From Prehabilitation to Rehabilitation: A Systematic Review of Resistance Training as a Strategy to Combat Sarcopenia in Pre- and Post-Liver Transplant Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
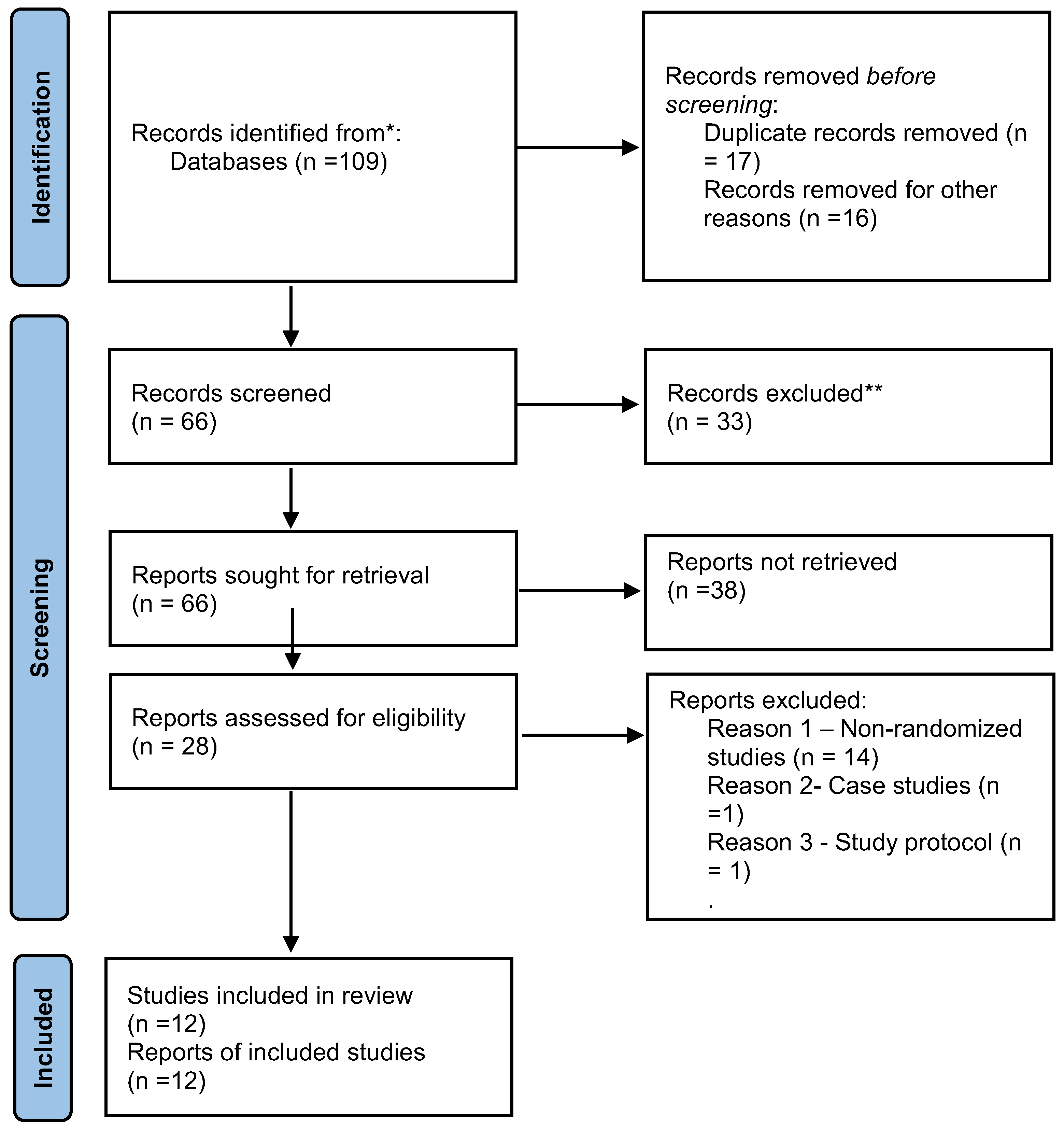
3. Results
3.1. Systematic Review Results
3.1.1. Pre-Liver Transplantation Patients
3.1.2. Post-Liver Transplantation Patients
3.1.3. Risk of Bias Assessment
| Author (Year) | Number of Patients (IG/CG) | Design | Intervention | Outcomes Measured | Safety Outcomes | Outcome Data (IG/CG) |
|---|---|---|---|---|---|---|
| Al-Judaibi et al. (2019) [58] | 258/200 | Retrospective cohort | Comprehensive in/outpatient program, 5×/week; physiotherapy, home, hospital, and online support | 90-day readmission, LOS, post-op surgical/vascular/medical complications | Surgical, vascular, infectious complications (UTI, pneumonia, C. diff) | Readmission: 45 vs. 41; MSK rehab: 4 vs. 1; LOS: 14 vs. 17 d; surgical: 72 vs. 55; vascular: 10 vs. 12; medical: 63 vs. 35 |
| Aamann et al. (2020) [63] | 20/19 | RCT | Supervised resistance training, 3×/week, 12 weeks | Muscle strength, CSA (MRI), lean mass, 6MWD | No safety events reported | Strength: +11 vs. 0 N·m; CSA: +4.4 vs. 0 cm2; lean mass: +1.7 vs. 0 kg; 6MWD: +18.8 vs. 0 m |
| Zenith et al. (2014) [55] | 9/10 | RCT | Cycle ergometer, 3×/week, 8 weeks, 60–80% VO2 peak | Peak VO2, quad circumference, 6MWD, QoL | No variceal bleeding or hepatic decompensation | VO2: +5.3 vs. 0 mL/kg/min; circumference: +1.05 vs. 0 cm; 6MWD: +23.5 vs. 0 m; QoL: +20.4 vs. 0 pts |
| Kruger et al. (2018) [47] | 20/20 | RCT | Moderate-high intensity cycling, 3×/week, 8 weeks | Peak VO2, 6MWD, thigh circumference and muscle thickness | No MSK injuries, variceal bleeding, or liver decompensation | VO2: +2.9 vs. 0.2 mL/kg/min; 6MWD: +30.4 vs. −20.4 m; thigh: +1.8 vs. 0.6 cm; thickness: +0.6 vs. 0.06 cm/m2 |
| Wallen et al. (2019) [44] | 8/9 | RCT | Hospital- and home-based aerobic + resistance, 8 weeks | VT, VO2 peak, 6MWD, grip strength, global strength | No adverse events, encephalopathy, or MSK injuries | VT: +1.2 mL/kg/min; VO2: +1.5; 6MWD: +16 m; Grip: +0.4 kg; global strength: −51.1 N (no CG data) |
| Sirisunhirun et al. (2022) [61] | 20/20 | RCT | Home-based moderate aerobic/isotonic training, 4×/week, 12 weeks | 6MWD, QoL, thigh mass, HVPG | No variceal bleeding or new ascites | 6MWD: +18.8 m; compression index: +0.08 cm/m2; HVPG: −0.04 kPa; QoL: +5.6 pts |
| Lai et al. (2021) [41] | 58/25 | RCT | STRIVE strength training + coaching, 12 weeks | Liver Frailty Index, CLDQ QoL, steps/day | No adverse events; liver function unchanged | LFI: −3.8 vs. −3.7 pts; QoL: +0.4 vs. 0.0 pts; steps/day: +4451 vs. +3569 |
| Aamann et al. (2023) [59] | 20/19 | RCT | Supervised resistance training, followed 3 years | Hospitalization, mortality, CirCom score, 6MWD | No major adverse events reported | Admission: 9 vs. 15; mortality: 1 vs. 5; CirCom ≥1: 6 vs. 4; 6MWD: +32.4 vs. +11.3 m |
| Rossi et al. (2022) [19] | 13/12 | RCT | Supervised face-to-face vs. non-supervised home-based aerobic training, 2×/week, 12 weeks | Fatigue, respiratory/pulmonary strength, 6MWD, SF-36 QoL | No adverse events noted | Fatigue: −1.86 vs. +0.1; PiMax: −23 vs. −2.5; torque: +28.5 vs. −0.65 N·m; 6MWD: +97.8 vs. +11.7 m; QoL: functional +19.7 vs. −1.17 pts |
| Author (Year) | Number of Patients (IG/CG) | Design | Intervention | Outcomes Measured | Safety Outcomes | Outcome Data (IG/CG) |
|---|---|---|---|---|---|---|
| Moya-Nájera et al. (2017) [64] | 21/20 | RCT | Supervised resistance and aerobic training, 3×/week, 12 weeks | Aerobic capacity, muscle strength, QoL, liver enzymes, body composition | No adverse events reported | VO2 ↑; hip/elbow strength ↑; SF-36 ↑; no change in LFTs/body composition |
| Ergene et al. (2022) [65] | 25/25 | RCT | Respiratory muscle training + physical rehab, 8 weeks | 6MWD, respiratory pressures (MIP/MEP), deltoid strength, fatigue, QoL | No adverse events reported | 6MWD: +97 m; fatigue score ↓ 3.7 pts; MIP/MEP ↑; QoL ↑ |
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of Outcome | Selection of Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Aamann et al. (2020) [63] | Low | Low | Low | Low | Low | Low |
| Zenith et al. (2014) [55] | Some concerns | Low | Low | Some concerns | Low | Some concerns |
| Kruger et al. (2018) [47] | Low | Low | Low | Low | Low | Low |
| Wallen et al. (2019) [44] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Sirisunhirun et al. (2022) [61] | Low | Low | Low | Low | Low | Low |
| Lai et al. (2021) [41] | Low | Low | Some concerns | Low | Low | Low |
| Aamann et al. (2023) [59] | Low | Low | Low | Low | Low | Low |
| Rossi et al. (2022) [19] | Low | Low | Low | Low | Low | Low |
| Moya-Nájera et al. (2017) [64] | Low | Low | Low | Low | Low | Low |
| Ergene et al. (2022) [65] | Low | Low | Low | Low | Low | Low |
| Al-Judaibi et al. (2019) [58] | Not applicable | Not applicable | High | High | High | High (observational) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis-aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013, 3, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef] [PubMed]
- Soldera, J. Exercise and Sarcopenia in Cirrhosis. Gastroenterol. Med. Res. 2018, 2–3. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Yilmaz, O.; Kılıç, C.; Oren, M.M.; Karan, M.A. Performance of SARC-F in Regard to Sarcopenia Definitions, Muscle Mass and Functional Measures. J. Nutr. Health Aging 2018, 22, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Health, Aging and Body Composition Study. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging And Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Aguirre, E.; López-Teros, T.; Gutiérrez-Robledo, L.; Vandewoude, M.; Pérez-Zepeda, M. Availability and use of dual energy X-ray absorptiometry (DXA) and bio-impedance analysis (BIA) for the evaluation of sarcopenia by Belgian and Latin American geriatricians. J. Cachexia Sarcopenia Muscle 2014, 5, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Heymsfield, S.B. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: What are we really estimating? J. Cachexia Sarcopenia Muscle 2017, 8, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gonzalez, M.C.; Lu, J.; Jia, G.; Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 2015, 74, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; Baudry, S.; Bauer, J.; Beckwée, D.; De Cock, A.M.; Hobbelen, H.; Jager-Wittenaar, H.; Kasiukiewicz, A.; Landi, F.; Marco, E.; et al. Application of ultrasound for muscle assessment in sarcopenia: Towards standardized measurements. Eur. Geriatr. Med. 2018, 9, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Glüer, C.C.; Bosy-Westphal, A.; Müller, M.J. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; D’Avila, A.F.; Galant, L.H.; Marroni, C.A. Exercise in the Physical Rehabilitation of Cirrotics: A Randomized Pilot Study. Arq. Gastroenterol. 2022, 59, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and appendicular lean mass reference ranges for Australian men and women: The Geelong osteoporosis study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Beaudart, C.; Reginster, J.; Buckinx, F.; Schoene, D.; Hirani, V.; Cooper, C.; Kanis, J.; Rizzoli, R.; Mccloskey, E.; et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice: An international survey. Eur. Geriatr. Med. 2016, 7, 243–246. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Faustini Pereira, J.L.; Galant, L.H.; Rossi, D.; Telles da Rosa, L.H.; Garcia, E.; de Mello Brandão, A.B.; Marroni, C.A. Functional Capacity, Respiratory Muscle Strength, and Oxygen Consumption Predict Mortality in Patients with Cirrhosis. Can. J. Gastroenterol. Hepatol. 2016, 2016, 6940374. [Google Scholar] [CrossRef] [PubMed]
- Peel, N.M.; Kuys, S.S.; Klein, K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Ceda, G.P.; Ticinesi, A.; De Vita, F.; Gelmini, G.; Costantino, C.; Meschi, T.; Kressig, R.W.; Cesari, M.; Fabi, M.; et al. Instrumental and Non-Instrumental Evaluation of 4-Meter Walking Speed in Older Individuals. PLoS ONE 2016, 11, e0153583. [Google Scholar] [CrossRef] [PubMed]
- Henrique, D.M.N.; Mourao-Junior, C.A.; Pace, F.; Oliveira, T.M.D.; Malaguti, C.; Chebli, J. Six-minute walk test predicts future decompensation in patients with compensated liver cirrhosis. Rev. Assoc. Médica Bras. 2022, 68, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Stähelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a cut-off point for normal mobility: A comparison of the timed ’up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Simonsick, E.M.; Naydeck, B.L.; Boudreau, R.M.; Kritchevsky, S.B.; Nevitt, M.C.; Pahor, M.; Satterfield, S.; Brach, J.S.; Studenski, S.A.; et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006, 295, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014, 20, 1424. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl. 2012, 18, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.; Garber, A.; Narayanan, A.; Shah, S.N.; Barnes, D.; Eghtesad, B.; Fung, J.; McCullough, A.J.; Dasarathy, S. Post-liver transplantation sarcopenia in cirrhosis: A prospective evaluation. J. Gastroenterol. Hepatol. 2014, 29, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Meza-Junco, J.; Montano-Loza, A.J.; Baracos, V.E.; Prado, C.M.; Bain, V.G.; Beaumont, C.; Esfandiari, N.; Lieffers, J.R.; Sawyer, M.B. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J. Clin. Gastroenterol. 2013, 47, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Watanabe, S.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Sarcopenia Impairs Prognosis of Patients with Hepatocellular Carcinoma: The Role of Liver Functional Reserve and Tumor-Related Factors in Loss of Skeletal Muscle Volume. Nutrients 2017, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Low, G.; Mourtzakis, M.; Zenith, L.; Myers, R.P.; Abraldes, J.G.; Shaheen, A.A.; Qamar, H.; Mansoor, N.; Carbonneau, M.; et al. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1473–1480.e3. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Sonnenday, C.J.; Tapper, E.B.; Duarte-Rojo, A.; Dunn, M.A.; Bernal, W.; Carey, E.J.; Dasarathy, S.; Kamath, B.M.; Kappus, M.R.; et al. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am. J. Transpl. Transplant. 2019, 19, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Van Jacobs, A.C. Frailty Assessment in Patients with Liver Cirrhosis. Clin. Liver Dis. 2019, 14, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.R.; Berzigotti, A.; Lord, J.M.; Lai, J.C.; Armstrong, M.J. Review article: Impact of exercise on physical frailty in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2019, 50, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Dodge, J.L.; Kappus, M.R.; Wong, R.; Mohamad, Y.; Segev, D.L.; McAdams-DeMarco, M. A Multicenter Pilot Randomized Clinical Trial of a Home-Based Exercise Program for Patients With Cirrhosis: The Strength Training Intervention (STRIVE). Am. J. Gastroenterol. 2021, 116, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Coombes, J.S.; Macdonald, G.A. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012, 18, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Morkane, C.M.; Kearney, O.; Bruce, D.A.; Melikian, C.N.; Martin, D.S. An Outpatient Hospital-based Exercise Training Program for Patients With Cirrhotic Liver Disease Awaiting Transplantation: A Feasibility Trial. Transplantation 2020, 104, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Wallen, M.P.; Keating, S.E.; Hall, A.; Hickman, I.J.; Pavey, T.G.; Woodward, A.J.; Skinner, T.L.; Macdonald, G.A.; Coombes, J.S. Exercise Training Is Safe and Feasible in Patients Awaiting Liver Transplantation: A Pilot Randomized Controlled Trial. Liver Transpl. 2019, 25, 1576–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Ferrando, A.; White, M.G.; Dennis, R.A.; Xie, J.; Pauly, M.; Park, S.; Bartter, T.; Dunn, M.A.; Ruiz-Margain, A.; et al. Home-Based Physical Activity and Diet Intervention to Improve Physical Function in Advanced Liver Disease: A Randomized Pilot Trial. Dig. Dis. Sci. 2020, 65, 3350–3359. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.R.; Vallance, A.; Faulkner, T.; Towey, J.; Durman, S.; Kyte, D.; Elsharkawy, A.M.; Perera, T.; Holt, A.; Ferguson, J.; et al. Home-Based Exercise in Patients Awaiting Liver Transplantation: A Feasibility Study. Liver Transpl. 2019, 25, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.; McNeely, M.L.; Bailey, R.J.; Yavari, M.; Abraldes, J.G.; Carbonneau, M.; Newnham, K.; DenHeyer, V.; Ma, M.; Thompson, R.; et al. Home Exercise Training Improves Exercise Capacity in Cirrhosis Patients: Role of Exercise Adherence. Sci. Rep. 2018, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Albillos, A.; Villanueva, C.; Genescá, J.; Ardevol, A.; Augustín, S.; Calleja, J.L.; Bañares, R.; García-Pagán, J.C.; Mesonero, F.; et al. Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology 2017, 65, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Terziyski, K.; Andonov, V.; Marinov, B.; Kostianev, S. Exercise performance and ventilatory efficiency in patients with mild and moderate liver cirrhosis. Clin. Exp. Pharmacol. Physiol. 2008, 35, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Galant, L.H.; Forgiarini, L.A., Jr.; Dias, A.S. The aerobic capacity and muscle strength are correlated in candidates for liver transplantation. Arq. Gastroenterol. 2011, 48, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Bay Nielsen, H.; Secher, N.H.; Clemmesen, O.; Ott, P. Maintained cerebral and skeletal muscle oxygenation during maximal exercise in patients with liver cirrhosis. J. Hepatol. 2005, 43, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lunzer, M.; Newman, S.P.; Sherlock, S. Skeletal muscle blood flow and neurovascular reactivity in liver disease. Gut 1973, 14, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Zenith, L.; Meena, N.; Ramadi, A.; Yavari, M.; Harvey, A.; Carbonneau, M.; Ma, M.; Abraldes, J.G.; Paterson, I.; Haykowsky, M.J.; et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1920–1926.e2. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.F.M.G.; Amaral, A.C.C.; Gonzalez, A.M.; Lai, M.; Mota, D.O.; Ferraz, M.L.G.; Junior, W.M.; Kondo, M. Six-minute walking test performance is associated with survival in cirrhotic patients. World J. Hepatol. 2021, 13, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Cox-Flaherty, K.; Moutchia, J.; Krowka, M.J.; Al-Naamani, N.; Fallon, M.B.; DuBrock, H.; Forde, K.A.; Krok, K.; Doyle, M.F.; Kawut, S.M.; et al. Six-Minute walk distance predicts outcomes in liver transplant candidates. Liver Transpl. 2023, 29, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Al-Judaibi, B.; Alqalami, I.; Sey, M.; Qumosani, K.; Howes, N.; Sinclair, L.; Chandok, N.; Eddin, A.H.; Hernandez-Alejandro, R.; Marotta, P.; et al. Exercise Training for Liver Transplant Candidates. Transpl. Transplant. Proc. 2019, 51, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Aamann, L.; Dam, G.; Jepsen, P.; Borre, M.; Drljevic-Nielsen, A.; Overgaard, K.; Andersen, H.; Vilstrup, H.; Aagaard, N.K. Reduced 3-year risk of hospital admission and mortality after 12-week resistance training of cirrhosis patients: A follow-up of a randomized clinical trial. J. Gastroenterol. Hepatol. 2023, 38, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, S.R.; Chachá, S.G.F.; Libardi, C.A. Resistance training combined with blood flow restriction in cirrhosis: Study protocol for a randomized controlled trial. Trials 2020, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- Sirisunhirun, P.; Bandidniyamanon, W.; Jrerattakon, Y.; Muangsomboon, K.; Pramyothin, P.; Nimanong, S.; Tanwandee, T.; Charatcharoenwitthaya, P.; Chainuvati, S.; Chotiyaputta, W. Effect of a 12-week home-based exercise training program on aerobic capacity, muscle mass, liver and spleen stiffness, and quality of life in cirrhotic patients: A randomized controlled clinical trial. BMC Gastroenterol. 2022, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, A.J.; Polineni, P.; Morrissey, S.; Belfanti, K.; Nizamuddin, M.; Siddiqui, O.; Daud, A.; Simpson, D.C.; Levitsky, J.; Flores, A.M.; et al. Home-based LIver FrailTy Intervention (LIFT) in Transplant Candidates: A Feasibility Study. Transplantation 2025, 109, e202–e212. [Google Scholar] [CrossRef] [PubMed]
- Aamann, L.; Dam, G.; Borre, M.; Drljevic-Nielsen, A.; Overgaard, K.; Andersen, H.; Vilstrup, H.; Aagaard, N.K. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 1179–1187.e6. [Google Scholar] [CrossRef] [PubMed]
- Moya-Nájera, D.; Moya-Herraiz, Á.; Compte-Torrero, L.; Hervás, D.; Borreani, S.; Calatayud, J.; Berenguer, M.; Colado, J.C. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl. 2017, 23, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Yüksel Ergene, T.; Karadibak, D.; Dönmez, R.; Polat, K.Y. Effects of Early Resistance Training After Liver Transplantation Procedures: A Randomized Controlled Pilot Trial. Turk. J. Gastroenterol. 2022, 33, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, S.; Patel, K.V.; Bandinelli, S.; Ferrucci, L.; Guralnik, J.M. Characteristics of 400-meter walk test performance and subsequent mortality in older adults. Rejuvenation Res. 2009, 12, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Soldera, J.; Freitas, L.F.; Rech, A.; Rossi, D.; Onzi, G.; Heinrich, C.F.; Turella, D.J.P.; Weber, T.A.S.; Oliveira, M.L. Resistance training effects on quality of life and fatigue for compensated cirrhotic patients: Preliminary results from a randomized controlled trial. UEG Week Virtual 2020. United Eur. Gastroenterol. J. 2020, 8 (Suppl. S1). Available online: https://www.nxtbook.com/ueg/UEG/Abstracts/index.php (accessed on 29 March 2025).
- Soldera, J.; Rech, A.; Rossi, D.; Freitas, L.F.; Onzi, G.; Heinrich, C.F.; Turella, D.J.P.; Weber, T.A.S.; Oliveira, M.L.; Lopez, P.; et al. Resistance training effects on muscle strength and muscle mass in compensated cirrhotic patients: Preliminary results from a randomized controlled trial. UEG Week Virtual 2020. United Eur. Gastroenterol. J. 2020, 8 (Suppl. S1). Available online: https://www.nxtbook.com/ueg/UEG/Abstracts/index.php#/p/619/OnePage (accessed on 29 March 2025).
- Soldera, J.; Rossi, D.; d’Avila, A.F.; Galant, L.H.; Marroni, C.A. Physical rehabilitation in the cirrhotic patient: Results from a clinical trial. UEG Week Virtual 2020. United Eur. Gastroenterol. J. 2020, 8 (Suppl. S1). Available online: https://www.nxtbook.com/ueg/UEG/Abstracts/index.php#/p/623/OnePage (accessed on 29 March 2025).
- Wongtrakul, W.; Bandidniyamanon, W.; Charatcharoenwitthaya, P. Relationship between Sarcopenia and minimal hepatic encephalopathy in patients with cirrhosis: A prospective observational study. BMC Gastroenterol. 2025, 25, 88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellatt, S.; Soldera, J. From Prehabilitation to Rehabilitation: A Systematic Review of Resistance Training as a Strategy to Combat Sarcopenia in Pre- and Post-Liver Transplant Patients. Livers 2025, 5, 25. https://doi.org/10.3390/livers5020025
Vellatt S, Soldera J. From Prehabilitation to Rehabilitation: A Systematic Review of Resistance Training as a Strategy to Combat Sarcopenia in Pre- and Post-Liver Transplant Patients. Livers. 2025; 5(2):25. https://doi.org/10.3390/livers5020025
Chicago/Turabian StyleVellatt, Sooraj, and Jonathan Soldera. 2025. "From Prehabilitation to Rehabilitation: A Systematic Review of Resistance Training as a Strategy to Combat Sarcopenia in Pre- and Post-Liver Transplant Patients" Livers 5, no. 2: 25. https://doi.org/10.3390/livers5020025
APA StyleVellatt, S., & Soldera, J. (2025). From Prehabilitation to Rehabilitation: A Systematic Review of Resistance Training as a Strategy to Combat Sarcopenia in Pre- and Post-Liver Transplant Patients. Livers, 5(2), 25. https://doi.org/10.3390/livers5020025






