Abstract
The sleep–wake rhythm, regulated by the suprachiasmatic nucleus (SCN) and peripheral clocks, is influenced by light, nutrition, stress, and exercise. Recent findings suggest that estrogen receptors in the SCN may link ovarian hormone regulation to circadian rhythms, highlighting the need to consider hormonal fluctuations throughout the menstrual cycle when examining the effects of exercise on the sleep–wake rhythm in females. Therefore, we conducted a scoping review to compile existing studies involving human participants, aiming to provide a foundation for research focused on the specific physiological phenomena in females. Based on 228 literature sources, only 6 met the inclusion criteria. Despite variations in the exercise type, intensity, and duration, transient exercise demonstrated a significant impact on the expression of the clock genes BMAL1, PER2, and CRY1, suggesting their susceptibility to such activities. This review highlights the absence of studies examining the effects of exercise on clock gene expression in females, particularly in relation to menstrual cycles and ovarian hormone fluctuations, emphasizing the need for future investigations that incorporate these factors.
1. Introduction
The sleep–wake rhythm, which fluctuates over a 24 h cycle, is regulated by the central clock located in the suprachiasmatic nucleus (SCN) and peripheral clocks expressed locally. Various factors, including light intensity [1,2], nutrition [3], exercise [4,5], and stress [6], can influence the cycle length, phase shift, and amplitude by altering the expression levels of clock genes. Engaging in physical activity or maintaining a regular exercise routine is an important factor in the establishment of the circadian rhythm [7], highlighting the significance of moderate exercise for promoting good sleep quality. Wolff et al. showed that the phase of peak PER2∷LUC bioluminescence in skeletal muscle advanced during four weeks of exercise in mice and that exercise affects the molecular circadian clock in peripheral tissues independently of light stimulation [4]. Furthermore, an animal study by Saracino et al. demonstrated that acute aerobic exercise can activate the protein called Regulated in Development and DNA Damage 1 (REDD1) in skeletal muscle through the release of adrenal stress hormones, such as glucocorticoids, aldosterone, and epinephrine, which increases the mRNA expression of the clock gene Per1 [8]. However, this study did not carry out a times series. This suggests that acute exercise can induce changes in clock gene expression and may, in turn, cause phase shifts in circadian rhythms. However, many of these studies were performed mostly in rodents and, furthermore, have not accounted for the sex of the participants.
The suprachiasmatic nucleus (SCN), a neuronal structure located in the hypothalamus, plays a central role in the regulation of sleep/wake cycles. The release of many hormones, neurotransmitters and other mediators which is rhythmic or regulated by circadian rhythms or displays rhythmicity, is under the control of circadian rhythms [9]. Menstruation, a physiological phenomenon unique to females, is regulated by the secretion of two ovarian hormones (estrogen and progesterone), which follow this circadian rhythm. Estrogen and progesterone, ovarian hormones, fluctuate over a roughly one-month cycle, which is broadly divided into the menstrual phase, follicular phase, ovulatory phase, and luteal phase [10]. Both hormones are at low concentrations during the menstrual phase (the period when menstruation occurs), but after menstruation, during the follicular phase, estrogen levels gradually increase. Following ovulation, both hormones rise, and as the next menstruation approaches, their levels decline again. These fluctuations in female hormone levels across phases are regulated by the hypothalamic–pituitary–gonadal (HPG) axis. Recent studies have reported the presence of estrogen receptors in the SCN of humans [11], suggesting that the SCN, which is the key player of sleep/wake cycles, may regulate the secretion of ovarian hormones and present the possibility that the clock genes can be affected by estrogen. While exercise has been reported to influence clock gene expression, it has also been shown to increase the levels of female hormones, such as estrogen [12] and progesterone [13]. Although the baseline levels of these hormones vary depending on the phase of the menstrual cycle, the elevated concentrations of these hormones are likely to have a significant impact on clock gene expression. Disruptions in the synchronization between the SCN and peripheral clocks, potentially resulting from the combination of exercise and ovarian hormones, may lead to metabolic imbalances. This misalignment has been linked to various health issues, including insulin resistance and metabolic syndrome [14]. Therefore, it is crucial to consider the influence of ovarian hormone levels in females when investigating the effects of exercise on clock gene expression and changes in the phase shift in the sleep–wake rhythm.
We hypothesized that the expression levels of clock genes and the phase shift observed before and after exercise may vary across different phases of the menstrual cycle, even when the same female performs the same exercise. To explore the effects of exercise on clock gene expression in relation to fluctuations in ovarian hormones, we conducted a scoping review. This review aims to summarize the experiments and studies involving human participants to date and provide foundational material for future research in females.
2. Results
After the initial screening, 228 articles were identified (185 in PubMed written all in English and 43 in Ichu-shi written all in Japanese). Of these, six studies examined the expression of clock genes before and after acute exercise. Five of the studies involved male participants exclusively, with one not disclosing the information of the participants’ sex (Table 1).

Table 1.
Overview of studies included in scoping review.
Yeung et al. [15], Small et al. [17], and Zambon et al. [18] collected biopsies from the patellar tendon, vastus lateralis muscle, and lateral portion of the vastus lateralis muscle, respectively. In the experiments conducted by Yeung et al. [15] and Zambon et al. [18], a single-leg exercise was performed, and biopsies were taken from both the rested and exercised legs. Yeung et al. [15] collected biopsies at three time points—2 h, 6 h, and 26 h post-exercise—which were assigned randomly. Zambon et al. [18] obtained biopsies from the same participants at 6 h and 18 h post-exercise, also from both the rested and exercised legs. Small et al. [17] collected biopsies prior to a cycling exercise and 60 min after exercise completion.
Tanaka et al. [16] and de Souza Teixeira et al. [20] used venous blood samples. Tanaka et al. analyzed peripheral blood mononuclear cells, whereas de Souza Teixeira et al. examined effector-memory CD4 + and CD8 + T-cells. Tanaka et al. implemented a washout period of at least one week and conducted both an exercise and a non-exercise session. All participants were assessed every 3 h (6:00, 9:00, 12:00, 15:00, 18:00, 21:00, 23:00, and 6:00 on the second day) during each session [16]. De Souza Teixeira et al. measured samples before exercise, 10 min post-exercise, and 60 min post-exercise [20].
Okamoto et al. [19] collected hair follicle cells by extracting facial hair roots at seven time points (12:00, 16:00, 20:00, 24:00, 4:00, 8:00, and 12:00) every 4 h over 4 days, following the athlete’s training schedule.
2.1. Clock Genes
Only PER2 was included in the analysis of all six studies. BMAL1 and CRY1 were analyzed in five of the six studies, PER1 and REV-ERBα (NR1D1) in four, CLOCK, CRY2, and PER3 in three, REV-ERBβ (NR1D2) in two, and Nfil3, C/EBPb, DBP, ARNTL, RORa, RORb, and RORc were analyzed in only one of the six studies. The details of the clock genes analyzed and the primers used in these studies are provided in Supplementary Table S1.
2.2. Participant Characteristics
Although five studies included only male participants, the age groups were inconsistent across the studies. Yeung et al. included 31 healthy young males, with ages ranging from 23.5 ± 3.0 years in group A, 23.5 ± 3.0 years in group B, and 23.9 ± 2.0 years in group C [15]. Tanaka et al. included 11 healthy young males aged 24.5 ± 0.8 years, with a weight of 67.3 ± 7.3 kg [16]. Small et al. enrolled 16 males aged 18–21 years, mainly in their 20s [17]. In contrast, Zambon et al. included four males aged 31–51 years [18], Okamoto et al. examined one male aged 34 years [19], and de Souza Teixeira et al. evaluated eighteen master athletes (MA) with an average age of 53.56 ± 9.25 years [20]. Furthermore, Okamoto et al. and de Souza Teixeira et al. assessed athletes [19,20], while the participants in the other four studies were physically active but did not participate in competitive exercise [15,16,17,18].
2.3. Exercise Intensity
Three of the six studies used a cycling ergometer to assess exercise intensity: one study evaluated a 60% VO2 max for 60 min [16], another assessed an 80% VO2 max for 15 min [17], and a third used an all-out effort [20]. Yeung et al. used a cycling ergometer with one-leg pedaling and a 67% Wmax load for 1 h [15]. In the study by Zambon et al., participants performed resistance exercises consisting of 10 sets of eight isotonic knee extension exercises at 80% 1-RM [18]. In contrast, Okamoto et al. evaluated professional fighters who performed 2 h of daily resistance exercises but did not provide further details about the specific exercises [19].
2.4. Sampling Points and Time
Yeung et al. analyzed 31 healthy young males who performed a one-leg kicking exercise, collecting samples from three groups at 2, 6, and 26 h after the exercise [15]. Zambon et al. collected samples at 6 h (20:00) and 18 h (8:00 the next morning) after exercise [18]. Small et al. collected samples twice: immediately before and 60 min after the end of the exercise [17]. de Souza Teixeira et al. collected samples before the exercise as well as 10 min and 1 h after the exercise [20]. These four studies examined the expression levels of clock genes before and after exercise but did not assess the effects on the phase of the circadian rhythms.
In contrast, Tanaka et al. studied the phase of clock genes, with eight sampling points obtained [16]. Okamoto et al. examined the effect on the phase of circadian rhythms, establishing seven sampling points every 4 h (12:00, 16:00, 20:00, 24:00, 4:00, 8:00, and 12:00) [19].
2.5. Sample Type
Not all six studies included in this review used the same specimens. One study used tendon samples, two used the gastrocnemius muscle, two used lymphocytes, and one used hair follicles.
Yeung et al. used patellar tendons as specimens and compared the results at each time point between the exercised and non-exercised (control) sides [15]. Two studies performed muscle biopsies on the vastus lateralis muscle [17,18]. Tanaka et al. and de Souza Teixeira et al. used leukocytes, including lymphocytes, by collecting and analyzing blood samples [16,20]. Okamoto et al. collected hair follicles from the tips of the chin (approximately five hair follicles) [19].
3. Discussion
This review aimed to scope the effects of (acute) exercise on the expression levels of clock genes and the sleep–wake rhythm regulated by these genes in humans. Although exercise-induced alterations in clock gene expression have been reported, studies considering sex differences and female-specific physiological phenomena remain limited. Notably, estrogen, one of the ovarian hormones, has been implicated in the regulation of sleep mechanisms and may influence both clock gene expression and the sleep–wake cycle [11]. This review highlights that the included studies were conducted with male participants (including one study unknown) and did not account for physiological phenomena specific to females. It underscores the importance of considering these factors in future research.
The reported exercise-induced changes in clock genes suggest that intense or acute exercise affects the expression of circadian clock genes across various tissues. The expression levels of clock-related genes, such as BMAL1, PER2, and CRY1, were observed to be regulated by exercise. In a study analyzing the post-exercise expression levels of clock genes in tissues, such as tendons, skeletal muscle, immune cells, and follicular cells, individual differences in the clock gene expression and variability were observed under different conditions (e.g., young versus old tendons and exercised versus unexercised muscles). These findings provide valuable insights for designing future experiments, as follows.
3.1. Sample Size and Selection
The authors of all six studies acknowledged limitations related to the sample size and selection criteria. The authors noted that the small sample size limited the generalizability of the results. In addition, control groups often consisted of healthy individuals, which may not fully represent the broader population, particularly in studies involving older individuals.
3.2. Tissue-Specific Differences
The studies selected from the screening of the primary and secondary literature focused on the effects of exercise on circadian clock gene expression and circadian rhythms in various tissues, particularly skeletal muscles, immune cells, and hair follicle cells. These studies aimed to understand how exercise affects the molecular clock mechanisms across different tissues and how changes in the clock gene expression are related to overall health and athletic performance.
Yeung et al. examined clock gene expression using the patellar tendon as a specimen and reported that the expression of clock genes before and after transient exercise was less sensitive to detection [15]. In a 2014 report, Yeung et al. compared clock gene expression levels in tendons using the same analytical method and tissue samples from the same animals to measure clock gene expression in cartilage tissue [21]. They reported that circadian rhythms differed between tissues, even within the same individual. However, there are only a few reports on the expression levels of clock genes in human tendons, raising concerns about the validity of the use of tendons as a sample for future studies.
Two of the included studies used vastus lateralis muscle biopsies [17,18]. While the discovery of clock genes in mice has been reported [22], the study by Zambon et al. was the first to report that, in humans, exercise upregulates the expression of a specific clock gene [18]. However, the limitations of using biopsies to examine clock gene expression levels are evident. Biopsies cannot provide insights into the overall effects of clock genes on the entire body and do not allow for frequent sample collection, which is necessary for phase delineation.
Basti et al. investigated the efficacy of peripheral blood mononuclear cells (PBMCs), hair roots, and saliva samples for assessing clock gene expression [23]. While approximately 10 hair samples were collected at a time, the detection sensitivity was low in females, indicating the potential for sex differences. This suggests that when using hair samples collected from females, the number of samples collected should be higher. In contrast, the expression cycles of BMAL1 and PER2 range from 20 to 28 h in both hair samples and PBMCs. The similarity in the expression levels of clock genes across saliva and hair samples collected at three different time points (early 9:00, middle 17:00, and late 21:00) suggests that saliva samples may be useful for clock gene analysis.
The studies highlight that the response of clock genes may vary across tissues, emphasizing the importance of considering the specific tissues being analyzed in each study. It is also crucial to recognize that the biological clock maintains its rhythm under the influence of various zeitgebers. These include exercise [4,5], feeding [24], temperature variations [25], and bright light [25], which is considered the most powerful. While bright light serves as the most potent zeitgeber for the central clock, peripheral clocks are influenced by factors such as feeding patterns, exercise, and temperature changes [26]. The studies included in this review primarily focus on peripheral clocks, and, thus, we cannot discuss the extent to which exercise affects the SCN or central clock. Nevertheless, when the synchronization between the SCN and peripheral clocks is disrupted, for example, due to mismatched feeding times or inconsistent sleep patterns, it can result in metabolic imbalances. This lack of alignment has been associated with a variety of health issues, such as insulin resistance and metabolic syndrome [14]. Therefore, as research on females progresses, it is important to focus on examining exercise as a zeitgeber for regulating peripheral clock gene expression directly, without involving the central clock.
3.3. Type of Exercise and Timing of Measurements
When considering the impact of exercise on clock genes, the results may vary depending on the type, intensity, and timing of the exercise. The six included in this review used different methods of exercise stress. Yeung et al. [15], Small et al. [17], and Zambon et al. [18] conducted evaluations using muscle biopsies, and each used different exercise modalities. Yeung et al. and Zambon et al. used resistance exercises on lower limbs, while Small et al. used high-intensity aerobic exercise. Limited exploration exists regarding the potential impact of different exercise modalities, intensities, and timings on clock genes. Additionally, the presence or absence of participants’ exercise habits must be considered, as this factor may influence the choice of exercise stress tests used. In 2020, the guidelines from the World Health Organization on physical activity and sedentary behavior emphasized the crucial role of regular physical activity in preventing and managing non-communicable diseases. The guidelines recommend that adults engage in at least 150–300 min of moderate-intensity or 75–150 min of vigorous-intensity aerobic physical activity per week [27]. Consequently, most research exploring the connection between exercise and the circadian rhythm has focused on aerobic exercises of moderate to high intensity, lasting more than 30 min per day. Furthermore, the phase-shifting effects of exercise vary depending on the time of day. It has been reported that exercise in the early active phase (from morning to early afternoon) advances the circadian clock, whereas exercise in the late inactive phase (after 7 PM) delays it [26,28]. Additionally, a study in which participants were kept under complete darkness for three days while following a strict daily schedule, including 10 min of cycling exercise, demonstrated rhythm entrainment [29]. Another study found that only the group that performed cycling exercises under low-light conditions showed enhanced entrainment to the daily schedule, suggesting that exercise after waking strengthens phase adjustment [30]. However, the specific factors in physical exercise that influence circadian rhythms remain largely unknown. While exercise affects various physiological functions, many of its effects are mediated by the autonomic nervous system.
3.4. Inflammation and Methodology
Two studies have highlighted potential issues associated with inflammation, particularly with regard to blood sample collection [16,20]. In night-shift workers with circadian rhythm disorders, circadian rhythm disruption leads to impaired inflammatory responses [31] and the upregulation of inflammatory markers [32].
Tanaka et al. [16] used PBMCs as specimens collected from intravenous catheters. This method may increase inflammatory biomarkers, including interleukin-6 (IL-6), due to the puncture from the blood collection needle [33]. However, Tanaka et al. reported that the clock gene expression at 6:00 on day 2 was similar to that at 6:00 on day 3, suggesting that the inflammation caused by the puncture is unlikely to have affected the clock gene expression.
de Souza Teixeria et al. reported differences in inflammatory profiles and clock gene expression after intense exercise between MA and non-exercising healthy individuals [20]. After acute exercise sessions, the expression of CRY1 and REV-ERBα (NR1D1) in effector memory CD4 + T cells was increased in MA. Additionally, the expression of REV-ERBα (NR1D1) positively correlates with the maximal oxygen uptake, exercise duration, and power. In contrast, in non-exercisers, the expression of CLOCK and BMAL1 in effector memory CD4 + T cells increased after exercise. In addition, in non-exercisers, the REV-ERBβ (NR1D2) expression in effector memory CD8 + T cells is lower. While the main conclusion of this study is that lifelong exercise may help ameliorate age-related inflammatory conditions, it highlights that clock genes can be regulated in lymphocytes after acute exercise, regardless of the type of exercise training.
Both authors emphasized the importance of considering inflammatory markers when interpreting the results. In addition, the use of specific molecular assays and their detection limits have been discussed as methodological concerns.
3.5. Comparison with Animal Models
In a few studies, the results of human experiments have been compared with similar experiments in animal models, particularly rodents [16,17,18]. In a report by Small et al., experiments in young males showed that after 1 h of intense exercise (80% VO2 max on a bicycle ergometer), the PER1 and PER2 expression in the vastus lateralis muscle increased by approximately 1.5-fold [17]. In contrast, in experiments where the soleus muscle of mice was excised and contracted in vitro, the Per2 expression increased approximately 1.4-fold 1 h after the contractile stimulus. Furthermore, an in vitro model using the electrical pulse stimulation (EPS) of cultured C2C12 myotubes confirmed that the EPS-induced contraction increased the Per2 gene expression. These findings suggest that, in humans, exercise affects the expression of PER1, PER2, REV-ERBα (NR1D1), and CRY1, while in in vitro experiments with mice, contraction mainly affects the Per2 expression. This suggests that the Per2 expression may be directly regulated by muscle contraction, while the expression of other clock genes may be influenced by systemic factors, such as hormones and the nervous system, associated with exercise.
Similarly, Zambon et al. reported a species difference in the circadian rhythm gene expression between human and mouse skeletal muscle [18]. PER1 (Per1) and PER2 (Per2) were upregulated in the morning in human skeletal muscles, while in mouse skeletal muscles, these genes were downregulated in the morning. This observation may reflect an opposite phase of the core clock gene expression between human and mouse peripheral tissues. However, they were unable to determine the exact phase of the circadian rhythm genes in human skeletal muscle because human samples were measured at only two time points in their protocol.
In experiments with mice, the exercise load directly affects the peripheral clock in the muscles without affecting the SCN [4,34,35]. For instance, the disruption of CLOCK, one of the central clock genes, in mice did not inhibit the improvements in running ability, glucose tolerance, mitochondrial protein expression, and mitochondrial enzyme activity induced by aerobic training, suggesting that CLOCK is not essential for these skeletal muscle adaptations [36]. Cross-species and cross-tissue comparisons will likely offer a more comprehensive understanding of circadian gene regulation and the tissue-specific responses to exercise.
The included studies shared common concerns regarding the sample size, tissue-specific differences, timing of measurements, assessment of protein levels, inflammation, and benefits of cross-species comparisons. Recognizing and addressing these issues is crucial for advancing our understanding of circadian rhythms, the effects of exercise, and the regulation of clock genes in humans.
3.6. Relationship Between Estrogen and Clock Gene Expression
Receptors for estrogen, one of the female hormones, are expressed in the SCN, which regulates sleep–wake rhythms and other functions of the SCN [11]. This suggests that the SCN is involved in the regulation of ovarian hormone secretion and is affected by the binding of estrogen. Estrogen affects the circadian clock gene expression by directly and indirectly modulating the expression of several circadian clock genes [37,38,39]. Estrogen regulates gene expression via the estrogen response elements (EREs), which are specific DNA sequences [40].
Several circadian clock genes contain EREs and are regulated by estrogen, among which the PER2 and CLOCK genes are directly regulated by estrogen via EREs [41,42].
Estrogen affects the circadian clock gene expression through ERE- or E-box-independent mechanisms. For example, in human breast cancer MCF-7 cells, PER2 directly binds to ERα, promoting its degradation, whereas PER2 knockdown leads to ERα stabilization [43]. Similarly, CLOCK interacts directly with ERα and enhances the estrogen-ERα transcriptional activity [44]. Furthermore, estrogen regulates the CLOCK transcriptional activity by inducing its post-translational modifications [44]. Estrogen treatment increases CLOCK sumoylation (which is a post-translational modification that alters the localization, stability, or function of proteins), which in turn enhances the CLOCK-mediated transcription of a luciferase reporter [44]. Thus, estrogen influences the circadian clock gene expression through the direct regulation of PER2 and CLOCK genes via EREs and other mechanisms, including direct binding and post-translational modifications between ER and circadian clock proteins.
Therefore, it is essential to consider the varying levels of estrogen, as well as the different phases of the menstrual cycle, when examining the effects of exercise on the expression of clock genes and circadian rhythms in females.
3.7. The Examination of Other Relevant Factors in the Effect of Exercise on the Expression of Clock Genes
Firstly, the presence of melatonin is another factor involved in the effects of exercise on circadian rhythms. Melatonin is primarily secreted by the pineal gland and plays a crucial role in the regulation of circadian rhythms through melatonin receptors (MTNR1A, MTNR1B) expressed in tissues such as the pancreas, hypothalamus, and retina. In relation to exercise, it has been shown that the timing of exercise significantly affects the secretion rhythm of melatonin. Buxton et al. reported that the response to exercise varies by the time of day, with morning exercise delaying the phase of melatonin secretion, while evening exercise advances the phase [45]. Additionally, Youngstedt et al. demonstrated that even just 1 h of moderate exercise for 3 days induces a significant shift in the onset time of key metabolites, such as 6-sulfatoxymelatonin (the primary metabolite of melatonin), in urine [46]. Regarding sex differences in melatonin secretion, there is evidence suggesting that sex differences exist and that the menstrual cycle should be considered [47]. Women tend to have an earlier melatonin secretion onset compared to men (women: 22:49 ± 1.45 h, men: 23:28 ± 1.27 h) [48]. Furthermore, it has been suggested that women exhibit greater melatonin suppression than men when exposed to the same intensity of light (400 lux: women 93.7 ± 9.6% suppression, men 83.2 ± 18.7% suppression; 2000 lux: women 99.5 ± 1.0% suppression, men 96.9 ± 4.3% suppression) [49]. Given these findings, it is important to consider the time of exercise, standardize the timing of the exercise session, and control the light intensity. Additionally, because no studies have considered the menstrual cycle [47], it is essential to examine the potential involvement of melatonin secretion rhythms in the effects of exercise on circadian rhythms.
Secondly, is the testosterone level. Testosterone levels have been reported to increase with exercise [50]. However, it has also been reported that testosterone concentrations in men are nearly 15 times higher than in women [51], and testosterone levels in healthy women are relatively low. Moreover, studies focusing on women have consistently reported a mean difference of 0.5 nmol/L in testosterone levels following exercise interventions, regardless of the exercise type (endurance, isokinetic, or strength) [52]. In the prostate mesenchyme of rats, a decrease in testosterone levels due to castration resulted in the reduced expression of Bmal1 and Clock transcripts [53]. Furthermore, the addition of testosterone significantly increased the transcripts of Bmal1, Clock, and Casp3 in cultured cells, while the Per2 and Nr1d1 transcripts were significantly inhibited.
In women, polycystic ovary syndrome (PCOS), characterized by high androgen levels (including elevated testosterone), has been shown to have significantly lower BMAL1 expression in granulosa cells compared to women without PCOS [54]. This suggests that testosterone itself can influence the expression of clock genes. Based on these findings, it is likely that the increase in testosterone levels due to exercise could influence the expression of clock genes (e.g., increased Bmal1 and Clock and decreased Per2 and Nr1d1 in mice). Even though testosterone levels are relatively low in healthy women, and the increase in testosterone levels following exercise is consistent across different types of exercise, it is important to evaluate testosterone levels as part of the assessment over the menstrual cycle since there has a few studies on this.
4. Materials and Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Reviews checklist [55].
A comprehensive literature search was performed on PubMed, along with a thorough review of the Japanese biomedical literature (Table 2), on 10 December 2024.

Table 2.
Database search strategies.
4.1. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) the text was written in English or Japanese and (2) the study design was observational (cross-sectional, cohort, and case–control studies). The exclusion criteria were as follows: (1) the full text was not available, (2) the participants were not human, (3) healthy participants, (4) the characteristics of the participants were not described, (5) clock genes were not evaluated, and (6) the relationship between exercise and clock genes was not examined.
4.2. Study Selection
The literature review was conducted independently by two authors. The process involved first assessing the title and abstract of each article, followed by a full-text analysis of the relevant studies in accordance with the inclusion and exclusion criteria.
The PRISMA flowchart in Figure 1 illustrates the selection process and the number of articles identified at each stage.
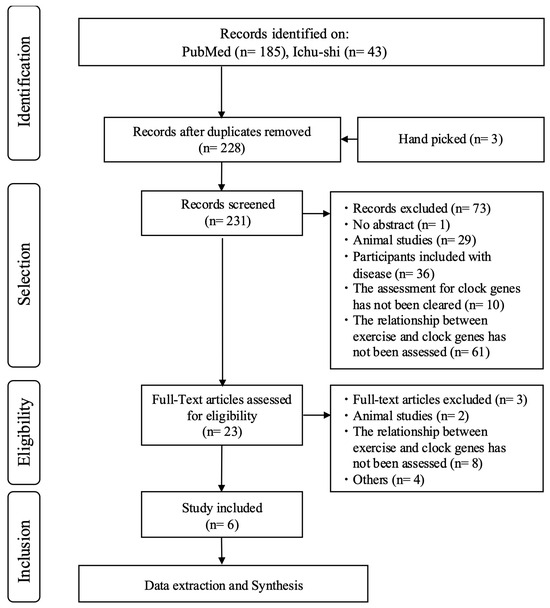
Figure 1.
PRISMA flowchart of selected studies.
4.3. Quality Assessment
In accordance with the guidelines for scoping reviews, the quality of the included studies and the risk of bias were not assessed.
5. Conclusions
The findings of this review contribute to our understanding of how exercise affects the expression of circadian clock genes in various tissues in humans (males). The results highlight the importance of factors such as the timing, duration, and individual training status when examining exercise-induced changes in circadian rhythms. However, no studies have yet examined the effects of exercise on clock gene expression in females. Designing experiments that consider these factors may provide meaningful insights into the interplay between exercise, circadian rhythms, and overall health when considering sex differences.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/women5020015/s1, Table S1: Primers used for gene amplification in included studies.
Author Contributions
A.S. conceived of the study. A.S. and T.S. participated in the design of the study. A.S. and T.S. undertook the literature review process. A.S. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Japan Society for the Promotion of Sciences (grant number: JP 20K19501).
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| SCN | suprachiasmatic nucleus |
| REDD1 | Regulated in Development and DNA Damage 1 |
| HPG | hypothalamic-pituitary-gonadal |
References
- Meijer, J.H.; Schwartz, W.J. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythm. 2003, 18, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Kavcic, P.; Rojc, B.; Dolenc-Groselj, L.; Claustrat, B.; Fujs, K.; Poljak, M. The impact of sleep deprivation and nighttime light exposure on clock gene expression in humans. Croat. Med. J. 2011, 52, 594–603. [Google Scholar] [CrossRef]
- Tognini, P.; Murakami, M.; Liu, Y.; Eckel-Mahan, K.L.; Newman, J.C.; Verdin, E.; Baldi, P.; Sassone-Corsi, P. Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metab. 2017, 26, 523–538.e5. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Esser, K.A. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med. Sci. Sports Exerc. 2012, 44, 1663–1670. [Google Scholar] [CrossRef]
- Sasaki, H.; Hattori, Y.; Ikeda, Y.; Kamagata, M.; Iwami, S.; Yasuda, S.; Tahara, Y.; Shibata, S. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in per2::LUC mice. Sci. Rep. 2016, 6, 27607. [Google Scholar] [CrossRef]
- Imamura, K.; Yoshitane, H.; Hattori, K.; Yamaguchi, M.; Yoshida, K.; Okubo, T.; Naguro, I.; Ichijo, H.; Fukada, Y. ASK family kinases mediate cellular stress and redox signaling to circadian clock. Proc. Natl Acad. Sci. USA 2018, 115, 3646–3651. [Google Scholar] [CrossRef]
- Tranel, H.R.; Schroder, E.A.; England, J.; Black, W.S.; Bush, H.; Hughes, M.E.; Esser, K.A.; Clasey, J.L. Physical activity, and not fat mass is a primary predictor of circadian parameters in young men. Chronobiol. Int. 2015, 32, 832–841. [Google Scholar] [CrossRef]
- Saracino, P.G.; Rossetti, M.L.; Steiner, J.L.; Gordon, B.S. Hormonal regulation of core clock gene expression in skeletal muscle following acute aerobic exercise. Biochem. Biophys. Res. Commun. 2019, 508, 871–876. [Google Scholar] [CrossRef]
- Lateef, O.M.; Akintubosun, M.O. Sleep and reproductive Health. J. Circadian Rhythm. 2020, 18, 1. [Google Scholar] [CrossRef]
- Schmalenberger, K.M.; Tauseef, H.A.; Barone, J.C.; Owens, S.A.; Lieberman, L.; Jarczok, M.N.; Girdler, S.S.; Kiesner, J.; Ditzen, B.; Eisenlohr-Moul, T.A. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology 2021, 123, 104895. [Google Scholar] [CrossRef]
- Kruijver, F.P.M.; Swaab, D.F. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology 2002, 75, 296–305. [Google Scholar] [CrossRef]
- Moradpour, R. The effects of regular aerobic exercise on primary dysmenorrhea in young girls. J. Phys. Act. Horm. 2019, 3, 67–82. [Google Scholar]
- Elbandrawy, A.M.; Elhakk, S.M. Comparison between the effects of aerobic and isometric exercises on primary dysmenorrhea. Acta Gymnica. 2021, 51, e2021.014. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.C.; Schjerling, P.; Heinemeier, K.M.; Boesen, A.P.; Dideriksen, K.; Kjær, M. Investigating circadian clock gene expression in human tendon biopsies from acute exercise and immobilization studies. Eur. J. Appl. Physiol. 2019, 119, 1387–1394. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ogata, H.; Kayaba, M.; Ando, A.; Park, I.; Yajima, K.; Araki, A.; Suzuki, C.; Osumi, H.; Zhang, S.; et al. Effect of a single bout of exercise on clock gene expression in human leukocyte. J. Appl. Physiol. (1985) 2020, 128, 847–854. [Google Scholar] [CrossRef]
- Small, L.; Altıntaş, A.; Laker, R.C.; Ehrlich, A.; Pattamaprapanont, P.; Villarroel, J.; Pillon, N.J.; Zierath, J.R.; Barrès, R. Contraction influences Per2 gene expression in skeletal muscle through a calcium-dependent pathway. J. Physiol. 2020, 598, 5739–5752. [Google Scholar] [CrossRef]
- Zambon, A.C.; McDearmon, E.L.; Salomonis, N.; Vranizan, K.M.; Johansen, K.L.; Adey, D.; Takahashi, J.S.; Schambelan, M.; Conklin, B.R. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 2003, 4, R61. [Google Scholar] [CrossRef]
- Okamoto, A.; Yamamoto, T.; Matsumura, R.; Node, K.; Akashi, M. An out-of-lab trial: A case example for the effect of intensive exercise on rhythms of human clock gene expression. J. Circadian Rhythm. 2013, 11, 10. [Google Scholar] [CrossRef]
- de Souza Teixeira, A.A.; Minuzzi, L.G.; Lira, F.S.; Gonçalves, A.S.V.P.; Martinho, A.; Rosa Neto, J.C.; Teixeira, A.M. Improvement in the anti-inflammatory profile with lifelong physical exercise is related to clock genes expression in effector-memory CD4+ T cells in master athletes. Exerc. Immunol. Rev. 2021, 27, 67–83. [Google Scholar]
- Yeung, C.Y.C.; Gossan, N.; Lu, Y.; Hughes, A.; Hensman, J.J.; Bayer, M.L.; Kjær, M.; Kadler, K.E.; Meng, Q.J. Gremlin-2 is a BMP antagonist that is regulated by the circadian clock. Sci. Rep. 2014, 4, 5183. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.H.; McDearmon, E.L.; Panda, S.; Hayes, K.R.; Zhang, J.; Andrews, J.L.; Antoch, M.P.; Walker, J.R.; Esser, K.A.; Hogenesch, J.B.; et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl Acad. Sci. USA 2007, 104, 3342–3347. [Google Scholar] [CrossRef]
- Basti, A.; Yalçin, M.; Herms, D.; Hesse, J.; Aboumanify, O.; Li, Y.; Aretz, Z.; Garmshausen, J.; El-Athman, R.; Hastermann, M.; et al. Diurnal variations in the expression of core-clock genes correlate with resting muscle properties and predict fluctuations in exercise performance across the day. BMJ Open Sport Exerc. Med. 2021, 7, e000876. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.K. Feeding rhythms and the circadian regulation of metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Schurhoff, N.; Toborek, M. Circadian rhythms in the blood–brain barrier: Impact on neurological disorders and stress responses. Mol. Brain 2023, 16, 5. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Entrainment of the mouse circadian clock: Effects of stress, exercise, and nutrition. Free Radic. Biol. Med. 2018, 119, 129–138. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Shibata, S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front. Neurosci. 2017, 11, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Klerman, E.B.; Rimmer, D.W.; Dijk, D.J.; Kronauer, R.E.; Rizzo, J.F., 3rd; Czeisler, C.A. Nonphotic entrainment of the human circadian pacemaker. Am. J. Physiol. 1998, 274 Pt 2, R991–R996. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hashimoto, S.; Masubuchi, S.; Honma, S.; Honma, K.I. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am. J. Physiol. 2001, 281, R197–R205. [Google Scholar] [CrossRef]
- Castanon-Cervantes, O.; Wu, M.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, S.; Viitasalo, K.; Härmä, M. Effect of shiftwork on systemic markers of inflammation. Chronobiol. Int. 2011, 28, 528–535. [Google Scholar] [CrossRef]
- Chabot, K.; Lavoie, M.E.; Bastard, J.P.; Rabasa-Lhoret, R. Intravenous catheters induce a local inflammatory response. Cytokine 2018, 111, 470–474. [Google Scholar] [CrossRef]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Honma, S.; Honma, K. Scheduled exposures to a novel environment with a running-wheel differentially accelerate re-entrainment of mice peripheral clocks to new light-dark cycles. Genes Cells 2008, 13, 497–507. [Google Scholar] [CrossRef]
- Pastore, S.; Hood, D.A. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. J. Appl. Physiol. (1985) 2013, 114, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Kriegsfeld, L.J.; Silver, R. The regulation of neuroendocrine function: Timing is everything. Horm. Behav. 2006, 49, 557–574. [Google Scholar] [CrossRef]
- Williams, W.P., 3rd; Kriegsfeld, L.J. Circadian control of neuroendocrine circuits regulating female reproductive function. Front. Endocrinol. 2012, 3, 60. [Google Scholar] [CrossRef]
- Miller, B.H.; Takahashi, J.S. Central circadian control of female reproductive function. Front. Endocrinol. 2013, 4, 195. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef]
- Rossetti, S.; Corlazzoli, F.; Gregorski, A.; Azmi, N.H.A.; Sacchi, N. Identification of an estrogen-regulated circadian mechanism necessary for breast acinar morphogenesis. Cell Cycle 2012, 11, 3691–3700. [Google Scholar] [CrossRef]
- Xiao, L.; Chang, A.K.; Zang, M.X.; Bi, H.; Li, S.; Wang, M.; Xing, X.; Wu, H. Induction of the CLOCK gene by E2-ERalpha signaling promotes the proliferation of breast cancer cells. PLoS ONE 2014, 9, e95878. [Google Scholar] [CrossRef]
- Gery, S.; Virk, R.K.; Chumakov, K.; Yu, A.; Koeffler, H.P. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene 2007, 26, 7916–7920. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Ao, X.; Chang, A.K.; Yang, C.; Zhao, F.; Bi, H.; Liu, Y.; Xiao, L.; Wu, H. CLOCK is a substrate of SUMO and SUMOylation of CLOCK upregulates the transcriptional activity of estrogen receptor-α. Oncogene 2013, 32, 4883–4891. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Lee, C.W.; L’Hermite-Baleriaux, M.; Turek, F.W.; Van Cauter, E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R714–R724. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human circadian phase-response curves for exercise. J. Physiol. 2019, 597, 2253–2268. [Google Scholar] [CrossRef]
- Lok, R.; Qian, J.; Chellappa, S.L. Sex differences in sleep, circadian rhythms, and metabolism: Implications for precision medicine. Sleep Med. Rev. 2024, 75, 101926. [Google Scholar] [CrossRef]
- Cain, S.W.; Dennison, C.F.; Zeitzer, J.M.; Guzik, A.M.; Khalsa, S.B.S.; Santhi, N.; Schoen, M.W.; Czeisler, C.A.; Duffy, J.F. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythm. 2010, 25, 288–296. [Google Scholar] [CrossRef]
- Vidafar, P.; McGlashan, E.M.; Burns, A.C.; Anderson, C.; Shechter, A.; Lockley, S.W.; Phillips, A.J.K.; Cain, S.W. Greater sensitivity of the circadian system of women to bright light, but not dim to-moderate light. J. Pineal Res. 2024, 76, e12936. [Google Scholar] [CrossRef]
- Hackney, A.C.; Willett, H.N. Testosterone responses to intensive, prolonged endurance Exercise in Women. Endocrines 2020, 1, 119–124. [Google Scholar] [CrossRef]
- Clark, R.V.; Wald, J.A.; Swerdloff, R.S.; Wang, C.; Wu, F.C.W.; Bowers, L.D.; Matsumoto, A.M. Large divergence in testosterone concentrations between men and women: Frame of reference for elite athletes in sex-specific competition in sports, a narrative review. Clin. Endocrinol. 2019, 90, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Shahid, W.; Rabiya, N.; Muhammad, S.B. Effects of exercise on sex steroid hormones (estrogen, progesterone, testosterone) in eumenorrheic females: A systematic to review and meta-analysis. BMC Womens Health 2024, 24, 354. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Tasaki, H.; Misawa, I.; Chu, G.; Yamauchi, N.; Hattori, M.A. Contribution of testosterone to the clock system in rat prostate mesenchyme cells. Andrology 2014, 2, 225–233. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhu, K.; Hong, Y.; Sun, Y.; Zhao, X.; Du, Y.; Chen, Z.J. Effects of BMAL1-SIRT1-positive cycle on estrogen synthesis in human ovarian granulosa cells: An implicative role of BMAL1 in PCOS. Endocrine 2016, 53, 574–584. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).