A Metabolomics-Based Approach for Diagnosing NAFLD and Identifying Its Pre-Condition Along the Potential Disease Spectrum
Abstract
1. Introduction
2. Materials and Methods
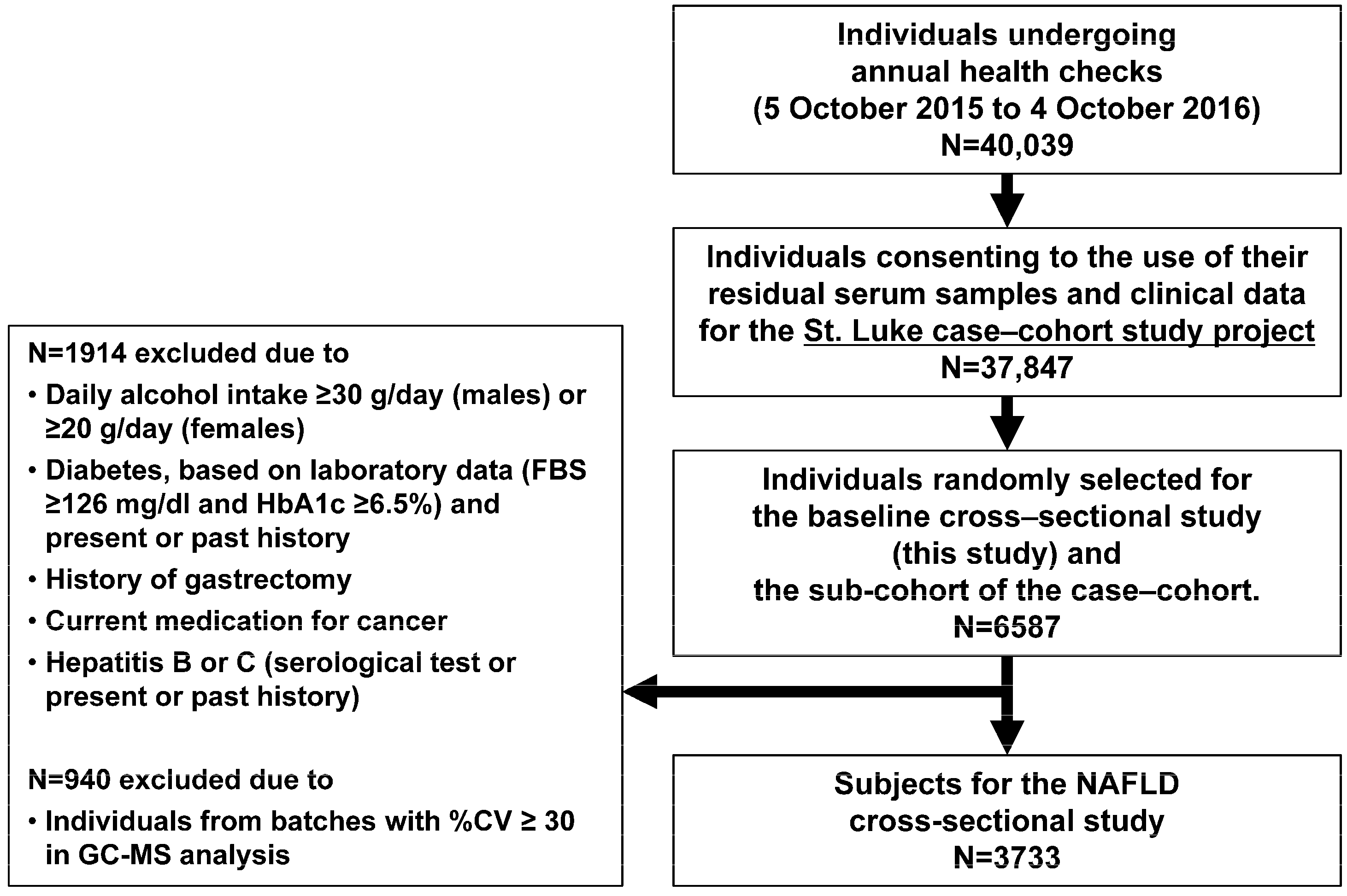
2.1. Study Design and Population
2.2. Data Collection and Fatty Liver Diagnosis
2.3. Sample Collection
2.4. Metabolome Analysis
2.5. Statistical Analysis
3. Results
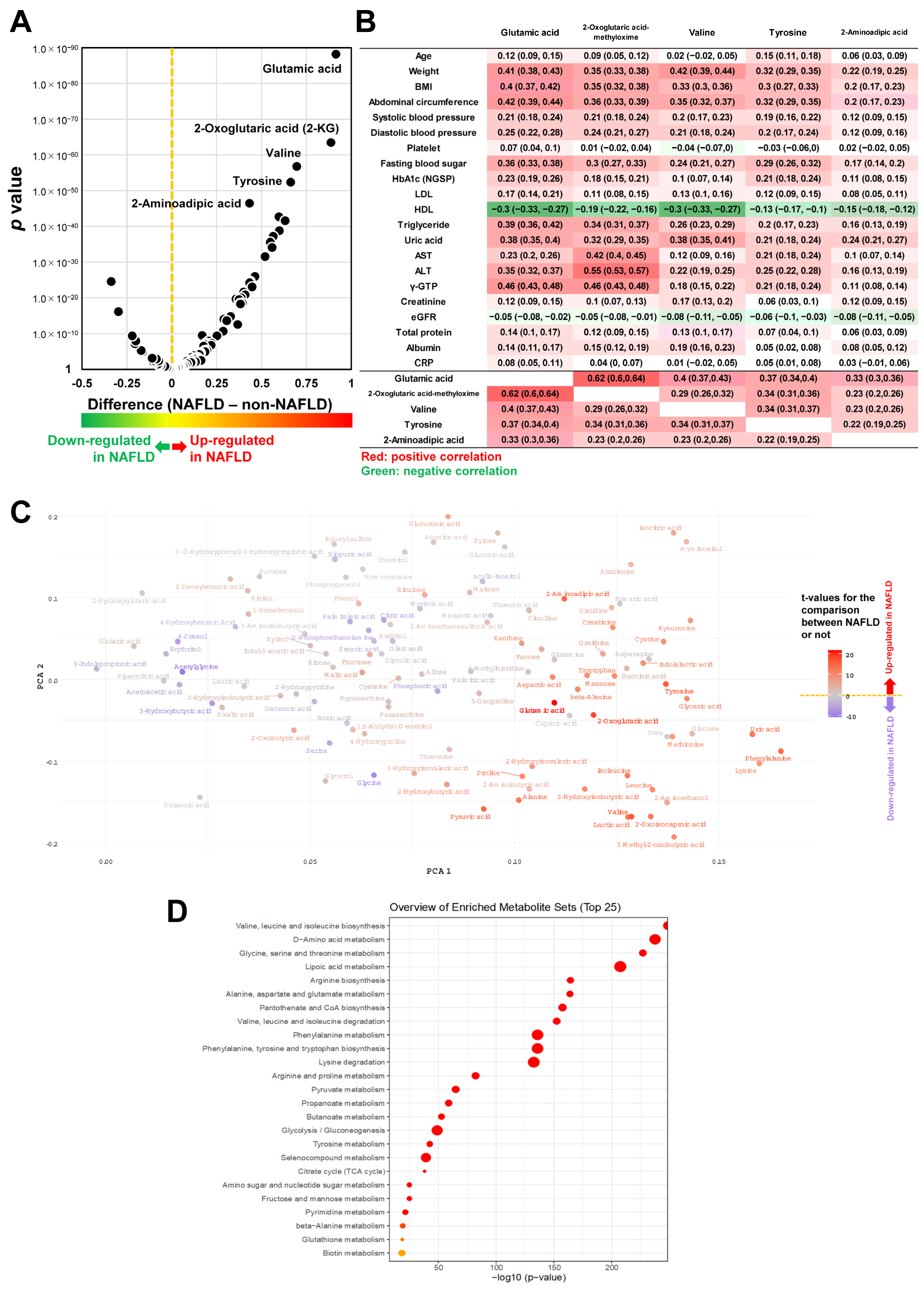
3.1. The Association Bewteen Metabolites and NAFLD
3.2. MSEA
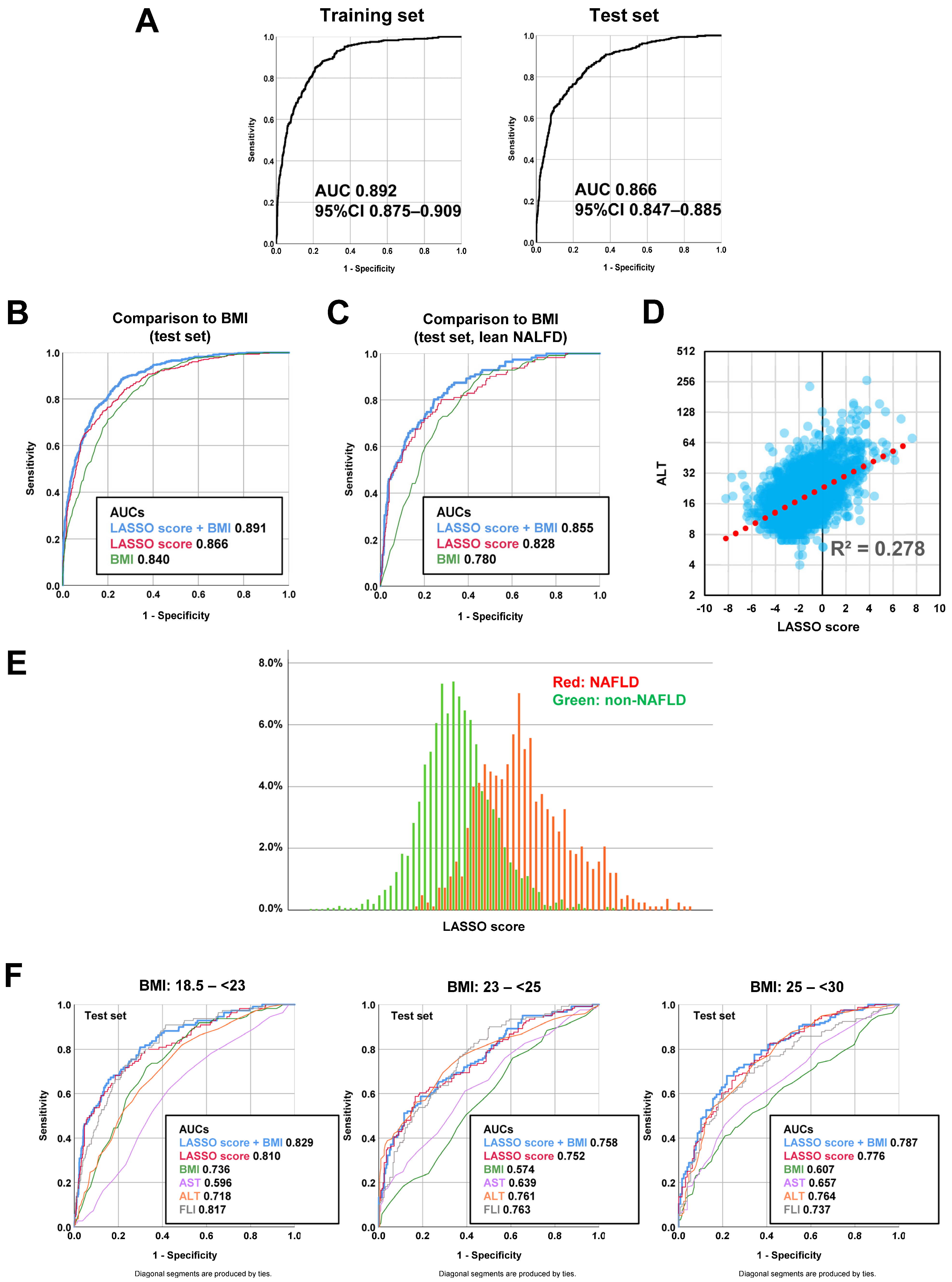
3.3. Diagnostic Model Development Using a Machine Learning Method
3.4. Predictive Advantage of LASSO Model Independent from BMI
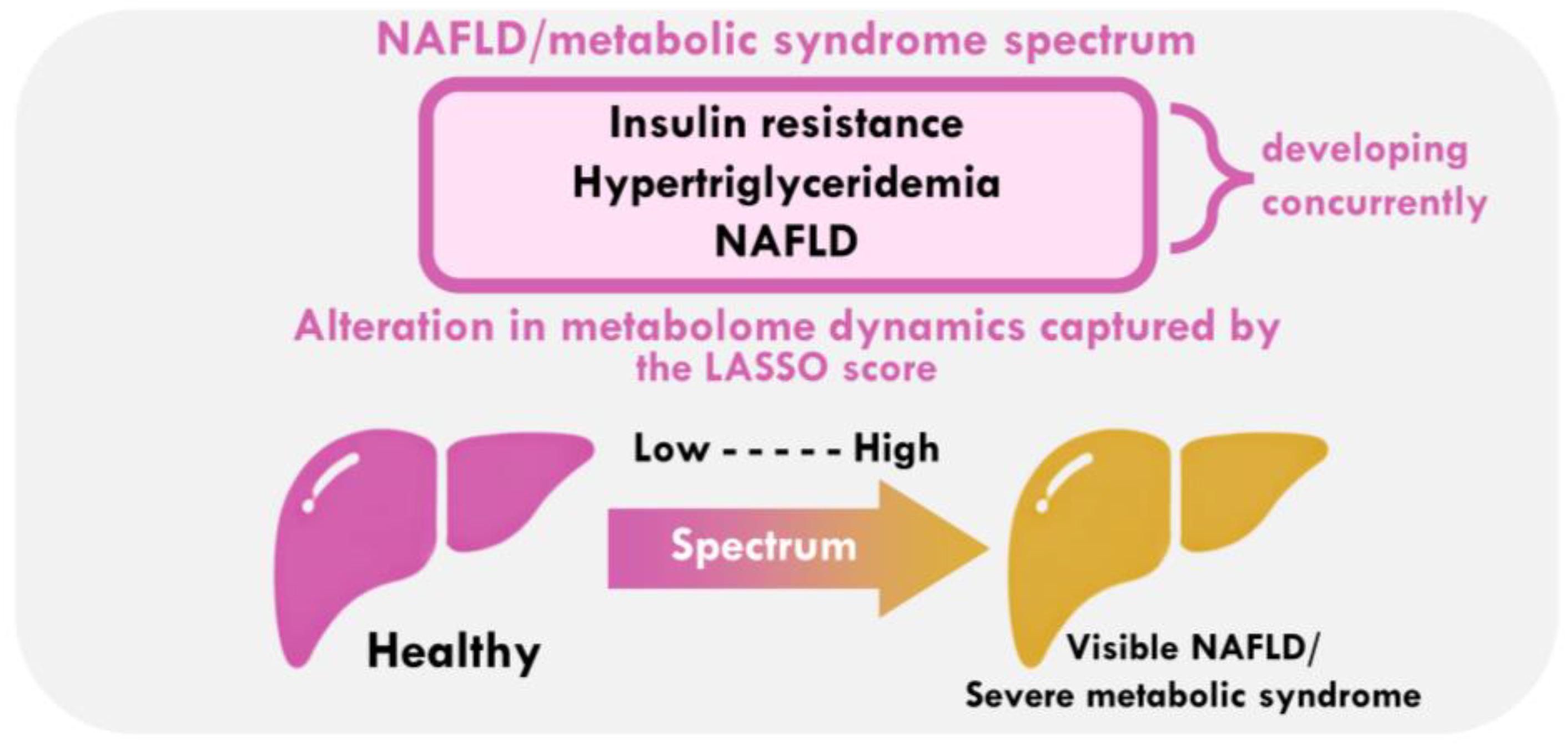
3.5. What Does the LASSO Score Reflect Clinically?
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Razavi, H.; Negro, F.; Nakajima, A.; Marchesini, G.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: A meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targer, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef]
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Chang, H.S.; Bae, I.Y. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2017, 68, 140–146. [Google Scholar] [CrossRef]
- Wang, A.Y.; Dhaliwal, J.; Mouzaki, M. Lean non-alcoholic fatty liver disease. Clin. Nutr. 2019, 38, 975–981. [Google Scholar] [CrossRef]
- Albhaisi, S.; Chowdhury, A.; Sanyal, A.J. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019, 1, 329–341. [Google Scholar] [CrossRef]
- Golabi, P.; Paik, J.; Fukui, N.; Locklear, C.T.; de Avilla, L.; Younossi, Z.M. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin. Diabetes 2019, 37, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Govaere, O.; Petta, S.; Miele, L.; Tiniakos, D.; Burt, A. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: Time for reappraisal of BMI-driven approach? Gut 2022, 71, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres DMShaw, J.; Contreras, M. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Aretz, I.; Meierhofer, D. Advantages and pitfalls of mass spectrometry-based metabolome profiling in systems biology. Int. J. Mol. Sci. 2016, 17, 632. [Google Scholar] [CrossRef]
- Klassen, A.; Faccio, A.T.; Canuto, G.A.B.; Rocha da Cruz, P.L.; Ribeiro, H.C.; Tavares, M.F.M. Metabolomics: Definitions and significance in systems biology [internet]. Adv. Exp. Med. Biol. 2017, 965, 3–17. [Google Scholar]
- Lindon, J.C.; Nicholson, J.K.; Holmes, E. (Eds.) The Handbook of Metabolic Phenotyping; Elsevier: Amsterdam, The Netherlands, 2019; Available online: https://linkinghub.elsevier.com/retrieve/pii/C20160031650 (accessed on 22 November 2021).
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Scalbert, A.; Ferrari, P. Biomarker discovery. In Metabolomics for Biomedical Research; Adamski, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kimura, T.; Deshpande, G.A.; Urayama, K.Y.; Masuda, K.; Fukui, T.; Matsuyama, Y. Association of weight gain since age 20 with non-alcoholic fatty liver disease in normal weight individuals. J. Gastroenterol. Hepatol. 2015, 30, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Bugianesi, E. NASH in lean individuals. Semin. Liver Dis. 2019, 39, 86–95. [Google Scholar] [CrossRef]
- Nishiumi, S.; Shinohara, M.; Ikeda, A.; Yoshie, T.; Hatano, N.; Kakuyama, S. Serum metabolomics as a novel diagnostic approach for pancreatic cancer. Metabolomics 2010, 6, 518–528. [Google Scholar] [CrossRef]
- Brial, F.; Alzaid, F.; Sonomura, K.; Kamatani, Y.; Meneyrol, K.; Le Lay, A. The natural metabolite 4-cresol improves glucose homeostasis and enhances β-cell function. Cell Rep. 2020, 30, 2306–2320. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, 2016. Available online: https://www.R-project.org (accessed on 3 March 2022).
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald PEWishart, D.S.; Li, S.; Xia, J. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Masoodi, M.; Gastaldelli, A.; Hyötyläinen, T.; Arretxe, E.; Alonso, C.; Gaggini, M. Metabolomics and lipidomics in NAFLD: Biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 835–856. [Google Scholar] [CrossRef]
- Guerra, S.; Mocciaro, G.; Gastaldelli, A. Adipose tissue insulin resistance and lipidome alterations as the characterizing factors of non-alcoholic steatohepatitis. Eur. J. Clin. Invest. 2022, 52, e13695. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Latta, V.D.; Ciociaro, D.; Abate, M.L.; Gastaldelli, A.; Bugianesi, E.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Lee, H.J.; Jang, H.B.; Kim, W.H.; Park, K.J.; Kim, K.Y.; Park, S.I.; Lee, H.J. 2-Aminoadipic acid (2-AAA) as a potential biomarker for insulin resistance in childhood obesity. Sci. Rep. 2019, 9, 13610. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gallego, E.; Guirro, M.; Riera-Borrull, M.; Hernandez-Aguilera, A.; Marine-Casado, R.; Fernández-Arroyo, S.; Beltrán-Debón, R.; Sabench, F.; Hernández, M.; del Castillo, D.; et al. Mapping of the circulating metabolome reveals α-ketoglutarate as a predictor of morbid obesity-associated non-alcoholic fatty liver disease. Int. J. Obes. 2015, 39, 279–287. [Google Scholar] [CrossRef]
- Cabré, N.; Luciano-Mateo, F.; Baiges-Gayà, G.; Fernandez-Arroyo, S.; Rodriguez-Tomas, E.; Hernández-Aguilera, A.; París, M.; Sabench, F.; Del Castillo, D.; López-Miranda, J.; et al. Plasma metabolic alterations in patients with severe obesity and non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2020, 51, 374–387. [Google Scholar] [CrossRef]
- Lind, L.; Johansson, L.; Ahlström, H.; Eriksson, J.W.; Larsson, A.; Risérus, U.; Kullberg, J.; Oscarsson, J. Comparison of four non-alcoholic fatty liver disease detection scores in a Caucasian population. World J. Hepatol. 2020, 12, 149–159. [Google Scholar] [CrossRef]
- Hsu, C.L.; Wu, F.Z.; Lin, K.H.; Chen, Y.H.; Wu, P.C.; Chen, Y.H.; Chen, C.-S.; Wang, W.-H.; Mar, G.-Y.; Yu, H.-C. Role of fatty liver index and metabolic factors in the prediction of nonalcoholic fatty liver disease in a lean population receiving health checkup. Clin. Transl. Gastroenterol. 2019, 10, e00042. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirwan, J.A. Translating metabolomics into clinical practice. Nat. Rev. Bioeng. 2023, 1, 228–229. [Google Scholar] [CrossRef]

| Metabolites Most Upregulated in Participants with NAFLD | Univariate Analysis (Welch’s t-Test) | ROC Analysis | |||
|---|---|---|---|---|---|
| p Value | Difference NAFLD- non-NAFLD a | 95% CI | AUC | 95% CI | |
| Glutamic acid | 6.4 × 10−89 | 0.914 | 0.996–0.833 | 0.759 | 0.740–0.778 |
| 2-Oxoglutaric acid | 3.5 × 10−64 | 0.887 | 0.982–0.791 | 0.729 | 0.709–0.750 |
| Valine | 1.6 × 10−57 | 0.696 | 0.777–0.615 | 0.699 | 0.678–0.719 |
| Tyrosine | 4.4 × 10−53 | 0.662 | 0.743–0.582 | 0.685 | 0.664–0.705 |
| 2-Aminoadipic acid | 3.3 × 10−47 | 0.433 | 0.491–0.376 | 0.729 | 0.710–0.748 |
| Phenylalanine | 2.1 × 10−43 | 0.597 | 0.678–0.516 | 0.667 | 0.646–0.688 |
| Pyruvic acid | 2.7 × 10−42 | 0.632 | 0.719–0.544 | 0.671 | 0.649–0.692 |
| Uric acid | 1.6 × 10−39 | 0.599 | 0.685–0.513 | 0.666 | 0.645–0.687 |
| 2-Oxoisocaproic acid | 5.8 × 10−38 | 0.565 | 0.648–0.482 | 0.659 | 0.638–0.680 |
| Alanine | 2.6 × 10−36 | 0.549 | 0.632–0.466 | 0.654 | 0.633–0.675 |
| BMI | 5.0 × 10−164 | 1.264 | 1.199–1.330 | 0.853 | 0.840–0.867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nojima, M.; Kimura, T.; Aoki, Y.; Fujimoto, H.; Hayashi, K.; Ohtake, J.; Kimura-Asami, M.; Suzuki, K.; Urayama, K.; Matsuura, M.; et al. A Metabolomics-Based Approach for Diagnosing NAFLD and Identifying Its Pre-Condition Along the Potential Disease Spectrum. Livers 2025, 5, 12. https://doi.org/10.3390/livers5010012
Nojima M, Kimura T, Aoki Y, Fujimoto H, Hayashi K, Ohtake J, Kimura-Asami M, Suzuki K, Urayama K, Matsuura M, et al. A Metabolomics-Based Approach for Diagnosing NAFLD and Identifying Its Pre-Condition Along the Potential Disease Spectrum. Livers. 2025; 5(1):12. https://doi.org/10.3390/livers5010012
Chicago/Turabian StyleNojima, Masanori, Takeshi Kimura, Yutaka Aoki, Hirotaka Fujimoto, Kuniyoshi Hayashi, Junya Ohtake, Mariko Kimura-Asami, Kazuhiko Suzuki, Kevin Urayama, Masaaki Matsuura, and et al. 2025. "A Metabolomics-Based Approach for Diagnosing NAFLD and Identifying Its Pre-Condition Along the Potential Disease Spectrum" Livers 5, no. 1: 12. https://doi.org/10.3390/livers5010012
APA StyleNojima, M., Kimura, T., Aoki, Y., Fujimoto, H., Hayashi, K., Ohtake, J., Kimura-Asami, M., Suzuki, K., Urayama, K., Matsuura, M., Sato, T.-A., & Masuda, K. (2025). A Metabolomics-Based Approach for Diagnosing NAFLD and Identifying Its Pre-Condition Along the Potential Disease Spectrum. Livers, 5(1), 12. https://doi.org/10.3390/livers5010012





