Abstract
Non-alcoholic fatty liver disease (NAFLD), recently redefined as metabolic dysfunction-associated steatotic liver disease (MASLD), is the most common cause of chronic liver disease worldwide. Characterized by excessive hepatic fat accumulation, this disease encompasses a spectrum from simple steatosis to more severe forms, including steatohepatitis, fibrosis, and cirrhosis. Emerging evidence highlights the pivotal role of gut dysbiosis in the pathogenesis of MASLD. Dysbiosis disrupts the gut–liver axis, an intricate communication network that regulates metabolic, immune, and barrier functions. Alterations in gut microbiota composition, increased gut permeability, and translocation of pro-inflammatory metabolites/factors have been shown to trigger liver inflammatory and fibrotic cascades, exacerbating hepatic inflammation and injury. Recent studies have identified microbiome signatures associated with MASLD, offering promise as non-invasive diagnostic biomarkers and paving the way for new potential therapeutic strategies targeting gut dysbiosis. This review explores the crucial role of the gut microbiota in MASLD pathogenesis and highlights the need for further targeted research in this field to validate microbial biomarkers and optimize therapeutic strategies. Comprehensive understanding of the gut–liver axis may enable innovative diagnostic and therapeutic approaches, transforming the clinical management of MASLD.
1. Introduction
Non-alcoholic fatty liver disease (NALFD) is characterized by the abnormal accumulation of fat within hepatocytes (>5%) in the absence of other common causes, such as significant alcohol consumption, autoimmune or viral hepatitis, amongst others [1]. It is currently the most common cause of chronic liver disease globally, affecting up to 25–30% of the general adult population and posing a major public health concern with a considerable clinical and economic burden [2,3]. Given the established relationship of NAFLD with cardio-metabolic diseases and risk factors, in 2023, a Delphi consensus statement introduced the term metabolic dysfunction-associated steatotic liver disease (MASLD) to underscore the association of NAFLD with metabolic dysfunction, which may co-exist with liver disease, and remove the term “fatty” from the relevant nomenclature due to its association with perceived stigma [4,5]. Notably, in contrast to the diagnosis of NAFLD, which is defined by the absence of other liver conditions, the proposed diagnostic criteria for MASLD are dependent on hepatic steatosis and the presence of at least one of five cardio-metabolic risk factors, namely elevated body mass index (BMI), fasting glucose, triglycerides, blood pressure, and low HDL cholesterol [4]. The proposal of the new MASLD nomenclature has also raised a debate regarding its interchangeable use with the term NAFLD, and, accordingly, the inter-application of related scientific evidence [6,7]. Although similar pathophysiological mechanisms are believed to likely underpin both of these entities, research specifically exploring MASLD and the impact of the new MASLD diagnostic criteria in comparison to NAFLD is now growing, but is still limited [6,7,8]. For the purposes of the present review in the following sections, the term MASLD is used to describe the disease and related pathophysiological mechanisms; however, the term NAFLD is used where the related primary research study was before the introduction of the MASLD nomenclature/definition and/or refers to a NAFLD diagnosis/model.
MASLD encompasses a disease spectrum ranging from simple steatosis to metabolic-associated steatohepatitis (MASH)—previously referred to as non-alcoholic steatohepatitis (NASH)—which may then progress to hepatic fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC) [8]. The exact pathophysiology underlying the progression of this disease remains to be completely understood, as its course can vary significantly amongst individuals [9,10]. In contrast to previous assumptions that suggested a linear disease progression, recent insights reveal a more complex disease trajectory, where the disease may progress to MASH or cirrhosis, whereas others may see stabilization or even regression of their condition [9,10]. As such, the pathogenesis of MASLD is a complex interplay of genetic, metabolic, and environmental factors [8,9,10]. For example, genome wide association studies have identified the role of specific genes in association with MASLD, including PNPLA3 (patatin-like phospholipase containing 3), TM6SF2 (transmembrane 6 superfamily member 2), MBOAT7 (membrane bound O acyltransferase 7), and GCKR (glucokinase regulator) [11]. Furthermore, lifestyle factors, such as high-fat/high-sugar diets, and a sedentary lifestyle with low levels of physical activity, have been implicated in promoting the development and progression of MASLD [12,13]. Relating to lifestyle factors, it is also noteworthy that the aforementioned 2023 Delphi consensus statement introduced the term metabolic and alcohol related/associated liver disease (MetALD) to describe MASLD in those with a weekly intake of alcohol greater than 210–420 g and 140–350 g for males and females, respectively [4]. Indeed, alcohol consumption is linked to increased risk of multiple cancer types, including HCC, through various underlying mechanisms which appear to include increased inflammation, oxidative stress, reactive oxygen species, acetaldehyde toxicity and DNA damage [14,15].
Recent evidence has also suggested the role of gut dysbiosis in the pathogenesis of MASLD [16,17,18]. The human microbiome describes the entirety of microorganisms that inhabit the human body, and since these reside mostly in the gut, it is often referred to as the gut microbiome [16,17,19]. The adult gut bacteria belong to two main phyla, namely Gram-positive Firmicutes and Gram-negative Bacteroidetes [16,17,19]. Overall, the gut microbiome is responsible for the regulation of the micro-environment and immune-system reactions in the gut and is therefore involved in maintaining physiological homeostasis [18]. However, changes secondary to genetic and environmental factors may create an imbalance between the gut microbiota and the host, resulting in modulation of the structure or diversity of the gut microbiota, known as “gut dysbiosis” [20]. This dysbiosis may then trigger a cascade of metabolic derangements implicated in the pathogenesis of various disease states, including obesity, type 2 diabetes mellitus (T2DM), and MASLD [20]. Understanding the pathogenic mechanisms behind MASLD and the contribution of the microbiome may aid our understanding of the disease’s natural history and risk of progression, which may improve diagnostics and promote the identification of novel therapeutic targets.
Based on the increasing body of relevant translational and clinical evidence, the present review aims to summarize recent data on the role of gut microbiota in the pathogenesis of MASLD, including implicated microbial signatures and factors that promote dysbiosis. Insights are also highlighted regarding the potential of microbial-based diagnostic biomarkers and the alteration of gut microbiota through therapeutic interventions, such as the use of probiotics, prebiotics, synbiotics, phages, fecal microbiota transplantation (FMT) and novel targeted therapies.
2. Gut Microbiota Dysbiosis and Microbial Signatures Associated with MASLD
Recent research has evolved our understanding of the gut microbiome, revealing it to be a dynamic ecosystem of microorganisms which plays an active role in the digestion and absorption of nutrients, whilst interacting with the host through endocrine/immune-mediated mechanisms [20,21]. The gut microbiota modulates the host’s immune system through the release of microbial-derived metabolites which are transported via the portal circulation and ultimately systemic circulation [20]. These microbial-derived metabolites affect antigen recognition, immune cell recruitment and proliferation and induce both pro- and anti-inflammatory cascades [22]. The association between compositional and functional alterations of the gut microbiota and MASLD, including severe disease states, such as cirrhosis and HCC, have long been described in the literature [20,23]. Referred to as gut dysbiosis, this often entails either a reduction in commensal bacteria, complete loss of microbial species, or overgrowth of pathogenic commensals, which then results in immune-mediated changes contributing/causing metabolic derangements and disorders [21]. Of note, the outgrowth of pathobionts (pathogenic commensals), such as Proteobacteria, particularly the Enterobacteriaceae family, is observed in prevalent metabolic disorders (e.g., obesity, T2DM and MASLD), and is therefore considered a potential diagnostic marker of gut dysbiosis [24,25].
Gut dysbiosis is driven by the complex interactions between the genetic background of the host (including their health status and drug medications) and lifestyle habits (e.g., diet and physical activity/exercise) [21]. Changes in diet have demonstrated significant shifts in the composition of the gut microbiota, with diets rich in fat and sugars exhibiting an association with intestinal inflammation and weakening of the gut barrier in both animal models and human studies [26,27]. Food additives and preservatives, which are now integral to most processed food items in Western-type diets, have also been shown to promote the overgrowth of Proteobacteria and directly alter the gut microbial composition, inducing inflammation of the gastrointestinal system [21,28,29].
Strong evidence for NAFLD and increasing evidence for MASLD show that this disease spectrum occurs in the context of metabolic dysfunction often in tandem with obesity and poor dietary habits. Alterations in the gut microbiota have been implicated in NAFLD/MASLD, including NASH/MASH, as demonstrated in animal models and human studies [18,19,21]. Of note, Rabot et al. described that germ-free mice showed lower lipid levels in the liver compared to conventional mice [30]. This was further reinforced by the transfer of gut microbiome into germ-free mice, which resulted in the development of fasting hyperglycemia, and NAFLD when compared to healthy mice [31]. Certain bacterial species, such as Lachnospiraceae spp. and Barnesiella intestinihominis, were positively associated with NAFLD in mice compared to controls, while Bacteroides vulgatus was negatively under-represented in the NAFLD animal model [31]. In most human studies of NAFLD or MASLD, gut dysbiosis is hypothesized to occur through the malfunction of the defense mechanisms of the gut–liver axis where invading pathogens and toxins induce systemic inflammation [21]. The most accepted microbial signature amongst NAFLD patients includes an overgrowth of Bacteroidetes spp. and differences in the composition of Firmicutes spp., resulting in a decreased Firmicutes spp. to Bacteroidetes spp. ratio [32,33]. An important consideration is that this ratio only represents a crude estimate given the huge diversity of microorganisms within these phyla [19]. Additionally, differences in the molecular methods used to identify these species may yield significantly different results. Species that have demonstrated a positive association with NAFLD patients include the Enterobacteriaceae family, Escherichia ssp., Clostridium spp., Anaerobacter spp., Streptococcus spp., and Lactobacillus spp., whereas Rikenellaceae, Ruminococcaceae, Faecalibacterium spp., Coprococcus spp., Anaerosporobacter spp., and Eubacterium spp. have become less prominent [21,34]. Amongst NASH patients when compared to healthy controls Proteobacteria, Enterobacteriaceae, and Escherichia demonstrated increased abundance, while Faecalibacterium prausnitzii (F. prausnitzii) and Akkermansia muciniphila were comparatively reduced [19,35,36]. Functionally, there is a shift from beneficial to harmful microbes leading to the promotion of a pro-inflammatory state which is the result of gut barrier dysfunction, exposing the liver to microbiota-derived factors and culminating in disease progression [36]. Unsurprisingly, the NAFLD associated microbial signatures also overlap with other metabolic diseases, where reduced levels of F. prausnitzii were observed in cirrhosis, obesity, T2DM, and inflammatory bowel disease [37,38,39,40]. F. prausnitzii is considered a beneficial microbe given its role in promoting anti-inflammatory pathways [38]. Conversely, increased levels of B. vulgatus were associated with advanced fibrosis, insulin resistance, and T2DM, promoting pro-inflammatory cascades [32].
Differences in microbiome composition between patients with NAFLD or MASLD based on their BMI have also been documented [2,41]. In one cohort lower levels of Ruminococcaceae and increased levels of Veillonellaceae with lower species diversity were noted amongst NAFLD patients with obesity compared to those that were lean [42]. These differences are not limited to bacterial species alone, but also viruses and fungal species [43,44]. Furthermore, Lang et al. demonstrated that patients with NAFLD and an increased disease activity score exhibited decreased bacteriophage diversity when compared to patients with lower disease activity scores [43]. It should be noted that, given the recent introduction of the MASLD nomenclature, most of the relevant human studies have been conducted under the NAFLD diagnostic criteria. Studies in this field which adopt the MASLD nomenclature/criteria are now emerging, with one such study showing a different microbial signature for MASLD [45], which was characterized by a higher abundance of Ruminococcaceae, Bilophila spp., and Sellimonas spp., and a lower presence of Defluviitaleaceae, Lachnospiraceae and Coprobacter spp. This highlights the need for further targeted studies exploring the impact of the newly defined MASLD diagnostic criteria on the gut microbiome to build a robust body of evidence specifically for MASLD which will supplement and may even show differences from that for NAFLD.
Collectively, most of the evidence discussed originates from association studies, which have demonstrated that correlations between gut microbial compositions and NAFLD or MASLD exist. However, current evidence is significantly limited by the lack of studies on potential underlying causal links, and by the marked heterogeneity between the relevant studies due to differences in geographical location, diet and co-morbidities which limit the reproducibility of results between cohorts [22]. Thus, potential underlying causal links remain to be determined, exploring whether gut dysbiosis is a direct cause rather than a disease-associated effect resulting from the host’s immune and endocrine/metabolic systems.
3. Mechanisms of Microbiome Modulation
The gut–liver axis refers to the bi-directional interplay between the gut epithelium, the biliary tract, immunological barriers, and the hepatic circulation (portal and systemic circulation). Gut-derived factors (e.g., microbial and by-products of dietary intake) regulate bile acid synthesis and liver metabolism, whilst liver-derived factors (e.g., bile acids) also affect gut microbial composition and function [21]. Any disruption of this regulated symbiotic relationship of the gut–liver axis (e.g., through gut dysbiosis or increased intestinal permeability) can result in failure to regulate the gut microbiota and promote hepatic pro-inflammatory changes [18,21]. Figure 1 summarizes key aspects of the relationship of the gut–liver axis with certain factors which relate to gut dysbiosis and have been implicated in the pathogenesis of NAFLD/MASLD.
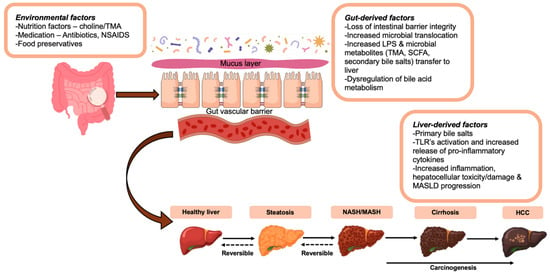
Figure 1.
The gut–liver axis and key factors contributing to gut dysbiosis in the pathogenesis of NAFLD/MASLD. These include factors relating to the environment, diet and medications (e.g., the use of food preservatives and antibiotics), gut-derived factors (e.g., loss of the intestinal barrier integrity) and liver-derived factors (e.g., increased hepatic inflammation) which are either directly or indirectly implicated in gut dysbiosis and the development and/or progression of NAFLD/MASLD. Abbreviations: LPS, lipopolysaccharide (endotoxin); MASLD, metabolic associated steatotic liver disease; NAFLD, non-alcoholic fatty liver disease; NSAIDS: Non-steroidal anti-inflammatory drugs; SCFA, short-chain fatty acids; TMA, trimethylamine; TLR, Toll-like receptors.
The gut barrier is composed of a mucus layer and an epithelial cell layer, which are held together via tight junction proteins, such as claudins and occludins [46]. These proteins play a significant role in maintaining the integrity of the gut barrier by hindering the translocation of microbial pathogens to the intestinal epithelial cells [18,46]. The gut barrier also consists of commensal bacteria, mucins secreted by goblet cells, and an immunological barrier composed of components of both cellular and humoral immunity [18,46]. Antimicrobial peptides and immunoglobulins, such as IgA, control the load and composition of microorganisms in the lumen, by binding to microbial antigens and toxins and transporting infiltrated bacteria to mesenteric lymph nodes to induce the priming of the adaptive immune response in the gut [21]. Emerging evidence has demonstrated that disruption of the gut barrier function (e.g., through disturbance of tight junctions) promotes intestinal permeability and excessive para-cellular leakage, which can lead to the development of NASH/MASH in patients [47,48,49]. A meta-analysis by Luther et al. has shown that NAFLD patients had altered gut permeability compared to healthy controls, with a stronger association in patients with NASH [49]. This is reinforced on a molecular level, where patients with NAFLD exhibited lower levels of expression of tight junction proteins such as zonula occludens-1 and junctional adhesions molecules [47,50]. An unhealthy diet composed of low fiber, high sugar and food additive content, as well as excessive alcohol consumption, and excessive exposure to antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs) have also been associated with tight junction disruption and increased intestinal permeability [51,52,53]. However, underlying causal links between gut barrier dysfunction and NAFLD/MASLD still remain unclear, with some evidence demonstrating that disrupted gut permeability is a consequence rather than a cause of the disease [22]. Indeed, in one animal model study alterations in tight junction proteins were only shown after initial hepatic injury [49]. Despite the inconsistency of evidence regarding the initial triggering pathogenetic event, there is consensus that impaired gut barrier function further exacerbates steatohepatitis [21,22]. According to Gabele et al., the induction of inflamed and disrupted gut permeability led to the translocation of microbial derived factors, such as lipopolysaccharide (LPS; or endotoxin), to systemic circulation, which resulted in worsening hepatic inflammation and fibrosis [54].
The immunological tolerance of the liver is also important in the understanding of the pathogenic mechanisms underpinning the modulation of the gut microbiome. The liver is capable of processing low-level exposure to innocuous molecules, such as microbial antigens, by hepatic antigen presenting cells [55]. Antigen presentation by this collection of cells results in the suppression of T-cell responses and induces anti-inflammatory cytokines (e.g., transforming growth factor-beta and interleukin-10) [55]. This mechanism protects the liver from immune-mediated injury and induces hepatic tolerance. However, when there is a dysfunction of the gut barrier function, the liver is overwhelmed with gut-mediated antigens, resulting in the loss of immunological tolerance and the development of a pro-inflammatory state [56]. Activation of pattern recognition receptors on hepatic macrophages by microbial antigens results in increased production of inflammatory and fibrogenic cytokines (e.g., tumor necrosis factor-alpha, interleukin-1, interleukin-6), and activation of CD8+ cytotoxic T-cells, which contributes to inflammatory-mediated hepatic injury [55,56]. The activation and maintenance of hepatic inflammation may then lead to fibrosis, cirrhosis, or even HCC [56].
Various microbiome-derived compounds have been implicated in the pathogenesis of NAFLD/MASLD, including short-chain fatty acids (SCFAs), LPS, choline, and choline metabolites. SCFAs are fatty acids with fewer than six carbons, and commonly include acetate, propionate and butyrate [21]. Produced via anaerobic fermentation by gut microbiota from non-digestible starch and fiber, these SCFAs play an important role in promoting intestinal integrity, supporting glucose (gluconeogenesis) and lipid (lipogenesis) metabolism, as well as immune-modulation [57]. Notably, increased fecal levels of SCFAs (acetate and propionate) were found to be associated with NAFLD and hepatic fibrosis [32]. This impact is mediated through the functioning of these SCFAs as signaling molecules via the activation of G-protein coupled receptors (GPR41 and GPR43), triggering the secretion of peptide-YY from gut entero-endocrine cells which in turn slows gastric emptying, thus increasing nutrient absorption and hepatic lipid accumulation and gluconeogenesis [58]. However, some SCFAs, such as butyrate, have demonstrated anti-inflammatory properties and may play a protective role against diet-induced obesity, hepatic steatosis, and insulin resistance [59,60]. Therefore, the metabolic and immune-modulatory effects of SCFAs have the potential for both harmful and protective impact; although the majority of published data favor the induction of anti-inflammatory properties, but under certain conditions pro-inflammatory cascades are also induced [61,62]. Human studies have also reflected this, with circulating butyrate measured in patients with cirrhosis being inversely correlated to inflammatory makers in one study, whereas in another study increased levels of butyrate were associated with mild or moderate NAFLD [63,64]. Animal models of NAFLD have shown that supplementation of SCFAs (butyrate) in mice fed a high-fat diet resulted in reductions in hepatic and adipose tissue inflammation, and reduced endotoxin-releasing bacteria in the gut microbiome [57,65]. This may suggest that SCFA supplementation may have beneficial metabolic effects, although large-scale human studies of SCFA supplementation amongst patients with NAFLD or MASLD are lacking to support this. Variations in diet, and other factors, as well as in SCFA sample processing, are likely to have contributed to the observed differences amongst clinical studies, highlighting the need for further, well-designed research to determine the potential contribution of SCFAs in the pathogenesis of MASLD and discriminate whether the net impact is positive or negative.
Another important gut-derived factor to consider is choline, which is mainly obtained from dietary sources such as eggs, cheese, and red meat [66]. Notably, choline is required for the synthesis of phosphatidylcholine, a component of cell membranes, whilst it is also necessary for the production of both the neurotransmitter acetylcholine and very low-density lipoproteins (VLDLs) [21]. Given the latter, choline deficiency results in reduced production of VLDLs and the consequent accumulation of triglycerides in the liver [67]. As such, choline-deficient diets have been used as a method to induce NASH/MASH in animal models, with choline supplementation reversing the disease process [68]. However, under certain conditions choline may play a role in the development of NAFLD/MASLD. Specific gut microbiota can convert choline into trimethylamine (TMA), which is then oxidized by hepatic monooxygenases to trimethylamine N-oxide (TMAO) that is considered a harmful metabolite [67,68]. Choline conversion by the gut microbiota is considered to play a role in NAFLD/MASLD pathogenesis through direct and indirect mechanisms [21]. Animal models have shown that higher rates of conversion of choline to TMA by the gut microbiota leads to reduced bioavailability of choline, resulting in hepatic inflammation and lipid accumulation mediated by lower VLDL levels [66,69]. Additionally, TMAO may act directly on the liver contributing to impaired glucose metabolism through the reduced activity of CYP7A1 and CYP27A1 enzymes, decreasing the conversion of cholesterol into bile acids and promoting hepatic steatosis as demonstrated in both preclinical and clinical studies [70,71]. Compared to healthy controls, patients with NAFLD have exhibited higher levels of TMAO, and this was also correlated with disease severity [72]. Similar associations have been shown between TMAO and cardio-metabolic diseases, such as T2DM and cardiovascular disease [73]. TMAO promotes macrophage migration and their transformation into foam cells and has been associated with endothelial dysfunction and thrombus formation, increasing the risk of atherosclerosis and cardiovascular disease [74]. Notably, TMAO has been established as a prognostic marker for both short- and long-term cardiovascular events in patients with acute coronary syndrome [75]. However, the potential of serum TMAO levels as a biomarker of MASLD progression and prognosis or even as a therapeutic target for atherosclerosis remains to be elucidated in future studies.
Bile acids have also been implicated in the pathogenesis of NAFLD/MASLD in relation to gut microbiome modulation [76]. Bile acids are synthesized in the liver from cholesterol and are secreted in the form of conjugated bile salts via the bile duct [76]. They are classified as primary (e.g., cholic acid and chenodeoxycholic acid) and secondary bile acids (e.g., deoxycholic and lithocholic acid) [76]. Primary bile acids are conjugated in the gall bladder before being secreted into the intestines where they aid in the absorption of lipids and lipid-soluble vitamins, whilst they also prevent bacterial overgrowth and maintain the composition of the microbiome [77]. Gut microbiota deconjugate primary bile acids into secondary bile acids, which are then reabsorbed in the distal ileum and are recycled to the liver through the portal circulation [76,77]. In the presence of dietary triggers, changes in the secretion of bile acids may result in alterations of the gut microbiome, which has been associated with steatosis/steatohepatitis [77]. According to Chen et al., increased levels of primary bile acids (chenodeoxycholic acid) were noted in NASH patients and correlated with the underlying histological severity and fibrosis grade [78]. This is likely mediated through the signaling capabilities of bile acids, where they bind to cellular receptors, such as the nuclear farnesoid X receptor (FXR) and TGR5 (G protein-coupled bile acid receptor 1; GPBAR-1) [79]. A plausible hypothesis is that gut dysbiosis results in modification of the primary and secondary bile acid balance, resulting in disruption of FXR signaling and the promotion of metabolic and immune modulatory responses, including lipid and glucose dysregulation [17,79]. However, due to the dearth of animal and human studies regarding the role of bile acids in the pathogenesis of NAFLD/MASLD, it is difficult to draw robust conclusions. Notably, bile acids can be influenced by diet (e.g., by dietary protein and fiber content) [80]; hence, more translational and clinical research is required to elucidate the potential pathophysiologic links between bile acids and MASLD and how these may be addressed by therapeutic interventions, such as certain dietary changes. A detailed description of the molecular pathways (e.g., bile acids signaling, FXR regulation and TLR4 signaling) which are implicated in the pathophysiologic mechanisms that appear to mediate the development of obesity, MASDL and other obesity-related diseases is beyond the scope of the present review as these have been discussed in detail in other reviews [81,82,83,84,85,86,87,88].
4. Microbiota-Based Biomarkers and Therapeutic Implications
Our increasing understanding of the gut microbiome has resulted in its emergence as a potential diagnostic and therapeutic target for MASLD. Currently, liver biopsy is the gold standard for diagnosis, but given its invasive nature there is a growing drive for reliable and non-invasive markers [8]. The gut microbiome represents a rich source of metabolite biomarkers, which has prompted clinical exploration into the utility of gut, serum and fecal microbiota and metabolite biomarkers. According to Loomba et al. a 37-bacterial strain panel was able to accurately predict the presence of fibrosis in patients with NAFLD [32]. Progression of NAFLD may also be monitored by the integration of multiple measurements of blood serum metabolites, as highlighted in the study by Hoyles et al., where data from metagenomics, liver transcriptomics and metabolomics were integrated as a potential diagnostic tool to determine disease stages [89]. Additionally, associations have been identified between the presence of Veillonellaceae bacteria and metabolites produced by NAFLD-associated microbes, such as Bacteroidaceae and Prevotella spp. [42,90]. These associations were found to be elevated in fecal samples from patients, suggesting their potential utility as diagnostic markers for MASLD [42,90]. However, the use of fecal samples as a biomarker source for MASLD is challenged by the confounding effects of age, sex, diet, medication, hormonal and lifestyle factors on the gut microbiota and the associated high cost of sample processing [18]. Moreover, the feasibility of using the gut microbiota as a potential therapeutic target is gaining substantial attention given its role in modulating host metabolism and immunity. Emerging research has explored the potential in mitigating hepatic inflammation and metabolic dysfunction through restoration of microbial balance by interventions like targeted therapies, probiotics, prebiotics, synbiotics, bacteriophages and FMT.
Therapeutic drugs proposed for NAFLD/MASLD include drugs targeting bile acid regulation, such as FXR agonists and antagonists, as well as fibroblast growth factor (FGF) mimetics [22,91]. Other innovative treatments, including peroxisome proliferator-activated receptor (PPAR) agonists, glucagon like pepetide-1 (GLP-1) agonists, and thyroid hormone receptor beta (THR-β) agonists, are also being actively explored [22,92]. These therapies are at various stages of clinical development, with several showing encouraging results in terms of improving hepatic steatosis, reducing fibrosis, and addressing systemic metabolic dysfunction [92,93]. Table 1 summarizes these pertinent therapeutic agents for the treatment of NAFLD/MASLD (under investigation/development or approved) which should also be explored regarding their impact on gut microbiota. These drugs and their targets have been discussed extensively in other reviews [92,93,94,95,96,97].

Table 1.
Selected pertinent therapeutic agents for the treatment of NAFLD/MASLD (Abbreviations: ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; FGF, fibroblast growth factor, FXR, farnesoid X receptor; GGT, gamma-glutamyl transferase; GLP-1, glucagon-like peptide 1; MASLD, metabolic associated steatotic liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptor; THR-β, thyroid hormone receptor beta).
Given the contribution of gut dysbiosis to disease pathogenesis, probiotics (defined as live microorganisms that confer health benefits when consumed in adequate amounts) have been proposed for the treatment of NAFLD/MASLD [110,111]. According to the International Scientific Association for Probiotics and Prebiotics consensus statement, probiotics have demonstrated potential in improving hepatic function and lipid profiles in patients with NAFLD [110,111]. However, the evidence remains inconsistent, with most studies conducted on animal models [18,110]. The most widely used probiotics include Bifidobacterium and Lactobacillus species or a combination of them, but next generation probiotics, such as F. prausnitzii, A. muciniphila, or Clostridium strains, have also demonstrated beneficial results [3,41]. Notably, combination probiotics, such as the high concentration probiotic mixture VSL#3 with eight probiotic strains of the genera Lactobacillus, Bifidobacterium, and Streptococcus [112], have proven superior to single probiotics [18]. Animal models have demonstrated that VSL#3 has a protective effect against NAFLD by inhibiting inflammatory pathways (e.g., c-Jun N-terminal kinase and nuclear factor-kappa B signaling), and by maintaining the integrity of the gut-barrier (therefore improving gut dysbiosis through these effects) [113,114]. However, in another animal study, VSL#3 did not alter hepatic inflammation, although some improvement in hepatic fibrosis was observed [115]. According to a randomized controlled trial (RCT) patients with NAFLD receiving VSL#3 exhibited improved triglyceride levels and inflammatory markers when compared with controls [116]. Similarly, another RCT demonstrated that the administration of a 16-strain probiotic mixture to patients with NAFLD resulted in amelioration of histological findings, such as reduced hepatocellular ballooning and fibrosis along with improved hepatic function, when compared to placebo [117]. In contrast, a further RCT, which evaluated a probiotic mixture containing strains of Lactobacillus and Bifidobacterium, did not demonstrate any significant improvement in hepatic steatosis or function amongst patients with NAFLD [118]. This was also echoed by the findings of other RCTs where no improvement in hepatic function, steatosis or fibrosis was observed amongst patients receiving combination probiotics [119,120].
Contrary to probiotics, which include live organisms, prebiotics constitute non-digestible compounds, predominantly carbohydrates, which may lead to beneficial changes to the host through influencing the composition and/or activity of the gut microbiota [111]. Thus, prebiotics are defined as substrates which are selectively utilized by host microorganisms and confer a health benefit, working primarily by promoting the growth of beneficial bacteria via their degradation by enzymes in the gut, and this definition has been expanded to include non-carbohydrate substances as well [111]. For example, they increase the production of Lactobacillus and Bifidobacterium, which confers positive alterations in the composition and function of the gut microbiota [21]. Prebiotics have also been associated with increased production of SCFAs, which as aforementioned have anti-inflammatory properties and may promote the intestinal barrier integrity [121]. Overall it is considered that, by modulating the composition of the gut microbiota, prebiotics modify the concentrations of SCFAs, bile acids, and other microbial-derived molecules transported to the liver to alleviate hepatic inflammation and steatosis associated with NAFLD/MASLD [121]. The exact potential of prebiotics in NAFLD/MASLD treatment is limited by the scarcity of clinical studies, with the need for further research to fully elucidate their role as an impactful therapeutic modality.
Synbiotics are a combination of probiotics and prebiotics which have also demonstrated therapeutic potential for NAFLD/MASLD, by way of reducing de novo lipogenesis and stimulating fatty acid beta-oxidation [17]. Although not definite, there are data indicating that synbiotics supplementation in patients with NAFLD have a positive impact on BMI and hepatic function [122]. Other studies have also described the role of synbiotics in improving liver stiffness and promoting anti-inflammatory and antioxidant properties [122,123]. However, similar to probiotics and prebiotics, not all results have been consistent, and further, large-scale clinical research studies are needed to investigate their therapeutic potential.
The utilization of bacteriophages and FMT in NAFLD/MASLD is considered to be in its early stages when compared with the use of pro-/pre-/syn-biotics as relevant therapeutic options. Bacteriophages are viruses that infect and kill bacteria, which have been proposed as a tool for targeting dysbiotic parts of the gut microbiota in patients with NAFLD/MASLD [18]. However, so far, these have been found effective only in alcoholic liver disease, targeting E. faecalis, which is overrepresented in such patients, resulting in improved hepatic function and inflammation [124]. Concerns regarding the safety and cost of phage therapy may also represent a challenge for their future application [17]. Similarly, FMT has only been used widely in the context of Clostridium Difficile infections and to a lesser extent in hepatic encephalopathy [125,126]. Animal studies have demonstrated the beneficial effects of FMT on hepatic lipid accumulation and its success in patients with cirrhosis and alcoholic hepatitis [127,128]. An RCT by Xue et al. in patients with NAFLD, showed that FMT from healthy donors resulted in reductions in hepatic lipid accumulation and improved lipid profiles through amelioration of gut dysbiosis [129]. Another study by Stols-Goncalves et al. also confirmed alterations in the gut microbiome following FMT [130]; however, two patients in the study developed extended-spectrum beta-lactamase infections, raising safety concerns particularly amongst immunocompromised patients and highlighting the importance of ‘healthy’ donor selection. Rigorous experimental protocols and well-designed RCTs are still required to study the potential efficacy (including long-term effects) of FMT in the treatment of NAFLD/MASLD, as has also been shown by a recent systematic review [131,132]. Indeed, that systematic review of RCTs showed that supplementation with probiotics and synbiotics appears promising for improving hepatic steatosis, though additional RCTs are required to study the efficacy of FMT approaches in patients with MASLD [132]. For such microbiome-targeted therapies, and particularly for FMT, challenges relating to potential adverse effect risks (e.g., potential pathogen transmission associated with FMT) and variability in patient responses, as well as the scalability of these therapeutic interventions should be further explored. Table 2 presents RCTs with interventions targeting the gut microbiota in the management of hepatic steatosis/steatohepatitis, such as probiotics, prebiotics, synbiotics and FMT. As a common limitation of these existing RCTs is their relatively small sample size and/or short duration, large scale and longer-term RCTs are still required before these interventions can be introduced in routine clinical practice.

Table 2.
Selected randomized controlled trials on interventions targeting (directly or indirectly) the gut microbiota in the management of hepatic steatosis/steatohepatitis (Abbreviations: ALT, Alanine Aminotransferase; APRI, Aspartate Aminotransferase to Platelet Ratio Index; AST, Aspartate Aminotransferase; BMI, body mass index; CFU: colony-forming units; CK-18, cytokeratin-18; CRP, C-reactive protein; FGF19, fibroblast growth factor 19; FMT, fecal microbiota transplantation; GGT, gamma glutamine transferase; HDL, High-Density Lipoprotein; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; IL-6, interleukin-6; IR, insulin resistance; LDL, low-density lipoprotein; MASLD, metabolic associated steatotic liver disease; MRI, Magnetic Resonance Imaging; NAFLD, non-alcoholic fatty liver disease; NAS: non-alcoholic fatty liver activity score; NASH, non-alcoholic steatohepatitis; RCT: randomized controlled trial; TLR-4,Toll-like receptor 4; TNF-α, tumor necrosis factor-alpha).
5. Future Perspectives and Research Needs to Exploit/Target the Gut Microbiota in the Context of MASLD
In recent decades, remarkable progress has been achieved in our understanding of the MASLD pathophysiology which has rightly attracted the intense focus of translational and clinical research given its increasing prevalence and the significant related burden on healthcare systems globally [151]. Elucidating the complex interplay of the underlying pathophysiology of MASLD, particularly regarding the role of novel mediators such as that of the gut microbiota and the gut–liver axis, can further advance more effective and tailored diagnostic and management strategies for this chronic liver condition. Thus, further research focus is currently required on how insight into the role that the gut microbiota plays in maintaining or impairing liver health can support more personalized approaches for the diagnosis and treatment of MASLD, and, especially, of MASH [152,153]. In this context, future research should also address various barriers and constraints which presently prohibit or delay the integration of gut microbiome-based diagnostic approaches and microbiome-targeted therapies to routine clinical practice. Such constraints relate to both the validation/standardization (e.g., issues relating to sample collection, as well as to the duration and method of testing) and the feasibility and availability/scalability (e.g., the clinical applicability, safety, and scalability of FMT) of the relevant diagnostic methods and treatments.
Overall, progress in the detection/analysis of gut microbiome-based biomarkers is expected to aid in the early diagnosis and effective management of MASLD in patients at high risk for MASH and related complications. Indeed, establishing validated and readily available gut microbiome-based biomarkers, together with omics technologies (e.g., metabolomics, lipidomics and transcriptomics) [154], could lead to novel algorithms for prompt diagnosis of MASLD/MASH in routine clinical practice. These, for example, could take into account the bi-directional links within the adipose tissue–liver–gut axis (e.g., adipokines and adipose tissue biomarkers which are implicated in hepatic steatosis and/or inflammation and the development and/or progression of MASLD) [155], as well as the interplay between the gut microbiome and specific MASLD phenotypes, thus helping the early identification of the individuals at high risk for MASH and disease progression within the MASLD spectrum based on their metabolic and gut-microbiome profiles [152,153]. It should also be highlighted that MAFLD is linked to markedly increased risk for a number of additional cardio-metabolic conditions, including T2DM and cardiovascular disease, with overlapping pathophysiologic processes [156]. Thus, omics and gut microbiome-based biomarkers in combination with polygenic risk scores may offer valuable novel screening and diagnostic tools for the prompt and effective monitoring of the broader cardiovascular-liver-metabolic health [156].
Moreover, non-invasive gut microbiome profiling holds strong potential for the development of personalized dietary/nutritional interventions and pharmacological treatments for MASLD [152,153]. Of note, although MASLD is associated with increasing morbidity and mortality [151], progress in specific pharmacotherapy for MASLD/MASH faces various challenges, whilst drugs specifically for steatohepatitis are now being approved/developed [157,158,159]. As such, the primary management recommendations of the current MASLD clinical guidelines rely on dietary interventions/modifications to manage weight loss and hepatic inflammation [160,161,162]. In this context, insight into the complex crosstalk between dietary factors and the gut-microbiota may help the development of personalized nutrition approaches for patients with MASLD/MASH which would be supported in practice by gut microbiome profiling/biomarkers [152,153]. For example, large-scale and long-term trials are required to provide evidence on whether the Mediterranean diet, which has established cardio-metabolic benefits compared to Western dietary patterns [153,163], could be further tailored based on gut microbiome-based biomarkers to better meet the needs of patients with MASLD/MASH or those with both MASDL and T2DM. Similar clinical trials can also explore how the profiling of the gut microbiome could help to tailor the optimum diet for such patients (e.g., in the context of very low-calorie, very low carbohydrate and ketogenic diets, as well as in relationship to intermittent fasting) [163,164], potentially combined with supplementation with probiotics, prebiotics, or synbiotics. The evidence from such studies could be further combined with genomic data to better predict MASLD phenotypes and their responses to specific dietary interventions and pharmacological treatments as part of a more holistic approach to precision nutrition/medicine for the management of MASLD (Figure 2) [163,164,165]. As such, there is a need for more studies in this field that can support evidence-based recommendations for a shift in routine clinical practice from generalized approaches to personalized management strategies, which would incorporate genetic, multi-omics and gut microbiome-based biomarkers, for the treatment of MASLD/MASH. This type of personalized/precision management may effectively decrease the development of MASLD and/or its progression to MASH, thus improving the overall cardiovascular-liver-metabolic health of these patients.
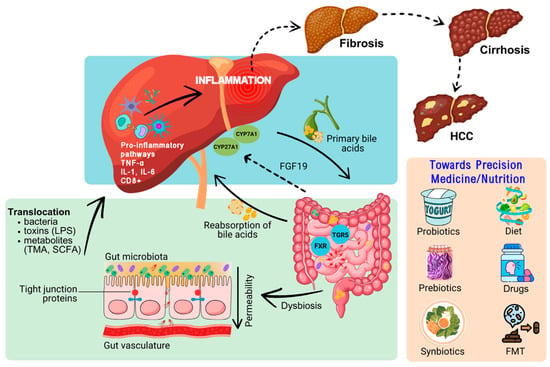
Figure 2.
Schematic representation of underlying mechanisms/links which implicate the gut microbiota and the gut–liver axis in the pathophysiology of steatosis/steatohepatitis and which could be leveraged for the management of these hepatic pathologies in the context of personalized medicine/nutrition therapeutic approaches. Dysbiosis of the microbiome leads to damage and permeability of the gut barrier which enables translocation of bacteria, toxins, and metabolites into the liver via the portal vein. This may trigger pro-inflammatory pathways/cascades which can lead to fibrosis, cirrhosis, and even HCC. Conjugated primary bile acids are secreted from the liver into the small intestine. In the gut, bile acids interact with microbiota and cellular receptors, including the TGR5 and FXR. The interaction of bile acids with FXR induces FGF19 secretion which serves as a negative feedback loop for bile acid synthesis in hepatocytes. Dietary patterns (e.g., the Mediterranean diet), supplementation with probiotics, prebiotics and synbiotics, as well as interventions such as FMT and novel drugs, are examples of precision medicine/nutrition approaches which may target gut dysbiosis for the treatment of MASLD. Abbreviations used include the following: CYP27A1, Cytochrome P450, Family 27, Subfamily A, Polypeptide 1; CYP7A1, Cytochrome P450, Family 7, Subfamily A, Polypeptide 1; FGF19, fibroblast growth factor 19; FMT; fecal microbiota transplantation; FXR, farnesoid X receptor; HCC, hepatocellular carcinoma; MASLD, metabolic associated steatotic liver disease; LPS, lipopolysaccharide (endotoxin); SCFA, short-chain fatty acids; TGR5, takeda G-protein-coupled receptor 5; TMA, trimethylamine.
6. Conclusions
A growing body of recent scientific evidence has enhanced our understanding of the gut microbiota’s role in the pathogenesis of MASLD. The dynamic gut–liver axis facilitates the bi-directional communication and transport of both beneficial and harmful metabolites from the gut to the liver and vice versa. Within these interactions, when the liver is overwhelmed with harmful and pro-inflammatory molecules (e.g., due to unhealthy dietary habits), as well as gut dysbiosis, or increased gut permeability, hepatic function becomes impaired and MASLD pathogenetic mechanisms are triggered. Such hepatic dysfunction may also result in an inability to effectively regulate the gut microbiota through bile acids and other factors, further promoting gut dysbiosis and gut barrier dysfunction. This self-perpetuating vicious cycle may have far-reaching implications, exacerbating hepatic damage and overall health.
Recent clinical studies have uncovered promising NAFLD/MASLD-associated microbiome signatures which could potentially be used for non-invasive diagnosis and monitoring of disease progression. However, inconsistencies arising from variability between populations, disease stages, and confounding factors (e.g., ethnic background, dietary habits and comorbidities such as T2DM and obesity) complicate the establishment of definitive gut microbial signatures. Additionally, despite the growing prevalence of MASLD, current diagnostic methods remain sub-optimal. Therefore, there is an imperative need to explore new diagnostic, and therapeutic strategies which consider the interplay between the gut microbiota, its metabolites, and MASLD. Accordingly, robust validation through large, well-designed cohort studies and RCTs is required to fully evaluate the diagnostic potential of microbial signatures and biomarkers, as well as the therapeutic potential of probiotics, prebiotics, synbiotics, and other microbiota-based interventions. Future studies should also consider the dosing, feasibility and long-term safety of these interventions.
Author Contributions
Conceptualization, F.A., I.K. and H.S.R.; Writing—Original draft preparation, F.A.; Literature Search, F.A., A.M., C.K., L.L., A.D., N.N.T., E.K., I.K. and H.S.R.; Visualization, F.A., A.M., C.K., L.L., A.D., I.K. and H.S.R.; Writing-Reviewing and Editing, F.A., A.M., C.K., L.L., A.D., N.N.T., E.K., I.K. and H.S.R.; Supervision, I.K., and H.S.R., I.K. and H.S.R. have contributed equally to this work and are joint senior and corresponding co-authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
I.K. and H.S.R. would like to thank the University Hospitals Coventry and Warwickshire (UHCW) NHS Trust for their ongoing support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Singal, A.G.; Kono, Y.; Tan, D.J.H.; El-Serag, H.B.; Loomba, R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022, 34, 969–977.e2. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.; Lionis, C.; Kite, C.; Atkinson, L.; Lagojda, L.; Chaggar, S.S.; Kyrou, I.; Randeva, H.S. Challenges in the Management of Non-Alcoholic Fatty Liver Disease (NAFLD): Towards a Compassionate Approach. Livers 2023, 3, 434–447. [Google Scholar] [CrossRef]
- Lonardo, A.; Bril, F.; Caldwell, S.H.; Eslam, M.; Fan, J.G.; Gish, R.G.; Gronbaek, H.; Sanal, M.G.; Stefan, N.; Suzuki, A.; et al. Researchers call for more flexible editorial conduct rather than abruptly adopting only the new MASLD nomenclature. J. Hepatol. 2024, 80, e192–e194. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef]
- Abdelhameed, F.; Kite, C.; Lagojda, L.; Dallaway, A.; Chatha, K.K.; Chaggar, S.S.; Dalamaga, M.; Kassi, E.; Kyrou, I.; Randeva, H.S. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review from NAFLD to MAFLD and MASLD. Curr. Obes. Rep. 2024, 13, 510–531. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Mejia, M.M.; Jimenez-Gutierrez, C.; Eslam, M.; George, J.; Mendez-Sanchez, N. Breaking new ground: MASLD vs. MAFLD-which holds the key for risk stratification? Hepatol. Int. 2024, 18, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Wong, V.W. Implications of the new nomenclature of steatotic liver disease and definition of metabolic dysfunction-associated steatotic liver disease. Aliment. Pharmacol. Ther. 2024, 59, 150–156. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, B. Unraveling the pathogenesis of non-alcoholic fatty liver diseases through genome-wide association studies. J. Gastroenterol. Hepatol. 2023, 38, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Vilar, L.; Oliveira, C.P.; Faintuch, J.; Mello, E.S.; Nogueira, M.A.; Santos, T.E.; Alves, V.A.; Carrilho, F.J. High-fat diet: A trigger of non-alcoholic steatohepatitis? Preliminary findings in obese subjects. Nutrition 2008, 24, 1097–1102. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.B.; Yao, F. Alcohol and Hepatocellular Carcinoma. Clin. Liver Dis. 2024, 28, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Liu, J.; Dalamaga, M. What are the key points in the association between the gut microbiome and nonalcoholic fatty liver disease? Metabol. Open 2019, 1, 9–10. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kounatidis, D.; Psallida, S.; Vythoulkas-Biotis, N.; Adamou, A.; Zachariadou, T.; Kargioti, S.; Karampela, I.; Dalamaga, M. NAFLD/MASLD and the Gut-Liver Axis: From Pathogenesis to Treatment Options. Metabolites 2024, 14, 366. [Google Scholar] [CrossRef]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef]
- Betrapally, N.S.; Gillevet, P.M.; Bajaj, J.S. Gut microbiome and liver disease. Transl. Res. 2017, 179, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef] [PubMed]
- Hrncirova, L.; Hudcovic, T.; Sukova, E.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Human gut microbes are susceptible to antimicrobial food additives in vitro. Folia Microbiol. 2019, 64, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Hrncirova, L.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Food Preservatives Induce Proteobacteria Dysbiosis in Human-Microbiota Associated Nod2-Deficient Mice. Microorganisms 2019, 7, 383. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gerard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Cohen, T.S. Can You Trust Your Gut? Implicating a Disrupted Intestinal Microbiome in the Progression of NAFLD/NASH. Front Endocrinol. 2020, 11, 592157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergstrom, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Backhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Dore, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e585. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Demir, M.; Martin, A.; Jiang, L.; Zhang, X.; Duan, Y.; Gao, B.; Wisplinghoff, H.; Kasper, P.; Roderburg, C.; et al. Intestinal Virome Signature Associated with Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 159, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Lang, S.; Hartmann, P.; Duan, Y.; Martin, A.; Miyamoto, Y.; Bondareva, M.; Zhang, X.; Wang, Y.; Kasper, P.; et al. The fecal mycobiome in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Zazueta, A.; Valenzuela-Perez, L.; Ortiz-Lopez, N.; Pinto-Leon, A.; Torres, V.; Guinez, D.; Aliaga, N.; Merino, P.; Sandoval, A.; Covarrubias, N.; et al. Alteration of Gut Microbiota Composition in the Progression of Liver Damage in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int. J. Mol. Sci. 2024, 25, 4387. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Kamiya, T.; Kawada, N. Recent updates on the role of the gut-liver axis in the pathogenesis of NAFLD/NASH, HCC, and beyond. Hepatol. Commun. 2023, 7, e0241. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Volynets, V.; Kuper, M.A.; Strahl, S.; Maier, I.B.; Spruss, A.; Wagnerberger, S.; Konigsrainer, A.; Bischoff, S.C.; Bergheim, I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2012, 57, 1932–1941. [Google Scholar] [CrossRef]
- Luther, J.; Garber, J.J.; Khalili, H.; Dave, M.; Bale, S.S.; Jindal, R.; Motola, D.L.; Luther, S.; Bohr, S.; Jeoung, S.W.; et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Desai, C.; Iyer, S.S.; Thorn, N.E.; Kumar, P.; Liu, Y.; Smith, T.; Neish, A.S.; Li, H.; Tan, S.; et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology 2016, 151, 733–746.E12. [Google Scholar] [CrossRef]
- Johnson, R.J.; Rivard, C.; Lanaspa, M.A.; Otabachian-Smith, S.; Ishimoto, T.; Cicerchi, C.; Cheeke, P.R.; Macintosh, B.; Hess, T. Fructokinase, Fructans, Intestinal Permeability, and Metabolic Syndrome: An Equine Connection? J. Equine Vet. Sci. 2013, 33, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Spruss, A.; Bergheim, I. Dietary fructose and intestinal barrier: Potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2009, 20, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Weber, S.; Vos, M.; Kramer, S.; Volynets, V.; Kaserouni, S.; McClain, C.J.; Bischoff, S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: Role of endotoxin. J. Hepatol. 2008, 48, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Gabele, E.; Dostert, K.; Hofmann, C.; Wiest, R.; Scholmerich, J.; Hellerbrand, C.; Obermeier, F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 2011, 55, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Horst, A.K.; Neumann, K.; Diehl, L.; Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol. Immunol. 2016, 13, 277–292. [Google Scholar] [CrossRef]
- Doherty, D.G. Antigen-specific immune tolerance in the liver. Nat. Biomed. Eng. 2019, 3, 763–765. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Festuccia, W.T.; Crisma, A.R.; Alves, V.S.; Martins, A.R.; Amaral, C.L.; Fiamoncini, J.; Hirabara, S.M.; Sato, F.T.; et al. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E272–E282. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhanel, M.; Woting, A.; Petzke, K.J.; Gohlke, S.; Schulz, T.J.; Blaut, M.; et al. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e1-10. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Hwang, S.J.; O’Donnell, C.J.; Ellison, R.C.; Vasan, R.S.; D’Agostino, R.B., Sr.; Liang, T.J.; Fox, C.S. Parental obesity and offspring serum alanine and aspartate aminotransferase levels: The Framingham heart study. Gastroenterology 2008, 134, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, L.F.; Sun, J.; Singh, B.K.; Zhou, J.; Kaddai, V.A.; Lanni, A.; Yen, P.M.; Sinha, R.A. Short chain fatty acids induce UCP2-mediated autophagy in hepatic cells. Biochem. Biophys. Res. Commun. 2016, 480, 461–467. [Google Scholar] [CrossRef]
- Zhai, S.; Qin, S.; Li, L.; Zhu, L.; Zou, Z.; Wang, L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol. Lett. 2019, 366, fnz153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mehedint, M.G.; Zeisel, S.H. Choline’s role in maintaining liver function: New evidence for epigenetic mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 339–345. [Google Scholar] [CrossRef]
- Flessa, C.M.; Nasiri-Ansari, N.; Kyrou, I.; Leca, B.M.; Lianou, M.; Chatzigeorgiou, A.; Kaltsas, G.; Kassi, E.; Randeva, H.S. Genetic and Diet-Induced Animal Models for Non-Alcoholic Fatty Liver Disease (NAFLD) Research. Int. J. Mol. Sci. 2022, 23, 15791. [Google Scholar] [CrossRef]
- Dumas, M.E.; Barton, R.H.; Toye, A.; Cloarec, O.; Blancher, C.; Rothwell, A.; Fearnside, J.; Tatoud, R.; Blanc, V.; Lindon, J.C.; et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12511–12516. [Google Scholar] [CrossRef]
- Tang, W.H.; Hazen, S.L. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl. Res. 2017, 179, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Liu, Y.; Zhou, R.F.; Chen, X.L.; Wang, C.; Tan, X.Y.; Wang, L.J.; Zheng, R.D.; Zhang, H.W.; Ling, W.H.; et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.S.; Obeid, S.; Wang, Z.N.; Hazen, B.J.; Li, L.; Wu, Y.P.; Hurd, A.G.; Gu, X.D.; Pratt, A.; Levison, B.S.; et al. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur. Heart J. 2019, 40, 2700–2709. [Google Scholar] [CrossRef]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [PubMed]
- Ferslew, B.C.; Xie, G.; Johnston, C.K.; Su, M.; Stewart, P.W.; Jia, W.; Brouwer, K.L.; Barritt, A.S.t. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3318–3328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, M.; Liu, J.; Luo, Y.; Yang, W.; Yang, J.; Liu, J.; Zhou, J.; Xu, C.; Zhao, F.; et al. Ratio of Conjugated Chenodeoxycholic to Muricholic Acids is Associated with Severity of Nonalcoholic Steatohepatitis. Obesity 2019, 27, 2055–2066. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Hattersley, J.G.; Kyrou, I.; Arafat, A.M.; Rudovich, N.; Roden, M.; Nowotny, P.; von Loeffelholz, C.; Matysik, S.; Schmitz, G.; et al. Effects of supplemented isoenergetic diets varying in cereal fiber and protein content on the bile acid metabolic signature and relation to insulin resistance. Nutr. Diabetes 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Belka, M.; Gostynska-Stawna, A.; Stawny, M.; Krajka-Kuzniak, V. Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease. Int. J. Mol. Sci. 2024, 25, 11213. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Yi, Q.; Luo, L.; Xiong, Y. Regulation of bile acids and their receptor FXR in metabolic diseases. Front. Nutr. 2024, 11, 1447878. [Google Scholar] [CrossRef]
- Tang, Y.; Fan, Y.; Wang, Y.; Wang, D.; Huang, Q.; Chen, T.; Cao, X.; Wen, C.; Shen, X.; Li, J.; et al. A Current Understanding of FXR in NAFLD: The multifaceted regulatory role of FXR and novel lead discovery for drug development. Biomed. Pharmacother. 2024, 175, 116658. [Google Scholar] [CrossRef]
- Wu, D.; van de Graaf, S.F.J. Maladaptive regeneration and metabolic dysfunction associated steatotic liver disease: Common mechanisms and potential therapeutic targets. Biochem. Pharmacol. 2024, 227, 116437. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Tu, W.; Huang, G. Regulating bile acids signaling for NAFLD: Molecular insights and novel therapeutic interventions. Front. Microbiol. 2024, 15, 1341938. [Google Scholar] [CrossRef] [PubMed]
- Almeqdadi, M.; Gordon, F.D. Farnesoid X Receptor Agonists: A Promising Therapeutic Strategy for Gastrointestinal Diseases. Gastro Hep Adv. 2024, 3, 344–352. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, Z.; Chu, H.; Wang, W.; Jin, Y.; Yang, L. TRIF-dependent signaling and its role in liver diseases. Front. Cell Dev. Biol. 2024, 12, 1370042. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Zhu, L.; Tao, Y.; Lu, W.; Cheng, H. Role of targeting TLR4 signaling axis in liver-related diseases. Pathol. Res. Pract. 2023, 244, 154410. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernandez-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, Z.; Zhang, L. Bile acid regulation: A novel therapeutic strategy in non-alcoholic fatty liver disease. Pharmacol. Ther. 2018, 190, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Swamikkannu, D.M.; Dasarapu, S.; Siva, R.P.V.; Nallam, J.; Pabba, S. The gut-liver nexus: Exploring gut microbiota dysbiosis in non-alcoholic fatty liver disease and its therapeutic implications. Egypt. Liver J. 2024, 14, 28. [Google Scholar] [CrossRef]
- Ma, J.L.; Zhou, Q.H.; Li, H.K. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients 2017, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Yki-Jarvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatotic liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Bugianesi, E. Dietary and pharmacological treatment in patients with metabolic-dysfunction associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 20–27. [Google Scholar] [CrossRef]
- Ciardullo, S.; Muraca, E.; Vergani, M.; Invernizzi, P.; Perseghin, G. Advancements in pharmacological treatment of NAFLD/MASLD: A focus on metabolic and liver-targeted interventions. Gastroenterol. Rep. 2024, 12, goae029. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, B.; Rao, G.; Tang, Y.; Rodriguez, A.; Glass, L.C.; Hartman, M.L. Incretin-based investigational therapies for the treatment of MASLD/MASH. Diabetes Res. Clin. Pract. 2024, 211, 111675. [Google Scholar] [CrossRef] [PubMed]
- Chianelli, D.; Rucker, P.V.; Roland, J.; Tully, D.C.; Nelson, J.; Liu, X.D.; Bursulaya, B.; Hernandez, E.D.; Wu, J.N.; Prashad, M.; et al. Nidufexor (LMB763), a Novel FXR Modulator for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 2020, 63, 3868–3880. [Google Scholar] [CrossRef]
- Traussnigg, S.; Halilbasic, E.; Hofer, H.; Munda, P.; Stojakovic, T.; Fauler, G.; Kashofer, K.; Krssak, M.; Wolzt, M.; Trauner, M. Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wien. Klin. Wochenschr. 2021, 133, 441–451. [Google Scholar] [CrossRef]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients with Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Lopez, P.; Lawitz, E.J.; Lucas, K.J.; Loeffler, J.; Kim, W.; Goh, G.B.B.; Huang, J.F.; Serra, C.; Andreone, P.; et al. Tropifexor for nonalcoholic steatohepatitis: An adaptive, randomized, placebo-controlled phase 2a/b trial. Nat. Med. 2023, 29, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, T.S.; Mounika, N.; Vishwakarma, G.; Adela, R. Effect of obeticholic acid in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) patients: A systematic review and meta-analysis. RPS Pharm. Pharmacol. Rep. 2022, 1, rqac001. [Google Scholar] [CrossRef]
- Lin, X.; Mai, M.; He, T.; Huang, H.; Zhang, P.; Xia, E.; Guo, H. Efficiency of ursodeoxycholic acid for the treatment of nonalcoholic steatohepatitis: A systematic review and meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Ling, L.; Beuers, U.; DePaoli, A.M.; Lieu, H.D.; Harrison, S.A.; Hirschfield, G.M. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep. 2021, 3, 100255. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.; Neff, G.; Patil, R.; Behling, C.; Hu, C.; Shringarpure, R.; de Temple, B.; Fong, E.; et al. A randomized, double-blind, placebo-controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis. JHEP Rep. 2023, 5, 100563. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Samajdar, S.S.; Das, S. Effects of saroglitazar in the treatment of non-alcoholic fatty liver disease or non-alcoholic steatohepatitis: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102174. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Velayudham, A.; Dolganiuc, A.; Ellis, M.; Petrasek, J.; Kodys, K.; Mandrekar, P.; Szabo, G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 2009, 49, 989–997. [Google Scholar]
- Paolella, G.; Mandato, C.; Pierri, L.; Poeta, M.; Di Stasi, M.; Vajro, P. Gut-liver axis and probiotics: Their role in non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 15518–15531. [Google Scholar] [CrossRef]
- Govender, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; van Vuuren, S.; Pillay, V. A review of the advancements in probiotic delivery: Conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS PharmSciTech 2014, 15, 29–43. [Google Scholar] [PubMed]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014, 39, 1276–1285. [Google Scholar] [PubMed]
- Derosa, G.; Guasti, L.; D’Angelo, A.; Martinotti, C.; Valentino, M.C.; Di Matteo, S.; Bruno, G.M.; Maresca, A.M.; Gaudio, G.V.; Maffioli, P. Probiotic Therapy With VSL#3® in Patients With NAFLD: A Randomized Clinical Trial. Front. Nutr. 2022, 9, 846873. [Google Scholar]
- Duseja, A.; Acharya, S.K.; Mehta, M.; Chhabra, S.; Shalimar; Rana, S.; Das, A.; Dattagupta, S.; Dhiman, R.K.; Chawla, Y.K. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): A randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019, 6, e000315. [Google Scholar] [CrossRef]
- Mohamad Nor, M.H.; Ayob, N.; Mokhtar, N.M.; Raja Ali, R.A.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.; Laight, D.; Aspinall, R.J.; Higginson, A.; Cummings, M.H. A randomised placebo controlled trial of VSL#3® probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 144. [Google Scholar]
- Silva-Sperb, A.S.; Moraes, H.A.; Barcelos, S.T.A.; de Moura, B.C.; Longo, L.; Michalczuk, M.T.; Cerski, C.T.S.; Uribe-Cruz, C.; da Silveira, T.R.; Alvares-da-Silva, M.R.; et al. Probiotic supplementation for 24 weeks in patients with non-alcoholic steatohepatitis: The PROBILIVER randomized clinical trial. Front. Nutr. 2024, 11, 1362694. [Google Scholar] [CrossRef] [PubMed]
- Bomhof, M.R.; Parnell, J.A.; Ramay, H.R.; Crotty, P.; Rioux, K.P.; Probert, C.S.; Jayakumar, S.; Raman, M.; Reimer, R.A. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: A pilot clinical trial. Eur. J. Nutr. 2019, 58, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Obesity: Current Evidence, Controversies, and Perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, W.; Rupasinghe, H.P.V. Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction. Molecules 2024, 29, 709. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Pande, A.; Shasthry, S.M.; Jamwal, K.D.; Khillan, V.; Chandel, S.S.; Kumar, G.; Sharma, M.K.; Maiwall, R.; Jindal, A.; et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin. Gastroenterol. Hepatol. 2017, 15, 600–602. [Google Scholar] [CrossRef] [PubMed]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Kelly, C.R.; Kim, A.M.; Laine, L.; Wu, G.D. The AGA’s Fecal Microbiota Transplantation National Registry: An Important Step Toward Understanding Risks and Benefits of Microbiota Therapeutics. Gastroenterology 2017, 152, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Xue, L.; Deng, Z.; Luo, W.; He, X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef] [PubMed]
- Stols-Goncalves, D.; Mak, A.L.; Madsen, M.S.; van der Vossen, E.W.J.; Bruinstroop, E.; Henneman, P.; Mol, F.; Scheithauer, T.P.M.; Smits, L.; Witjes, J.; et al. Faecal Microbiota transplantation affects liver DNA methylation in Non-alcoholic fatty liver disease: A multi-omics approach. Gut Microbes 2023, 15, 2223330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Chen, M. Commentary: Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: A randomized clinical trial. Front. Cell Infect. Microbiol. 2022, 12, 1056394. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, S.; Zhang, L.; Zhao, W.; Qin, Y.; Liu, M. The effect of gut microbiome-targeted therapies in nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Front. Nutr. 2024, 11, 1470185. [Google Scholar] [CrossRef] [PubMed]
- Ayob, N.; Muhammad Nawawi, K.N.; Mohamad Nor, M.H.; Raja Ali, R.A.; Ahmad, H.F.; Oon, S.F.; Mohd Mokhtar, N. The Effects of Probiotics on Small Intestinal Microbiota Composition, Inflammatory Cytokines and Intestinal Permeability in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2023, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Won, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Li, K.C.; Chan, H.L. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [CrossRef]
- Sepideh, A.; Karim, P.; Hossein, A.; Leila, R.; Hamdollah, M.; Mohammad, E.G.; Mojtaba, S.; Mohammad, S.; Ghader, G.; Seyed Moayed, A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2016, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; De Luis, D.A.; Izaola, O.; Conde, R.; Gonzalez Sagrado, M.; Primo, D.; De La Fuente, B.; Gonzalez, J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1090–1095. [Google Scholar] [PubMed]
- Ahn, S.B.; Jun, D.W.; Kang, B.K.; Lim, J.H.; Lim, S.; Chung, M.J. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 5688. [Google Scholar] [CrossRef] [PubMed]
- Escouto, G.S.; Port, G.Z.; Tovo, C.V.; Fernandes, S.A.; Peres, A.; Dorneles, G.P.; Houde, V.P.; Varin, T.V.; Pilon, G.; Marette, A.; et al. Probiotic Supplementation, Hepatic Fibrosis, and the Microbiota Profile in Patients with Nonalcoholic Steatohepatitis: A Randomized Controlled Trial. J. Nutr. 2023, 153, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Reshef, N.; Gophna, U.; Reshef, L.; Konikoff, F.; Gabay, G.; Zornitzki, T.; Knobler, H.; Maor, Y. Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)-A Randomized Pilot Trial. Nutrients 2024, 16, 1571. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610.E7. [Google Scholar] [CrossRef]
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: A pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 2017, 117, 662–668. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, A.; Askari, G.; Esmailzade, A.; Feizi, A.; Mohammadi, V. The Effect of Symbiotic Supplementation on Liver Enzymes, C-reactive Protein and Ultrasound Findings in Patients with Non-alcoholic Fatty Liver Disease: A Clinical Trial. Int. J. Prev. Med. 2016, 7, 59. [Google Scholar] [PubMed]
- Abhari, K.; Saadati, S.; Yari, Z.; Hosseini, H.; Hedayati, M.; Abhari, S.; Alavian, S.M.; Hekmatdoost, A. The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: A randomized, placebo-controlled, clinical trial. Clin. Nutr. ESPEN 2020, 39, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sayari, S.; Neishaboori, H.; Jameshorani, M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2018, 24, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.C.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals With Steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef]
- Abdel-Razik, A.; Mousa, N.; Shabana, W.; Refaey, M.; Elzehery, R.; Elhelaly, R.; Zalata, K.; Abdelsalam, M.; Eldeeb, A.A.; Awad, M.; et al. Rifaximin in nonalcoholic fatty liver disease: Hit multiple targets with a single shot. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1237–1246. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Harrison, S.A.; Rinella, M.E.; Abdelmalek, M.F.; Trotter, J.F.; Paredes, A.H.; Arnold, H.L.; Kugelmas, M.; Bashir, M.R.; Jaros, M.J.; Ling, L.; et al. NGM282 for treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018, 391, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Fianchi, F.; Liguori, A.; Gasbarrini, A.; Grieco, A.; Miele, L. Nonalcoholic Fatty Liver Disease (NAFLD) as Model of Gut-Liver Axis Interaction: From Pathophysiology to Potential Target of Treatment for Personalized Therapy. Int. J. Mol. Sci. 2021, 22, 6485. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Schnabl, B.; Knight, R.; Loomba, R. Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease. Cell Metab. 2021, 33, 21–32. [Google Scholar] [CrossRef]
- Bourganou, M.V.; Chondorgianni, M.E.; Kyrou, I.; Flessa, C.-M.; Chatzigeorgiou, A.; Oikonomou, E.; Randeva, H.S.; Kassi, E. Unraveling Metabolic Dysfunction-Associated Steatotic Liver Disease Through the Use of Omics Technologies. Int. J. Mol. Sci. 2025, 26, 1589. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext [INTERNET]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Chew, N.W.S.; Mehta, A.; Goh, R.S.J.; Zhang, A.; Chen, Y.; Chong, B.; Chew, H.S.J.; Shabbir, A.; Brown, A.; Dimitriadis, G.K.; et al. Cardiovascular-Liver-Metabolic Health: Recommendations in Screening, Diagnosis, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease in Cardiovascular Disease via Modified Delphi Approach. Circulation 2025, 151, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Puengel, T.; Tacke, F. Pharmacotherapeutic options for metabolic dysfunction-associated steatotic liver disease: Where are we today? Expert. Opin. Pharmacother. 2024, 25, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Tsioulos, G.; Kassi, E.; Chatzigeorgiou, A. Current and experimental pharmacotherapy for the management of non-alcoholic fatty liver disease. Hormones 2024, 23, 621–636. [Google Scholar] [CrossRef]
- Zhi, Y.; Dong, Y.; Li, X.; Zhong, W.; Lei, X.; Tang, J.; Mao, Y. Current Progress and Challenges in the Development of Pharmacotherapy for Metabolic Dysfunction-Associated Steatohepatitis. Diabetes Metab. Res. Rev. 2024, 40, e3846. [Google Scholar] [CrossRef] [PubMed]
- Ivancovsky Wajcman, D.; Byrne, C.J.; Dillon, J.F.; Brennan, P.N.; Villota-Rivas, M.; Younossi, Z.M.; Allen, A.M.; Crespo, J.; Gerber, L.H.; Lazarus, J.V. A narrative review of lifestyle management guidelines for metabolic dysfunction-associated steatotic liver disease. Hepatology 2024, 10, 1058. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes. Facts 2024, 17, 374–444. [Google Scholar] [CrossRef]
- Allen, A.M.; Charlton, M.; Cusi, K.; Harrison, S.A.; Kowdley, K.V.; Noureddin, M.; Shubrook, J.H. Guideline-based management of metabolic dysfunction-associated steatotic liver disease in the primary care setting. Postgrad. Med. 2024, 136, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Grinshpan, L.S.; Ivancovsky-Wajcman, D.; Goldenshluger, A.; Gepner, Y. One size does not fit all; practical, personal tailoring of the diet to NAFLD patients. Liver Int. 2022, 42, 1731–1750. [Google Scholar] [CrossRef] [PubMed]
- Thakral, N.; Desalegn, H.; Diaz, L.A.; Cabrera, D.; Loomba, R.; Arrese, M.; Arab, J.P. A Precision Medicine Guided Approach to the Utilization of Biomarkers in MASLD. Semin. Liver Dis. 2024, 44, 273–286. [Google Scholar] [CrossRef]
- Mullins, V.A.; Bresette, W.; Johnstone, L.; Hallmark, B.; Chilton, F.H. Genomics in Personalized Nutrition: Can You “Eat for Your Genes”? Nutrients 2020, 12, 3118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).