Wnt Signaling Pathway Is among the Drivers of Liver Metastasis
Abstract
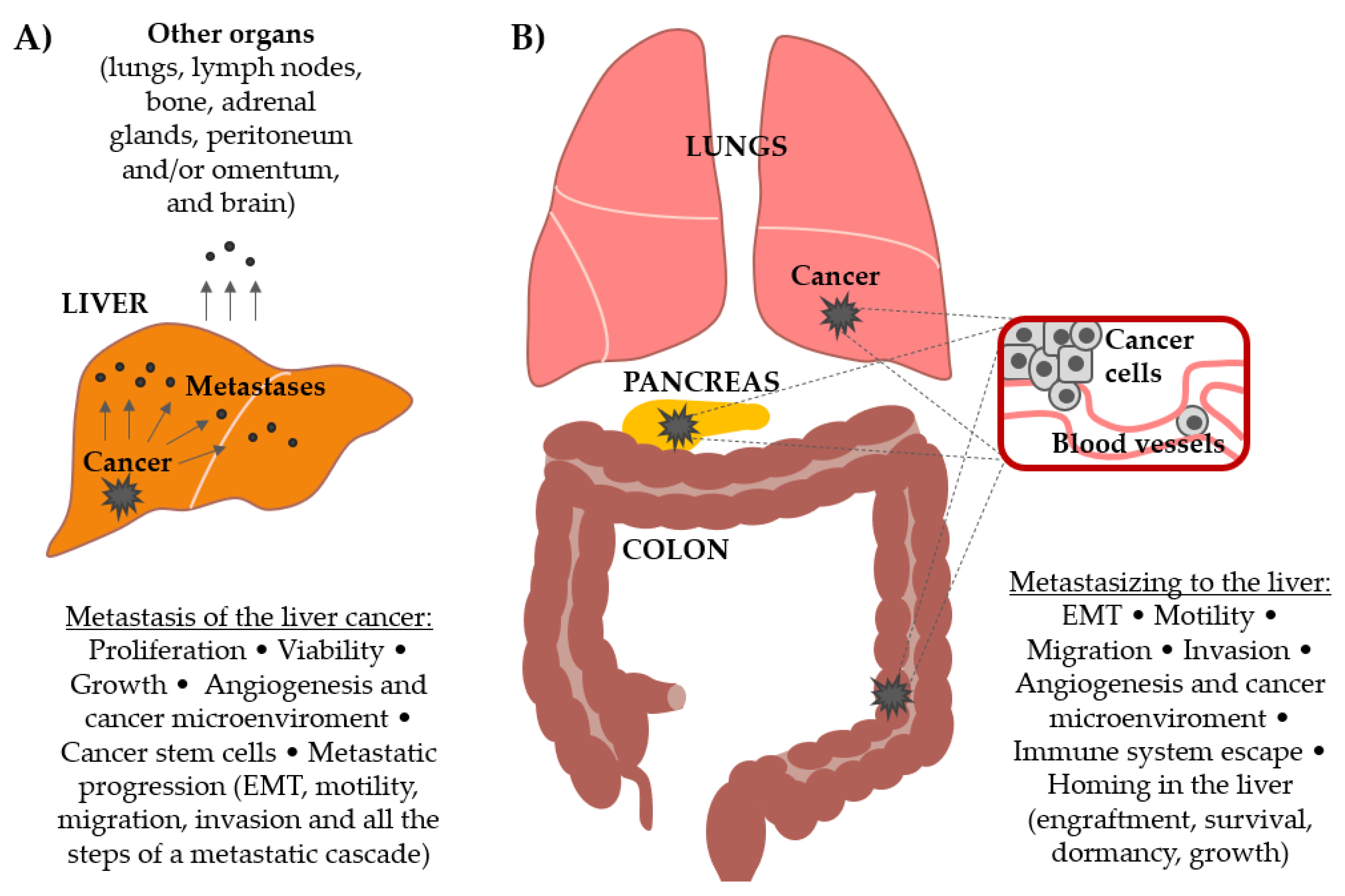
1. Introduction
2. Wnt Signaling Pathways
2.1. ‘Canonical’ Wnt Signaling Pathway
2.2. ‘Non-Canonical’ Wnt Signaling Pathways
2.3. Proteins That Modulate Wnt Signaling Pathways
3. Wnt Signaling Pathway Drives Hepatocellular Carcinoma Metastasis
3.1. Aberrant Expression of Wnt Signaling Pathway Components Correlates with the Ability of Liver Cancers to Metastasize
3.2. Mechanisms of Action of the Wnt Signaling Pathway in Liver Cancers Metastasis Process: The Roles in EMT, Migration, Invasion, and Metastasis Formation
Proteins That Converge to Wnt Signaling Pathway to Influence Liver Cancer Metastasis Formation
3.3. Wnt Signaling Pathway in Liver Cancer Stem Cell and Mesenchymal Stem Cell Biology
3.4. Wnt Signaling Pathway Affects the Communication between Different Components of the Liver Cancer Microenvironment That Promote Metastasis
3.5. Wnt Signaling Pathway Is Activated in Residual HCC Cells after Incomplete Radiofrequency Ablation
4. Wnt Signaling Pathway Drives Secondary Liver Cancers
4.1. Colorectal Cancer Liver Metastasis
4.1.1. Aberrant Expression of Wnt Signaling Pathway Components Correlates with the Ability of CRC to Metastasize to the Liver
4.1.2. Mechanisms of Action of the Wnt Signaling Pathway in CRC Metastasis Process
4.1.3. Wnt Signaling Pathway Affects the Communication between Different Components of the CRC Microenvironment That Promote Metastasis
4.2. Breast Cancer Liver Metastasis
4.3. Gastric Cancer Liver Metastasis
4.4. Lung Cancer Liver Metastasis
4.5. Melanoma Liver Metastasis
4.6. Pancreatic Cancer Liver Metastasis
4.7. Prostate Cancer Liver Metastasis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 14, 16018. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibañes, E.; Pawlik, T.M. Liver metastases. Nat. Rev. Dis. Primers 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mlodzik, M. Wnt-frizzled/planar cell polarity signaling: Cellular orientation by facing the wind (Wnt). Annu. Rev. Cell Dev. Biol. 2015, 13, 623–646. [Google Scholar] [CrossRef]
- De, A. Wnt/Ca 2 signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef]
- Kestler, H.A.; Kühl, M. From individual Wnt pathways towards a Wnt signalling network. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1333–1347. [Google Scholar] [CrossRef]
- Das, S.; Yu, S.; Sakamori, R.; Stypulkowski, E.; Gao, N. Wntless in Wnt secretion: Molecular, cellular and genetic aspects. Front. Biol. 2012, 7, 587–593. [Google Scholar] [CrossRef]
- Malinauskas, T.; Jones, E.Y. Extracellular modulators of Wnt signalling. Curr. Opin. Struct. Biol. 2014, 29, 77–84. [Google Scholar] [CrossRef]
- He, S.; Tang, S. WNT/β-catenin signaling in the development of liver cancers. Biomed. Pharmacother. 2020, 132, 110851. [Google Scholar] [CrossRef]
- Khalaf, A.M.; Fuentes, D.; Morshid, A.I.; Burke, M.R.; Kaseb, A.O.; Hassan, M.; Hazle, J.D.; Elsayes, K.M. Role of Wnt/β-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J. Hepatocell. Carcinoma 2018, 27, 61–73. [Google Scholar] [CrossRef]
- Kwee, S.A.; Tiirikainen, M. Beta-catenin activation and immunotherapy resistance in hepatocellular carcinoma: Mechanisms and biomarkers. Hepatoma Res. 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT signaling in tumors: The way to evade drugs and immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, V.; Turcios, L.; Marti, F.; Gedaly, R. Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment. World J. Gastroenterol. 2016, 22, 823–832. [Google Scholar] [CrossRef]
- Chen, J.; Rajasekaran, M.; Hui, K.M. Atypical regulators of Wnt/β-catenin signaling as potential therapeutic targets in Hepatocellular Carcinoma. Exp. Biol. Med. 2017, 242, 1142–1149. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Jin, R.; Shen, J.; Liang, Y.; Ma, R.; Lin, H.; Liang, X.; Yu, H.; Cai, X. Cytoplasmic and/or nuclear expression of β-catenin correlate with poor prognosis and unfavorable clinicopathological factors in hepatocellular carcinoma: A meta-analysis. PLoS ONE 2014, 9, e111885. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Yao, M.; Cai, Y.; Gu, J.J.; Yang, X.L.; Wang, L.; Yao, D.F. Oncogenic Wnt3a expression as an estimable prognostic marker for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Chen, X.; Zhou, Y.; Yin, B.; Ni, R.; Zhang, Y.; Liu, J. Overexpression of dishevelled 2 is involved in tumor metastasis and is associated with poor prognosis in hepatocellular carcinoma. Clin. Transl. Oncol. 2017, 19, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, X.D.; Liu, Y.K.; Wu, X.; Huang, X.W. Association of hTcf-4 gene expression and mutation with clinicopathological characteristics of hepatocellular carcinoma. World J. Gastroenterol. 2002, 8, 804–807. [Google Scholar] [CrossRef]
- Hyeon, J.; Ahn, S.; Lee, J.J.; Song, D.H.; Park, C.K. Prognostic significance of bcl9 expression in hepatocellular carcinoma. Korean J. Pathol. 2013, 47, 130–136. [Google Scholar] [CrossRef]
- Shi, Y.; Bai, J.; Guo, S.; Wang, J. Wntless is highly expressed in advanced-stage intrahepatic cholangiocarcinoma. Tohoku J. Exp. Med. 2018, 244, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Zucchini-Pascal, N.; Peyre, L.; Rahmani, R. Crosstalk between beta-catenin and snail in the induction of epithelial to mesenchymal transition in hepatocarcinoma: Role of the ERK1/2 pathway. Int. J. Mol. Sci. 2013, 14, 20768–20792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bai, X.; Chen, W.; Ma, T.; Hu, Q.; Liang, C.; Xie, S.; Chen, C.; Hu, L.; Xu, S.; et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis 2013, 34, 962–973. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Cheng, M.; Wang, J.; Liu, Z.; He, S.; Luo, X.; Huang, W.; Chen, T.; Yan, W.; et al. Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci. Rep. 2017, 7, 40446. [Google Scholar] [CrossRef] [PubMed]
- Steinway, S.N.; Zanudo, J.G.T.; Ding, W.; Rountree, C.B.; Feith, D.J.; Loughran, T.P.; Albert, R. Network modeling of TGFβ signaling in hepatocellular carcinoma epithelial-to-mesenchymal transition reveals joint sonic hedgehog and Wnt pathway activation. Cancer Res. 2014, 74, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Yamada, S.; Fuchs, B.C.; Takami, H.; Hayashi, M.; Sugimoto, H.; Fujii, T.; Tanabe, K.K.; Kodera, Y. Clinical implication of Frizzled 2 expression and its association with epithelial-to-mesenchymal transition in hepatocellular carcinoma. Int. J. Oncol. 2017, 50, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Gujral, T.S.; Chan, M.; Peshkin, L.; Sorger, P.K.; Kirschner, M.W.; Macbeath, G. A noncanonical frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell 2014, 159, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; He, Y.; Duan, J.; Yang, Y.; Zhong, C.; Zhang, J.; Liao, W.; Huang, X.; Zhu, R.; Li, M. Expression of Wnt3a in hepatocellular carcinoma and its effects on cell cycle and metastasis. Int. J. Oncol. 2017, 159, 844–856. [Google Scholar] [CrossRef]
- Huang, W.J.; Tian, X.P.; Bi, S.X.; Zhang, S.R.; He, T.S.; Song, L.Y.; Yun, J.P.; Zhou, Z.G.; Yu, R.M.; Li, M. The β-catenin/TCF-4-LINC01278-miR-1258-Smad2/3 axis promotes hepatocellular carcinoma metastasis. Oncogene 2020, 39, 4538–4550. [Google Scholar] [CrossRef]
- Cetin, G.O.; Toylu, A.; Atabey, N.; Sercan, Z.; Sakizli, M. Downregulation of VANGL1 inhibits cellular invasion rather than cell motility in hepatocellular carcinoma cells without stimulation. Genet. Test. Mol. Biomarkers 2015, 19, 283–287. [Google Scholar] [CrossRef]
- Jiang, G.X.; Liu, W.; Cui, Y.F.; Zhong, X.Y.; Tai, S.; Wang, Z.D.; Shi, Y.G.; Li, C.L.; Zhao, S.Y. Reconstitution of secreted frizzled-related protein 1 suppresses tumor growth and lung metastasis in an orthotopic model of hepatocellular carcinoma. Dig. Dis. Sci. 2010, 55, 2838–2843. [Google Scholar] [CrossRef]
- Song, G.; Cao, H.X.; Yao, S.X.; Li, C.T. Abnormal expression of WIF1 in hepatocellular carcinoma cells and its regulating effect on invasion and metastasis factors of TIMP-3 and caveolin-1 of hepatocellular carcinoma. Asian Pac. J. Trop. Med. 2015, 8, 958–963. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Li, Q.; Wang, C.J.; Xie, S.Q. DKK1 promotes hepatocellular carcinoma cell migration and invasion through β-catenin/MMP7 signaling pathway. Mol. Cancer 2013, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Fezza, M.; Moussa, M.; Aoun, R.; Haber, R.; Hilal, G. DKK1 promotes hepatocellular carcinoma inflammation, migration and invasion: Implication of TGF-β1. PLoS ONE 2019, 14, e0223252. [Google Scholar] [CrossRef]
- Tao, Y.M.; Liu, Z.; Liu, H.L. Dickkopf-1 (DKK1) promotes invasion and metastasis of hepatocellular carcinoma. Dig. Liver Dis. 2013, 45, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Yeh, C.T.; Huang, Y.H.; Wu, S.M.; Chi, H.C.; Tsai, M.M.; Tsai, C.Y.; Liao, C.J.; Tseng, Y.H.; Lin, Y.H.; et al. Dickkopf 4 positively regulated by the thyroid hormone receptor suppresses cell invasion in human hepatoma cells. Hepatology 2012, 55, 910–920. [Google Scholar] [CrossRef]
- Li, J.; Gong, W.; Li, X.; Wan, R.; Mo, F.; Zhang, Z.; Huang, P.; Hu, Z.; Lai, Z.; Lu, X.; et al. Recent Progress of Wnt Pathway Inhibitor Dickkopf-1 in Liver Cancer. J. Nanosci. Nanotechnol. 2018, 18, 5192–5206. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Su, Z.; Fei, H.; Liu, X.; Pan, Q. miR-25 promotes hepatocellular carcinoma cell growth, migration and invasion by inhibiting RhoGDI1. Oncotarget 2015, 6, 36231–36244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, J.; Yang, J.; Shen, W.; Zhang, C.; Mou, W.; Luo, J.; Yan, H.; Sun, P.; Luo, Y.; et al. miR-885-5p suppresses hepatocellular carcinoma metastasis and inhibits Wnt/β-catenin signaling pathway. Oncotarget 2016, 7, 75038–75051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Shi, Y.; Hong, D.F.; Song, M.; Huang, D.; Wang, C.Y.; Zhao, G. MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway. Sci. Rep. 2015, 5, 8087. [Google Scholar] [CrossRef]
- Tang, J.; Li, L.; Huang, W.; Sui, C.; Yang, Y.; Lin, X.; Hou, G.; Chen, X.; Fu, J.; Yuan, S.; et al. MiR-429 increases the metastatic capability of HCC via regulating classic Wnt pathway rather than epithelial-mesenchymal transition. Cancer Lett. 2015, 364, 33–43. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, P.; Lin, J.; Zheng, X.; Yang, F.; Zhang, G.; Chen, D.; Xie, J.; Gao, Z.; Peng, L.; et al. MicroRNA-197 promotes metastasis of hepatocellular carcinoma by activating wnt/β-catenin signaling. Cell. Physiol. Biochem. 2018, 51, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Wei, G.; Zhou, C.; Gao, Q.; Wu, Y.; Sun, X.; Li, X. Upregulation of MiR-212 inhibits migration and tumorigenicity and inactivates Wnt/β-catenin signaling in human hepatocellular carcinoma. Technol. Cancer Res. Treat. 2018, 17, 533034618765221. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Li, Y.; Wang, L.; Chen, T.; Niu, Y.; Liu, Q.; Liu, Z. MicroRNA-3194-3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by decreasing Wnt/β-catenin signaling through targeting BCL9. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3885–3895. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ou, J.; Jian, Z.; Ou, Y. miR-186 modulates hepatocellular carcinoma cell proliferation and mobility via targeting MCRS1-mediated Wnt/β-catenin signaling. J. Cell. Physiol. 2019, 234, 23135–23145. [Google Scholar] [CrossRef]
- Huang, F.I.; Chen, Y.L.; Chang, C.N.; Yuan, R.H.; Jeng, Y.M. Hepatocyte growth factor activates Wnt pathway by transcriptional activation of LEF1 to facilitate tumor invasion. Carcinogenesis 2012, 33, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wang, T.H.; Hsu, H.C.; Yuan, R.H.; Jeng, Y.M. Overexpression of CTHRC1 in hepatocellular carcinoma promotes tumor invasion and predicts poor prognosis. PLoS ONE 2013, 8, e70324. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shen, H.; Zhang, Y.; Zhong, F.; Liu, Y.; Qin, L.; Yang, P. CAV1 promotes HCC cell progression and metastasis through Wnt/β-catenin pathway. PLoS ONE 2014, 9, e106451. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, Y.D.; Fu, L.; Xu, W.; Liu, D.; Liang, Q.; Zhang, X.; Xu, L.; Guan, X.Y.; Wu, B.; et al. CLDN3 inhibits cancer aggressiveness via Wnt-EMT signaling and is a potential prognostic biomarker for hepatocellular carcinoma. Oncotarget 2014, 5, 7663–7676. [Google Scholar] [CrossRef]
- Srivastava, J.; Siddiq, A.; Gredler, R.; Shen, X.N.; Rajasekaran, D.; Robertson, C.L.; Subler, M.A.; Windle, J.J.; Dumur, C.I.; Mukhopadhyay, N.D.; et al. Astrocyte elevated gene-1 and c-Myc cooperate to promote hepatocarcinogenesis in mice. Hepatology 2015, 61, 915–929. [Google Scholar] [CrossRef]
- Bacigalupo, M.L.; Manzi, M.; Espelt, M.V.; Gentilini, L.D.; Compagno, D.; Laderach, D.J.; Wolfenstein-Todel, C.; Rabinovich, G.A.; Troncoso, M.F. Galectin-1 Triggers epithelial-mesenchymal transition in human hepatocellular carcinoma cells. J. Cell. Physiol. 2015, 230, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yu, C.; Chen, M.; Tian, S.; Sun, C. Over-expression of TRIM37 promotes cell migration and metastasis in hepatocellular carcinoma by activating Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2015, 464, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, S.; Wang, H.; Wu, J.; Chen, D.; Peng, B.; Zhou, Q. High expression of hexokinase domain containing 1 is associated with poor prognosis and aggressive phenotype in hepatocarcinoma. Biochem. Biophys. Res. Commun. 2016, 474, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.H.; Du, F.J.; Liu, X.J.; Chen, N. Knockdown of FRAT1 inhibits hypoxia-induced epithelialto-mesenchymal transition via suppression of the Wnt/β-catenin pathway in hepatocellular carcinoma cells. Oncol. Rep. 2016, 36, 2999–3004. [Google Scholar] [CrossRef]
- Ye, Y.; Long, X.; Zhang, L.; Chen, J.; Liu, P.; Li, H.; Wei, F.; Yu, W.; Ren, X.; Yu, J. NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 2016, 7, 70303–70322. [Google Scholar] [CrossRef]
- Tang, B.; Tang, F.; Wang, Z.; Qi, G.; Liang, X.; Li, B.; Yuan, S.; Liu, J.; Yu, S.; He, S. Overexpression of CTNND1 in hepatocellular carcinoma promotes carcinous characters through activation of Wnt/β-catenin signaling. J. Exp. Clin. Cancer Res. 2016, 35, 82. [Google Scholar] [CrossRef]
- Chen, J.; Rajasekaran, M.; Xia, H.; Zhang, X.; Kong, S.N.; Sekar, K.; Seshachalam, V.P.; Deivasigamani, A.; Goh, B.K.P.; Ooi, L.L.; et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut 2016, 65, 1522–1534. [Google Scholar] [CrossRef]
- Yan, Y.; Na, Z.; Zhu, J.; Hong, X.T.; Liu, H.; Ou, Y.R.; Su, F.; Rui, W.; Li, Y.M.; Wu, Q. Downregulated connexin32 promotes EMT through the Wnt/β-catenin pathway by targeting Snail expression in hepatocellular carcinoma. Int. J. Oncol. 2017, 50, 1977–1988. [Google Scholar] [CrossRef]
- Lin, J.; Lin, W.; Ye, Y.; Wang, L.; Chen, X.; Zang, S.; Huang, A. Kindlin-2 promotes hepatocellular carcinoma invasion and metastasis by increasing Wnt/β-catenin signaling. J. Exp. Clin. Cancer Res. 2017, 36, 134. [Google Scholar] [CrossRef]
- Sun, L.; Liu, T.; Zhang, S.; Guo, K.; Liu, Y. Oct4 induces EMT through LEF1/β-catenin dependent WNT signaling pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 2599–2606. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Z.; Liu, L.; Sun, J.; Tang, H.; Zhang, B.; Zou, Y.; Li, H. DDX39 promotes hepatocellular carcinoma growth and metastasis through activating Wnt/β-catenin pathway article. Cell Death Dis. 2018, 9, 675. [Google Scholar] [CrossRef]
- Cai, Z.; Qian, Z.Y.; Jiang, H.; Ma, N.; Li, Z.; Liu, L.Y.; Ren, X.X.; Shang, Y.R.; Wang, J.J.; Li, J.J.; et al. hPCL3s promotes hepatocellular carcinoma metastasis by activating b-catenin signaling. Cancer Res. 2018, 78, 2536–2549. [Google Scholar] [CrossRef]
- Lin, Z.; He, R.; Luo, H.; Lu, C.; Ning, Z.; Wu, Y.; Han, C.; Tan, G.; Wang, Z. Integrin-β5, a MIR-185-targeted gene, promotes hepatocellular carcinoma tumorigenesis by regulating β-catenin stability. J. Exp. Clin. Cancer Res. 2018, 37, 17. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Shi, X.; Ding, Y. JUB induces epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway in hepatocellular carcinoma cells. Int. J. Clin. Exp. Pathol. 2018, 11, 1374–1382. [Google Scholar]
- Shao, L.; Jing, W.; Wang, L.; Pan, F.; Wu, L.; Zhang, L.; Yang, P.; Hu, M.; Fan, K. LRP16 prevents hepatocellular carcinoma progression through regulation of Wnt/β-catenin signaling. J. Mol. Med. 2018, 96, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, X.; Sun, D.; Wu, Y.; Zhao, Z. ZIC5 facilitates the growth of hepatocellular carcinoma through activating Wnt/β-catenin pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Hou, B.; Wang, Y.; Deng, D.; Fu, Z.; Xu, Z. A SOX9-AS1/miR-5590-3p/SOX9 positive feedback loop drives tumor growth and metastasis in hepatocellular carcinoma through the Wnt/β-catenin pathway. Mol. Oncol. 2019, 13, 2194–2210. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, X.; Lin, C.; Zhang, X.; Ye, L.; Ren, L.; Chen, M.; Yang, M.; Li, Y.; Li, M.; et al. AKIP1 promotes early recurrence of hepatocellular carcinoma through activating the Wnt/β-catenin/CBP signaling pathway. Oncogene 2019, 38, 5516–5529. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.H.; Cui, Y.P.; He, Y.K.; Shu, R.H. FBXO17 promotes malignant progression of hepatocellular carcinoma by activating wnt/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8265–8273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lin, J.; Wu, H.; Mo, Z.; Lian, Y.; Wang, P.; Hu, Z.; Gao, Z.; Peng, L.; Xie, C. Forkhead box (FOX) G1 promotes hepatocellular carcinoma epithelial-mesenchymal transition by activating Wnt signal through forming T-cell factor-4/Beta-catenin/FOXG1 complex. J. Exp. Clin. Cancer Res. 2019, 38, 475. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, M.; Zhang, R.; Wang, Q.; Li, W.; Dong, X.; Chen, Y.; Lu, Y.; Liu, K.; Lin, B.; et al. GATA5 inhibits hepatocellular carcinoma cells malignant behaviours by blocking expression of reprogramming genes. J. Cell. Mol. Med. 2019, 23, 2536–2548. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Xiao, H.; Luo, C.; Chen, L.; Liu, X.; Hu, Z.; Zou, S.; Guan, J.; Yang, D.; Wang, K. GRP78 activates the Wnt/HOXB9 pathway to promote invasion and metastasis of hepatocellular carcinoma by chaperoning LRP6. Exp. Cell Res. 2019, 383, 111493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.R.; Wang, J.L.; Xu, C.; Li, Y.M.; Sun, B.; Yang, L.Y. HEG1 indicates poor prognosis and promotes hepatocellular carcinoma invasion, metastasis, and EMT by activating Wnt/β-catenin signaling. Clin. Sci. 2019, 133, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.K.; Zheng, H.Z.; Yuan, L.Y. High NDRG3 expression facilitates HCC metastasis by promoting nuclear translocation of β-catenin. BMB Rep. 2019, 52, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, C.; Zhang, B. Upregulation of musashi1 increases malignancy of hepatocellular carcinoma via the Wnt/β-catenin signaling pathway and predicts a poor prognosis. BMC Gastroenterol. 2019, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Li, P.; Wang, X.; Li, J.; Shi, J.; Qin, J.; Zhang, X.; Ma, Y.; Francia, G.; Zhang, J.Y. Overexpression of p62/IMP2 can promote cell migration in hepatocellular carcinoma via activation of the wnt/β-catenin pathway. Cancers 2020, 12, 7. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Gong, L.; Zhu, S.; Tian, J.; Zhang, F.; Zhu, Q.; Wang, J.; Lan, M.; Li, Y.; et al. RICH2, a potential tumor suppressor in hepatocellular carcinoma. Front. Biosci.-Landmark 2019, 24, 1363–1376. [Google Scholar] [CrossRef]
- Liao, S.; Chen, H.; Liu, M.; Gan, L.; Li, C.; Zhang, W.; Lv, L.; Mei, Z. Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/β-catenin pathway. Aging 2020, 12, 1527–1544. [Google Scholar] [CrossRef]
- Du, J.; Zhu, Z.; Xu, L.; Chen, X.; Li, X.; Lan, T.; Li, W.; Yuan, K.; Zeng, Y. ARHGEF11 promotes proliferation and epithelial-mesenchymal transition of hepatocellular carcinoma through activation of β-catenin pathway. Aging 2020, 12, 20235–20253. [Google Scholar] [CrossRef]
- Song, M.; Pan, Q.; Yang, J.; He, J.; Zeng, J.; Cheng, S.; Huang, Y.; Zhou, Z.Q.; Zhu, Q.; Yang, C.; et al. Galectin-3 favours tumour metastasis via the activation of β-catenin signalling in hepatocellular carcinoma. Br. J. Cancer 2020, 123, 1521–1534. [Google Scholar] [CrossRef]
- Wei, S.; Dai, M.; Zhang, C.; Teng, K.; Wang, F.; Li, H.; Sun, W.; Feng, Z.; Kang, T.; Guan, X.; et al. KIF2C: A novel link between Wnt/β-catenin and mTORC1 signaling in the pathogenesis of hepatocellular carcinoma. Protein Cell 2020, 12, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, S.; Xie, H.; Wang, C.; Gao, X.; Rong, Y.; Liu, Z.; Lu, Y. KIF18B promotes hepatocellular carcinoma progression through activating Wnt/β-catenin-signaling pathway. J. Cell. Physiol. 2020, 235, 6507–6514. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Peng, Y.; Hu, J.; Zhan, H.; Yang, L.; Gao, Q.; Jia, H.; Luo, R.; Dai, Z.; Tang, Z.; et al. Metadherin-PRMT5 complex enhances the metastasis of hepatocellular carcinoma through the WNT-ß-catenin signaling pathway. Carcinogenesis 2020, 41, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, M.; Xiang, Y.; Ru, X.; Ren, Y.; Liu, X.; Qiu, L.; Zhang, Y. Nrf1 is endowed with a dominant tumor-repressing effect onto the Wnt/β-catenin-dependent and Wnt/β-catenin-independent signaling networks in the human liver cancer. Oxid. Med. Cell. Longev. 2020, 2020, 5138539. [Google Scholar] [CrossRef]
- Qianqian, L.I.; Ningbo, L.I.; Zeng, Y.; Wang, X.; Jie, L.I.; Hongying, S.U.; Meiqin, G.A.O.; Huang, X. Nuclear receptor FXR impairs SK-Hep-1 cell migration and invasion by inhibiting the Wnt/β-catenin signaling pathway. Oncol. Lett. 2020, 20, 161. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Gao, C.; Sun, Y.; Zhou, K.; Wang, P.; Cheng, J.; Guo, W.; Ya, C.; Fan, J.; et al. USP1 Maintains the survival of liver circulating tumor cells by deubiquitinating and stabilizing TBLR1. Front. Oncol. 2020, 10, 554809. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.-M.; Sun, B.; Zhong, F.-J.; Yang, L.-Y. ATE1 Inhibits liver cancer progression through rgs5-mediated suppression of Wnt/β-catenin signaling. Mol. Cancer Res. 2021, 19, 1441–1453. [Google Scholar] [CrossRef]
- Zuo, Q.; He, J.; Zhang, S.; Wang, H.; Jin, G.; Jin, H.; Cheng, Z.; Tao, X.; Yu, C.; Li, B.; et al. PPARγ coactivator-1α suppresses metastasis of hepatocellular carcinoma by inhibiting Warburg effect by PPARγ–dependent WNT/β-catenin/pyruvate dehydrogenase kinase isozyme 1 axis. Hepatology 2021, 73, 644–660. [Google Scholar] [CrossRef]
- Feng, S.; Liu, J.; Hailiang, L.; Wen, J.; Zhao, Y.; Li, X.; Lu, G.; Gao, P.; Zeng, X. Amplification of RAD54B promotes progression of hepatocellular carcinoma via activating the Wnt/β-catenin signaling. Transl. Oncol. 2021, 14, 101124. [Google Scholar] [CrossRef]
- Li, L.Y.; Yang, J.F.; Rong, F.; Luo, Z.P.; Hu, S.; Fang, H.; Wu, Y.; Yao, R.; Kong, W.H.; Feng, X.W.; et al. ZEB1 serves an oncogenic role in the tumourigenesis of HCC by promoting cell proliferation, migration, and inhibiting apoptosis via Wnt/β-catenin signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1676–1689. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Savic, D.; Dudás, J.; Kvitsaridze, I.; Skvortsov, S.; Riechelmann, H.; Skvortsova, I.I. Cancer stem cells and their unique role in metastatic spread. Semin. Cancer Biol. 2020, 60, 148–156. [Google Scholar] [CrossRef]
- de Sousa e Melo, F.; Vermeulen, L. Wnt signaling in cancer stem cell biology. Cancers 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M. Wnt signaling in stem cells and cancer stem cells: A tale of two coactivators. Prog. Mol. Biol. Transl. Sci. 2018, 153, 209–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huan, H.; Liu, C.; Luo, Y.; Shen, J.; Zhuo, Y.; Zhang, Z.; Qian, C. Deacetylation of β-catenin by SIRT1 regulates self-renewal and oncogenesis of liver cancer stem cells. Cancer Lett. 2019, 463, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, H.Y.; Park, K.K.; Choi, Y.K.; Nam, J.S.; Hong, I.S. CWP232228 targets liver cancer stem cells through Wnt/β-catenin signaling: A novel therapeutic approach for liver cancer treatment. Oncotarget 2016, 7, 20395–20409. [Google Scholar] [CrossRef]
- Leung, C.O.N.; Mak, W.N.; Kai, A.K.L.; Chan, K.S.; Lee, T.K.W.; Ng, I.O.L.; Lo, R.C.L. Sox9 confers stemness properties in hepatocellular carcinoma through Frizzled-7 mediated Wnt/β-catenin signaling. Oncotarget 2016, 7, 29371–29386. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Zhuo, M.; Guo, P.; Chen, Q.; Mo, P.; Li, W.; Yu, C. Histone demethylase JMJD2D promotes the self-renewal of liver cancer stem-like cells by enhancing EpCAM and Sox9 expression. J. Biol. Chem. 2021, 296, 100121. [Google Scholar] [CrossRef]
- Lou, W.; Liu, J.; Gao, Y.; Zhong, G.; Ding, B.; Xu, L. MicroRNA regulation of liver cancer stem cells. Am. J. Cancer Res. 2018, 8, 1126–1141. [Google Scholar]
- Tang, J.; Tao, Z.H.; Wen, D.; Wan, J.L.; Liu, D.L.; Zhang, S.; Cui, J.F.; Sun, H.C.; Wang, L.; Zhou, J.; et al. MiR-612 suppresses the stemness of liver cancer via Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2014, 447, 210–215. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yang, Z.; Wu, L.; Xie, H.; Jiang, C.; Lin, B.; Chen, T.; Xing, C.; Liu, Z.; et al. MicroRNA-452 promotes stem-like cells of hepatocellular carcinoma by inhibiting sox7 involving wnt/β-catenin signaling pathway. Oncotarget 2016, 7, 28000–28012. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zuo, S.; Luo, R.; Li, Y.; Yu, G.; Zou, Y.; Zhou, Y.; Liu, Z.; Liu, Y.; Hu, Y.; et al. HBX-induced miR-5188 impairs FOXO1 to stimulate β-catenin nuclear translocation and promotes tumor stemness in hepatocellular carcinoma. Theranostics 2019, 9, 7583–7598. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Lin, J.; Qin, F.; Yang, Z.; Ding, Y.; Zhang, Y.; Han, L.; Zhu, X.; Zhang, Q. LncAPC drives Wnt/β-catenin activation and liver TIC self-renewal through EZH2 mediated APC transcriptional inhibition. Mol. Carcinog. 2018, 57, 408–418. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Chen, J.; Dai, H.; Wang, L.; Xiong, Y.; Zhong, Y.; Zhang, L. SAMMSON drives the self-renewal of liver tumor initiating cells through EZH2-dependent Wnt/β-catenin activation. Oncotarget 2017, 8, 103785–103796. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Yao, L.; Liu, Y.; Huang, L.; Yan, Z.; Zhao, W.; Zhu, P.; Weng, H. LncFZD6 initiates Wnt/β-catenin and liver TIC self-renewal through BRG1-mediated FZD6 transcriptional activation. Oncogene 2018, 37, 3098–3112. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, D.; Wu, W.; Wu, S.; Qian, J.; Hao, Y.; Yan, F.; Zhu, P.; Wu, J.; Huang, G.; et al. Mesenchymal stem cells promote hepatocarcinogenesis via lncRNA–MUF interaction with ANXA2 and miR-34a. Cancer Res. 2017, 77, 6704–6716. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, N.; Zhu, P.; Chang, J.; Du, Y.; Shen, W. Irradiated mesenchymal stem cells support stemness maintenance of hepatocellular carcinoma stem cells through Wnt/β-catenin signaling pathway. Cell Biosci. 2020, 10, 93. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.L.; Wang, H.T.; Li, D.W.; Dai, H.J.; Zhang, Q.Q.; Zhang, J.; Ma, Y.; Xia, Q.; Bian, J.M.; et al. Overexpression of hepatocyte nuclear factor 4α in human mesenchymal stem cells suppresses hepatocellular carcinoma development through Wnt/β-catenin signaling pathway downregulation. Cancer Biol. Ther. 2016, 17, 558–565. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.C.; Chen, Y.; Zhao, J.L.; Gao, C.C.; Han, H.; Liu, W.C.; Qin, H.Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef]
- Tian, X.; Wu, Y.; Yang, Y.; Wang, J.; Niu, M.; Gao, S.; Qin, T.; Bao, D. Long noncoding RNA LINC00662 promotes M2 macrophage polarization and hepatocellular carcinoma progression via activating Wnt/β-catenin signaling. Mol. Oncol. 2020, 14, 462–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Fu, Y.P.; Gan, W.; Liu, G.; Zhou, P.Y.; Zhou, C.; Sun, B.Y.; Guan, R.Y.; Zhou, J.; Fan, J.; et al. Hepatic stellate cells promote the progression of hepatocellular carcinoma through microRNA-1246-RORα-Wnt/β-Catenin axis. Cancer Lett. 2020, 476, 140–151. [Google Scholar] [CrossRef]
- Olsen, J.J.; Pohl, S.; Öther, G.; Deshmukh, A.; Visweswaran, M.; Ward, N.C.; Arfuso, F.; Agostino, M.; Dharmarajan, A. The role of Wnt signalling in angiogenesis. Clin. Biochem. Rev. 2017, 38, 131–142. [Google Scholar]
- Samarzija, I.; Sini, P.; Schlange, T.; MacDonald, G.; Hynes, N.E. Wnt3a regulates proliferation and migration of HUVEC via canonical and non-canonical Wnt signaling pathways. Biochem. Biophys. Res. Commun. 2009, 386, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Inverso, D.; Shi, J.; Lee, K.H.; Jakab, M.; Ben-Moshe, S.; Kulkarni, S.R.; Schneider, M.; Wang, G.; Komeili, M.; Vélez, P.A.; et al. A spatial vascular transcriptomic, proteomic, and phosphoproteomic atlas unveils an angiocrine Tie–Wnt signaling axis in the liver. Dev. Cell 2021, 56, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Leibing, T.; Géraud, C.; Augustin, I.; Boutros, M.; Augustin, H.G.; Okun, J.G.; Langhans, C.D.; Zierow, J.; Wohlfeil, S.A.; Olsavszky, V.; et al. Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology 2018, 68, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, Y.; Cheng, F.; Hu, Y.; Yang, S.; Rao, J.; Wang, X. Mir-1301 inhibits hepatocellular carcinoma cell migration, invasion, and angiogenesis by decreasing wnt/β-catenin signaling through targeting bcl9. Cell Death Dis. 2017, 8, e2999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Chai, Z.T.; Zhu, Z.M.; Zhu, X.D.; Ma, D.N.; Zhang, Q.B.; Zhao, Y.M.; Wang, M.; Ao, J.Y.; et al. Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating β-catenin signaling. PLoS ONE 2014, 9, e115949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, H.; Qin, C.; Ma, D.; Zhao, Y.; Zhu, W.; Wang, L. Insufficient radiofrequency ablation promotes the metastasis of residual hepatocellular carcinoma cells via upregulating flotillin proteins. J. Cancer Res. Clin. Oncol. 2019, 145, 895–907. [Google Scholar] [CrossRef]
- Schatoff, E.M.; Leach, B.I.; Dow, L.E. WNT Signaling and Colorectal Cancer. Curr. Colorectal Cancer Rep. 2017, 13, 101–110. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef]
- Zhu, G.X.; Gao, D.; Shao, Z.Z.; Chen, L.; Ding, W.J.; Yu, Q.F. Wnt/β-catenin signaling: Causes and treatment targets of drug resistance in colorectal cancer (Review). Mol. Med. Rep. 2021, 23, 105. [Google Scholar] [CrossRef]
- Katoh, M. WNT2 and human gastrointestinal cancer (review). Int. J. Mol. Med. 2003, 12, 811–816. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, Y.; Luo, Q.; Chen, H.; Fan, W.; Pan, Z.; Wang, X.; Zhang, L. High WNT6 expression indicates unfavorable survival outcome for patients with colorectal liver metastasis after liver resection. J. Cancer 2019, 10, 2619–2627. [Google Scholar] [CrossRef]
- Suzuki, H.; Masuda, N.; Shimura, T.; Araki, K.; Kobayashi, T.; Tsutsumi, S.; Asao, T.; Kuwano, H. Nuclear β-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res. 2008, 28, 1821–1830. [Google Scholar]
- Cheng, H.; Liang, H.; Qin, Y.; Liu, Y. Nuclear beta-catenin overexpression in metastatic sentinel lymph node is associated with synchronous liver metastasis in colorectal cancer. Diagn. Pathol. 2011, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Michl, M.; Heinemann, V.; Jung, A.; Engel, J.; Kirchner, T.; Neumann, J. Expression of cancer stem cell markers in metastatic colorectal cancer correlates with liver metastasis, but not with metastasis to the central nervous system. Pathol. Res. Pract. 2015, 211, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Kim, J.H.; Kang, J.; Park, Y.; Park, S.J.; Cheon, J.H.; Kim, W.H.; Kim, H.; Park, J.J.; Kim, T. Il mTOR signaling combined with cancer stem cell markers as a survival predictor in stage II colorectal cancer. Yonsei Med. J. 2020, 61, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Au, K.Y.; Lo, R.C.L. An immunohistochemical study of β-catenin expression and immune cell population in metastatic carcinoma to the liver. Pathol. Oncol. Res. 2021, 27, 1609752. [Google Scholar] [CrossRef]
- Kraus, S.; Vay, C.; Baldus, S.; Knoefel, W.T.; Stoecklein, N.H.; Vallbohmer, D. Expression of wingless-type mouse mammary tumor virus integration site family pathway effectors in lymphatic and hepatic metastases of patients with colorectal cancer: Associations with the primary tumor. Oncol. Lett. 2015, 10, 863–868. [Google Scholar] [CrossRef][Green Version]
- Kirana, C.; Smith, E.; Ngo, D.T.; Trochsler, M.I.; Hewett, P.J.; Stubbs, R.S.; Hardingham, J.E.; Maddern, G.J.; Hauben, E. High preoperative levels of circulating SFRP5 predict better prognosis in colorectal cancer patients. Futur. Oncol. 2020, 16, 2499–2509. [Google Scholar] [CrossRef]
- Ueno, K.; Hazama, S.; Mitomori, S.; Nishioka, M.; Suehiro, Y.; Hirata, H.; Oka, M.; Imai, K.; Dahiya, R.; Hinoda, Y. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br. J. Cancer 2009, 101, 1374–1381. [Google Scholar] [CrossRef]
- Qi, J.; Yu, Y.; Öztürk, Ö.A.; Holland, J.D.; Besser, D.; Fritzmann, J.; Wulf-Goldenberg, A.; Eckert, K.; Fichtner, I.; Birchmeier, W. New Wnt/β-catenin target genes promote experimental metastasis and migration of colorectal cancer cells through different signals. Gut 2016, 65, 1690–1701. [Google Scholar] [CrossRef]
- Li, Y.; Lv, Z.; He, G.; Wang, J.; Zhang, X.; Lu, G.; Ren, X.; Wang, F.; Zhu, X.; Ding, Y.; et al. The SOX17/miR-371-5p/SOX2 axis inhibits EMT, stem cell properties and metastasis in colorectal cancer. Oncotarget 2015, 6, 9099–9112. [Google Scholar] [CrossRef]
- Liang, J.; Chen, M.; Hughes, D.; Chumanevich, A.A.; Altilia, S.; Kaza, V.; Lim, C.U.; Kiaris, H.; Mythreye, K.; Pena, M.M.; et al. CDK8 selectively promotes the growth of colon cancer metastases in the liver by regulating gene expression of TIMP3 and matrix metalloproteinases. Cancer Res. 2018, 78, 6594–6606. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-S.; Shen, F.; Feng, Z.; Chen, J.-W.; Liu, Q.-C.; Li, E.-M.; Xu, B.; Cao, J. Downregulation of CDK-8 inhibits colon cancer hepatic metastasis by regulating Wnt/β-catenin pathway. Biomed. Pharmacother. 2015, 74, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.S.; Wang, J.; Zhang, X.P.; Wang, L.Z.; Wang, Y.; Li, X.X.; Jin, K.Z.; Hu, Z.Q.; Wang, W.J. Hepatocyte nuclear factor 4α suppresses the aggravation of colon carcinoma. Mol. Carcinog. 2016, 55, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.K.; Fan, C.C.; Lin, P.S.; Liao, P.Y.; Tung, J.C.; Hsieh, C.H.; Hung, M.C.; Chen, C.H.; Chang, W.C. Sciellin mediates mesenchymal-to-epithelial transition in colorectal cancer hepatic metastasis. Oncotarget 2016, 7, 25742–25754. [Google Scholar] [CrossRef]
- Dong, X.; Liao, W.; Zhang, L.; Tu, X.; Hu, J.; Chen, T.; Dai, X.; Xiong, Y.; Liang, W.; Ding, C.; et al. RSPO2 suppresses colorectal cancer metastasis by counteracting the Wnt5a/Fzd7-driven noncanonical Wnt pathway. Cancer Lett. 2017, 402, 153–165. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Zhao, N.; Zhu, B.; Zhang, Q.; Yang, X.; Sheng, D.; Hou, J.; Guo, S.; Wei, L.; et al. Inhibition of DNMT suppresses the stemness of colorectal cancer cells through down-regulating Wnt signaling pathway. Cell. Signal. 2018, 47, 79–87. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, C.; Li, H.; Zhang, D.; Liu, G. E2A attenuates tumor-initiating capacity of colorectal cancer cells via the Wnt/beta-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 276. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.X.; Sun, L.G.; Dai, F.H.; Shao, H.S.; Zheng, N.G.; Cai, H.Y. Inhibition of PRRX2 suppressed colon cancer liver metastasis via inactivation of Wnt/β-catenin signaling pathway. Pathol. Res. Pract. 2019, 215, 152593. [Google Scholar] [CrossRef]
- Xu, C.; Tian, G.; Jiang, C.; Xue, H.; Kuerbanjiang, M.; Sun, L.; Gu, L.; Zhou, H.; Liu, Y.; Zhang, Z.; et al. NPTX2 promotes colorectal cancer growth and liver metastasis by the activation of the canonical Wnt/β-catenin pathway via FZD6. Cell Death Dis. 2019, 10, 217. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J. FOXP3 promotes colorectal carcinoma liver metastases by evaluating MMP9 expression via regulating S-adenosylmethionine metabolism. Ann. Transl. Med. 2020, 8, 592. [Google Scholar] [CrossRef]
- Tian, G.A.; Xu, C.J.; Zhou, K.X.; Zhang, Z.G.; Gu, J.R.; Zhang, X.L.; Wang, Y.H. MPC1 Deficiency Promotes CRC Liver Metastasis via Facilitating Nuclear Translocation of β-Catenin. J. Immunol. Res. 2020, 2020, 8340329. [Google Scholar] [CrossRef]
- Agarwal, S.; Behring, M.; Kim, H.G.; Chandrashekar, D.S.; Chakravarthi, B.V.S.K.; Gupta, N.; Bajpai, P.; Elkholy, A.; Al Diffalha, S.; Datta, P.K.; et al. TRIP13 promotes metastasis of colorectal cancer regardless of p53 and microsatellite instability status. Mol. Oncol. 2020, 14, 3007–3029. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, Z.; Zhou, R.; An, H.; Cao, G.; Ye, J.; Huang, C.; Wu, D. Numb negatively regulates the epithelial-to-mesenchymal transition in colorectal cancer through the Wnt signaling pathway. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G841–G853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Yang, C.N.; Kuo, M.Y.P.; Lai, W.T.; Wu, T.S.; Lin, B.R. ATMIN Suppresses metastasis by altering the WNT-signaling pathway via PARP1 in MSI-high colorectal cancer. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, M.; Li, Z. FOSL1 promotes tumorigenesis in colorectal carcinoma by mediating the FBXL2/Wnt/β-catenin axis via Smurf1. Pharmacol. Res. 2021, 165, 105405. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Han, Z.; Wu, X.; Wang, Y.; Zhou, L.; Yang, L.; Liu, N.; Sui, H.; Cai, J.; Ji, Q.; et al. β-arrestin1 promotes colorectal cancer metastasis through GSK-3β/β-catenin signaling- mediated epithelial-to-mesenchymal transition. Front. Cell Dev. Biol. 2021, 9, 650067. [Google Scholar] [CrossRef]
- Yin, H.; Gao, T.; Xie, J.; Huang, Z.; Zhang, X.; Yang, F.; Qi, W.; Yang, Z.; Zhou, T.; Gao, G.; et al. FUBP1 promotes colorectal cancer stemness and metastasis via DVL1-mediated activation of Wnt/β-catenin signaling. Mol. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, L.; Wang, Y.; Peng, Q.; Li, H.; Zhang, X.; Su, Z.; Song, J.; Sun, Q.; Sayed, S.; et al. The CK1δ/ϵ-AES axis regulates tumorigenesis and metastasis in colorectal cancer. Theranostics 2021, 11, 4421–4435. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.; Nakayama, M.; Oshima, H.; Kouyama, Y.; Niida, A.; Fujii, S.; Ochiai, A.; Nakayama, K.I.; Mimori, K.; Suzuki, Y.; et al. Combined mutation of Apc, Kras, and Tgfbr2 effectively drives metastasis of intestinal cancer. Cancer Res. 2018, 78, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hong, S.; Gao, J.; Li, J. Whole-exome sequencing characterized the landscape of somatic mutations and pathways in colorectal cancer liver metastasis. J. Oncol. 2019, 2019, 2684075. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Arlt, F.; Smith, J.; Sack, U.; Herrmann, P.; Walther, W.; Lemm, M.; Fichtner, I.; Shoemaker, R.H.; Schlag, P.M. Intervening in β-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia 2011, 13, 131–144. [Google Scholar] [CrossRef]
- Sack, U.; Walther, W.; Scudiero, D.; Selby, M.; Kobelt, D.; Lemm, M.; Fichtner, I.; Schlag, P.M.; Shoemaker, R.H.; Stein, U. Novel effect of antihelminthic niclosamide on s100a4-mediated metastatic progression in colon cancer. J. Natl. Cancer Inst. 2011, 103, 1018–1036. [Google Scholar] [CrossRef]
- Haase, G.; Gavert, N.; Brabletz, T.; Ben-Ze’ev, A. The wnt target gene L1 in colon cancer invasion and metastasis. Cancers 2016, 8, 48. [Google Scholar] [CrossRef]
- Gavert, N.; Vivanti, A.; Hazin, J.; Brabletz, T.; Ben-Ze’ev, A. L1-Mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol. Cancer Res. 2011, 9, 14–24. [Google Scholar] [CrossRef]
- Wu, Z.; Wei, D.; Gao, W.; Xu, Y.; Hu, Z.; Ma, Z.; Gao, C.; Zhu, X.; Li, Q. TPO-Induced Metabolic Reprogramming Drives Liver Metastasis of Colorectal Cancer CD110+ Tumor-Initiating Cells. Cell Stem Cell 2015, 17, 47–59. [Google Scholar] [CrossRef]
- Takahashi, H.; Ishii, H.; Nishida, N.; Takemasa, I.; Mizushima, T.; Ikeda, M.; Yokobori, T.; Mimori, K.; Yamamoto, H.; Sekimoto, M.; et al. Significance of Lgr5+ve cancer stem cells in the colon and rectum. Ann. Surg. Oncol. 2011, 18, 1166–1174. [Google Scholar] [CrossRef]
- Xu, L.; Lin, W.; Wen, L.; Li, G. Lgr5 in cancer biology: Functional identification of Lgr5 in cancer progression and potential opportunities for novel therapy. Stem Cell Res. Ther. 2019, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019. [Google Scholar] [CrossRef]
- Berdiel-Acer, M.; Bohem, M.E.; López-Doriga, A.; Vidal, A.; Salazar, R.; Martínez-Iniesta, M.; Santos, C.; Sanjuan, X.; Villanueva, A.; Molleví, D.G. Hepatic carcinoma-associated fibroblasts promote an adaptative response in colorectal cancer cells that inhibit proliferation and apoptosis: Nonresistant cells die by nonapoptotic cell death. Neoplasia 2011, 18, 91. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Wei, Z.; Feng, M.; Zhu, Y.; Chen, Y.; Zhu, D. Pharmacological inhibition and genetic knockdown of BCL9 modulate the cellular landscape of cancer-associated fibroblasts in the tumor-immune microenvironment of colorectal cancer. Front. Oncol. 2021, 11, 603556. [Google Scholar] [CrossRef]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 2020, 23, 159–177. [Google Scholar] [CrossRef]
- Song, J.; Shu, H.; Zhang, L.; Xiong, J. Long noncoding RNA GAS5 inhibits angiogenesis and metastasis of colorectal cancer through the Wnt/β-catenin signaling pathway. J. Cell. Biochem. 2019, 120, 6937–6951. [Google Scholar] [CrossRef]
- Kasprzak, A. Angiogenesis-related functions of wnt signaling in colorectal carcinogenesis. Cancers 2020, 12, 3601. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Zhao, Z.; Yang, M.; Chen, M.; Liu, C.; Ji, J.; Zhu, D. Single-cell transcriptome analysis reveals tumor immune microenvironment heterogenicity and granulocytes enrichment in colorectal cancer liver metastases. Cancer Lett. 2020, 470, 84–94. [Google Scholar] [CrossRef]
- Park, J.; Schlederer, M.; Schreiber, M.; Ice, R.; Merkel, O.; Bilban, M.; Hofbauer, S.; Kim, S.; Addison, J.; Zou, J.; et al. AF1q is a novel TCF7 co-factor which activates CD44 and promotes breast cancer metastasis. Oncotarget 2015, 6, 20697–20710. [Google Scholar] [CrossRef]
- Corda, G.; Sala, G.; Lattanzio, R.; Iezzi, M.; Sallese, M.; Fragassi, G.; Lamolinara, A.; Mirza, H.; Barcaroli, D.; Ermler, S.; et al. Functional and prognostic significance of the genomic amplification of frizzled 6 (FZD6) in breast cancer. J. Pathol. 2017, 241, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, A.; Conradi, L.C.; Menck, K.; Schmick, N.A.; Schubert, A.; Rietkötter, E.; Arackal, J.; Middel, P.; Schambony, A.; Liersch, T.; et al. β-catenin-independent WNT signaling and Ki67 in contrast to the estrogen receptor status are prognostic and associated with poor prognosis in breast cancer liver metastases. Clin. Exp. Metastasis 2016, 33, 309–323. [Google Scholar] [CrossRef]
- Fatima, I.; El-Ayachi, I.; Playa, H.C.; Alva-Ornelas, J.A.; Khalid, A.B.; Kuenzinger, W.L.; Wend, P.; Pence, J.C.; Brakefield, L.; Krutilina, R.I.; et al. Simultaneous multi-organ metastases from chemo-resistant triple-negative breast cancer are prevented by interfering with WNT-signaling. Cancers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.P.A.; Yu, J.; Hoffmann, J.; Rocco, A.; Röcken, C.; Kahmann, S.; Müller, O.; Korc, M.; Sung, J.J.; Malfertheiner, P. Loss of beta-catenin expression in metastatic gastric cancer. J. Clin. Oncol. 2003, 21, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.Q.; Li, J.H.; Wen, J.F.; Zhou, Y.H.; Hu, Y.B.; Zhou, J.H. Effect and mechanism of the Twist gene on invasion and metastasis of gastric carcinoma cells. World J. Gastroenterol. 2008, 14, 2487–2493. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, H.; Yamamoto, H.; Sakane, H.; Matsumoto, S.; Ohdan, H.; Sato, A.; Kikuchi, A. An anti-Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol. Cancer Ther. 2012, 11, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, Y.; Huang, J.; Cai, Z.; Wang, Y. RYK, a receptor of noncanonical Wnt ligand Wnt5a, is positively correlated with gastric cancer tumorigenesis and potential of liver metastasis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G352–G360. [Google Scholar] [CrossRef] [PubMed]
- Ara, H.; Takagishi, M.; Enomoto, A.; Asai, M.; Ushida, K.; Asai, N.; Shimoyama, Y.; Kaibuchi, K.; Kodera, Y.; Takahashi, M. Role for Daple in non-canonical Wnt signaling during gastric cancer invasion and metastasis. Cancer Sci. 2016, 107, 133–139. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, M.; Xia, W.; Xu, Y.; Mao, Q.; Wang, J.; Dong, G.; Xu, L.; Yang, X.; Yin, R. Glypican-5 suppresses epithelial-mesenchymal transition of the lung adenocarcinoma by competitively binding to Wnt3a. Oncotarget 2016, 7, 79736–79746. [Google Scholar] [CrossRef]
- Wang, B.; Tang, Z.; Gong, H.; Zhu, L.; Liu, X. Wnt5a promotes epithelial-to-mesenchymal transition and metastasis in non-small-cell lung cancer. Biosci. Rep. 2017, 37, BSR20171092. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chang, W.J.; Jia, C.Y.; Qiao, L.; Zhou, J.; Chen, Q.; Zheng, X.W.; Zhang, J.H.; Li, H.C.; Yang, Z.Y.; et al. Destrin contributes to lung adenocarcinoma progression by activating Wnt/β-catenin signaling pathway. Mol. Cancer Res. 2020, 18, 1789–1802. [Google Scholar] [CrossRef]
- Sinnberg, T.; Levesque, M.P.; Krochmann, J.; Cheng, P.F.; Ikenberg, K.; Meraz-Torres, F.; Niessner, H.; Garbe, C.; Busch, C. Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Mol. Cancer 2018, 17, 59. [Google Scholar] [CrossRef]
- Chetty, R.; Serra, S.; Asa, S.L. Loss of membrane localization and aberrant nuclear E-cadherin expression correlates with invasion in pancreatic endocrine tumors. Am. J. Surg. Pathol. 2008, 32, 413–419. [Google Scholar] [CrossRef]
- Sannino, G.; Armbruster, N.; Bodenhöfer, M.; Haerle, U.; Behrens, D.; Buchholz, M.; Rothbauer, U.; Sipos, B.; Schmees, C. Role of BCL9L in transforming growth factor-β (TGF-β)-induced epithelial-to-mesenchymal-transition (EMT) and metastasis of pancreatic cancer. Oncotarget 2016, 7, 73725–73738. [Google Scholar] [CrossRef]
- Saxena, S.; Purohit, A.; Varney, M.L.; Hayashi, Y.; Singh, R.K. Semaphorin-5A maintains epithelial phenotype of malignant pancreatic cancer cells. BMC Cancer 2018, 18, 1283. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Z.; Mei, D.; Gu, H.; Zhang, J. Long noncoding RNA DLX6-AS1 promotes tumorigenesis by modulating miR-497-5p/FZD4/ FZD6/Wnt/β-catenin pathway in pancreatic cancer. Cancer Manag. Res. 2019, 11, 4209–4221. [Google Scholar] [CrossRef]
- Han, Y.; Ru, G.Q.; Mou, X.; Wang, H.J.; Ma, Y.; He, X.L.; Yan, Z.; Huang, D. AUTS2 is a potential therapeutic target for pancreatic cancer patients with liver metastases. Med. Hypotheses 2015, 85, 203–206. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Meng, Z.; Yang, C.; Li, Z.; Li, M.; Zhang, Z.; Gan, Y.; Tu, H. Cadherin 13 inhibits pancreatic cancer progression and epithelial-mesenchymal transition by Wnt/β-catenin signaling. J. Cancer 2020, 11, 2101–2112. [Google Scholar] [CrossRef]
- Chen, T.; Lei, S.; Zeng, Z.; Zhang, J.; Xue, Y.; Sun, Y.; Lan, J.; Xu, S.; Mao, D.; Guo, B. Linc00261 inhibits metastasis and the WNT signaling pathway of pancreatic cancer by regulating a miR-552-5p/FOXO3 axis. Oncol. Rep. 2020, 43, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Samaržija, I. Site-specific and common prostate cancer metastasis genes as suggested by meta-analysis of gene expression data. Life 2021, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.L.; Daignault, S.D.; Shah, R.B.; Pienta, K.J.; Keller, E.T. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 2008, 68, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Kaliberov, S.; Sohn, R.E.; Kaliberova, L.; Du, Y.; Prior, J.L.; Leib, D.J.; Chauchereau, A.; Sehn, J.K.; Curiel, D.T.; et al. A new model of multi-visceral and bone metastatic prostate cancer with perivascular niche targeting by a novel endothelial specific adenoviral vector. Oncotarget 2017, 8, 12272–12289. [Google Scholar] [CrossRef] [PubMed]
| Protein | Mechanism | Effect | Ref. |
|---|---|---|---|
| HGF | Activates Wnt pathway by transcriptional activation of LEF1 | Facilitates in vitro tumor migration and invasion | [47] |
| CTHRC1 | Activates the PCP pathway of Wnt signaling | Promotes in vitro tumor migration and invasion and cell-matrix adhesion | [48] |
| CAV1 | Induces Wnt/β-catenin pathway through nuclear accumulation of β-catenin | Enhances EMT, invasiveness, and lung metastasis in vitro and in vivo | [49] |
| CLDN3 | Inactivates the Wnt/β-catenin-EMT axis through downregulation of GSK3B, CTNNB1, SNAI2, and CDH2 | Inhibits cell motility and invasiveness in vitro and in vivo | [50] |
| AEG-1 | Transcriptionally regulated by c-Myc and induces c-Myc by activating the Wnt/β-catenin pathway | Activates prosurvival and EMT–signaling pathways and induces in vivo lung metastasis | [51] |
| GAL1 | Promotes β-catenin nuclear translocation, TCF4/LEF1 transcriptional activity and CCND1 and c-Myc expression | Triggers EMT in vitro | [52] |
| TRIM37 | Activates the Wnt/β-catenin pathway | Promotes in vitro and in vivo cell migration and metastasis by inducing EMT | [53] |
| HKDC1 | Downregulation represses β-catenin and c-Myc expression | Associated with aggressive phenotype | [54] |
| FRAT1 | Knockdown suppresses Wnt/β-catenin pathway by partially suppressing the expression levels of β-catenin, CCND1, and c-Myc | Knockdown inhibits in vitro hypoxia-induced EMT, migration, and invasion | [55] |
| NTR1 | NTS/NTR1 co-expression correlates with the activation of the Wnt/β-catenin signaling pathway | NTS/NTR1 co-expression enhances EMT, invasion, and in vivo metastasis formation | [56] |
| CTNND1 | Acts, at least in part, by indirectly enhancing Wnt/β-catenin signaling | Promotes in vitro migration, invasion, and in vivo metastasis formation | [57] |
| PRC1 | Inhibits APC stability, and promotes β-catenin release from the APC complex | Promotes in vitro migration and invasion | [58] |
| CX32 | Its inhibition enhances Snail expression through activation of Wnt/β-catenin signaling | Regulates EMT, migration, and invasion in vitro and inhibits tumor metastasis in vivo | [59] |
| FERMT2 | Activates Wnt/β-catenin signaling and increases β-catenin expression (especially non-phosphorylated form) | Promotes in vitro invasion and metastasis | [60] |
| OCT4 | Upregulates LEF1, a key component of the WNT signaling pathway | Induces EMT in vitro | [61] |
| DDX39 | Activates Wnt/β-catenin pathway by increasing β-catenin levels in the nucleus | Promotes tumor growth, migration, invasion, and in vivo metastasis | [62] |
| PCL3 | Inhibits β-catenin degradation, and activates β-catenin/TCF signaling | Positively regulates the migration, invasion, and in vivo metastasis formation | [63] |
| ITGB5 | Directly interacts with β-catenin and inhibits its degradation, thus leading to Wnt/β-catenin activity | Elevated ITGB5 facilitates in vitro cell migration | [64] |
| JUB | Activates β-catenin in the nuclei | Induces in vitro EMT and migration | [65] |
| LRP16 | Its overexpression could prevent β-catenin from entering the nucleus | Attenuates cell migration, and invasion in vitro, and metastasis in vivo | [66] |
| ZIC5 | Increases the expression of β-catenin and CCND1 and promotes β-catenin to enter the nucleus | Promotes proliferation, migration, and invasion in vitro and in vivo | [67] |
| SOX9 | SOX9-AS1/miR-5590-3p/SOX9 positive feedback acts through the Wnt/β-catenin pathway | Aggravates HCC progression and metastasis in vitro and in vivo | [68] |
| AKIP1 | Interacts with and sustains β-catenin in the nucleus by blocking its interaction with APC; enhances phosphorylation of β-catenin | Promotes invasion and increases intrahepatic and lung metastasis in vivo | [69] |
| FBXO17 | Its silencing might function through downregulating the expression of proteins in Wnt/β-catenin pathway | In vitro metastasis ability in the anti-FBXO17 group is decreased | [70] |
| FOXG1 | Activates Wnt signaling through forming TCF4/β-catenin/FOXG1 complex | Promotes EMT and aggressiveness in vitro and enhances metastasis in vivo | [71] |
| GATA5 | Co-localizes with β-catenin in the cytoplasm, preventing β-catenin from entering the nucleus | Inhibits in vitro cell growth, colony formation, migration, and invasion | [72] |
| GRP78 | Activates the Wnt/HOXB9 pathway by chaperoning LRP6 | Promotes in vitro and in vivo invasion and metastasis | [73] |
| HEG1 | Promotes β-catenin expression and maintains its stability, leading to its accumulation and nuclear translocation | Promotes EMT and in vitro and in vivo invasion and metastasis | [74] |
| NDRG3 | Promotes nuclear translocation of β-catenin | Enhances metastasis and angiogenesis in vitro and in vivo | [75] |
| MSI1 | Activates Wnt/β-catenin signaling pathway (downregulation reduces the expression of phospho-β-catenin and CCND1 and elevates the protein expression of DKK1 and APC) | Affects in vitro cancer cell viability, migration, and invasiveness | [76] |
| p62/IMP2 | Activates Wnt/β-catenin pathway | Promotes in vitro EMT and migration | [77] |
| RICH2 | Overexpression positively correlates with the expression of WNT5a and inversely correlates with β-catenin | Inhibits formation of filopodia and invasion and proliferation in vitro | [78] |
| AQP9 | Overexpression reduces the levels of DVL2, GSK-3β, CCND1, and β-catenin | Overexpression suppresses in vitro migration, invasion, and EMT | [79] |
| ARHGEF11 | Induces β-catenin nuclear translocation and upregulates ZEB1 | Promotes EMT and migration in vitro | [80] |
| GAL3 | Activates the PI3K-Akt-GSK-3β-β-catenin signaling cascade | Regulates in vitro angiogenesis and EMT and favors tumor lung metastasis in vivo | [81] |
| KIF2C | Direct target of the Wnt/β-catenin pathway that mediates the crosstalk between Wnt/β-catenin and mTORC1 signaling | Promotes migration, invasion, and metastasis both in vitro and in vivo | [82] |
| KIF18B | The knockdown downregulates the expression of c-Myc, CCND1, β-catenin, and p-GSK-3β | Knockdown might suppressproliferation, migration, and invasion in vitro | [83] |
| MTDH | Its overexpression induces PRMT5 translocation from the nucleus to the cytoplasm and translocation of β-catenin from the cytoplasm to the nucleus which upregulates WNT/β-catenin signaling pathway | PRMT5 and β-catenin play a pivotal role in MTDH-mediated HCC in vivo metastasis | [84] |
| NRF1 | Enhances ubiquitination of β-catenin for targeting proteasomal degradation | Promotes invasion and metastasis to the lung and liver in in vivo models | [85] |
| FXR | Decreases expression of β-catenin target genes and reduces nuclear translocation of β-catenin proteins in vitro and in vivo | Suppresses migration and invasion in vitro and inhibits local invasion and lung metastasis in vivo | [86] |
| USP1 | Its knockout impairs expression of Wnt target genes | Frequently upregulated in liver circulating tumor cells and expression correlates with metastasis | [87] |
| ATE1 | Accelerates degradation of β-catenin and inhibits Wnt signaling by regulating turnover of RGS5 | Knockdown promotes cancer growth, migration, and disease progression in vitro and in vivo | [88] |
| PGC1α | Inhibits Warburg effect by PPARγ–dependent WNT/β-catenin/PDK1 axis | Suppresses in vitro and in vivo metastasis | [89] |
| RAD54B | Increases nuclear β-catenin and up-regulates Wnt/β-catenin downstream target genes (c-Myc, CCND1, MMP7, CD44, VEGF, c-Jun) | Increases in vitro cell viability and motility, and in vivo intrahepatic metastasis | [90] |
| ZEB1 | Could activate the Wnt/β-catenin signaling pathway by upregulating the protein expression levels of β-catenin, c-Myc, and CCND1 | Promotes in vitro cell proliferation and migration and inhibits apoptosis | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaržija, I. Wnt Signaling Pathway Is among the Drivers of Liver Metastasis. Livers 2021, 1, 180-200. https://doi.org/10.3390/livers1040015
Samaržija I. Wnt Signaling Pathway Is among the Drivers of Liver Metastasis. Livers. 2021; 1(4):180-200. https://doi.org/10.3390/livers1040015
Chicago/Turabian StyleSamaržija, Ivana. 2021. "Wnt Signaling Pathway Is among the Drivers of Liver Metastasis" Livers 1, no. 4: 180-200. https://doi.org/10.3390/livers1040015
APA StyleSamaržija, I. (2021). Wnt Signaling Pathway Is among the Drivers of Liver Metastasis. Livers, 1(4), 180-200. https://doi.org/10.3390/livers1040015






