Abstract
Urinary tract infections (UTIs) are among the most prevalent infectious diseases in older women, especially those over 65 years of age. Physiological changes related to aging, comorbidities, and frequent use of medical devices such as urinary catheters increase susceptibility. Increasing antimicrobial resistance further complicates treatment strategies. This study aims to describe the epidemiological profile of UTI in women over 65 years of age, focusing on the characterization of etiological agents, observed antimicrobial resistance patterns, and commonly reported risk factors. We conducted a retrospective analysis of microbiological and clinical data from elderly women diagnosed with UTIs. Bacterial isolates were identified and antimicrobial susceptibility profiles were evaluated over a specified period. A statistical analysis was performed to determine the prevalence of different pathogens and antibiotic resistance trends. Escherichia coli was the predominant uropathogen, consistent across different clinical scenarios and patient conditions. The four most common bacterial strains—E. coli, Klebsiella pneumoniae, Proteus mirabilis, and Enterococcus faecalis—aligned with global epidemiological data. In Escherichia coli a significant increase in resistance to nitrofurantoin was observed, possibly indicating excessive empirical use, while resistance to other antibiotics, such as amoxicillin/clavulanic acid and ertapenem, remained stable or decreased. Institutional antibiotic stewardship programs likely contributed to this trend. The study highlights E. coli as the main etiological agent in elderly women with UTIs. The observed resistance patterns emphasize the need for localized antimicrobial surveillance and personalized therapeutic approaches. Continuous microbiological monitoring and rational use of antibiotics are crucial to optimize treatment outcomes and control the development of resistance.
1. Introduction
Urinary tract infections (UTIs) represent one of the most prevalent infectious conditions in elderly populations, particularly among women aged over 65 years [1]. This demographic is increasingly vulnerable due to a combination of age-related physiological, immunological, and anatomical changes that predispose to colonization and infection of the urinary tract. The shorter female urethra, postmenopausal alterations in the vaginal and urinary microbiota, and the natural decline in mucosal immunity all contribute to an increased risk of both acute and recurrent infections in this population [2,3]. Moreover, the prevalence of comorbidities such as diabetes mellitus, urinary incontinence, cognitive decline, and immobility further exacerbates this risk and complicates the management of UTIs in older women [4]. These factors are often compounded by medical interventions, such as urinary catheterization, which introduce or promote the growth of resistant microorganisms, increasing the likelihood of complicated infections [5,6].
As life expectancy continues to rise globally, the prevalence and clinical burden of UTIs in elderly women have become a significant public health concern. UTIs in this age group are often associated with severe complications, including pyelonephritis, urosepsis, and acute renal failure, which can lead to prolonged hospitalizations, increased emergency admissions, and higher healthcare costs [6,7]. Furthermore, elderly women frequently present with nonspecific or atypical symptoms of UTIs, such as confusion or functional decline, which can delay diagnosis and treatment [6,7]. This, in turn, can result in adverse clinical outcomes, including the progression to more severe systemic infections that are difficult to manage.
From a microbiological perspective, Escherichia coli remains the predominant etiological agent of UTIs in women of all age groups, including the elderly [1,5,8]. However, studies also report the frequent isolation of other Gram-negative pathogens such as Proteus mirabilis and Pseudomonas aeruginosa, as well as Gram-positive cocci, such as Enterococcus faecalis, particularly in complicated or hospital-acquired infections [1,5,8]. The increasing resistance of these uropathogens to first-line antibiotics, including fluoroquinolones, nitrofurantoin, and third-generation cephalosporins, presents a growing challenge to effective empirical therapy, highlighting the urgent need for ongoing epidemiological surveillance and updated antimicrobial resistance (AMR) data to inform treatment guidelines [8,9].
Globally, antimicrobial resistance (AMR) has been recognized as one of the greatest threats to public health. The World Health Organization (WHO) emphasizes the necessity of localized, real-time resistance data to guide empirical treatment protocols and to mitigate the spread of multidrug-resistant organisms [9,10,11]. Elderly women, as a particularly vulnerable group, are exposed to frequent antibiotic use, often empirically prescribed without precise microbiological data, which accelerates the emergence of resistant strains. Therefore, a tailored antimicrobial stewardship approach, based on localized epidemiological data, is crucial to optimizing therapeutic outcomes and minimizing the unnecessary use of broad-spectrum antibiotics.
In addition to clinical and microbiological complexities, UTIs in elderly women must be considered within the broader context of the One Health framework, which recognizes the interconnectedness of human, animal, and environmental health. The transmission of antimicrobial resistance is influenced not only by human antibiotic consumption but also by agricultural practices, environmental contamination, and animal reservoirs. The widespread presence of antimicrobial agents in agricultural runoff, animal husbandry, and hospital effluents has been identified as a significant factor contributing to the development and dissemination of resistant pathogens, including those responsible for UTIs in elderly populations [12,13]. Thus, an integrated approach that combines human healthcare, veterinary medicine, and environmental monitoring is essential to combat the growing threat of AMR.
The aim of the present study was to conduct a comprehensive epidemiological and microbiological analysis of UTIs in women aged 65 years and older. Specifically, this study seeks to characterize the etiological agents of UTIs, analyze resistance patterns over a five-year period, and identify the key clinical and demographic factors associated with these infections. By presenting real-world data from two hospital centers in Central Portugal, this research aims to contribute to the regional understanding of UTI dynamics in elderly women and to inform more effective, evidence-based, and geographically relevant therapeutic strategies. Given the demographic shift and the increasing burden of UTIs in elderly populations, this study exclusively focuses on women aged 65 and older, thereby providing a more targeted epidemiological and microbiological analysis.
2. Materials and Methods
This was a descriptive, retrospective observational study based on all positive urine cultures collected between January 2018 and December 2022, from women aged 65 years or older, processed in the microbiology laboratories of two public hospital centers located in Central Portugal. A total of 5603 records were retrieved and reviewed. No random sampling method was applied, as all eligible positive urine cultures during the study period were included.
2.1. Data Source and Variables
The data were obtained from the hospitals’ electronic laboratory information management systems (LIMS), and included the following: patient age and sex, origin of the sample (emergency, inpatient, outpatient, day hospital), presence or absence of urinary catheter, isolated microorganisms, and antimicrobial susceptibility test (AST) results. Only records corresponding to positive cultures from women aged ≥65 were included in the analysis. Duplicate isolates from the same patient within a 30-day interval were excluded to avoid overrepresentation. Details regarding sample collection methodology (e.g., midstream clean-catch, catheterization, or suprapubic aspiration) were not recorded in the LIMS and therefore could not be assessed.
2.2. Microbiological Identification and Susceptibility Testing (Contextual Description)
All microbiological analyses—including organism identification and antibiotic susceptibility testing—were conducted as part of routine hospital diagnostic procedures by the respective hospital microbiology laboratories. The identification of bacterial isolates was typically performed using automated systems (e.g., VITEK® 2 Compact, bioMérieux, based in Marcy-l’Étoile, France) and confirmed, where applicable, by MALDI-TOF MS. Antimicrobial susceptibility testing was carried out using disc diffusion and/or automated MIC methods, in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines valid at the time of analysis.
The antibiotics evaluated for Escherichia coli isolates included the following: amoxicillin/clavulanic acid, ceftazidime, cefuroxime axetil, ertapenem, nitrofurantoin, piperacillin/tazobactam, and trimethoprim/sulfamethoxazole. Resistance trends were assessed across the five-year study period (2018–2022).
2.3. Statistical Analysis
The data were analyzed using IBM SPSS Statistics version 29.0.1 for macOS. Descriptive statistics (frequencies and percentages) were used to characterize the distribution of bacterial species and resistance patterns. Continuous variables were presented as means with standard deviations. The data were analyzed using descriptive statistics (frequencies and percentages). No inferential statistical tests were applied, and resistance data were described across the five-year period without performing observation of changes.
2.4. Ethical Considerations
This study was approved on 19/04/2023 by the Ethics Committee of the University of Beira Interior (approval reference: CE-UBI-Pj-2023-020 and authorized by the respective institutional Data Protection Officer. As it involved secondary analysis of anonymized data collected for routine clinical purposes, the requirement for informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki and institutional regulations.
This work is part of the ITUCIP project (Urinary Tract Infections in Central Portugal), which aims to generate epidemiological evidence to inform local public health strategies and empirical antibiotic treatment guidelines.
3. Results
A total of 5603 positive urine cultures from women aged 65 years and older were analyzed, corresponding to all bacteriologically confirmed samples received at two hospital centers in Central Portugal between January 2018 and December 2022 (60 months). This dataset provides a comprehensive representation of the elderly female population affected by urinary tract infections (UTIs) in this regional healthcare context.
It was observed that most of the samples originated from the emergency department (Figure 1).
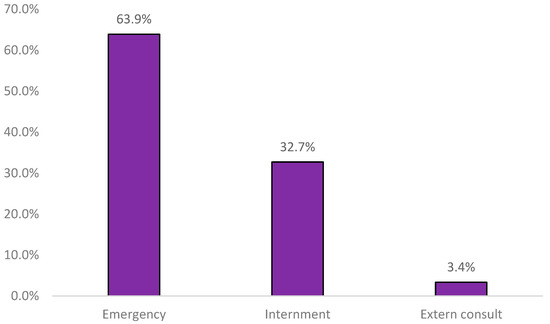
Figure 1.
Sample distribution by origin (n = 5603).
The most prevalent microorganism was Escherichia coli, with over 18 distinct bacterial species identified (Figure 2).
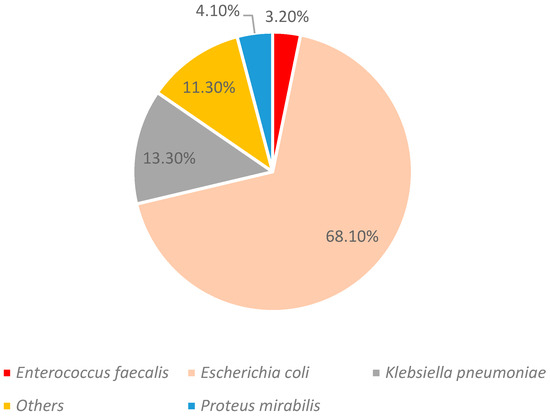
Figure 2.
Identified strains (n = 5603).
Among the women analyzed, 132 were catheterized. Among these, the most prevalent strain was again Escherichia coli (Figure 3).
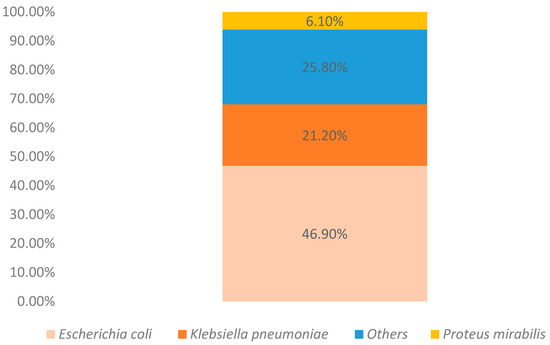
Figure 3.
Identified strains in catheterized patients (n = 132).
Resistance data for Escherichia coli isolates were described across the five-year interval from 2018 to 2022, as shown in Table 1. No statistical comparisons were performed; observed differences are presented descriptively.

Table 1.
E. coli resistance patterns to antibiotics between 2018 and 2022 (n = 3815).
4. Discussion
The exclusive focus on elderly women provides a detailed characterization of this high-risk group, which is frequently underrepresented in broader UTI studies. Given the unique clinical challenges posed by this population—such as polypharmacy, catheterization, and immunosenescence—our findings offer valuable insights for age-specific empirical treatment strategies and antimicrobial stewardship policies.
The data demonstrate that Escherichia coli remains the dominant microorganism in UTIs among elderly women, a finding that is consistent with numerous international studies [12]. This high prevalence, regardless of the collection site—whether in the emergency department, among hospitalized patients, or even among bedridden individuals—reinforces the central role of this bacterium in the etiology of UTIs [13,14]. Accordingly, when considering the most frequently isolated bacteria, the prevalence of the four most common strains (Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Enterococcus faecalis) aligns perfectly with the majority of studies addressing this subject. This distribution pattern underscores the predominant role of E. coli as the principal etiological agent of UTIs in elderly women, often associated with high virulence and an enhanced ability to adhere to the uroepithelium. Moreover, the significant presence of Gram-negative bacilli, such as K. pneumoniae and P. mirabilis, raises additional concerns regarding both intrinsic and acquired resistance to broad-spectrum antimicrobials, making it essential to consider these factors when formulating empirical treatment strategies. The detection of E. faecalis, a Gram-positive bacterium frequently linked to hospital-acquired infections, also suggests the possibility of previous colonizations or more complex clinical scenarios in this population [14,15,16,17].
Another thematic area described longitudinally is the description of antimicrobial resistance profiles between 2018 and 2022, which reveals distinct observations for different antibiotic classes associated with the most prevalent bacterium (E. coli). Several international studies underscore the imperative need for geographically localized research to enhance scientific knowledge in specific regions [18]. E. coli was tested in 2018 and again in 2022 against seven antibiotics, showing an increase in resistance to only one—nitrofurantoin. The rise in resistance to nitrofurantoin can be interpreted as an indicator of its intensive and possibly inappropriate empirical use, suggesting that selective pressure may have favored more resistant strains, suggesting that selective pressure may have favored more resistant strains. This occurs when antibiotic exposure eliminates susceptible bacteria, allowing resistant variants to thrive and become dominant within the population. It is well established that primary resistance is associated with mutations in the genes encoding two cytoplasmic nitroreductases (NfsA and NfsB) [19]. In other research, a description of similar studies reveals that during the COVID-19 pandemic period, there was an increase in the sensitivity of urinary E. coli isolates to nitrofurantoin [20]. The observed discrepancy, despite our study covering the same chronological period, may be attributed to geographic differences, as the cited article was conducted in India. Once again, this reinforces the absolute and indispensable need to conduct microbiological studies in specific geographic settings [18]. Nonetheless, nitrofurantoin remains an extremely important drug in the treatment of E. coli-associated urinary infections [20].
In contrast, the observed decrease in resistance to amoxicillin/clavulanic acid, cefuroxime axetil, trimethoprim/sulfamethoxazole, and ertapenem may reflect the effectiveness of antibiotic stewardship strategies implemented at the institutional level. These strategies, based on rational prescribing and periodic review of therapeutic protocols, aim to reduce the unnecessary or inappropriate use of antibiotics by promoting more judicious choices tailored to the local epidemiological profile. These results reinforce the hypothesis that structured interventions—such as prescription audits, the continuous education of healthcare professionals, and the support of clinical microbiology in therapeutic guidance—can indeed contribute to reversing or stabilizing resistance rates. This observation is further corroborated by several recent studies which demonstrate that the adoption of antimicrobial control policies not only improves clinical outcomes but also reduces the emergence of multidrug-resistant strains, even in contexts of high antibiotic pressure. Furthermore, the reduction in the use of broad-spectrum antibiotics, such as ertapenem, may have an indirect positive effect by minimizing the selection of bacteria with more complex resistance mechanisms, such as carbapenemase production [21,22,23,24].
Additionally, the stability observed in resistance indices for ceftazidime and piperacillin/tazobactam may indicate that the determinants of resistance for these antibiotics follow a more complex dynamic, possibly influenced by multiple factors such as their specific pharmacokinetic and pharmacodynamic properties, their more restricted use in selected clinical contexts, and the molecular mechanisms characteristic of the E. coli strains present in this specific population. These mechanisms may include alterations in porin systems, efflux pumps, and the presence or absence of extended-spectrum β-lactamases (ESBLs) and carbapenemases. This finding underscores the importance of continuous microbiological surveillance and in-depth studies that integrate both genotypic and phenotypic analyses to investigate specific mutations, mobile genetic elements (such as plasmids and integrons), and clonal patterns [25,26,27]. Understanding these variables may provide valuable insights into the evolution of resistance and guide more precise therapeutic decisions adapted to the local microbiological reality, as previously noted [18].
Clearly, E. coli plays a fundamental role in urinary infections among women over 65, as it was consistently the most isolated bacterium, independent of bedridden status—a factor that in itself constitutes an additional risk for urinary infections [28]. These findings are further corroborated by other studies, in which, under similar conditions, E. coli was also identified as the predominant pathogen with very similar percentage values (45.9%) in urinary infections among bedridden patients [29].
Although clinical data such as comorbidities or previous antibiotic exposure were not available in the current dataset, the large sample size and the consistent microbiological methodology across the study period provide reliable indicators of local resistance dynamics.
This study has some limitations. One limitation is that the data were obtained from a hospital-based population, which may not be fully representative of the general elderly female population. Furthermore, the descriptive design does not allow for the description of causal risk factors. Future studies with analytical designs are recommended to better explore the risk factors associated with UTIs in elderly women.
Additionally, the retrospective nature of the study and the absence of clinical data such as symptom severity or treatment outcomes constitute further limitations.
Given the interconnectedness of human, animal, and environmental health, the findings reinforce the importance of a One Health approach to antimicrobial stewardship, especially in addressing the spread of resistant uropathogens. A One Health perspective calls for integrated surveillance systems that encompass human, veterinary, and environmental health, thus ensuring a coordinated approach to tackling the growing threat of antimicrobial resistance.
5. Conclusions
This study unequivocally demonstrates that Escherichia coli is the primary etiological agent of urinary tract infections (UTIs) in women over 65, regardless of the clinical context, thereby reinforcing its central role in the pathogenesis of these infections. The longitudinal description of antimicrobial resistance profiles revealed a significant increase in resistance to nitrofurantoin, possibly associated with its intensive empirical use, while other antibiotics showed stable or even reduced resistance rates. These observed tendencies suggest that institutional interventions based on antibiotic stewardship—especially when combined with rational prescribing practices and continuous microbiological surveillance—can positively impact the containment of resistance. In contrast, the stability in resistance profiles for antibiotics such as ceftazidime and piperacillin/tazobactam points to more complex molecular mechanisms, which warrant further investigation through comprehensive genomic and phenotypic analyses. Overall, the results of this study underscore the importance of localized approaches to controlling antimicrobial resistance and highlight the need for therapeutic policies tailored to the specific epidemiological profile of each population.
Author Contributions
M.C.-B., conception and design of the study, data collection, review and approval of the final manuscript; P.C., S.M. and J.M., data analysis, and review and approval of the final manuscript; F.J.B.R., conception and design of the study, data collection, and review and approval of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved on 19/04/2023 by the Ethics Committee of the University of Beira Interior (CE-UBI-Pj-2023-020) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to it being a retrospective study, with data consultation on a hospital basis, without access to patient identification.
Data Availability Statement
Due to ethical restrictions and the protection of participants’ confidentiality, the data are not publicly available. However, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest in relation to this work.
References
- Matsumoto, T. Urinary tract infections in the elderly. Curr. Urol. Rep. 2001, 2, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, M.; Palomo, I.; Montecino-Garrido, H.; Wehinger, S.; Rodriguez-Mañas, L.; Trostchansky, A.; Fuentes, E. Physiological changes associated with aging: Identification of novel biomarkers for frailty syndrome in women. Free Radic. Biol. Med. 2024, 223, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Query, H.; Carroll, A.; Klausner, A.P.; Burkett, L.S. A Review for Clinical Practice in the Treatment and Prevention of Recurrent Urinary Tract Infections in Women over Age 65. J. Women’s Health 2024, 33, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Sako, A.; Yasunaga, H.; Matsui, H.; Fushimi, K.; Yanai, H.; Gu, Y.; Ohmagari, N. Hospitalization for urinary tract infections in Japan, 2010–2015: A retrospective study using a national inpatient database. BMC Infect Dis. 2021, 21, 1048. [Google Scholar] [CrossRef]
- Rodrigues, F.; Barroso, A. Etiologia e sensibilidade bacteriana em infecções do trato urinário. Rev. Port. Saúde Pública 2011, 29, 123–131. [Google Scholar] [CrossRef]
- Newlands, A.F.; Kramer, M.; Roberts, L.; Maxwell, K.; Price, J.L.; Finlay, K.A. Evaluating the quality of life impact of recurrent urinary tract infection: Validation and refinement of the Recurrent UTI Impact Questionnaire (RUTIIQ). Neurourol. Urodyn. 2024, 43, 902–914. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L. Urinary tract infections in the elderly: A review of disease characteristics and current treatment options. Drugs Context 2020, 9, 2020-4-13. [Google Scholar] [CrossRef]
- Harding, C.; Mossop, H.; Homer, T.; Chadwick, T.; King, W.; Carnell, S.; Lecouturier, J.; Abouhajar, A.; Vale, L.; Watson, G.; et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: Multicentre, open label, randomised, non-inferiority trial. BMJ 2022, 376, e068229. [Google Scholar] [CrossRef]
- Luu, T.; Albarillo, F.S. Asymptomatic Bacteriuria: Prevalence, Diagnosis, Management, and Current Antimicrobial Stewardship Implementations. Am. J. Med. 2022, 135, e236–e244. [Google Scholar] [CrossRef]
- Rodrigues, F.; Coelho, P.; Mateus, S.; Caseiro, A.; Eideh, H.; Gonçalves, T.; Branco, M.C. Decoding Urinary Tract Infection Trends: A 5-Year Snapshot from Central Portugal. Clin. Pract. 2025, 15, 14. [Google Scholar] [CrossRef]
- Jover-Sáenz, A.; Ramírez-Hidalgo, M.; Bellés, A.B.; Murillo, E.R.; Bosch, M.B.; Cabanillas, J.C.; Garrido-Calvo, S.; Vilas, M.I.G.; Navés, L.G.; Caudevilla, M.J.J.; et al. Impact of a Primary Care Antimicrobial Stewardship Program on Bacterial Resistance Control and Ecological Imprint in Urinary Tract Infections. Antibiotics 2022, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Droste, L.R.; Ratliff, C.R. Characteristics of Urinary Tract Infections and the Use of Cranberry Products in Patients With Urinary Diversions: A Narrative Review. J. Wound Ostomy Cont. Nurs. 2024, 51, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Yin, R.; Nercelles, P.; Rivera-Molina, S.E.; Jyoti, S.; Dongol, R.; Aguilar-De-Moros, D.; Tumu, N.; Alarcon-Rua, J.; Stagnaro, J.P.; et al. International Nosocomial Infection Control Consortium (INICC) report of health care associated infections, data summary of 45 countries for 2015 to 2020, adult and pediatric units, device-associated module. Am. J. Infect. Control. 2024, 52, 1002–1011. [Google Scholar] [CrossRef]
- Shmoury, A.H.; Hanna, W.; Zakhour, J.; Zahreddine, N.K.; Kanj, S.S. Epidemiology and microbiology of catheter-associated urinary tract infections: A 14-year surveillance study at a tertiary care center in Lebanon. J. Infect. Public Health 2024, 17, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Filev, R.; Lyubomirova, M.; Bogov, B.; Kolevski, A.; Pencheva, V.; Kalinov, K.; Rostaing, L. Urinary Tract Infections Caused by Klebsiella pneumoniae and Prolonged Treatment with Trimethoprim/Sulfamethoxazole. Microorganisms 2025, 13, 422. [Google Scholar] [CrossRef]
- Phiri, R.M.; Ertuğrul, M.B.; Bozdoğan, B.; Hoşbul, T. Evaluation of virulence genes in Proteus strains isolated from diabetic foot infections and urinary tract infections. J. Infect. Dev. Ctries. 2024, 18, 1559–1565. [Google Scholar] [CrossRef]
- Ruhal, R.; Sahu, A.; Koujalagi, T.; Das, A.; Prasanth, H.; Kataria, R. Biofilm-specific determinants of enterococci pathogen. Arch. Microbiol. 2024, 206, 397. [Google Scholar] [CrossRef]
- Torabian, P.; Singh, N.; Crawford, J.; Gonzalez, G.; Burgado, N.; Videva, M.; Miller, A.; Perdue, J.; Dinu, M.; Pietropaoli, A.; et al. Effect of clinically relevant antibiotics on bacterial extracellular vesicle release from Escherichia coli. Int. J. Antimicrob. Agents. 2025, 65, 107384. [Google Scholar] [CrossRef]
- Dulyayangkul, P.; Sealey, J.E.; Lee, W.W.Y.; Satapoomin, N.; Reding, C.; Heesom, K.J.; Williams, P.B.; Avison, M.B. Improving nitrofurantoin resistance prediction in Escherichia coli from whole-genome sequence by integrating NfsA/B enzyme assays. Antimicrob. Agents Chemother. 2024, 68, e0024224. [Google Scholar] [CrossRef]
- Venugopal, S.; Chunchanur, S.; Panigrahy, R.; Tak, V.; Yadav, M.; Chauhan, A.; Srinivasamurthy, H.; Rajendran, J.; Pundir, S.; Bhatt, S.; et al. Changes in antimicrobial resistance of Escherichia coli isolated from community-associated urinary tract infection before and during the COVID-19 pandemic in India. J. Glob. Antimicrob. Resist. 2024, 37, 165–167. [Google Scholar] [CrossRef]
- Maaland, M.G.; Jakobsen, L.; Guardabassi, L.; Frimodt-Møller, N. Pharmacokinetic and pharmacodynamic evaluation of nitrofurantoin against Escherichia coli in a murine urinary tract infection model. APMIS 2024, 132, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.C.; Coelho, P.; Rodrigues, F. Urinary Tract Infections in a Single Hospital in Central Portugal, a 5-Year Analysis. Microbiol. Res. 2024, 15, 850–863. [Google Scholar] [CrossRef]
- Grigoryan, L.; Trautner, B.W. Antibiotic Stewardship Interventions for Urinary Tract Infections in Outpatient Settings: A Narrative Review. Infect. Dis. Clin. N. Am. 2024, 38, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.K.; Septimus, E.; Kleinman, K.; Varma, N.; Avery, T.R.; Heim, L.; Rahm, R.; Cooper, W.S.; Cooper, M.; McLean, L.E.; et al. Stewardship Prompts to Improve Antibiotic Selection for Urinary Tract Infection: The INSPIRE Randomized Clinical Trial. JAMA 2024, 331, 2018–2028. [Google Scholar] [CrossRef]
- Cabello, M.; Hernández-García, M.; Maruri-Aransolo, A.; Michelena, M.; Pérez-Viso, B.; Ponce-Alonso, M.; Cantón, R.; Ruiz-Garbajosa, P. Occurrence of multi-carbapenemase-producing Enterobacterales in a tertiary hospital in Madrid (Spain): A new epidemiologic scenario. J. Glob. Antimicrob. Resist. 2024, 38, 281–291. [Google Scholar] [CrossRef]
- Lee, Y.L.; Liu, C.E.; Wang, W.Y.; Tan, M.C.; Chen, P.J.; Shiau, Y.R.; Wang, H.Y.; Lai, J.F.; Huang, I.W.; Yang, Y.S.; et al. Antimicrobial resistance among imipenem-non-susceptible Escherichia coli and Klebsiella pneumoniae isolates, with an emphasis on novel β-lactam/β-lactamase inhibitor combinations and tetracycline derivatives: The Taiwan surveillance of antimicrobial resistance program, 2020–2022. J. Microbiol. Immunol. Infect. 2025, 58, 219–225. [Google Scholar] [CrossRef]
- Akinpelu, S.; Ajayi, A.; Smith, S.I.; Adeleye, A.I. Genotypic and phenotypic characterization of determinants that mediate antimicrobial resistance in Escherichia coli strains of clinical origin in South-Western Nigeria. J. Infect. Prev. 2024, 25, 126–133. [Google Scholar] [CrossRef]
- Fletke, K.J.; Jeong, D.H.; Herrera, A.V. Urinary Catheter Management. Am. Fam. Physician 2024, 110, 251–258. [Google Scholar]
- Asmare, Z.; Erkihun, M.; Abebe, W.; Ashagre, A.; Misganaw, T.; Feleke, S.F. Catheter-associated urinary tract infections in Africa: Systematic review and meta-analysis. Infect. Dis. Health 2024, 29, 172–179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).