Abstract
With an expected incidence of more than 1 million cases by 2025, liver cancer remains a problem for world health. With over 90% of cases, hepatocellular carcinoma (HCC) is the most prevalent kind of liver cancer. In this review, we presented the range of experimental therapeutics for patients with advanced HCC, the successes and failures of new treatments, areas for future development, the evaluation of dose-limiting toxicity in different drugs, and the safety profile in patients with liver dysfunction related to the underlying chronic liver disease. In addition to the unmet demand for biomarkers to guide treatment decisions and the burgeoning fields of immunotherapy and systemic therapy in hepatocellular carcinoma, the development of old and new drugs, including their failures and current advancements, has been reviewed. This review aims to evaluate the updated optimal clinical treatment of unresectable hepatocellular carcinomas in clinical practice, mainly through targeted therapy. Although surgical treatment can significantly enhance the survival probability of early and intermediate-stage patients, it is unsuitable for most HCC patients due to a lack of donors. Due to their severe toxicity, the few first-line anti-HCC drugs, such as sorafenib, are often reserved for advanced HCC patients for whom other therapies have failed. The second-line drugs are usually alternatives for patients with intolerance or resistance. Consequently, the ongoing growth of possible preclinical drugs and studies on miRNAs, lncRNAs, and numerous other signaling pathway targets for developing novel drugs may introduce additional treatment prospects for HCC.
1. Introduction
Following the most recent guidelines of the European Association for the Study of Liver/European Institute for Cancer Research and Treatment research organization [1], liver cancer is the sixth most frequent cancer and the third leading cause of cancer-related mortality [2]. Among the most frequently occurring forms of primary liver cancer, hepatocellular carcinoma (HCC) is responsible for more than 90% of the global health burden due to liver cancer [3]. Males appear to be more susceptible to HCC than females [4]. Hepatocellular carcinoma is most frequently reported in people with chronic liver disorders, such as cirrhosis caused by hepatitis B or C infection or a genetic disorder (e.g., hemochromatosis) [5]. Additionally, HCC is more prevalent in those who use a considerable amount of alcohol and have a fatty liver [6]. Although multifocality and vascular invasion are prevalent, HCC tends to stay inside the liver [7]. In comparison to liver metastases developing in a noncirrhotic liver, the common existence of underlying liver disease enhances the risks associated with all HCC therapy [8].
Over the last decade, the management of HCC has improved considerably due to an increased understanding of the natural history, advancements in staging methods and treatment algorithms, as well as the development of therapeutic options. Despite advances in treatment, liver cancer remains one of the most challenging types of cancer to cure [9]. Surgery, local destructive therapy, and liver transplantation all have the potential to cure individuals with early HCC. Additionally, conventional systemic chemotherapy has limited effectiveness and provides few survival advantages [10].
Generally, HCC treatment has been classified as curative or palliative. Curative procedures such as percutaneous ablation, liver transplantation, and resection all result in complete remission in many cases and are likely to enhance survival [11]. While palliative treatments are not intended to cure, they can enhance response rates and even survival in some patients [12]. When treating patients with liver cancer, it is common to use a combination of multidisciplinary approaches that take into account the patient’s specific condition, including the severity of their liver disease and overall health. Liver cancers are treated differently in different specialties and in different parts of the world [13]. Additionally, most people with HCC have an incurable illness. Traditionally, these individuals have had few therapy choices because HCC displays resistance to currently available systemic treatments [14]. This investigation aimed to summarize the many classes of treatments that have been shown to be beneficial in the treatment of unresectable hepatocellular carcinoma.
2. HCC Stages and Treatment
HCC therapy options include resection and liver transplantation (LT), both surgical procedures. Stereoscopic body radiotherapy (SBRT) and other non-catheter-based treatments, including chemoembolization and radioembolization [15], make medical treatment of HCC difficult due to the disease’s pathophysiologic complexity. Maintaining liver function while providing adequate tumor therapy has been challenging [16]. Generally, patients with HCC are treated according to their BCLC clinical stage (Figure 1) [17]. The 5-year survival rates for various curative methods such as surgical excision, orthotopic liver transplantation, and local ablation] are generally between 50 and 70% [18]. As a consequence of the operations’ adverse effects on liver activity and the consequent scarcity of organ donors, they are rarely employed as first-choice therapies, as reported by Lin et al. “for small HCC (tumor number ≤ 3, maximum diameter of each ≤3 cm) or early-stage HCC (single tumor ≤ 5 cm in diameter, or tumor number ≤ 3, the maximum diameter of each ≤3 cm)”. As represented in Figure 1, local ablation is now advised for patients with early-stage HCC that is unresectable because of co-morbidities, the patient’s wish to maintain liver function, or the patient’s reluctance to undergo surgical therapy [19].
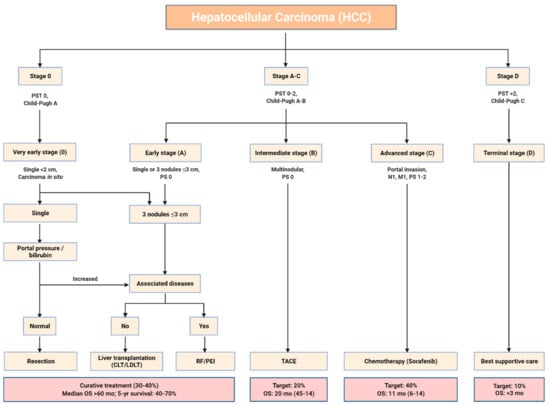
Figure 1.
Barcelona Clinic Liver Cancer (BCLC) staging classification. Abbreviation: PST: performance status test; PS: performance status; CLT: cadaveric liver transplantation; LDLT: living donor liver transplantation; RF: radio-frequency ablation; PEI: percutaneous ethanol injection; TACE: trans-arterial chemoembolization; OS: overall survival.
3. Targeted Therapy and Recent Drug Treatments
Chemotherapy is mainly reserved for cases not eligible for curative therapy for hepatocellular carcinoma such as resection, transplantation, or ablation, and so serves mainly as a palliative measure [20]. Numerous chemotherapeutic drugs have been assessed in the treatment of HCC. Few are consistently linked to antitumor responses [21]. Chemotherapy can be given systemically or locally. Regional chemotherapy may also comprise intra-arterial treatment, which has a similar effect to chemoembolization. HCC is typically linked with cirrhosis, which limits the dose and response rate of systemic chemotherapy (generally fewer than 25% of objective responses) [22]. Antiangiogenic medicines have significant potential for treating HCC, owing to the tumor’s vascularity [23].
Chemotherapy has not been frequently utilized in patients with advanced HCC since HCC has generally been regarded as a chemotherapy-resistant malignancy, and systemic chemotherapy is typically not tolerated well in patients with significant underlying liver disease [24]. Chemotherapy might still be indicated in some patients, especially those with an underlying non-cirrhotic liver. The Eastern Cooperative Oncology Group Performance Status Scale recommends systemic treatment for progressed stage (C) HCC with portal hypertension, extrahepatic dissemination, and intact liver activity [24]. Cases involving advanced-stage (C) patients with HCC who are ineligible for TACE therapy may be a better match for this approach. For the first time, targeted therapy and immunotherapy have emerged as therapeutic options for all stages of the disease as shown in Figure 2 [25]. Current FDA-approved agents for targeted therapy in advanced HCC are summarized in Table 1.

Figure 2.
Agents used in targeted therapy of HCC and their different pathways. Abbreviations: VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor; PDGF: platelet-derived growth factor; PDGFR: platelet-derived growth factor receptor; FGF: fibroblast growth factor; FGFR: fibroblast growth factor receptors; Tie-2: an angiopoietin receptor; FL: Fms-like tyrosine kinase 3 ligand; FLT3: Fms-like tyrosine kinase 3; SCF: stem cell factor; HGF: hepatocyte growth factor; c-Met: mesenchymal-epithelial transition factor; GDNF: glial cell-derived neurotrophic factor; JAK: Janus kinases; STAT: signal transducer and activator of transcription proteins; Ras: rat sarcoma virus; Raf: rapidly accelerated fibrosarcoma; MEK: mitogen-activated protein kinase; MAPK: mitogen activated protein kinases; PI3K: phosphoinositide 3-kinases; AKT: protein kinase B; mTOR: mammalian target of rapamycin; PLCγ: phospholipase C γ; DAG: diacylglycerol; PKC: protein kinase C.

Table 1.
Selected first-line and second-line FDA-approved agents for targeted therapies in advanced HCC.
3.1. First Line
3.1.1. Sorafenib
Sorafenib, an orally administered multikinase inhibitor, is the first systemic drug demonstrated to prolong survival in advanced HCC [37,38]. It delays disease progression by influencing two key signaling pathways. By inhibiting molecular components of the Raf, MEK, and ERK signaling pathways, it leads to diminished tumor growth, and by suppressing VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR-β, it stops neovascularization [39]. Sorafenib monotherapy extended overall survival (OS) and delayed the time to progression (TTP) in patients with HCC, according to the results of two well-designed, randomized, double-blind, placebo-controlled, and multinational phase III trials. The findings of the SHARP study, which was conducted in Europe, America, Australia, and New Zealand and included a few Asian patients, were subsequently confirmed by the Asia-Pacific study results. Both trials showed no significant difference between sorafenib and placebo in the time to symptomatic progression (TTSP). The disease control rate was significantly higher with sorafenib versus placebo in both studies. No complete responses were seen in either trial, and partial response rates were very low. Overall, both studies indicated that sorafenib was effective in prolonging 3 months of median overall survival in patients with late-stage HCC [37,38]. GIDEON was a large, prospective, observational study conducted in 39 countries among 2708 HCC patients that revealed regional variations in the management of HCC and in patient outcomes. The study showed that the safety profile of sorafenib was consistent across patients with preserved liver function and those in whom the liver was not functioning properly, suggesting that sorafenib may be a valid treatment for some patients with liver impairment [40,41,42]. The STELLA and SOFIA studies conducted in Italy and the INSIGHT studies conducted in Germany and Austria were prospective, multicenter, and non-interventional studies that demonstrated the efficacy of sorafenib in HCC patients in real-world settings [43,44,45]. Sorafenib was generally well tolerated in patients with aHCC, with a manageable adverse effect profile. The most common sorafenib treatment-related adverse events were diarrhea, hand-foot skin reactions, hypertension, anorexia, alopecia, weight loss, dry skin, and abdominal pain [38]. However, drug resistance limits the therapeutic effect of sorafenib, so that only about 30% of HCC patients acquired benefits from sorafenib and the development of resistance within 6 months occurred in HCC patients. The acquisition of resistance to sorafenib is complex, and the contributing mechanism is still unknown [46]. The limited therapeutic impact of sorafenib and the complex molecular pathophysiology of HCC have made it necessary to conduct novel research projects on sorafenib combinations with other molecular targeting drugs. Sorafenib has been coupled with antiangiogenic drugs, MEK/ERK pathway inhibitors, EGF/EGFR pathway inhibitors, and inhibitors of the HGF/c-Met pathway [47]. Overall, sorafenib prolongs overall survival by approximately 3 months in patients with aHCC and remains one of the best first-line treatment options with an acceptable tolerability and safety profile.
3.1.2. Sunitinib
Sunitinib is an orally administered multitargeted tyrosine kinase inhibitor with anticancer and antiangiogenic activity toward VEGFRs, PDGFRs, and several other related tyrosine kinases [48]. Only one phase III trial (SUN1170) evaluated the drug’s efficacy as a first-line medication for HCC; however, it was terminated because of side effects. In any case, sunitinib appears to be less effective than sorafenib in terms of overall survival (7.9 vs. 10.2 months, p = 0.0014). Based on existing evidence, sunitinib is not a feasible treatment option as a replacement for sorafenib [49]. Comparisons between sunitinib and sorafenib demonstrated that sunitinib had a significantly lower overall survival rate, despite no significant difference in progression-free survival.
3.1.3. Brivanib
Brivanib is a synthetic drug that inhibits both VEGFR and FGFR tyrosine kinase activity [50]. In the BRISK-FL study, the drug was compared to sorafenib as a first-line treatment, and subsequently to a placebo as a second-line treatment for patients unable to tolerate or respond to sorafenib in the BRISK-PS trial. However, despite similar outcomes in terms of overall survival (time to progression), objective response rate (ORR), and disease control (DC), the intended non-inferiority criterion for overall survival was not met [51].
Overall survival did not differ significantly between brivanib and sorafenib (9.5 vs. 9.9 months) in the BRISK-FL study and with placebo (9.4 vs. 8.2 months) in the BRISK-PS study. Although currently we cannot consider brivanib as a deserving alternative to sorafenib, better tumor progression times resulting from the BRISK-PS trial and the absence of cross-tolerance with sorafenib have made brivanib an appropriate option for further trials [52].
3.1.4. Linifanib
Linifanib is a newly developed ATP-competitive suppressor of all VEGF and PDGF RTKs with no effect on cytosolic TKs or serine/threonine kinases. Due to the high rates of vascularization caused by VEGF overexpression, angiogenic TKIs are thought to be a deserving option in aHCC therapy [53]. Prior preclinical studies showed some anti-proliferative and pro-apoptotic effects of linifanib against tumor cells by suppressing TKRs such as FLT-3. In an open-label, phase II trial, linifanib monotherapy in patients with advanced HCC resulted in a median TTP of 5.4 months and a median OS of 9.7 months among the trial participants, with 89% of Asian ethnicity, which compared favorably with the corresponding results for patients in the phase III sorafenib study of the Asia-Pacific region [37,54]. In a large open-label, randomized Phase III trial (LiGHT) among 1035 patients with advanced or metastatic HCC, results indicated that linifanib was associated with longer TTP (5.4 vs. 4.0 months), higher ORR (13.0% vs. 6.9%), and more frequent adverse events (54% vs. 38%), including grades 3 and 4 hypertension, encephalopathy, ascites, and hyperbilirubinemia. The median OS was 9.1 months for linifanib (95% CI, 8.1 to 10.2) and 9.8 months for sorafenib (95% CI, 8.3 to 11.0), with no significant difference [55]. Despite similar overall survival and a statistically significant increase in time to progression in the linifanib arm compared to the sorafenib arm, the pre-defined non-inferiority margin for overall survival was not exceeded. Overall, linifanib cannot be considered the best treatment option due to the issues it faces in terms of efficacy and toxicity.
3.1.5. Lenvatinib
One of the most frequently prescribed drugs in the world, lenvatinib is an orally active inhibitor of multiple receptors of the tyrosine kinase, including VEGFR 1–3, FGFR 1,2–4, PDGFRα [56], and KIT. Lenvatinib was recently approved as a first-choice therapy for non-excisable HCC (August 2018) [57].
For more than a decade, sorafenib was the only effective first-line therapy available until Lenvatinib was recently demonstrated to be comparable to sorafenib in terms of overall survival [28]. For advanced HCC patients, the drug demonstrated promising anticancer activity, with a response rate of 23.9%, a median progression time of 9.4 months, and a survival time of 18.3 months in Phase II studies [58]. A worldwide Phase III trial (REFLECT) comparing lenvatinib with sorafenib as a first-line treatment was performed as a non-inferiority study based on the outcomes of this Phase II trial [28]. Among 954 participants, lenvatinib users lived an average of 13.6 months longer, in comparison with 12.3 months for sorafenib users. Overall response rate and progression-free survival were markedly better in the lenvatinib group.
A higher incidence of hypertension, proteinuria, and hepatic encephalopathy was reported in the lenvatinib group. According to the trial’s results, sorafenib was shown to be non-inferior but not greater than lenvatinib when it came to overall survival. Lenvatinib cannot yet completely replace sorafenib as a standard of therapy, despite both medications being deemed standard of care [59]. Some oncologists may choose sorafenib due to the lesser side effects, while others may pick lenvatinib owing to the antitumor activity or cost-effectiveness. Lenvatinib 12 mg/day costs less than half as much as sorafenib 800 mg/day based on the results of a trial conducted in Japan, since the suggested dosage of lenvatinib (12 mg/day) for patients with advanced HCC is less than half that indicated for cases having other sorts of malignancy, such as thyroid cancer (24 mg/day) [4]. Administering the most often employed drug in first-line HCC therapy will depend on market conditions following lenvatinib authorization. Lenvatinib was not tested in patients with significant portal vein thrombosis, bile duct invasion, or liver involvement of more than 50% [60]. Further research on lenvatinib’s effectiveness in combination with other classes of drugs is required. Lenvatinib, an FDA-approved therapy option for HCC, has similar adverse effects as sorafenib, and thus the choice of drug may be based on cost effectiveness for the patient.
3.1.6. Donafenib
Donafenib, a derivative of sorafenib, is a novel multikinase inhibitor of multiple receptor kinases, including VEGFR, PDGFR, and Raf kinases, leading to suppressed tumoral growth and angiogenesis. Donafenib has been demonstrated to be efficient and safe in some preclinical phase Ia and Ib trials [61,62]. In an open-label, randomized phase II/III trial [63] among the donafenib and sorafenib groups, results showed a significant increase in overall survival (12.1 and 10.3 months, respectively). Although the median time to progression (3.7 months vs. 3.6 months), the objective response rate (4.6% vs. 2.7%), and the disease control rate (30.8% vs. 28.7%) were not significantly different. Donafenib demonstrated better safety and tolerability compared to sorafenib; drug-related AEs of grade ≥ 3 were experienced by 37.5% of patients in the donafenib group, versus 49.7% in the sorafenib group, and the incidence of treatment interruptions caused by drug-related AEs was 30.3% in the donafenib group, compared to 42.5% in the sorafenib group. Overall, donafenib improves OS significantly compared to sorafenib with a better safety and tolerability profile. Therefore, donafenib has the potential to be an effective first-line treatment for advanced HCC; however, further research through larger scale trials is necessary to accurately assess its efficacy and safety.
3.1.7. Atezolizumab + Bevacizumab
An immune checkpoint inhibitor, atezolizumab, aims for programmed cell death ligand 1 (PD-L1), while bevacizumab is a vascular endothelial growth factor (VEGF) monoclonal antibody. There is emerging evidence to support the application of these two drugs together for the treatment of metastatic HCC [64].
For healthy individuals with no worse than Child–Turcotte–Pugh class A cirrhosis (Table 1), an excellent performance status, no contraindications to bevacizumab, and no post-liver transplantation relapse, atezolizumab plus bevacizumab combination therapy is suggested rather than sorafenib monotherapy. This recommendation is consistent with the 2020 guideline from ASCO [65], the 2021 guidelines from the Society for Immunotherapy of Cancer [66], and a position paper from the European Association for the Study of the Liver [22].
Combination therapy was directly compared to sorafenib monotherapy in an open-label phase III trial called IMBrave150 among 501 previously untreated patients with advanced, unresectable HCC who did not have worse than Child–Turcotte–Pugh class A cirrhosis. Atezolizumab (1200 mg intravenous (IV) every three weeks) plus bevacizumab (15 mg/kg IV every three weeks after Atezolizumab) were administered to 336 patients, and sorafenib (400 mg orally twice daily) to 165 patients [29]. IMbrave150 research indicated that atezolizumab plus bevacizumab outperformed sorafenib with a median OS of 19.2 vs. 13.4 months and an ORR of 29.8% vs. 11.3%, respectively, with a higher rate of complete responses in the combination therapy group (CR = 7.7%). This combination regimen has demonstrated the longest overall survival rate ever seen in first-line phase III studies, further affirming its potential to become the standard of care for patients with advanced HCC who have not received prior systemic therapy. In the case of adverse effects, a similar number of patients in both groups (57 vs. 55 percent) experienced grade 3 or 4 side effects, showing a tolerable safety profile. Although combination therapy was associated with higher rates of hypertension, pyrexia, alanine transaminase elevation, and proteinuria. The FDA has authorized atezolizumab plus bevacizumab for unresectable or advanced metastatic HCC in patients who have not previously completed systemic therapy in light of these findings [67].
3.1.8. Sintilimab + Bevacizumab
Sintilimab is an IgG4 monoclonal antibody that boosts T-cell anticancer activity by binding to programmed cell death receptor-1 (PD-1) and suppressing the interaction of PD-1 with its ligands (PD-L1 and PL-L2). Sintilimab was found to outperform sorafenib in the Chinese ORIENT-32 study in combination with IBI305 (bevacizumab biosimilar) [68]. ORIENT-32 was a randomized, open-label, phase II–III trial conducted at 50 Chinese clinical locations among 595 patients with unresectable HCC. Combination therapy showed a significantly longer overall survival than did sorafenib (median not reached) vs. 10·4 months. In phase II of the study 7 (29%) of the patients experienced grade 3 or worse adverse events during treatment. In phase III, the sintilimab–bevacizumab biosimilar group showed a significantly longer median PFS (4·6 months) than the sorafenib group (2·8 months). The most common grade 3–4 treatment-emergent adverse events in phase III were hypertension and hand-foot syndrome [69]. Overall, sintilimab plus IBI305 significantly improved overall survival and PFS versus sorafenib in patients with unresectable, HBV-associated HCC with a tolerable safety level. This combination therapy regimen could be an effective option for such patients [68].
3.1.9. Cediranib
Cediranib is an orally administered suppressor of RTKs that specially affects vascular endothelial growth factor-A (VEGF-A or VEGF), and it was developed for the purpose of suppressing tumor growth, neovascularization, and metastasis [70]. A phase II trial was conducted in 2006 among 28 patients with unresectable or metastatic HCC. Patients received 45 mg of cediranib orally, once daily, for 28-day cycles. All 28 patients were evaluable for efficacy outcomes. Twelve patients (42.9%) survived 6 months, 15 (53.6%) died within 6 months, and one (3.6%) was lost to follow-up before 6 months. The median OS was 5.8 months (95% CI: 3.4–7.3 months). No partial or complete response was observed. The median TTP was 2.8 months (95% CI: 2.3–4.4 months). Twenty-six patients (93%) experienced a grade 3+ adverse event (AE), with the most common AEs being fatigue (46%), anorexia (25%), hypertension (21%), and elevated alanine aminotransferase (ALT) (18%). This phase II trial showed stable disease in 25% of the patients treated. However, with respect to the lower tolerance and rate of response compared to sorafenib, further development of cediranib with the dose and schedule used in this trial was not justifiable [71]. In the SHARP trial, the use of sorafenib resulted in a 71% rate of stable disease [37]. Another phase II trial was conducted in 2009 among 17 patients with advanced HCC, and sufficient hematologic, hepatic, and renal functions were observed in those who received cediranib 30 mg/d (4 weeks/cycle). With a median follow-up time of 17 months, the median PFS of this cohort was 5.3 months [95% CI: 3.5–9.7 months], and the median OS was 11.7 months [95% CI: 7.5–13.6 months]. The estimated three-month PFS rate was 77% [95% CI: 60–99%] [71]. However, in the previous phase II study in advanced HCC, the use of cediranib at 45 mg daily led to toxicity in 93% of the patients, including grade 3 or above adverse events, including fatigue (46%), anorexia (25%), and hypertension (21%) [70]. In this study, a different tolerability profile was observed. Grade 3 events of fatigue and anorexia were low (5% and 0%, respectively). A high incidence of grade 3 toxicities, including hypertension (29%), hyponatremia (29%), and hyperbilirubinemia (18%), was observed. Only modest evidence of antitumor activity (disease stabilization) was found in this small cohort of 17 HCC patients treated with cediranib [71]. The median PFS (5.3 months) and OS (11.7 months) in this group of patients compared favorably to data reported with 45 mg/d dosing of cediranib in advanced HCC (TTP of 2.8 months and OS of 5.8 months). Overall, cediranib at either a 30 mg or 45 mg daily dosage showed a high incidence of toxicity and preliminary evidence of antitumor activity in advanced HCC and cannot be considered an appropriate option for HCC therapy.
3.1.10. Nintedanib
Nintedanib is an orally administered triple angiokinase inhibitor of VEGFR1-3, PDGFRα and β, FGFR1-3, Flt-3, Lck, Lyn, and Src, with anti-tumor and anti-angiogenic activity in preclinical models of HCC [56]. In a phase-II trial, 93 patients were randomized in a 2:1 ratio to receive nintedanib 200 mg bid (n = 62) or sorafenib 400 mg bid (n = 31) continuously in 28-day cycles, until intolerable AEs or disease progression (PD) [72]. Median TTP was 5.5 vs. 4.6 months, median OS was 11.9 vs. 11.4 months, and median PFS was 5.3 vs. 3.9 months, respectively. Dose intensity and tolerability favored nintedanib. Fewer patients on nintedanib (87.1%) vs. sorafenib (96.8%) had drug-related adverse events (AEs) or grade ≥ 3 AEs (67.7% vs. 90.3%), but more patients on nintedanib (28 (45.2%)) had AEs leading to drug discontinuation compared to those on sorafenib (7 (22.6%)). Approximately a quarter of patients on nintedanib experienced GI AEs of grade 3 or higher. Nausea (48.4% vs. 29.0%), vomiting (38.7% vs. 29.0%), and upper abdominal pain (25.8% vs. 12.9%) occurred >10% more frequently with nintedanib compared with sorafenib. Hand-foot syndrome (35.5% vs. 1.6%), alopecia (35.5% vs. 4.8%) and rash (22.6% vs. 9.7%) were more frequent with sorafenib compared with nintedanib. Overall, nintedanib may have similar efficacy to sorafenib in an HCC with a tolerable and different safety profile but with higher VEGF-related toxicity. The results suggest that nintedanib could be a suitable option for combination studies in HCC.
3.1.11. Dovitinib
Dovitinib, a strong inhibitor of FGFRs, VEGFRs, and PDGFR, exhibits anticancer efficacy through antiproliferative and antiangiogenic mechanisms [73]. A phase II randomized trial of Asian-Pacific patients with advanced HCC comparing the clinical activity of dovitinib (500 mg/day, 5 days on, 2 days off; n = 82) vs. sorafenib (400 mg twice daily; n = 83) showed comparable OS and median time to progression. The median overall survival (mOS) was 8.0 months for dovitinib vs. 8.4 months for sorafenib, and the median time to advance on investigator assessment was 4.1 months for dovitinib vs. 4.1 months for sorafenib. Common adverse events included diarrhea (62%), decreased appetite (43%), nausea (41%), vomiting (41%), fatigue (35%), rash (34%), and pyrexia (30%) for dovitinib; hand-foot syndrome (66%) and decreased appetite (31%) for sorafenib [74,75]. Another phase II trial among 24 patients with early and intermediate-stage HCC who received neoadjuvant oral dovitinib 500 mg daily (5 days on/2 days off) for 4 weeks after locoregional therapy showed a decrease in intratumoral blood flow and a mild anticancer response. The most frequent grade 3–4 adverse events that emerged in 88% of patients were hypertension (54%), fatigue (25%), and thrombocytopenia (21%) [76]. Overall, dovitinib may not be a vigorous rival for sorafenib as the frontline systemic drug used for HCC treatment, but it might be used as a systemic neoadjuvant therapy after dose adjustments due to poor tolerability.
3.1.12. Everolimus
Everolimus is an orally administered mTOR inhibitor [77]. The mammalian target of rapamycin (mTOR), which is located in the downstream of the PI3K AKT pathway, is important in angiogenesis, cell cycle progression, and proliferation of hepatic tumor cells. Activation of the mTOR pathway is observed in various solid cancers, including 30–40% of HCC [78]. EVOLVE-1 was a randomized, double-blind, phase III study conducted among 546 adults from 17 countries with BCLC stage B or C HCC whose disease progressed during or after sorafenib or who were intolerant of sorafenib. A total of 362 patients were randomized to the 7.5 mg/d everolimus group and 184 patients to the placebo group. No significant difference in OS was seen between treatment groups, with 303 deaths (83.7%) in the everolimus group and 151 deaths (82.1%) in the placebo group (HR = 1.05; 95% CI, 0.86–1.27; p = 0.68; median OS, 7.6 months with everolimus, 7.3 months with placebo). The median TTP with everolimus and placebo was 3.0 months and 2.6 months, respectively (HR, 0.93; 95% CI, 0.75–1.15), and the disease control rate was 56.1% and 45.1%, respectively (p = 0.01). The most common grade 3/4 adverse events for everolimus vs. placebo were anemia (7.8% vs. 3.3%, respectively), asthenia (7.8% vs. 5.5%, respectively), and decreased appetite (6.1% vs. 0.5%, respectively) [79]. In the SAKK77/08 and SASL29 trials among 106 patients with unresectable or metastatic HCC, comparing the efficacy of (800 mg/d sorafenib) monotherapy against (800 mg/d sorafenib + 5 mg/d everolimus) combination therapy, no evidence was found that combination therapy improves efficacy compared to monotherapy. Even so, it can be more toxic [80]. In another single-arm phase I/II study among 25 patients with advanced HCC, 10 mg/day of everolimus was administered, and some preliminary antitumor activity was observed [81]. Grade 3–4 adverse events included lymphopenia (n = 3), aspartate transaminase (n = 3), hyponatremia (n = 2), and 1 patient each with anemia, alanine transaminase, hyperglycemia, proteinuria, rash, and hypoxia. One patient (4%) had a partial response (95% confidence interval [CI], 0.9–19.6%). The median PFS and overall survival were 3.8 months (95% CI, 2.1–4.6) and 8.4 months (95% CI, 3.9–21.1), respectively. The estimated PFS rate at 24 weeks was 28.6% (95% CI, 7.9–49.3%). Overall, everolimus is not able to improve OS in patients with advanced HCC, and it cannot be considered a first-line treatment for such patients, but it can delay tumor progression and can be expected as a second-line therapy in HCC resistant to sorafenib.
3.1.13. Tislelizumab
The programmed cell death-1/programmed cell death ligand-1 (PD-1/PD-L1) axis plays a central role in suppressing antitumor immunity, and axis dysregulation can be used by cancer cells to evade the immune system [82]. Tislelizumab, an investigational humanized IgG4 monoclonal antibody with high affinity and binding specificity for PD-1, was engineered to minimize binding to FcγR on macrophages to limit antibody-dependent phagocytosis, a potential mechanism of resistance to anti-PD-1 therapies [83]. A preliminary report of the HCC cohort consisted of 50 previously treated HCC patients that were treated with tislelizumab 5 mg/kg every 3 weeks, and the most common treatment-emergent AEs were decreased appetite, rash, decreased weight, and cough. Overall, the safety profile of tislelizumab included mostly mild-to-moderate AEs. This preliminary safety profile and antitumor activity support the continued development of tislelizumab as a single agent or in combination with other agents in patients with unresectable HCC [84]. RATIONALE 301 was a worldwide Phase III open-label, randomized, multicenter trial comparing the effectiveness and safety of tislelizumab as first-line treatment compared to sorafenib in 674 patients with unresectable HCC who had no history of prior systemic therapy. Tislelizumab monotherapy demonstrated clinically meaningful and noninferior overall survival (mOS: 15.9 vs. 14.1 months, respectively), a higher ORR (14.3% vs. 5.4%), more durable responses (mDoR: 36.1 vs. 11.0 months), and fewer grade ≥ 3 adverse effects compared to sorafenib in patients with unresectable HCC. Overall, tislelizumab showed a noteworthy therapeutic advantage over sorafenib with a tolerable and manageable safety profile as a first-line treatment for patients with unresectable HCC [85].
3.1.14. Regorafenib
Regorafenib is a multitargeted TKI derived from sorafenib, and its molecular structure is modified by an added fluorine atom in the central phenyl ring of sorafenib. This great change makes Regorafenib stronger in terms of its inhibitory profile and pharmacological activity. Regorafenib influences a variety of tyrosine kinases involved in angiogenesis and tumor growth [86,87,88]. As a second choice of medication following progression despite using sorafenib, regorafenib can be used instead of nivolumab/ipilimumab or pembrolizumab monotherapy. Among 36 cases having advanced hepatocellular carcinoma (HCC), sorafenib was shown to be safe and effective (72.2 percent disease control rate, median time to progression, and median survival of 13.8 months) [89]. Because of this, a global Phase III trial (RESORCE) comparing regorafenib to placebo was conducted in patients with advanced HCC who developed illness while receiving sorafenib treatment or shortly afterward [32]. Sorafenib tolerance was defined as using 400 mg/day or more of sorafenib for at least 20 of the previous 28 days of therapy and being in Child-Pugh class A. A total of 573 individuals were randomized and assigned to receive regorafenib or a placebo in a 2:1 ratio. Once a day, one 160 mg dose was given for four weeks, with a one-week break in between the third and fourth weeks. The most critical metric was the capacity to survive. Those who received regorafenib had a median survival time of 10.6 months, while those who received a placebo had a median survival time of 7.8 months, a statistically significant difference (hazard ratio, 0.62; p < 0.001). Patients in the regorafenib category had a remarkable median survival duration of 26 months after commencing sorafenib [90]. Overall response rate, duration of progression, and progression-free survival were all significantly different between the two therapy groups. Adverse effects were all similar to the toxicity record of regorafenib, including hand–foot syndrome, weariness, high blood pressure, liver dysfunction, and hypophosphatemia [91]. As a result of these findings, in April 2017, the FDA extended the eligibility for regorafenib to cover patients previously treated with sorafenib who have been diagnosed with HCC. Regorafenib trials are reasonable for patients who have progressed after receiving first-line sorafenib, who have good performance status and adequate liver function, and who are willing to accept treatment-related morbidity in exchange for the possibility of a small increase in median overall survival, despite the fact that the proper patient classification for second-line regorafenib has not yet been established [32]. Overall, regorafenib is the only systemic treatment shown to provide a survival benefit in HCC patients progressing on sorafenib treatment.
3.2. Second Line
Patients whose malignancies develop during the first-line therapy and who have adequate liver function may take advantage of second-line therapy. The best regimen is not established, and it depends on what was administered as first-line [18,92].
3.2.1. Immune Checkpoint Inhibitors
The preferred option for second-line medication in cases treated with sorafenib that have not yet undergone liver transplantation is immunotherapy with either nivolumab plus ipilimumab or pembrolizumab monotherapy. Immunotherapy approaches should be avoided in patients who recur after orthotopic liver transplantation due to high rates of allograft rejection [93].
Tumor cells’ ability to resist immune impairment has been illustrated as a hallmark of the disease. HCC has been demonstrated to be immunogenic, and resistant therapy techniques have been developed to target tumor cells specifically by activating or enhancing the pre-existing tumor-specific immune response. Ipilimumab, an anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody, has been recognized for the treatment of metastatic melanoma with hopeful outcomes [94]. PD-1, a co-inhibitory receptor protein produced on initiated T and B cells, is essential for the modulation of peripheral immunological tolerance. One of the most important mechanisms of tumor microenvironment immunosuppression is the interaction between PD-1 and its ligands, B7H1 and B7-DC, known as the programmed death-ligands 1 and 2 (PD-L1 and PD-L2) (Figure 3). Cancer aggressiveness and postoperative recurrence have been linked to PD-L1 overexpression in individuals with hepatocellular carcinoma (HCC). Predictors of disease progression and post-operative relapse in HCC patients were found to have elevated PD-1 CD8 T cells, according to the findings by Shi et al. [95]. PD-1 antagonist antibodies, such as nivolumab, pembrolizumab, and pidilizumab, have been developed and are now being tested in clinical studies for various cancers. Nivolumab is one of the anti-PD-1 antibodies now being studied in a phase I trial for patients with advanced HCC, whether or not they have viral hepatitis, and it is an entirely human monoclonal IgG4 antibody that binds PD-1 with high affinity and prevents the binding with B7-H1 and B7-DC [96]. Tumor-associated antigens (TAAs) identified by cytotoxic T lymphocytes (CTLs) have also been used to increase host protection against HCC in other immunotherapeutic approaches. These include cyclophilin B, SART2, SART3, AFP, human telomerase reverse transcriptase (hTERT), glypican-3 (GPC3), NYESO-1, and SSX-2, as well as MAGE-A, which is a melanoma antigen gene A (MAGE-A) [97]. HCC patients in early clinical trials have been given immunotherapy using autologous dendritic cells that have been pre-loaded with specific TAA (tumor vaccines) [98,99].
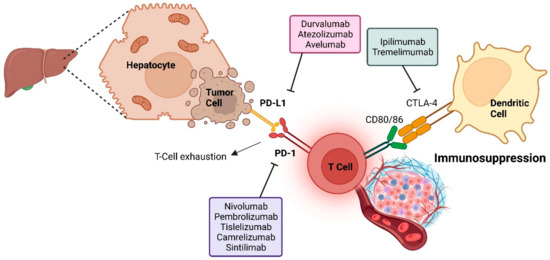
Figure 3.
Immune checkpoint inhibitors mechanism of action against HCC tumor cells.
One method of anticancer therapy is to alter the patient’s immune system so it recognizes specific antigens on cancer cells. In contrast, other techniques involve strengthening immune activity by restricting immunosuppressive signaling checkpoints, cancer vaccines that avoid infection or inflammation, and non-specific cancer immunotherapeutic medications that enhance the immune system [100]. As ramucirumab and bevacizumab, two monoclonal antibodies targeting VEGF receptor-2, are now being tested in combination therapy with various cancer treatments, clinical trials are now taking place [101]. The human immune system can target cancer cells that manufacture immune checkpoint blockers with the help of these inhibitors (Figure 4). Programed cell death protein 1 (PD-1) and programed cell death-ligand 1 (PD-L1) engage with cells to trigger these checkpoints. PD-1 is observed on T cells, B cells, Treg cells, natural killer cells, myeloid cells, monocytes, dendritic cells, antigen-presenting cells, myeloid-derived suppressor cells, and some tumor cells; PD-L1 is discovered on a wide range of immune and nonimmune cells, including B cells, T cells, dendritic cells, macrophages, antigen-presenting cells, tumor cells, epithelial, and endothelial cells. PD-1 and PD-L1 interaction raises tumor evasion from the immune system and reinforces cancer development. PD-L1 expressed by APCs and tumor cells binds to PD-1 on activated T cells and deteriorates their function, neutralizes them, and boosts IL-10 production in the tumor microenvironment [102]. By inhibiting T-cell activity and decreasing the generation of interferon, interleukin-2, and other cytokines owing to their interaction with PD-1 and PD-L1, a patient’s ability to generate an anticancer immune response is weakened. The outcome is a transient decrease in the body’s ability to fight off infection. Tumor aggressiveness and recurrence are connected to increased apoptosis of tumor-specific T cells when PDL1 is expressed. Thus, PD-L1 can be considered a biomarker. T-cell evasion is improved when a patient’s PD-L1 level is elevated. Patients with high levels of PD-L1 expression (PD-L1 positive) have a worse prognosis than those with low levels of PD-L1 expression (PD-L1 negative). The recurrence rate is twice as high for PD-L1 positive individuals, and the frequency of tumors with vascular invasion is much higher in these patients than in PD-L1 negative ones [103]. The mixture of anti-PD-1, anti-CXCR4, and sorafenib reduces metastasis and HCC development and shows that immunosuppression improves treatment results. Histocompatibility complex class C risk can be reduced by targeting PD-L1/PD-1 inhibition in hepatitis B and C infections. Antiviral therapy may prevent HCC relapses because viral infections are the primary cause of recurrence. An overactive immune system bypassing the PD-L1 and PD-L2 systems may lead to autoimmune disease, and it is necessary to look at the relationship between chronic hepatitis type C and autoimmune liver illnesses. Despite this danger, patients’ immune systems are strengthened and cancer cells are destroyed by checkpoint inhibitors, which show no indication of triggering autoimmune diseases [104]. Since then, the FDA has given the checkpoint inhibitor, known clinically as nivolumab, a thumbs up for treating many malignancies, including HCC. A CTLA-4 negative regulator, nivolumab, is being administered in conjunction with ipilimumab in a clinical study for HCC. In the context of past great clinical results, Ipilimumab was authorized by the FDA to treat melanoma, colorectal cancer, and renal cell carcinoma. In cancer treatment, monoclonal antibodies (mAbs) target particular tumor antigens to kill cancer cells. These synthetic antibodies must attach to the appropriate antigens to be more functional [105]. Few tumors can benefit from this strategy due to the wide range of cancer types. Antibody-antigen interactions differ with cancer type, even if they are optimized. Apoptosis of T cells, antitumor immunity, and cell proliferation can all be improved by creating mAbs that target the immune checkpoint regulator PD-1. In the battle against cancer, anti-PD-1 and anti-PD-L1 drugs help activate T cells and boost the immune system. Tumor cells cannot engage with immune checkpoint inhibitors such as nivolumab and ipilimumab, which empower the immune system to target them. Sorafenib is now being compared to various immune checkpoint inhibitors in clinical studies. Multidrug resistance can be reduced by targeting cancer stem-like cells/cancer-originating cells, which are resistant to cancer treatments and implicated in metastases and tumor relapse. This applies to a large number of HCC treatment strategies. It is possible to extend the effectiveness of existing therapies by killing HCC stem cells and animal models with Annexin A3-transfected dendritic cells. Virus-induced malignancies benefit from vaccinations. Vaccines against the hepatitis B and C viruses are recommended for those at high risk to prevent liver cancer. It is also possible that some viruses induce cancerous cell proliferation [106].

Figure 4.
Methods of treatment and the mechanisms through which they function. When upstream signaling molecules are genetically altered, the Ras/Raf, MEK, ERK, and PI3K/Akt/mTOR pathways can be activated. Abbreviations: HCC, hepatocellular carcinoma; VEGFR, vascular endothelial growth factor receptor 2; PDGFR, platelet-derived growth factor; FLT3, FMS-like tyrosine kinase 3; PD-L1, programmed death ligand 1; FGFR, fibroblast growth factor receptors; hepatocyte growth factor receptor (HGFR); Tie2, a tyrosine kinase receptor; PD-1, programmed cell death protein; MET, tyrosine-protein kinase met.
Nivolumab
Nivolumab is an IgG4 monoclonal antibody that boosts T-cell responses and improves antitumoral immunity by suppressing nivolumab, the programmed cell death protein 1 (PD1) immunological checkpoint [107]. In the phase I/II trial, the safety and effectiveness of nivolumab in cases suffering from HCC and/or chronic viral hepatitis were demonstrated [108]. Patients who had previously received sorafenib treatment were enrolled in this research and showed an overall survival rate of 15.6 months. Forty-eight participants in the dose-escalation portion of the study and 214 in the expansion cohort were included in the combined report. A maximum dose of 3 mg/kg of nivolumab was provided intravenously every two weeks for two years during the dose-escalation phase; however, the expansion cohort was not HBV-infected [109,110]. Patients receiving nivolumab experienced an ORR of 20% during the dosage-expansion phase and 15% during the dose-escalation phase. In the dose-escalation group, the median response period was 17 months (95% CI 6–24), and the median overall survival was 15 months. In the expansion cohort, the median period of response was 9.9 months, and the data were insufficiently mature for median survival; however, 74 percent of patients remained alive at nine months.
Immune-related side effects are the most prevalent adverse events linked with immune checkpoint inhibitors. Pneumonitis is a dangerous side effect that can be deadly. Enterocolitis and an increase in liver enzymes are also frequent adverse effects [111]. Hepatitis produced by the immunological system, hypothyroidism, and hypophysitis are unusual side effects [112]. The FDA granted nivolumab expedited approval in September 2017 for sorafenib-pretreated patients with HCC according to the outcomes of the CheckMate-040 phase I/II dosage escalation and expansion cohort [113]. However, this approval was removed in 2021 because of the negative findings of the phase III trial directly comparing nivolumab versus sorafenib as the choice of medication. Phase III studies, on the other hand, have failed so far to yield results that are statistically significant [114].
Nivolumab + Ipilimumab
Ipilimumab is an inhibitor of a different immune checkpoint (cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)), and mixed therapy with nivolumab effectively targets two various immune checkpoints that restrain the adaptive immune response [115]. CheckMate040 is a phase 1/2 randomized clinical study that is multicenter, open-label, and multicohort. Patients were assigned to one of three dosage arms in a 1:1:1 ratio. Every three weeks, arm A received nivolumab 1 mg/kg + ipilimumab 3 mg/kg (4 doses). Every three weeks, arm B received nivolumab 3 mg/kg + ipilimumab 1 mg/kg (4 doses). Nivolumab 240 mg intravenously was given every two weeks to both arms A and B. Arm C received nivolumab 3 mg/kg every two weeks, as well as ipilimumab 1 mg/kg every six weeks [36]. In subjects who received sorafenib, nivolumab combination therapy with ipilimumab produced a significant clinical benefit, with ORRs of 16 out of 50 (32%) in arm A, 15 out of 49 (31%) in arm B, and 15 out of 49 (31%) in arm C, (according to the BICR per RECIST version 1.1) (seven patients had complete responses). Patients in arm A had the maximum comprehensive response rate, the most encouraging median overall survival of 22.8 months, 12-month survival rates of 61 percent, 24-month survival rates of 48 percent, and 30-month overall survival rates of 44 percent, all with better health conditions than patients in the other two arms. The more remarkable overall survival in arm A compared to arms B and C might be attributed to the larger ipilimumab initial dosage in arm A. Immune-mediated side effects were also more prevalent in this group (10 of 49, or 20%), with a median start time of 1.3 months. High-dose glucocorticoids were administered to 70 percent (median 14 days), and complete resolution occurred in 70 percent. Of the four patients who restarted treatment after symptom improvement, none had a recurrence of hepatitis. Other immune-mediated side effects included rash in 35 percent (17 of 49; median time to onset: 15 days), adrenal insufficiency in 18 percent (9 of 49; median time to onset: 2.8 months), hypothyroidism or thyroiditis in 22 percent (5 of 49; median time to onset: 1.4 months), colitis in 10 percent (5 of 49; median time to onset: 2 months), pneumonitis in 10 percent (5 of 49; median time to onset: 8.3 months), and infusion-related reactions in 8 percent (4 of 49) [116].
In this randomized clinical trial, nivolumab with ipilimumab demonstrated acceptable toxicity, good objective response rates, and long-lasting responses. The “bicep” in the United States, a treatment plan based on these findings (four doses of nivolumab 1 mg/kg with ipilimumab 3 mg/kg every three weeks, then nivolumab 240 mg every two weeks) was given expedited approval [36]. Recently, a follow-up long-term analysis of the trial population with a minimum follow-up of 44 months was published. The median OS and 3-year survival rates were 22.2 months and 42% for arm A; 12.5 months and 26% for arm B; and 12.7 months and 30% for arm C [117].
Pembrolizumab
As a second-choice therapy for HCC subjects who had received sorafenib, the highly selective monoclonal antibody IgG4/kappa isotype pembrolizumab has recently been granted accelerated approval. It works by inhibiting the bonding between PD-1 and its receptors, programmed death ligand 1 (PD-L1) and PD-L2 [118]. Pembrolizumab, such as nivolumab, was granted rapid approval by the FDA in November 2018 as a second choice drug for the treatment of HCC following sorafenib therapy [119]. Results from the phase II Keynote-224 trial of pembrolizumab in cases previously receiving sorafenib implicate benefits for this alternative PD-1 inhibitor (objective response rate 17 percent, with 44 percent stable disease) [30]. The median period of pembrolizumab therapy was 4.2 months (interquartile range 2.1 to 7.7 months).
These results were confirmed in the international phase III KEYNOTE-240 trial of best supportive care combined with either pembrolizumab or a placebo for second-line therapy of advanced HCC with Child–Turcotte–Pugh class A cirrhosis after radiographic progression/intolerance of sorafenib [120]. Overall, 413 cases were randomized and allocated in a 2:1 ratio to pembrolizumab or placebo. The improvements in median overall survival (13.9 versus 10.6 months, HR 0.78, 95% CI 0.61–0.998) and progression-free survival (3 versus 2.8 months) were not significant because prespecified efficacy boundaries were not reached. However, the objective response rate was higher for pembrolizumab (18.3 versus 4.4 percent), there were more complete responders with pembrolizumab (six versus none), and responses were durable (median duration of response: 13.8 months, range: 1.5 to 23.6+ months). Although the trial did not meet its predetermined level of statistical significance, both OS and PFS showed clinically meaningful improvement over a placebo, similar to the results shown in the KEYNOTE-224 trial. Overall, pembrolizumab is demonstrated to be effective and tolerable as second-line treatment in patients with advanced HCC who had prior therapy with sorafenib [30,121].
Avelumab
Avelumab is an anti-PD-L1 monoclonal antibody approved for advanced urothelial, renal cell, and Merkel cell cancer. Efficacy in 30 cases of patients with progressed HCC and no worse than Child-Pugh class A cirrhosis who were previously treated with sorafenib was demonstrated in a phase II trial, in which there were three partial responses (10 percent) and 19 patients (63 percent) with prolonged stable disease [122]. Tumoral overexpression of PD-L1 did not affect the antitumor response. The median time to tumor progression and overall survival durations were 4.4 and 14.2 months, respectively. The medication was well tolerated, with fewer than five grade 3 side effects. An additional study evaluates the safety and effectiveness of avelumab in conjunction with axitinib, a VEGF-targeting TKI. Avelumab + axitinib was administered to patients until progression, unacceptable toxicity, or discontinuation from the study. A minimum of 24 weeks of follow-up on the public study data set was used to evaluate the preliminary results [123]. Avelumab and axitinib were used to treat 22 individuals in this study. When RECIST and mRECIST criteria were used, ORR emerged in 13.6 percent (95% CI: 2.9–34.9) and 31.8 percent (95% CI: 13.9–54.9) of cases, respectively. Hypertension was the most prevalent grade 3 treatment-related AE in 11 (50.0%) of patients, followed by hand-foot syndrome in five (22.7%). No grade 4/5 AEs recorded as a result of the therapy. Currently, no patients have stopped using the medication due to side effects. Because OS analyses were still in their infancy at the time of the data cutoff, they were not performed. According to the researchers, the early safety of avelumab + axitinib in HCC is tolerable and consistent with the known safety profiles of avelumab and axitinib when used as monotherapies. The combination has anticancer efficacy in HCC, according to this research [124]. Overall, either avelumab used as monotherapy or in combination with axitinib showed moderate antitumor activity with an acceptable toxicity profile.
Tremelimumab + Durvalumab
Combinations of the anti-CTLA-4 monoclonal antibody tremelimumab plus durvalumab, an anti-PD-L1 monoclonal antibody, appear to be tolerable and clinically active among individuals who developed resistance to or rejected sorafenib as their disease advanced [125]. In a phase I/II study, 332 patients with HCC who had progressed on, were intolerant to, or refused sorafenib were randomized to four arms: (T300 + D, N = 75); (T monotherapy, N = 69); (D monotherapy, N = 104); and (T75 + D, N = 84). Tolerability was acceptable across arms, with grade ≥ 3 treatment-related adverse events occurring in 37.8%, 20.8%, 43.5%, and 24.4%, respectively. The confirmed ORRs (95% CI) were 24.0% (14.9 to 35.3), 10.6% (5.4 to 18.1), 7.2% (2.4 to 16.1), and 9.5% (4.2 to 17.9), respectively. The median (95% CI) overall survival was 18.7 (10.8 to 27.3), 13.6 (8.7 to 17.6), 15.1 (11.3 to 20.5), and 11.3 (8.4 to 15.0) months in the T300 + D, durvalumab, tremelimumab, and T75 1 D arms, respectively [126]. Overall, the T300 + D regimen showed the lowest toxicity and highest efficacy compared with other regimens. T300 + D also stimulated CD8+ T-cell production, enhancing response and efficacy.
Durvalumab
One of the most critical immunological checkpoints tumor cells exploit to inhibit antitumor responses is PD-1/PD-L1. Durvalumab is a human IgG1 monoclonal antibody that prevents PD-L1 from linking to PD-1 (IC50 0.1 nM) and CD80 (IC50 0.5 nM) (B7.1; IC50 0.04 nM) [127].
Patients with HCC in a group of 40 who received durvalumab monotherapy for their disease responded well to the treatment, with a 10% response rate and a median survival duration of 13.2 months recorded within a phase I/II trial. Medication-related adverse events (AEs) affected 80% of subjects in many ways. Fatigue (27.5%), itchiness (25.0%), and increased aspartate aminotransferase were the most common (AST; 22.5%). Twenty percent of patients experienced side effects of grade 3/4 related to their treatment. Increased AST (7.5%) and elevated ALT (7.5%) were the most common (5.0 percent). There are no therapy-related adverse events (AEs) to blame for the discontinuation of seven individuals (17.5 percent). Due to therapy, no one died as a result. Overall, durvalumab showed a tolerable safety profile and promising antitumor activity and overall survival in patients with HCC, particularly HCV+ patients [128].
Camrelizumab
Camrelizumab is a humanized anti-PD-1 monoclonal antibody that blocks the binding of PD-1 to PD-L1 and consequently inhibits tumor cells evasion of the immune system [129]. A multicenter phase III trial to treat advanced liver cancer found that the combination of camrelizumab and apatinib was safe and effective when used as the primary choice of drug treatment. First-line treatment for advanced HCC or cholangiocarcinoma (BTC) with camrelizumab plus FOLFOX-4 or GEMOX resulted in an ORR of 26.5% and a DCR of 79.4 percent, although the median overall survival was not met. HCC patients experienced 85.3% of treatment-related adverse events at level 3 or higher. However, only 5.9% of these were immune-related adverse events, indicating that the camrelizumab monoclonal antibody plus FOLFOX4 or GEMOX chemotherapy are well-tolerated when used in conjunction. For advanced HCC, this combination is predicted to be successful. Camrelizumab and FOLFOX4 systemic chemotherapy are being reported in a randomized, multicenter, controlled phase III trial for subjects with advanced HCC [130].
Spartalizumab
Humanized IgG4 monoclonal antibody spartalizumab targets human programmed cell death-1 (PD-1) [131]. In an open-label, multicenter study among 18 patients diagnosed with solid tumors who had worsened after conventional therapy, those who were unresponsive to therapy, or those who had no standard therapy available, spartalizumab was administered intravenously once every two weeks (Q2W), until disease advancement based on immune-related response criteria (irRC), unacceptable toxicities, or termination of treatment by the investigator or patient. The starting dose of spartalizumab used in the trial was 1 mg/kg Q2W and it turned out to be safe for a dose of up to 10 mg/kg Q2W. Maculopapular rash, malaise, and a rise in serum alkaline phosphatase were the most frequent drug-related adverse effects. 10 mg/kg Q2W was established as the recommended dose for subsequent studies [132]. Spartalizumab was administered IV to 58 patients at doses of 1, 3, or 10 mg/kg every 2 weeks (Q2W) or 3 or 5 mg/kg every 4 weeks (Q4W) in phase I of a trial. Patients had a variety of tumor types, most commonly sarcoma (28%), metastatic RCC (10%), and 2 out of 58 patients had HCC. Most patients (93%) had received previous anticancer treatment. There were no dose-limiting toxicities. The most common treatment-related adverse events of any grade were fatigue (22%), diarrhea (17%), pruritus (14%), hypothyroidism (10%), and nausea (10%). Spartalizumab was well tolerated at all doses tested in patients with advanced solid tumors and a history of prior therapy. Overall, spartalizumab dose escalation for subsequent studies has been conducted already, and it is also being tested in combination with several targeted treatments and other immunotherapies [131]. ACROPOLI is an open-label, single-arm, non-randomized, multicenter phase II study to evaluate the efficacy of Spartalizumab in monotherapy in metastatic patients with Programmed Death-1 (PD1)-high-expressing tumors [133].
3.2.2. Other Targeting Agents
Ramucirumab
Ramucirumab is a recombinant human IgG1 monoclonal antibody that binds to the extracellular domain of human VEGFR-2 and prevents it from interacting through its ligands, inhibiting endothelial proliferation and migration [134]. The first study of ramucirumab first-line monotherapy in aHCC was a single-arm study among 42 patients with a mPFS of 4.0 months (95% CI: 2.6–5.7), an ORR of 9.5% (95% CI: 2.7–22.6) with four partial responses, and a mOS of 12.0 months (95% CI: 6.1–197.7). Survival benefit was more noticeable in BCLC stage C and Child-Pugh type A cirrhotic patients [135]. These promising results led to the enrollment of the Phase III REACH trial, which compared ramucirumab + best supportive care (BSC) to placebo + BSC in the second-line treatment of patients with prior sorafenib therapy [136]. Despite acceptable adverse events, OS (9.2 vs. 7.6 months) and median PFS (2.9 vs. 2.1 months) had no significant improvement. Similar to prior studies, in this study some survival benefits were also noticed in Child-Pugh A cirrhotic patients. However, a subgroup analysis of patients with AFP ≥ 400 ng/mL showed a significantly higher efficacy for ramucirumab (n = 119) compared to placebo (n = 131), with an mOS (7.8 vs. 4.2 months; HR 0.67; p < 0.001) and an mPFS of (2.7 vs. 1.5 months; HR: 0.70) [137]. Subsequently, a phase III trial (REACH-2) was enrolled to evaluate the efficacy of ramucirumab as a second-line treatment after sorafenib in AFP ≥ 400 ng/mL, aHCC patients. 197 patients on ramucirumab and 95 patients on placebo had a mOS (8.5 vs. 7.3 months; HR 0.71; p = 0.0199) and mPFS (2.8 vs. 1.6 months; HR 0.452; p < 0.0001) [35]. This survival benefit (OS and PFS) of ramucirumab was confirmed via pooled analysis of patients with AFP ≥ 400 ng/mL from REACH and REACH-2, with a mOS (8.1 vs. 5.0 months; HR 0.69; p = 0.0002) and mPFS (2.8 vs. 1.5 months; p < 0.0001) [138,139]. The most common treatment-related adverse events in these trials included: nausea, fatigue, anorexia, peripheral edema, diarrhea, headache, and abdominal pain. Grade ≥ 3 adverse events were hypertension and hyponatremia in ≥ 5% of patients [35,136]. Subsequently, ramucirumab was approved for the treatment of HCC patients with an AFP ≥ 400 ng/mL and a prior sorafenib therapy. Overall, ramucirumab prolongs the survival of advanced HCC in the second-line setting and has an overall tolerable and manageable safety profile [140].
Cabozantinib
Cabozantinib is a tyrosine kinase inhibitor that inhibits the phosphorylation of MET, AXL, and VEGFR 1, 2, and 3 [141]. At both the first and second lines of the drug in individuals with HCC, a Phase II randomized discontinuation survey showed excellent clinical activity (percent disease control; median progression-free survival of 4.4 months; median survival of 15.1 months) [142]. Thus, a Phase III study (CELESTIAL) was initiated in HCC cases that have progressed following prior systemic therapy (NCT01908426), in which 707 patients with progressing HCC and no worse than Child-Turcotte-Pugh class A cirrhosis were randomized to receive either cabozantinib (60 mg/d) or a placebo [31]. The median overall survival of cabozantinib was substantially greater (10.2 versus 8.0 months) in subjects who received a second- or third-line regimen after prior sorafenib intervention, and the distinction was even more pronounced once the evaluation was limited to patients who had only received sorafenib as their only prior therapy (median overall survival 11.3 versus 7.2 months). Hand-foot syndrome (17 against 0% in the placebo group), hypertension (16 versus 2%), elevated aspartate aminotransferase (12 versus 7%), tiredness (10 versus 4%), and diarrhea (10 versus 2%) were the most prevalent grade 3 or 4 side effects after cabozantinib (10 versus 2 percent) [31].
Cabozantinib was authorized in January 2019 to manage patients with HCC who had prior sorafenib therapy based on such findings. In addition, in a retrospective multicenter cohort study, cabozantinib showed comparable efficacy (OS beyond 9 months in Child-Pugh A patients) and safety in patients with preserved liver function and confirmed the reported findings of the CELESTIAL trial [143].
Apatinib
Apatinib is a tyrosine kinase inhibitor that exclusively inhibits VEGFR-2, blocking its signaling pathways, leading to reduced vascular endothelial cell migration, tumoral angiogenesis, and vessel density [144,145,146,147]. A randomized, open-label, nationwide, multicenter, phase II trial of apatinib as second-line therapy for advanced HCC was conducted among 121 patients with aHCC in a 1:1 ratio between the 850 mg dose group and the 750 mg dose group. The results confirmed that the efficacy of both groups was nearly the same: mTTP and mOS were not significantly different (4.2 mo vs. 3.3 mo, p > 0.05; 9.7 mo vs. 9.8 mo, p > 0.05). The DCRs of the two groups were 48.6% and 37.3% (p > 0.05), and the ORRs were 8.6% and 0 (p > 0.05), respectively. The incidence of adverse events was also similar. Drug-related toxicities in the 850 mg dose group were more than those in the 750 mg dose group but not significantly more, including hand-foot skin reaction (HFSR), elevated aminotransferase, and elevated bilirubin. In grades 3 and above, drug-related side effects included hypertension, proteinuria, HFSR, fatigue, and peripheral blood cell reduction. Overall, 750 mg/d was considered the recommended dose for subsequent studies [148]. AHELP was a randomized, double-blind, multicenter, placebo-controlled, phase III trial among 393 eligible patients with aHCC who were unresponsive or intolerant to prior therapy and were randomized to receive apatinib (750 mg/d, n = 261) or a placebo (n = 132). Overall survival was significantly improved in the apatinib group vs. the placebo group (median 8.7 months [95% CI [7.5–9.8] vs. 6.8 months [5.7–9.1]; HR = 0·785 [95% CI 0.617–0.998], p = 0.048). The most common treatment-related adverse events of grades 3 or 4 were hypertension, hand-foot syndrome, and thrombocytopenia. Apatinib significantly improved overall survival in patients with pretreated aHCC with an acceptable safety profile [149].
Further small molecules are currently being investigated for use in the treatment of hepatocellular carcinoma (HCC). Several are aimed at targeting angiogenesis-related proteins such as vascular endothelial growth factor (VEGF), while others act on pathways that are already the target of other drugs, such as mitogen-activated protein kinase (MEK) and c-MET [150]. It is essential to emphasize medications that focus on c-MET because they may be more effective in HCC with increased receptor expression [151]. Further phase III clinical trials are necessary to determine their clinical value in comparison to sorafenib [52].
3.3. Systemic Chemotherapy
Conventional chemotherapy medicines, especially after locoregional therapy, have had minimal efficacy in treating HCC when applied at a more advanced stage of the illness [152]. This evaluation is based on an initial investigation of single-arm, open-label research assessing the application of specific chemotherapeutic drugs, which did not result in the extension of this class of pharmaceuticals and their usage to palliative care in recent years. However, innovative chemotherapy medicines, such as oxaliplatin, have lately proven efficacy in treating digestive system tumors (stomach, colon, and pancreas) [153]. Most of the mentioned therapies have been explored for use in the treatment of advanced HCC, considering these encouraging results.
Since the management of HCC started, cytotoxic chemotherapy has been tried routinely, although it has not been able to enhance overall survival in most medical studies undertaken so far [154]. The existence of liver cirrhosis complicates antitumor therapy in HCC. In addition to increasing the toxicity of chemotherapy drugs, cirrhosis also impairs their metabolism [155]. Additionally, HCC is relatively resistant to many cytotoxic anti-cancer treatments. In a new randomized experiment, doxorubicin exhibited a tumor response rate < 10% and a slight increase in overall survival rate (10.6 weeks) when compared to no treatment [156]. Notably, 25% of patients died due to complications related to doxorubicin, including septicemia and cardiotoxicity. In clinical and historical studies, other chemotherapeutic drugs such as gemcitabine, oxaliplatin, and capecitabine showed little antitumor effect, with less than 20% clinical responses [157]. Combination therapies such as PIAF (cisplatin, interferon, adriamycin, and fluorouracil) and FOLFOX (5-fluorouracil, folic acid, and oxaliplatin) did not significantly enhance survival when compared to doxorubicin in randomized controlled studies [158]. Additionally, the PIAF group was observed to have a significant risk of myelotoxicity. So far, no cytotoxic chemotherapy treatment has been shown to increase the survival of HCC patients significantly, and there is no indication for chemotherapy as a regular treatment for the disease in this population. Nevertheless, a recent retrospective study found that ECF (epirubicin, cisplatin, and 5-fluorouracil) co-administration significantly extended survival time in sorafenib-refractory patients with metastatic HCC who had a tumor response; average survival intervals had been 20.4 months in responders and 4.9 months in non-responders (p < 0.001) [159]. As a result, although more prospective trials are necessary, ECF may be a feasible alternative or rescue treatment for individuals who have failed to react to sorafenib. In Asian nations, hepatic arterial infusion chemotherapy (HAIC) has been applied to treat advanced HCC with portal vein thrombosis [160]. Table 2 and Table 3 represent the clinical studies of systemic chemotherapy in HCC. Figure 5 represents the efficacy of agents used in HCC targeted therapy and immunotherapy based on survival rate. Similarly, Table 4 represents the clinical trials used for targeted therapy and immunotherapy in patients with unresectable HCC.

Table 2.
Phase II and III studies of systemic chemotherapy in hepatocellular carcinoma.

Table 3.
Systemic therapy plus targeted therapy.
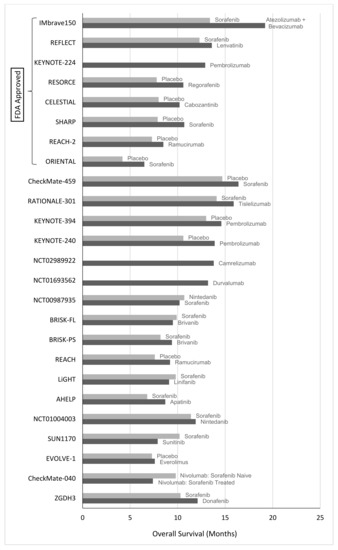
Figure 5.
Efficacy of agents used in HCC targeted therapy and immunotherapy based on survival rate.

Table 4.
Clinical trials used for targeted therapy and immunotherapy of patients with unresectable HCC.
3.4. Herbal Management Role in HCC
In recent years, there has been growing interest in the potential role of herbal medicine in the prevention and treatment of hepatocellular carcinoma (HCC). While chemotherapy and immunotherapy have shown promise in slowing the progression of HCC, there is still a need for more effective and less toxic treatments. Here, we discuss some recent advances in the use of herbal medicine for HCC management. TCM has a long history of using herbal medicine for the treatment of liver diseases, including HCC. Recent studies have identified several herbal compounds that may have anti-tumor effects and be effective in slowing the progression of HCC [185] For example, the compound curcumin, derived from the turmeric plant, has been shown to have anti-inflammatory and anti-tumor properties and may help to induce apoptosis (cell death) in HCC cells. Another herb commonly used in TCM, milk thistle, contains a compound called silymarin that may help to protect the liver and slow the growth of HCC. Ayurvedic medicine is a traditional system of medicine that originated in India and has been used for thousands of years. Several Ayurvedic herbs have been studied for their potential in HCC management. For example, the herb Phyllanthus niruri, also known as “stonebreaker”, has been shown to have anti-tumor properties and may help to inhibit the growth of HCC cells. Another herb commonly used in Ayurvedic medicine, Andrographis paniculata, contains compounds that have been shown to induce apoptosis in HCC cells and may help to reduce inflammation [186].
In addition to TCM and Ayurvedic medicine, several other herbal medicines have been studied for their potential role in HCC management. For example, the herb ginseng has been shown to have anti-tumor effects and may help improve liver function in HCC patients. The herb Astragalus membranaceus has also been studied for its potential in HCC management and may help to reduce inflammation and promote apoptosis in HCC cells. While these herbal medicines show promise in HCC management, it is important to note that more research is needed to determine their safety and efficacy. Additionally, it is important for patients to consult with a healthcare provider before using any herbal remedies, as they may interact with other medications or have potential side effects. Therefore, recent advances in herbal medicine suggest that certain herbs may have a role in the prevention and treatment of HCC. While more research is needed to confirm their efficacy, the use of herbal medicines may offer a promising complementary approach to traditional chemotherapy and immunotherapy for HCC management [187].
4. Conclusions
Hepatocellular carcinoma is the most frequently occurring primary liver cancer. Curative medication for HCC remains a challenge, and various trials have been conducted worldwide to investigate potential HCC medications. The management of HCC is stage-dependent, and regrettably, HCC is frequently discovered at an advanced stage. As a result, screening for HCC, particularly in patients with higher risk, such as cirrhotic patients, should be evaluated early in the disease’s progression, as curative therapy is only successful in the early stages. In conclusion, thanks to intensive scientific and clinical research, effective treatment drugs for advanced hepatocellular carcinoma have evolved significantly over the previous decade. On the other hand, tumor heterogeneity due to multifactorial risk factors impedes the development of effective medicines for HCC, and this issue has hampered the treatment of HCC patients. The management of HCC has undergone a revolution to overcome the growing morbidity and mortality associated with the disease. Fortunately, nowadays there has been a notable increase in the range of monotherapy and combination therapy regimens that have been approved for the treatment of advanced HCC. The rest have failed due to their high toxicity profiles. The miRNAs, lncRNAs, and many other signaling pathways are among the unexplored aspects of HCC treatment that should be worked on. Additionally, novel combination therapy regimens might be the key to success and should be considered for upcoming studies. The combination of atezolizumab and bevacizumab has been found to be superior to sorafenib, which was the former standard of care for unresectable HCC, in terms of overall survival (OS), progression-free survival (PFS), quality of life, and adverse events. Consequently, it is now assumed to be the first-line therapy of choice. As atezolizumab and bevacizumab are antibodies, their combination is unlikely to adversely affect liver function. Therefore, it is estimated that 70–80% of patients who have received first-line therapy with atezolizumab plus bevacizumab will qualify for second-line treatment. At present, Lenvatinib appears to be the most promising second-line treatment and is expected to produce higher response rates, a longer PFS, and a longer OS than other targeted agents due to its effectiveness on FGFR4 overexpressed HCC. After failure of atezolizumab plus bevacizumab combination therapy, a variety of agents and sequences may be used for third-line or later therapies. Regorafenib, Cabozantinib, and Ramucirumab have been shown to increase overall survival and may be used as second-line treatments in some cases. Real-world clinical settings are needed to determine the best sequence of therapies.
Author Contributions
Conceptualization, B.F.F. and N.F.H.; investigation, D.R., P.F., P.H. and B.F.F.; resources, N.F.H.; writing—original draft preparation, B.F.F., D.R., P.F. and P.H.; writing—review and editing, A.K.R., B.F.F., N.B.L. and M.R.N.-J.; visualization, B.F.F.; supervision, M.R.N.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are thankful to Amir Ali Sohrabpour for his kind help and comments. We are also thankful to Biorender.com for helping us draw figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Childs, A.; Meyer, T. Hepatocellular Carcinoma: Treatment. In Evidence-Based Gastroenterology and Hepatology 4e; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 703–714. [Google Scholar]
- Kweon, S.-S. Updates on cancer epidemiology in Korea, 2018. Chonnam Med. J. 2018, 54, 90–100. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Morizane, C.; Ueno, M.; Okusaka, T.; Ishii, H.; Furuse, J. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Jpn. J. Clin. Oncol. 2018, 48, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Perisetti, A.; Goyal, H.; Yendala, R.; Thandassery, R.B.; Giorgakis, E. Non-cirrhotic hepatocellular carcinoma in chronic viral hepatitis: Current insights and advancements. World J. Gastroenterol. 2021, 27, 3466–3482. [Google Scholar] [CrossRef]
- Geh, D.; Manas, D.M.; Reeves, H.L. Hepatocellular carcinoma in non-alcoholic fatty liver disease—A review of an emerging challenge facing clinicians. Hepatobiliary Surg. Nutr. 2021, 10, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.; Ryan, M.; Howell, J. Epidemiology of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A western perspective. Hepatoma Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Saitta, C.; Pollicino, T.; Raimondo, G. Obesity and liver cancer. Ann. Hepatol. 2019, 18, 810–815. [Google Scholar] [CrossRef]
- Chew, S.A.; Moscato, S.; George, S.; Azimi, B.; Danti, S. Liver cancer: Current and future trends using biomaterials. Cancers 2019, 11, 2026. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chen, K.-F.; Chen, P.-J. Treatment of liver cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a021535. [Google Scholar] [CrossRef]
- Petrowsky, H.; Fritsch, R.; Guckenberger, M.; De Oliveira, M.L.; Dutkowski, P.; Clavien, P.-A. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 755–772. [Google Scholar] [CrossRef]
- Llovet, J.M.; Burroughs, A.; Bruix, J. Hepatocellular carcinoma. Lancet 2003, 362, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Shen, L.; Li, J.; Zhou, Z.-W.; Liang, H.; Zhang, X.-T.; Tang, L.; Xin, Y.; Jin, J.; Zhang, Y.-J. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2019, 39, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.-M.; Lewandowski, R.-J.; Sato, K.-T.; Gates, V.-L.; Kulik, L.; Mulcahy, M.-F.; Ryu, R.-K.; Omary, R.-A.; Salem, R. Radioembolization for the treatment of unresectable hepatocellular carcinoma: A clinical review. World J. Gastroenterol. 2008, 14, 1664–1669. [Google Scholar] [CrossRef]
- Zhong, Q.; Shuai, Y.; Luo, Q.; Feng, G.; Wu, M.; Fan, E.; Chen, Q.; Yue, G.; Zhang, G. A novel five-gene signature for predicting prognosis in liver cancer. 2020. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018, 412, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.K.; Brown Jr, R.S.; Siegel, A.B. Hepatocellular carcinoma: Review of current treatment with a focus on targeted molecular therapies. Ther. Adv. Gastroenterol. 2010, 3, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment strategies for hepatocellular carcinoma—A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef]
- Lin, S.M. Local Ablation for Hepatocellular Carcinoma in Taiwan. Liver Cancer 2013, 2, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Serper, M.; Parikh, N.D.; Thiele, G.; Ovchinsky, N.; Mehta, S.; Kuo, A.; Ho, C.; Kanwal, F.; Volk, M.; Asrani, S.K. Patient-Reported Outcomes in HCC: A Scoping Review by the Practice Metrics Committee of the American Association for the Study of Liver Diseases. Hepatology 2022, 76, 251–274. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Morine, Y.; Yamada, S.; Saito, Y.; Ikemoto, T.; Tokuda, K.; Takasu, C.; Miyazaki, K.; Shimada, M. Nrf2 signaling promotes cancer stemness, migration, and expression of ABC transporter genes in sorafenib-resistant hepatocellular carcinoma cells. PLoS ONE 2021, 16, e0256755. [Google Scholar] [CrossRef] [PubMed]
- Exposito, M.J.; Akce, M.; Alvarez, J.M.; Assenat, E.; Balart, L.; Baron, A.; Decaens, T.; Heurgue-Berlot, A.; Martin, A.; Paik, S. CA209-9DX: Phase III, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann. Oncol. 2018, 29, viii267–viii268. [Google Scholar] [CrossRef]
- Rimassa, L.; Santoro, A. Sorafenib therapy in advanced hepatocellular carcinoma: The SHARP trial. Expert Rev. Anticancer Ther. 2009, 9, 739–745. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Guan, Z.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Yang, T.-S.; Tak, W.Y.; Pan, H.; Yu, S.J.E.J.o.C. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: Subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur. J. Cancer 2012, 48, 1452–1465. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracian, A.C.; Acosta-Rivera, M.; Choo, S.P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.F.; et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J. Clin. Oncol. 2019, 37, 327. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Lencioni, R.; Kudo, M.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.F.; de Guevara, L.L.; Papandreou, C.; et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): Second interim analysis. Int. J. Clin. Pract. 2014, 68, 609–617. [Google Scholar] [CrossRef]
- Lencioni, R.; Kudo, M.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.F.; Ladrón de Guevara, L.; Papandreou, C.; et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int. J. Clin. Pract. 2012, 66, 675–683. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.H.; de Guevara, L.L.; et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef]
- Ganten, T.M.; Stauber, R.; Schott, E.; Malfertheiner, P.; Buder, R.; Galle, P.R.; Goehler, T.; Bernard, I.; Gerken, G. Final Analysis of Overall Survival Per Subgroups of Hcc Patients in the Prospective, Non-Interventional Insight Study Treated with Sorafenib. Ann. Oncol. 2014, 25, iv246. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Sacco, R.; de Stefano, G.; Montesarchio, V.; Cabibbo, G.; Zolfino, T.; Carucci, P.; Pisconti, S.; De Vita, F.; Giovanis, P.; et al. Safety and efficacy of sorafenib in stella study, a Multicenter, Observational, Phase IV Study In Italian Centers. Ann. Oncol. 2015, 26, vi95. [Google Scholar] [CrossRef]
- Iavarone, M.; Cabibbo, G.; Piscaglia, F.; Zavaglia, C.; Grieco, A.; Villa, E.; Cammà, C.; Colombo, M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011, 54, 2055–2063. [Google Scholar] [CrossRef]
- Ford, R.; Schwartz, L.; Dancey, J.; Dodd, L.E.; Eisenhauer, E.A.; Gwyther, S.; Rubinstein, L.; Sargent, D.; Shankar, L.; Therasse, P.; et al. Lessons learned from independent central review. Eur. J. Cancer 2009, 45, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yang, X.-R.; Chung, W.-Y.; Dennison, A.R.; Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target. Ther. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Hironaka, S. Anti-angiogenic therapies for gastric cancer. Asia-Pac. J. Clin. Oncol. 2019, 15, 208–217. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Lin, D.Y.; Park, J.W.; Kudo, M.; Qin, S.; Chung, H.C.; Song, X.; Xu, J.; Poggi, G.; et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J. Clin. Oncol. 2013, 31, 4067–4075. [Google Scholar] [CrossRef]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef]
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.-H.; Heo, J.; Xu, J. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef]
- Zhong, Y.; Qiu, R.-Z.; Sun, S.-L.; Zhao, C.; Fan, T.-Y.; Chen, M.; Li, N.-G.; Shi, Z.-H. Small-molecule Fms-like tyrosine kinase 3 inhibitors: An attractive and efficient method for the treatment of acute myeloid leukemia. J. Med. Chem. 2020, 63, 12403–12428. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.C.; Chen, P.-J.; Carr, B.I.; Knox, J.J.; Gill, S.; Ansell, P.; McKeegan, E.M.; Dowell, B.; Pedersen, M.; Qin, Q.; et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2012, 119, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Cainap, C.; Qin, S.; Huang, W.T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.K.; Chen, P.J.; Toh, H.C.; et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Marzin, K.; Kretschmar, G.; Luedtke, D.; Kraemer, S.; Kuelzer, R.; Schlenker-Herceg, R.; Schmid, U.; Schnell, D.; Dallinger, C. Pharmacokinetics of nintedanib in subjects with hepatic impairment. J. Clin. Pharmacol. 2018, 58, 357–363. [Google Scholar] [CrossRef]
- Maucort-Boulch, D.; de Martel, C.; Franceschi, S.; Plummer, M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int. J. Cancer 2018, 142, 2471–2477. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsunaga, S.; Ohno, I.; Hashimoto, Y.; Takahashi, H.; Watanabe, K.; Umemoto, K.; Okusaka, T. Systemic Chemotherapy for Advanced Hepatocellular Carcinoma: Past, Present, and Future. Diseases 2015, 3, 360–381. [Google Scholar] [CrossRef]
- Al-Salama, Z.T.; Syed, Y.Y.; Scott, L.J. Lenvatinib: A review in hepatocellular carcinoma. Drugs 2019, 79, 665–674. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, T.-H.; Yau, T.; Hsu, C. Novel systemic therapy for hepatocellular carcinoma. Hepatol. Int. 2020, 14, 638–651. [Google Scholar] [CrossRef]
- Li, X.; Qiu, M.; Wang, S.; Zhu, H.; Feng, B.; Zheng, L. A Phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharm. 2020, 85, 593–604. [Google Scholar] [CrossRef]
- Bi, F.; Qiu, M.; Chai, X.; Niu, J.; Ding, Y.; Bai, Y.; Wu, L.; Shentu, J.-z.; Hao, P.; Chen, J.; et al. A multicenter phase II study of donafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2017, 35, e15682. [Google Scholar] [CrossRef]
- Bi, F.; Qin, S.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib versus sorafenib as first-line therapy in advanced hepatocellular carcinoma: An open-label, randomized, multicenter phase II/III trial. J. Clin. Oncol. 2020, 38, 4506. [Google Scholar] [CrossRef]
- Cui, X.; Jia, H.; Xin, H.; Zhang, L.; Chen, S.; Xia, S.; Li, X.; Xu, W.; Chen, X.; Feng, Y.; et al. A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front. Immunol. 2021, 12, 778978. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Abou-Alfa, G.K.; Cheng, A.-L.; Duffy, A.G.; El-Khoueiry, A.B.; Finn, R.S.; Galle, P.R.; Goyal, L.; He, A.R.; Kaseb, A.O. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, e002794. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Deininger, S.; Törzsök, P.; Oswald, D.; Lusuardi, L. Current Systemic Treatment Options in Metastatic Urothelial Carcinoma after Progression on Checkpoint Inhibition Therapy-A Systemic Review Combined with Single-Group Meta-Analysis of Three Studies Testing Enfortumab Vedotin. Cancers 2021, 13, 3206. [Google Scholar] [CrossRef]
- Alberts, S.R.; Fitch, T.R.; Kim, G.P.; Morlan, B.W.; Dakhil, S.R.; Gross, H.M.; Nair, S. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: A phase II north central cancer treatment group (NCCTG) Clinical Trial. Am. J. Clin. Oncol. 2012, 35, 329. [Google Scholar] [CrossRef]
- Zhu, A.X.; Ancukiewicz, M.; Supko, J.G.; Sahani, D.V.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Abrams, T.A.; McCleary, N.J.; Bhargava, P.; Muzikansky, A.; et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: A phase II study. Clin. Cancer Res. 2013, 19, 1557–1566. [Google Scholar] [CrossRef]
- Palmer, D.H.; Ma, Y.T.; Peck-Radosavljevic, M.; Ross, P.; Graham, J.; Fartoux, L.; Deptała, A.; Studeny, M.; Schnell, D.; Hocke, J.; et al. A multicentre, open-label, phase-I/randomised phase-II study to evaluate safety, pharmacokinetics, and efficacy of nintedanib vs. sorafenib in European patients with advanced hepatocellular carcinoma. Br. J. Cancer 2018, 118, 1162–1168. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar] [PubMed]
- Gardini, A.C.; Santini, D.; Aprile, G.; Silvestris, N.; Felli, E.; Foschi, F.G.; Ercolani, G.; Marisi, G.; Valgiusti, M.; Passardi, A.J.O. Antiangiogenic agents after first line and sorafenib plus chemoembolization: A systematic review. Oncotarget 2017, 8, 66699–66708. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Thongprasert, S.; Lim, H.Y.; Sukeepaisarnjaroen, W.; Yang, T.-S.; Wu, C.-C.; Chao, Y.; Chan, S.L.; Kudo, M.; Ikeda, M.; et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology 2016, 64, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Woei-A-Jin, F.S.H.; Weijl, N.I.; Burgmans, M.C.; Sarasqueta, A.F.; van Wezel, J.T.; Wasser, M.N.; Coenraad, M.J.; Burggraaf, J.; Osanto, S. Neoadjuvant Treatment with Angiogenesis-Inhibitor Dovitinib Prior to Local Therapy in Hepatocellular Carcinoma: A Phase II Study. Oncologist 2021, 26, 854–864. [Google Scholar] [CrossRef]
- Houghton, P.J. Everolimus. Clin. Cancer Res. 2010, 16, 1368–1372. [Google Scholar] [CrossRef]
- Efeyan, A.; Sabatini, D.M. mTOR and cancer: Many loops in one pathway. Curr. Opin. Cell Biol. 2010, 22, 169–176. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.K.; Lim, H.Y.; Poon, R.T.; Blanc, J.F.; Vogel, A.; Chen, C.L.; et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA 2014, 312, 57–67. [Google Scholar] [CrossRef]
- Koeberle, D.; Dufour, J.F.; Demeter, G.; Li, Q.; Ribi, K.; Samaras, P.; Saletti, P.; Roth, A.D.; Horber, D.; Buehlmann, M.; et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): A randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann. Oncol. 2016, 27, 856–861. [Google Scholar] [CrossRef]
- Zhu, A.X.; Abrams, T.A.; Miksad, R.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Zheng, H.; Muzikansky, A.; Clark, J.W.; Kwak, E.L.; Schrag, D.; et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer 2011, 117, 5094–5102. [Google Scholar] [CrossRef]
- Zhang, T.; Song, X.; Xu, L.; Ma, J.; Zhang, Y.; Gong, W.; Zhang, Y.; Zhou, X.; Wang, Z.; Wang, Y.; et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol. Immunother. 2018, 67, 1079–1090. [Google Scholar] [CrossRef]
- Dahan, R.; Sega, E.; Engelhardt, J.; Selby, M.; Korman, A.J.; Ravetch, J.V. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015, 28, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Deva, S.; Lee, J.-S.; Lin, C.-C.; Yen, C.-J.; Millward, M.; Chao, Y.; Keam, B.; Jameson, M.; Hou, M.-M.; Kang, Y.-K. A phase Ia/Ib trial of tislelizumab, an anti-PD-1 antibody (ab), in patients (pts) with advanced solid tumors. Ann. Oncol. 2018, 29, x24–x25. [Google Scholar] [CrossRef]
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, M.; Macarulla, T.M.; Tomasello, G.; Boisserie, F.; Hou, J.; et al. RATIONALE 301 study: Tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019, 15, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- de la Fouchardière, C. Regorafenib in the treatment of metastatic colorectal cancer. Future Oncol. 2018, 14, 2239–2246. [Google Scholar] [CrossRef]
- Ferraro, D.; Zalcberg, J. Regorafenib in gastrointestinal stromal tumors: Clinical evidence and place in therapy. Ther. Adv. Med. Oncol. 2014, 6, 222–228. [Google Scholar] [CrossRef]
- Mei, L.; Du, W.; Idowu, M.; von Mehren, M.; Boikos, S.A. Advances and Challenges on Management of Gastrointestinal Stromal Tumors. Front. Oncol. 2018, 8, 135. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Tao, C.-C.; Tao, Z.-G.; Zhang, K.; Wu, A.-K.; Wu, J.-X.; Rong, W.-Q. Research progress regarding programmed cell death 1/programmed cell death ligand 1 inhibitors combined with targeted therapy for treating hepatocellular carcinoma. World J. Gastrointest. Surg. 2021, 13, 1136. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Hirooka, Y.; Fujishiro, M. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol. Res. 2019, 49, 1054–1065. [Google Scholar] [CrossRef]
- Grothey, A.; George, S.; Van Cutsem, E.; Blay, J.-Y.; Sobrero, A.; Demetri, G.D. Optimizing treatment outcomes with regorafenib: Personalized dosing and other strategies to support patient care. Oncologist 2014, 19, 669–680. [Google Scholar] [CrossRef]
- Pathak, S.; Sonbol, M. Second-Line Treatment Options for Hepatocellular Carcinoma: Current Landscape and Future Direction. J. Hepatocell. Carcinoma 2021, 8, 1147–1158. [Google Scholar] [CrossRef]
- Kumar, V.; Shinagare, A.B.; Rennke, H.G.; Ghai, S.; Lorch, J.H.; Ott, P.A.; Rahma, O.E. The Safety and Efficacy of Checkpoint Inhibitors in Transplant Recipients: A Case Series and Systematic Review of Literature. Oncologist 2020, 25, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and novel combinations in the treatment of melanoma—An update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.; Yang, L.; Li, Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J. Hematol. Oncol. 2013, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanloo, A.; Soltani, A.; Modaresi, S.M.S.; Hashemy, S.I. Recent advances in the clinical development of immune checkpoint blockade therapy. Cell. Oncol. 2019, 42, 609–626. [Google Scholar] [CrossRef]
- Kitahara, M.; Mizukoshi, E.; Terashima, T.; Nakagawa, H.; Horii, R.; Iida, N.; Arai, K.; Yamashita, T.; Sakai, Y.; Yamashita, T. Safety and long-term outcome of intratumoral injection of ok432-stimulated dendritic cells for hepatocellular carcinomas after radiofrequency ablation. Transl. Oncol. 2020, 13, 100777. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, Y.; Lee, M.; Heo, M.K.; Song, J.-S.; Kim, K.-H.; Lee, H.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br. J. Cancer 2015, 113, 1666–1676. [Google Scholar] [CrossRef]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.-C.; Lee, W.-B.; Lee, H.-S.; Bae, Y.-S. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Chang, R.; Yan, X. Supramolecular Cancer Photoimmunotherapy Based on Precise Peptide Self-Assembly Design. Chem. Commun. 2022, 58, 2247–2258. [Google Scholar] [CrossRef]
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2. [Google Scholar] [CrossRef]
- Zhulai, G.; Oleinik, E. Targeting regulatory T cells in anti-PD-1/PD-L1 cancer immunotherapy. Scand. J. Immunol. 2021, 95, e13129. [Google Scholar] [CrossRef]
- Kojima, K.; Sakamoto, T.; Kasai, T.; Kagawa, T.; Yoon, H.; Atagi, S. PD-L1 expression as a predictor of postoperative recurrence and the association between the PD-L1 expression and EGFR mutations in NSCLC. Sci. Rep. 2021, 11, 17522. [Google Scholar] [CrossRef]
- Dietz, H.; Weinmann, S.C.; Salama, A.K. Checkpoint Inhibitors in Melanoma Patients with Underlying Autoimmune Disease. Cancer Manag. Res. 2021, 13, 8199–8208. [Google Scholar] [CrossRef]
- Lisi, L.; Lacal, P.M.; Martire, M.; Navarra, P.; Graziani, G. Clinical experience with CTLA-4 blockade for cancer immunotherapy: From the monospecific monoclonal antibody ipilimumab to probodies and bispecific molecules targeting the tumor microenvironment. Pharmacol. Res. 2022, 175, 105997. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Mahn, R.; Vogt, A.; Kupczyk, P.; Sadeghlar, F.; van Beekum, K.; Hüneburg, R.; Meyer, C.; Toma, M.; Ahmadzadehfar, H.; Essler, M. Programmed cell death protein 1 (PD-1)-inhibition in hepatocellular carcinoma (HCC): A single center experience. Scand. J. Gastroenterol. 2020, 55, 1057–1062. [Google Scholar] [CrossRef]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.-P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B. CheckMate 040 Cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021, 5, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Hammers, H.; Lipson, E.J. Nivolumab: Targeting PD-1 to bolster antitumor immunity. Future Oncol. 2015, 11, 1307–1326. [Google Scholar] [CrossRef]
- Tella, S.H.; Mahipal, A.; Kommalapati, A.; Jin, Z. Evaluating the Safety and Efficacy of Nivolumab in Patients with Advanced Hepatocellular Carcinoma: Evidence to Date. OncoTargets Ther. 2019, 12, 10335–10342. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef]
- Ji, H.-h.; Tang, X.-w.; Dong, Z.; Song, L.; Jia, Y.-t. Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: Analysis of spontaneous reports submitted to FAERS. Clin. Drug Investig. 2019, 39, 319–330. [Google Scholar] [CrossRef]
- Stein, A.; Moehler, M.; Trojan, J.; Goekkurt, E.; Vogel, A. Immuno-oncology in GI tumours: Clinical evidence and emerging trials of PD-1/PD-L1 antagonists. Crit. Rev. Oncol./Hematol. 2018, 130, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: A nonrandomized, open-label, phase II study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Azoury, S.C.; Straughan, D.M.; Shukla, V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr. Cancer Drug Targets 2015, 15, 452–462. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Yau, T.; Kang, Y.-K.; Kim, T.-Y.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J. Clin. Oncol. 2021, 39, 269. [Google Scholar] [CrossRef]
- Feun, L.G.; Li, Y.-Y.; Wu, C.; Wangpaichitr, M.; Jones, P.D.; Richman, S.P.; Madrazo, B.; Kwon, D.; Garcia-Buitrago, M.; Martin, P.; et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 2019, 125, 3603–3614. [Google Scholar] [CrossRef] [PubMed]
- Bangaru, S.; Marrero, J.A.; Singal, A.G. New therapeutic interventions for advanced hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 51, 78–89. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Pembrolizumab for the treatment of hepatocellular carcinoma. Liver Cancer 2019, 8, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, Y.J.; Yoon, J.H.; Kim, T.Y.; Han, S.W.; Oh, D.Y.; Im, S.A.; et al. Phase II Study of Avelumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib. Clin. Cancer Res. 2021, 27, 713–718. [Google Scholar] [CrossRef]
- Kudo, M.; Motomura, K.; Wada, Y.; Inaba, Y.; Sakamoto, Y.; Kurosaki, M.; Umeyama, Y.; Kamei, Y.; Yoshimitsu, J.; Fujii, Y.; et al. Avelumab in Combination with Axitinib as First-Line Treatment in Patients with Advanced Hepatocellular Carcinoma: Results from the Phase 1b VEGF Liver 100 Trial. Liver Cancer 2021, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Motomura, K.; Wada, Y.; Inaba, Y.; Sakamoto, Y.; Kurosaki, M.; Umeyama, Y.; Kamei, Y.; Yoshimitsu, J.; Fujii, Y.; et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100). J. Clin. Oncol. 2019, 37, 4072. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.-K.; Qin, S.; Tai, D.W.-M.; Lim, H.Y.; Yau, T.; et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Abou-Alfa, G.K.; Bendell, J.C.; Kim, T.-Y.; Borad, M.J.; Yong, W.-P.; Morse, M.; Kang, Y.-K.; Rebelatto, M.; Makowsky, M.; et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J. Clin. Oncol. 2017, 35, 4073. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sangro, B.; Morse, M.; Zhu, A.X.; Kim, R.D.; Cheng, A.L.; Kudo, M.; Kang, Y.K.; Chan, S.L.; Antal, J.; et al. Phase 1/2 study of durvalumab and tremelimumab as monotherapy and in combination in patients with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2016, 34, TPS3103. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Segal, N.H.; Jaeger, D.; Lee, K.-H.; Marshall, J.; Antonia, S.J.; Butler, M.; Sanborn, R.E.; Nemunaitis, J.J.; Carlson, C.A. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. 2017, 35, 4071. [Google Scholar] [CrossRef]
- Markham, A.; Keam, S.J. Camrelizumab: First Global Approval. Drugs 2019, 79, 1355–1361. [Google Scholar] [CrossRef]
- Qin, S.; Chen, Z.; Liu, Y.; Xiong, J.; Ren, Z.; Meng, Z.; Gu, S.; Wang, L.; Zou, J. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J. Clin. Oncol. 2019, 37, 4074. [Google Scholar] [CrossRef]
- Naing, A.; Gainor, J.F.; Gelderblom, H.; Forde, P.M.; Butler, M.O.; Lin, C.C.; Sharma, S.; Ochoa de Olza, M.; Varga, A.; Taylor, M.; et al. A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti-PD-1 antibody, in patients with advanced solid tumors. J. Immunother. Cancer 2020, 8, e000530. [Google Scholar] [CrossRef]
- Minami, H.; Doi, T.; Toyoda, M.; Imamura, Y.; Kiyota, N.; Mitsuma, A.; Shimokata, T.; Naito, Y.; Matsubara, N.; Tajima, T.; et al. Phase I study of the antiprogrammed cell death-1 Ab spartalizumab (PDR001) in Japanese patients with advanced malignancies. Cancer Sci. 2021, 112, 725–733. [Google Scholar] [CrossRef]
- Prat, A.; Paz-Ares, L.; Juan, M.; Felip, E.; Garralda, E.; González, B.; Arance, A.; Martín-Liberal, J.; Gavilá, J.; López-González, A.; et al. SOLTI-1904 ACROPOLI TRIAL: Efficacy of spartalizumab monotherapy across tumor-types expressing high levels of PD1 mRNA. Future Oncol. 2022, 18, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.L.; Chintharlapalli, S.; Uhlik, M.T.; Pytowski, B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol. Ther. 2016, 164, 204–225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Mulcahy, M.; Gurtler, J.; Sun, W.; Schwartz, J.D.; Dalal, R.P.; Joshi, A.; Hozak, R.R.; Xu, Y.; et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin. Cancer Res. 2013, 19, 6614–6623. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Poon, R.; Pastorelli, D.; Blanc, J.F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Baron, A.D.; Malfertheiner, P.; Kudo, M.; Kawazoe, S.; Pezet, D.; Weissinger, F.; Brandi, G.; Barone, C.A.; Okusaka, T.; et al. Ramucirumab as Second-Line Treatment in Patients With Advanced Hepatocellular Carcinoma: Analysis of REACH Trial Results by Child-Pugh Score. JAMA Oncol. 2017, 3, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Finn, R.; Galle, P.; Llovet, J.; Blanc, J.F.; Okusaka, T.; Chau, I.; Abada, P.; Hsu, Y.; Kudo, M. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: Pooled efficacy and safety across two global randomized Phase 3 studies (REACH-2 and REACH). Ann. Oncol. 2018, 29, v122. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Kang, Y.K.; Yen, C.J.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Motomura, K.; Ohno, I.; et al. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. Br. J. Cancer 2021, 124, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Choucair, K.; Kamran, S.; Saeed, A. Clinical Evaluation of Ramucirumab for the Treatment of Hepatocellular Carcinoma (HCC): Place in Therapy. Onco Targets Ther. 2021, 14, 5521–5532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, X.; Jiang, Y.; Guo, M.; Zhang, S.; Li, J.; He, J.; Liu, J.; Wang, J.; Ouyang, L. Recent advances in the development of dual VEGFR and c-Met small molecule inhibitors as anticancer drugs. Eur. J. Med. Chem. 2016, 108, 495–504. [Google Scholar] [CrossRef]
- Goyal, L.; Zheng, H.; Abrams, T.A.; Miksad, R.; Bullock, A.J.; Allen, J.N.; Yurgelun, M.B.; Clark, J.W.; Kambadakone, A.; Muzikansky, A. A phase II and biomarker study of sorafenib combined with modified FOLFOX in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2019, 25, 80–89. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Scheiner, B.; Leyh, C.; Best, J.; Fründt, T.W.; Czauderna, C.; Beutel, A.; Bettinger, D.; Weiß, J.; Meischl, T.; et al. Cabozantinib in Advanced Hepatocellular Carcinoma: Efficacy and Safety Data from an International Multicenter Real-Life Cohort. Liver Cancer 2021, 10, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Apatinib for molecular targeted therapy in tumor. Drug Des. Dev. Ther. 2015, 9, 6075–6081. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Messersmith, W.A.; Jimeno, A. Apatinib: A promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today 2015, 51, 223–229. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Chen, L.; Guo, H.; Lv, F.; Jia, K.; Yv, K.; Wang, F.; Li, C.; Qian, J.; et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010, 10, 529. [Google Scholar] [CrossRef]
- Tian, S.; Quan, H.; Xie, C.; Guo, H.; Lü, F.; Xu, Y.; Li, J.; Lou, L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011, 102, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Qin, S. Apatinib in Chinese patients with advanced hepatocellular carcinoma: A phase II randomized, open-label trial. J. Clin. Oncol. 2014, 32, 4019. [Google Scholar] [CrossRef]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef]
- García-Hernández, L.; García-Ortega, M.B.; Ruiz-Alcalá, G.; Carrillo, E.; Marchal, J.A.; García, M.Á. The p38 MAPK Components and Modulators as Biomarkers and Molecular Targets in Cancer. Int. J. Mol. Sci. 2022, 23, 370. [Google Scholar] [CrossRef]
- Thomas, B.J.; Porciani, D.; Burke, D.H. Cancer immunomodulation using bispecific aptamers. Mol. Ther.-Nucleic Acids 2022, 27, 894–915. [Google Scholar] [CrossRef]
- Yang, T.; Huo, J.; Xu, R.; Su, Q.; Tang, W.; Zhang, D.; Zhu, M.; Zhan, Y.; Dai, B.; Zhang, Y. Selenium sulfide disrupts the PLAGL2/C-MET/STAT3-induced resistance against mitochondrial apoptosis in hepatocellular carcinoma. Clin. Transl. Med. 2021, 11, e536. [Google Scholar] [CrossRef]
- Raoof, M.; Malhotra, G.; Kohut, A.; O’Leary, M.; Frankel, P.; Tran, T.; Fakih, M.; Chao, J.; Lim, D.; Woo, Y. PIPAC for the treatment of gynecologic and gastrointestinal peritoneal metastases: Technical and logistic considerations of a Phase 1 trial. Ann. Surg. Oncol. 2022, 29, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.S.; Hanley, K.L.; Liang, Y.; Lin, X. Improving the efficacy of liver cancer immunotherapy: The power of combined preclinical and clinical studies. Hepatology 2021, 73, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Brahma, M.K.; Gilglioni, E.H.; Zhou, L.; Trépo, E.; Chen, P.; Gurzov, E.N. Oxidative stress in obesity-associated hepatocellular carcinoma: Sources, signaling and therapeutic challenges. Oncogene 2021, 40, 5155–5167. [Google Scholar] [CrossRef] [PubMed]
- Mabed, M.; Esmaeel, M.; El-Khodary, T.; Awad, M.; Amer, T. A randomized controlled trial of transcatheter arterial chemoembolization with lipiodol, doxorubicin and cisplatin versus intravenous doxorubicin for patients with unresectable hepatocellular carcinoma. Eur. J. Cancer Care 2009, 18, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Talati, C.; Kim, R. Hepatocellular carcinoma (HCC): Beyond sorafenib—Chemotherapy. J. Gastrointest. Oncol. 2017, 8, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.J.; Suh, S.J.; Um, S.H. Current management of hepatocellular carcinoma: An Eastern perspective. World J. Gastroenterol. 2015, 21, 3826–3842. [Google Scholar] [CrossRef]
- Lee, J.E.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; You, Y.K.; Lee, M.A. Epirubicin, cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 235–241. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.-P.; Zhong, B.-Y.; Lau, W.Y.; Madoff, D.C.; Davidson, J.C.; Qi, X.; Cheng, S.-Q.; Teng, G.-J. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: Comparing east and west. Lancet Gastroenterol. Hepatol. 2019, 4, 721–730. [Google Scholar] [CrossRef]
- Louafi, S.; Boige, V.; Ducreux, M.; Bonyhay, L.; Mansourbakht, T.; de Baere, T.; Asnacios, A.; Hannoun, L.; Poynard, T.; Taïeb, J. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC) results of a phase II study. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 1384–1390. [Google Scholar]
- Chia, W.K.; Ong, S.; Toh, H.C.; Hee, S.W.; Choo, S.P.; Poon, D.Y.; Tay, M.H.; Tan, C.K.; Koo, W.H.; Foo, K.F. Phase II trial of gemcitabine in combination with cisplatin in inoperable or advanced hepatocellular carcinoma. Ann. Acad. Med. Singap. 2008, 37, 554–558. [Google Scholar] [CrossRef]
- Lombardi, G.; Zustovich, F.; Farinati, F.; Cillo, U.; Vitale, A.; Zanus, G.; Donach, M.; Farina, M.; Zovato, S.; Pastorelli, D. Pegylated liposomal doxorubicin and gemcitabine in patients with advanced hepatocellular carcinoma: Results of a phase 2 study. Cancer 2011, 117, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Bai, Y.; Lim, H.Y.; Thongprasert, S.; Chao, Y.; Fan, J.; Yang, T.-S.; Bhudhisawasdi, V.; Kang, W.K.; Zhou, Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 2013, 31, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.O.; Kim, W.S.; Park, S.H.; Park, K.W.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2004, 54, 385–390. [Google Scholar] [CrossRef]
- Ikeda, M.; Okusaka, T.; Ueno, H.; Takezako, Y.; Morizane, C. A phase II trial of continuous infusion of 5-fluorouracil, mitoxantrone, and cisplatin for metastatic hepatocellular carcinoma. Cancer 2005, 103, 756–762. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.; Han, S.H.; Kwon, S.Y.; Kwon, O.S.; Kim, S.S.; Kim, J.H.; Park, Y.H.; Lee, J.N.; Bang, S.-M. Systemic chemotherapy with doxorubicin, cisplatin and capecitabine for metastatic hepatocellular carcinoma. BMC Cancer 2006, 6, 3–6. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tsuji, A.; Morita, S.; Horimi, T.; Shirasaka, T.; Kanematsu, T. A phase II study of LFP therapy (5-FU (5-fluorourasil) continuous infusion (CVI) and Low-dose consecutive (Cisplatin) CDDP) in advanced biliary tract carcinoma. BMC Cancer 2006, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Park, J.-W.; Nam, B.H.; Lee, W.J.; Kim, C.-M. Efficacy of combination chemotherapy with capecitabine plus cisplatin in patients with unresectable hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2009, 63, 459–467. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Oh, D.; Kim, J.; Im, S.; Kim, T.; Bang, Y.-J. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann. Oncol. 2009, 20, 1402–1407. [Google Scholar] [CrossRef]
- Patt, Y.Z.; Hassan, M.M.; Aguayo, A.; Nooka, A.K.; Lozano, R.D.; Curley, S.A.; Vauthey, J.N.; Ellis, L.M.; Schnirer, I.I.; Wolff, R.A. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 101, 578–586. [Google Scholar] [CrossRef]
- Porta, C.; Moroni, M.; Nastasi, G.; Arcangeli, G. 5-Fluorouracil and d, l-leucovorin calcium are active to treat unresectable hepatocellular carcinoma patients: Preliminary results of a phase II study. Oncology 1995, 52, 487–491. [Google Scholar] [CrossRef]
- Olweny, C.L.; Toya, T.; Katongole-Mbidde, E.; Mugerwa, J.; Kyalwazi, S.K.; Cohen, H. Treatment of hepatocellular carcinoma with adriamycin. Preliminary communication. Cancer 1975, 36, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.S.; Lin, Y.C.; Chen, J.S.; Wang, H.M.; Wang, C.H. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2000, 89, 750–756. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Clark, J.W.; Ryan, D.P.; Kulke, M.H.; Kim, H.; Earle, C.C.; Vincitore, M.; Mayer, R.J.; Stuart, K.E. A phase II trial of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer 2002, 94, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Kubicka, S.; Rudolph, K.L.; Tietze, M.K.; Lorenz, M.; Manns, M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepato-Gastroenterol. 2001, 48, 783–789. [Google Scholar]
- O’Reilly, E.M.; Stuart, K.E.; Sanz-Altamira, P.M.; Schwartz, G.K.; Steger, C.M.; Raeburn, L.; Kemeny, N.E.; Kelsen, D.P.; Saltz, L.B. A phase II study of irinotecan in patients with advanced hepatocellular carcinoma. Cancer 2001, 91, 101–105. [Google Scholar] [CrossRef]
- Boige, V.; Taïeb, J.; Hebbar, M.; Malka, D.; Debaere, T.; Hannoun, L.; Magherini, E.; Mignard, D.; Poynard, T.; Ducreux, M. Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma: A multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur. J. Cancer 2006, 42, 456–459. [Google Scholar] [CrossRef]
- Leung, T.W.; Patt, Y.Z.; Lau, W.-y.; Ho, S.K.; Simon, C.; Chan, A.T.; Mok, T.S.; Yeo, W.; Liew, C.-t.; Leung, N.W. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin. Cancer Res. 1999, 5, 1676–1681. [Google Scholar]
- Abou-Alfa, G.K.; Niedzwieski, D.; Knox, J.J.; Kaubisch, A.; Posey, J.; Tan, B.R.; Kavan, P.; Goel, R.; Murray, J.J.; Bekaii-Saab, T.S.; et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J. Clin. Oncol. 2016, 34, 192. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Yang, T.; Hsu, C.; Toh, H.; Epstein, R.; Hsiao, L.; Chen, P.; Lin, Z.; Chao, T.-Y.; Cheng, A. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br. J. Cancer 2010, 102, 981–986. [Google Scholar] [CrossRef]
- Sun, W.; Sohal, D.; Haller, D.G.; Mykulowycz, K.; Rosen, M.; Soulen, M.C.; Caparro, M.; Teitelbaum, U.R.; Giantonio, B.; O’Dwyer, P.J. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011, 117, 3187–3192. [Google Scholar] [CrossRef]
- Asnacios, A.; Fartoux, L.; Romano, O.; Tesmoingt, C.; Louafi, S.S.; Mansoubakht, T.; Artru, P.; Poynard, T.; Rosmorduc, O.; Hebbar, M. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: Results of a multicenter phase 2 study. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 112, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Blaszkowsky, L.S.; Ryan, D.P.; Clark, J.W.; Muzikansky, A.; Horgan, K.; Sheehan, S.; Hale, K.E.; Enzinger, P.C.; Bhargava, P. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2006, 24, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Abass, S.A.; Mohamed, A.A.; Muneam Hamid, D. Herbal management of hepatocellular carcinoma through cutting the pathways of the common risk factors. Biomed. Pharmacother. 2018, 107, 1246–1258. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Zaghloul, R.A.; Abdelghany, A.M.; El Gayar, A.M. Selenium nanoparticles and quercetin suppress thioacetamide-induced hepatocellular carcinoma in rats: Attenuation of inflammation involvement. J. Biochem. Mol. Toxicol. 2022, 36, e22989. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhao, L.; Wang, Y.; Wang, Y. Treatment for hepatocellular carcinoma is enhanced when norcantharidin is encapsulated in exosomes derived from bone marrow mesenchymal stem cells. Mol. Pharm. 2021, 18, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).