28, 43. [Google Scholar] [CrossRef]
- Ye, Z.; Gao, M.; Ge, C.; Lin, W.; Zhang, L.; Zou, Y.; Peng, Q. Association between albumin infusion and septic patients with coronary heart disease: A retrospective study based on medical information mart for intensive care III database. Front. Cardiovasc. Med. 2022, 9, 982969. [Google Scholar] [CrossRef] [PubMed]
- Maiwall, R.; Kumar, A.; Pasupuleti, S.S.R.; Hidam, A.K.; Tevethia, H.; Kumar, G.; Sahney, A.; Mitra, L.G.; Sarin, S.K. A randomized-controlled trial comparing 20% albumin to plasmalyte in patients with cirrhosis and sepsis-induced hypotension [ALPS trial]. J. Hepatol. 2022, 77, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Bauer, M.; Nierhaus, A.; Kluge, S.; Schumacher, U.; Putensen, C.; Fichtner, F.; Petros, S.; Scheer, C.; Jaschinski, U.; et al. Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): Protocol for a randomized controlled trial. Trials 2020, 21, 1002. [Google Scholar] [CrossRef]
- Djoussé, L.; Rothman, K.J.; Cupples, L.A.; Levy, D.; Ellison, R.C. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation 2002, 106, 2919–2924. [Google Scholar] [CrossRef]
- Talukder, A.; Siraj, M.M.; Khondokar, M.N.; Habib, S.M.A.; Salim, M.A.; Rahman, M.W.; Banerjee, S.K.; Ahsan, S.A.; Hoque, M.H.; Rahman, F. Outcome of Albumin Infusion in Heart Failure Patients. Univ. Heart J. 2019, 15, 47–53. [Google Scholar] [CrossRef]
- Kugiyama, K.; Kerns, S.A.; Morrisett, J.D.; Roberts, R.; Henry, P.D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature 1990, 344, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Joles, J.A.; Willekes-Koolschijn, N.; Koomans, H.A. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997, 52, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.D.; De Kimpe, S.; De Roos, R.; Rabelink, T.J.; Koomans, H.A.; Joles, J.A. Albumin restores lysophosphatidylcholine-induced inhibition of vasodilation in rat aorta. Kidney Int. 2001, 60, 1088–1096. [Google Scholar] [CrossRef]
- Valeriani, E.; Pannunzio, A.; Palumbo, I.M.; Bartimoccia, S.; Cammisotto, V.; Castellani, V.; Porfidia, A.; Pignatelli, P.; Violi, F. Risk of venous thromboembolism and arterial events in patients with hypoalbuminemia: A comprehensive meta-analysis of more than 2 million patients. J. Thromb. Haemost. 2024, 22, 2823–2833. [Google Scholar] [CrossRef]
- Basili, S.; Carnevale, R.; Nocella, C.; Bartimoccia, S.; Raparelli, V.; Talerico, G.; Stefanini, L.; Romiti, G.F.; Perticone, F.; Corazza, G.R.; et al. Serum Albumin Is Inversely Associated With Portal Vein Thrombosis in Cirrhosis. Hepatol. Commun. 2019, 3, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Paar, M.; Rossmann, C.; Nusshold, C.; Wagner, T.; Schlagenhauf, A.; Leschnik, B.; Oettl, K.; Koestenberger, M.; Cvirn, G.; Hallström, S. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS ONE 2017, 12, e0182997. [Google Scholar] [CrossRef]
- Rasmussen, K.C.; Højskov, M.; Johansson, P.I.; Kridina, I.; Kistorp, T.; Salling, L.; Nielsen, H.B.; Ruhnau, B.; Pedersen, T.; Secher, N.H. Impact of Albumin on Coagulation Competence and Hemorrhage During Major Surgery: A Randomized Controlled Trial. Medicine 2016, 95, e2720. [Google Scholar] [CrossRef]
- Galanakis, D.K. Anticoagulant albumin fragments that bind to fibrinogen/fibrin: Possible implications. Semin. Thromb. Hemost. 1992, 18, 44–52. [Google Scholar] [CrossRef]
- Aibiki, M.; Fukuoka, N.; Shiro, B.; Matsumoto, H.; Ohshita, M.; Maekawam, S.; Takebe, J. 25% Albumin Infusion Maintains Antithrombin III (AT) Activity after AT AgentAdministration in Critically Ill Patients with Disseminated IntravascularCoagulation (DIC). J. Blood Disord. Transfus. 2014, 5, 1000208. [Google Scholar]
- Lam, F.W.; Cruz, M.A.; Leung, H.-C.E.; Parikh, K.S.; Smith, C.W.; Rumbaut, R.E. Histone induced platelet aggregation is inhibited by normal albumin. Thromb. Res. 2013, 132, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.W.; Zollinger, R.M.; Moore, R.; Ellison, E.H. The Use of Human Serum Albumin in the Management of Acute Pancreatitis: Experimental and Clinical Observations. Gastroenterology 1955, 28, 563–592. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wan, J.; He, W.; Zhu, Y.; Zeng, H.; Liu, P.; Liu, J.; Xia, L.; Liu, F.; Zhu, Y.; et al. Albumin infusion may decrease the mortality of hypoalbuminemia patients with severe acute pancreatitis: A retrospective cohort study. BMC Gastroenterol. 2023, 23, 195. [Google Scholar] [CrossRef]
- Abdo, E.E.; Coelho, A.M.M.; Patzina, R.A.; Sampietre, S.N.; Cunha, J.E.M.; Machado, M.C.C.; D’Albuquerque, L.A.C. Nitric oxide synthase inhibition reduces albumin induced lung damage in acute pancreatitis. Pancreatology 2013, 13, 225–229. [Google Scholar] [CrossRef]
- Kiaer, C.; Thams, P. Serum albumin protects from cytokine-induced pancreatic beta cell death by a phosphoinositide 3-kinase-dependent mechanism. Endocrine 2009, 35, 325–332. [Google Scholar] [CrossRef]
- Header, D.A.; Aboelwafa, R.A.; Elkeleny, M.R.; Bedewy, E.S.; Ellakany, A.I. C-reactive protein/albumin ratio (CAR) as a marker for detecting acute severe ulcerative colitis in Egyptian patients. Rev. Gastroenterol. México 2022, 87, 447–454. [Google Scholar] [CrossRef]
- Dusunceli, I.; Gok, S.Z.; Umut, C.; and Sargin, F. The ability of C-reactive protein-albumin ratio to predict disease activity in ulcerative colitis. Biomark. Med. 2025, 19, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Furukawa, S.; Shiraishi, K.; Miyake, T.; Tange, K.; Hashimoto, Y.; Kitahata, S.; Kawamura, T.; Ninomiya, T.; Mori, K.; et al. The albumin to globulin ratio is associated with clinical outcome in Japanese patients with ulcerative colitis. Ann. Coloproctol. 2023, 39, 155–163. [Google Scholar] [CrossRef]
- Steinfeld, J.L.; Davidson, J.D.; Gordon, R.S.; Greene, F.E. The mechanism of hypoproteinemia in patients with regional enteritis and ulcerative colitis. Am. J. Med. 1960, 29, 405–415. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, W.; Zhang, W.; Ren, F.; Wang, P. Pea Albumin Attenuates Dextran Sulfate Sodium-Induced Colitis by Regulating NF-κB Signaling and the Intestinal Microbiota in Mice. Nutrients 2022, 14, 3611. [Google Scholar] [CrossRef]
- Yang, X.; Mao, Z.; Huang, Y.; Yan, H.; Yan, Q.; Hong, J.; Fan, J.; Yao, J. Reductively modified albumin attenuates DSS-Induced mouse colitis through rebalancing systemic redox state. Redox Biol. 2021, 41, 101881. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.H.; Gustavson, L.E.; Sperelakis, R.; Lam, N.P.; El-Shourbagy, T.; Qian, J.X.; Layden, T. Pharmacokinetics and Safety of Tiagabine in Subjects with Various Degrees of Hepatic Function. Epilepsia 1997, 38, 445–451. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Hennig, B.; Ott, L.G.; Goldblum, S.; Young, A.B. Mechanisms and implications of hypoalbuminemia in head-injured patients. J. Neurosurg. 1988, 69, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, F.; Erill, S. Abnormal serum protein binding of acidic drugs in diabetes mellitus. Clin. Pharmacol. Ther. 1984, 36, 691–695. [Google Scholar] [CrossRef]
- Baraka-Vidot, J.; Guerin-Dubourg, A.; Bourdon, E.; Rondeau, P. Impaired drug-binding capacities of In Vitro and In Vivo glycated albumin. Biochimie 2012, 94, 1960–1967. [Google Scholar] [CrossRef]
- El Kadi, N.; Taulier, N.; Le Huérou, J.Y.; Gindre, M.; Urbach, W.; Nwigwe, I.; Kahn, P.C.; Waks, M. Unfolding and Refolding of Bovine Serum Albumin at Acid pH: Ultrasound and Structural Studies. Biophys. J. 2006, 91, 3397–3404. [Google Scholar] [CrossRef]
- Takeda, K.; Wada, A.; Yamamoto, K.; Moriyama, Y.; Aoki, K. Conformational change of bovine serum albumin by heat treatment. J. Protein Chem. 1989, 8, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.R.; Zunszain, P.A.; Hamilton, J.A.; Curry, S. Location of High and Low Affinity Fatty Acid Binding Sites on Human Serum Albumin Revealed by NMR Drug-competition Analysis. J. Mol. Biol. 2006, 361, 336–351. [Google Scholar] [CrossRef]
- Zsila, F. Subdomain IB is the third major drug binding region of human serum albumin: Toward the three-sites model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [CrossRef]
- Wanwimolruk, S.; Birkett, D.J.; Brooks, P.M. Structural requirements for drug binding to site II on human serum albumin. Mol. Pharmacol. 1983, 24, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Baler, K.; Martin, O.A.; Carignano, M.A.; Ameer, G.A.; Vila, J.A.; Szleifer, I. Electrostatic Unfolding and Interactions of Albumin Driven by pH Changes: A Molecular Dynamics Study. J. Phys. Chem. B 2014, 118, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Wilting, J.; van der Giesen, W.F.; Janssen, L.H.; Weideman, M.M.; Otagiri, M.; Perrin, J.H. The effect of albumin conformation on the binding of warfarin to human serum albumin. The dependence of the binding of warfarin to human serum albumin on the hydrogen, calcium, and chloride ion concentrations as studied by circular dichroism, fluorescence, and equilibrium dialysis. J. Biol. Chem. 1980, 255, 3032–3037. [Google Scholar] [CrossRef] [PubMed]
- van der Giesen, W.F.; Wilting, J. Consequences of the N-B transition of albumin for the binding of warfarin in human serum. Biochem. Pharmacol. 1983, 32, 281–285. [Google Scholar] [CrossRef]
- Morotti, A.; Charidimou, A.; Phuah, C.-L.; Jessel, M.J.; Schwab, K.; Ayres, A.M.; Romero, J.M.; Viswanathan, A.; Gurol, M.E.; Greenberg, S.M.; et al. Association Between Serum Calcium Level and Extent of Bleeding in Patients with Intracerebral Hemorrhage. JAMA Neurol. 2016, 73, 1285–1290. [Google Scholar] [CrossRef]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Chen, R.; Li, Y.; Xiong, Y.; Li, C. The study of interaction between human serum albumin and alternaria toxins using multi-spectroscopy, molecular docking and molecular dynamic. J. Mol. Struct. 2024, 1315, 138774. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef]
- Austermeier, S.; Pekmezović, M.; Porschitz, P.; Lee, S.; Kichik, N.; Moyes, D.L.; Ho, J.; Kotowicz, N.K.; Naglik, J.R.; Hube, B.; et al. Albumin Neutralizes Hydrophobic Toxins and Modulates Candida albicans Pathogenicity. mBio 2021, 12, e0053121. [Google Scholar] [CrossRef]
- Jalan, R.; Kapoor, D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin. Sci. 2004, 106, 467–474. [Google Scholar] [CrossRef]
- Suzuki, Y.; Taguchi, K.; Okamoto, W.; Enoki, Y.; Komatsu, T.; Matsumoto, K. Methemoglobin-albumin clusters for the treatment of hydrogen sulfide intoxication. J. Control. Release 2022, 349, 304–314. [Google Scholar] [CrossRef]
- Solomon, H.M.; Schrogie, J.J.; Williams, D. The displacement of phenylbutazone-14C and warfarin-14C from human albumin by various drugs and fatty acids. Biochem. Pharmacol. 1968, 17, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Soligard, H.T.; Nilsen, O.G.; Bratlid, D. Displacement of bilirubin from albumin by ibuprofen In Vitro. Pediatr. Res. 2010, 67, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Takadate, A.; Otagiri, M. Characterization of binding site of uremic toxins on human serum albumin. Biol. Pharm. Bull. 1995, 18, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Takamura, N.; Maruyama, T.; Otagiri, M. Effects of uremic toxins and fatty acids on serum protein binding of furosemide: Possible mechanism of the binding defect in uremia. Clin. Chem. 1997, 43, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Boss, K.; Waterstradt, K.; Schnurr, K.; Paar, M.; Stolpe, S.; Ickerott, P.; Wieneke, U.; Spitthöver, R.; Oettl, K.; Kribben, A. Binding and detoxification efficiency of albumin decline after haemodialysis. Nephrol. Dial. Transplant. 2024, 39, 215–221. [Google Scholar] [CrossRef]
- Paudel, K.R.; Panth, N.; Kim, D.W.; Karki, R. Chungtaejeon (CTJ) inhibits adhesion and migration of VSMC through cytoskeletal remodeling pathway. Heliyon 2024, 10, e38508. [Google Scholar] [CrossRef]
- Paudel, K.R.; Singh, M.; De Rubis, G.; Kumbhar, P.; Mehndiratta, S.; Kokkinis, S.; El-Sherkawi, T.; Gupta, G.; Singh, S.K.; Malik, M.Z.; et al. Computational and biological approaches in repurposing ribavirin for lung cancer treatment: Unveiling antitumorigenic strategies. Life Sci. 2024, 352, 122859. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Chanu, N.R.; Jha, S.K.; Khanal, P.; Paudel, K.R. In silico design and evaluation of a multiepitope vaccine targeting the nucleoprotein of Puumala orthohantavirus. Proteins Struct. Funct. Bioinform. 2024, 92, 1161–1176. [Google Scholar] [CrossRef]
- Rimac, H.; Dufour, C.; Debeljak, Ž.; Zorc, B.; Bojić, M. Warfarin and Flavonoids Do Not Share the Same Binding Region in Binding to the IIA Subdomain of Human Serum Albumin. Molecules 2017, 22, 1153. [Google Scholar] [CrossRef]
- Poór, M.; Li, Y.; Kunsági-Máté, S.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Molecular displacement of warfarin from human serum albumin by flavonoid aglycones. J. Lumin. 2013, 142, 122–127. [Google Scholar] [CrossRef]
- Setoguchi, N.; Takamura, N.; Fujita, K.; Ogata, K.; Tokunaga, J.; Nishio, T.; Chosa, E.; Arimori, K.; Kawai, K.; Yamamoto, R. A diclofenac suppository-nabumetone combination therapy for arthritic pain relief and a monitoring method for the diclofenac binding capacity of HSA site II in rheumatoid arthritis. Biopharm. Drug Dispos. 2013, 34, 125–136. [Google Scholar] [CrossRef]
- Takamura, N.; Maruyama, T.; Chosa, E.; Kawai, K.; Tsutsumi, Y.; Uryu, Y.; Yamasaki, K.; Deguchi, T.; Otagiri, M. Bucolome, a potent binding inhibitor for furosemide, alters the pharmacokinetics and diuretic effect of furosemide: Potential for use of bucolome to restore diuretic response in nephrotic syndrome. Drug Metab. Dispos. 2005, 33, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, H.G.; Labby, D.H.; Ahrens, E.H.; Shank, R.E.; Hoagland, C.L. The Use of Concentrated Human Serum Albumin in the Treatment of Cirrhosis of the Liver. J. Clin. Investig. 1948, 27, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Losowsky, M.S.; Atkinson, M. Intravenous albumin in the treatment of diuretic-resistant ascites in portal cirrhosis. Lancet 1961, 2, 386–389. [Google Scholar] [CrossRef]
- Wilkinson, P.; Sherlock, S. The effect of repeated albumin infusions in patients with cirrhosis. Lancet 1962, 2, 1125–1129. [Google Scholar] [CrossRef]
- McCormick, P.A.; Mistry, P.; Kaye, G.; Burroughs, A.K.; McIntyre, N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut 1990, 31, 204–207. [Google Scholar] [CrossRef]
- Romanelli, R.G.; La Villa, G.; Barletta, G.; Vizzutti, F.; Lanini, F.; Arena, U.; Boddi, V.; Tarquini, R.; Pantaleo, P.; Gentilini, P.; et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: An unblinded randomized trial. World J. Gastroenterol. 2006, 12, 1403–1407. [Google Scholar] [CrossRef]
- Caraceni, P.; Riggio, O.; Angeli, P.; Alessandria, C.; Neri, S.; Foschi, F.G.; Levantesi, F.; Airoldi, A.; Boccia, S.; Svegliati-Baroni, G.; et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): An open-label randomised trial. Lancet 2018, 391, 2417–2429. [Google Scholar] [CrossRef]
- Lombardo, A.; Capodicasa, L.; Alaimo, D.; Mercurio, F.; Zimbardo, A.; Simone, F.; Alessi, N.; Celsa, C.; Pennisi, G.; Cabibbo, G.; et al. Long-term therapy with intravenous human albumin increase survival in patients with decompensated cirrhosis and refractory ascites. Dig. Liver Dis. 2024, 56, S69. [Google Scholar] [CrossRef]
- Instituto Grifols, S.A. (Ed.) Prevention of Mortality with Long-Term Administration of Human Albumin in Subjects with Decompensated Cirrhosis and Ascites; ClinicalTrials.gov: Bethesda, MD, USA, 2018. [Google Scholar]
- Serramontmany, E.; Muñoz, M.; Fernández-Polo, A.; Morillo, M.; Gómez-Ganda, L.; Cañete-Ramírez, C.; Ariceta, G. Home Albumin Infusion Therapy, Another Alternative Treatment in Patients With Congenital Nephrotic Syndrome of the Finnish Type. Front. Pediatr. 2020, 8, 614535. [Google Scholar] [CrossRef] [PubMed]
- China, L.; Freemantle, N.; Forrest, E.; Kallis, Y.; Ryder Stephen, D.; Wright, G.; Portal Andrew, J.; Becares Salles, N.; Gilroy Derek, W.; O’Brien, A. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N. Engl. J. Med. 2021, 384, 808–817. [Google Scholar] [CrossRef]
- Callum, J.; Skubas, N.J.; Bathla, A.; Keshavarz, H.; Clark, E.G.; Rochwerg, B.; Fergusson, D.; Arbous, S.; Bauer, S.R.; China, L.; et al. Use of Intravenous Albumin: A Guideline From the International Collaboration for Transfusion Medicine Guidelines. Chest 2024, 166, 321–338. [Google Scholar] [CrossRef]
- Moctezuma-Velazquez, C.; Castro-Narro, G.; Simó, P.; Viayna, E.; Aceituno, S.; Soler, M.; Torre, A. Economic evaluation of long-term albumin use in cirrhosis patients from the Mexican healthcare system perspective. Ann. Hepatol. 2022, 27, 100673. [Google Scholar] [CrossRef] [PubMed]
- Guidet, B.; Ghout, I.; Ropers, J.; Aegerter, P. Economic model of albumin infusion in septic shock: The EMAISS study. Acta Anaesthesiol. Scand. 2020, 64, 781–788. [Google Scholar] [CrossRef]
- Cavallin, M.; Kamath, P.S.; Merli, M.; Fasolato, S.; Toniutto, P.; Salerno, F.; Bernardi, M.; Romanelli, R.G.; Colletta, C.; Salinas, F.; et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology 2015, 62, 567–574. [Google Scholar] [CrossRef]
- Hung, T.H.; Ko, P.H.; Wang, C.Y.; Tsai, C.C.; Lee, H.F. Effect of hypoalbuminemia on mortality in cirrhotic patients with spontaneous bacterial peritonitis. Tzu Chi Med. J. 2024, 36, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, F.; Giorgi, A.; Riggio, O.; De Santis, A.; Laviano, A.; Rossi-Fanelli, F. Is spontaneous bacterial peritonitis an inducer of vasopressin analogue side-effects? A case report. Dig. Liver Dis. 2003, 35, 503–506. [Google Scholar] [CrossRef]
- Salerno, F.; Navickis, R.J.; Wilkes, M.M. Albumin treatment regimen for type 1 hepatorenal syndrome: A dose-response meta-analysis. BMC Gastroenterol. 2015, 15, 167. [Google Scholar] [CrossRef]
- Wong, F.; Pappas, S.C.; Curry, M.P.; Reddy, K.R.; Rubin, R.A.; Porayko, M.K.; Gonzalez, S.A.; Mumtaz, K.; Lim, N.; Simonetto, D.A.; et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N. Engl. J. Med. 2021, 384, 818–828. [Google Scholar] [CrossRef]
- Sort, P.; Navasa, M.; Arroyo, V.; Aldeguer, X.; Planas, R.; Ruiz-del-Arbol, L.; Castells, L.; Vargas, V.; Soriano, G.; Guevara, M.; et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 1999, 341, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, T.; Shao, W.; Huang, S.; Ma, H. Association mechanism of remimazolam-serum albumin nano-drug for potential clinical application. Process Biochem. 2022, 114, 156–162. [Google Scholar] [CrossRef]
- Cheng, L.; Niu, M.M.; Yan, T.; Ma, Z.; Huang, K.; Yang, L.; Zhong, X.; Li, C. Bioresponsive micro-to-nano albumin-based systems for targeted drug delivery against complex fungal infections. Acta Pharm. Sin. B 2021, 11, 3220–3230. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Le, T.M.D.; Janarthanan, G.; Ngo, P.-K.T.; Lee, D.S.; Thambi, T. Development of bioresorbable smart injectable hydrogels based on thermo-responsive copolymer integrated bovine serum albumin bioconjugates for accelerated healing of excisional wounds. J. Ind. Eng. Chem. 2021, 96, 345–355. [Google Scholar] [CrossRef]
- Kremer, P.; Hartung, G.; Bauder-Wüst, U.; Schrenk, H.H.; Wunder, A.; Heckl, S.; Zillmann, U.; Sinn, H. Efficacy and tolerability of an aminopterin-albumin conjugate in tumor-bearing rats. Anti-Cancer Drugs 2002, 13, 615–623. [Google Scholar] [CrossRef]
- Guindani, C.; Feuser, P.E.; Cordeiro, A.P.; de Meneses, A.C.; Possato, J.C.; da Silva Abel, J.; Machado-de-Ávila, R.A.; Sayer, C.; de Araújo, P.H.H. Bovine serum albumin conjugation on poly (methyl methacrylate) nanoparticles for targeted drug delivery applications. J. Drug Deliv. Sci. Technol. 2020, 56, 101490. [Google Scholar] [CrossRef]
- Lei, Y.; Nosoudi, N.; Vyavahare, N. Targeted chelation therapy with EDTA-loaded albumin nanoparticles regresses arterial calcification without causing systemic side effects. J. Control. Release 2014, 196, 79–86. [Google Scholar] [CrossRef]
- Karamched, S.R.; Nosoudi, N.; Moreland, H.E.; Chowdhury, A.; Vyavahare, N.R. Site-specific chelation therapy with EDTA-loaded albumin nanoparticles reverses arterial calcification in a rat model of chronic kidney disease. Sci. Rep. 2019, 9, 2629. [Google Scholar] [CrossRef] [PubMed]
- Pertusa, J.A.G.; León-Quinto, T.; Berná, G.; Tejedo, J.R.; Hmadcha, A.; Bedoya, F.J.; Martín, F.; Soria, B. Zn2+ chelation by serum albumin improves hexameric Zn2+-insulin dissociation into monomers after exocytosis. PLoS ONE 2017, 12, e0187547. [Google Scholar] [CrossRef]
- Kovalik, S.G.; Ledgerwood, A.M.; Lucas, C.E.; Higgins, R.F. The cardiac effect of altered calcium homeostasis after albumin resuscitation. J. Trauma 1981, 21, 275–279. [Google Scholar] [CrossRef]
- Ip, M.M.; Masso-Welch, P.A.; Shoemaker, S.F.; Shea-Eaton, W.K.; Ip, C. Conjugated Linoleic Acid Inhibits Proliferation and Induces Apoptosis of Normal Rat Mammary Epithelial Cells in Primary Culture. Exp. Cell Res. 1999, 250, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Alsabeeh, N.; Chausse, B.; Kakimoto, P.A.; Kowaltowski, A.J.; Shirihai, O. Cell culture models of fatty acid overload: Problems and solutions. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2018, 1863, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef] [PubMed]
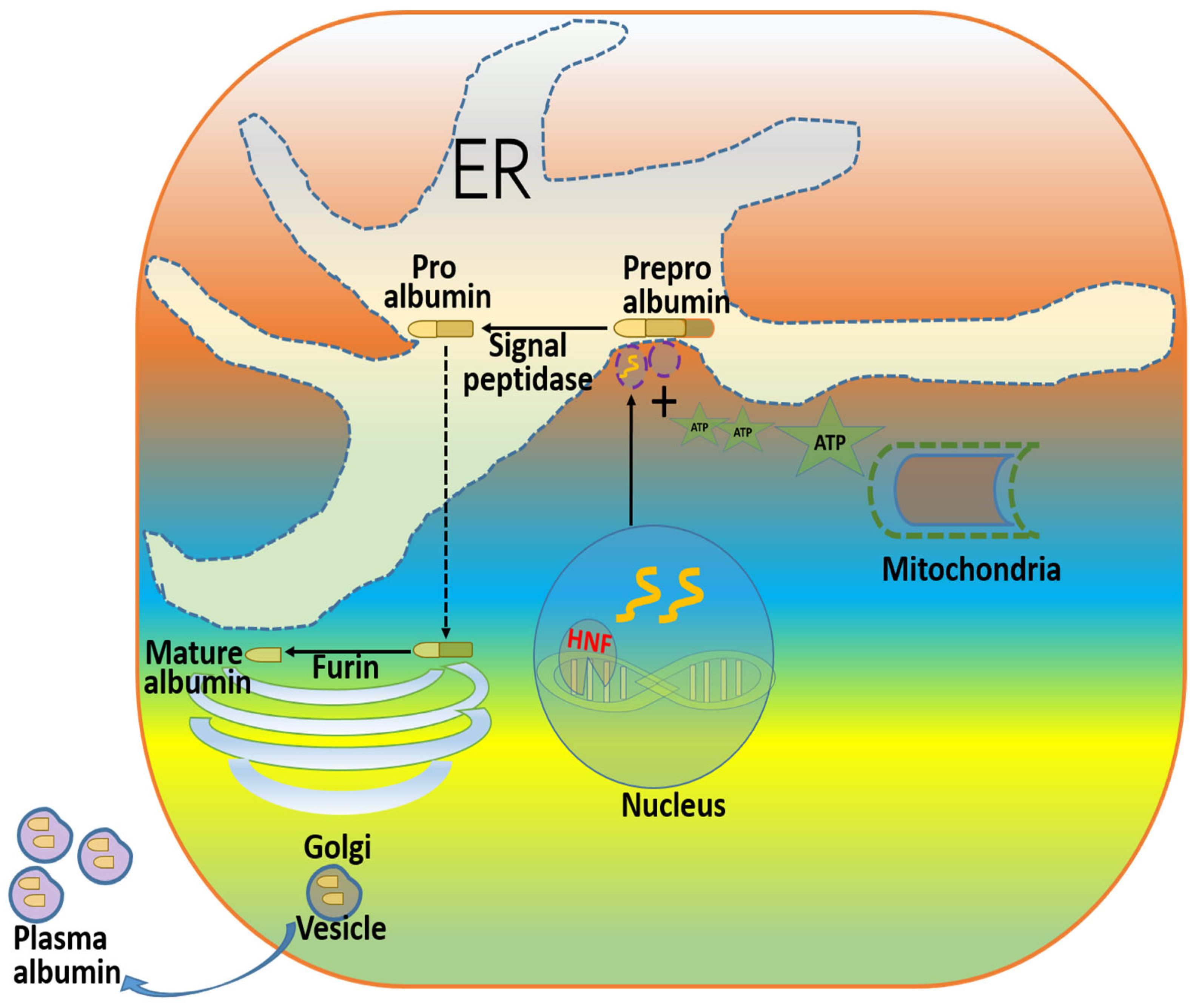

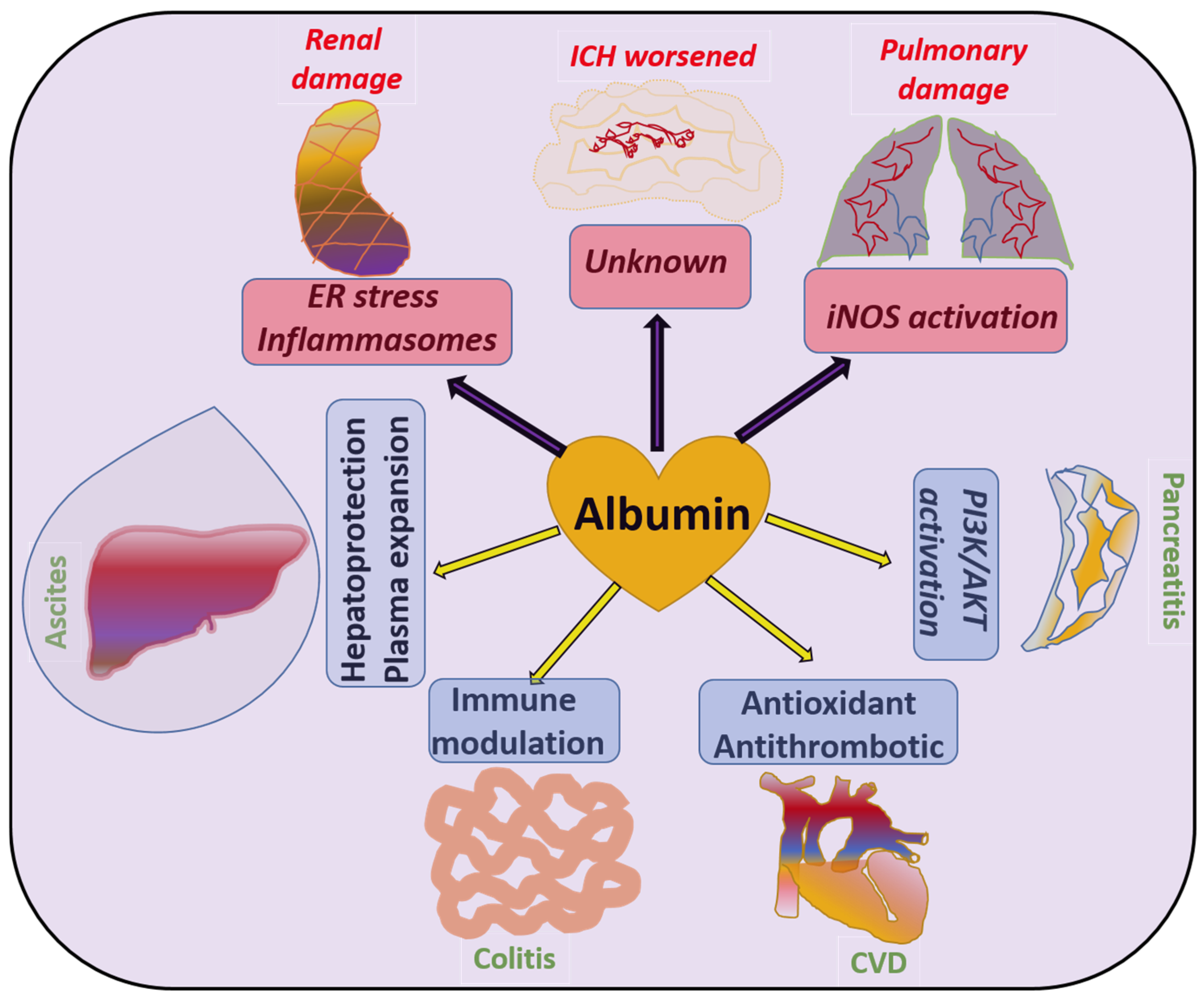
| Pathology | Albumin Administration | Mechanisms Involved | Refs. |
|---|---|---|---|
| Ascites | Improves | Albumin prevents the abnormal leakage of ions and fluids by sustaining the colloidal osmotic pressure and also reduces systemic inflammation | [102,105] |
| CVD | Improves | Due to the antioxidant properties of albumin, it prevents endothelial injury and also suppresses histones and fibrinogen-dependent blood clotting to prevent CVD | [132,149,151] |
| Pancreatitis | Improves | Studies indicate that albumin can activate survival kinase pathways in pancreatic cells to prevent inflammatory cell death and preserve pancreatic function | [155] |
| Alzheimer | Improves | Clearance of amyloid-β plaques off the brain via direct interaction, and also preventing hyperphosphorylation of Tau in order to prevent neuronal apoptosis | [112,114] |
| SHA | Improves | Experimental evidence suggests that the suppression of inflammatory pathways such as IL-1β by albumin exerts neuroprotective effects during SHA | [115] |
| NAFLD/ MASLD | Unsure but may improve | Mitochondrial protection by albumosomes formed by pre-albumin’s interaction with CPT2 to prevent fat accumulation in the liver | [109] |
| Sepsis | Controversial | Mainly due to the restoration in osmotic pressure, it can improve hypovolemic shock and lactate clearance in sepsis, but on the other hand can result in adverse effects in the lungs | [138] |
| Renal disease | Worsens | Activation of the NLRP3 inflammasome pathway causes renal damage by albumin | [94] |
| IHC | Worsens | By a yet-unclear mechanism, a large multicenter study involving 1275 subjects concluded albumin as an infective agent for the management of stroke and worsened IHC in some patients | [117] |
| Pulmonary edema | Worsens | Activation of iNOS has been reported to be involved in certain cases of adverse pulmonary pathologies such as fibrosis, and in a study, albumin-activated iNOS was found to cause pulmonary edema | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baral, A. Albumin: Bountiful Arrow in the Quiver of Liver and Its Significance in Physiology. Livers 2025, 5, 27. https://doi.org/10.3390/livers5020027
Baral A. Albumin: Bountiful Arrow in the Quiver of Liver and Its Significance in Physiology. Livers. 2025; 5(2):27. https://doi.org/10.3390/livers5020027
Chicago/Turabian StyleBaral, Ananda. 2025. "Albumin: Bountiful Arrow in the Quiver of Liver and Its Significance in Physiology" Livers 5, no. 2: 27. https://doi.org/10.3390/livers5020027
APA StyleBaral, A. (2025). Albumin: Bountiful Arrow in the Quiver of Liver and Its Significance in Physiology. Livers, 5(2), 27. https://doi.org/10.3390/livers5020027





