Rationalizing Polysaccharide Extraction with Deep Eutectic Solvents: From Supramolecular Architecture to Emerging AI-Guided Solvent Design
Abstract
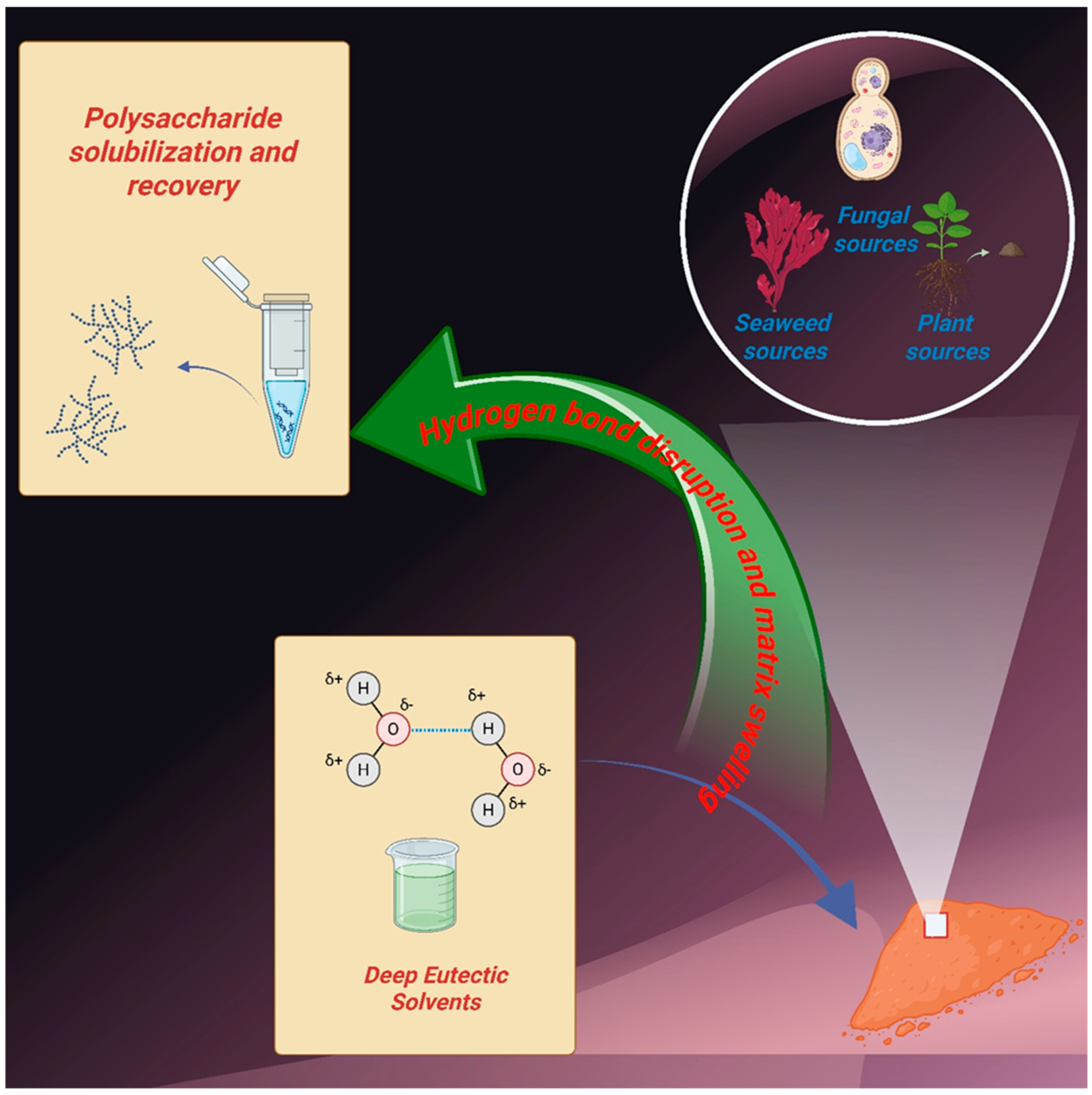
1. Introduction
2. Fundamentals of DESs Relevant to Polysaccharide Systems
Physicochemical Properties of DESs: Implications for Biopolymer Interactions
3. Hydrogen-Bonding Networks and Viscosity Modulation in Polysaccharide-Compatible DESs
4. Tailoring Polarity and Solvent Microenvironments for Polysaccharide Affinity
5. Polysaccharide Organization and Aggregation in Biological Matrices: Structural Implications for DES Extraction
6. Mechanistic Insights into DES–Polysaccharide Interactions
7. Role of DES Composition in Breaking Glycosidic and Hydrogen-Bonding Networks
8. Enhancing Polysaccharide Solubility and Dispersibility Using DESs
9. In Situ vs. Pre-Formulated DES
10. AI, ML, and Biotechnological Innovations in DES–Polysaccharide Research
11. Comparative Evaluation of AI-Guided and Traditional DES Design Strategies
12. Challenges and Future Directions
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Md Yusoff, M.H.; Shafie, M. Pioneering polysaccharide extraction with deep eutectic solvents: A review on impacts to extraction yield, physicochemical properties and bioactivities. Int. J. Biol. Macromol. 2025, 306, 141469. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Nakaweh, A.; Al-Akayleh, F.; Al-Remawi, M.; Abdallah, Q.; Agha, A.S.A. Deep eutectic system-based liquisolid nanoparticles as drug delivery system of curcumin for in-vitro colon cancer cells. J. Pharm. Innov. 2024, 19, 18. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Alkhawaja, B.; Al-Zoubi, N.; Abdelmalek, S.M.; Daadoue, S.; AlAbbasi, D.; Al-Masri, S.; Agha, A.S.A.; Olaimat, A.R.; Woodman, T. Novel therapeutic deep eutectic system for the enhancement of ketoconazole antifungal activity and transdermal permeability. J. Mol. Liq. 2024, 413, 125975. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Alkhawaja, B.; Al-Remawi, M.; Al-Zoubi, N.; Nasereddin, J.; Woodman, T.; Nisrein, J.; Ahmad, M.I.A.; AbuQatouseh, L.; Omari, D.; et al. An Investigation into the Preparation, Characterization, and Therapeutic Applications of Novel Gefitinib/Capric Acid Deep Eutectic Systems. J. Pharm. Innov. 2024, 19, 79. [Google Scholar] [CrossRef]
- Alsoy Altinkaya, S. A perspective on cellulose dissolution with deep eutectic solvents. Front. Membr. Sci. Technol. 2024, 3, 1382054. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, G.; Chen, L.; Liu, Y. Polysaccharides extraction from Ganoderma lucidum using a ternary deep eutectic solvents of choline chloride/guaiacol/lactic acid. Int. J. Biol. Macromol. 2024, 263, 130263. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.-N.; Woo, H.-C.; Chun, B.-S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Nie, J.-Y.; Chen, D.; Lu, Y. Deep Eutectic Solvents Based Ultrasonic Extraction of Polysaccharides from Edible Brown Seaweed Sargassum horneri. J. Mar. Sci. Eng. 2020, 8, 440. [Google Scholar] [CrossRef]
- Shang, X.-C.; Chu, D.; Zhang, J.-X.; Zheng, Y.; Li, Y. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep. Purif. Technol. 2020, 259, 118169. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, S.; Zhai, X.; Sun, J.-S.; Hu, X.; Pei, H.-S.; Chen, G. Green and Efficient Extraction of Polysaccharides From Poria cocos F.A. Wolf by Deep Eutectic Solvent. Nat. Prod. Commun. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Y.; Yu, W.; Wang, C.; Li, F.; Tan, Z. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Clean. Prod. 2020, 274, 123047. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, C.Y.; Yoon, K.Y. Application of Taguchi Method to Optimize Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Polysaccharides from Maca and Its Biological Activity. Food Bioprocess Technol. 2025, 18, 2709–2720. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, X.; Liu, Y.; Hu, J.; Hu, K.; Liu, Y.; Deng, Q.; Yang, S.; Hao, F.; Wen, X. Investigation of natural deep eutectic solvent for the extraction of crude polysaccharide from Polygonatum kingianum and influence of metal elements on its immunomodulatory effects. Talanta 2024, 271, 125721. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, J. Optimization of Ultrasound-Assisted Deep Eutectic Solvent Extraction, Characterization, and Bioactivities of Polysaccharide from Pericarpium Citri Reticulatae. Appl. Biochem. Biotechnol. 2024, 196, 8700–8723. [Google Scholar] [CrossRef]
- Qu, H.; Wu, Y.; Luo, Z.; Dong, Q.; Yang, H.; Dai, C. An efficient approach for extraction of polysaccharide from abalone (Haliotis Discus Hannai Ino) viscera by natural deep eutectic solvent. Int. J. Biol. Macromol. 2023, 244, 125336. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, H.; Yang, G.; Chen, Y.; Zhong, X.; Li, S.; Pan, M.; Li, Y.; Zhang, J. Enhancing the black truffle polysaccharide extraction efficiency using a combination of natural deep eutectic solvents and ultrasound-assisted techniques. J. Food Meas. Charact. 2025, 19, 2208–2219. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, Y.; Bao, Y.; Li, W.-J.; Hong, B.; Zhao, M. A high selective separation method for high-purity polysaccharides from dandelions by density-oriented deep eutectic solvent ultrasonic-assisted system. Sustain. Chem. Pharm. 2024, 42, 101844. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z.; Liu, G.; Liang, L.; Wen, C.; Liu, X.; Li, Y.; Ji, T.; Liu, D.; Ren, J.; et al. Subcritical Water Enhanced with Deep Eutectic Solvent for Extracting Polysaccharides from Lentinus edodes and Their Antioxidant Activities. Molecules 2022, 27, 3612. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zha, F.; Zhang, J.; Zhong, Q.; Tian, H.; Guo, X. Simultaneous extraction of Saponin and Polysaccharide from Acanthopanax senticosus fruits with three-component Deep eutectic solvent and the extraction mechanism analysis. J. Mol. Liq. 2024, 396, 123977. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.-Y.; Zhou, R.-R.; Fang, L.-Z.; Zhao, D.; Cai, P.; Yu, R.; Zhang, S.-H.; Huang, J.-H. The extraction of phenolic acids and polysaccharides from Lilium lancifolium Thunb. using a deep eutectic solvent. Anal. Methods 2021, 13, 1226–1231. [Google Scholar] [CrossRef]
- Wen, Y.; Yan, X.; Chen, H. Optimization of ultrasonic-assisted deep eutectic solvent extraction, characterization, and bioactivities of polysaccharides from Eucommia ulmoides. Prep. Biochem. Biotechnol. 2025, 55, 577–589. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Bi, W.; Chen, D.D.Y. Plant polysaccharide itself as hydrogen bond donor in a deep eutectic system-based mechanochemical extraction method. Food Chem. 2023, 399, 133941. [Google Scholar] [CrossRef]
- Meng, J.; Guan, C.; Chen, Q.; Pang, X.; Wang, H.; Cui, X.; Ye, R.; Zhang, X. Structural Characterization and Immunomodulatory Activity of Polysaccharides from Polygonatum sibiricum Prepared with Deep Eutectic Solvents. J. Food Qual. 2023, 2023, 3147644. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, Y.; Cai, C.; Tan, Z. Extraction of Lycium barbarum polysaccharides using temperature-switchable deep eutectic solvents: A sustainable methodology for recycling and reuse of the extractant. J. Mol. Liq. 2023, 383, 122063. [Google Scholar] [CrossRef]
- Xia, B.; Liu, Q.; Sun, D.; Wang, Y.; Wang, W.; Liu, D. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Polysaccharides from Anji White Tea: Characterization and Comparison with the Conventional Method. Foods 2023, 12, 588. [Google Scholar] [CrossRef]
- Chen, X.; Wang, R.; Tan, Z. Extraction and purification of grape seed polysaccharides using pH-switchable deep eutectic solvents-based three-phase partitioning. Food Chem. 2023, 412, 135557. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Su, J.-S.; Wang, X.; Zhang, R.; Li, X.; Li, Y.; Ding, Y.; Chu, X. Eco-Friendly and Efficient Extraction of Polysaccharides from Acanthopanax senticosus by Ultrasound-Assisted Deep Eutectic Solvent. Molecules 2024, 29, 942. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, L.; Zhao, M. Simultaneously efficient dissolution and structural modification of chrysanthemum pectin: Targeting at proliferation of Bacteroides. Int. J. Biol. Macromol. 2024, 267, 131469. [Google Scholar] [CrossRef]
- Pan, X.; Xu, L.; Meng, J.; Chang, M.; Cheng, Y.; Geng, X.; Guo, D.; Liu, R. Ultrasound-Assisted Deep Eutectic Solvents Extraction of Polysaccharides From Morchella importuna: Optimization, Physicochemical Properties, and Bioactivities. Front. Nutr. 2022, 9, 912014. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sui, X.; Pan, X.; Zhang, X.; Sui, H.; Xu, T.; Zhang, H.; Liu, T.; Liu, J.; Ge, P. Density-oriented deep eutectic solvent-based system for the selective separation of polysaccharides from Astragalus membranaceus var. Mongholicus under ultrasound-assisted conditions. Ultrason. Sonochem. 2023, 98, 106522. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, J.; Luo, X.; Xu, Z.; Liu, K.; Chen, T.; Zhou, L.; Ding, C. Green extraction of polysaccharides from Camellia oleifera fruit shell using tailor-made deep eutectic solvents. Int. J. Biol. Macromol. 2023, 253, 127286. [Google Scholar] [CrossRef]
- Luo, L.; Fan, W.; Qin, J.; Guo, S.; Xiao, H.; Tang, Z. Study on Process Optimization and Antioxidant Activity of Polysaccharide from Bletilla striata Extracted via Deep Eutectic Solvents. Molecules 2023, 28, 5538. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-Y.; Cai, Z.-H.; Zhao, P.-Q.; Wang, J.-D.; Fu, L.-N.; Gu, Q.; Fu, Y.-J. Application of a novel and green temperature-responsive deep eutectic solvent system to simultaneously extract and separate different polar active phytochemicals from Schisandra chinensis (Turcz.) Baill. Food Res. Int. 2023, 165, 112541. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Agha, A.S.A.; Olaimat, A.R.; Qinna, N.A. Rationalizing Polysaccharide Extraction with Deep Eutectic Solvents: From Supramolecular Architecture to Emerging AI-Guided Solvent Design. Preprints 2025, 2025070571. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Al-Remawi, M.; Agha, A.; Abu-Nameh, E. Applications and risk assessments of ionic liquids in chemical and pharmaceutical domains: An updated overview. Jordan J. Chem. 2023, 18, 53–76. [Google Scholar]
- Alkhawaja, B.; Al-Akayleh, F.; Al-Rubaye, Z.; Bustami, M.; Smairat, M.a.; Agha, A.S.; Nasereddin, J.; Qinna, N.; Michael, A.; Watts, A.G. Dissecting the stability of Atezolizumab with renewable amino acid-based ionic liquids: Colloidal stability and anticancer activity under thermal stress. Int. J. Biol. Macromol. 2024, 270, 132208. [Google Scholar] [CrossRef]
- Al-Mawla, L.; Al-Akayleh, F.; Daadoue, S.; Mahyoob, W.; Al-Tameemi, B.; Al-Remawi, M.; Adwan, S.; Agha, A.S.A. Development, characterization, and ex vivo permeation assessment of diclofenac diethylamine deep eutectic systems across human skin. J. Pharm. Innov. 2023, 18, 2196–2209. [Google Scholar] [CrossRef]
- Alkhawaja, B.; Al-Akayleh, F.; Nasereddin, J.; Kamran, M.; Woodman, T.; Al-Rubaye, Z.; Qinna, N.; Al-Remawi, M.; Olaimat, A.R. Structural insights into novel therapeutic deep eutectic systems with capric acid using 1D, 2D NMR and DSC techniques with superior gut permeability. RSC Adv. 2024, 14, 14793–14806. [Google Scholar] [CrossRef]
- Crespo, E.A.; Silva, L.P.; Martins, M.A.R.; Bülow, M.; Ferreira, O.; Sadowski, G.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. The Role of Polyfunctionality in the Formation of [Ch]Cl-Carboxylic Acid-Based Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2018, 57, 11195–11209. [Google Scholar] [CrossRef]
- Aragón-Tobar, C.F.; Endara, D.; de la Torre, E. Dissolution of Metals (Cu, Fe, Pb, and Zn) from Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Three Deep Eutectic Solvents Based on Choline Chloride. Molecules 2024, 29, 290. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Bai, Y.; Feng, M.; Zhou, F.; Lu, Y.; Guo, Y.; Zhang, Y.; Mu, T. Mild and efficient recovery of lithium-ion battery cathode material by deep eutectic solvents with natural and cheap components. Green Chem. Eng. 2023, 4, 303–311. [Google Scholar] [CrossRef]
- Cicci, A.; Sed, G.; Bravi, M. Potential of Choline Chloride-based Natural Deep Eutectic Solvents (nades) in the Extraction of Microalgal Metabolites. Chem. Eng. Trans. 2017, 57, 61–66. [Google Scholar]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Krawczyk, P.; Banach, M. Equilibrium, kinetics and thermodynamics of metal oxide dissolution based on CuO in a natural deep eutectic solvent. Chem. Eng. Res. Des. 2024, 202, 365–376. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, G.; Sun, H.; Xu, C.; Wu, B.; Huang, C.; Lei, B. A novel strategy to intensify the dissolution of cellulose in deep eutectic solvents by partial chemical bonding. BioResources 2022, 17, 4167–4185. [Google Scholar] [CrossRef]
- Gamare, J.S.; Vats, B.G. A Hydrophobic Deep Eutectic Solvent for Nuclear Fuel Cycle: Extraction of Actinides and Dissolution of Uranium Oxide. Eur. J. Inorg. Chem. 2023, 26, e202300441. [Google Scholar] [CrossRef]
- Guajardo, N.; Carlesi, C.; Aracena, Á. Toluene Oxidation by Hydrogen Peroxide in Deep Eutectic Solvents. ChemCatChem 2015, 7, 2451–2454. [Google Scholar] [CrossRef]
- Gupta, R.; Gamare, J.S.; Gupta, S.K.; Kumar, S. Direct dissolution of uranium oxides in deep eutectic solvent: An insight using electrochemical and luminescence study. J. Mol. Struct. 2020, 1215, 128266. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Z.; Ren, S.; Wu, W. The applications of deep eutectic solvents in the separation of mixtures. Chin. Sci. Bull. 2015, 60, 2490–2499. [Google Scholar] [CrossRef]
- Kianpoor, A.; Sadeghi, R. Novel acidic deep eutectic solvents: Synthesis, phase diagram, thermal behavior, physicochemical properties and application. J. Iran. Chem. Soc. 2024, 21, 2385–2399. [Google Scholar] [CrossRef]
- Kumari, T.; Chauhan, R.; Sharma, N.; Kaur, K.; Krishnamurthy, A.; Pandey, P.; Aggarwal, S. Zinc Chloride as Acetamide based Deep Eutectic Solvent. DU J. Undergrad. Res. Innov. 2016, 2, 203–210. [Google Scholar]
- Liu, Q.; Che, J.; Yu, Y.; Chu, D.; Zhang, H.; Zhang, F.; Zhao, M.; Yin, H. Dissolving Chitin by Novel Deep Eutectic Solvents for Effectively Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2024, 196, 8410–8428. [Google Scholar] [CrossRef]
- Manasi, I.; King, S.; Edler, K.J. Cationic micelles in deep eutectic solvents: Effects of solvent composition. Faraday Discuss. 2024, 253, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, V.; Fazio, G.; Pollastri, S.; Gentili, A.; Tomai, P.; Tavani, F.; D’Angelo, P. Solubilization properties and structural characterization of dissociated HgO and HgCl2 in deep eutectic solvents. J. Mol. Liq. 2021, 329, 115505. [Google Scholar] [CrossRef]
- Mu, L.; Gao, J.; Zhang, Q.; Kong, F.; Zhang, Y.; Ma, Z.; Sun, C.; Lv, S. Research Progress on Deep Eutectic Solvents and Recent Applications. Processes 2023, 11, 1986. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. New chloroacetic acid-based deep eutectic solvents for solubilizing metal oxides. J. Mol. Liq. 2021, 347, 118393. [Google Scholar] [CrossRef]
- Popović, B.M.; Uka, D.; Alioui, O.; Ždero Pavlović, R.; Benguerba, Y. Experimental and COSMO-RS theoretical exploration of rutin formulations in natural deep eutectic solvents: Solubility, stability, antioxidant activity, and bioaccessibility. J. Mol. Liq. 2022, 359, 119266. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.-X.; Wang, D.; Xu, C.; Zhang, Q.; Jian, L.; Li, Y. Dissolution-electrodeposition pathway and bulk porosity on the impact of in situ reduction of solid PbO in deep eutectic solvent. Electrochim. Acta 2016, 196, 56–66. [Google Scholar] [CrossRef]
- Sakhno, T.; Barashkov, N.N.; Irgibaeva, I.S.; Mendigaliyeva, S.; Bostan, D.S. Ionic Liquids and Deep Eutectic Solvents and Their Use for Dissolving Animal Hair. Adv. Chem. Eng. Sci. 2020, 10, 40–51. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Sturabotti, E.; Giacomo Marrani, A.; Simonetti, G.; Pagnanelli, F. Decomposition of Deep Eutectic Solvent Aids Metals Extraction in Lithium-Ion Batteries Recycling. ChemSusChem 2022, 15, e202200966. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.K.; Sequeira, R.A.; Pereira, M.M.; Maity, T.K.; Chudasama, N.A.; Prasad, K. Are ionic liquids and deep eutectic solvents the same?: Fundamental investigation from DNA dissolution point of view. J. Mol. Liq. 2021, 328, 115386. [Google Scholar] [CrossRef]
- Sharma, A.; Park, Y.R.; Garg, A.; Lee, B.-S. Deep Eutectic Solvents Enhancing Drug Solubility and Its Delivery. J. Med. Chem. 2024, 67, 14807–14819. [Google Scholar] [CrossRef]
- Söldner, A. Deep Eutectic Solvents as Extraction, Reaction and Detection Media for Inorganic Compounds. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 2020. [Google Scholar]
- Söldner, A.; Zách, J.; Iwanow, M.; Gärtner, T.; Schlosser, M.; Pfitzner, A.; König, B. Preparation of Magnesium, Cobalt and Nickel Ferrite Nanoparticles from Metal Oxides using Deep Eutectic Solvents. Chemistry 2016, 22, 13108–13113. [Google Scholar] [CrossRef]
- Zinov’eva, I.V.; Fedorov, A.; Milevskii, N.A.; Zakhodyaeva, Y.A.; Voshkin, A.A. Dissolution of Metal Oxides in a Choline Chloride–Sulphosalicylic Acid Deep Eutectic Solvent. Theor. Found. Chem. Eng. 2021, 55, 663–670. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, C.; Hao, S.; Xue, K.; Zhang, J.; Sun, Z.; Xiao, L.; Ren, W.; Yang, J.; Cao, B.; et al. Green synthesis of multifunctional cellulose-based eutectogel using a metal salt hydrate-based deep eutectic solvent for sustainable self-powered sensing. Chem. Eng. J. 2025, 506, 159636. [Google Scholar] [CrossRef]
- Kundu, D.; Rao, P.S.; Banerjee, T. First-principles prediction of Kamlet–Taft solvatochromic parameters of deep eutectic solvent using the COSMO-RS model. Ind. Eng. Chem. Res. 2020, 59, 11329–11339. [Google Scholar] [CrossRef]
- Ren, H.; Chen, C.; Wang, Q.; Zhao, D.; Guo, S. The Properties of Choline Chloride-based Deep Eutectic Solvents and their Performance in the Dissolution of Cellulose. Bioresources 2016, 11, 5435–5451. [Google Scholar] [CrossRef]
- Md Yusoff, M.H.; Shafie, M.H. Microwave-assisted extraction of polysaccharides from Micromelum minutum leaves using citric acid monohydrate-glycerol based deep eutectic solvents and evaluation of biological activities. Anal. Chim. Acta 2024, 1331, 343351. [Google Scholar] [CrossRef]
- Bing, Y.; Zhang, D.-Q.; Xie, C.; Yu, F.; Yu, S.-T. Hydration of α-pinene catalyzed by oxalic acid/polyethylene glycol deep eutectic solvents. J. Fuel Chem. Technol. 2021, 49, 330–338. [Google Scholar]
- Duan, C.; Tian, C.; Feng, X.; Tian, G.; Liu, X.; Ni, Y. Ultrafast process of microwave-assisted deep eutectic solvent to improve properties of bamboo dissolving pulp. Bioresour. Technol. 2023, 370, 128543. [Google Scholar] [CrossRef]
- Chourasia, V.R.; Bisht, M.; Pant, K.K.; Henry, R.J. Unveiling the Potential of Water as a Co-solvent in Microwave-assisted Delignification of Sugarcane Bagasse using Ternary Deep Eutectic Solvents. Bioresour. Technol. 2022, 351, 127005. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar]
- Rifai, A.; Bayan, A.; Faisal, A.-A.; Mayyas, A.-R.; Jehad, N.; Safwan, A.R.; Tim, W.; Ali Agha, A.S.A. Eutectic-based self-emulsifying drug delivery system for enhanced oral delivery of risperidone. J. Dispers. Sci. Technol. 2025, 1–12. [Google Scholar] [CrossRef]
- Chatterjee, S.; Deshmukh, S.H.; Bagchi, S. Does Viscosity Drive the Dynamics in an Alcohol-Based Deep Eutectic Solvent? J. Phys. Chem. B 2022, 126, 8331–8337. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Sebbah, T.; Liu, Y.; Cao, X. Terpenoid-capric acid based natural deep eutectic solvent: Insight into the nature of low viscosity. Clean. Eng. Technol. 2021, 3, 100116. [Google Scholar] [CrossRef]
- Chatterjee, S.; Deshmukh, S.H.; Chowdhury, T.; Bagchi, S. Viscosity effects on the dynamics of diols and diol-based deep eutectic solvents. Photochem. Photobiol. 2024, 100, 946–955. [Google Scholar] [CrossRef]
- Fan, C.; Liu, Y.; Sebbah, T.; Cao, X. A Theoretical Study on Terpene-Based Natural Deep Eutectic Solvent: Relationship between Viscosity and Hydrogen-Bonding Interactions. Glob. Chall. 2021, 5, 2000103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Xu, X.; Song, J.; Chen, Y.; Kan, Z.; Li, C. Molecular dynamics simulations of choline chloride and ascorbic acid deep eutectic solvents: Investigation of structural and dynamics properties. J. Mol. Graph. Model. 2024, 130, 108784. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, X.D.; Yang, R.-J.; Qin, X.; Zhou, X.; Zhang, H.; Kong, W.; Zhang, J.; Wang, J. Deep eutectic solvents boosting solubilization and Se-functionalization of heteropolysaccharide: Multiple hydrogen bonds modulation. Carbohydr. Polym. 2022, 284, 119159. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.B.; Sinclair, N.S.; Wainright, J.S.; Savinell, R.F. (Invited) Effects of Halide Anion Type and Alkyl Chain Length on Hydrogen Bonding in Eutectic Solvent System. ECS Meet. Abstr. 2022, MA2022-02, 1102. [Google Scholar] [CrossRef]
- D’agostino, C.; Harris, R.C.; Abbott, A.P.; Gladden, L.F.; Mantle, M.D. Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21383–21391. [Google Scholar] [CrossRef]
- Ren, H.; Chen, C.; Guo, S.; Zhao, D.; Wang, Q. Synthesis of a Novel Allyl-Functionalized Deep Eutectic Solvent to Promote Dissolution of Cellulose. Bioresources 2016, 11, 8457–8469. [Google Scholar] [CrossRef]
- Florindo, C.; Florindo, C.; McIntosh, A.J.S.; Welton, T.; Branco, L.C.; Marrucho, I.M.; Marrucho, I.M. A closer look into deep eutectic solvents: Exploring intermolecular interactions using solvatochromic probes. Phys. Chem. Chem. Phys. 2017, 20, 206–213. [Google Scholar] [CrossRef]
- Teles, A.R.R.; Capela, E.V.; Carmo, R.S.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Solvatochromic parameters of deep eutectic solvents formed by ammonium-based salts and carboxylic acids. Fluid Phase Equilib. 2017, 448, 15–21. [Google Scholar] [CrossRef]
- He, C.; Shen, F.; Tian, D.; Huang, M.; Zhao, L.; Yu, Q.; Shen, F. Lewis acid/base mediated deep eutectic solvents intensify lignocellulose fractionation to facilitate enzymatic hydrolysis and lignin nanosphere preparation. Int. J. Biol. Macromol. 2023, 254, 127853. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Chen, T.; Guo, G.-M.; Shen, D.; Tang, Y.R. Metal Salt-Based Deep Eutectic Solvent Pretreatment of Moso Bamboo to Improve Enzymatic Hydrolysis. Fermentation 2023, 9, 618. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, L.; Shen, F.; Hu, J.; Huang, M.; He, J.; Zhang, Y.; Deng, S.-H.; Li, Q.; Tian, D.-J. Insights into lignocellulosic waste fractionation for lignin nanospheres fabrication using acidic/alkaline deep eutectic solvents. Chemosphere 2021, 286 Pt 2, 131798. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zhang, Y.; Wang, T.; Jiang, B.; Liu, M.; Zhao, L.; Hu, J.; Shen, F. The swelling-induced fractionation strategy to mediate cellulose availability and lignin structural integrity. Chem. Commun. 2025, 61, 2277–2280. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Wang, X.; Cui, M.; An, B.; Pu, J.; Liu, J.; Zhang, B.; Ma, G.; Zhong, C. Diatom-inspired multiscale mineralization of patterned protein–polysaccharide complex structures. Natl. Sci. Rev. 2021, 8, nwaa191. [Google Scholar] [CrossRef]
- Kasaai, M.R. Calculation of viscometric constants, hydrodynamic volume, polymer–solvent interaction parameter, and expansion factor for three polysaccharides with different chain conformations. Carbohydr. Res. 2008, 343, 2266–2277. [Google Scholar] [CrossRef]
- Jarvis, M.C. Hydrogen bonding and other non-covalent interactions at the surfaces of cellulose microfibrils. Cellulose 2023, 30, 667–687. [Google Scholar] [CrossRef]
- Schmid, F.; Stone, B.A.; McDougall, B.M.; Bacic, A.; Martin, K.L.; Brownlee, R.T.C.; Chai, E.; Seviour, R.J. Structure of epiglucan, a highly side-chain/branched (1→3;1→6)-β-glucan from the micro fungus Epicoccum nigrum Ehrenb. ex Schlecht. Carbohydr. Res. 2001, 331, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Boughanmi, R.; Oelmann, M.; Steinbach, C.; Schwarz, S. Sustainable polyelectrolyte complexes of pectin and chitosan as adsorbents for heavy metal ions from surface water. J. Polym. Sci. 2025, 63, 133–145. [Google Scholar] [CrossRef]
- Volod’ko, A.V.; Davydova, V.N.; Glazunov, V.P.; Likhatskaya, G.N.; Yermak, I.M. Influence of structural features of carrageenan on the formation of polyelectrolyte complexes with chitosan. Int. J. Biol. Macromol. 2016, 84, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Dulong, V.; Thebault, P.; Karakasyan, C.; Picton, L.; le Cerf, D. Polyelectrolyte complexes of chitosan and hyaluronic acid or carboxymethylpullulan and their aminoguaiacol derivatives with biological activities as potential drug delivery systems. Carbohydr. Polym. 2024, 341, 122330. [Google Scholar] [CrossRef]
- Rodriguez-Velazquez, E.; Alatorre-Meda, M.; Mano, J.F. Polysaccharide-based nanobiomaterials as controlled release systems for tissue engineering applications. Curr. Pharm. Des. 2015, 21, 4837–4850. [Google Scholar] [CrossRef]
- Wang, T.; Salazar, A.; Zabotina, O.A.; Hong, M. Structure and Dynamics of Brachypodium Primary Cell Wall Polysaccharides from Two-Dimensional 13C Solid-State Nuclear Magnetic Resonance Spectroscopy. Biochemistry 2014, 53, 2840–2854. [Google Scholar] [CrossRef]
- Rinaudo, M. Non-Covalent Interactions in Polysaccharide Systems. Macromol. Biosci. 2006, 6, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 57–112. [Google Scholar]
- Yahaya, N.; Mohamed, A.H.; Sajid, M.; Zain, N.N.M.; Liao, P.-C.; Chew, K.W. Deep eutectic solvents as sustainable extraction media for extraction of polysaccharides from natural sources: Status, challenges and prospects. Carbohydr. Polym. 2024, 338, 122199. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Han, J.; Tang, H.; Sun, Y.; Li, L.; Kong, W.; Liang, J.; Zhang, J.; Wang, J. The dissolution of galactomannans in type II/III deep eutectic solvents: Effect of polysaccharide structure and interaction mechanism. Food Hydrocoll. 2025, 158, 110552. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.J.; Budtova, T.; de Wever, P.; Fardim, P.; Gericke, M.; Groth, T.; Heinze, T.; Höfte, H.; Huber, A.; Ikkala, O. EPNOE, Research Roadmap 2040; EPNOE: Leuven, Belgium, 2022. [Google Scholar]
- Huang, J.; Chang, P.R.; Dufresne, A. Polysaccharide Nanocrystals: Current Status and Prospects in Material Science; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Liu, H.; Lin, J.; Hu, Y.; Lei, H.; Zhang, Q.; Tao, X.; Zhang, D.; Niu, H. DES-Assisted Extraction of Pectin from Ficus carica Linn. peel: Optimization, Partial Structure Characterization, Functional and Antioxidant Activities. J. Sci. Food Agric. 2024, 104, 5149–5162. [Google Scholar] [CrossRef]
- Shafie, M.H.; Gan, C.-Y. Could choline chloride-citric acid monohydrate molar ratio in deep eutectic solvent affect structural, functional and antioxidant properties of pectin? Int. J. Biol. Macromol. 2020, 149, 835–843. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Zhang, W.; Li, M.; Peng, F.; Bian, J. Alkaline deep eutectic solvents as novel and effective pretreatment media for hemicelluloses dissociation and enzymatic hydrolysis enhancement. Int. J. Biol. Macromol. 2021, 193, 1610–1616. [Google Scholar] [CrossRef]
- Marullo, S.; Raia, G.; Bailey, J.J.; Gunaratne, H.Q.N.; D’Anna, F. Inulin Dehydration to 5-HMF in Deep Eutectic Solvents Catalyzed by Acidic Ionic Liquids Under Mild Conditions. ChemSusChem 2025, 18, e202402522. [Google Scholar] [CrossRef]
- Cheng, W.-Y.; Lam, K.-L.; Pik-Shan Kong, A.; Chi-Keung Cheung, P. Prebiotic supplementation (beta-glucan and inulin) attenuates circadian misalignment induced by shifted light-dark cycle in mice by modulating circadian gene expression. Food Res. Int. 2020, 137, 109437. [Google Scholar] [CrossRef]
- Ling, Z.; Tang, W.; Su, Y.; Shao, L.; Wang, P.; Ren, Y.; Huang, C.; Lai, C.; Yong, Q. Promoting enzymatic hydrolysis of aggregated bamboo crystalline cellulose by fast microwave-assisted dicarboxylic acid deep eutectic solvents pretreatments. Bioresour. Technol. 2021, 333, 125122. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Wu, C. Probing the evolutionary mechanism of the hydrogen bond network of cellulose nanofibrils using three DESs. Int. J. Biol. Macromol. 2023, 234, 123694. [Google Scholar] [CrossRef]
- Soleimanzadeh, H.; Bektashi, F.M.; Ahari, S.Z.; Salari, D.; Olad, A.; Ostadrahimi, A. Optimization of cellulose extraction process from sugar beet pulp and preparation of its nanofibers with choline chloride–lactic acid deep eutectic solvents. Biomass Convers. Biorefin. 2022, 13, 14457–14469. [Google Scholar] [CrossRef]
- Ghasemi, M.F.; Tsianou, M.; Alexandridis, P. Solvent-Induced Decrystallization of Cellulose: Thermodynamics, Transport, and Kinetics. In Proceedings of the 2014 AIChE Annual Meeting, Atlanta, GA, USA, 16–21 November 2014. [Google Scholar]
- Wang, X.; Zhou, P.; Lv, X.-Y.; Liang, Y.L. Insight into the structure-function relationships of the solubility of chitin/chitosan in natural deep eutectic solvents. Mater. Today Commun. 2021, 27, 102374. [Google Scholar] [CrossRef]
- Gupta, V.; Thakur, R.; Das, A.B. Effect of natural deep eutectic solvents on thermal stability, syneresis, and viscoelastic properties of high amylose starch. Int. J. Biol. Macromol. 2021, 187, 575–583. [Google Scholar] [CrossRef]
- Xiao, Q.; Dai, M.; Zhou, H.; Huang, M.; Lim, L.T.; Zeng, C. Formation and structure evolution of starch nanoplatelets by deep eutectic solvent of choline chloride/oxalic acid dihydrate treatment. Carbohydr. Polym. 2022, 282, 119105. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.; Li, K.; Jung, D.; Park, K.; Lee, J. Preparation and characterization of various chitin-glucan complexes derived from white button mushroom using a deep eutectic solvent-based ecofriendly method. Int. J. Biol. Macromol. 2020, 169, 122–129. [Google Scholar] [CrossRef]
- Zeng, C.; Zhao, H.; Wan, Z.; Xiao, Q.; Xia, H.; Guo, S. Highly biodegradable, thermostable eutectogels prepared by gelation of natural deep eutectic solvents using xanthan gum: Preparation and characterization. RSC Adv. 2020, 10, 28376–28382. [Google Scholar] [CrossRef]
- Hiemstra, I.S.A.; Meinema, J.T.; Eppink, M.H.M.; Wijffels, R.H.; Kazbar, A. Choline chloride-based solvents as alternative media for alginate extraction from brown seaweed. LWT 2024, 214, 117174. [Google Scholar] [CrossRef]
- Afifah, N.; Sarifudin, A.; Purwanto, W.W.; Krisanti, E.A.; Mulia, K. Glucomannan isolation from porang (Amorphophallus muelleri Blume) flour using natural deep eutectic solvents and ethanol: A comparative study. Food Chem. 2024, 453, 139610. [Google Scholar] [CrossRef]
- Pedro, S.N.; Valente, B.F.A.; Vilela, C.; Oliveira, H.; Almeida, A.; Freire, M.G.; Silvestre, A.J.D.; Freire, C.S.R. Switchable adhesive films of pullulan loaded with a deep eutectic solvent-curcumin formulation for the photodynamic treatment of drug-resistant skin infections. Mater. Today Bio 2023, 22, 100733. [Google Scholar] [CrossRef] [PubMed]
- Depoorter, J.; Mourlevat, A.; Sudre, G.; Morfin, I.; Prasad, K.; Serghei, A.; Bernard, J.; Fleury, E.; Charlot, A. Fully Biosourced Materials from Combination of Choline Chloride-Based Deep Eutectic Solvents and Guar Gum. ACS Sustain. Chem. Eng. 2019, 7, 16747–16756. [Google Scholar] [CrossRef]
- Keerthashalini, P.; Sobanadevi, V.; Uppuluri, K.B. Deep eutectic solvent assisted recovery of high molecular weight levan from an isolated Neobacillus pocheonensis BPSCM4. Prep. Biochem. Biotechnol. 2023, 54, 407–418. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Separation of Polysaccharides by SEC Utilizing Deep Eutectic Solvent Modified Mesoporous Siliceous Materials. Chromatographia 2017, 80, 1161–1169. [Google Scholar] [CrossRef]
- Das, A.K.; Sharma, M.; Mondal, D.; Prasad, K. Deep eutectic solvents as efficient solvent system for the extraction of κ-carrageenan from Kappaphycus alvarezii. Carbohydr. Polym. 2016, 136, 930–935. [Google Scholar] [CrossRef]
- Tang, P.L.; Hao, E.; Du, Z.; Deng, J.; Hou, X.; Qin, J. Polysaccharide extraction from sugarcane leaves: Combined effects of different cellulolytic pretreatment and extraction methods. Cellulose 2019, 26, 9423–9438. [Google Scholar] [CrossRef]
- Pan, M.; Zhao, G.; Ding, C.; Wu, B.; Lian, Z.; Lian, H. Physicochemical transformation of rice straw after pretreatment with a deep eutectic solvent of choline chloride/urea. Carbohydr. Polym. 2017, 176, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hsueh, P.-H.; Ulfadillah, S.A.; Wang, S.-T.; Tsai, M.-L. Exploring the Sustainable Utilization of Deep Eutectic Solvents for Chitin Isolation from Diverse Sources. Polymers 2024, 16, 3187. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pan, X.-C.; Li, D.; Wang, Z.; Tan, L.; Chang, M.; Feng, C.; Cheng, Y.; Geng, X.; Meng, J. Structural characterization, rheological characterization, hypoglycemic and hypolipidemic activities of polysaccharides from Morchella importuna using acidic and alkaline deep eutectic solvents. LWT 2024, 193, 115742. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Hyypiö, K.; Asaadi, S.; Junka, K.; Liimatainen, H. High-strength cellulose nanofibers produced via swelling pretreatment based on a choline chloride–imidazole deep eutectic solvent. Green Chem. 2020, 22, 1763–1775. [Google Scholar]
- Ashworth, C.; Matthews, R.P.; Welton, T.; Hunt, P.A. Doubly ionic hydrogen bond interactions within the choline chloride-urea deep eutectic solvent. Phys. Chem. Chem. Phys. 2016, 18, 18145–18160. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Fan, C.; Cao, X. One-step extraction and hydrolysis of crocin into crocetin by recyclable deep eutectic solvents. Ind. Crops Prod. 2024, 209, 117969. [Google Scholar] [CrossRef]
- Santra, S.; Das, M.; Karmakar, S.; Banerjee, R. NADES assisted integrated biorefinery concept for pectin recovery from kinnow (Citrus reticulate) peel and strategic conversion of residual biomass to L(+) lactic acid. Int. J. Biol. Macromol. 2023, 250, 126169. [Google Scholar] [CrossRef]
- Zhao, J.; Pedersen, C.M.; Chang, H.; Hou, X.; Wang, Y.; Qiao, Y. Switchable product selectivity in dehydration of N-acetyl-d-glucosamine promoted by choline chloride-based deep eutectic solvents. iScience 2023, 26, 106980. [Google Scholar]
- Lee, C.B.T.L.; Wu, T.Y.; Yong, K.J.; Cheng, C.K.; Siow, L.F.; Jahim, J.M. Investigation into Lewis and Brønsted acid interactions between metal chloride and aqueous choline chloride-oxalic acid for enhanced furfural production from lignocellulosic biomass. Sci. Total Environ. 2022, 827, 154049. [Google Scholar]
- AlZahrani, Y.M.; Britton, M.M. Probing the influence of Zn and water on solvation and dynamics in ethaline and reline deep eutectic solvents by 1H nuclear magnetic resonance. Phys. Chem. Chem. Phys. 2021, 23, 21913–21922. [Google Scholar] [PubMed]
- Wang, J.; Qin, C.; Xu, B.; Yan, L. Fast Dissolution of Chitin in Amino Acids Based Deep Eutectic Solvents Under the Assistance of Microwave. Macromol. Rapid Commun. 2024, 46, e2400685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, J.; Lan, P.; Yang, H.; Lu, J.; Wang, Z. Study on the Dissolution Mechanism of Cellulose by ChCl-Based Deep Eutectic Solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Feng, Y.; Zhang, X.; Xu, F. Deep eutectic solvent (TMAH·5H2O/Urea) with low viscosity for cellulose dissolution at room temperature. Carbohydr. Polym. 2024, 339, 122260. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.; Ren, J.; He, B.-H. Effect of Ternary Deep Eutectic Solvents on Bagasse Cellulose and Lignin Structure in Low-Temperature Pretreatment. Processes 2022, 10, 778. [Google Scholar] [CrossRef]
- Ling, Z.; Guo, Z.; Huang, C.; Yao, L.; Xu, F. Deconstruction of oriented crystalline cellulose by novel levulinic acid based deep eutectic solvents pretreatment for improved enzymatic accessibility. Bioresour. Technol. 2020, 305, 123025. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, J.; Wang, P.; Ling, Z.; Yong, Q. Natural arginine-based deep eutectic solvents for rapid microwave-assisted pretreatment on crystalline bamboo cellulose with boosting enzymatic conversion performance. Bioresour. Technol. 2023, 385, 129438. [Google Scholar] [CrossRef]
- Nor, N.A.M.; Mustapha, W.A.W.; Hassan, O. Deep eutectic solvent (DES) as a pretreatment for oil palm empty fruit bunch (OPEFB) in production of sugar. Procedia Chem. 2015, 18, 147–154. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, L.; Huang, B.-N.; Yu, Y.; Liu, J.; Li, T. Synthesis of Green Deep Eutectic Solvents for Pretreatment Wheat Straw: Enhance the Solubility of Typical Lignocellulose. Sustainability 2022, 4, 657. [Google Scholar] [CrossRef]
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules 2021, 26, 6556. [Google Scholar] [CrossRef]
- Pereda-Cruz, D.; Macías-Salinas, R. Viscosity Modelling of Deep Eutectic Solvents via the Use of a Residual-entropy Scaling. Chem. Eng. Trans. 2023, 100, 499–504. [Google Scholar]
- Benguerba, Y.; Alnashef, I.; Erto, A.; Balsamo, M.; Ernst, B. A quantitative prediction of the viscosity of amine based DESs using Sσ-profile molecular descriptors. J. Mol. Struct. 2019, 1184, 357–363. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Zhang, Y.; Bi, W. Switching from deep eutectic solvents to deep eutectic systems for natural product extraction. Green Chem. Eng. 2025, 6, 36–53. [Google Scholar] [CrossRef]
- Lim, J.J.Y.; Hoo, D.Y.; Tang, S.Y.; Manickam, S.; Yu, L.J.; Tan, K.W. One-pot extraction of nanocellulose from raw durian husk fiber using carboxylic acid-based deep eutectic solvent with in situ ultrasound assistance. Ultrason. Sonochem. 2024, 106, 106898. [Google Scholar] [CrossRef] [PubMed]
- Koigerova, A. Estimation of efficiency of ultrasonic extraction using deep eutectic solvents for extraction of biologically active compounds from plant raw materials. E3S Web Conf. 2024, 486, 04012. [Google Scholar] [CrossRef]
- Meles Neguse, S.; Yoon, S.; Lim, H.; Jang, J.; Baek, S.; Jöckel, D.M.; Widenmeyer, M.; Balke-Grünewald, B.; Weidenkaff, A. The Pitfalls of Deep Eutectic Solvents in the Recycling of Lithium-Ion Batteries. Energy Technol. 2024, 12, 2301213. [Google Scholar] [CrossRef]
- Elizondo Sada, O.M.; Hiemstra, I.S.A.; Chorhirankul, N.; Eppink, M.; Wijffels, R.H.; Janssen, A.E.M.; Kazbar, A. Pressure-driven membrane processes for the recovery and recycling of deep eutectic solvents: A seaweed biorefinery case study. Biotechnol. Rep. 2024, 43, e00849. [Google Scholar] [CrossRef]
- Mariño, M.A.; Paredes, M.G.; Martinez, N.; Millan, D.; Tapia, R.A.; Ruiz, D.; Isaacs, M.; Pavez, P. A ternary eutectic solvent for cellulose nanocrystal production: Exploring the recyclability and pre-pilot scale-up. Front. Chem. 2023, 11, 1233889. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Jeliński, T.; Przybyłek, M.; Mai, A.; Kułak, J. Experimental and Machine-Learning-Assisted Design of Pharmaceutically Acceptable Deep Eutectic Solvents for the Solubility Improvement of Non-Selective COX Inhibitors Ibuprofen and Ketoprofen. Molecules 2024, 29, 2296. [Google Scholar] [PubMed]
- Abbas, U.L.; Zhang, Y.; Tapia, J.; Md, S.; Chen, J.; Shi, J.; Shao, Q. Machine-Learning-Assisted Design of Deep Eutectic Solvents Based on Uncovered Hydrogen Bond Patterns. Engineering 2024, 39, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Jetti, K.D.; Smith, M.D.; Demerdash, O.N.; Kidder, M.K.; Smith, J.C. Accurate Machine Learning for Predicting the Viscosities of Deep Eutectic Solvents. J. Chem. Theory Comput. 2024, 20, 3911–3926. [Google Scholar] [CrossRef]
- Firouzi, M.; Siddiqua, S.; Kazemian, H.; Kiamahalleh, M.V. Green solvent-based extraction of cellulose from hemp bast fibers: From treatment efficacy to characterizations and optimization. Int. J. Biol. Macromol. 2025, 288, 138689. [Google Scholar] [CrossRef]
- Luu, R.K.; Wysokowski, M.; Buehler, M. Generative discovery of de novo chemical designs using diffusion modeling and transformer deep neural networks with application to deep eutectic solvents. Appl. Phys. Lett. 2023, 122, 234103. [Google Scholar] [CrossRef]
- Al-Remawi, M.; Abdel-Rahem, R.A.; Al-Akayleh, F.; Aburub, F.; Agha, A.S.A. Transforming Obesity Care Through Artificial Intelligence: Real-Case Implementations and Personalized Solutions. In Proceedings of the 2025 1st International Conference on Computational Intelligence Approaches and Applications (ICCIAA), Amman, Jordan, 28–30 April 2025. [Google Scholar]
- Aburub, F.; Al-Remawi, M.; Abdel-Rahem, R.A.; Al-Akayleh, F.; Agha, A.S.A. AI-Driven Whole-Exome Sequencing: Advancing Variant Interpretation and Precision Medicine. In Proceedings of the 2025 1st International Conference on Computational Intelligence Approaches and Applications (ICCIAA), Amman, Jordan, 28–30 April 2025. [Google Scholar]
- Aburub, F.; Al-Akayleh, F.; Abdel-Rahem, R.A.; Al-Remawi, M.; Agha, A.S.A. AI-Driven Transcriptomics: Advancing Gene Expression Analysis and Precision Medicine. In Proceedings of the 2025 1st International Conference on Computational Intelligence Approaches and Applications (ICCIAA), Amman, Jordan, 28–30 April 2025. [Google Scholar]
- Al-Akayleh, F.; Abdel-Rahem, R.A.; Al-Remawi, M.; Aburub, F.; Al-Adham, I.S.; Agha, A.S.A. AI-Driven Tools and Methods for Wound Healing: Towards Precision Wound Care and Optimized Outcomes. In Proceedings of the 2025 1st International Conference on Computational Intelligence Approaches and Applications (ICCIAA), Amman, Jordan, 28–30 April 2025. [Google Scholar]
- Al-Akayleh, F.; Al-Remawi, M.; Abdel-Rahem, R.A.; Al-Adham, I.S.; Aburub, F.; Agha, A.S.A. AI-Driven Strategies in Prebiotic Research: Addressing Challenges and Advancing Human Health. In Proceedings of the 2025 1st International Conference on Computational Intelligence Approaches and Applications (ICCIAA), Amman, Jordan, 28–30 April 2025. [Google Scholar]
- Al-Remawi, M.; Aburub, F.; Al-Akayleh, F.; Abdel-Rahem, R.A.; Agha, A.S.A. Artificial Intelligence in Lipidomics: Advancing Biomarker Discovery, Pathway Analysis, and Precision Medicine. In Proceedings of the 2025 1st International Conference on Computational Intelligence Approaches and Applications (ICCIAA), Amman, Jordan, 28–30 April 2025. [Google Scholar]
- Al-Akayleh, F.; Ali Agha, A.S.; Abdel Rahem, R.A.; Al-Remawi, M. A mini review on the applications of artificial intelligence (AI) in surface chemistry and catalysis. Tenside Surfactants Deterg. 2024, 61, 285–296. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Agha, A.S.A. Trust, ethics, and user-centric design in AI-integrated genomics. In Proceedings of the 2024 2nd International Conference on Cyber Resilience (ICCR), Dubai, United Arab Emirates, 26–28 February 2024. [Google Scholar]
- Al-Remawi, M.; Agha, A.S.A.; Al-Akayleh, F.; Aburub, F.; Abdel-Rahem, R.A. Artificial intelligence and machine learning techniques for suicide prediction: Integrating dietary patterns and environmental contaminants. Heliyon 2024, 10, e40925. [Google Scholar] [CrossRef]
- Ghunaim, L.; Agha, A.S.A.A.; Aburjai, T. Integrating artificial intelligence and advanced genomic technologies in unraveling autism spectrum disorder and gastrointestinal comorbidities: A multidisciplinary approach to precision medicine. Jordan J. Pharm. Sci. 2024, 17, 567–581. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Al-Remawi, M.; Agha, A.S.A. AI-driven physical rehabilitation strategies in post-cancer care. In Proceedings of the 2024 2nd International Conference on Cyber Resilience (ICCR), Dubai, United Arab Emirates, 26–28 February 2024. [Google Scholar]
- Aburub, F.; Agha, A.S.A. AI-driven psychological support and cognitive rehabilitation strategies in post-cancer care. In Proceedings of the 2024 2nd International Conference on Cyber Resilience (ICCR), Dubai, United Arab Emirates, 26–28 February 2024. [Google Scholar]
- Liu, Y.; Kashima, H. Chemical property prediction under experimental biases. Sci. Rep. 2020, 12, 8206. [Google Scholar] [CrossRef]
- Jia, X.; Lynch, A.; Huang, Y.; Danielson, M.L.; Lang’at, I.; Milder, A.; Ruby, A.E.; Wang, H.; Friedler, S.A.; Norquist, A.J.; et al. Anthropogenic biases in chemical reaction data hinder exploratory inorganic synthesis. Nature 2019, 573, 251–255. [Google Scholar] [CrossRef]
- Ghunaim, L.; Agha, A.S.A.; Al-Samydai, A.; Aburjai, T. The Future of Pediatric Care: AI and ML as Catalysts for Change in Genetic Syndrome Management. Jordan Med. J. 2024, 58, 510–528. [Google Scholar] [CrossRef]
- Halder, A.K.; Haghbakhsh, R.; Ferreira, E.S.C.; Duarte, A.R.C.; Cordeiro, M.N.D.S. Machine learning-driven prediction of deep eutectic solvents’ heat capacity for sustainable process design. J. Mol. Liq. 2025, 418, 126707. [Google Scholar] [CrossRef]
- Darwish, A.S.; Lemaoui, T.; AlYammahi, J.; Taher, H.; Benguerba, Y.; Banat, F.; AlNashef, I.M. Molecular insights into potential hydrophobic deep eutectic solvents for furfural extraction guided by COSMO-RS and machine learning. J. Mol. Liq. 2023, 379, 121631. [Google Scholar] [CrossRef]
- Ge, H.; Bai, Y.; Zhou, R.; Liu, Y.; Wei, J.; Wang, S.; Li, B.; Xu, H. Explicable Machine Learning for Predicting High-Efficiency Lignocellulose Pretreatment Solvents Based on Kamlet–Taft and Polarity Parameters. ACS Sustain. Chem. Eng. 2024, 12, 7578–7590. [Google Scholar] [CrossRef]
- Sun, C.; Chen, H.; Madadi, M.; Song, G.; Meng, X.; Zhuang, X.; Song, X.; Tan, X.; Sun, F.; Ragauskas, A.J. Understanding the role of porous particle characteristics and water state distribution at multi-scale variation on cellulase hydrolysis of lignocellulosic biomass. Energy 2024, 310, 133263. [Google Scholar] [CrossRef]
- Isci, A.; Kaltschmitt, M. Recovery and recycling of deep eutectic solvents in biomass conversions: A review. Biomass Convers. Biorefinery 2021, 12, 197–226. [Google Scholar]
- Wang, D.; Zhang, M.; Lim Law, C.; Zhang, L. Natural deep eutectic solvents for the extraction of lentinan from shiitake mushroom: COSMO-RS screening and ANN-GA optimizing conditions. Food Chem. 2023, 430, 136990. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2020, 120, 340–350. [Google Scholar]
| Source | Polysaccharide Type | DES Extraction Method/System | Benefit/Remarks vs. Conventional | Reference |
|---|---|---|---|---|
| Dioscorea opposita | Crude polysaccharides | Choline chloride + 1,4-butanediol (ultrasound assisted) | Higher yield than hot water or water–ultrasound extraction | [8] |
| Ganoderma lucidum | β-glucan-rich polysaccharides | Choline chloride + guaiacol + lactic acid (ternary DES) | 94.7 mg/g yield; stable reuse; superior due to strong hydrogen bonding | [9] |
| Saccharina japonica | Alginate and fucoidan | DES + subcritical water hydrolysis | High yields of alginate (28.1%) and fucoidan (14.9%) | [10] |
| Sargassum horneri | Sulfated polysaccharides | Choline chloride + 1,2-propanediol (ultrasound assisted) | Better removal of impurities and higher antioxidant activity | [11] |
| Fucus vesiculosus | Sulfated fucose-rich polysaccharides | Microwave-assisted DES: choline chloride + 1,4-butanediol | 116.3 mg/g yield; strong antioxidant and anticancer activities | [12] |
| Poria cocos | (1→3)-β-D-glucan-rich branched glucans | Choline chloride + oxalic acid DES | 8.6× yield over hot water; good recyclability | [13] |
| Ganoderma lucidum | Acidic heteropolysaccharides composed primarily of glucose, galactose, and glucuronic acid | Temperature-responsive DES | Polysaccharides recovered at UCST; green and recyclable system | [14] |
| Maca | Crude maca polysaccharides (unspecified heteropolysaccharide mixture) | Choline chloride + urea (ultrasound assisted) | 2× yield over water; strong antioxidant and prebiotic benefits | [15] |
| Polygonatum kingianum | Crude polysaccharide (uncharacterized) | Choline chloride/glycerol (1:2), NADES | 2.5× higher yield than water; boosts IL-6 and iNOS in macrophages | [16] |
| Pericarpium Citri Reticulatae | Acidic heteropolysaccharide (PCRPs-1) rich in galactose, rhamnose, and uronic acids | Ultrasound-assisted DES | 5.41% yield vs. 3.92% (water); antioxidant and antidiabetic effects | [17] |
| Abalone viscera | Marine-derived acidic heteropolysaccharide rich in galactose and glucuronic acid (AVP) | Choline chloride + ethylene glycol (1:3 molar ratio), 25% water; ultrasound assisted | Higher yield (17.32%), enhanced glucuronic acid content, lower Mw (53.33 kDa), and stronger antioxidant activity than hot-water extraction | [18] |
| Black truffle | Crude black truffle polysaccharide (uncharacterized) | Betaine + citric acid NADES (ultrasound assisted) | 11× yield over ethanol; antioxidant and anti-aging bioactivities | [19] |
| Dandelion | Crude dandelion polysaccharides (likely inulin-type fructans, arabinogalactans, and/or pectic polysaccharides) | Ultrasound-assisted NADES (choline chloride/oxalic acid 1:2; 60% water) | Higher yield (68.5 mg/g) and purity (0.88 mg/mg); outperforming traditional methods; green and cost effective | [20] |
| Lentinus edodes | Heteropolysaccharide (glucose/galactose/mannose ≈ 32.9:1:2.54) | Subcritical water extraction (SWE) + ChCl–malonate (1:2) DES | 19.2% more yield than SWE; better antioxidant profile | [21] |
| Acanthopanax senticosus | Glucose-based heteropolysaccharide | 3c-DES (betaine/triethanolamine/MgCl2·6H2O = 1:4:0.08, molar ratio); ethanol precipitation | Simultaneous extraction of saponins and polysaccharides | [22] |
| Lilium lancifolium | Crude heteropolysaccharides (glucose, galactose, arabinose, or mannose containing) | Choline chloride–ethylene glycol (ChEtgly, 1:2) with 20% water; ultrasound assisted at 50 °C for 40 min | Comparable yield to hot-water extraction in 1/3 the time; simultaneous phenolic acid co-extraction; green solvent advantage | [23] |
| Eucommia ulmoides | Acidic heteropolysaccharides (mannose, rhamnose, and galacturonic) | Choline chloride + oxalic acid (ultrasound assisted) | 2.3× yield vs. water; strong antioxidant and enzyme inhibition | [24] |
| Dendrobium devonianum | Glucose-based heteropolysaccharide (α-/β-glucans) | Mechanochemical self-forming DESys | No external HBD needed; high efficiency and bioactivity | [25] |
| Polygonatum sibiricum | Galactose- and mannose-rich heteropolysaccharide (DPSP-3) | Choline chloride/oxalic acid (1:1, m/m) DES at 70 °C for 40 min | 15.62% yield (1.53× higher than water extraction); enriched in galactose (65.75%) and mannose (19.76%); improved immunomodulatory activity (ROS, NO, IL-6, and TNF-α release in RAW264.7) | [26] |
| Lycium barbarum | Low-MW heteropolysaccharides (glucose-rich LBP) | Temperature-switchable DES (tetracaine/lauric acid, 1:1; 70 wt%) | 465 mg/g yield; recyclable; strong antioxidant profile | [27] |
| Anji white tea | Acidic arabinogalactan-type heteropolysaccharide | Choline chloride + 1,6-hexanediol (ultrasound assisted) | Higher yield and antioxidant activity vs. water | [28] |
| Grape seed | Heteropolysaccharide (mannose, glucose, galactose, and arabinose) | pH-switchable DES: dodecanoic acid + octanoic acid | 98 mg/g yield; reusable 25×; green alternative to t-butanol | [29] |
| Acanthopanax senticosus root | Acidic heteropolysaccharide (rich in galacturonic acid, arabinose, and galactose) | L-malic acid + L-proline (ultrasound assisted) | 2.6× higher yield than hot water; strong antioxidant activity | [30] |
| Chrysanthemum morifolium | Pectin (Rhamnogalacturonan-I (RG-I) rich) | DES (urea/choline chloride or 1,2-PG:ChCl) | D2: 83.5% RG-I domain; low GalA; enhanced prebiotic activity vs. inulin | [31] |
| Morchella importuna | Acidic heteropolysaccharide (GlcN, Gal, Glc, and Man; 0.39:1.88:3.82:3.91) | Choline chloride + oxalic acid (2:1), 90% H2O | 4.5× higher yield than HWE; higher carbohydrate (85.3%) and sulfate content (34.2%); enhanced antioxidant and α-glucosidase inhibitory effects | [32] |
| Astragalus membranaceus | Astragalus polysaccharides (APS); heteropolysaccharides containing Glc, Gal, Ara, Rha, and Man | Choline chloride + oxalic acid (ultrasound assisted) | Increased yield and reduced impurities vs. conventional | [33] |
| Camellia oleifera | Pectic-like heteropolysaccharide (rich in Ara, Glc, Gal, Rha, GalA, and GlcA) | Choline chloride + propionic acid + 1,3-butanediol (DES-28; ternary) | 1.5× higher yield than hot water; enhanced antioxidant and hypoglycemic activities | [34] |
| Bletilla striata | Glucomannan | Choline chloride + urea | ↑ Yield (36.77%), ↑ antioxidant activity (DPPH, ABTS, and FRAP), and recyclable DES | [35] |
| Schisandra chinensis | Galacturonic acid-rich pectic polysaccharide | Ethanolamine/4-Methoxyphenol (1:1) | 1.39× higher yield vs. water; recyclable TRDES; simultaneous lignanoid extraction | [36] |
| Soluble Substances | Mechanism/Insight | Reference |
|---|---|---|
| Cu, Fe, Pb, and Zn (oxides, sulfates, and sulfides) | Sulfates dissolve best; solubility ~100× higher due to enhanced coordination in DES. | [43] |
| LiCoO2 (lithium cobalt oxide) | Reductive dissolution via ascorbic acid and PEG-based DES with 84.2% Co leaching. | [44] |
| Lipids, proteins, and carbohydrates | NaDES polarity and viscosity enhance biomolecule extraction. | [45] |
| DNA, starch, gluten, and bioactives | Natural DESs dissolve biopolymers via extensive hydrogen-bonding networks. | [46] |
| CuO, ZnO, MgO, CaO, and Fe2O3 | Thermodynamic favorability and morphology changes improve solubility. | [47] |
| Cellulose | Partial bonding and enhanced H-bonding increase cellulose solubility in ChCl–resorcinol DES. | [48] |
| Uranium oxide (UO3) | Coordination with TOPO and HTTA in hydrophobic DESs achieves high solubility. | [49] |
| Toluene (reaction medium) | DESs activate H2O2 via H-bonding and low viscosity, enhancing oxidation reactions. | [50] |
| UO2, U3O8, and UO3 | Strong hydrogen bonding in PTSA:ChCl DES enables uranium oxide dissolution. | [51] |
| CO2, SO2, H2S, and aromatic bioactives | DESs solvate via selective polarity and hydrogen bonding matched to target compounds. | [52] |
| PbO, CuO, Fe2O3, and ZnO | Acidic DESs use H-bond networks and phase behavior to dissolve metal oxides. | [53] |
| Metal oxides, salts, and polar organics | Ionic interactions and hydrogen bonds enhance solubility of diverse substances. | [54] |
| Chitin | Novel DESs using TMBAC and acids dissolved chitin up to 12% and enhanced enzymatic hydrolysis 2×. | [55] |
| CnTAB surfactants (micelles) | Micelle formation in DESs depends on solvent microstructure and hydrogen bonding. | [56] |
| HgO and HgCl2 | Complete dissociation via Cl− coordination in DES; H-bond donors do not replace chloride ligands. | [57] |
| Metal oxides, drugs, flavonoids, and phenols | Broad solubility via strong hydrogen bonding, high polarity, and solvent customization. | [58] |
| Fe3O4, CuO, ZnO, and PbO | Chloroacetic acid DESs with ammonium bromides dissolve oxides through optimized H-bonding. | [59] |
| Rutin | High solubility in ChCl/propanediol/urea DES due to hydrogen bonding and polarity. | [60] |
| PbO | [PbO·Cl·EG]− species formation drives dissolution in ChCl–EG DES. | [61] |
| Keratin (animal hair) | Sulfur-containing DESs disrupt protein structure, achieving up to 79% solubility. | [62] |
| Metal oxides from lithium-ion batteries | DES decomposition products (e.g., Cl3−) promote oxidative dissolution. | [63] |
| DNA | Solubility depends on hydrogen-bonding strength and ionic conductivity in DES. | [64] |
| Bioactive pharmaceutical ingredients | DES polarity and hydrogen bonding tailored to drug properties, improving solubility. | [65] |
| Metal salts, oxides, and phosphates | Solubility varies with DES pH and polarity; acidic DESs dissolve oxides effectively. | [66] |
| MgFe2O4, ZnFe2O4, CoFe2O4, and NiFe2O4 | DESs enable low-temp synthesis and precursor dissolution for ferrite nanoparticles. | [67] |
| Co, Cu, Zn, Fe, Ni, and Mn oxides | Temp/time-dependent coordination and solubilization in choline chloride–acid DES. | [68] |
| Cellulose | ZnCl2 hydrate–acrylic acid DES disrupts cellulose H-bonding for efficient dissolution. | [69] |
| APIs | THEDES systems can dissolve APIs by transforming the crystalline drug into a supramolecular liquid mixture. | [40] |
| Polysaccharide | Biological Source | Dominant Domain(s) | Structural Features | Implication for DES Extraction | Key Reference |
|---|---|---|---|---|---|
| Cellulose | Plant cell walls | Crystalline > interfacial | Linear β(1→4)-Glc; extensive hydrogen bonding; microfibrillar | Requires strong HBAs or heat/ultrasound; limited solubility in mild DESs | [117,118,119] |
| Pectin (RG-I, Homogalacturonan (HG)) | Plant middle lamella | Amorphous | Galacturonic acid rich; HG linear, RG-I branched | Readily extracted by acidic DESs; mild DESs preserve structure and promote bioactivity | [110,111] |
| Hemicellulose | Secondary plant walls | Amorphous + interfacial | Heterogeneous; short chains; variable composition | Extractable under mild DESs; solubility depends on sugar composition and structure | [112] |
| Chitin | Fungi and crustaceans | Crystalline | β(1→4)-GlcNAc; highly ordered, strong H-bonding | Requires acidic/basic DESs; needs thermal/ultrasonic pretreatment | [55] |
| Chitosan | Deacetylated chitin | Amorphous + interfacial | Linear, partially cationic; degree of deacetylation influences solubility | Soluble in acidic DESs (e.g., choline chloride–lactic acid); forms gels and films | [120] |
| Starch (amylose) | Plant storage tissues | Semi-crystalline | Linear α(1→4)-Glc; helical; forms double helices | Requires thermal gelatinization to be solubilized in DESs | [121] |
| Starch (amylopectin) | Plant storage tissues | Amorphous (contributes to semi-crystalline lamellae in native starch) | Highly branched α(1→4)/α(1→6) Glc | Easily solubilized by polar DESs under mild conditions | [122] |
| Inulin | Chicory and dahlia | Amorphous | Linear and branched fructans (β(2→1)-linked) | Fully soluble in polar DESs; enhances bioactive film formation | [113,114] |
| β-Glucan | Oats, barley, and yeast | Amorphous | Mixed β(1→3)/(1→4)-Glc; gel forming | Soluble in neutral DESs; used in functional food and pharma | [123] |
| Xanthan gum | Bacterial EPS | Amorphous | β(1→4)-linked glucose backbone with charged side chains | Highly soluble in DESs; enables shear-thinning formulations | [124] |
| Alginate | Brown seaweed | Amorphous | Linear mannuronic and guluronic acid blocks; polyanionic | Acidic DESs shield charges and promote solubilization | [125] |
| Fucoidan | Brown seaweed | Amorphous | Sulfated, branched α(1→3)/α(1→4)-L-fucose | Soluble in ionic and polar DESs; mild extraction preserves bioactivity | [10] |
| Glucomannan | Porang (Amorphophallus muelleri Blume) | Amorphous | Linear β(1→4)-linked glucose and mannose | Highly extractable under mildly polar DESs | [126] |
| Pullulan | Fungi (Aureobasidium) | Amorphous | Linear α(1→6)-linked maltotriose units; non-ionic and water soluble | Compatible with polar DESs; maintains solubility across solvents | [127] |
| Galactomannan | Legumes (e.g., guar) | Amorphous | β(1→4)-linked mannan with α(1→6)-galactose side chains | Easily solubilized in polar DESs; can be enzymatically modified | [128] |
| Levan | Bacterial (e.g., Bacillus) | Amorphous | β(2→6)-linked fructose units; highly branched and water soluble | Readily soluble in polar DESs; useful in prebiotic applications | [129] |
| Dextran | Bacterial (Leuconostoc) | Amorphous | Linear α(1→6)-Glc backbone with α(1→3/1→4) branches | Soluble in mild, neutral DESs; applicable in food and pharma | [130] |
| Carrageenan | Red algae | Amorphous | Sulfated galactans; alternating α(1→3)/β(1→4)-linked units; gelling | Soluble in ionic DESs; gelation influenced by ions and solvent polarity | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Akayleh, F.; Ali Agha, A.S.A.; Olaimat, A.R.; Qinna, N.A. Rationalizing Polysaccharide Extraction with Deep Eutectic Solvents: From Supramolecular Architecture to Emerging AI-Guided Solvent Design. Polysaccharides 2025, 6, 82. https://doi.org/10.3390/polysaccharides6030082
Al-Akayleh F, Ali Agha ASA, Olaimat AR, Qinna NA. Rationalizing Polysaccharide Extraction with Deep Eutectic Solvents: From Supramolecular Architecture to Emerging AI-Guided Solvent Design. Polysaccharides. 2025; 6(3):82. https://doi.org/10.3390/polysaccharides6030082
Chicago/Turabian StyleAl-Akayleh, Faisal, Ahmed S. A. Ali Agha, Ali R. Olaimat, and Nidal A. Qinna. 2025. "Rationalizing Polysaccharide Extraction with Deep Eutectic Solvents: From Supramolecular Architecture to Emerging AI-Guided Solvent Design" Polysaccharides 6, no. 3: 82. https://doi.org/10.3390/polysaccharides6030082
APA StyleAl-Akayleh, F., Ali Agha, A. S. A., Olaimat, A. R., & Qinna, N. A. (2025). Rationalizing Polysaccharide Extraction with Deep Eutectic Solvents: From Supramolecular Architecture to Emerging AI-Guided Solvent Design. Polysaccharides, 6(3), 82. https://doi.org/10.3390/polysaccharides6030082







