Endogenous Alcohol and Auto-Brewery Syndrome Complicating Liver Transplantation: A Case Report and Literature Review
Abstract
1. Introduction
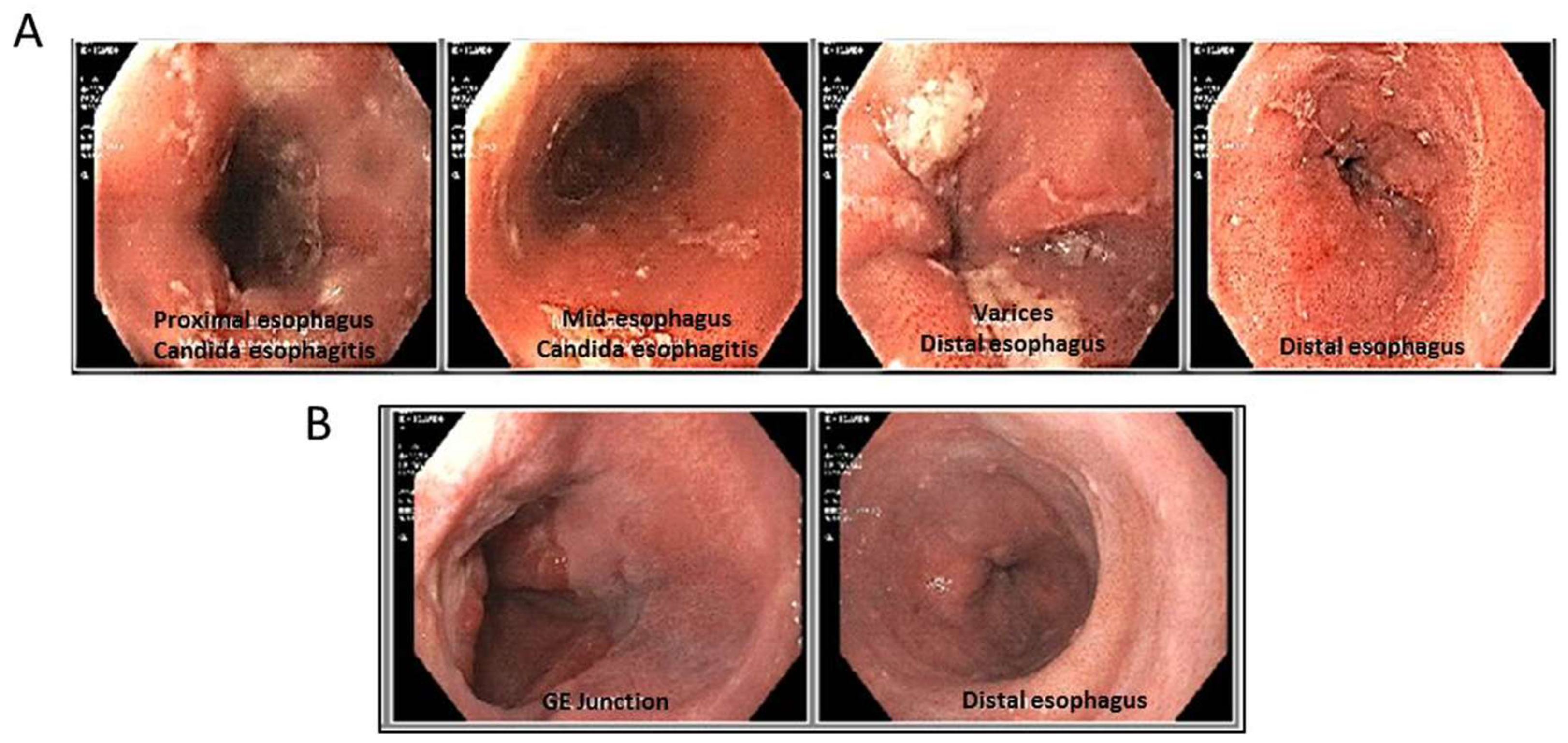
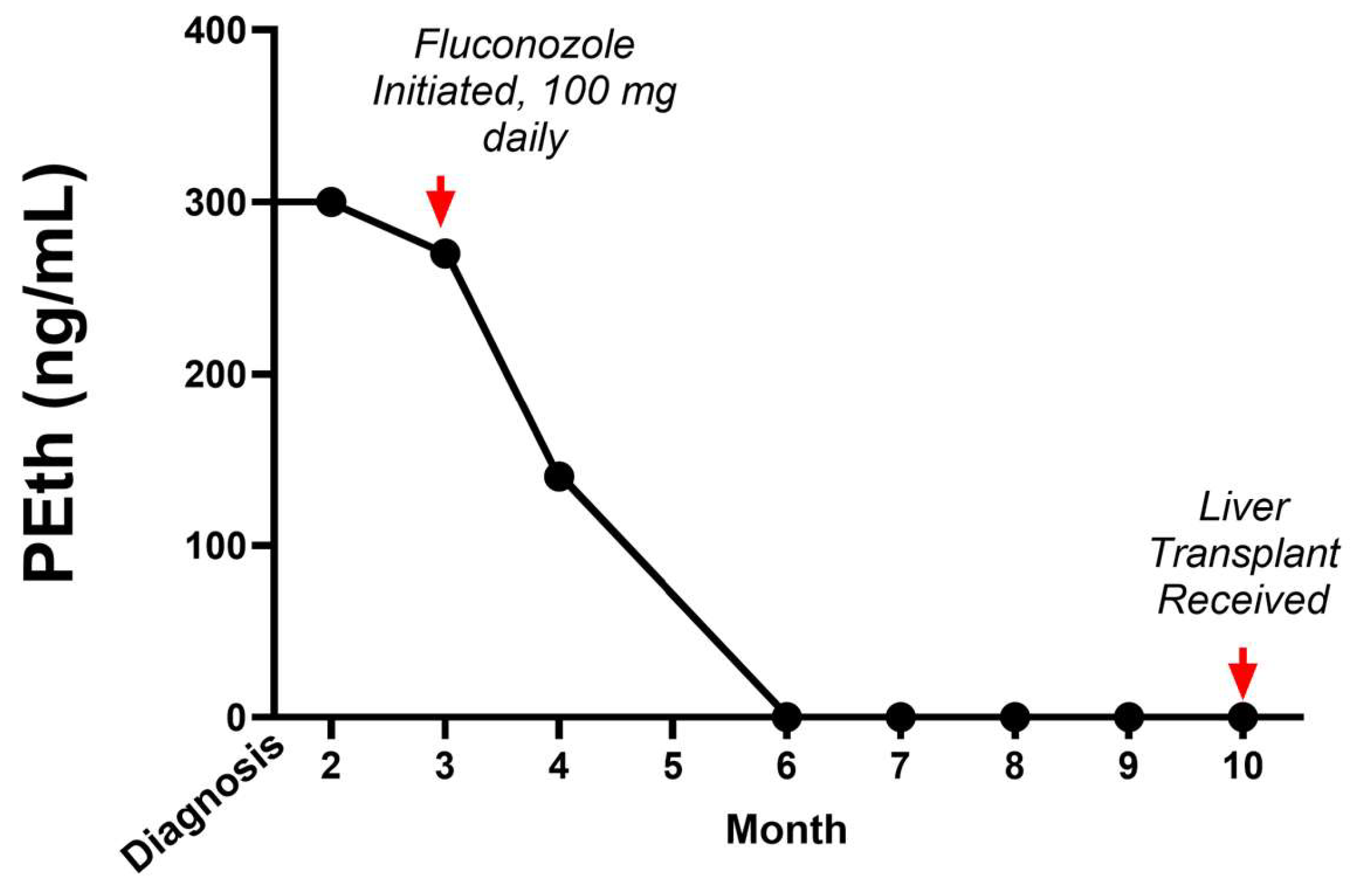
2. Case Report
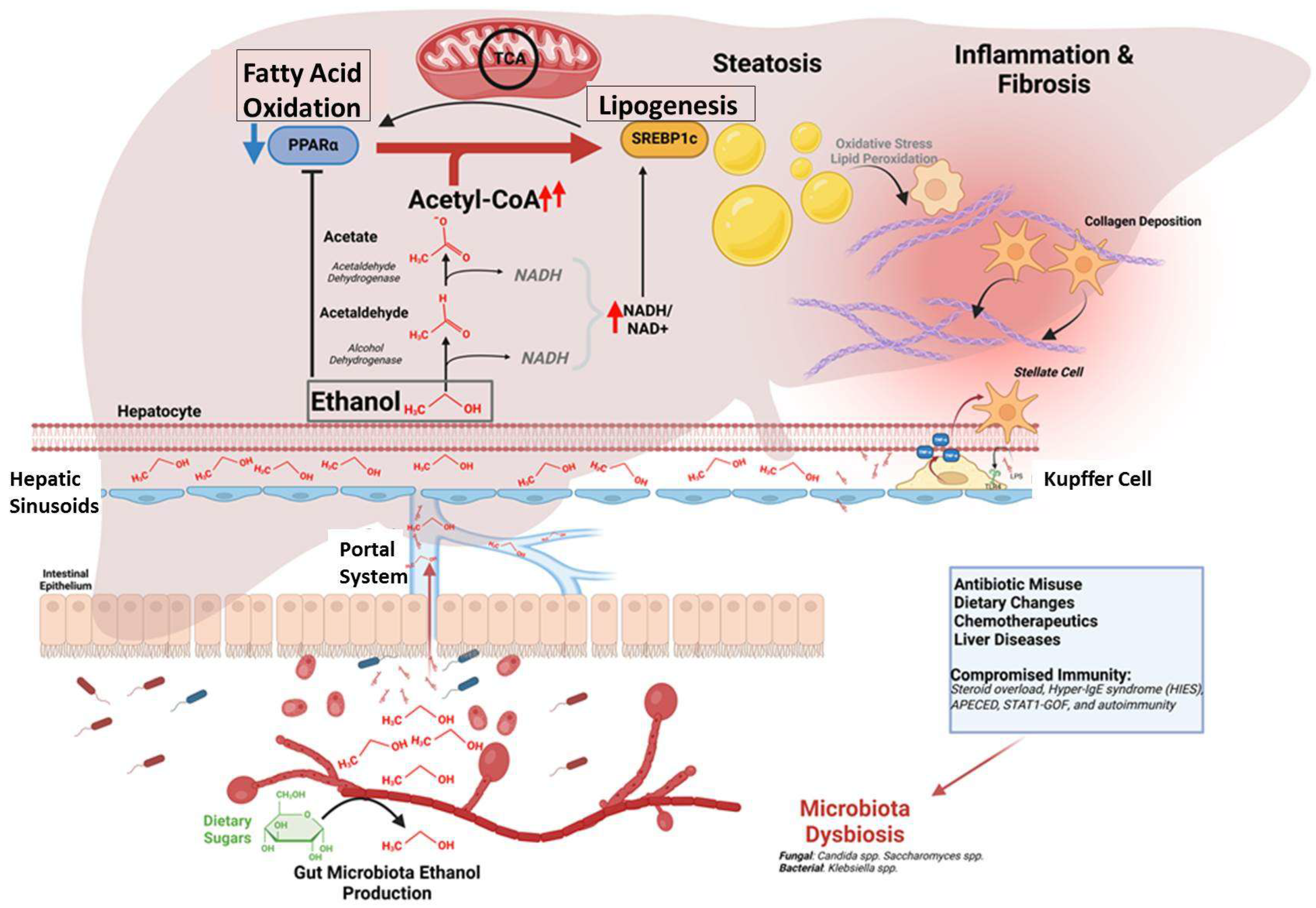
3. Discussion
| Etiology | Organism | Type of Report | Number of Patients | Reference | Note |
|---|---|---|---|---|---|
| Gastrointestinal | K. pneumoniae, C.albicans, C. glabrata, S. cerevisiae, C. intermedia, C. parapsilosis, C. kefyr. | Review | N = 17 | [23] | Seven of the subjects had prior antibiotic use |
| GI dysbiosis | Candida | Survey | N = 28 | [24] | Those with ABS had higher incidence of allergies |
| Metabolic-associated steatohepatitis | Pichia kudriavzevii; C. glabrata, C. albicans, Galactomyces geotrichum; Klebsiella Pneumoniae | Article | N = 10 | [25] | Measured fecal ethanol by Mass Spec compared to healthy controls |
| Gastrointestinal | C. krusei C. parapsilosis | Case report | N = 1 | [26] | History of hemicolectomy and constipation |
| Short bowel syndrome | C. glabrata S.cerevisiae | Case report | N = 1 | [27] | 13 year old |
| Crohn’s disease | Candida glabrata | Case report | N = 1 | [5] | Bowel obstruction and antibiotics |
| Gastrointestinal- MASLD | K. pneumoniae, K. quasi pneumoniae, K. variicola | Case study | N = 5 | [12] | Subjects did not respond to anti-fungal medications |
| Urinary | C. glabrata | Case report | N = 1 | [13] | Waiting liver TX, Urinary ETOH + plasma neg |
| Oral | Geotrichum candidum | Case report | N = 1 | [14] | Occurred after eating honey |
| Oral | Case report | N = 1 | [15] | Chocolate |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paramsothy, J.; Gutlapalli, S.D.; Ganipineni, V.D.P.; Okorie, I.J.; Ugwendum, D.; Piccione, G.; Ducey, J.; Kouyate, G.; Onana, A.; Emmer, L.; et al. Understanding Auto-Brewery Syndrome in 2023: A Clinical and Comprehensive Review of a Rare Medical Condition. Cureus 2023, 15, e37678. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.; Simms, L.; Al Rubaye, A.; Marcus, A.J. S2183 Auto-brewery Syndrome: Drunk on Carbohydrates. Off. J. Am. Coll. Gastroenterol. 2021, 116, S936. [Google Scholar] [CrossRef]
- Malik, F.; Wickremesinghe, P.; Saverimuttu, J. Case report and literature review of auto-brewery syndrome: Probably an underdiagnosed medical condition. BMJ Open Gastroenterol. 2019, 6, e000325. [Google Scholar] [CrossRef]
- Hafez, E.M.; Hamad, M.A.; Fouad, M.; Abdel-Lateff, A. Auto-brewery syndrome: Ethanol pseudo-toxicity in diabetic and hepatic patients. Hum. Exp. Toxicol. 2017, 36, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.T.; Coelho Prabhu, N.; Walkoff, L.; Trenkner, S.W. Auto-brewery Syndrome in the Setting of Long-standing Crohn’s Disease: A Case Report and Review of the Literature. J. Crohn’s Colitis 2016, 10, 1448–1450. [Google Scholar] [CrossRef] [PubMed]
- Saverimuttu, J.; Malik, F.; Arulthasan, M.; Wickremesinghe, P. A Case of Auto-brewery Syndrome Treated with Micafungin. Cureus 2019, 11, e5904. [Google Scholar] [CrossRef]
- Moon, A.M.; Curtis, B.; Mandrekar, P.; Singal, A.K.; Verna, E.C.; Fix, O.K. Alcohol-Associated Liver Disease Before and After COVID-19-An Overview and Call for Ongoing Investigation. Hepatol. Commun. 2021, 5, 1616–1621. [Google Scholar] [CrossRef]
- Hernández-Évole, H.; Jiménez-Esquivel, N.; Pose, E.; Bataller, R. Alcohol-associated liver disease: Epidemiology and management. Ann. Hepatol. 2024, 29, 101162. [Google Scholar] [CrossRef]
- Stamation, R. Endogenous Ethanol Production in the Human Alimentary Tract: A Literature Review. J. Gastroenterol. Hepatol. 2025, 1–8. [Google Scholar] [CrossRef]
- Meijnikman, A.S.; Nieuwdorp, M.; Schnabl, B. Endogenous ethanol production in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 556–571. [Google Scholar] [CrossRef]
- Tamama, K.; Kruckenberg, K.M.; DiMartini, A.F. Gut and bladder fermentation syndromes: A narrative review. BMC Med. 2024, 22, 26. [Google Scholar] [CrossRef]
- Xue, G.; Feng, J.; Zhang, R.; Du, B.; Sun, Y.; Liu, S.; Yan, C.; Liu, X.; Du, S.; Feng, Y.; et al. Three Klebsiella species as potential pathobionts generating endogenous ethanol in a clinical cohort of patients with auto-brewery syndrome: A case control study. EBioMedicine 2023, 91, 104560. [Google Scholar] [CrossRef] [PubMed]
- Kruckenberg, K.M.; DiMartini, A.F.; Rymer, J.A.; Pasculle, A.W.; Tamama, K. Urinary Auto-brewery Syndrome: A Case Report. Ann. Intern. Med. 2020, 172, 702–704. [Google Scholar] [CrossRef]
- Smędra, A.; Trzmielak, M.; Góralska, K.; Dzikowiec, M.; Brzeziańska-Lasota, E.; Berent, J. Oral form of auto-brewery syndrome. J. Forensic Leg. Med. 2022, 87, 102333. [Google Scholar] [CrossRef]
- Van Lieshout, A. Chocolate and the auto-brewery syndrome. Lancet 1990, 336, 1131. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Y.; Eljabbour, T.; Lee, H.; Batool, A. Possible Risk Factors for Candida Esophagitis in Immunocompetent Individuals. Gastroenterol. Res. 2018, 11, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Gaffen, S.L. IL-17–Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J. Immunol. 2015, 195, 780–788. [Google Scholar] [CrossRef]
- Frede, N.; Rojas-Restrepo, J.; Caballero Garcia de Oteyza, A.; Buchta, M.; Hubscher, K.; Gamez-Diaz, L.; Proietti, M.; Saghafi, S.; Chavoshzadeh, Z.; Soler-Palacin, P.; et al. Genetic Analysis of a Cohort of 275 Patients with Hyper-IgE Syndromes and/or Chronic Mucocutaneous Candidiasis. J. Clin. Immunol. 2021, 41, 1804–1838. [Google Scholar] [CrossRef]
- Capalbo, D.; De, M.L.; Giardino, G.; Di Mase, R.; Di Donato, I.; Parenti, G.; Vajro, P.; Pignata, C.; Salerno, M. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: Insights into genotype-phenotype correlation. Int. J. Endocrinol. 2012, 2012, 353250. [Google Scholar] [CrossRef]
- Eren Akarcan, S.; Ulusoy Severcan, E.; Edeer Karaca, N.; Isik, E.; Aksu, G.; Migaud, M.; Evin Gurkan, F.; Azarsiz, E.; Puel, A.; Casanova, J.-L.; et al. Gain-of-Function Mutations in STAT1: A Recently Defined Cause for Chronic Mucocutaneous Candidiasis Disease Mimicking Combined Immunodeficiencies. Case Rep. Immunol. 2017, 2017, 2846928. [Google Scholar] [CrossRef]
- Chascsa, D.M.; Ferré, E.M.N.; Hadjiyannis, Y.; Alao, H.; Natarajan, M.; Quinones, M.; Kleiner, D.E.; Simcox, T.L.; Chitsaz, E.; Rose, S.R.; et al. APECED-Associated Hepatitis: Clinical, Biochemical, Histological and Treatment Data from a Large, Predominantly American Cohort. Hepatology 2021, 73, 1088–1104. [Google Scholar] [CrossRef] [PubMed]
- Zinser, E.; Tan, K.-L.; Kim, D.-I.S.; O’brien, R.; Winstanley, A.; Yong, P.F.K. Differential Diagnosis: Hepatic Complications in Inborn Errors of Immunity. J. Clin. Med. 2023, 12, 7480. [Google Scholar] [CrossRef]
- Bayoumy, A.B.; Mulder, C.J.J.; Mol, J.J.; Tushuizen, M.E. Gut fermentation syndrome: A systematic review of case reports. United Eur. Gastroenterol. J. 2021, 9, 332–342. [Google Scholar] [CrossRef]
- Cordell, B.; Kanodia, A.; Miller, G.K. Factors in an Auto-Brewery Syndrome group compared to an American Gut Project group: A case-control study. F1000Research 2021, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Mbaye, B.; Borentain, P.; Magdy, W.R.; Alou, M.T.; Armstrong, N.; Mottola, G.; Meddeb, L.; Ranque, S.; Gerolami, R.; Million, M.; et al. Endogenous Ethanol and Triglyceride Production by Gut Pichia kudriavzevii, Candida albicans and Candida glabrata Yeasts in Non-Alcoholic Steatohepatitis. Cells 2022, 11, 3390. [Google Scholar] [CrossRef]
- Ser, M.H.; Çalıkuşu, F.Z.; Erener, N.; Destanoğlu, O.; Kıykım, E.; Siva, A. Auto brewery syndrome from the perspective of the neurologist. J. Forensic Leg. Med. 2023, 96, 102514. [Google Scholar] [CrossRef]
- Dahshan, A.; Donovan, K. Auto-Brewery Syndrome in a Child with Short Gut Syndrome: Case Report and Review of the Literature. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 214–215. [Google Scholar] [CrossRef]
- Semova, I.; Biddinger, S.B. Triglycerides in Nonalcoholic Fatty Liver Disease: Guilty Until Proven Innocent. Trends Pharmacol. Sci. 2021, 42, 183–190. [Google Scholar] [CrossRef]
- Jasirwan, C.O.M.; Lesmana, C.R.A.; Hasan, I.; Sulaiman, A.S.; Gani, R.A. The role of gut microbiota in non-alcoholic fatty liver disease: Pathways of mechanisms. Biosci. Microbiota Food Health 2019, 38, 81–88. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Woodhouse, C.A.; Patel, V.C.; Singanayagam, A.; Shawcross, D.L. Review article: The gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 2018, 47, 192–202. [Google Scholar] [CrossRef]
- Rahman, K.; Desai, C.; Iyer, S.S.; Thorn, N.E.; Kumar, P.; Liu, Y.; Smith, T.; Neish, A.S.; Li, H.; Tan, S.; et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology 2016, 151, 733–746.e12. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Toth, E.; Cherrington, N.J. Alcohol Metabolism in the Progression of Human Nonalcoholic Steatohepatitis. Toxicol. Sci. 2018, 164, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Iruzubieta, P.; Medina, J.M.; Fernández-López, R.; Crespo, J.; de la Cruz, F. A Role for Gut Microbiome Fermentative Pathways in Fatty Liver Disease Progression. J. Clin. Med. 2020, 9, 1369. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwaki, M.; Nakajima, A.; Nogami, A.; Yoneda, M. Current Research on the Pathogenesis of NAFLD/NASH and the Gut-Liver Axis: Gut Microbiota, Dysbiosis, and Leaky-Gut Syndrome. Int. J. Mol. Sci. 2022, 23, 11689. [Google Scholar] [CrossRef]
- Babuta, M.; Morel, C.; de Carvalho Ribeiro, M.; Calenda, C.; Ortega-Ribera, M.; Nagesh, P.T.; Copeland, C.; Zhuang, Y.; Wang, Y.; Cho, Y.; et al. Neutrophil extracellular traps activate hepatic stellate cells and monocytes via NLRP3 sensing in alcohol-induced acceleration of MASH fibrosis. Gut 2024, 73, 1854–1869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drda, J.C.; Smith, J.P. Endogenous Alcohol and Auto-Brewery Syndrome Complicating Liver Transplantation: A Case Report and Literature Review. Livers 2025, 5, 13. https://doi.org/10.3390/livers5010013
Drda JC, Smith JP. Endogenous Alcohol and Auto-Brewery Syndrome Complicating Liver Transplantation: A Case Report and Literature Review. Livers. 2025; 5(1):13. https://doi.org/10.3390/livers5010013
Chicago/Turabian StyleDrda, Jack C., and Jill P. Smith. 2025. "Endogenous Alcohol and Auto-Brewery Syndrome Complicating Liver Transplantation: A Case Report and Literature Review" Livers 5, no. 1: 13. https://doi.org/10.3390/livers5010013
APA StyleDrda, J. C., & Smith, J. P. (2025). Endogenous Alcohol and Auto-Brewery Syndrome Complicating Liver Transplantation: A Case Report and Literature Review. Livers, 5(1), 13. https://doi.org/10.3390/livers5010013







