Development and Evaluation of Hyaluronic Acid-Chitosan Coated Liposomes for Enhanced Delivery of Resveratrol to Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liposome Formulations
2.2.1. Blank Liposomes
2.2.2. RES-Loaded Liposomes
2.3. Characterization of Liposomes
2.3.1. Particle Size and Zeta Potential Analysis
2.3.2. Morphology Study
2.3.3. Conformational Properties
2.3.4. Thermal Analysis
2.4. Determination of the Entrapment Capacity of Liposomes
2.5. Release Behavior of RES in Different Release Media
2.6. Antioxidant Activity
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.7. In Vitro Cell Study
2.7.1. Biocompatibility Study
2.7.2. Cytotoxicity
2.8. Stability Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Liposomes
3.2. Encapsulation Capacity of Liposomes
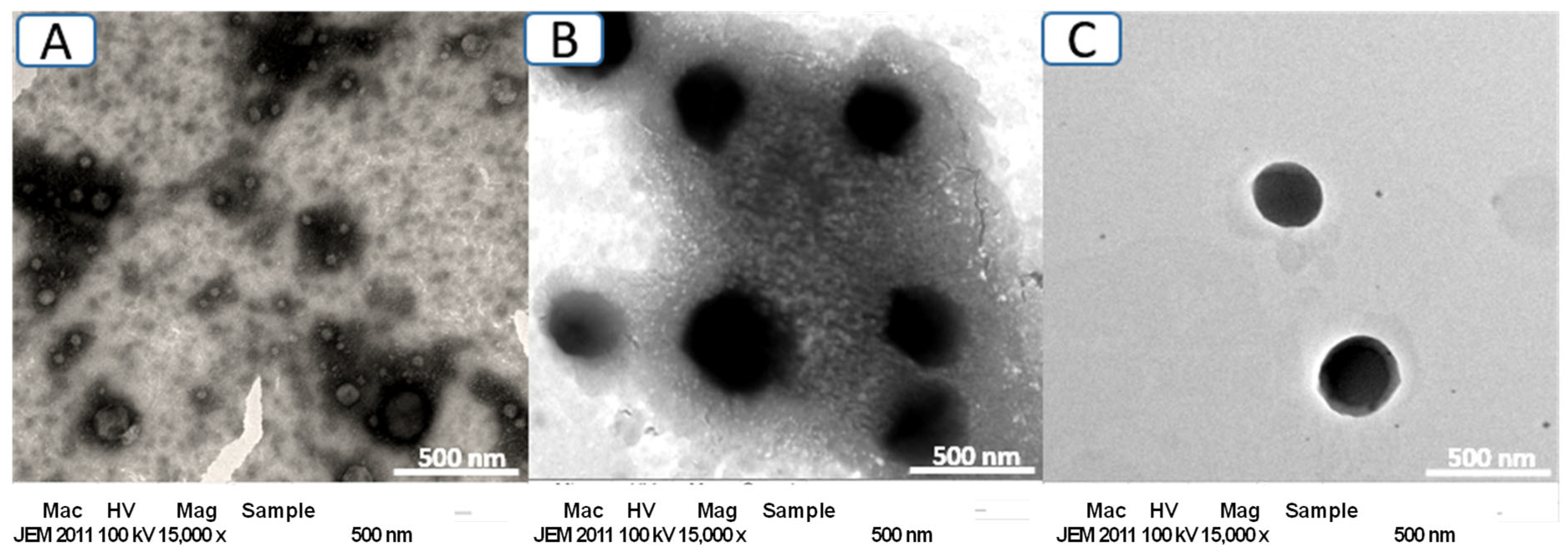
3.3. Morphology Study
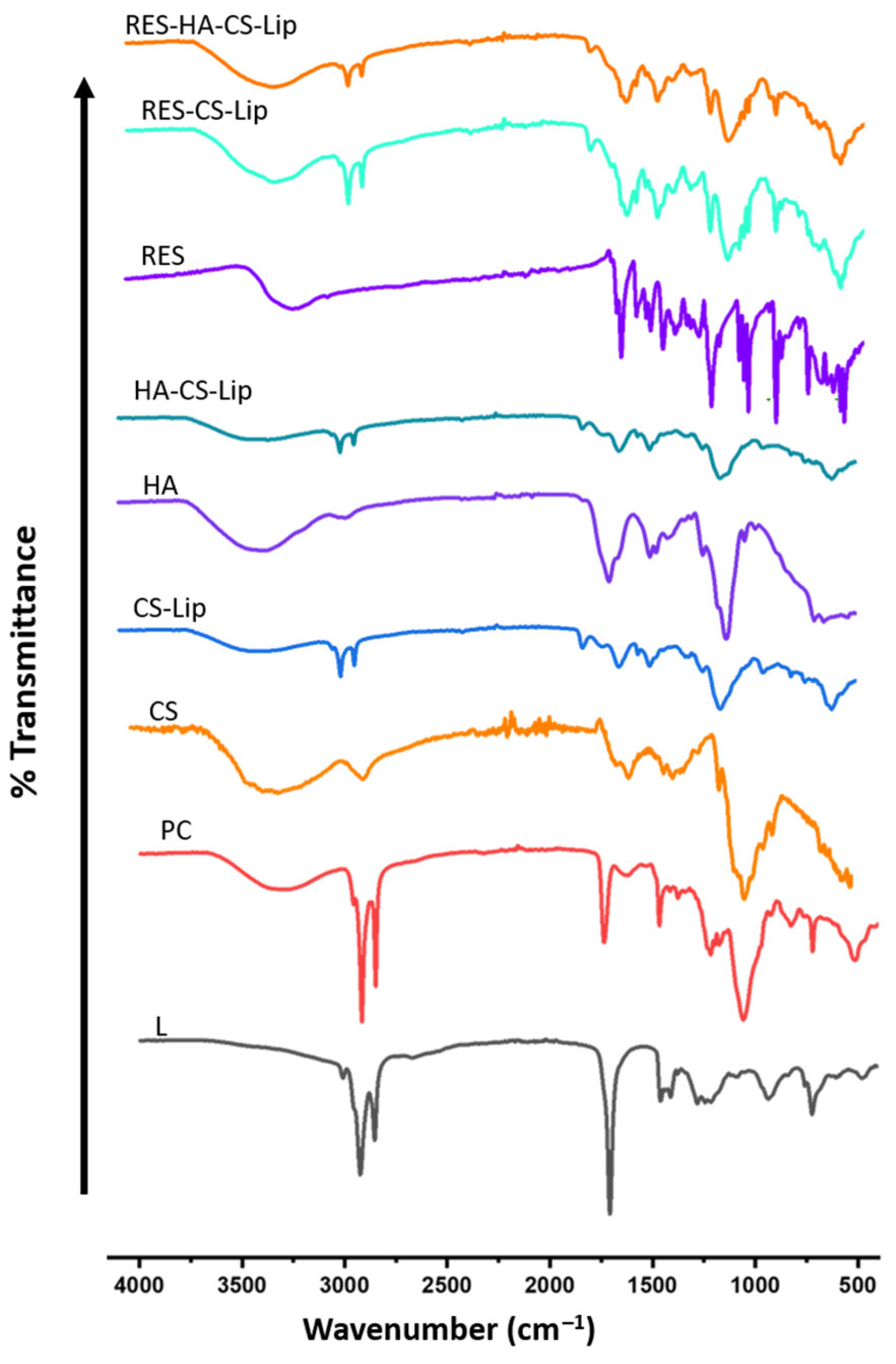
3.4. Conformational Properties
3.5. Thermal Analysis
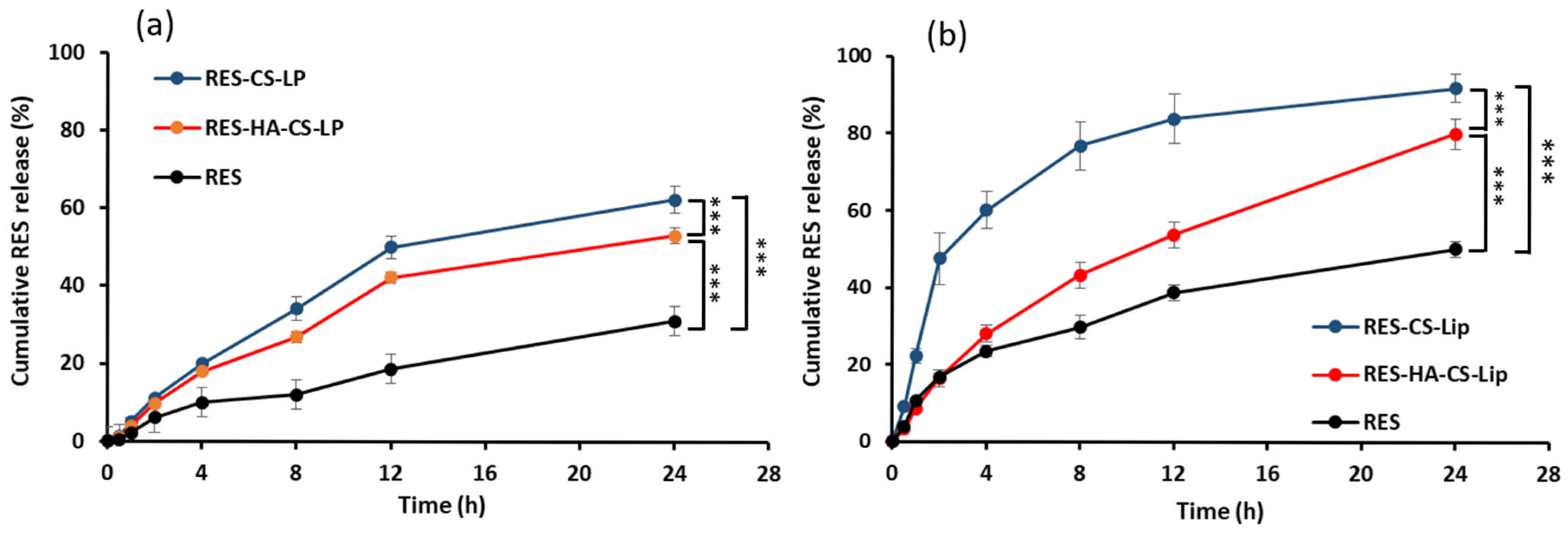
3.6. Release Behavior of RES in Different Release Media
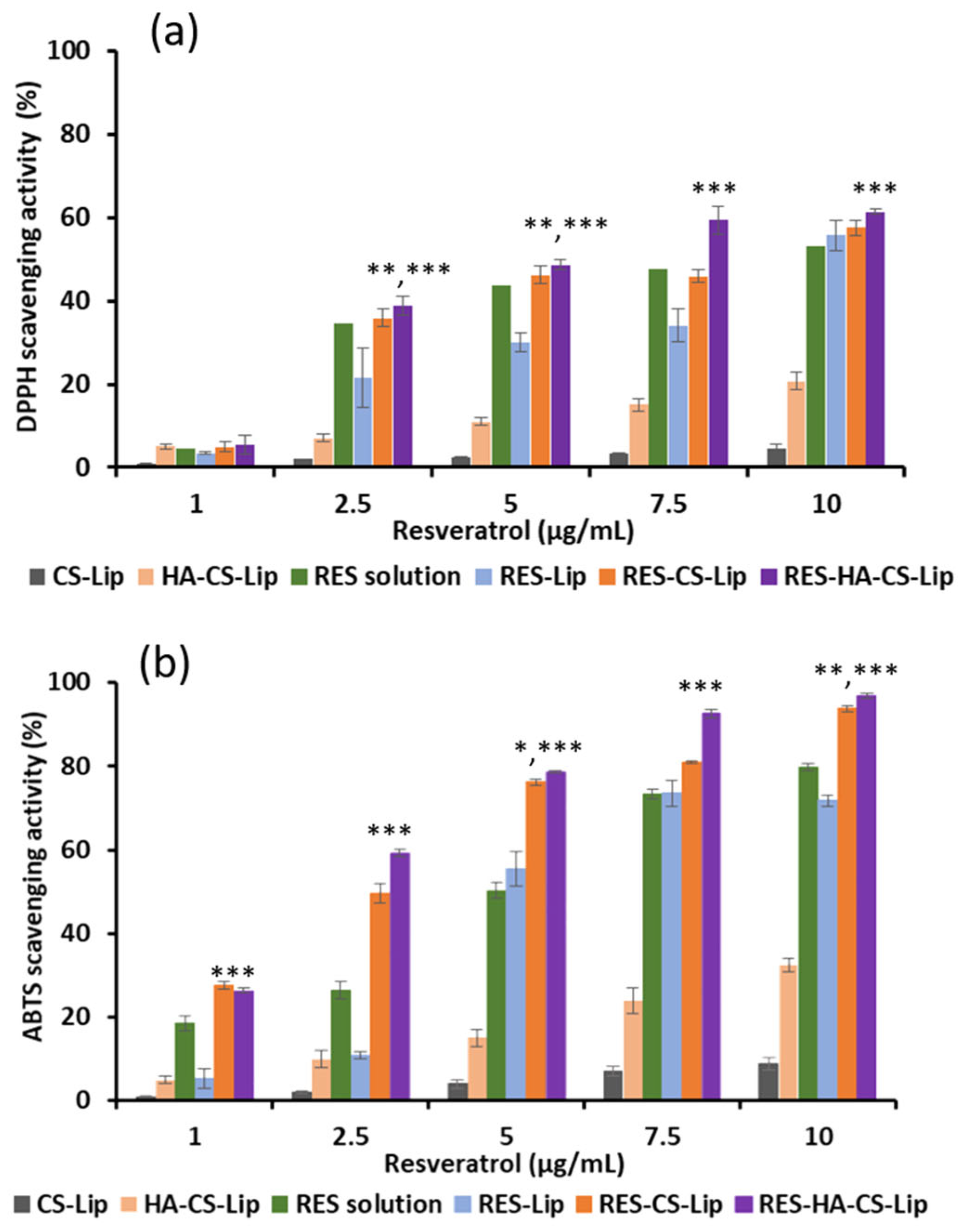
3.7. Antioxidant Activity of RES
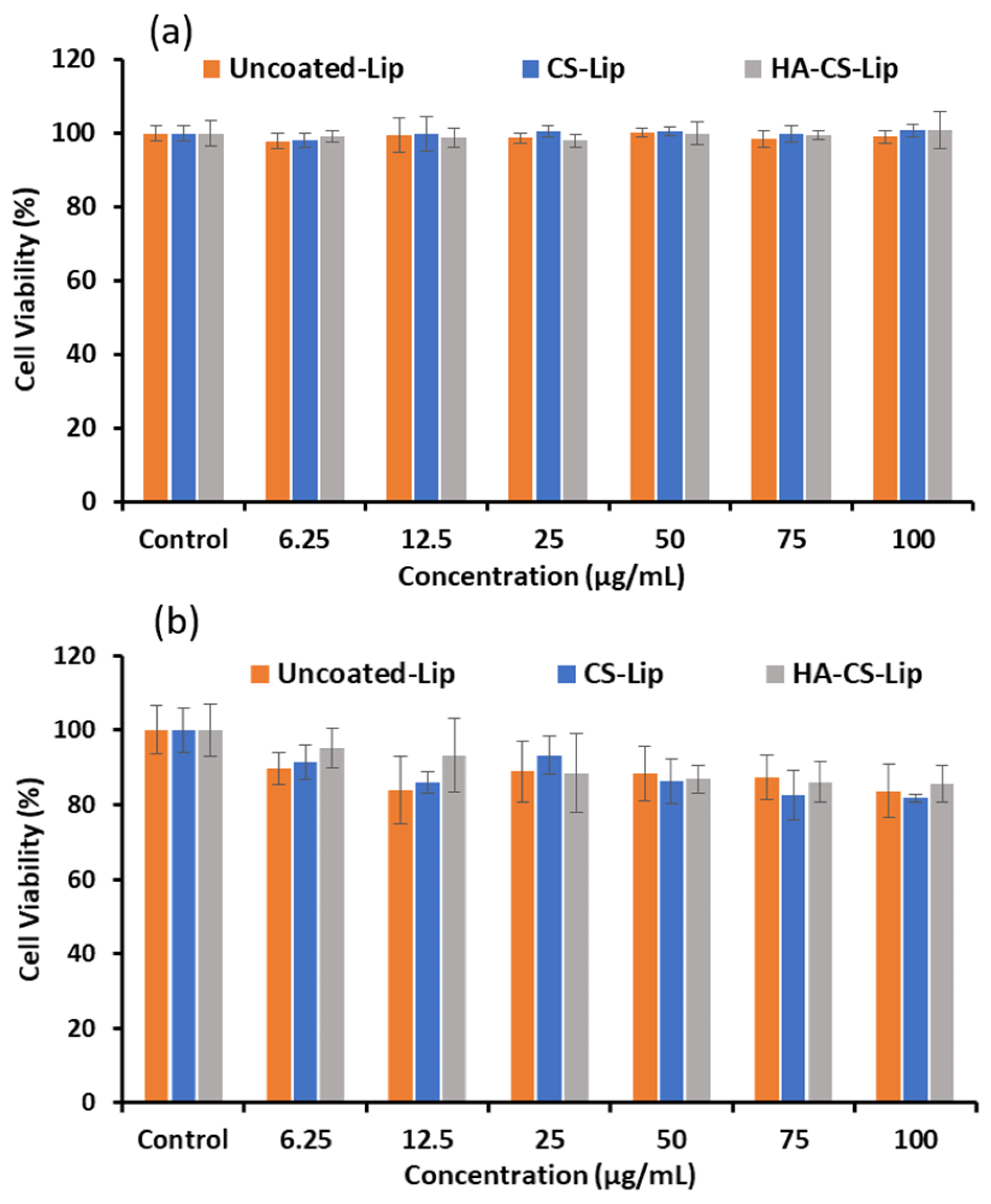
3.8. Biocompatibility Study
3.9. Cytotoxicity Study
3.10. Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid |
| ATR-FTIR | Attenuated total reflectance Fourier-transformed infrared spectroscopy |
| CS | Chitosan |
| CS-Lip | Blank liposomes coated with chitosan |
| DLS | Dynamic light scattering |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FTIR | Fourier-transformed infrared spectroscopy |
| HA | Hyaluronic acid |
| HA-CS-Lip | Blank CS-Lip coated with hyaluronic acid |
| L | Linoleic acid |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PC | Phosphatidylcholine |
| RES | Resveratrol |
| RES-CS-Lip | CS-Lip loaded with resveratrol |
| RES-HA-CS-Lip | HA-CS-Lip loaded with resveratrol |
| TEM | Transmission electron microscope |
References
- Kundu, J.K.; Surh, Y.-J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008, 269, 243–261. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Azios, N.G.; Krishnamoorthy, L.; Harris, M.; Cubano, L.A.; Cammer, M.; Dharmawardhane, S.F. Estrogen and Resveratrol Regulate Rac and Cdc42 Signaling to the Actin Cytoskeleton of Metastatic Breast Cancer Cells. Neoplasia 2007, 9, 147–158. [Google Scholar] [CrossRef]
- Ito, Y.; Mitani, T.; Harada, N.; Isayama, A.; Tanimori, S.; Takenaka, S.; Nakano, Y.; Inui, H.; Yamaji, R. Identification of Carbonyl Reductase 1 as a Resveratrol-Binding Protein by Affinity Chromatography Using 4’-Amino-3,5-dihydroxy-trans-stilbene. J. Nutr. Sci. Vitaminol. 2013, 59, 358–364. [Google Scholar] [CrossRef][Green Version]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The Use of Micro- and Nanocarriers for Resveratrol Delivery into and across the Skin in Different Skin Diseases-A Literature Review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Bohara, R.A.; Tabassum, N.; Singh, M.P.; Gigli, G.; Ragusa, A.; Leporatti, S. Recent Overview of Resveratrol’s Beneficial Effects and Its Nano-Delivery Systems. Molecules 2022, 27, 5154. [Google Scholar] [CrossRef] [PubMed]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Sun, X.; He, Z.; Lu, R.; Liu, Z.; Chiampanichayakul, S.; Anuchapreeda, S.; Jiang, J.; Tima, S.; Zhong, Z. Hyaluronic acid-modified liposomes Potentiated in-vivo anti-hepatocellular carcinoma of icaritin. Front. Pharmacol. 2024, 15, 1437515. [Google Scholar] [CrossRef]
- Louderbough, J.M.; Schroeder, J.A. Understanding the dual nature of CD44 in breast cancer progression. Mol. Cancer Res. 2011, 9, 1573–1586. [Google Scholar] [CrossRef]
- Bonechi, C.; Tamasi, G.; Donati, A.; Leone, G.; Consumi, M.; Cangeloni, L.; Volpi, V.; Magnani, A.; Cappelli, A.; Rossi, C. Physicochemical Characterization of Hyaluronic Acid and Chitosan Liposome Coatings. Appl. Sci. 2021, 11, 12071. [Google Scholar] [CrossRef]
- Yang, K.K.; Kong, M.; Wei, Y.N.; Liu, Y.; Cheng, X.J.; Li, J.; Park, H.J.; Chen, X.G. Folate-modified–chitosan-coated liposomes for tumor-targeted drug delivery. J. Mater. Sci. 2013, 48, 1717–1728. [Google Scholar] [CrossRef]
- Rodwattanagul, S.; Sasarom, M.; Riangjanapatee, P.; Anuchapreeda, S.; Okonogi, S. Antioxidant activity of Sophora exigua and liposome development of its powerful extract. Drug Discov. Ther. 2024, 18, 150–159. [Google Scholar] [CrossRef]
- Sasarom, M.; Wanachantararak, P.; Chaijareenont, P.; Okonogi, S. Antioxidant, antiglycation, and antibacterial of copper oxide nanoparticles synthesized using Caesalpinia Sappan extract. Drug Discov. Ther. 2024, 18, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Hussain, A.; Qamar, W.; Gilani, S.J.; Zafar, A.; Alruwaili, N.K.; Alanazi, S.; Almutairy, B.K. Formulation of Piperine–Chitosan-Coated Liposomes: Characterization and In Vitro Cytotoxic Evaluation. Molecules 2021, 26, 3281. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.L.; Francis, D.M.; Sis, M.J.; Kidambi, S. Ionic Driven Embedment of Hyaluronic Acid Coated Liposomes in Polyelectrolyte Multilayer Films for Local Therapeutic Delivery. Sci. Rep. 2015, 5, 14683. [Google Scholar] [CrossRef]
- Park, S.N.; Jo, N.R.; Jeon, S.H. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J. Ind. Eng. Chem. 2014, 20, 1481–1485. [Google Scholar] [CrossRef]
- Channarong, S.; Chaicumpa, W.; Sinchaipanid, N.; Mitrevej, A. Development and evaluation of chitosan-coated liposomes for oral DNA vaccine: The improvement of Peyer’s patch targeting using a polyplex-loaded liposomes. AAPS PharmSciTech 2011, 12, 192–200. [Google Scholar] [CrossRef]
- Milkova, V.; Vilhelmova-Ilieva, N.; Gyurova, A.; Kamburova, K.; Dimitrov, I.; Tsvetanova, E.; Georgieva, A.; Mileva, M. Remdesivir-Loaded Nanoliposomes Stabilized by Chitosan/Hyaluronic Acid Film with a Potential Application in the Treatment of Coronavirus Infection. Neurol. Int. 2023, 15, 1320–1338. [Google Scholar] [CrossRef]
- Gu, H.; Chen, P.; Liu, X.; Lian, Y.; Xi, J.; Li, J.; Song, J.; Li, X. Trimethylated chitosan-coated flexible liposomes with resveratrol for topical drug delivery to reduce blue-light-induced retinal damage. Int. J. Biol. Macromol. 2023, 252, 126480. [Google Scholar] [CrossRef]
- Myat, Y.Y.; Vollrath, M.K.; Sahatspan, N.; Ngawhirunpat, T.; Pornpitchanarong, C.; Patrojanasophon, P. Development of mucoadhesive chitosan-iodoacetamide buccal tablets for local delivery of dextromethorphan. Nat. Life Sci. Commun. 2023, 22, e2023041. [Google Scholar] [CrossRef]
- Yeo, S.; An, J.; Park, C.; Kim, D.; Lee, J. Design and Characterization of Phosphatidylcholine-Based Solid Dispersions of Aprepitant for Enhanced Solubility and Dissolution. Pharmaceutics 2020, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Vongsvivut, J.; Heraud, P.; Zhang, W.; Kralovec, J.A.; McNaughton, D.; Barrow, C.J. Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chem. 2012, 135, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, X.; Yang, J.; Tan, C. Hyaluronic Acid-Coated Nanoliposomes as Delivery Systems for Fisetin: Stability, Membrane Fluidity, and Bioavailability. Foods 2024, 13, 2406. [Google Scholar] [CrossRef]
- Balanč, B.; Trifković, K.; Đorđević, V.; Marković, S.; Pjanović, R.; Nedović, V.; Bugarski, B. Novel resveratrol delivery systems based on alginate-sucrose and alginate-chitosan microbeads containing liposomes. Food Hydrocoll. 2016, 61, 832–842. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, P.; Kawish, S.M.; Ahmad, S.; Iqbal, Z.; Vohora, D.; Kohli, K. Novel Chitosan-Coated Liposomes Coloaded with Exemestane and Genistein for an Effective Breast Cancer Therapy. ACS Omega 2024, 9, 9735–9752. [Google Scholar] [CrossRef]
- Ramasamy, T.; Haidar, Z.S.; Tran, T.H.; Choi, J.Y.; Jeong, J.-H.; Shin, B.S.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014, 10, 5116–5127. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Physical properties of crosslinked hyaluronic acid hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 3335–3343. [Google Scholar] [CrossRef]
- de Souza, J.B.; de Lacerda Coriolano, D.; Dos Santos Silva, R.C.; da Costa Júnior, S.D.; de Almeida Campos, L.A.; Cavalcanti, I.D.L.; Lira Nogueira, M.C.B.; Pereira, V.R.A.; Brelaz-de-Castro, M.C.A.; Cavalcanti, I.M.F. Ceftazidime and Usnic Acid Encapsulated in Chitosan-Coated Liposomes for Oral Administration against Colorectal Cancer-Inducing Escherichia coli. Pharmaceuticals 2024, 17, 802. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surf. B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Chen, S.; Zhao, J.; Lei, Y.; Geng, L. Nano-Resveratrol Liposome: Physicochemical Stability, In Vitro Release, and Cytotoxicity. Appl. Biochem. Biotechnol. 2023, 195, 5950–5965. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Crayton, S.H.; Thawani, J.P.; Amirshaghaghi, A.; Tsourkas, A.; Cheng, Z. A pH-Responsive Drug-Delivery Platform Based on Glycol Chitosan-Coated Liposomes. Small 2015, 11, 4870–4874. [Google Scholar] [CrossRef] [PubMed]
- Beheshtkhoo, N.; Jadidi Kouhbanani, M.A.; Daghighi, S.M.; Shakouri Nikjeh, M.; Esmaeili, Z.; Khosravani, M.; Adabi, M. Effect of oral resveratrol-loaded nanoliposomes on hyperlipidemia via toll-like receptor 3 and TIR domain-containing adaptor inducing interferon-β protein expression in an animal model. J. Liposome Res. 2025, 35, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; McShane, M.; Lvov, Y.M.; Ji, Q.; Hill, J.P. Layer-by-layer assembly for drug delivery and related applications. Expert. Opin. Drug Deliv. 2011, 8, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Z.; Zhang, W.; Wang, L.; Zhao, P.; Lv, X.; Guo, P.; Chen, J. Surface modification by chitosan for improving stability and antioxidative activity of astaxanthin-loaded liposomes. LWT 2024, 198, 116033. [Google Scholar] [CrossRef]
- Machado, N.D.; Gutiérrez, G.; Matos, M.; Fernández, M.A. Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes. Foods 2021, 10, 988. [Google Scholar] [CrossRef]
- Sarma, S.; Agarwal, S.; Bhuyan, P.; Hazarika, J.; Ganguly, M. Resveratrol-loaded chitosan-pectin core-shell nanoparticles as novel drug delivery vehicle for sustained release and improved antioxidant activities. R. Soc. Open Sci. 2022, 9, 210784. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef]
- Alomrani, A.; Badran, M.; Harisa, G.I.; Lshehry, A.M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef]
- Hasan, M.; Elkhoury, K.; Belhaj, N.; Kahn, C.; Tamayol, A.; Barberi-Heyob, M.; Arab-Tehrany, E.; Linder, M. Growth-Inhibitory Effect of Chitosan-Coated Liposomes Encapsulating Curcumin on MCF-7 Breast Cancer Cells. Mar. Drugs 2020, 18, 217. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-A.; Wu, H.-G. Characterization of chitosan-hyaluronic acid blended membranes and their biocompatibility with keratocytes. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; Volume 4, pp. 1650–1654. [Google Scholar]
- Dana, P.; Thumrongsiri, N.; Tanyapanyachon, P.; Chonniyom, W.; Punnakitikashem, P.; Saengkrit, N. Resveratrol Loaded Liposomes Disrupt Cancer Associated Fibroblast Communications within the Tumor Microenvironment to Inhibit Colorectal Cancer Aggressiveness. Nanomaterials 2022, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Firouzi Amandi, A.; Bahmanyar, Z.; Dadashpour, M.; Lak, M.; Natami, M.; Döğüş, Y.; Alem, M.; Adeli, O.A. Fabrication of magnetic niosomal platform for delivery of resveratrol: Potential anticancer activity against human pancreatic cancer Capan-1 cell. Cancer Cell Int. 2024, 24, 46. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Elamin, K.M.; Wathoni, N. Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells. Polymers 2023, 15, 1025. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Mukazhanova, Z.; Knut, E.; Turgumbayeva, A.; Kipchakbayeva, A.; Seitimova, G.; Mahomoodally, M.F.; Lobine, D.; Koay, A.; et al. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front. Mol. Biosci. 2021, 8, 649395. [Google Scholar] [CrossRef]


| Lipids | Molar Ratio | CS (mg/mL) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| PC:L | 15:2 | 0.0 | 111.7 ± 4.1 | 0.31 ± 0.14 | −41.13 ± 2.4 |
| PC:L | 15:2 | 1.0 | 189.03 ± 13.3 | 0.3 ± 0.06 | −13.4 ± 1.4 |
| PC:L | 15:2 | 1.5 | 206.5 ± 0.6 * | 0.23 ± 0.17 | +24.8 ± 2.5 * |
| PC:L | 15:2 | 2.0 | 306.6 ± 5.1 | 0.27 ± 0.24 | +17.63 ± 0.2 |
| PC:L | 15:2 | 2.5 | 368.9 ± 1.7 | 0.25 ± 0.01 | +13.77 ± 0.5 |
| PC:L | 15:2 | 3.0 | 501.1 ± 5.9 | 0.35 ± 0.05 | +5.98 ± 1.1 |
| Liposomes | HA (mg/mL) | CS (mg/mL) | RES (mg/mL) |
|---|---|---|---|
| S1 | 0 | 1.5 | 0.50 |
| S2 | 0 | 1.5 | 0.75 |
| S3 | 0 | 1.5 | 1.00 |
| S4 | 0 | 1.5 | 1.25 |
| S5 | 0 | 1.5 | 1.50 |
| F1 | 2 | 1.5 | 0.50 |
| F2 | 2 | 1.5 | 0.75 |
| F3 | 2 | 1.5 | 1.00 |
| F4 | 2 | 1.5 | 1.25 |
| F5 | 2 | 1.5 | 1.50 |
| Liposomes | Size (nm) | PDI | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|
| S1 | 205.4 ± 14.7 | 0.28 ± 0.01 | +32.3 ± 0.2 | 27.01 ± 0.05 |
| S2 | 213.3 ± 4.1 | 0.19 ± 0.01 | +29.4 ± 0.6 | 28.23 ± 0.03 |
| S3 | 217.5 ± 5.4 | 0.21 ± 0.02 | +35.6 ± 0.5 | 53.41 ± 0.02 |
| S4 | 258.9 ± 11.7 | 0.42 ± 0.04 | +34.8 ± 1.3 | 80.92 ± 0.02 |
| S5 | 246.6 ± 7.3 | 0.22 ± 0.01 | +32.1 ± 1.6 | 80.73 ± 0.31 * |
| F1 | 215.4 ± 1.5 | 0.15 ± 0.00 | +1.7 ± 0.2 | 60.53 ± 0.01 |
| F2 | 228.4 ± 1.4 | 0.17 ± 0.01 | +8.0 ± 0.6 | 59.28 ± 0.15 |
| F3 | 234.2 ± 1.2 | 0.21 ± 0.00 | +9.6 ± 0.2 | 60.52 ± 2.75 |
| F4 | 176.0 ± 0.5 | 0.22 ± 0.00 | +11.6 ± 1.3 | 64.05 ± 4.64 |
| F5 | 212.5 ± 7.3 | 0.16 ± 0.01 | +9.0 ± 1.0 | 82.16 ± 0.03 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myat, Y.Y.; Gyi, K.K.; Riangjanapatee, P.; Chittasupho, C.; Anuchapreeda, S.; Okonogi, S. Development and Evaluation of Hyaluronic Acid-Chitosan Coated Liposomes for Enhanced Delivery of Resveratrol to Breast Cancer Cells. Polysaccharides 2025, 6, 93. https://doi.org/10.3390/polysaccharides6040093
Myat YY, Gyi KK, Riangjanapatee P, Chittasupho C, Anuchapreeda S, Okonogi S. Development and Evaluation of Hyaluronic Acid-Chitosan Coated Liposomes for Enhanced Delivery of Resveratrol to Breast Cancer Cells. Polysaccharides. 2025; 6(4):93. https://doi.org/10.3390/polysaccharides6040093
Chicago/Turabian StyleMyat, Yin Yin, Khin Khin Gyi, Pornthida Riangjanapatee, Chuda Chittasupho, Songyot Anuchapreeda, and Siriporn Okonogi. 2025. "Development and Evaluation of Hyaluronic Acid-Chitosan Coated Liposomes for Enhanced Delivery of Resveratrol to Breast Cancer Cells" Polysaccharides 6, no. 4: 93. https://doi.org/10.3390/polysaccharides6040093
APA StyleMyat, Y. Y., Gyi, K. K., Riangjanapatee, P., Chittasupho, C., Anuchapreeda, S., & Okonogi, S. (2025). Development and Evaluation of Hyaluronic Acid-Chitosan Coated Liposomes for Enhanced Delivery of Resveratrol to Breast Cancer Cells. Polysaccharides, 6(4), 93. https://doi.org/10.3390/polysaccharides6040093









