Abstract
Wheat is often used as a raw material in the brewing of special styles of beer. Hydrocolloids naturally present in wheat are called pentosans. They constitute approximately 2% of wheat flour. Arabinoxylans (pentosanes) and β-glucan are common compounds in wheat and are mostly found in the cell wall. Hydrocolloids are commonly used to retain moisture in bread and baked goods. Besides the moisture content, they affect the texture and retrogradation enthalpy of starch molecules. In the baking industry, they can be useful and improve the dough properties, but in the brewing industry, they are commonly designated as problematic compounds. Namely, to a certain extent, they can improve the foam stability; however, they can hinder the filtration process. This review paper aims to give an overview of non-starch compounds and their properties and to emphasize the significance of these macromolecules in the malting and brewing industries, especially in wheat varieties. The objective of this review is to gather information by searching different databases with scientific papers to broaden knowledge on arabinoxylans and β-glucans in brewing.
1. Introduction
Wheat, as a common, but not primary, raw material for brewing, is often used as an un-malted or malted adjunct. Different countries fostered different recipes regarding wheat beers. According to the modern version of the German Beer Purity Law (Vorläufiges Biergesetz), wheat beers brewed in Germany must be top-fermented and produced with at least 50% malted wheat, with the lowest original gravity of 11 °P. Before the feudal purity law (Reinheitsgebot), which was declared in 1516, any available raw material could have been and was used for brewing. The updated version of Reinheitsgebot currently conditions the composition and physical–chemical properties of “white beers”. According to this, white beers must be produced from at least 50% malted wheat and are mandatory to be fermented using top-fermenting yeast [1]. The original gravity (°P) of 1.047–1.056 (11.8–14 °P), apparent extract/final gravity (°P) of 1.008–1.016 (2–4 °P), alcohol by weight (volume) of 3.9–4.4% (4.9–5.5%), bitterness of 10–15 IBU, and color of SRM (EBC) of 3–9 (6–18 EBC) are characteristic of the south German-style Hefeweizen. This beer style has to exhibit the characteristic flavor and aroma [2], commonly reminiscent of banana, clove, and fruits due to esters, furanones, and phenols. The pronounced clove aroma originates from 4-vinylguaiacol, and the fruity, aromatic, banana-like flavor is usually correlated with isoamyl acetate. The estery component originates from ethyl acetate [3]. Another country that fosters the tradition of wheat beers is Belgium. However, the classic Belgian wheat beer style, “wit” in Flemish and “bière blanche” in French, is brewed with up to 50–50% un-malted wheat and barley malt with the addition of coriander and bitter orange peel [1,4].
However, despite its centuries-long use in brewing, there are still no specifically designed wheat varieties that yield optimal results in malting and brewing. According to Faltermeier [1], today, wheat varieties used for malting and brewing give satisfactory results in both processes. There are certain requirements that wheat varieties have to fulfill in order to be considered suitable for brewing, and they mostly regard protein content, but there are other parameters that should be followed. The acceptable parameters for wheat for malting vary from author to author, but they mostly range from the following [5]:
Water content: 4.5–5.0%
Protein: 11.0–13.0%
Extract (d.m.): >83%
Viscosity, mPa*s: <1.800
Final attenuation, app.: >79%
Soluble N mg/100 g malt (d.m.): 650–780
Kolbach index: 37–40%
FAN mg/100 g malt (d.m.): 90–120
Some authors recommend different but near values: crude protein (TP) max. 11–12%; 1000 kernel mass (TKW) as high as possible; starch content as high as possible (preferably >70% d.m. in grains) with as large starch granules as possible; low grain hardness NIR-hardness < 50; endosperm vitreousness as low as possible with as high a transient vitreousness as possible; and a low content of soluble pentosans [6,7]. The indicators most often taken into consideration are TP, TKW, NIR-hardness, and starch content. The negative effects of using wheat and wheat malt include increased protein content (+0.5–2.5%) in wort, a longer saccharification time (+2–15 min), an increased content of high-molecular HMW and medium-molecular MMW protein fractions (+3–15 mg/100 mL), a lower FAN content (20–50 mg/L) and an increase in wort viscosity (+0.1–1.5 mPa × s), and poorer filterability [1,8].
Xylan is designated as the second-most abundant polysaccharide in plant cell walls. Xylans, originating from cereals, contain large shares of L-arabinose and thus, are often referred to as arabinoxylans [9]. Xylans commonly occur as heteropolysaccharides, differing in substituent groups in the backbone chain and in the side chain [10]. The common substituents found on the backbone of xylans are acetyl, arabinosyl, and glucuronosyl residues [11]. Wheat generally contains much higher content of arabinoxylans (AXs) than β-glucans in the endosperm cell wall. Pentosanes can be classified as water-soluble and water-insoluble. Water-soluble pentosans are cca. 40% arabinoxylans and often can be bound to proteins, while water-insoluble pentosans are composed of approximately 30% arabinoxylans with bonds to residual starch [12]. As can be noticed from the above-mentioned recommendation for brewing varieties, soluble pentosan levels are not quantified but are recommended to be as low as possible.
To this day, the only diversification of European wheat varieties is the classification into quality groups according to the requirements of the baking industry. These groups are marked as E, A, B, and C. E-grade (elite) wheats are characterized by the highest quality, followed by A-grade (quality), B-grade (bread making), and C-grade (not usable for baking) wheat [13]. Since Germany is a traditional wheat-brewing country, the term “brauweizen” has been introduced into this classification [14]. Brauweizen varieties show desirable malting parameters and are mainly related to the North-European agro-climatic region. Their utilization for malting and brewing greatly relies on the year-long experience of a certain malting house. They commonly recognize a selection of varieties based on many years of practical experience. Varieties known as white soft wheat varieties, and additionally confectionery varieties (group C) are the most relevant to the required properties [14]. The south-eastern Europe area does not support the growth of white soft or confectionary wheat varieties. This region, however, responds very well to the cultivation of red hard wheat varieties with a hybrid endosperm. Moreover, the conducted studies indicate that certain varieties show potential for very good malting properties [15,16], not only as malted but as un-malted raw material as well [17].
Thus, it would certainly be useful to dissect the pentosane content, especially AXs, in wheat varieties, particularly the ones intended for malting. There are many studies on pentosans in wheat, but they commonly concern dough properties and their influence on bread quality. Since there is not much research on pentosanes in beer, specifically in wheat malt and beer, this review aims to give a close insight into the significance of pentosanes in malting and brewing with wheat and to elaborate on the relationship between different classes of wheat and their brewing properties concerning AXs content. The objective of this review is to gather information about the application of wheat in malting and brewing by searching different databases with scientific papers with the purpose of broadening the knowledge on arabinoxylans, and β-glucans in brewing.
The research methodology included the following databases: PubMed/MEDLINE, ScienceDirect, SpringerLink, LinkedIn, and ResearchGate. Keywords for databases included “wheat pentosanes brewing”, “wheat beer”, “arabinoxylans beer”, and “beta-glucans brewing”.
2. Hydrocolloids in Wheat
2.1. Arabinoxylans
Pentosanes are defined as non-starch, large hemicellulose molecules [18,19] and can take up about 1.5–2.5% (or, according to some authors, 1–3%) of wheat flour [20]. They are composed of polymers of two 5-carbon (pentose) sugars, arabinose and xylose. Arabinoxylans are the major constituents of pentosans in wheat, and they can be found in the endosperm as one of the main non-starchy polysaccharides of the cell walls, which makes up approximately 66% of the total polysaccharides in endosperm cell walls [1,21,22].
AXs (Figure 1), found in the endosperm cell walls of wheat and other cereals, have an xylopyranosyl skeleton to which the arabinofuranosyl residues are bound [23]. So far, not much data are available about the molecular characteristics and distribution of arabinoxylans among different parts of wheat grain. Even though AXs and β-glucans are the most pronounced pentosans in wheat, wheat is still very poor in β-d-glucans [1,18] (0.5–2%, 3–7%). Moreover, not only is the pentosane content in wheat higher than in barley, but its pentosane solubility varies between 1 and 1.5%, which is almost two-fold higher than that of barley (0.7%) [1]. However, it should be noted that even though wheat shows scantier in pentosan than barley, wheat beer is made from max. 50% of wheat malt, so pentosans present a potential problem in malting and brewing, in general.
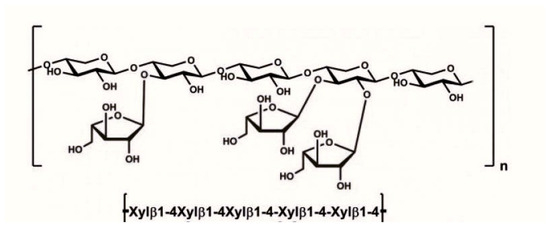
Figure 1.
Structure of wheat arabinoxylan.
As mentioned before, pentosanes or, more precisely, arabinoxylans can be water-soluble (or water-extractable/WEAX) and water-insoluble [24,25]. The terminology, such as water-extractable, water-soluble, and water-insoluble, varies in different papers; sometimes they reference pentosanes and sometimes AXs. However, since AX comprises the majority of pentosans, the extraction always refers to AX. Thus, to simplify reading and understanding, in this review, the terminology will refer to AXs.
The solubility is affected by the molecular weight of pentosan and the number of branches of arabinose. Namely, the solubility of AX increases with a higher degree of arabinose substitution or a shorter chain length of the xylan backbone [26]. According to Medcalf et al. [16], the latter are generally correlated with the starch tailing portion of flour. Water-soluble pentosane fractions are also called wheat gums. They display gum-like properties and reduce the mixing time in dough production [27,28]. Water-soluble pentosanes play a significant role in forming a gel structure and increasing the viscosity of wort. According to Arif et al. [29], WEP (water-extractable pentosanes) displayed a significant effect on pasting properties of wheat flour as opposed to WIP (water-insoluble pentosanes). The wheat variety also had a low impact on this. Some authors reported the increasing effect of pentosans on the peak viscosity of flour, and the increase was greater with the addition of water-soluble pentosans. Water-soluble pentosans had little effect [30]. However, another study reported the opposite; they found no correlation between pentosans and the pasting properties of wheat flour [31]. Such major differences in results could be caused by variations in the material (varieties) from which the pentosanes were isolated [29]. There are differences between the types of wheat, such as durum, hard red winter, hard red spring, soft red winter, and club wheats, in causing changes in the peak viscosity. Namely, for durum wheat, pentosan addition decreases peak viscosity, while for other types, it has no effect or an increase in peak viscosity was noted. This could also be a matter of isolating material, but there are many elements such as the isolating process of pentosanes, the level of supplementation, and the quality of base flours [29,32], variety, irrigation process, and sowing time [33,34,35,36]. In any case, this could be important for future research on increasing pasting temperatures during mashing, meaning that climatic changes potentially affect the content or the ratio of soluble–insoluble AXs and cause changes in pasting temperatures. This is certainly something that needs to be further and more deeply examined.
Hong et al. [37] correlated grain hardness with pentosanes content in wheat varieties. They reported that hard wheats had significantly higher levels of water-soluble pentosans than soft wheats. Also, in the water-soluble fraction, soft white winter wheat had lower molecular weight pentosan and less branched arabinose than hard red spring wheat. According to this research, harder grains may be dependent on environmental conditions for the changes in water-soluble pentosan and endosperm protein levels. Several studies reported that both water-soluble pentosans (WSPs) and water-insoluble pentosans (WIPs) could affect the water absorption of wheat flour [38,39,40,41].
Hong et al. [37] classify wheat pentosans, according to the classification Hashimoto et al. established in their research [42], into water-soluble, enzyme-extractable, and total pentosans. Water-soluble and non-soluble AXs comprise O-1,4-linked d-xylopyranosyl residues of branched with single residues, α-l-arabinofuranose, substituted at the C(O)3 and C(O)2 positions [1,8]. Ferulic acid can be added via ester linkage to the C(O)5 position. A principal property-determining indicator is the arabinose-to-xylose ratio, which is 0.5–0.6 in the wheat endosperm. However, water-insoluble pentosanes have β-1,4-linked xylopyranosyl as backbone units to which arabinofuranose [(some of which are esterified by phenolic acids, ferulic acid (FA)] is linked at the C(O)3 and C(O)2 positions [43,44,45,46].
Another very important piece of information is the location of arabinoxylans in wheat grain because different locations condition variations in their structure. For example, AXs isolated from the outer layer of the grain do not have as many arabinose substitutes as the ones from the inner endosperm layer [20]. According to Faltermeier et al. [1,8], approximately 30% of wheat bran is made up of glucuronoarabinoxylans, incorporating methylated glucuronic acid into the arabinoxylan’s complex.
2.2. Glucans
Glucans are non-pentosane compounds that can be found in plant cell walls. Generally, they consist of glucose polymers that can be found predominantly in barley and malt. Linear-mixed (1→4) (1→3)-linked β-d-glucans (Figure 2), in which blocks of (1→4)-linked β-Glcp units are separated by single (1→3)-linkages, are widely present in plants, mainly cereals [47].
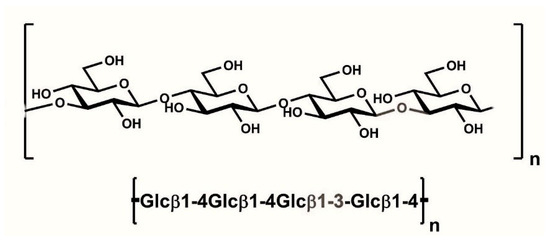
Figure 2.
Structure of β-glucan.
Mainly, the segments are tri- and tetramers, but longer (1→4)-linked oligomers may form the chains. Cereal β-glucans are known to differ in their tri- to tetramer ratio, the share of longer cellulosic fragments, and the ratio between the two types of glycosidic bonds [48]. Glucans are also found in wheat and wheat malt but to a much lesser extent. However, since they represent pentosans as well, this section will briefly mention the significance of glucans in wheat. They generally undergo degradation during malting and mashing, but if they “survive” these stages, they can end up in wort and beer. The commonly found glucans in wheat and wheat malt are β-glucans. β-glucans are β-linked (1,3, and 1,4) glucose polymers, while α-glucans are α-linked glucose polymers, colloquially called dextrins. Glucans can be found in cell walls and the endosperm, just like AXs. However, during the malting process, the cell wall undergoes degradation via enzymatic activity (β-glucanase), and β-glucan molecules are released from the cell wall. The endosperm cell walls are complex structures made up of hemicelluloses and gums. These chemically similar compounds are diverse in molecular weight and solubility. For example, hemicelluloses dissolve in diluted alkali and gums in warm water [49].
If the process of kilning malt, or later on mashing, is not conducted in a manner respectful to enzymatic activity (high temperatures), glucans fail to degrade and afterwards can cause problems during mash filtration (slowing it down or causing losses in extract efficiency). Further problems can be caused if they enter the fermenter; they can hinder the beer filtration process, and, later on, they destabilize the colloidal properties of finished beer, causing haze [49].
3. Hydrocolloids in Malting and Brewing with Wheat
Arabinoxylan and β-glucan both have water-extractable/soluble fractions that can end up in wort. On the other hand, water-inextractable (insolubilized by enzymes) arabinoxylan and β-glucan fractions end up in the spent grains [50,51,52,53,54,55,56,57,58]. However, as mentioned before, if the kilning stage or the mashing stage were not conducted properly, then glucans can cause problems in further production. Nevertheless, studies have shown that malting can significantly decrease the total concentration of non-starch polysaccharides in cereals [53,59,60]. Shaluk et al. [59] reported that reducing the grain moisture content from ~46% to ~39% increases the soluble β-glucan but, on the other hand, slightly reduces soluble arabinoxylans during malting. They also reported that grist moisture content can influence arabinoxylan and β-glucan extraction during the mashing. Namely, a slight decrease in the grist moisture content during mashing can significantly increase the soluble β-glucan extracted into the wort, along with a slight decrease in soluble AX [59].
3.1. Effect of Pentosanes on Malting and Brewing
As the main component of the wheat cell wall [60], AXs can be related to many problems in brewing. Namely, an increased amount of wheat in the grist usually negatively affects the wheat beer quality parameters, such as the haze formation [56], increased wort viscosity [57], and, according to Li et al. [58], the general sensory profile. Several authors reported results of excellent studies about the content and changes in AXs during malting, mashing, and brewing [52,56,58,59,60,61,62,63]. Krahl et al. [52] followed up AXs and fructan content during malting and mashing. They also determined the AXs in beer. According to their report, water-soluble arabinoxylan (WSAX) showed a rapid increase during malting, while the brewing and fermentation stages seemed to have no statistically significant influence on the content of WSAX in the finished beer. No changes in the AX content could be observed after the end of the malting process. The malting process has a significant influence on the amount of WSAXs in malt, and, consequently, in beer and malt-based beverages made from it. Changes in the malting parameters, such as moisture content and germination time, can influence the WSAX content of barley malts. Thus, WSAX and viscosity were correlated negatively, leaving no reason to use malts with low WSAX contents. The highest WSAX content was noted before original gravity reached maximum values. During lautering and wort boiling, their levels are directly linked to original gravity. Considering their research, it seems that the AX content in beer is mainly influenced by the choice of raw materials, while the mashing and brewing process and the brewing equipment have no influence.
Currently, arabinoxylans are not considered a quality indicator for the evaluation of malt quality due to the lack of an evaluation system. Even though many methods for the determination of AX are available (in grains and wort), there is still not one standardized method. Thus, the results obtained from different studies are not comparable since every study used a different method [64].
3.2. β-Glucans and AXs in Malting Wheat
When it comes to β-glucan in wheat, it is considered that 70–90% of β-glucan is adequately degraded during wheat production, and it will not affect further production stages, while with arabinoxylans, scholars still do not recognize the efficient changes in AX during the malting process. Different theories can apply, according to different research groups. Some claim that changes in AXs during the malting process can be classified as degradation of water-soluble AXs, degradation of WSAXs released from the endosperm cell wall, and finally, AXs synthesized in the embryo [65]. Other studies [66,67] report that WSAXs are completely degraded during the malting process, while WUAXs are only partially degraded. Lee et al. [68] carried out research on the changes in AXs and β-glucan during the malting process. They tested 16 barley varieties, which they subjected to germination for 4 days. They reported that cca. 90% of the β-glucan and 20% of AXs were degraded. Debyser et al. [69] reported the content of AXs in six types of malt. The results showed that malt contains between 6.4% and 6.9%. Water-soluble AXs amounted to 0.49–0.69%. Both Leclerq [70] and Dervilly [71] concluded that the content of water-soluble AXs doubles during wheat preparation. Water-soluble AXs in barley and malt can be categorized according to their molecular weight: high molecular weight HMW (molecular weights of about 1000 kDa), medium molecular weight MMW (molecular weight is about 350 kDa), and low molecular weight LMW (molecular weight is about 50–100 kDa). It seems that high molecular weight bait (HMW) in water-soluble arabinoxylans is also at least partially degraded during wheat (malt) production.
3.3. β-Glucans and AXs in Brewing
As the application of wheat as a raw material came to be more accepted, the influence of AXs on the mashing process became more important. Problems such as reduced extraction rate, increased wort viscosity, and extended filtration time came into the spotlight. Vietor et al. [66] reported that AXs, which are not fully degraded during the mashing process, end up as polymers in wort. The addition of endoxylanase and β-glucanase can reduce the viscosity of wort and shorten the filtration time Ducroo et al. [72]. According to Debyser et al. [73], the solubility of AXs during mashing indicates that the AXs in the wort are mainly derived from the water-soluble AXs in the malt. A smaller quantity originates from the dissolution of insoluble AXs during the mashing process. Authors reported [50] that reducing the initial mashing temperature can extend the activity time of endoxylanase. Xylanase activity is at its peak at 50 °C. It rapidly decreases above this temperature and is almost lost at the end of the mashing stage, at 72 °C. During mashing, the arabinosidase activity rapidly decreases at temperatures above 50 °C. Lee et al. [68] reported that the AX content in malt is positively correlated with the AX content in the wort. Moreover, this study claimed that AX content in malt is related to the wort viscosity.
Determination of AX in wort and its correlation to AX in malt produced further studies, which broadened the question of AX to its existence in beer. Schwarz et al. [74] reported that β-glucan in different beers ranged from 0.3 to 247.7 mg/L, and the content of AXs ranged from 514 to 4211 mg/L. It is obvious that AX content in beer is much higher than β-glucan. The content of AXs in wheat beer reportedly amounted to 4, 211 mg/L, and this certainly correlates with the increased share of AXs in wheat malt. Differences in the brewing production process contribute to different shares of AX in beers. For example, in light beer, the AX content was noted to be much lower, 514 mg/L, with a corresponding β-glucan content of 0.3 mg/L. However, the original wort concentration of AX and β-glucan was not reported; the results cannot be compared. Further research [75] found that Ara/Xyl value is between 0.70 and 0.72 in finished beer, and that AXs makes up about 70% of the total NSP. In general, German wheat beers have a higher content of AXs and β-glucan. β-glucan effects on beer filtration have been elucidated, but little knowledge exists about AXs’ impact on beer membrane filtration [76]. Kunze [77] explained that AXs have a slight effect when it comes to beer membrane filtration. Stewart et al. [78] reported that beer viscosity is positively correlated with the AX content in the beer. A more detailed evaluation of this matter was conducted by Sadosky et al. [79]. They evaluated the effects of AXs, β-glucan, and dextrin (α-glucan) on the viscosity and membrane filtration of model beer. They separated three different molecular weights (1600 kDa, 800 kDa, and 300 kDa) of β-glucan and AX of different molecular weights (1000 kDa and 80 kDa), isolated from wheat. According to this research, dextrin showed a significant influence on beer viscosity. The share of dextrin in beer is about 100× higher than that of non-starch polysaccharides; thus, this does not indicate that the contribution of dextrin to beer viscosity is more significant than that of non-starch polysaccharides. However, both Sadosky et al. [79] and Narziss et al. [80], reported that the molecular weight and concentration of β-glucan could affect the viscosity of beer, while the viscosity of beer was not significantly affected by the molecular weight and concentration of AXs. On the other hand, Narziss et al. [80] did not acknowledge that the total AX content in beer was related to beer filtration capacity. This research found that the filtration capacity of beer was significantly affected by 750 kDa AXs, showing that the filtration capacity of beer was affected by the molecular weight of AX. Sadosky et al. [79] also found that adding AXs and β-glucans to beer could affect the MFE value. According to their paper, dextrin has no effect, while AX has the greatest impact, and both properties, molecular weight and concentration, have a significant influence on beer membrane filtration.
However, it should be noted that this paper does not offer explicit results of starting wheat parameters by varieties, number of varieties, or cultivars and does not report changes during the different malting stages. In any case, it would be interesting to check wheat varieties grown under the conditions of forced maturation, a climatic environment that corresponds to south-eastern countries.
4. Analytical Methods
AXs can be determined and quantified with various methods, but colorimetric and chromatographic analysis are the most commonly used. However, it mostly depends on the matrix.
4.1. Determination in Wheat and Wheat Flour
Determination in wheat and wheat flour (or cereals in general) usually involves extraction, purification, and quantification as shown in Figure 3.
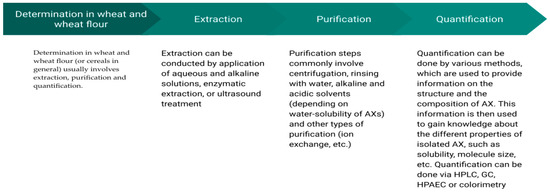
Figure 3.
Schematic overview of analytical stages in AX determination.
4.1.1. Extraction of AX
Extraction can be conducted by application of aqueous and alkaline solutions, enzymatic extraction, or ultrasound treatment [81,82].
As WEAX is weakly bound to the plant tissue cell wall, they are easily extracted/solubilized in water. Water-unextractable AXs have a different degree of arabinose-substituents, substitution pattern of AX, and degree of cross-links between FA and other cell wall components. To destroy these bonds, solutions with higher pH have to be utilized. Thus, to extract the WUAX from the cell wall, chemicals such as NaOH, Ba(OH)2, e.g., or certain enzymes (xylanase and esterase), or ultrasound application are necessary [80,82]. To gain optimal results of extraction methods, additional steps have been introduced, such as stirring, heating, or enzyme treatments.
Cereal matrix is a complex structure consisting mainly of starch, non-starch polysaccharides, different proteins, lipids, and other compounds. To minimize the impedance of the matrix, it is necessary to eliminate any unwanted components that could affect the extraction of the AXs. A general overview of applicable methods can be found in Table 1.

Table 1.
General overview of applicable methods for extraction.
4.1.2. Water-Soluble AX
Since water-extractable AX are only weakly embedded in the cell wall tissue of the plant, they can easily be isolated by moderate extraction methods in which the principal agent is water. Extraction methods differ in temperature, time, and the number of purification steps. Purification steps commonly involve centrifugation of the aqueous suspensions. WEAX is commonly retained in the aqueous supernatant, and, according to some authors, it can be directly used for the analysis of WEAX. An overview of the extraction methods is shown in Table 2.

Table 2.
Overview of extraction methods for water-extractable AX.
The addition of ethanol is not confirmed by some authors. Namely, Cleemput et al. [91] reported that a concentration of 65% (v/v) ethanol resulted in the best separation between the two pentosan polysaccharides, AX and AG (arabinogalactan), but Loosveld et al. [93] published that no separation of WEAX and water-extractable arabinogalactan-peptides occurred when either of the ethanol concentrations applied.
AX can be separated via adsorption of water-soluble hemicelluloses with tris-HCl buffer on a diethylaminoethyl (DEAE)-Sepharose (CL-6B) column. Following these steps, the obtained solution was subjected to freezing and freeze-dried [98]. Nilsson et al. [88] used the same technique for the fractionation of a crude AX extract.
Extraction using water is a simple method to extract water-extractable AXs. It is fairly easy; no special tools and chemicals are required, and it is cost friendly. Water-extractable AXs display different functional properties in comparison with water-unextractable AXs. This mainly refers to water absorption ability, dough development, and gel formation. Usually, levels of WEAX are lower than WUAX and are related to the source of isolation/extraction [82].
4.1.3. Water-Insoluble AX
Water-insoluble AXs require more sophisticated extraction methods. Several parameters need to be adjusted, such as longer stirring times and higher temperatures. Purification yield and purity are dependent on the applied extraction solvents, which can be roughly divided into alkaline and acidic. Table 3 gives an overview of applicable methods and operations for the extraction of water-unextractable AX.

Table 3.
An overview of applicable methods for the extraction of water-unextractable AX.
The application of Ba(OH)2 yields approx. 80% of AX is present in wheat flour and wheat bran. To obtain the same amounts of AX, NaOH can be used as well. DMSO and urea resulted in lower amounts of extracted AX. A combination of NH2OH and HCl resulted in a spectrum of yields depending on the pH. At pH 7.2, more AX were extracted than at pH 5.0 [99]. pH affects the structure of AX, which consequently influences the functional properties. The main goal of all these extraction operations is to obtain the supernatant, which can then be purified with any of the filtration methods (microfiltration, dialysis), then subjected to dry-spray or remixed with water and analyzed.
4.1.4. Enzymatic Extraction of AX
AXs can be extracted via enzymatic treatment, a more expensive method of extraction. Enzymatic extraction of AX ensures the degradation into its molecular components, namely xylose and arabinose. For degradation enzymes such as (1→4)-β-endo-xylanase (EC 3.2.1.8), β-d-xylosidase (EC 3.2.1.37), α-l-arabinofuranosidase (EC 3.2.1.55), or feruloyl-esterase (EC 3.1.1.2) [102] can be applied. Endoxylanases degrade the AX backbone and change the functionality of the polymers [103] by transferring water-insoluble AX to water-soluble AX, which are then degraded into arabinose and xylose [104,105]. According to a study conducted by [102], in the sugar content in the supernatant obtained after enzymatic reaction (degradation), the amount of obtained carbohydrates accounted for 63% of the total dry matter, of which 40% were arabinose and xylose and 50% of the wheat bran AX was solubilized by enzymatic treatments. Similar results were reported by [106].
A multicomponent enzyme system [37] using cellulases, β-glucosidase, xylanase, β-xylosidase, and α-l-arabinosidase, combined with a method described by [37,42] resulted in enzyme-extractable and total pentosans. In this case, the pentosan content instead of the AX content was determined, and by further degradation, it would be possible to determine the actual AX content.
Generally, to enzymatically isolate AXs, they have to first be made available by splitting them from lignin in the cell wall. Destroying the ester linkage between arabinose and ferulic acid using the feruloyl-esterase is the first step. By this specific reaction, which requires a water solution and moderate temperatures, the content of accessible WEAX and WUAX in the matrix increased. The content of AX at this stage can be considered as the whole AX content in the grain. To transform WUAX into WEAX, endoxylanases, xylanases, and arabinofuranosidases can be applied, and the success of the transformation depends on the reaction time and concentration of the chosen enzymes. After the ferulic acid is cleaved, the WEAX and WUAX are then accessible without intermolecular linkages and can be isolated for further use in the quantification of AX. The next step is the application of arabinofuranosidase, and the last step is the use of xylanases. This ensures a total liquefaction of AX into arabinose and xylose molecules, which can be further determined quantitatively and represent the total amount of AX. This method is very effective in isolating AX from different plant tissues; however, it is an expensive method and not available on a large scale [81]. Additionally, the application of enzymes in the extraction of AXs can cause problems when treating AXs with high ratios of ara/xyl and highly branched AXs, because the enzyme activity can be hindered by the side chains [107,108], and the degree of hydrolysis of the AX structure depends on the specific enzyme [105]. Another shortage in enzymatic application is that compounds such as lignin are known to hinder the accessibility of enzymes in the tissue, and the extraction yields of AX are lower in comparison with others [102,107].
4.1.5. Extraction of AX via Ultrasound
The application of ultrasound in AX extraction was tested for non-water-extractable Glucurono-AX from rye bran [81]. The rye was milled, purified, and the WEAX was removed. The sample was subjected to US in combination with alkaline hydrogen peroxide (NaOH-H2O2) and 3% NaOH (NaOH). As a comparison, an extraction of WUAX without the application of ultrasound was performed. However, the application of ultrasound as a single means of extraction does not give better results when it comes to the extraction quantities. Nevertheless, it has a significant effect on reaction times.
4.1.6. Quantification of AX
The final step in AX determination is the quantification. Quantification can be achieved by various methods, which are used to provide information on the structure and the composition of AX. This information is then used to gain knowledge about the different properties of isolated AX, such as solubility, molecule size, etc. Table 4 shows an overview of applicable methods for the quantification of isolated AXs.

Table 4.
An overview of applicable methods for the quantification of isolated AXs.
According to [19], the sample is dispersed in a buffer (sodium acetate and acetic acid or tris(hydroxymethyl)-amino-methane and hydrochloric acid (HCl)), treated with HCl, and hydrolyzed. After cooling, the sample is neutralized by the addition of NaOH. Samples with starch were treated with glucose oxidase/catalase to convert glucose into gluconic acid. This is an important step since a peak overlay between glucose and pentosan monosaccharides can occur. This results in a longer retention time of gluconic acid by the HPAEC.
Hydrolysis of AXs with trifluoroacetic acid was described by [98]. Trifluoroacetic acid was added to the AX isolates and aerated with nitrogen for one minute. The oven was then heated to 105 °C for 2 h for hydrolyzing AX, and the solution was evaporated at 50 °C. Before injection into the instrument, the sample was dissolved in distilled water and filtered. Monosaccharides were analyzed by HPLC at 80 °C. In similar studies, sulfuric acid for the hydrolysis of WEAX was used, with small variations in time and temperature [26,92,106,111].
GC analysis works for sample hydrolysates treated with different chemicals for converting the monosaccharides into alditol acetates (highly volatile).
Colorimetry is also one of the methods used in quantification. This can be performed via the phloroglucinol method. The samples were treated with a special extraction solution and placed in a boiling water bath for 25 min. After rapid cooling in cold water, the absorbance at 552 nm and 510 nm was measured. Calculation was performed as described by [82]. Nevertheless, the most commonly used method for the detection of AX is via its molecular weight. This method ensures information about molecule size, which differs from plant to plant [82].
Sedimentation test, gel filtration, chromatography, and size exclusion chromatography (SEC) with different detectors such as a laser light scattering analyzer or a refractometer can be applied [82]. Various researchers have adopted Douglas’s phloroglucinol method [112] for it can be applied to a broad range of cereal-based products [113,114,115,116,117], and it has been proven as the most efficient method for AX quantification. Additionally, it is quick and easy to perform. Thus, it was chosen as the basic method for the high-throughput approach [25]. Today, the Douglas method was adjusted from a reference-beam photometer to a multi-mode microplate reader to avoid the instability of the colored reaction product and to obtain the necessary high throughput of samples to save time and costs.
5. Hydrocolloids in Beer
Recent studies attempted to consolidate several methods in order to determine multiple non-starch polysaccharides in beer. Hydrocolloids, or non-starch polysaccharides such as arabinoxylans and β-glucans, in general, occur in standard and low/nonalcoholic beer with the average range of 0.5–4.0 g/L [118]. Wheat beers commonly contain higher amounts of total non-starch polysaccharides ranging from 1.5 to 4.0 g L−1 due to a naturally higher concentration of arabinoxylans, their higher average molecular weight, and reduced enzymatic activity of wheat [119,120].
6. Drawbacks and Scope of Improvements
Since no wheat variety is completely designated as a brewing variety, the use of wheat in brewing demands complex insight into acceptable brewing properties. Whether malted or un-malted, wheat has to satisfy several basic parameters in order to be acceptable for malting and brewing. One of them is the low as possible content of pentosanes. Mainly, this regards the arabinoxylans since they are the most abundant pentosanes in wheat. So far, there have been studies that confirmed the positive and negative influence of AXs content in malt to wort and beer. Their influence on beer foam is surely one of the positive properties, but the influence they have on the filtration process is not.
Improvements can surely be provided through wheat variety breeding, careful selection of varieties that can satisfy most quality demands for malting and brewing. Continuous monitoring of quality parameters and their influence on malting and brewing processes is one of the most important actions that can result in optimization of malting and brewing.
7. Conclusions
Novel methods of determination and quantification of pentosanes in raw material, analyses during the production phases, and in the finished products can effectively result in important information about the nature and importance of pentosanes in brewing. Further insight is needed into molecular structure and position of the molecule with regard to starch molecules, since studies showed a relation between pasting temperature and AX type in certain wheat varieties. AXs could be very important in elucidating the increasing pasting temperature of starch. Thus, deeper insight into AX’s importance and relation to climatic changes is necessary.
Author Contributions
Conceptualization, K.H. and V.K.; methodology, M.K.B.; software, K.M.; validation, K.D. and D.H.; formal analysis, K.M. and V.K.; investigation, M.K.B.; resources, K.H.; data curation, V.K.; writing—original draft preparation, M.K.B.; writing—review and editing, K.H.; visualization, K.H.; supervision, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AXs | arabinoxylans |
| WEAXs | water-extractable arabinoxylans |
| WEP | water-extractable pentosanes |
| WUP | water-insoluble pentosanes |
| AG | arabinogalactan |
| DEAE | diethylaminoethyl |
| WSAX | water-soluble arabinoxylans |
References
- Faltermaier, A.E. Fundamental Studies of the Application of Wheat for Malting and Brewing. Ph.D. Thesis, University College Cork, Cork, Ireland, 2015. [Google Scholar]
- Narziß, L. Zur Geschichte des Bayerischen Weizenbieres im Allgemeinen und des Weihenstephaner im Besonderen. Weihenstephaner 1995, 4, 234–236. [Google Scholar]
- Langos, L.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Two Bavarian Wheat Beers by Means of the Sensomics Approach. J. Agric. Food Chem. 2013, 61, 11303–11311. [Google Scholar] [CrossRef]
- Papazian, C. Beer Styles: Their Origins and Classification. In Handbook of Brewing, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 35–53. [Google Scholar]
- Narziß, L. Abriss der Bierbrauerei, 7th ed.; Wiley-VCH Verlag GmbH&Co.KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Titze, J.; Faltermaier, A.; Schnitzenbaumer, B. Theoretical Study on a Statistical Method for the Simple and Reliable Pre-Selection of Wheat Malt Types for Brewing Purposes based on Generally Accepted Quality Characteristics. J. Am. Soc. Brew. Chem. 2013, 71, 67–75. [Google Scholar] [CrossRef]
- Back, W. Ausgewählte Kapitel der Brautechnologie; Hans Carl GmbH: Nürnberg, Germany, 2008; pp. 249–254. ISBN 978-3-418-00910-0. [Google Scholar]
- Faltermaier, A.; Waters, D.; Becker, T.; Arendt, E.; Gastl, M. Common wheat (Triticum aestivum L.) and its use as a brewing cereal—A review. J. Inst. Brew. 2014, 120, 1–15. [Google Scholar] [CrossRef]
- Butt, M.S.; Tahir-Nadeem, M.; Ahmad, Z.; Tauseef Sultan, M. Xylanases in Baking Industry. Food Technol. Biotechnol. 2008, 46, 22–31. [Google Scholar]
- Biely, P. Microbial xylanolytic systems. Trends Biotechnol. 1985, 3, 286–290. [Google Scholar] [CrossRef]
- Whistler, R.L.; Richards, E.L. Hemicelluloses. In The Carbohydrates; Academic Press: New York, NY, USA, 1970; pp. 447–469. [Google Scholar]
- Faurot, A.-L.; Saulnier, L.; Bérot, S.; Ppineau, Y.; Petit, M.-D.; Rouau, X.; Thibault, J.-F. Large scale isolation of water-soluble and water-insoluble pentosans from wheat flour. LWT 1995, 28, 436–441. [Google Scholar] [CrossRef]
- Laidig, F.; Piepho, H.P.; Rentel, D.; Drobek, T.; Meyer, U.; Huesken, A. Breeding progress, environmental variation and correlation of winter wheat yield and quality traits in German official variety trials and on-farm during 1983–2014. Theor. Appl. Genet. 2017, 130, 223–245. [Google Scholar] [CrossRef]
- Sacher, B. Über den Einfluß von Sorte, Umwelt, Agronomischen Maßnahmen und Mälzungstechnologie Auf Die Wertbestimmenden Eigenschaften von Winterweizenmalzen. Ph.D Thesis, TU München, Munich, Germany, 1997. [Google Scholar]
- Krstanović, V.; Habschied, K.; Mastanjević, K. Research of Malting Procedures for Winter Hard Wheat Varieties—Part II. Foods 2021, 10, 147. [Google Scholar] [CrossRef]
- Krstanović, V.; Habschied, K.; Dvojković, K.; Mastanjević, K. Research of Malting Procedures for Winter Hard Wheat Varieties—Part I. Foods 2021, 10, 52. [Google Scholar] [CrossRef]
- Krstanović, V.; Habschied, K.; Lukinac, J.; Jukić, M.; Mastanjević, K. The Influence of partial substitution of malt with unmalted wheat in grist on quality parameters of Lager beer. Beverages 2020, 6, 7. [Google Scholar] [CrossRef]
- Mares, D.J.; Stone, B.A. Studies on wheat endosperm. 1. Chemical compositions and ultrastructure of cell-walls. Aust. J. Biol. Sci. 1973, 26, 793–812. [Google Scholar]
- Houben, R.; De Ruijter, C.F.; Brunt, K. Determination of the Pentosan Content of Wheat Products by Hydrolysis, Glucose Oxidase Treatment and Analysis by HPAEC/PAD. J. Cereal Sci. 1997, 26, 37–46. [Google Scholar] [CrossRef]
- Kornbrust, B.A.; Forman, T.; Matveeva, I. Applications of enzymes in breadmaking. In Breadmaking Improving Quality, 3rd ed.; Woodhead Publishing Limited: Sawston, UK, 2012; pp. 415–440. [Google Scholar]
- Delcour, J.A.; VanWin, H.; Grobet, P.J. Distribution and structural variation of arabinoxylans in common wheat mill streams. J. Agric. Food Chem. 1999, 47, 271–275. [Google Scholar] [CrossRef]
- Lazaridou, A.; Chornick, T.; Biliaderis, C.G.; Izydorczyk, M.S. Sequential solvent extraction and structural characterization of polysaccharides from the endosperm cell walls of barley grown in different environments. Carbohydr. Polym. 2008, 73, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Rosentrater, K.A.; Evers, A.D. Chemical components and nutrition. In Kent’s Technology of Cereals, 5th ed.; Woodhead Publishing Limited: Sawston, UK, 2018; pp. 267–368. [Google Scholar]
- Medcalf, D.G.; D’Appolonia, B.L.; Gills, K.A. Comparison of chemical composition and properties between hard red spring and durum wheat endosperm pentosan. Cereal Chem. 1968, 45, 539. [Google Scholar]
- Steiner, J.; Kupetz, M.; Becker, T. Advancing Quantification of Water-Extractable Arabinoxylan in Beer: A High-Throughput Approach. Polymers 2023, 15, 3959. [Google Scholar] [CrossRef]
- Hartmann, G.; Piber, M.; Koehler, P. Isolation and chemical characterisation of water-extractable arabinoxylans from wheat and rye during breadmaking. Eur. Food Res. Technol. 2005, 221, 487–492. [Google Scholar] [CrossRef]
- Kulp, K.; Bechtel, W.G. Effect of Water-insoluble pentosane fraction of wheat endosperm on the quality of white bread. Cereal Chem. 1963, 66, 369–373. [Google Scholar]
- Bailey, G.H. The Constituents of Wheat and Wheat Products; Reinhold: New York, NY, USA, 1944; p. 146. [Google Scholar]
- Arif, S.; Ali, T.M.; ul Afzal, Q.; Ahmed, M.; Siddiqui, A.J.; Hasnain, A. Effect of pentosans addition on pasting properties of flours of eight hard white spring wheat cultivars. J. Food Sci. Technol. 2014, 51, 1066–1075. [Google Scholar] [CrossRef]
- El-Wakeil, F.A.; Abdel-Akher, M.; Awad, A.A.; Morad, M.M. Wheat proteins and pentosans, their isolation and their effect on the rheological properties of dough and bread. I. identification of wheat flour pentosans and their effect on dough rheology. Deut Lebensm-Rundsch. 1976, 71, 317–320. [Google Scholar]
- Kim, S.K.; D’Appolonia, B.L. Effect of pentosans on the retrogradation of wheat starch gels. Cereal Chem. 1977, 54, 150–160. [Google Scholar]
- Tao, R.P.; Pomeranz, Y. Water-soluble pentosans in flours varying widely in bread-making potential. J. Food Sci. 1967, 32, 162–168. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K.; Gupta, S.K.; Kaur, N. Effect of sowing time on protein quality and starch pasting characteristics in wheat (Triticum aestivum L.) genotypes grown under irrigated and rain-fed conditions. Food Chem. 2010, 122, 559–565. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; MacRitchie, F. Relationship of polymeric proteins with pasting, gel dynamic and dough empirical-rheology in different Indian wheat varieties. Food Hydrocolloid. 2011, 25, 19–24. [Google Scholar] [CrossRef]
- Massaux, C.; Sindic, M.; Lenartz, J.; Sinnaeve, G.; Bodson, B.; Falisse, A.; Dardenne, P.; Deroanne, C. Variation in physicochemical and functional properties of starches extracted from European soft wheat (Triticum aestivum L.): The importance to preserve the varital identity. Carbohydr. Polym. 2008, 71, 32–41. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Jafari-Shabestari, J.; Qualset, C.O.; Corke, H. Diversity of starch pasting properties in Iranian hexapoloid wheat landraces. Cereal Chem. 1997, 74, 417–423. [Google Scholar] [CrossRef]
- Hong, B.H.; Rubenthaler, G.L.; Allan, R.E. Wheat Pentosans. I. Cultivar Variation and Relationship to Kernel Hardness. Cereal Chem. 1989, 40, 493–504. [Google Scholar]
- Jelaca, S.L.; Hlynka, I. Water-binding capacity of wheat flour crude pentosans and their relation to mixing characteristics of dough. Cereal Chem. 1971, 48, 211. [Google Scholar]
- Hoseney, R.C. Functional properties of pentosans in baked goods. Food Technol. 1984, 1, 114. [Google Scholar]
- Amado, R.; Neukom, H. Minor constituents of wheat flour: The pentosans. In New Approaches to Research on Cereal Carbohydrates; Hill, R.D., Munck, I., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1985; p. 249. [Google Scholar]
- Neukom, H. Chemistry and properties of the non-starch polysaccharides (NSP) of wheat flour. Lebensm. Wiss. Technol. 1976, 9, 143. [Google Scholar]
- Hashimoto, S.; Shogren, M.D.; Bolte, L.C.; Pomeranz, Y. Cereal Pentosans: Their Estimation and Significance. III. Pentosans in Abraded Grains and Milling By-Products. Am. Assoc. Cereal Chem. 1987, 64, 39–41. [Google Scholar]
- Adams, G.A. Constitution of a hemicellulose from bran. Canad. J. Chem. 1955, 33, 56–67. [Google Scholar] [CrossRef]
- Ring, S.G.; Selvendran, R.R. Isolation and analysis of cell wall material from beeswing wheat bran (Triticum aestivum). Phytochemistry 1980, 19, 1723–1730. [Google Scholar] [CrossRef]
- Smith, M.M.; Hartley, R.D. Occurrence and nature of ferulic acid substitution of cell-wall polysaccharides in graminaceous plants. Carbohydr. Res. 1983, 118, 65–80. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocol. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Structural and functional aspects of cereal arabinoxylans and β-glucans. In Novel Macromolecules in Food Systems; Doxastakis, G., Kiosseoglou, V., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2000; pp. 361–384. [Google Scholar]
- Lewis, M.J.; Tom, W.Y. Brewing; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001. [Google Scholar]
- Debyser, W.; Delvaux, F.; Delcour, J.A. Activity of arabinoxylan hydrolyzing enzymes during mashing with barley malt or barley malt and unmalted wheat. J. Agric. Food Chem. 1998, 46, 4836–4841. [Google Scholar] [CrossRef]
- Han, J.Y.; Schwarz, P.B. Arabinoxylan composition in barley, malt, and beer. J. Am. Soc. Brew. Chem. 2018, 54, 216–220. [Google Scholar] [CrossRef]
- Krahl, M.; Müller, S.; Zarnkow, M.; Back, W.; Becker, T. Arabinoxylan and fructan in the malting and brewing process. Qual. Assur. Saf. Crop Foods 2009, 1, 246–255. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Carbohydrate content and structure during malting and brewing: A mass balance study. J. Inst. Brew. 2020, 126, 253–262. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Gu, G. Control of arabinoxylan solubilization and hydrolysis in mashing. Food Chem. 2005, 90, 101–108. [Google Scholar] [CrossRef]
- Pomeranz, Y. Wheat: Chemistry and Technology; American Association of Cereal Chemists: St. Paul, MN, USA, 1998. [Google Scholar]
- Fangel, J.U.; Eiken, J.; Sierksma, A.; Schols, H.A.; Willats, W.; Harholt, J. Tracking polydaccharides through the brewing process. Carbohydr. Polym. 2018, 196, 465–473. [Google Scholar] [CrossRef]
- Cyran, M.R.; Ceglinska, A. Genetic variation in the extract viscosity of rye (Secale cereale L.) bread made from endosperm and wholemeal flour: Impact of high-molecular weight AXs, starch and protein. J. Sci. Food Agric. 2011, 91, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, J. Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer. Molecules 2019, 24, 1230. [Google Scholar] [CrossRef] [PubMed]
- Shaluk, D.; Bazin, S.; Chepurna, A.; Izydorczyk, M.S. Effects of variable grain hydration during steeping on the content and physicochemical properties of non-starch polysaccharides in malt and wort. Food Res. Int. 2019, 116, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Alfeo, V.; De Francesco, G.; Sileoni, V.; Blangiforti, S.; Palmeri, R.; Aerts, G.; Perretti, G.; Todaro, A. Physicochemical properties, sugar profile, and non-starch polysaccharides characterization of old wheat malt landraces. J. Food Comp. Anal. 2021, 102, 103997. [Google Scholar] [CrossRef]
- Guo, M.M.; Du, J.H.; Zhang, K.L.; Jin, Y.H. Content and molecular weight of water-extractable arabinoxylans in wheat malt and wheat malt-based wort with different kolbach indices. J. Sci. Food Agric. 2014, 94, 2794–2800. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y. Effects of AX solubilization on wort viscosity and filtration when mashing with grist containing wheat and wheat malt. Food Chem. 2006, 98, 164–170. [Google Scholar] [CrossRef]
- Li, J.; Du, J.H.; Wu, X.Y.; Zhang, Z.A.; Zhang, K.L. Changes in crude AX during cloudy wheat beer brewing on a production scale. J. Inst. Brew. 2017, 123, 192–198. [Google Scholar] [CrossRef]
- He, Z.; Xu, F.; Wu, P. Arabinoxylans in cereal grain and beer brewing. J. Food Technol. Pres. 2021, 5, 1–14. [Google Scholar]
- Steiner, W.; Lafferty, R.; Gomes, I.; Esterbauer, H. Studies on a wild strain of Schizophyllum commune: Cellulase and xylanase production and formation of the extracellular polysaccharide schizophyllan. Biotechnol. Bioeng. 1987, 30, 169–178. [Google Scholar] [CrossRef]
- Viëtor, V.A.; Angelino, S. Composition of non-starch polysaccharides in wort and spent grain from brewing trials with malt from a good malting quality barley and a feed barley. J. Inst. Brew. 1993, 99, 243–248. [Google Scholar] [CrossRef]
- Viëtor, V.A.; Angelino, S.; Pilnik, W. Non-starch polysaccharides in barley and malt a mass balance of flour fractionation. J. Cereal Sci. 1991, 14, 73–83. [Google Scholar] [CrossRef]
- Lee, A.G.; Greig, R.; Dunn, C.; Stuart, M. The effect of (1→ 3, 1→ 4)-β-glucan and Arabinoxylans on malt quality and brewhouse performance. In Proceedings of the 25th Convention of the Institute of Brewing, Pacific Section, Perth, Australia, 26–30 March 1989; Institute of Brewing: London, UK, 1998; pp. 33–37. [Google Scholar]
- Debyser, W.; Schooneveld, M.; Derdelinckx, G. Nuclear magnetic resonance and methylation analysis-derived structural features of water-extractable Arabinoxylans from barley (Hordeum vulgare L.) malts. Agric. Food Chem. 1997, 45, 2914–2918. [Google Scholar] [CrossRef]
- Leclercq, C.; Dervilly, G.; Saulnier, L.; Dallies, N.; Zimmermann, D.; Roue, C. Barley and malt pentosans: Structure and functionalities in the brewing industry. EBC 1999, 27, 429–436. [Google Scholar]
- Dervilly, G.; Leclercq, C.; Zimmermann, D. Isolation and characterization of high molar mass water-soluble Arabinoxylans from barley and barley malt. Carbohydr. Polym. 2002, 47, 143–149. [Google Scholar] [CrossRef]
- Ducroo, P.; Frelon, P.G. Improvement of beer production by the use of β-glucanase- pentosanase from Disporotrichum dimorphosporum. In European Brewery Convention; Oxford University Press, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Debyser, W.; Derdelinckx, G.; Delcour, J. Arabinoxylans and Arabinoxylans hydrolysing activities in barley malts and worts derived from them. J. Cereal Sci. 1997, 26, 67–74. [Google Scholar] [CrossRef]
- Schwarz, P.; Han, J. Arabinoxylans content of commercial beers. ASBC 1995, 53, 157–159. [Google Scholar] [CrossRef]
- Han, J.Y.; Schwarz, P. Arabinoxylans composition in barley malt and beer. J. ASBC 1996, 54, 216–220. [Google Scholar] [CrossRef]
- Aaman, P.; Hesselman, K. Analysis of starch and other main constituents of cereal grains. J. Agric. Res. 1984, 14, 135–139. [Google Scholar]
- Kunze, W. Brewing Malting; VLB: Berlin, Germany, 2004; pp. 18–52. [Google Scholar]
- Stewart, D.C.; Hawthorne, D.; Evans, D. Cold sterile filtration: A small scale filtration test and investigation of membrane plugging. J. Inst. Brew. 1998, 104, 321–326. [Google Scholar] [CrossRef]
- Sadosky, P.; Schwarz, P.B.; Horsley, R. Effect of Arabinoxylans, β -glucans, and dextrins on the viscosity and membrane filterability of a beer model solution. ASBC 2002, 60, 153162. [Google Scholar] [CrossRef]
- Narziss, L.; Reicheneder, E.; Edney, M. Studying beer filtration with an accurate beta-glucan assay. Monatsschrift Brauwiss. 1989, 42, 277–285. [Google Scholar]
- Elbegzaya, N.; Hollmann, J.; Lindhauer, M.G.; Pawelzik, E. Einfluss von Ultraschall auf Extrahierbarkeit und Struktur von Glukuronrarabinoxylanen aus Roggenkleie. Cereal Technol. 2010, 64, 162–175. [Google Scholar]
- Döring, C.; Jekle, M.; Becker, T. Technological and Analytical Methods for Arabinoxylan Quantification from Cereals. Crit. Rev. Food Sci. Nutr. 2015, 56, 999–1011. [Google Scholar] [CrossRef]
- Cleemput, G.; Booij, C.; Hessing, M.; Gruppen, H.; Delcour, J.A. Solubilisation and Changes in Molecular Weight Distribution of Arabinoxylans and Protein in Wheat Flours During Bread-Making, and the Effects of Endogenous Arabinoxylan Hydrolysing Enzymes. J. Cereal Sci. 1997, 26, 55–66. [Google Scholar] [CrossRef]
- Fincher, G.B.; Stone, B.A. Chemistry of Nonstarch Polysaccharides. In Encyclopedia of Grain Science; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 206–222. [Google Scholar]
- Courtin, C.M.; Delcour, J.A. Physicochemical and bread-making properties of low molecular weight wheat-derived arabinoxylans. J. Agric. Food Chem. 1998, 46, 4066–4073. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocol. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Rimsten, L.; Saulnier, L.; Andersson, R.; Åman, P. Water-extractable Arabinoxylan from Pearled Flours of Wheat, Barley, Rye and Triticale. Evidence for the Presence of Ferulic Acid Dimers and their Involvement in Gel Formation. J. Cereal Sci. 2001, 34, 207–214. [Google Scholar] [CrossRef]
- Nilsson, M.; Saulnier, L.; Andersson, R.; Åman, P. Water unextractable polysaccharides from three milling fractions of rye grain. Carbohydr. Polym. 1996, 30, 229–237. [Google Scholar] [CrossRef]
- Hansen, H.B.; Rasmussen, C.V.; Knudsen, K.E.B.; Hansen, Å. Effects of genotype and harvest year on content and composition of dietary fibre in rye (Secale cereale L.) grain. J. Sci. Food Agric. 2003, 83, 76–85. [Google Scholar] [CrossRef]
- Lempereur, I.; Rouau, X.; Abecassis, J. Genetic and Agronomic Variation in Arabinoxylan and Ferulic Acid Contents of Durum Wheat (Triticum durum L.) Grain and Its Milling Fractions. J. Cereal Sci. 1997, 25, 103–110. [Google Scholar] [CrossRef]
- Cleemput, G.; Roels, S.P.; Van Oort, M.; Grobet, P.J.; Delcour, J.A. Heterogeneity In The Structure of Water-Soluble Arabinoxylans in European Wheat Flours of Variable Bread-Making Quality. Cereal Chem. 1993, 70, 324–329. [Google Scholar]
- Rattan, O.; Izydorczyk, M.S.; Biliaderis, C.G. Structure and Rheological Behavior of Arabinoxylans from Canadian Bread Wheat Flours. Food Sci. Technol.-Lebensm.-Wiss. Technol. 1994, 27, 550–555. [Google Scholar] [CrossRef]
- Loosveld, A.M.A.; Grobet, P.J.; Delcour, J.A. Contents and structural features of water-extractable arabinogalactan in wheat flour fractions. J. Agric. Food Chem. 1997, 45, 1998–2002. [Google Scholar] [CrossRef]
- Vinkx, C.J.A.; Stevens, I.; Gruppen, H.; Grobet, P.J.; Delcour, J.A. Physicochemical and Functionalproperties of Rye Nonstarch Polysaccharides. 5. Variability in The Structure of Water-Soluble Arabinoxylans. Cereal Chem. 1993, 70, 311–317. [Google Scholar]
- Ragaee, S.M.; Campbell, G.L.; Scoles, G.J.; McLeod, J.G.; Tyler, R.T. Studies on rye (Secale cereale L.) lines exhibiting a range of extract viscosities. 1. Composition, molecular weight distribution of water extracts, and biochemical characteristics of purified water-extractable arabinoxylan. J. Agric. Food Chem. 2001, 49, 2437–2445. [Google Scholar] [CrossRef]
- Cleemput, G.; van Oort, M.; Hessing, M.; Bergmans, M.E.F.; Gruppen, H.; Grobe, P.J.; Delcour, J.A. Variation in the degree of D-Xylose substitution in arabinoxylans extracted from a European wheat flour. J. Cereal Sci. 1995, 22, 73–84. [Google Scholar] [CrossRef]
- Nilsson, M.; Andersson, R.; Andersson, R.E.; Autio, K.; Åman, P. Heterogeneity in a water-extractable rye arabinoxylan with a low degree of disubstitution. Carbohydr. Polym. 2000, 41, 397–405. [Google Scholar] [CrossRef]
- Shiiba, K.; Yamada, H.; Hara, H.; Okada, K.; Nagao, S. Purification And Characterization Of 2 Arabinoxylans From Wheat Bran. Cereal Chem. 1993, 70, 209–214. [Google Scholar]
- Gruppen, H.; Hamer, R.J.; Voragen, A.G.J. Barium hydroxide as a tool to extract pure arabinoxylans from water-insoluble cell wall material of wheat flour. J. Cereal Sci. 1991, 13, 275–290. [Google Scholar] [CrossRef]
- Bergmans, M.E.F.; Beldman, G.; Gruppen, H.; Voragen, A.G.J. Optimisation of the Selective Extraction of (Glucurono)arabinoxylans from Wheat Bran: Use of Barium and Calcium Hydroxide Solution at Elevated Temperatures. J. Cereal Sci. 1996, 23, 235–245. [Google Scholar] [CrossRef]
- Bataillon, M.; Mathaly, P.; Nunes Cardinali, A.-P.; Duchiron, F. Extraction and purification of arabinoxylan from destarched wheat bran in a pilot scale. Ind. Crops Prod. 1998, 8, 37–43. [Google Scholar] [CrossRef]
- Benamrouche, S.; Crônier, D.; Debeire, P.; Chabbert, B. A Chemical and Histological Study on the Effect of (1→4)-[beta]-endo-xylanase Treatment on Wheat Bran. J. Cereal Sci. 2002, 36, 253–260. [Google Scholar] [CrossRef]
- Dornez, E.; Gebruers, K.; Delcour, J.A.; Courtin, C.M. Grain-associated xylanases: Occurrence, variability, and implications for cereal processing. Trends Food Sci. Technol. 2009, 20, 495–510. [Google Scholar] [CrossRef]
- Petit-Benvegnen, M.D.; Saulnier, L.; Rouau, X. Solubilization of arabinoxylans from isolated water-unextractable pentosans and wheat flour doughs by cell-wall-degrading enzymes. Cereal Chem. 1998, 75, 551–556. [Google Scholar] [CrossRef]
- Courtin, C.M.; Delcour, J.A. Arabinoxylans and Endoxylanases in Wheat Flour Bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
- Beaugrand, J.; Reis, D.; Guillon, F.; Debeire, P.; Chabbert, B. Xylanase-Mediated Hydrolysis of Wheat Bran: Evidence for Subcellular Heterogeneity of Cell Walls. Int. J. Plant Sci. 2004, 165, 553–563. [Google Scholar] [CrossRef]
- Lequart, C.; Nuzillard, J.-M.; Kurek, B.; Debeire, P. Hydrolysis of wheat bran and straw by an endoxylanase: Production and structural characterization of cinnamoyl-oligosaccharides. Carbohydr. Res. 1990, 319, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Dervilly-Pinel, G.; Tran, V.; Saulnier, L. Investigation of the distribution of arabinose residues on the xylan backbone of water-soluble arabinoxylans from wheat flour. Carbohydr. Polym. 2004, 55, 171–177. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Biliaderis, C.G.; Bushuk, W. Comparison of the Structure and Composition of Water-Soluble Pentosans from Different Wheat Varieties. Cereal Chem. 1991, 68, 139–144. [Google Scholar]
- Rantanen, H.; Virkki, L.; Tuomainen, P.; Kabel, M.; Schols, H.; Tenkanen, M. Preparation of arabinoxylobiose from rye xylan using family 10 Aspergillus aculeatus endo-1,4-beta-D-xylanase. Carbohydr. Polym. 2007, 68, 350–359. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984, 109, 937–942. [Google Scholar] [CrossRef]
- Douglas, S.G. A rapid method for the determination of pentosans in wheat flour. Food Chem. 1981, 7, 139–145. [Google Scholar] [CrossRef]
- Bettge, A.D.; Morris, C.F. Oxidative gelation measurement and influence on soft wheat batter viscosity and end-use quality. Cereal Chem. 2007, 84, 237–242. [Google Scholar] [CrossRef]
- Bettge, A.D.; Morris, C.F. Relationships among grain hardness, pentosan fractions, and end-use quality of wheat. Cereal Chem. 2000, 77, 241–247. [Google Scholar] [CrossRef]
- Finnie, S.M.; Bettge, A.D.; Morris, C.F. Influence of cultivar and environment on water-soluble and water-insoluble arabinoxylans in soft wheat. Cereal Chem. 2006, 83, 617–623. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E. Barley _-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products—A Review. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Jacobs, M.; Dexter, J.E. Distribution and structural variation of nonstarch polysaccharides in milling fractions of hull-less barley with variable amylose content. Cereal Chem. 2003, 80, 645–653. [Google Scholar] [CrossRef]
- Reid, J.E.S.J.; Yakubov, G.E.; Lawrence, S.J. Non-starch polysaccharides in beer and brewing: A review of their occurrence and significance. Crit. Rev. Food Sci. Nutr. 2022, 64, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, J.; Zhang, K. Profiling of carbohydrates in commercial beers and their influence on beer quality. J. Sci. Food Agric. 2020, 100, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, J.; Zheng, Y. Non-starch polysaccharides in wheat beers and barley malt beers: A comparative study. Foods 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).