Abstract
More than 90% of all liver malignancies are hepatocellular carcinomas (HCCs), for which chemotherapy and immunotherapy are the ideal therapeutic choices. Hepatocellular carcinoma is descended from other liver diseases, such as viral hepatitis, alcoholism, and metabolic syndrome. Normal cells and tissues may suffer damage from common forms of chemotherapy. In contrast to systemic chemotherapy, localized chemotherapy can reduce side effects by delivering a steady stream of chemotherapeutic drugs directly to the tumor site. This highlights the significance of controlled-release biodegradable hydrogels as drug delivery methods for chemotherapeutics. This review discusses using hydrogels as drug delivery systems for HCC and covers thermosensitive, pH-sensitive, photosensitive, dual-sensitive, and glutathione-responsive hydrogels. Compared to conventional systemic chemotherapy, hydrogel-based drug delivery methods are more effective in treating cancer.
1. Introduction
Hydrogels are three-dimensional polymeric networks that absorb considerable water while conserving their structural integrity [1]. Hydrogels are typically divided into two categories: physical gels and chemical hydrogels. Hydrogels have gained significant attention and are utilized in different fields, including tissue engineering, implantable devices, biosensors, and drug delivery [2]. Hydrogels are especially important in cancer therapy, where chemotherapeutic agents are usually highly toxic yet poorly specific [3]. These drugs cannot differentiate between normal and cancerous tissues. Thus, conventional chemotherapy administration is known to cause side effects. Moreover, in conventional methods, most drug content is released immediately after administration, causing drug levels to increase and drop rapidly, resulting in unacceptable side effects and a short therapeutic course. Given this inadequate period of action, injections should be repeated, exacerbating the adverse effects. The most prevalent deadly cancer worldwide is liver cancer. Patients are typically diagnosed with advanced stages of liver cancer, resulting in an adverse prognosis. Over ninety percent of all liver cancers are hepatocellular carcinomas (HCCs), for which chemotherapy and immunotherapy are the most effective treatments [4,5].
Various techniques and approaches can be utilized in liver cancer therapy depending on its stage, including surgery, chemotherapy, radiotherapy, immunotherapy, gene therapy, and targeted therapy [4,6]. Although chemotherapy is vital in managing cancer, its inherent toxicity usually leads to various adverse effects. Therefore, cancer therapy has been moving toward targeted therapies [7]. Hydrogels have been utilized extensively in liver cancer to enable controlled drug release at the tumor site. Specifically, the nanocomposite hydrogel drug delivery system not only increases the drug concentration at the tumor site for a sufficient period but also prevents the metastasis of the remaining tumor cells [8]. Localized drug delivery can enhance the efficacy of extensively metabolized drugs relative to systemic administration and ensure sustained drug delivery at the tumor sites, reducing the risk of toxic drugs causing severe side effects [9]. In this way, drug molecules can be incorporated into the gel matrix of hydrogels to generate reservoirs that deliver bioactive agents [10]. Studies on the effects of enclosing cells in biomaterials on immune response and transplanted cell efficacy and viability have yielded encouraging findings [11]. Hydrogels have several benefits for encapsulating cells over other biomaterials because of their structural similarity to the extracellular matrix [12]. In the field of liver cancer therapy, hydrogels have been used to deliver various therapeutic agents, including doxorubicin [13], cisplatin [14], paclitaxel [15], and embelin [16] to cancerous liver tissues. These hydrogels have mainly been applied as stimuli-responsive hydrogels, which will be discussed in detail. Currently, there are many studies on the construction of hydrogel drug delivery systems. However, there are few comprehensive articles to highlight the findings. Herein, we review the synthesis methods and benefits of hydrogels for liver cancer therapy.
The research methodology for this review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A comprehensive search of the relevant literature was conducted using several databases, including PubMed, Embase, and the Cochrane Library. The search was limited to studies published in the English language and included both randomized controlled trials and observational studies from 2000 to 2023. Studies that examined the effectiveness of the intervention being reviewed met the inclusion criteria. Any discrepancies were resolved through discussion and consensus. The data were extracted from the included studies using a predefined data extraction form, and the risk of bias was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias. The results were synthesized narratively, and meta-analysis was performed where appropriate.
2. Structural Characteristics of Hydrogels
Hydrogels are networks of polymer chains linked together by either chemical or physical crosslinks. Non-covalent interactions include chain entanglement, weak molecular interactions (such as hydrogen bonding and the van der Waals interaction), and microcrystal regions, some of which are temporary and are created or destroyed in response to a condition [17]. The noteworthy point is that for a polymer to be called a hydrogel, at least one component of its polymer chains must be hydrophilic. At the same time, the polymer network is insoluble in water due to crosslinks. Regarding the swelling response, some hydrogels become more solid or liquid under various circumstances [18].
Hydrogels have 3D structures that can absorb a large amount of water with their crosslinked and insoluble network. Fundamentally, crosslinked hydrogels are organized based on the non-covalent interplay of the ingredients within the structure [19]. These hydrogels have several advantages, such as lower drug degradation throughout the synthesis and decreased toxicity, because they do not need an initiator. The main characteristic of hydrogels is that they are a huge network with high hydration percentages made up of several polymer chains which are irresoluble with crosslinked links [20]. To form crosslinked hydrogels, polymers interact with themselves or other polymers in pure hydrogel systems, and this formation can be seen in nanoparticle hydrogels shaped by interacting nanoparticles and polymer links [21].
Nanoparticle–hydrogel composites, made by nanoparticles and polymer chain interactions, have physical crosslinked forms, and they do not need to utilize the extra crosslinking factors, which are often toxic, with this synthesis method [7]. Nanoparticles with properties such as degradation, elimination, and propagation could help hydrogels purge and degrade toxicity from the body [22]. Regarding the detoxification of nanoparticles that could help the combination with biopolymers, it is possible to form a superstructure hydrogel with a high capacity for the loading of drugs that contribute to assisting clinical individuals in many fields such as biodetoxification and drug delivery [23].
One of the most important materials that plays an essential role in bioprocesses is DNA, a self-replicating material that is present in nearly all living organisms. This material could incorporate biopolymers to form biological material for various goals [24]. DNA-based hydrogels are created by physical crosslinking and have made the highest contribution in recent investigations, particularly in the biomedical field, because of their adjustable mechanical features, responsiveness to outside stimuli, and ease of functionalization [25]. By limiting the enzymes and ligases in the special parts of the structure of DNA biopolymers, they are easily monitored and controlled for the handling of hydrogel degradation and the piloting of the extrication kinetics of remediation [26]. In this case, Wang et al. formed a DNA hydrogel by utilizing the SERS method and specific encapsulation for detecting α-Fetoprotein (AFP), a tumor marker of liver cancer [27].
On the other hand, a promising investigation into DNA-based hydrogels and nanoparticle combinations has brought about formed materials that are utilized in surgical processes due to their unique structures and has brought attention to these materials. Silver nanoparticles (Ag-NPs) are one of the successful combinations for forming antibacterial hydrogels which place these nanoparticles into 3D crosslinks and introduce functional material [28]. In the future, more complex materials could be seen because of the high flexibility of the DNA-based hydrogel structure.
Understanding the kinds of interactions among nanoparticles is necessary for creating crosslinked bonds with colloidal hydrogels [29]. This method uses charge-based gravitation to preserve the hydrogels’ structure. For instance, in a study by Bidkar et al., both the negative and the positive charges of two materials, red blood cell membrane-coated nanoparticles (RBC-NPs) and chitosan-functionalized poly (lactic-co-glycolic-acid) nanoparticles (Chi-NPs), incorporate with each other [30]. After this combination, a cohesive network by electrostatics forces is shaped.
Furthermore, there is another type of bond in the structure of hydrogels called covalent crosslinking, which is different from the physical ones. The gelation in hydrogels has been boosted with these chemical bonds throughout the polymerization of the precursor constructing blocks [31]. This crosslinking leads to the creation of several benefits, such as increased mechanical features and good stability, and they improve them more than physically crosslinked hydrogels by crosslinker concentration. Similarly, these two types depend on environmental sense for the controlling of drug release in new extra layers [32]. The improvement of the synthesis methods to prevent the undesirable degradation of drug payloads and the exploration methods that modify the facilitation of prescriptions for medical purposes are two important issues that should be considered in the hydrogel fields [33].
For the creation of nanoparticle combination superstructures, biological gels, which are shaped by environmental intergrades, have been utilized. Hydrogels, which are enzymatic crosslinked productions, allow some positive properties, such as strong covalent linking and the acceleration of the gelation process under physiological circumstances in different enzyme concentrations [19]. In this case, thrombin and fibrinogen interact, and the final production is utilized in tissue engineering and wound healing. The combination and interaction of fibrinogen, a glycoprotein complex, with Factor XIII, a transglutaminase, create this enzyme gel. In the initial step, the end peptides of fibrinogen are released by the protease thrombin to produce fibrin monomers that are crosslinked with each other [34]. The spray formation of this production reconciles with the surgical process [35]. Moreover, some investigations used nanoparticles with hydrogels for screening for cancer drugs. For instance, Zhu et al. illustrated the fact that hierarchical hydrogels could work with micro-nanostructures for cancer construction on a chip. This integrated system has good potential for utilization in cancer drug screening, particularly as an ideal method for screening liver cancer drugs. In addition, the hierarchical hydrogel system has shown accuracy and good function throughout the processes and has many applications in the medical fields [36]. Therefore, hydrogel could play an essential role in improving performance in the medical fields and could bring several functional merits to their processes.
There are many methods for obtaining chemical crosslinking, and the popular one uses methacrylate precursors. These reagents, by the effectiveness of their carbon double bonds, could form hydrogels during the free radical polymerization process. This process leads to the ease and acceleration of the synthesis of heterogeneous network structures [37]. Moreover, based on the study by Bingol et al. on forming stimuli-responsive hydrogels, methacrylate combines with other polymers and produces responsive hydrogels [38]. It is thought that methacrylate could have more unknown benefits in the hydrogels field when combined with other materials such as nanoparticles and that this could add some new futures to hydrogels.
Crosslinkers play a significant role in cancer treatment, particularly in the therapy of liver cancer. Inchingolo et al. reported that transcatheter arterial chemoembolization (TACE) is a classical locoregional method used in chemotherapy with a high rate of success in cancer treatment in comparison to other ways [39]. Cytotoxic drugs by this method transfer to the closest site of the targeted tumor to create a hot spot near it [40,41]. In addition, injectable hydrogels, which are environmentally responsive, are utilized for liver cancer treatment by assisting the TACE method. Figure 1 represents the rationale for super selective cTACE and DEB-TACE/bland embolization in a gelatin-based hydrogel drug delivery system.
Several hydrogels could work with the TACE method, such as physically crosslinked hydrogels, especially the stimuli-responsive ones. In an investigation conducted by Nguyen and colleagues, they formed a hydrogel with a combination of poly (ε-caprolactone-co-lactide) (PCLA) and poly (urethane sulfide sulfamethazine) (PUSSM), which is named PCLA-PUSSM. This hydrogel could remain in the liquid phase at pH 8.5, and by increasing the pH, it converted to a solid. Moreover, this hydrogel underwent its animal model test successfully. In this study, PCLA-PUSSM prevents tumor growth by releasing doxorubicin (DOX) and chemoembolization effectively [42].
In another study, Yan et al. indicated that they had created a magnetic hydrogel with thermosensitive properties and powerful adhesion; the hydrogel worked in moisture situations, biodegradation, and high magnetic hyperthermia. This hydrogel could be applied by the TACE method and could embolize tumor arterial vessels by responding to external magnetic fields and the temperature of the body for liver cancer therapy [43]. This method, by assisting in the structures of physical crosslinking hydrogels, has a bright future in cancer treatment.
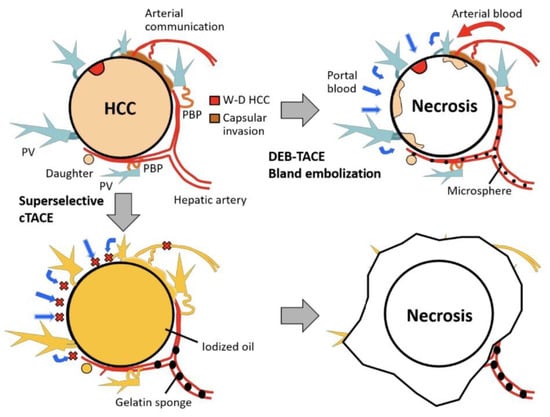
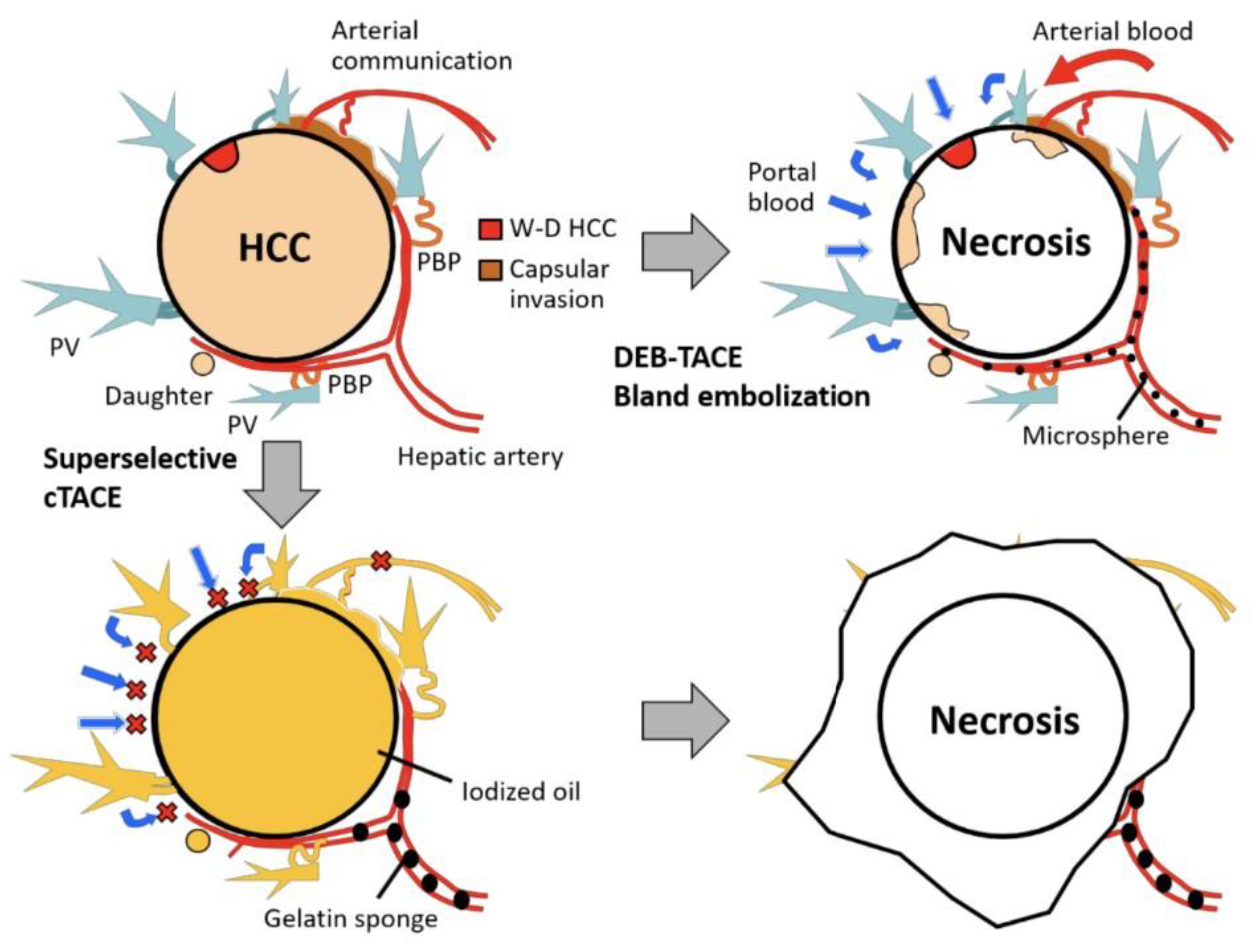

Figure 1.
Schematic representation of rationale for super selective cTACE and DEB-TACE/bland embolization in gelatin-based hydrogel drug delivery system. Abbreviations: PBP, peribiliary vascular plexus; W-D, well-differentiated; PV, portal vein. cTACE (conventional transarterial chemoembolization) is a widely used technique for treating patients with inoperable HCC. DEB-TACE (drug-eluting bead transarterial chemoembolization) is a variation of cTACE based on the utilization of microspheres to release chemotherapeutic agents within a desired site with controlled pharmacokinetics. Figure is reproduced with permission from an Open Access article (CC BY 4.0) [44].
Figure 1.
Schematic representation of rationale for super selective cTACE and DEB-TACE/bland embolization in gelatin-based hydrogel drug delivery system. Abbreviations: PBP, peribiliary vascular plexus; W-D, well-differentiated; PV, portal vein. cTACE (conventional transarterial chemoembolization) is a widely used technique for treating patients with inoperable HCC. DEB-TACE (drug-eluting bead transarterial chemoembolization) is a variation of cTACE based on the utilization of microspheres to release chemotherapeutic agents within a desired site with controlled pharmacokinetics. Figure is reproduced with permission from an Open Access article (CC BY 4.0) [44].

3. Hydrogel Synthesis for Drug Delivery Systems (DDS) Applications
Different monomeric or polymeric materials, such as cellulose, starch, ALG, gums, cellulose, rice husk, and acrylamide polysaccharides, could form hydrogels [45]. Hydrogels are divided into two kinds depending on their synthesis: physical and chemical crosslinking. Regarding the structure and linkage of hydrogels, they can be irreversible, due to their chemical links, or reversible, which is related to feeble bonds, such as hydrogen bonding [46]. Crosslinked graft hydrogels have more specific and improved properties compared to conventional hydrogels, such as mechanical properties and special electrical, thermal, and variable molecular weight-specific functionality with favorable chemical content. Alginate-methylcellulose hydrogels could be a successful cell delivery system with the highest performance in encapsulation because of their formation by a crosslinked structure [47]. Some interactions, such as electrostatic interaction, lead to the multilayering of the polyelectrolytes and the forming of macroscopic hydrogels. In addition, some useful biomedical properties such as self-healing are found in polyelectrolyte complexes [48]. In addition, Zixin Liu and his colleagues investigated pisocyanide (PIC) hydrogels, which are nonlinear and tunable to liver carcinoma cell therapy. They utilized the biomimetic hydrogel linkages of the Gly-Arg-Gly-Asp-Ser (GRGDS) peptide with PIC that have specific mechanical properties such as tunable nonlinearity for checking their effects on liver carcinoma cells. In conclusion, these hydrogels with PIC could affect the liver cancer cells positively with their strain-stiffening property in the calcium-activated potassium channel [49].
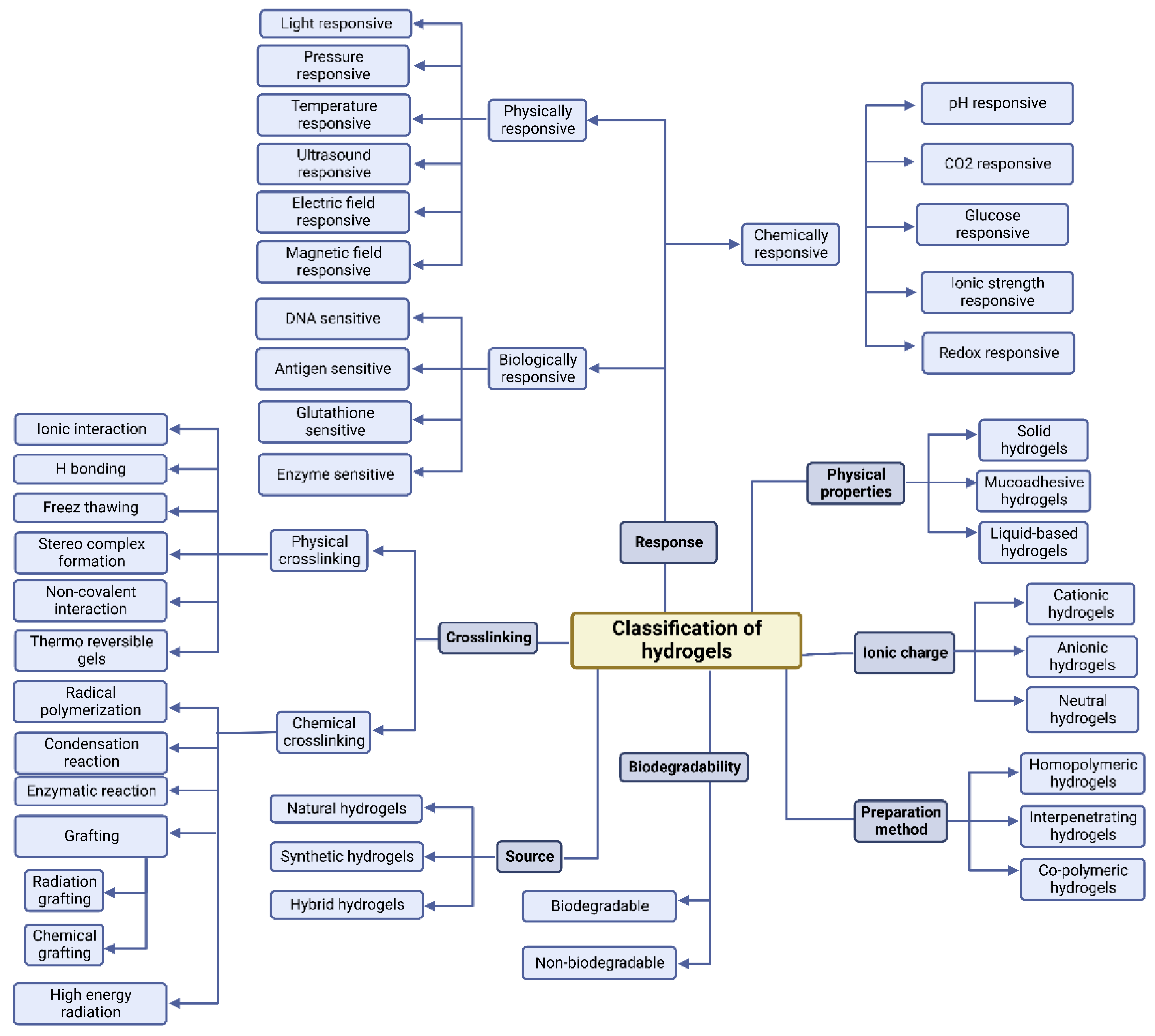
3.1. Hydrogels Classification Based on Their Preparation
Hydrogels can be classified in terms of how they are synthesized. Hydrogels can be either homopolymer or copolymer in structure, semi-interpenetrating networks (semi-IPN), or fully IPN (IPN) [50]. Homopolymer hydrogels are formed by only one kind of monomer, which is natural; their formations could have crosslinked structures depending on their polymerization method. However, copolymer hydrogels have two types of monomers in their formation, one of which is naturally hydrophilic. Conversely, a semi-IPN is formed when a linear polymer interpenetrates another crosslinked network without the presence of chemical links between the two networks [51]. In fact, because of the deficiency of a limiting interpenetrating elastic network, this type can more efficiently maintain quick kinetic response rates to pH or temperature when it still has key properties such as slow drug release and rectified pore size [52]. Moreover, when two polymers in the solution and the latter, crosslinked in situ or synthesized, are combined they form IPNs. The formation of IPNs has two main steps: a solution of monomers and initiators is made in the first step; the second one involves plunging a pre-polymerized hydrogel into this solution. In this type, the surface and pore properties could improve the handling and monitoring of drug release conditions and the mechanical characteristics [53]. Figure 2 represents the classification of the hydrogels according to their various characteristics.
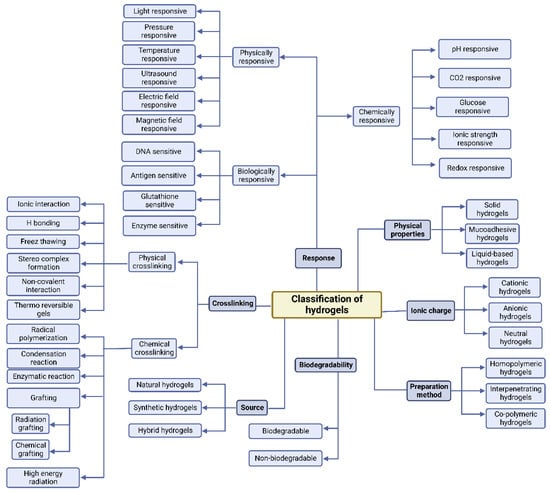
Figure 2.
Schematic representation of hydrogels classification according to their various characteristics. Reproduced with permission from an Open Access article (CC BY 4.0) [18].
Several polymers could be utilized for cancer treatment, such as PCEC copolymers. Their complex synthesis begins with ε-PCL polymerization by utilizing polyethene glycol (PEG) with a weight proportion of 20:1, respectively [54]. PCEC has a wide range of usage in many chemotherapeutic drugs, with successful feedback in anticancer delivery due to its biocompatibility, biodegradability, and low toxicity. Gao et al. prepared norcantharidin (NCTD)-loaded PCEC nanoparticles (NCTD-NPs) to enhance the solution in the water feature of NCTD and to improve its antitumor effect. Then, a thermosensitive hydrogel based on Pluronic F127 (PF127) was formed by the co-encapsulation of NCTD-NPs and DOX to create a dual drug-loaded hydrogel system capable of confronting H22 tumors via intratumoral administration [55]. This effort defines a promising liver cancer treatment and could have a bright future in cancer therapy. Table 1 presents representative examples of the synthesis methods and applications of hydrogels.

Table 1.
Examples of synthesis methods and applications of hydrogels.
3.2. Synthesis of Physically Crosslinked Hydrogel
There are six main factors in the physical formation of physically crosslinked hydrogels in polymeric chains: crystallization, protein interactions, hydrogen linkages, ionic/electrostatic interactions, amphiphilic polymer self-assembly, and metal consonance [64,65]. For instance, after the formation of polyacrylic and polymethacrylic acids and the creation of hydrogen linkages, this combination is protonated, and a pH-sensitive hydrogel is created. Additionally, this combination is formed when there is a carboxylic acid group [66]. Chemical crosslinking is preferred in in vivo applications. The deficiency of sufficient stability in physically crosslinked hydrogels, when used in biological and physiological areas such as the human body, is their main negative point for medical usage [67].
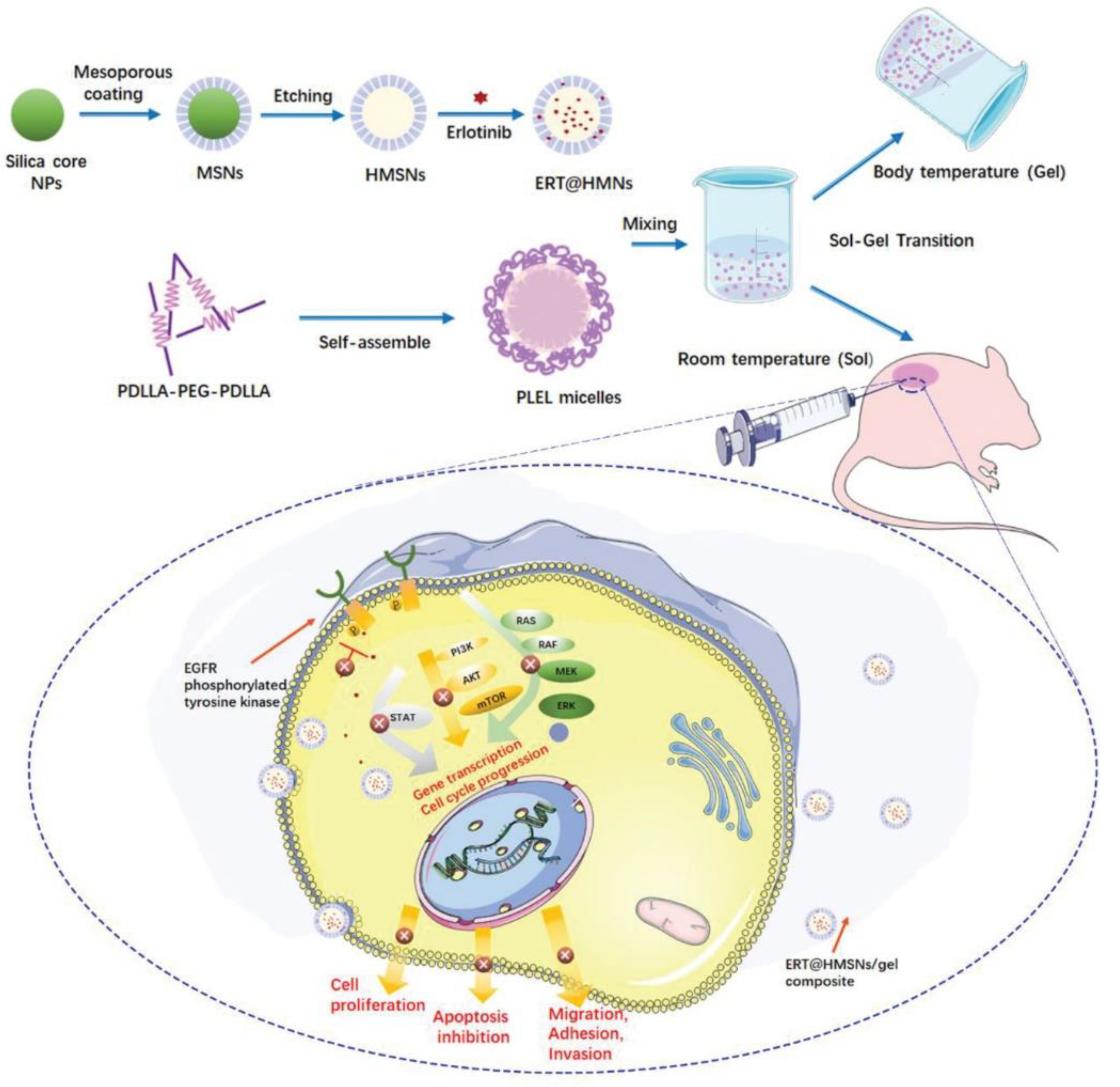
Figure 3 represents the hollow mesoporous silica nanoparticles (HMSNs/gel), a physically crosslinked hydrogel employed for localization and sustained transportation in an in situ drug delivery platform. In cancer treatment, HMSNs have been developed to encapsulate erlotinib. Erlotinib is a crucial targeted drug in the treatment of lung cancer; however, it has poor solubility, low oral bioavailability, and a high risk of toxicity, which restrict its clinical use. HMSNs could be successfully used for increasing drug loading and boosting the solubility to solve the incompatibility of the hydrophobic agents in hydrogel systems. This approach led to longer intratumoral and peritumoral drug retention [68].
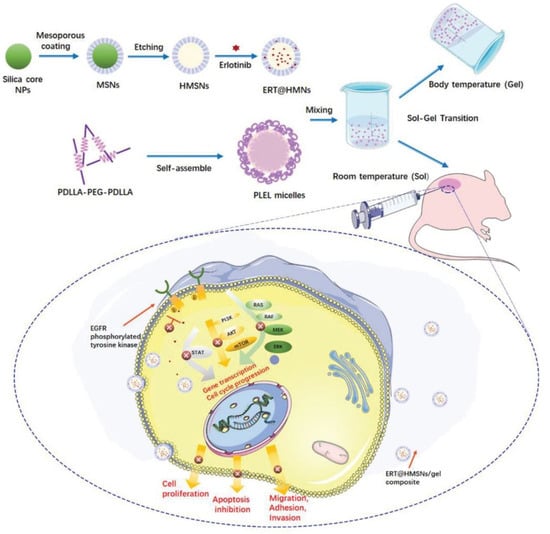
Figure 3.
Schematic representation of HMSNs/gel employed for localization and sustained transportation in in situ drug delivery platform. HMSNs (hollow mesoporous silica nanoparticles) have been developed to encapsulate erlotinib for increasing drug loading and boosting the solubility to solve the incompatibility of hydrophobic agents in hydrogel systems. Reproduced with permission from an Open Access article (CC BY 4.0) [68].
3.3. Synthesis of Chemically Crosslinked Hydrogel
Chemically crosslinked hydrogels have shown unique mechanical properties, leading to their biophysical adaptation because of the covalent bonds between the polymeric chains. Furthermore, their degradation is monitorable and controllable in irreversible and reversible interactions. Compared to physically crosslinked hydrogels, there is a significant demerit that demands catalysts and organic solvents, bringing many concerns about the biological adaptability and the environmental effects of chemically crosslinked hydrogels [19]. Schiff base formation, photopolymerization, Michael addition or the Diels–Alder reaction, reversible addition-fragmentation chain transfer (RAFT) polymerization, free radical polymerization (FRP), and enzyme-catalyzed reactions are all methods that can be used to advance and increase covalent bonds in chemically crosslinked hydrogels [69]. The Schiff base, formed when an amine reacts nucleophilically with the electrophilic carbon of ketones or aldehydes, is a chemical compound commonly used to produce hydrogels. For example, a successful hydrogel is an anticancer delivery drug made by a combination of glycol CST and polyethene glycol (PEG) [19,70]. Therefore, covalent bonds in chemically crosslinked hydrogels could easily form hydrogels with medical applications due to their adaptions to the biological environment, their controllable degradation, and their mechanical properties [71].
4. Chemical Properties of Hydrogels in DDS
Due to the hydrophilic polymer chains, hydrogels can change their shapes to desirable forms [72,73]. Several chemical and physical crosslinked bonds in every hydrogel lead to the creation of 3D networks in their structures, and they can have incredible water absorption with this formation [74]. The existence of hydrophobic groups such as amine (–NH2), carboxylic (–COOH), amide (–CONH, –CONH2), and sulfonic (–SO3H) groups in the polymeric chains brings about hydrophilic interactions, which bring about the appropriate stability in hydrogel structures [73]. In addition, there are more factors, such as ionic and hydrogen bonding, for stabilizing hydrogels as secondary forces [75]. Moreover, chemical crosslinking with covalent linkages and physical crosslinking with hydrogen linkages and complexing ions assist hydrogels in improving their properties, such as biodegradation, solubility, and reforming [76]. Furthermore, the rectification of content in hydrogels and the detoxification are enhanced by the absence of chemical crosslinking in hydrogels [77]. Accordingly, hydrogels can easily upgrade their functions and properties through their extraordinary structures.
In addition, hydrophobic polymers play an important role in drug delivery, especially in cancer treatment. For example, Gajendiran et al. reported that a hydrophobic polymer-based crosslinked 3D network might be utilized to localize drugs. This method works by transferring drug cargo by hydrogel to the closest site of the tumor. Then, the drug toxicity for controlling or preventing the growth of tumors is applied after the releasing of the drug by the degradation of the hydrogels. To enhance the performance of this procedure, several materials such as polyethylene glycol (PEG), polyphosphazene (PPZ), and poly (lactic-co-glycolic acid) (PLGA) are used [78].
Furthermore, Wane et al. conducted research that led to the production of PNAx triblock polymers by combining DOX and D-PNAx, a nanomedicine, simultaneously for localized HCC chemotherapy [46]. By combining D-PNAx and a thermosensitive hydrogel, an effective anticancer drug was formed to destroy H22 tumors. Moreover, the combination of DOX hydrochloride with PNAx polymers improves and upgrades the effectiveness of D-PNAx nanomedicine. For instance, the rate of DOX release was 9.4% in the first 24 h and reached 60% after 10 days in one case in which D-PNA100 nanomedicine was employed. This nanomedicine also indicated a successful confrontation against H22 tumors as an anticancer drug. On the other side, the low cumulation of the anticancer drug is always a restriction in systematic chemotherapy for HCC. Hydrogels that are thermally responsive could address this problem in local administration. Gao et al. formed an injectable thermally responsive hydrogel for the internal administration of norcantharidin-loaded nanoparticles (NCTD-NPs) and DOX for treating HCC tumors in an H22 tumor-bearing mouse model. The results showed that drug-loaded hydrogel remarkably suppressed tumor growth, diminished side effects, and prolonged the survival time of the mice. This drug solves the limitation by its unique properties and operation capabilities and can be used as a local–regional therapy for HCC thorough intratumoral administration [55].
Hydrogels have good potential for utilization as drug carriers because of their special chemistry. The existence of amine and aldehyde groups in hydrogel formations leads to the addition of some biological properties such as biodegradation and biocompatibility for use in bio-adhesives [79]. Due to the interaction between tissue amines and aldehydes, which are derived from dextran macromolecules, the adhesion of these amines is formed. In addition, an agglutinate mass is created by the interaction of unreacted aldehydes and dendrimer amines [80,81]. To avoid the adhesion of hydrogels to undesirable parts, dendrimer amines and dextran aldehyde crosslinks, which are unreactive, decrease the amount of free aldehyde [79]. In conclusion, hydrogels have a promising future because they are capable of combination with other biological materials to produce applicable materials in this field.
Moreover, nanoparticles could be employed in hydrogel structures to improve their properties. Due to their functional groups, hydrogels such as dendrimer–dextran hydrogels could transfer nanoparticles into themselves and move to the targeted site in HCC [82]. Xu et al. reported that they aggregate by particular stimuli such as pH or temperature in desirable sites and release the employed nanoparticles after degradation. Anticancer drug delivery for some cancer treatments such as liver cancer could operate with this method, and hydrogels transfer to specific tissues or organs by sensing stimuli [83]. Additionally, this method brings some advantages, such as detoxication due to the nano-based hydrogel’s nature which could assist in the attainment of a better treatment process. The toxicity of drugs during cancer treatment processes such as chemotherapy often kill the patient during the procedure, and these kinds of hydrogels do not have dangerous toxicity in this field.
5. Polymeric Hydrogels in DDS
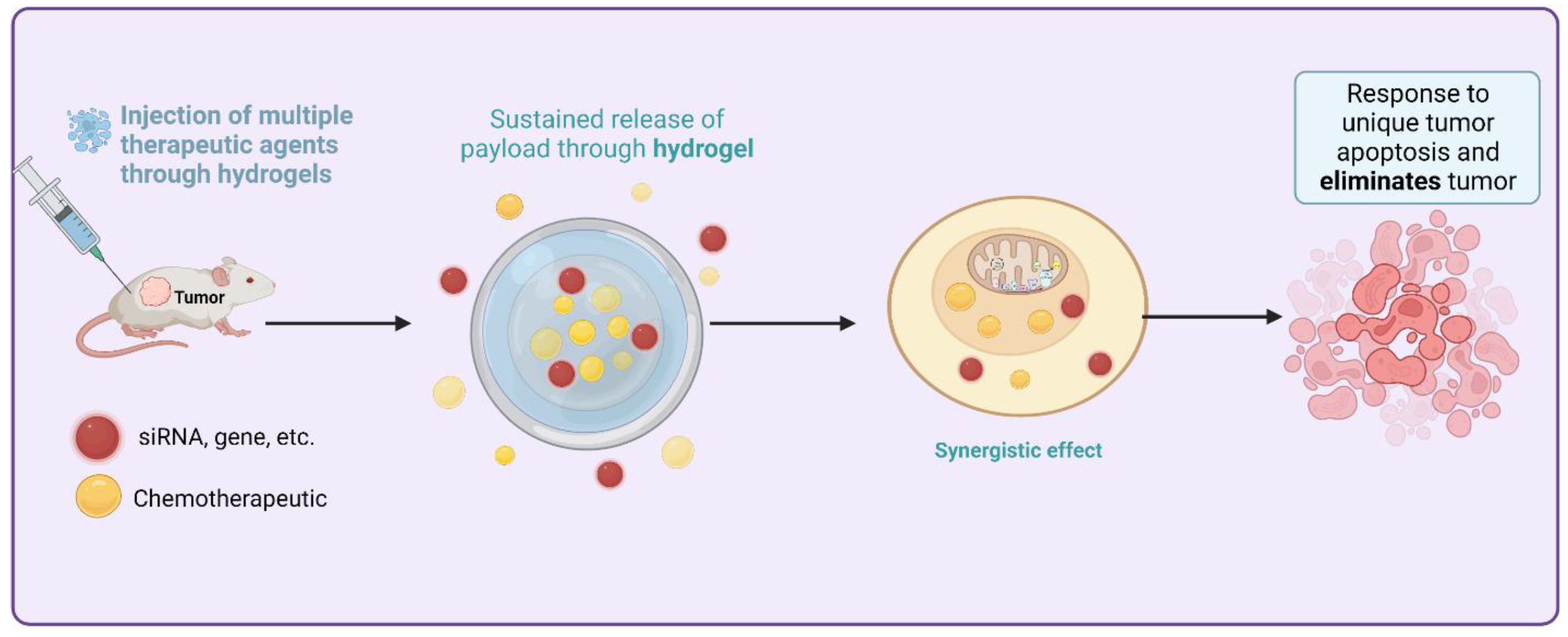
The crosslinker and the solvent contribute to the viscoelastic properties and the network structure of the polymer hydrogels, respectively. Then, they developed cutting-edge medicinal applications. Hydrogels can be broken down into two distinct categories, “physical” and “chemical,” based on their binding mechanisms [84]. Most physical hydrogels can be reverted to their original state when subjected to high temperatures or other stimuli. As opposed to biological hydrogels, chemical hydrogels typically exhibit stability because of the covalently crosslinked networks inside them [85]. Hydrogels can be classified as either natural or synthetic depending on where their components are derived from. Polysaccharide- and peptide- or protein-based hydrogels have received the most research attention among natural hydrogels [86]. Figure 4 provides the sustained and targeted method to treat HCC by utilizing siRNA thermosensitive injectable hydrogels and loading desirable cargo to release at the closest site.
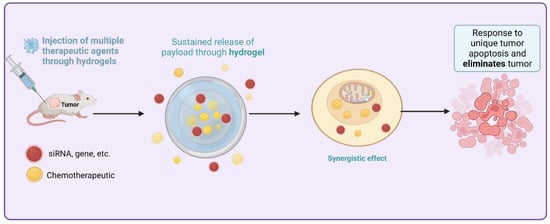
Figure 4.
Sustained and targeted method to treat HCC by utilizing siRNA thermosensitive injectable hydrogels and loading desirable cargo to release at the closest site.
5.1. Protein-Based Hydrogels
Proteins are the most significant macromolecules in all living systems, and they have specialized during evolutionary time to carry out a wide variety of metabolic, mechanical, and structural functions. Opportunities to employ proteins in novel ways have emerged as research into their structure and function has progressed, and new methods of manipulating proteins have been developed. Several proteins have been tested to see how well they perform as biomaterials due to their many desirable qualities, including biocompatibility, ease of large-scale synthesis via recombinant DNA technology, and ease of manipulation via chemical or enzymatic techniques [87]. Proteins often contain several domains that assist in cell signaling through their interaction with other proteins or ligands. Thus, the biomaterials prepared from them might also show this function [88]. Protein–protein interactions are crucial for the normal functioning of biological pathways, including cell-to-cell signaling and metabolic and developmental control [89]. This has made proteins a desirable source of biomaterials. Ongoing efforts are targeted toward developing hydrogels to fully mimic natural signaling networks to control biological processes [90].
Different types of protein-based hydrogels have been used for targeting tumor cells. In particular, these products are of great interest in the field of hepatocellular carcinoma (HCC) therapy. Protein-based hydrogels can be used to facilitate transarterial chemoembolization by providing a deeper permeation of tumor vasculature. Using this concept, Poursaid et al. designed a DOX/sorafenib-incorporated silk-elastin-like protein polymer, which resulted in the successful controlled release of these drugs at the HCC tumor site [91]. Furthermore, Qian et al. introduced an injectable silk fibroin hydrogel that could effectively cause the magnetic hyperthermia ablation of deep liver tumors [92]. Table 2 provides a classification of the protein-based hydrogels with diverse utilizations.

Table 2.
Protein-based hydrogels with diverse utilizations.
5.2. Polysaccharide-Based Hydrogels
Polysaccharides can be obtained from naturally occurring resources, including plants and marine life. Because of its ease, low cost, and scalability, the polysaccharide-based synthesis of hydrogels is a viable option for industrial applications. [110]. Polysaccharides are widely used in biological materials due to their distinct physical and chemical features, such as biocompatibility, biodegradability, and the absence of immunological responses [111]. Polysaccharides have recently gained attention as promising biological tools for various uses, including drug delivery and gene delivery [112]. In addition, the crosslinking or elasticity of matrices in polysaccharide composites allows for enhanced mechanical performances or processibilities in the ensuing hydrogels [113].
The application of polysaccharide hydrogels for cancer therapy has been associated with promising results (Table 3). Polysaccharides have been found to interact with the immune system to enhance its response and relieve the toxic side effects of cancer immunotherapy [114]. With respect to the benefits of polysaccharide-based polymers in liver cancer therapy, Na et al. synthesized a hydrogel from carboxymethylated (CM)-curdlan, substituted with a sulfonylurea for targeting hepatoma cell lines [115]. The regulated release of the anti-cancer medication, the presence of ligand receptor-mediated specific interactions, and the possible immunological enhancing activities of CM-curdlan in the body all prove that this hydrogel is an effective drug carrier for the treatment of liver cancer. Moreover, Xu et al. developed injectable hyaluronic acid-tyramine hydrogels, including interferon-2a, and found that they effectively suppressed angiogenesis in liver cancer tumor tissues [116].

Table 3.
Crosslinked hydrogels and their properties for drug delivery systems in cancer.
6. Stimuli-Responsive Hydrogels
Smart hydrogels, which can undergo a reversible yet discontinuous volume phase change in response to different external physicochemical factors, have attracted a lot of interest for decades due to their unusual properties and the promising technological and biomedical applications they lead to [125]. Molecular interactions between polymer chains and solutes in a system are affected by chemical signals, including pH, metabolites, and ionic variables. According to Hoque et al., external factors such as temperature and electrical potential may serve as alternative energy sources for modifying molecular interactions [126]. According to Coelho et al., polymeric materials will undergo various changes due to these interactions, including modifications to their solubility, swelling behavior, conformational change configurations, redox (reduction–oxidation) state, and crystalline/amorphous transition. The potential utility of such “smart gels” in the biomedical and pharmaceutical fields is generally accepted [127]. In addition, some investigations into thermosensitive hydrogels have led to progress in the cancer treatment fields. For example, Wen et al. made thermosensitive Pluronic hydrogels, which were used in liver cancer ascites treatment. This novel method can transfer anti-tumor cargo for confronting H22 tumors [128]. In another case, Zhao et al. demonstrated that Pin1 (peptidyl-prolyl cis/trans isomerase) siRNA is a promising targeted and a sustained way to cure HCC. They utilized injectable thermosensitive hydrogels to deliver siRNA/DP7-C nanoparticles to treat liver cancer [129]. Furthermore, Xiao et al. formed a novel thermosensitive hydrogel and loaded norethindrone nanoparticles (NPs) and oxaliplatin (N/O/Hydrogel) into it for confronting H22 tumors and hepatocellular carcinoma therapy. This composite is capable of intraperitoneal administration for malignant ascites of HCC [130].
6.1. Temperature-Induced Sol-Gel and Gel-Sol Phase Transition
Because of their low toxicity, high biocompatibility, and sensitivity to temperature changes, thermosensitive block copolymers have been identified as a promising class of drug carriers. Amphiphilic copolymers typically consist of two or three blocks of hydrophilic polyethylene glycol (PEG) and hydrophobic aliphatic polyester [131]. According to the research of Shi et al., amphiphilic block copolymers in water can self-assemble into micelles, which then form a micellar network following heating, ultimately leading to gelation. The PEG/polyester thermal in particular shows two phase transitions: sol-gel and gel-precipitate or sol-gel [132]. This sol-gel characteristic allows for the simple, low-temperature combining of drugs with PEG/polyester copolymer solutions. The resulting semisolid gel at body temperature allows for the subcutaneous injection of the mixed solutions. Therefore, PEG/polyester thermogels may be used as injectable drug carriers to treat diabetes, inflammation, and malignancies [133].
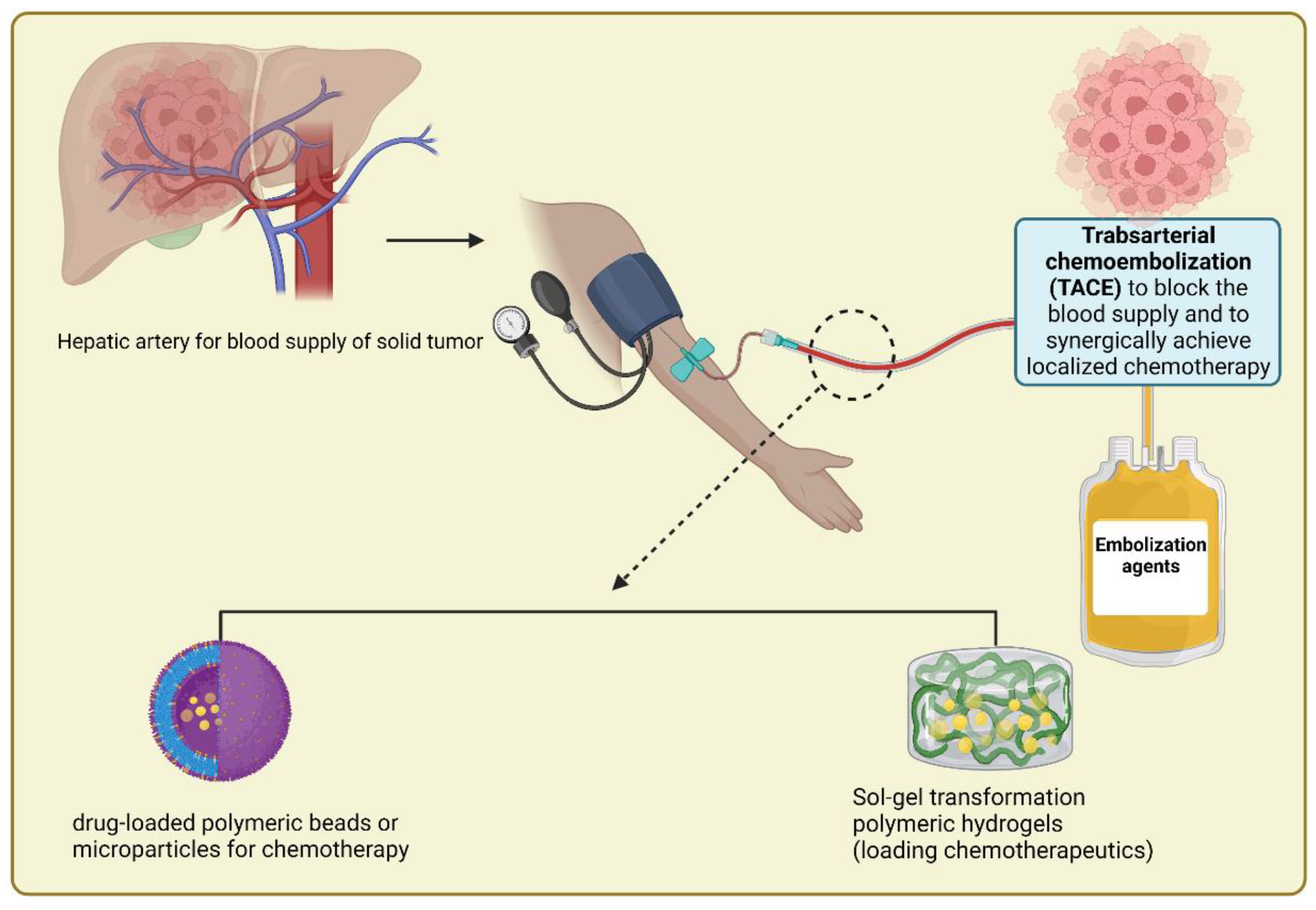
Huynh et al. described the synthesis of polyamino ester urethane block copolymers for treating liver cancer. The copolymers displayed a phase transition from sol to gel or gel to sol in an aqueous solution as the pH or temperature changed. The results in rabbits with HCC were promising when Huynh et al. produced a polymeric hydrogel to manufacture injectable radiopaque embolic materials [134]. Additionally, doxorubicin- and near-infrared dye-loaded zwitterionic nanogels were produced by Li et al. This method increased the DOX blood circulation time, tumor accumulation, and tumor penetration [135]. In another study, an in situ thermally sensitive magnetic hydrogel developed for HCC multidisciplinary therapy recently embolized the arterial arteries of a rabbit liver tumor using a vascular intervention procedure which showed a promising result in treating tumors [43]. Figure 5 depicts the design of polymeric beads or hydrogels used in transarterial chemoembolization (TACE) to occlude blood flow to a liver tumor and synergistically provide localized treatment with minimal toxicity to the surrounding healthy organs.
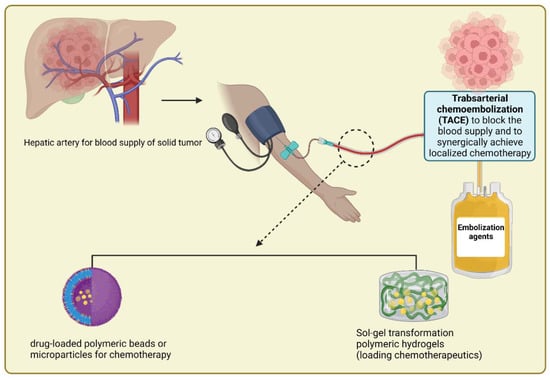
Figure 5.
Schematic depiction of the design of polymeric beads or hydrogels used in TACE to occlude blood flow to a liver tumor and so synergistically provide localized treatment with minimal toxicity to surrounding healthy organs.
6.2. pH-Dependent Anticancer Drug Release
There is a common requirement for nanoscale therapeutic systems to reach the tumor microenvironment while treating cancer. As nanoscale drug delivery systems can only enter cells via endocytosis, these carriers frequently end up in lysosomes, where they are exposed to low pH and proteolytic enzymes. Using stimuli-responsive polymers, this lysosomal microenvironment can be accessed to trigger a response drug release from the carrier [136]. Using this dual-stimuli technique, some researchers have targeted tumor cells. In a study, Salimi et al. created a cisplatin-loaded nanocomposite that was much more lethal to liver tumor cells than free cisplatin at 40 °C vs. 37 °C and pH 5.7 vs. pH 7.4 [14]. Moreover, Gu et al. employed pH-dependent release profiles of PEGylated mesoporous silica nanoparticles (MSN) to target DOX administration to liver cancer cells [137]. The subsequent tests confirmed that the cellular absorption of MSNs-P/G was substantially more significant than that of MSNs exhibiting galactose receptor-mediated endocytosis [138]. Raza et al. designed a pH-sensitive FER-8 peptide hydrogel for targeting liver cancer. The pH-sensitive features of the drug enabled it to be activated by the acidic pH environment at tumor locations, to provide sustained drug administration, and to increase tumor inhibition [15].
6.3. Photoinitiated Combinational Liver Cancer Therapy
With its ability to provide unprecedented spatial and temporal control over the development of the material, photoinitiated polymerization stands out as a promising technology for the in situ synthesis of hydrogels. As photopolymerizations can be initiated without the requirement for high temperatures or extreme pH conditions in a non-purged environment, they are also generally favorable for cellular encapsulation because they allow for simple encapsulation without considerable cell settling. Furthermore, unlike spontaneously polymerizing hydrogels, the material’s applicability is not limited by premature gelation because polymerization may be regulated temporally and launched on demand [139]. In recent years, photoinitiated polymerization has gained great attention in the targeting of tumor cells [140]. During the exposure of gel to radiation, light energy is converted to heat energy to disturb the structure of the gel and the drug release and destroy the cells associated with the tumor. This method has also been suggested for liver tumors. Wang et al. designed a hybrid hydrogel comprising poly (ethylene glycol) double acrylates, polyethylene glycol 400, phthalocyanine zinc, and phosphotungstic acid based on in situ photopolymerization [141]. This technique showed good biocompatibility, swelling, and drug retention abilities at the liver tumor site. Regarding combinational therapy in metastatic liver lesions, Spring et al. reported a photoinitiated release of cabozantinib and photodynamic therapy using photoactivable multi-inhibitor nanoliposomes (PMIL); this has shown an efficient reduction in tumor growth in a mouse model based on photothermal and multikinase inhibition effects. PMIL also decreased metastatic tumor lesions and reduced intratumoral micro-vessel growth [142].
6.4. Glutathione and pH-Responsive Hydrogels
Hydrogels sensitive to intracellular glutathione content and pH enable the controlled release of several drugs. Mahmoodzadeh et al. developed a chitosan-based, glutathione-responsive nanohydrogel as a possible nanoplatform for regulated DOX delivery. Positive findings were found in the studies of biocompatibility, chemical structures, DOX loading capacity, drug content discharged, and in vitro cytotoxicity effects [143]. Previously, the benefits of glutathione/pH-responsive nano-sponges were discovered for enhancing strigolactone delivery to prostate cancer cells [144]. In the case of liver cancer, although a polysaccharide system was designed to conjugate paclitaxel to hyaluronic acid via a glutathione-responsive disulfide linkage [145], the role of dual glutathione/pH-responsive hydrogels remains to be investigated.
6.5. pH–Temperature Dual Responsiveness
The unusual thermo-responsive feature of some synthetic polymers, the sol-gel transition near the body temperature of 37 °C, is targeted toward a broad range of drug delivery applications [146]. Injectable radiopaque embolic hydrogel made from a sulfamethazine-based pH-sensitive copolymer shows promise as a treatment for HCC. At a pH 8.2 transition boundary, PCLA-PUSSM showed a sol-to-gel phase change in an aqueous solution. There was evidence of the sol state in polymer solutions at elevated pH levels, such as pH 8.5. A gel-to-sol phase transition was detected at the pH range of the gel formation. The gel areas of the copolymer aqueous solution encompassed physiological or hepatic tumoral settings, suggesting the possibility of gel formation for in vivo investigation [42].
Fathi et al. reported a dual thermo- and pH-sensitive injectable hydrogel based on chitosan with poly (N-isopropylacrylamide-co-itaconic acid) for chemotherapeutic delivery [147]. Wang et al. developed a method for the simultaneous administration of docetaxel and granzyme B, which is loaded in pH-sensitive micro-micelles and embedded in a thermosensitive hydrogel [148]. Micelles are able to enter the tumor and release their payload at pH 5.5 after being liberated from the hydrogel by proteinase degradation.
7. Biocompatibility of Hydrogels in DDS
The basic characteristics of the hydrogels include their biocompatibility, biodegradability, and ease of injection in vitro for growth under certain conditions. They may easily be modified for usage in other locations and are a great way to transfer nutrients during the development phase [149]. They also have several limitations, such as limited mechanical strength, difficulty in handling, a high need for sterilization, and high treatment costs.
Hydrogels have recently gained popularity as a platform for biological applications (Table 4) [150]. The naturally occurring polymer known as silk fibroin (SF), which is generated from silkworm cocoons, has many inherent advantages, including biocompatibility and biodegradability, minimal inflammatory reaction, and controllable mechanical properties. Numerous hydrogels based on silk fibroin have also been created, showing strong clinical translational potential. By utilizing the universal interaction with different molecules and biocompatibility, SF-based composite hydrogels for bone regeneration, drug delivery, cancer treatment, and 3D bioprinting of organ structures were subsequently developed [92]. SF hydrogels must gradually functionalize in response to external stimuli to increase their applications in biomedicine. The use of biomaterials in vivo raises serious safety concerns. Omenetto and Kaplan reported that natural polymer silk fibroin, a biocompatible suture material used for millennia, was the major ingredient in their synthesized ferrimagnetic hydrogel [151]. Moreover, according to previous studies, the iron oxide nanoparticles with sufficient coating showed high biocompatibility for chemotherapeutic delivery [152].
It is evident that the physicochemical characteristics of 2DMs encourage their usage in biomedicine. For instance, g-C3N4 and BP are considered more advanced in the biomedical sector without any surfactants due to their better water dispersibility and colloidal stability [153]. In contrast, electrically inert h-boron nitrite sheets seem to be more biocompatible and better suited for the administration of drugs despite their poor stability in aqueous dispersions [154]. The creation of novel covalent functionalizations will be an intriguing endeavor for future research to enhance the biocompatibility of hydrogels as well as control their solubility and biodistribution.
Due to its good biocompatibility and adjustable transition temperature, Pluronic, a triblock copolymer comprising PEG and PPG, is a well-known thermo-gelling solution authorized by the Food and Drug Administration (FDA) for decades [155]. Pluronic gels, on the other hand, have reportedly been shown to have weak mechanical qualities; they are prone to erosion and often only last for one day in vivo. The fact that they are not biodegradable and often need a high critical gelation concentration (CGC) might have unfavorable side effects. Due to these drawbacks, the Pluronic systems’ prospective applications were limited. As a result, much effort was put toward altering Pluronic copolymers. In this study, we created a brand-new polyurethane-based thermo-gelling copolymer by copolymerizing poly (pentadecalactone) (PPDL), which has been shown to have outstanding mechanical and biocompatibility qualities [156].
Additionally, after testing the biocompatibility of Ink H4-RGD in several different cancer cell lines, Gebeyehu et al. found that all the tested cell lines, including the lung cancer (NSCLC-PDX, H460, HCC-827, and A549), breast cancer (MDA-MB-231WT), and bladder cancer (RT4) lines, showed greater than 90% cell viability following post-printing evaluation [157]. The excellent tissue adherence and biocompatibility of chitosan make it a popular ingredient in the hydrogels used for medication release. However, since chitosan does not dissolve well in water, it is often combined with a tiny quantity of acetic or hydrochloric acid to dissolve it. This increases chitosan’s acid toxicity to healthy tissues and reduces the delivery of pH-sensitive drugs. In this regard, Qu et al. synthesized a pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy, and it was a promising drug delivery system.
Most hydrogels used to treat liver cancer generally have appropriate biocompatibility, which has motivated us to give hydrogels more consideration when comparing different pharmaceutical formulations for treating this and other diseases. The most commonly used hydrogels are given in Table 1, along with information about their biocompatibility.


Table 4.
Biocompatibility and biosafety of different drug delivery systems based on hydrogel composites for tumor therapy.
Table 4.
Biocompatibility and biosafety of different drug delivery systems based on hydrogel composites for tumor therapy.
| Type of Material | Biocompatibility Biosafety | Type of Cancer/Application | Results | Ref |
|---|---|---|---|---|
| Carbon dot-supported gold (Au/cds) | Ideal blood compatibility | Liver cancer (HEPG-2 tumor-bearing mice) 4t1 tumor cell | In subcutaneous and orthotopic patient-derived xenograft hepatocellular carcinoma models, carbon dot-supported atomically dispersed gold acts as a mitochondrial oxidative stress amplifier to prohibit tumor development. | [158] |
| Porphyrin-like single atom Fe (iii) | Remarkable biocompatibility with HeLa cells | Breast cancer (HeLa-bearing mice) | Under NIR illumination, a metal-organic framework (MOF) rich in single atom Fe (iii) centers that resemble porphyrins (p-MOF) might effectively trigger cancer death and allow photo-acoustic imaging of cancer cells. | [159] |
| Single-atom Ru supported by Mn3[Co(cn)6]2 MOF | Biosafety (blood biochemical and blood routine) | Breast cancer (41 tumor-bearing mice) | OxgeMCC-r SAE with single-atom Ru loading content could relieve the hypoxia condition of solid tumors, lead to enhanced ROS generation, and cause apoptotic cell death both in vitro and in vivo. OxgeMCC-r SAE could selectively accumulate within tumor sites for enhanced photodynamic therapy of cancer under the guidance of t1 MR imaging. | [160,161] |
| Carbon dot-protoporphyrin ix | Good | Liver cancer | Biocompatibility increased by decreasing cytotoxicity, and the fluorescence of the drug enhanced in comparison with its precursor. | [162] |
| Single-atom Cu coordinated in hollow N-doped carbon sphere | High biocompatibility (body mass) | Breast cancer (4t1 tumor-bearing mice) | The Cu-HNCS demonstrated significant toxicity against cancer cells in vitro. In addition, Cu-HNCS catalysts could effectively suppress tumor growth and significantly increase survival rates in vivo. | [163,164] |
| Single-atom Pt/ceo2 | Good | Brain trauma | The single-atom pt/ceo2 presents long-lasting catalytic activity. Additionally, it exhibited the nanozyme-based bandages that could decrease indicators of oxidative stress and inflammation responses in neuron cells and improve impaired neurocognition. | [163] |
| Single-atom catalysts | Minimum cytotoxicity | Liver cancer | Studies have shown that synthesized SACs effectively treat various types of cancer (especially liver carcinoma), treat a wide range of infections (bacterial and inflammatory), treat brain trauma, and protect cells against oxidative stress. | [165] |
| Hierarchical Ni (OH)2 nanosheets/n-doped carbon nanoboxes | Slight cytotoxicity | Tumors biomarkers, liver cancer | Assisting in cancer detection as a biomarker in the early stages of medical processes. | [166] |
| Fe–n4 immobilized on a carbon substrate (Fe-n/c SACs) | Good | HeLa cells | Fe–n/c SACs could be used for enzyme-mimicking properties, including peroxide (pod)-like, oxide-like, cat-like, and gpx-like activities. In addition, it could effectively scavenge intracellular ROS in HeLa cells. | [167] |
| Fe-sas/nc | Minimum cytotoxicity | HeLa cells, lung cancer cells | Fe-sas/nc catalysts containing bifunctional Fe-n4 active sites showed excellent cat-like and sod-like activities. Moreover, it could protect HeLa cells effectively against oxidative stress by eliminating H2O2 and O2. | [168,169] |
| Poly (lactic and glycolic) acid | Products degrade during metabolic pathway, localized inflammation | Liver cells | The nhap/pla scaffold favored adhesion, matrix sediment, and osteogenic segregation of HMSCs. Both hpm and pm contribute to mineralization and osteogenesis in the defect zone of rats for in vivo transplantation. | [170,171] |
| Polyethylene oxide and polyethylene glycol | Hydrolysis, mild foreign in PEO and minimal foreign in peg body reaction, no inflammation | Liver cells | This research explained the long term effect of the lira method in the drug delivery system and indicated positive impacts in this process throughout the period; good diffusion of drug was reported because of the hydrogels matrix; lira-loaded pcga-peg-pcga gel formulation used in this investigation. | [172] |
| Polycaprolactone | Hydrolysis, minimal inflammation | Skin, ligament, tendon, vessels, nerves, cartilage, bone, retina | Curdlan sulfate and heparin-modified poly (caprolactone) (PCL) hybrids were expanded by physically trapping these molecules on the PCL surface. This amendment method was conducted by reversible gelation of the PCL surface area following exposure to a solvent and nonsolvent mixture. The biomacromolecule entrapment process is capable of being practical on PCL to obtain enhanced blood adaptability and decrease inflammatory host response for its future blood contacting applications. | [173] |
8. Physical Properties of Hydrogel Composites for DDS
A hydrogel is a polymeric network that has been crosslinked through the reaction or conjugation of one or more monomers and has expanded due to the presence of water. Hydrogels can absorb anywhere from 10 to 20% to hundreds of times their dry weight in water due to the presence of hydrophilic functional groups, which bridge the gap between the macromolecules and have a high affinity for biological fluids [174]. Stimuli-responsive hydrogels change shape in response to stimuli such as heat, cold, light, ions, or a magnetic field [175]. These “smart” hydrogels can also be made by employing acid-labile chemical connections, which are stable at physiological (neutral) pH values but break down or hydrolyze at low pH levels (such as in the tumor microenvironment) [176]. pH-sensitive hydrogels cause cancer cells to develop an acidic extracellular environment while their cytoplasm becomes alkaline. Some studies have shown that an alkaline intracellular pH promotes glycolysis, increases cellular tolerance to hypoxia, and stimulates the expansion of cancer cells. To achieve a simultaneous anticancer effect at the tumor site, Liu et al. developed an injectable pH-responsive peptide hydrogel as a carrier material for the antitumor medicines gemcitabine (GEM) and paclitaxel (PTX) [177]. Taking advantage of the pH change in the tumor environment, new pH-sensitive hydrogels have been developed to release the deadly drug only in the region of cancer cells, reducing the risk of damaging side effects in healthy tissues. Hydrogels that are photosensitive undergo chemical and/or physical changes when exposed to ultraviolet (UV), visible, or near-infrared (NIR) light. For on-demand tumor therapy, Yuan et al. reported a nanocomposite hydrogel with NIR/magnet/enzyme multiple responses [178].
Volume expansion/contraction, chemical bond cleavage/isomerization, and free-radical polymerization process are all triggered by incident light. In contrast, porphyrins, cyanines, oxides of gold and silver, and carbon all make up photothermal agents. NIR-responsive hydrogels are of special interest in photothermally generated hydrogels because of their deep tissue penetration and safety; however, tissue damage owing to overheating must be carefully addressed before this technology can be employed in biomedicine [179]. Other factors, such as ionic strength or magnetic field, may also affect the physical and chemical properties of stimulus-responsive hydrogels.
Hydrogels can be made magnetically sensitive by including iron oxide nanoparticles. Their vibration in response to a magnetic field has the potential to considerably increase local temperatures, which could improve therapeutic efficacy through thermal ablation processes. In addition, these systems are frequently coupled with thermosensitive hydrogels, which release medications as the surrounding temperature rises, creating a synergistic impact between thermal and chemotherapeutic cytotoxicity. Using pH-responsive iron oxide nanocluster assemblies, Lu et al. reported a compassionate diagnosis of hepatocellular cancer [180].
This approach, like NIR, has minimal invasiveness, deep tissue penetration, and spatial and temporal activity [181]. Gao et al. developed a drug delivery system by incorporating ferromagnetic vortex-domain iron oxide nano-rings (FVIOs) into a hydrogel made of chitosan and PEG that was doped with DOX. In vitro and in vivo studies showed the synergistic therapeutic effectiveness of the DOX chemotherapeutic mechanism and the thermal activity of FVIOS with the application of a magnetic field. Twenty-one days after the operation, it was feasible to assess the system’s effectiveness in preventing tumor recurrence after the surgical excision of the tumor and the administration of the formulation [182].
Hydrogels that have been combined with fibers may achieve excellent load bearing and minimal friction. The composite hydrogels exhibit exceptional strength and toughness in the opposite direction thanks to the creation of a highly porous 3D fiber network. The microstructures of the hydrogel network must be tightly connected to every mechanical feature of the composite hydrogels. The bilayer hydrogel achieves strong load-bearing properties and ultralow friction at the same time as this structure. Due to its excellent biocompatibility and great mechanical strength, PEEK (polyetheretherketone), a thermoplastic polymer material, has been investigated as a viable material for artificial joints. The material 25 PEEK was chosen as the load-bearing structure’s hard substrate as a result [183]. When N, N-methylenebisacrylamide (MBAA) was added to the reaction, the compressive modulus of the hydrogels also rose. This was conducted to compare the mechanical strength of the various hydrogel samples. The mobility of free ferric ions and the reversible ionic bonding between ferric ions and polyacrylic acid-polyacrylamide-ferric ion (PAA-PAAm-Fe3+) chains in the damaged area of the hydrogels were the key factors influencing the ability of hydrogels to self-heal. The free Fe3+ ions’ mobility and the un-crosslinked AA chain segments’ impact on the hydrogels’ capacity for self-healing were also significant factors. Hydrogels with a higher MBAA concentration exhibited denser networks and greater mechanical strength. Though this caused the hydrogels to recover more slowly and contain less water, meaning the load support heavily depended on the solid phase and might have resulted in the rise in friction coefficients, it also reduced elastic energy dissipation during the friction process [184]. The ability of the dual-network (DN) hydrogel to withstand external loads in a variety of ways, including stretching, twisting, and knotting, 29 without breaking, 30 suggests that it has better mechanical capabilities. A 500 g load can be withstood by the hydrogel, which is astounding. Its fracture strength and strain, calculated at 32 9.73 MPa and 1250%, respectively, are similar to previous studies on 33 hydrogels with good mechanical properties.
In order to assess the self-recovery performance concerning the reversible nature of the physical crosslinks, the sequential loading–unloading test was also carried out at a fixed 200% strain [185]. Even in an atomic force microscope image of a very low CNF concentration (0.005% w/v), it has been shown that the nanofibrils are very densely packed and prevent the cells from working around the fibers. This indicates that the nanofibril network is constantly remodeling, as indicated by the relatively high resistance of the cellulose nanofibril (CNF) hydrogel to strain. The alternating strain experiment illustrated the gel’s capacity for self-healing [186].
9. Most Recent Clinical Trials
Although pre-clinical studies have shown the potential of hydrogels in the treatment of liver malignancies, further research is needed to advance the hydrogel-based therapies into clinical application [46]. In this section, we give an overview of the ongoing clinical trials in this field.
A phase II clinical trial (NCT02470533) is investigating the outcomes of chemoembolization via hydrogel-based microspheres loaded with the chemotherapeutic drug DOX compared with stereotactic body radiation therapy in patients with HCC [187]. Another clinical trial (NCT04803019) has been designed to evaluate the use of transarterial embolization (TAE) versus drug-eluting beads chemoembolization for the treatment of HCC. The TAE will be carried out via Embozene microspheres, which are spherical particles of hydrogel coated with a perfluorinated inorganic polymer. Moreover, the chemotherapeutic agent used in this trial is DOX, which will be delivered into the tumor by Embozene [188]. Another clinical trial (NCT02525380) is also investigating the safety and efficacy of the DOX-loading DC Bead device in the treatment of HCC. The DC Bead is produced from polyvinyl alcohol and consists of hydrogel microspheres that are biocompatible, hydrophilic, non-resorbable, and capable of loading DOX [189].
10. Toxicity of Hydrogel-Based DDS
As the first stage of biocompatibility studies, in vitro cytotoxicity experiments should be used to predict acute toxicity. Endpoints in cell quantity, shape, and cellular activity are just some of the ways that the International Organization for Standardization (ISO) 10,993 (1992) has prescribed cytotoxicity studies for medical devices and materials [190].
Numerous attempts have been made to construct hydrogel-based DDS to lower the undesired systemic toxicity and dose strength of the drugs. Numerous hydrogels with clinical approval are available at pharmacies. In DDS, the biocompatibility of a polymeric hydrogel with the surrounding tissues is the primary consideration. The prerequisites for hydrogel systems include the noncytotoxic nature of the precursors and the soaked products. Important evaluation criteria for biocompatibility include cell viability and proliferation. Only in situ hydrogels are utilized to encapsulate cells [191].
Ni et al. demonstrated that poly (caprolactone)-poly (ethylene glycol)-poly (caprolactone) is a biocompatible, chemically synthesized, amphiphilic tri-block copolymer with hydrophobic PCL chains that can encapsulate hydrophobic drugs and hydrophilic PEG chains with good water solubility (PCL-PEG-PCL, PCEC) [192]. PCEC is commonly used as a nanocarrier because of its biocompatibility, biodegradability, and low toxicity, making it ideal for delivering a variety of chemotherapeutic drugs to specific tumors. For anticancer drugs, including gefitinib, paclitaxel, and honokiol, PCEC nanoparticles have created a sustained drug release mechanism [193]. The cytotoxicity of control PCEC nanoparticles, free NCTD/Dox, and NCTD-NPs/Dox Gel was measured in HepG2 cells using the MTT in vitro assay. No cytotoxicity was detected from the blank PCEC nanohydrogels since the cell viability was greater than 80%, even at 800 μg/mL. According to these results, PCEC nanohydrogels could be a safe and effective medication delivery strategy for HCC [55].
Furthermore, many of the polymers utilized to make hydrogels for cancer therapy can be decomposed naturally, including natural and synthetic polymers such as chitosan [194], hyaluronic acid [195], alginate, polyesters [196], and polyphosphazene [197]. The use of non-toxic, biodegradable hydrogels based on multi-block copolymers as drug delivery systems is described in a US Patent [198]. They were constructed using a hydrophilic soft block and a biodegradable, hydrophobic hard block. They might be broken down by the hydrolysis of intramolecular amide and ester bonds, which takes place in the body naturally. The hydrophilic, non-biodegradable polymers employed in the aforementioned invention are copolymers of PEO and polypropylene oxide (PPO) and/or polyethylene oxide (PEO), which have molecular weights between 600 and 30,000 Da. In this context, biodegradable polymers include polyglycolide (PGA), polylactide (PLA), and a PLA/PGA copolymer [128]. Mice treated with free RES/DDP had minor splenic and pulmonary toxicity, as measured by stannous octoate (Sn(Oct)2), -caprolactone (-CL), poly (ethylene glycol) (PEG4000), and stannous octanoate (Sn(Oct)2) (splenic nodular atrophy and pulmonary hemorrhage). This could be connected to the drug’s toxicity and adverse effects. Other organs, however, showed no poisoning symptoms [199]. Because of its hydrophilicity and steric repulsion properties, PEG is now the gold standard for coating NPs to reduce their toxicity. However, this permits “stealth” NP carriers to stay in circulation long enough to reach or identify their therapeutic site of action, decreasing toxicity, and enabling picture capture [128].
Poly (ε-caprolactone) (PCL) is also a good candidate to use in a hydrogel composite due to the effectiveness, good mechanical properties, and low toxicity of this treatment, as well as the significance of the cargo’s synergistic influence on the outcomes, which were shown in in vivo tests on tumors [128].
In conclusion, hydrogels are mostly biocompatible for chemotherapeutic delivery, which has brought greater attention to the development of modern polymeric biomaterials. Hydrogels based on chitosan and other natural polymers constitute a significant source of the biocompatible, biodegradable matrices widely applied in biomedicine. The main assets of biomaterials include biocompatibility, the lack of allergic, toxic, and mutagenic reactions, and the lack of immune responses and inflammations [200].
11. Limitations of Hydrogels for Cancer Therapy
Despite their wide advantages, hydrogels also have some shortcomings. The low tensile strength of many hydrogels restricts their benefits for load-bearing applications; these hydrogels dissolve or flow away from a targeted site before exerting their effects [201].
The delivery route is another major concern. The oral delivery route damages the gastrointestinal tract. Regarding the intravenous route, many hydrogels cannot be injected. However, intravenous injection has also some limitations such as rapid renal clearance. [202,203].
Photo-crosslinking hydrogels mostly have low penetration depth, which limits their penetration into deeper tissues [204].
Magnetic hydrogels might overheat under an alternating magnetic field, which results in heat damage to the adjacent healthy tissue [9].
Given these shortcomings, the application of hydrogel-based therapies in clinical practice may be limited.
12. Conclusions and Future Perspectives
Hydrogels, with their unique qualities, such as the capacity to transform from a liquid to a solid, serve as great platforms for controlled drug delivery. Hydrogel formulations offer a potential method for reducing off-target toxicity with chemotherapeutic medicines, which is especially important given that the systemic administration of these treatments is often at a dose limited by toxicity in normal cells. We have reviewed the use of hydrogel-based drug delivery systems for chemotherapeutics to tumors, including thermosensitive, pH-sensitive, photosensitive, dual-sensitive, and glutathione-sensitive hydrogels. The advantages of injectable hydrogel systems include lower toxicity to normal tissues, localized and prolonged distribution of the medications in the tumor region, more efficient cell death, and prevention of tumor growth. Within highly organized and controlled in vitro settings, the efficacy of these hydrogels for localized chemotherapy has been thoroughly described. Because most ectopic tumor models are subcutaneous, direct placement of a hydrogel close to or within the tumor mass is simple, and ectopic tumor growth can be monitored with relative simplicity. The lack of a tumor-specific microenvironment is a significant drawback of ectopic tumor models. While this indicates the field’s natural evolution from the in vitro setting to the preclinical in vivo models, additional testing in orthotopic tumor models that more faithfully simulate the tumor environment within a certain tissue or organ is necessary. Clinical trials involving humans will then be able to assess the efficacy of these drug delivery platforms for local chemotherapy and cancer treatment, and such studies are important to validate the hydrogels as treatment choices.
Author Contributions
Conceptualization, B.F.F.; methodology, B.F.F.; investigation, B.F.F., A.K.R., A.P. and G.M.; writing—original draft preparation, B.F.F., G.M., A.P., M.N.A. and A.K.R.; writing—review and editing, B.F.F. and M.R.N.-J., A.A.I. and E.N.; visualization, B.F.F.; supervision, B.F.F.; project administration, M.R.N.-J.; funding acquisition, B.F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.d.S.S.; Freitas, M.M.; Cerqueira, M.Â.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Benny Mattam, L.; Bijoy, A.; Abraham Thadathil, D.; George, L.; Varghese, A. Conducting Polymers: A Versatile Material for Biomedical Applications. Chem. 2022, 7, e202201765. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Mehrarya, M.; Gharehchelou, B.; Haghighi Poodeh, S.; Jamshidifar, E.; Karimifard, S.; Farasati Far, B.; Akbarzadeh, I.; Seifalian, A. Niosomal formulation for antibacterial applications. J. Drug Target. 2022, 30, 476–493. [Google Scholar] [CrossRef]
- Reghupaty, S.C.; Sarkar, D. Current status of gene therapy in hepatocellular carcinoma. Cancers 2019, 11, 1265. [Google Scholar] [CrossRef]
- Garg, J.; Chiu, M.N.; Krishnan, S.; Tripathi, L.K.; Pandit, S.; Far, B.F.; Jha, N.K.; Kesari, K.K.; Tripathi, V.; Pandey, S. Applications of lignin nanoparticles for cancer drug delivery: An update. Mater. Lett. 2022, 311, 131573. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Wu, H.; Li, Z.L.; Xu, X.F.; Xing, H.; Wang, M.D.; Jia, H.D.; Liang, L.; Li, C.; Sun, L.Y. Responsive hydrogels based on triggered click reactions for liver cancer. Adv. Mater. 2022, 34, 2201651. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2019, 17, 373–391. [Google Scholar] [CrossRef]
- Sahrayi, H.; Hosseini, E.; Karimifard, S.; Khayam, N.; Meybodi, S.M.; Amiri, S.; Bourbour, M.; Farasati Far, B.; Akbarzadeh, I.; Bhia, M. Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression. Pharmaceuticals 2021, 15, 6. [Google Scholar] [CrossRef]
- Asadi, S.; Mortezagholi, B.; Hadizadeh, A.; Borisov, V.; Ansari, M.J.; Shaker Majdi, H.; Nishonova, A.; Adelnia, H.; Farasati Far, B.; Chaiyasut, C. Ciprofloxacin-loaded titanium nanotubes coated with chitosan: A promising formulation with sustained release and enhanced antibacterial properties. Pharmaceutics 2022, 14, 1359. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, Z.; Afshari, A.R.; Sabouri, Z.; Mostafapour, A.; Far, B.F.; Jalili-Nik, M.; Darroudi, M. Plant-based synthesis of cerium oxide nanoparticles as a drug delivery system in improving the anticancer effects of free temozolomide in glioblastoma (U87) cells. Ceram. Int. 2022, 48, 30441–30450. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Dilmaghani, K.A.; Alizadeh, E.; Akbarzadeh, A.; Davaran, S. Enhancing cisplatin delivery to hepatocellular carcinoma HepG2 cells using dual sensitive smart nanocomposite. Artif. Cells Nanomed. Biotechnol. 2018, 46, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Raza, F.; Zhu, Y.; Chen, L.; You, X.; Zhang, J.; Khan, A.; Khan, M.W.; Hasnat, M.; Zafar, H.; Wu, J. Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater. Sci. 2019, 7, 2023–2036. [Google Scholar] [CrossRef]
- Peng, M.; Xu, S.; Zhang, Y.; Zhang, L.; Huang, B.; Fu, S.; Xue, Z.; Da, Y.; Dai, Y.; Qiao, L. Thermosensitive injectable hydrogel enhances the antitumor effect of embelin in mouse hepatocellular carcinoma. J. Pharm. Sci. 2014, 103, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Eshrati Yeganeh, F.; Eshrati Yeganeh, A.; Fatemizadeh, M.; Farasati Far, B.; Quazi, S.; Safdar, M. In vitro cytotoxicity and anti-cancer drug release behavior of methionine-coated magnetite nanoparticles as carriers. Med. Oncol. 2022, 39, 252. [Google Scholar] [CrossRef] [PubMed]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on biomedical application of polysaccharide-based hydrogels with a focus on drug delivery systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Lim, K.S.; Martens, P.; Poole-Warren, L. Biosynthetic hydrogels for cell encapsulation. In Functional Hydrogels as Biomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–29. [Google Scholar]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef]
- Meis, C.M.; Grosskopf, A.K.; Correa, S.; Appel, E.A. Injectable supramolecular polymer-nanoparticle hydrogels for cell and drug delivery applications. JoVE (J. Vis. Exp.) 2021, 168, e62234. [Google Scholar] [CrossRef]
- Esmaeely Neisiany, R.; Enayati, M.S.; Sajkiewicz, P.; Pahlevanneshan, Z.; Ramakrishna, S. Insight into the current directions in functionalized nanocomposite hydrogels. Front. Mater. 2020, 7, 25. [Google Scholar] [CrossRef]
- Singh, A.; Bhatia, D. DNA hydrogels: Principles, synthesis, characterization and applications to cell biology. Methods Cell Biol. 2022, 169, 323–346. [Google Scholar] [PubMed]
- Li, F.; Tang, J.; Geng, J.; Luo, D.; Yang, D. Polymeric DNA hydrogel: Design, synthesis and applications. Prog. Polym. Sci. 2019, 98, 101163. [Google Scholar] [CrossRef]
- Xu, N.; Ma, N.; Yang, X.; Ling, G.; Yu, J.; Zhang, P. Preparation of intelligent DNA hydrogel and its applications in biosensing. Eur. Polym. J. 2020, 137, 109951. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Jiang, N.; Wang, J.; Yu, M.; Zhuang, X. Preparation of aptamer responsive DNA functionalized hydrogels for the sensitive detection of α-fetoprotein using SERS method. Bioconjugate Chem. 2020, 31, 813–820. [Google Scholar] [CrossRef]
- Sethi, S.; Thakur, S.; Kaith, B.S.; Sharma, N.; Ansar, S.; Pandey, S.; Kuma, V. Biopolymer starch-gelatin embedded with silver nanoparticle–based hydrogel composites for antibacterial application. Biomass Convers. Biorefinery 2022, 12, 5363–5384. [Google Scholar] [CrossRef]
- Ruiz-Franco, J.; Zaccarelli, E. On the role of competing interactions in charged colloids with short-range attraction. Annu. Rev. Condens. Matter Phys. 2021, 12, 51–70. [Google Scholar] [CrossRef]
- Bidkar, A.P.; Sanpui, P.; Ghosh, S.S. Red Blood Cell-Membrane-Coated Poly(Lactic-co-glycolic Acid) Nanoparticles for Enhanced Chemo- and Hypoxia-Activated Therapy. ACS Appl. Bio Mater. 2019, 2, 4077–4086. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Damiri, F.; Rahman, M.H.; Zehravi, M.; Awaji, A.A.; Nasrullah, M.Z.; Gad, H.A.; Bani-Fwaz, M.Z.; Varma, R.S.; Germoush, M.O.; Al-Malky, H.S. MXene (Ti3C2Tx)-Embedded Nanocomposite Hydrogels for Biomedical Applications: A Review. Materials 2022, 15, 1666. [Google Scholar] [CrossRef] [PubMed]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.; Das, C.M.; Ma, M.; Qiao, N.; Fan, T.; Zhang, H.; Xu, G.; Yong, K.-T. Bioengineering applications of black phosphorus and their toxicity assessment. Environ. Sci. Nano 2021, 8, 3452–3477. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shao, C.; Chen, H.; Chen, Z.; Zhao, Y. Hierarchical hydrogels with ordered micro-nano structures for cancer-on-a-chip construction. Research 2021, 2021, 9845679. [Google Scholar] [CrossRef]
- Ranganathan, N.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Synthesis and properties of hydrogels prepared by various polymerization reaction systems. In Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 487–511. [Google Scholar]
- Bingol, H.B.; Agopcan-Cinar, S.; Bal, T.; Oran, D.C.; Kizilel, S.; Kayaman-Apohan, N.; Avci, D. Stimuli-responsive poly (hydroxyethyl methacrylate) hydrogels from carboxylic acid-functionalized crosslinkers. J. Biomed. Mater. Res. Part A 2019, 107, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, R.; Posa, A.; Mariappan, M.; Spiliopoulos, S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J. Gastroenterol. 2019, 25, 4614. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Lym, J.S.; Huynh, C.T.; Kim, B.S.; Jae, H.J.; Kim, Y.I.; Lee, D.S. A novel sulfamethazine-based pH-sensitive copolymer for injectable radiopaque embolic hydrogels with potential application in hepatocellular carcinoma therapy. Polym. Chem. 2016, 7, 5805–5818. [Google Scholar] [CrossRef]
- Yan, X.; Sun, T.; Song, Y.; Peng, W.; Xu, Y.; Luo, G.; Li, M.; Chen, S.; Fang, W.-W.; Dong, L. In situ thermal-responsive magnetic hydrogel for multidisciplinary therapy of hepatocellular carcinoma. Nano Lett. 2022, 22, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S. Treatment strategy of transarterial chemoembolization for hepatocellular carcinoma. Appl. Sci. 2020, 10, 7337. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef]
- Ma, J.; Wang, B.; Shao, H.; Zhang, S.; Chen, X.; Li, F.; Liang, W. Hydrogels for localized chemotherapy of liver cancer: A possible strategy for improved and safe liver cancer treatment. Drug Deliv. 2022, 29, 1457–1476. [Google Scholar] [CrossRef]
- Surrao, D.C.; Arasu, Y.; Ekberg, J.A.; St John, J.A. Blended, crosslinked alginate-methylcellulose hydrogels for encapsulation and delivery of olfactory ensheathing cells. Materialia 2020, 10, 100654. [Google Scholar] [CrossRef]
- Barroso, N.; Guaresti, O.; Perez-Alvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L. Self-healable hyaluronic acid/chitosan polyelectrolyte complex hydrogels and multilayers. Eur. Polym. J. 2019, 120, 109268. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, J.; Yuan, H.; Ma, B.; Cao, Z.; Chen, Y.; Xing, C.; Niu, X.; Li, N.; Wang, H. Polyisocyanide hydrogels with tunable nonlinear elasticity mediate liver carcinoma cell functional response. Acta Biomater. 2022, 148, 152–162. [Google Scholar] [CrossRef]
- Kanaan, A.F.; Piedade, A.P.; de Sousa, H.C.; Dias, A.M. Semi-interpenetrating chitosan/ionic liquid polymer networks as electro-responsive biomaterials for potential wound dressings and iontophoretic applications. Mater. Sci. Eng. C 2021, 121, 111798. [Google Scholar] [CrossRef]
- Farasati Far, B.; Asadi, S.; Naimi-Jamal, M.R.; Abdelbasset, W.K.; Aghajani Shahrivar, A. Insights into the interaction of azinphos-methyl with bovine serum albumin: Experimental and molecular docking studies. J. Biomol. Struct. Dyn. 2021, 40, 11863–11873. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, F.E.; Yeganeh, A.E.; Yousefi, M.; Farasati Far, B.; Akbarzadeh, I.; Bokov, D.O.; Raahemifar, K.; Soltani, M. Formulation and Characterization of Poly (Ethylene Glycol)-Coated Core-Shell Methionine Magnetic Nanoparticles as a Carrier for Naproxen Delivery: Growth Inhibition of Cancer Cells. Cancers 2022, 14, 1797. [Google Scholar] [CrossRef]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Lu, Q.; Zhao, Y.; Cheng, C.; Zhang, S. Synthesis and self-assembly of the pH-responsive anionic copolymers for enhanced doxorubicin-loading capacity. Langmuir 2018, 34, 7877–7886. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Luo, J.; Liu, Y.; Su, S.; Fu, S.; Yang, X.; Li, B. Intratumoral administration of thermosensitive hydrogel co-loaded with norcantharidin nanoparticles and doxorubicin for the treatment of hepatocellular carcinoma. Int. J. Nanomed. 2021, 16, 4073. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties-A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, S.; Khan, K.U.; Sohail, M.; Abdullah, O.; Khalid, I.; Malik, N.S. Synthesis and evaluation of polyethylene glycol-4000-co-poly (AMPS) based hydrogel membranes for controlled release of mupirocin for efficient wound healing. Curr. Drug Deliv. 2022, 19, 1102–1115. [Google Scholar] [CrossRef]
- Binder, S.; Gerlach, G. Performance of force-compensated chemical sensors based on bisensitive hydrogels. Sens. Actuators B Chem. 2021, 342, 129420. [Google Scholar] [CrossRef]
- Jawad, S. A Review On Novel Approach Of Drug Delivery–Hydrogels. 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/35301948/ (accessed on 11 October 2022).
- Xu, T.; Li, H.; He, C.; Xu, H.; Song, X.; Ji, H.; Zhao, C. Semi-interpenetrating polymer network microspheres with superior dimensional stability as multifunctional antibacterial adsorbent materials. J. Environ. Chem. Eng. 2019, 7, 103393. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, L.; Hu, S.; Hu, J.; Fu, Y.; Hu, Y.; Yang, X. Carboxymethyl chitosan microspheres loaded hyaluronic acid/gelatin hydrogels for controlled drug delivery and the treatment of inflammatory bowel disease. Int. J. Biol. Macromol. 2021, 167, 1598–1612. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Zhao, Z.; Lan, W.; Zhang, X.; Fan, M. Highly alkaline stable fully-interpenetrating network poly (styrene-co-4-vinyl pyridine)/polyquaternium-10 anion exchange membrane without aryl ether linkages. Int. J. Hydrog. Energy 2022, 47, 16580–16596. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.; Li, M.; Liu, Z.; Wang, X.; Zhang, L.; Wang, Z. Hydrogel-Based Biomaterials Engineered from Natural-Derived Polysaccharides and Proteins for Hemostasis and Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 780187. [Google Scholar] [CrossRef] [PubMed]
- Ziarati, P.; Far, B.F.; Mashayekhi, E.; Sawicka, B. Removing Arsenic by Food-Processing Waste (Zizyphus Jujuba Seeds) and Study on its Adsorptive Properties; National University of Civil Defence of Ukraine: Kharkiv, Ukraine, 2019. [Google Scholar]
- Siqueira, N.M.; Cirne, M.F.; Immich, M.F.; Poletto, F. Stimuli-responsive polymeric hydrogels and nanogels for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 343–374. [Google Scholar]
- Mohammadi, M.; Karimi, M.; Malaekeh-Nikouei, B.; Torkashvand, M.; Alibolandi, M. Hybrid in situ-forming injectable hydrogels for local cancer therapy. Int. J. Pharm. 2022, 616, 121534. [Google Scholar] [CrossRef]
- Zhou, X.; He, X.; Shi, K.; Yuan, L.; Yang, Y.; Liu, Q.; Ming, Y.; Yi, C.; Qian, Z. Injectable Thermosensitive Hydrogel Containing Erlotinib-Loaded Hollow Mesoporous Silica Nanoparticles as a Localized Drug Delivery System for NSCLC Therapy. Adv. Sci. 2020, 7, 2001442. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yan, L.; Liu, S.; Tan, Y.; Xiao, J.; Cao, Y.; Chen, K.; Xiao, W.; Li, B.; Liao, X. Preparation of silk fibroin/hyaluronic acid hydrogels with enhanced mechanical performance by a combination of physical and enzymatic crosslinking. J. Biomater. Sci. Polym. Ed. 2021, 32, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qu, Y.; Zhang, Z.; Huang, H.; Xu, Y.; Shen, F.; Wang, L.; Sun, L. Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy. Gels 2022, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Kamkar, M.; Janmaleki, M.; Erfanian, E.; Sanati-Nezhad, A.; Sundararaj, U. Covalently cross-linked hydrogels: Mechanisms of nonlinear viscoelasticity. Can. J. Chem. Eng. 2022, 100, 3227–3239. [Google Scholar] [CrossRef]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and development of hybrid hydrogels for biomedical applications: Recent trends in anticancer drug delivery and tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Jiang, C.; Wang, M.; Zhu, C.; Zhao, N.; Xu, J. Skin-inspired double-hydrophobic-coating encapsulated hydrogels with enhanced water retention capacity. Adv. Funct. Mater. 2021, 31, 2102433. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, L.; Chiao, M.; Yang, W. Antibacterial hydrogel coating: Strategies in surface chemistry. Adv. Colloid Interface Sci. 2020, 285, 102280. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Barghbani, M.; Far, B.F.; Hosseinian, S.; Hosseini, S.M. Extract-mediated biosynthesis and characterization of gold nanoparticles: Exploring their protective effect against cyclophosphamide-induced oxidative stress in rat testis. J. Drug Deliv. Sci. Technol. 2022, 71, 103306. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. [Google Scholar]
- Gelain, F.; Luo, Z.; Zhang, S. Self-assembling peptide EAK16 and RADA16 nanofiber scaffold hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, M.; Jo, H.; Kim, K.; Balasubramanian, S. Green synthesis of multifunctional PEG-carboxylate π back-bonded gold nanoconjugates for breast cancer treatment. Int. J. Nanomed. 2019, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Willner, B.; Willner, I. Redox-responsive and light-responsive DNA-based hydrogels and their applications. React. Funct. Polym. 2021, 166, 104983. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Feng, H.; Ma, Y.; Zhang, Z.; Yang, S.; Ma, Z.; Zhang, Y.; Ma, S.; Yu, B.; Cai, M.; Pei, X. Reversing Hydrogel Adhesion Property via Firmly Anchoring Thin Adhesive Coatings. Adv. Funct. Mater. 2022, 32, 2111278. [Google Scholar] [CrossRef]
- Rezaei, T.; Rezaei, M.; Karimifard, S.; Beram, F.M.; Dakkali, M.S.; Heydari, M.; Afshari-Behbahanizadeh, S.; Mostafavi, E.; Bokov, D.O.; Ansari, M.J. Folic Acid-Decorated pH-Responsive Nanoniosomes With Enhanced Endocytosis for Breast Cancer Therapy: In Vitro Studies. Front. Pharmacol. 2022, 13, 851242. [Google Scholar] [PubMed]
- Xu, W.; Wang, J.; Li, Q.; Wu, C.; Wu, L.; Li, K.; Li, Q.; Han, Q.; Zhu, J.; Bai, Y. Cancer cell membrane-coated nanogels as a redox/pH dual-responsive drug carrier for tumor-targeted therapy. J. Mater. Chem. B 2021, 9, 8031–8037. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H. Recent progress of polysaccharide-based hydrogel interfaces for wound healing and tissue engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Gagner, J.E.; Kim, W.; Chaikof, E.L. Designing protein-based biomaterials for medical applications. Acta Biomater. 2014, 10, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N. Protein-protein interaction detection: Methods and analysis. Int. J. Proteom. 2014, 2014, 147648. [Google Scholar] [CrossRef] [PubMed]
- Keung, A.J.; Kumar, S.; Schaffer, D.V. Presentation counts: Microenvironmental regulation of stem cells by biophysical and material cues. Annu. Rev. Cell Dev. Biol. 2010, 26, 533–556. [Google Scholar] [CrossRef]
- Poursaid, A. Design and Development of Silk-Elastinlike Protein Polymer Liquid Embolics for Treatment of Hepatocellular Carcinoma; The University of Utah: Salt Lake City, UT, USA, 2016. [Google Scholar]
- Qian, K.-Y.; Song, Y.; Yan, X.; Dong, L.; Xue, J.; Xu, Y.; Wang, B.; Cao, B.; Hou, Q.; Peng, W. Injectable ferrimagnetic silk fibroin hydrogel for magnetic hyperthermia ablation of deep tumor. Biomaterials 2020, 259, 120299. [Google Scholar] [CrossRef]
- Heymer, A.; Haddad, D.; Weber, M.; Gbureck, U.; Jakob, P.M.; Eulert, J.; Nöth, U. Iron oxide labelling of human mesenchymal stem cells in collagen hydrogels for articular cartilage repair. Biomaterials 2008, 29, 1473–1483. [Google Scholar] [CrossRef]
- Sahrayi, H.; Hosseini, E.; Ramazani Saadatabadi, A.; Atyabi, S.M.; Bakhshandeh, H.; Mohamadali, M.; Aidun, A.; Farasati Far, B. Cold atmospheric plasma modification and electrical conductivity induction in gelatin/polyvinylidene fluoride nanofibers for neural tissue engineering. Artif. Organs 2022, 46, 1504–1521. [Google Scholar] [CrossRef]
- Moreno-Arotzena, O.; Meier, J.G.; Del Amo, C.; García-Aznar, J.M. Characterization of fibrin and collagen gels for engineering wound healing models. Materials 2015, 8, 1636–1651. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Yan, Y.; He, C.; Li, X. Fabrication of injectable hydrogels from silk fibroin and angiogenic peptides for vascular growth and tissue regeneration. Chem. Eng. J. 2021, 418, 129308. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, T.; Zhang, K.Q.; Meng, K.; Zhao, H. Silk fibroin/chitosan hydrogel with antibacterial, hemostatic and sustained drug-release activities. Polym. Int. 2021, 70, 1741–1751. [Google Scholar] [CrossRef]
- Başaran, D.D.A.; Gündüz, U.; Tezcaner, A.; Keskin, D. Topical delivery of heparin from PLGA nanoparticles entrapped in nanofibers of sericin/gelatin scaffolds for wound healing. Int. J. Pharm. 2021, 597, 120207. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabello, J.C.; de Torre, I.G.; Ibañez-Fonseca, A.; Alonso, M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018, 129, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Sarangthem, V.; Singh, T.D.; Dinda, A.K. Emerging Role of Elastin-Like Polypeptides in Regenerative Medicine. Adv. Wound Care 2021, 10, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Tut, T.A.; Cesur, S.; Ilhan, E.; Sahin, A.; Yildirim, O.S.; Gunduz, O. Gentamicin-loaded polyvinyl alcohol/whey protein isolate/hydroxyapatite 3D composite scaffolds with drug delivery capability for bone tissue engineering applications. Eur. Polym. J. 2022, 179, 111580. [Google Scholar] [CrossRef]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Elviri, L.; Bianchera, A.; Bergonzi, C.; Bettini, R. Controlled local drug delivery strategies from chitosan hydrogels for wound healing. Expert Opin. Drug Deliv. 2017, 14, 897–908. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Cortes, H.; Caballero-Florán, I.H.; Mendoza-Muñoz, N.; Córdova-Villanueva, E.N.; Escutia-Guadarrama, L.; Figueroa-González, G.; Reyes-Hernández, O.D.; González-Del Carmen, M.; Varela-Cardoso, M.; Magaña, J.J. Hyaluronic acid in wound dressings. Cell. Mol. Biol. 2020, 66, 191–198. [Google Scholar] [CrossRef]
- Grijalvo, S.; Nieto-Díaz, M.; Maza, R.M.; Eritja, R.; Díaz, D.D. Alginate hydrogels as scaffolds and delivery systems to repair the damaged spinal cord. Biotechnol. J. 2019, 14, 1900275. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Pharmaceutical and biomedical applications of cellulose nanofibers: A review. Environ. Chem. Lett. 2021, 19, 2043–2055. [Google Scholar] [CrossRef]
- Tan, H.-T.; Corbin, K.R.; Fincher, G.B. Emerging technologies for the production of renewable liquid transport fuels from biomass sources enriched in plant cell walls. Front. Plant Sci. 2016, 7, 1854. [Google Scholar] [CrossRef]
- Farasati Far, B.; Bokov, D.; Widjaja, G.; Setia Budi, H.; Kamal Abdelbasset, W.; Javanshir, S.; Seif, F.; Pazoki-Toroudi, H.; Dey, S.K. Metronidazole, acyclovir and tetrahydrobiopterin may be promising to treat COVID-19 patients, through interaction with interleukin-12. J. Biomol. Struct. Dyn. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Farasati Far, B.; Naimi-Jamal, M.R.; Jahanbakhshi, M.; Mohammed, H.T.; Altimari, U.S.; Ansari, J. Poly (3-thienylboronic acid) coated magnetic nanoparticles as a magnetic solid-phase adsorbent for extraction of methamphetamine from urine samples. J. Dispers. Sci. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomás, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef]
- Na, K.; Park, K.-H.; Kim, S.W.; Bae, Y.H. Self-assembled hydrogel nanoparticles from curdlan derivatives: Characterization, anti-cancer drug release and interaction with a hepatoma cell line (HepG2). J. Control. Release 2000, 69, 225–236. [Google Scholar] [CrossRef]
- Xu, K.; Lee, F.; Gao, S.J.; Chung, J.E.; Yano, H.; Kurisawa, M. Injectable hyaluronic acid-tyramine hydrogels incorporating interferon-α2a for liver cancer therapy. J. Control. Release 2013, 166, 203–210. [Google Scholar] [CrossRef]
- Liang, H.-F.; Hong, M.-H.; Ho, R.-M.; Chung, C.-K.; Lin, Y.-H.; Chen, C.-H.; Sung, H.-W. Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules 2004, 5, 1917–1925. [Google Scholar] [CrossRef]
- Wu, J.; Su, Z.-G.; Ma, G.-H. A thermo-and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int. J. Pharm. 2006, 315, 1–11. [Google Scholar] [CrossRef]
- Zaino, C.; Zambito, Y.; Mollica, G.; Geppi, M.; Serafini, M.F.; Carelli, V.; Di Colo, G. A novel polyelectrolyte complex (PEC) hydrogel for controlled drug delivery to the distal intestine. Open Drug Deliv. J. 2007, 1, 68–75. [Google Scholar]
- Păduraru, O.M.; Ciolacu, D.; Darie, R.N.; Vasile, C. Synthesis and characterization of polyvinyl alcohol/cellulose cryogels and their testing as carriers for a bioactive component. Mater. Sci. Eng. C 2012, 32, 2508–2515. [Google Scholar] [CrossRef]
- Denkbaş, E.; Seyyal, M.; Pişkin, E. Implantable 5-fluorouracil loaded chitosan scaffolds prepared by wet spinning. J. Membr. Sci. 2000, 172, 33–38. [Google Scholar] [CrossRef]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Benitez, C.A.; Kohane, D.S. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 2007, 28, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S. Photo-cross-linkable and thermo-responsive hydrogels containing chitosan and Pluronic for sustained release of human growth hormone (hGH). J. Biomater. Sci. Polym. Ed. 2007, 18, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Lin, S.-Y.; Liu, D.-Z.; Tyan, Y.-C.; Yang, J.-S. Sustained release of 5-FU from Poloxamer gels interpenetrated by crosslinking chitosan network. Int. J. Pharm. 2009, 382, 39–44. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Hoque, J.; Sangaj, N.; Varghese, S. Stimuli-Responsive Supramolecular Hydrogels and Their Applications in Regenerative Medicine. Macromol Biosci 2019, 19, e1800259. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. Epma J 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, Y.; Luo, J.; Xiong, K.; Lu, Y.; Wu, Z.; Wang, B.Q.; Wu, J.; Chen, Y.; Fu, S. Therapeutic efficacy of thermosensitive Pluronic hydrogel for codelivery of resveratrol microspheres and cisplatin in the treatment of liver cancer ascites. Int. J. Pharm. 2020, 582, 119334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhou, B.; Shi, K.; Zhang, R.; Dong, C.; Xie, D.; Tang, L.; Tian, Y.; Qian, Z.; Yang, L. Sustained and targeted delivery of siRNA/DP7-C nanoparticles from injectable thermosensitive hydrogel for hepatocellular carcinoma therapy. Cancer Sci. 2021, 112, 2481–2492. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, Y.; Ma, W.; Zhou, P.; Wang, B.; Wu, Z.; Wen, Q.; Xiong, K.; Liu, Y.; Fu, S. Intraperitoneal administration of thermosensitive hydrogel Co-loaded with norcantharidin nanoparticles and oxaliplatin inhibits malignant ascites of hepatocellular carcinoma. Drug Deliv. 2022, 29, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wu, Y.L.; Li, Z.; Loh, X.J. Recent Progress in Polyhydroxyalkanoates-Based Copolymers for Biomedical Applications. Biotechnol. J. 2019, 14, e1900283. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Wang, Y.L.; Qu, Y.; Liao, J.F.; Chu, B.Y.; Zhang, H.P.; Luo, F.; Qian, Z.Y. Synthesis, characterization, and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci. Rep. 2016, 6, 19077. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Shen, W.; Li, K.; Yu, L.; Chen, Q.; Ding, J. Controlled release of liraglutide using thermogelling polymers in treatment of diabetes. Sci. Rep. 2016, 6, 31593. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.T.; Nguyen, Q.V.; Lym, J.S.; Kim, B.S.; Huynh, D.P.; Jae, H.J.; Kim, Y.I.; Lee, D.S. Intraarterial gelation of injectable cationic pH/temperature-sensitive radiopaque embolic hydrogels in a rabbit hepatic tumor model and their potential application for liver cancer treatment. RSC Adv. 2016, 6, 47687–47697. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Bie, N.; Xu, Q.; Yong, T.; Wang, Q.; Gan, L.; Yang, X. Zwitterionic temperature/redox-sensitive nanogels for near-infrared light-triggered synergistic thermo-chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 23564–23573. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Jones, G.T.; Rnjak-Kovacina, J.; Lin, Y.; Kaplan, D.L. pH-dependent anticancer drug release from silk nanoparticles. Adv. Healthc Mater. 2013, 2, 1606–1611. [Google Scholar] [CrossRef]
- Yeganeh, F.E.; Yeganeh, A.E.; Far, B.F.; Mansouri, A.; Sibuh, B.Z.; Krishnan, S.; Pandit, S.; Alsanie, W.F.; Thakur, V.K.; Gupta, P.K. Synthesis and Characterization of Tetracycline Loaded Methionine-Coated NiFe2O4 Nanoparticles for Anticancer and Antibacterial Applications. Nanomaterials 2022, 12, 2286. [Google Scholar] [CrossRef]
- Gu, J.; Su, S.; Zhu, M.; Li, Y.; Zhao, W.; Duan, Y.; Shi, J. Targeted doxorubicin delivery to liver cancer cells by PEGylated mesoporous silica nanoparticles with a pH-dependent release profile. Microporous Mesoporous Mater. 2012, 161, 160–167. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef]
- Korpusik, A.B.; Tan, Y.; Garrison, J.B.; Tan, W.; Sumerlin, B.S. Aptamer-Conjugated Micelles for Targeted Photodynamic Therapy Via Photoinitiated Polymerization-Induced Self-Assembly. Macromolecules 2021, 54, 7354–7363. [Google Scholar] [CrossRef]
- Wang, Y.; Han, B.; Shi, R.; Pan, L.; Zhang, H.; Shen, Y.; Li, C.; Huang, F.; Xie, A. Preparation and characterization of a novel hybrid hydrogel shell for localized photodynamic therapy. J. Mater. Chem. B 2013, 1, 6411–6417. [Google Scholar] [CrossRef]
- Spring, B.Q.; Bryan Sears, R.; Zheng, L.Z.; Mai, Z.; Watanabe, R.; Sherwood, M.E.; Schoenfeld, D.A.; Pogue, B.W.; Pereira, S.P.; Villa, E. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat. Nanotechnol. 2016, 11, 378–387. [Google Scholar] [CrossRef]
- Mahmoodzadeh, F.; Ghorbani, M.; Jannat, B. Glutathione and pH-responsive chitosan-based nanogel as an efficient nanoplatform for controlled delivery of doxorubicin. J. Drug Deliv. Sci. Technol. 2019, 54, 101315. [Google Scholar] [CrossRef]
- Argenziano, M.; Lombardi, C.; Ferrara, B.; Trotta, F.; Caldera, F.; Blangetti, M.; Koltai, H.; Kapulnik, Y.; Yarden, R.; Gigliotti, L.; et al. Glutathione/pH-responsive nanosponges enhance strigolactone delivery to prostate cancer cells. Oncotarget 2018, 9, 35813–35829. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione responsive polymers and their application in drug delivery systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L.; Kan, C.-W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly (N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Tan, L.; Zhao, Q.; Luo, F.; Wei, Y.; Qian, Z. PEG-PCL based micelle hydrogels as oral docetaxel delivery systems for breast cancer therapy. Biomaterials 2014, 35, 6972–6985. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Pritchard, E.M.; Kaplan, D.L. Self-assembling doxorubicin silk hydrogels for the focal treatment of primary breast cancer. Adv. Funct. Mater. 2013, 23, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef]
- Zhu, K.; Ju, Y.; Xu, J.; Yang, Z.; Gao, S.; Hou, Y. Magnetic nanomaterials: Chemical design, synthesis, and potential applications. Acc. Chem. Res. 2018, 51, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Kostarelos, K.; Prato, M.; Bianco, A. Biomedical uses for 2D materials beyond graphene: Current advances and challenges ahead. Adv. Mater. 2016, 28, 6052–6074. [Google Scholar] [CrossRef]
- Roy, A.K.; Park, B.; Lee, K.S.; Park, S.Y.; In, I. Boron nitride nanosheets decorated with silver nanoparticles through mussel-inspired chemistry of dopamine. Nanotechnology 2014, 25, 445603. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Chen, X.; Wang, W.; Loh, X.J. Engineering bioresponsive hydrogels toward healthcare applications. Macromol. Chem. Phys. 2016, 217, 175–188. [Google Scholar] [CrossRef]
- Chan, G.G.; Koch, C.M.; Connors, L.H. Blood proteomic profiling in inherited (ATTRm) and acquired (ATTRwt) forms of transthyretin-associated cardiac amyloidosis. J. Proteome Res. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Surapaneni, S.K.; Huang, J.; Mondal, A.; Wang, V.Z.; Haruna, N.F.; Bagde, A.; Arthur, P.; Kutlehria, S.; Patel, N.; et al. Polysaccharide hydrogel based 3D printed tumor models for chemotherapeutic drug screening. Sci. Rep. 2021, 11, 372. [Google Scholar] [CrossRef]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-doped and hybrid carbon dots: A comprehensive review on their synthesis and biomedical applications. J. Control. Release 2021, 330, 132–150. [Google Scholar] [CrossRef]
- Zmerli, I.; Michel, J.-P.; Makky, A. Multifunctional polydopamine-based nanoparticles: Synthesis, physico-chemical properties and applications for bimodal photothermal/photodynamic therapy of cancer. Multifunct. Mater. 2021, 4, 022001. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 2020, 11, 357. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, D.; Xie, T.; Zhou, M. Biodegradable microalgae-based carriers for targeted delivery and imaging-guided therapy toward lung metastasis of breast cancer. Small 2020, 16, 2000819. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, F.; Liu, X.-Y.; Yan, Y.-J.; Zhou, X.-P.; Chen, Z.-L. The Photodynamic Anti-Tumor Effects of New PPa-CDs Conjugate with pH Sensitivity and Improved Biocompatibility. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. -Anti-Cancer Agents) 2022, 22, 1286–1295. [Google Scholar] [CrossRef]
- Lu, X.; Gao, S.; Lin, H.; Yu, L.; Han, Y.; Zhu, P.; Bao, W.; Yao, H.; Chen, Y.; Shi, J. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv. Mater. 2020, 32, 2002246. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Speranza, G. Functionalization of carbon nanomaterials for biomedical applications. C 2019, 5, 72. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, C.; Zou, T. Single-Atom Catalysts for Biotherapy Applications: A Systematic Review. Nanomaterials 2020, 10, 2518. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H. Hierarchical nickel hydroxide nanosheets grown on hollow nitrogen doped carbon nanoboxes as a high-performance surface substrate for alpha-fetoprotein cancer biomarkers electrochemical aptasensing. Talanta 2022, 237, 122924. [Google Scholar] [CrossRef]
- Lu, M.; Wang, C.; Ding, Y.; Peng, M.; Zhang, W.; Li, K.; Wei, W.; Lin, Y. Fe–N/C single-atom catalysts exhibiting multienzyme activity and ROS scavenging ability in cells. Chem. Commun. 2019, 55, 14534–14537. [Google Scholar] [CrossRef]
- Ma, W.; Mao, J.; Yang, X.; Pan, C.; Chen, W.; Wang, M.; Yu, P.; Mao, L.; Li, Y. A single-atom Fe–N 4 catalytic site mimicking bifunctional antioxidative enzymes for oxidative stress cytoprotection. Chem. Commun. 2019, 55, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, F.G. Emerging Single-Atom Catalysts/Nanozymes for Catalytic Biomedical Applications. Adv. Healthc. Mater. 2022, 11, 2101682. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Qian, X.; Tang, Z.; Hu, Q.; Chen, J.; Gao, C.; Tang, R.; Tong, X.; Wang, J. Biocompatibility and bone-repairing effects: Comparison between porous poly-lactic-co-glycolic acid and nano-hydroxyapatite/poly (lactic acid) scaffolds. J. Biomed. Nanotechnol. 2014, 10, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Wu, K.; Wu, X.; Tang, J.; Cui, S.; Cao, D.; Liu, R.; Peng, C.; Yu, L. Visualizing the in vivo evolution of an injectable and thermosensitive hydrogel using tri-modal bioimaging. Small Methods 2020, 4, 2000310. [Google Scholar] [CrossRef]
- Cheng, Y. Poly (ethylene glycol)-polypeptide copolymer micelles for therapeutic agent delivery. Curr. Pharm. Biotechnol. 2016, 17, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Van Abel, D. Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J. Cosmet. Laser Ther. 2015, 17, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-responsive hydrogels for cancer treatment: The role of pH, light, ionic strength and magnetic field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M.; et al. pH-Sensitive Peptide Hydrogels as a Combination Drug Delivery System for Cancer Treatment. Pharmaceutics 2022, 14, 652. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Liu, T.; Yu, X.; Bai, Y.; Zhang, Y.; Chen, X. Nanocomposite hydrogel with NIR/magnet/enzyme multiple responsiveness to accurately manipulate local drugs for on-demand tumor therapy. Biomaterials 2020, 262, 120357. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.; Li, F.; Wang, J.; Liu, J.; Kim, D.; Fan, C.; Hyeon, T.; Ling, D. Highly Sensitive Diagnosis of Small Hepatocellular Carcinoma Using pH-Responsive Iron Oxide Nanocluster Assemblies. J. Am. Chem. Soc. 2018, 140, 10071–10074. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels–synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xie, W.; Miao, Y.; Wang, D.; Guo, Z.; Ghosal, A.; Li, Y.; Wei, Y.; Feng, S.S.; Zhao, L. Magnetic hydrogel with optimally adaptive functions for breast cancer recurrence prevention. Adv. Healthc. Mater. 2019, 8, 1900203. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, L.; Xu, R.; Ma, S.; Ma, Z.; Liu, Y.; Wu, Y.; Feng, L.; Cai, M.; Zhou, F. Fibers reinforced composite hydrogels with improved lubrication and load-bearing capacity. Friction 2022, 10, 54–67. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, D. Self-healable polyacrylic acid-polyacrylamide-ferric ion dual-crosslinked hydrogel with good biotribological performance as a load-bearing surface. J. Appl. Polym. Sci. 2020, 137, 48499. [Google Scholar] [CrossRef]
- Wang, X.-H.; Song, F.; Xue, J.; Qian, D.; Wang, X.-L.; Wang, Y.-Z. Mechanically strong and tough hydrogels with excellent anti-fatigue, self-healing and reprocessing performance enabled by dynamic metal-coordination chemistry. Polymer 2018, 153, 637–642. [Google Scholar] [CrossRef]
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.A.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.J.; Kock, L.M.; Spee, B. Cellulose nanofibril hydrogel promotes hepatic differentiation of human liver organoids. Adv. Healthc. Mater. 2020, 9, 1901658. [Google Scholar] [CrossRef]
- Transarterial Chemoembolization Versus Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma (TRENDY). Available online: https://clinicaltrials.gov/ct2/show/NCT02470533 (accessed on 27 January 2021).
- Transarterial Embolization Alone Versus Drug-Eluting Beads Chemoembolization for Hepatocellular Carcinoma (RAD-18-TAcE). Available online: https://clinicaltrials.gov/ct2/show/NCT04803019 (accessed on 19 March 2021).
- Safety and Efficacy of Doxorubicin-Eluting-Bead Embolization in Patients With Advanced Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02525380 (accessed on 17 January 2018).
- Yin, L.; Zhao, X.; Cui, L.; Ding, J.; He, M.; Tang, C.; Yin, C. Cytotoxicity and genotoxicity of superporous hydrogel containing interpenetrating polymer networks. Food Chem. Toxicol. 2009, 47, 1139–1145. [Google Scholar] [CrossRef]
- Vashist, A.; Vashist, A.; Gupta, Y.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.L.; Chen, L.X.; Zhang, H.; Yang, B.; Xu, S.; Wu, M.; Liu, J.; Yang, L.L.; Chen, Y.; Fu, S.Z. In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer. Drug Deliv. 2017, 24, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ni, X.; Chen, L.; Zhang, H.; Ren, P.; Feng, Y.; Chen, Y.; Fu, S.; Wu, J. Honokiol-loaded polymeric nanoparticles: An active targeting drug delivery system for the treatment of nasopharyngeal carcinoma. Drug Deliv. 2017, 24, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Salehi Moghaddam, Z.; Hejabi, F.; Heidari, F.; Quazi, S.; Farasati Far, B. Niosomes: A novel targeted drug delivery system for cancer. Med. Oncol. 2022, 39, 240. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, S.; Parayath, N.; Leslie, F.; Nair, S.V.; Menon, D.; Amiji, M.M. Intraperitoneal chemotherapy for ovarian cancer using sustained-release implantable devices. Expert Opin. Drug Deliv. 2018, 15, 481–494. [Google Scholar] [CrossRef]
- Farooq, M.A.; Aquib, M.; Farooq, A.; Haleem Khan, D.; Joelle Maviah, M.B.; Sied Filli, M.; Kesse, S.; Boakye-Yiadom, K.O.; Mavlyanova, R.; Parveen, A. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: An overview. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Zhang, L.; Chae, B.-S.; Palla, C.S.; Furst, E.M.; Kiick, K.L. Growth factor mediated assembly of cell receptor-responsive hydrogels. J. Am. Chem. Soc. 2007, 129, 3040–3041. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef]
- Maspes, A.; Pizzetti, F.; Rossetti, A.; Makvandi, P.; Sitia, G.; Rossi, F. Advances in bio-based polymers for colorectal cancer treatment: Hydrogels and nanoplatforms. Gels 2021, 7, 6. [Google Scholar] [CrossRef]
- Sibuh, B.Z.; Gahtori, R.; Al-Dayan, N.; Pant, K.; Far, B.F.; Malik, A.A.; Gupta, A.K.; Sadhu, S.; Dohare, S.; Gupta, P.K. Emerging trends in immunotoxin targeting cancer stem cells. Toxicol. Vitr. 2022, 83, 105417. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Anwar, J.; Khan, S. Synthesis and characterization of biodegradable hydrogels for oral delivery of 5-fluorouracil targeted to colon: Screening with preliminary in vivo studies. Adv. Polym. Technol. 2018, 37, 221–229. [Google Scholar] [CrossRef]
- Wu, Q.; He, Z.; Wang, X.; Zhang, Q.; Wei, Q.; Ma, S.; Ma, C.; Li, J.; Wang, Q. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Klotz, B.J.; Lindberg, G.C.; Melchels, F.P.; Hooper, G.J.; Malda, J.; Gawlitta, D.; Woodfield, T.B. Visible light cross-linking of gelatin hydrogels offers an enhanced cell microenvironment with improved light penetration depth. Macromol. Biosci. 2019, 19, 1900098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).