Microwave–Assisted OSA–Faba Bean Starch Production for Probiotic Microencapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Faba Bean Hydrolysis and OSA Modification
2.3. Characterization of OSA Starches
2.3.1. Degree of Substitution (DS)
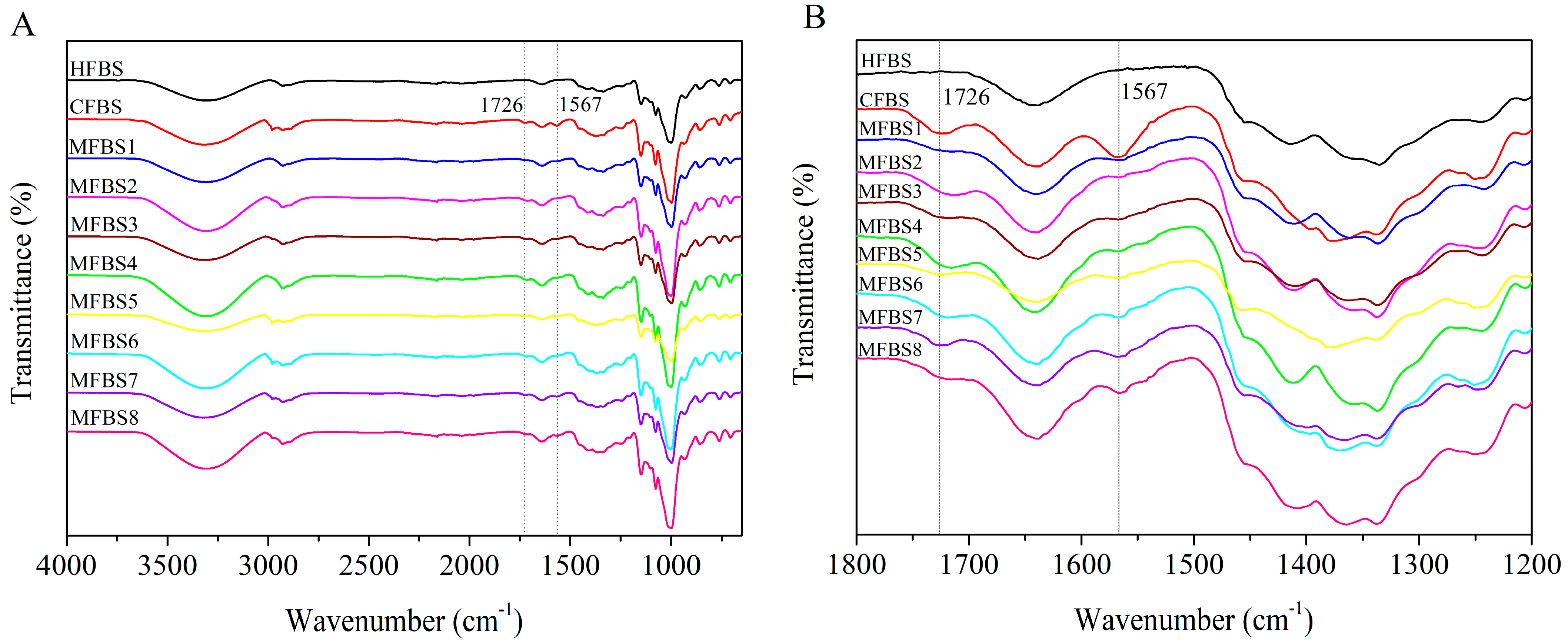
2.3.2. FTIR Analysis
2.3.3. Resistant Starch (RS), Amylose, Amylopectin, and Reducing Sugars
2.3.4. Viscosity Profiles
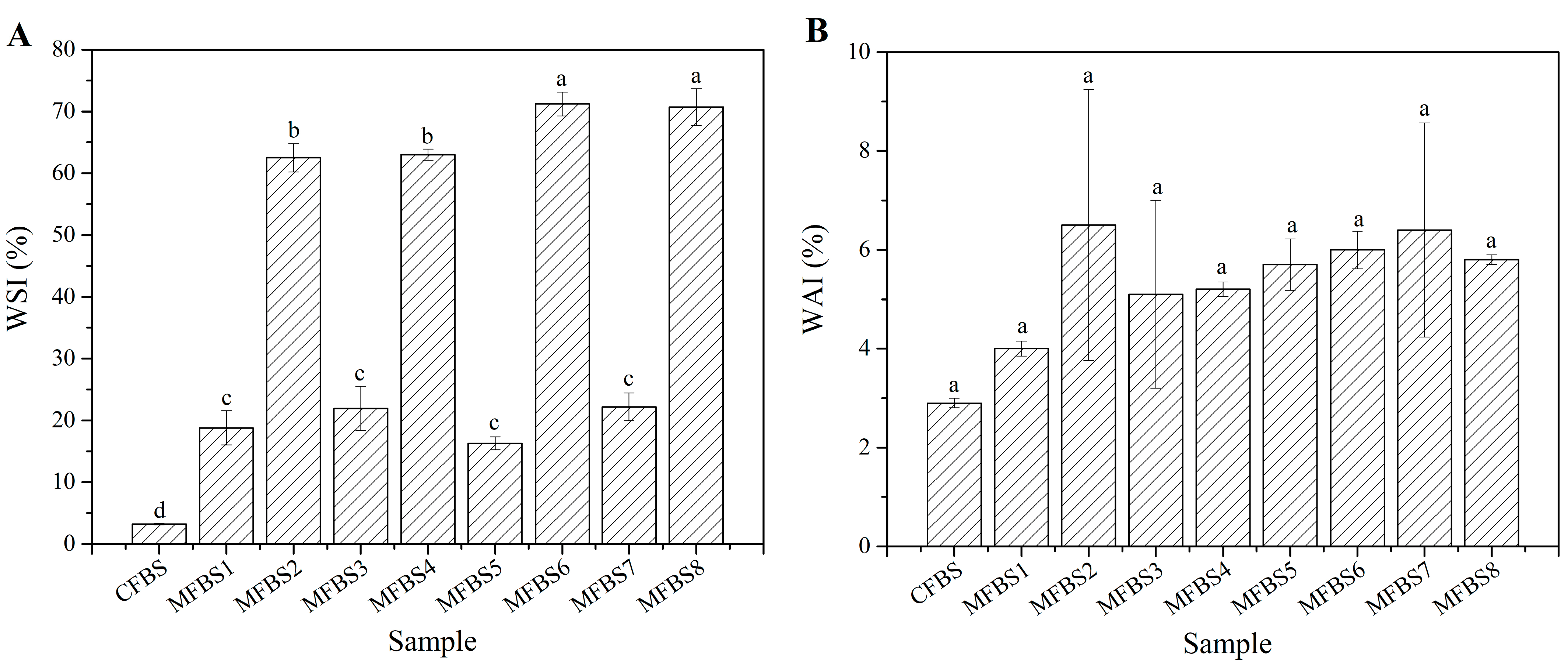
2.3.5. Water Absorption Index (WAI) and Water Solubility Index (WSI)
2.4. LGG Encapsulation by Spray Drying
2.5. Characterization of Microencapsulated LGG
2.5.1. Bacterial Count and Encapsulation Efficiency
2.5.2. Water Activity
2.5.3. Morphology of Microencapsulated LGG
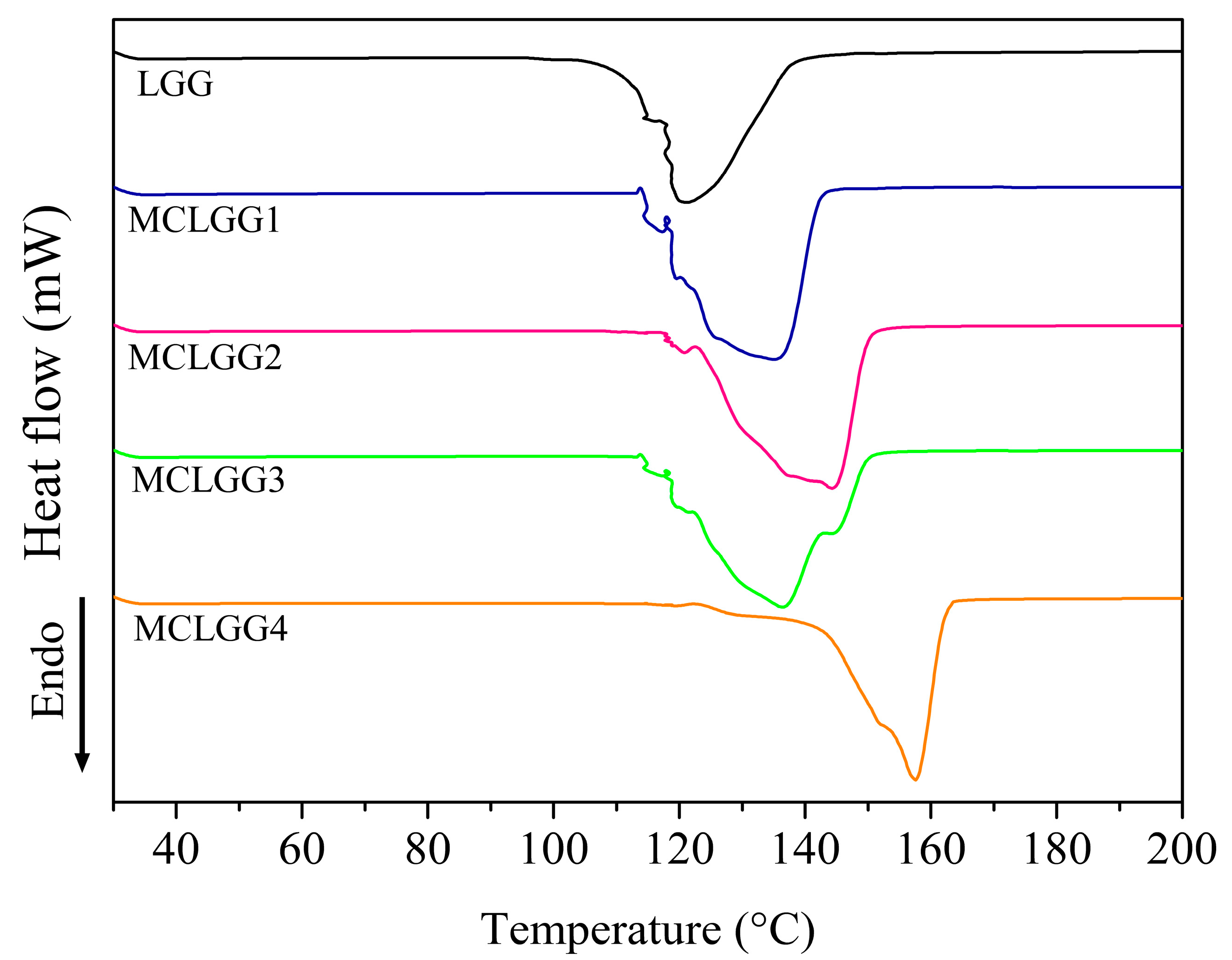
2.5.4. DSC
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Modified Starches
3.2. LGG Microencapsulation by Spray Drying and Microcapsule Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSA | Octenyl succinic anhydride |
| LGG | Lactobacillus rhamnosus GG |
| RS | Resistant starch |
| ME | Microwave energy |
| HFBS | Hydrolyzed faba bean starch |
| MFBS | Modified faba bean starch |
| CFBS | Conventionally esterified faba bean starch |
| DS | Degree of substitution |
| FTIR | Fourier transform infrared transmission spectroscopy |
| WSI | Water solubility index |
| WAI | Water absorption index |
| MCLGG | Spray-dried Lactobacillus rhamnosus GG microcapsules |
References
- Montoya-Yepes, D.F.; Jiménez-Rodríguez, A.A.; Aldana-Porras, A.E.; Velásquez-Holguin, L.F.; Méndez-Arteaga, J.J.; Murillo-Arango, W. Starches in the encapsulation of plant active ingredients: State of the art and research trends. Polym. Bull. 2024, 81, 135–163. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Ashwar, A.B.; Gani, A.; Gani, A.; Shah, A.; Massodi, A.F. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jiali, L.; Wu, Z.; Liu, L.; Yang, J.; Wang, L.; Li, Z.; Liu, L. The research advance of resistant starch: Structural characteristics, modification method, immunomodulatory function, and its delivery systems application. Crit. Rev. Food Sci. Nutr. 2024, 64, 10885–10902. [Google Scholar] [CrossRef]
- Muhammad, Z.; Ramzan, R.; Huo, G.C.; Tian, H.; Bian, X. Integration of polysaccharide-thermoprotectant formulations for microencapsulation of Lactobacillus plantarum, appraisal of survivability and physico-biochemical properties during storage of spray dried powders. Food Hydrocoll. 2017, 66, 286–295. [Google Scholar] [CrossRef]
- Nunes, G.L.; Motta, M.H.; Cichoski, A.J.; Wagner, R.; Muller, É.I.; Codevilla, C.F.; Menezes, C.R.D. Encapsulation of Lactobacillus acidophilus La-5 and Bifidobacterium Bb-12 by spray drying and evaluation of its resistance in simulated gastrointestinal conditions, thermal treatments and storage conditions. Cienc. Rural. 2018, 48, e20180035. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, N.; Zhang, H.; Li, H.; Guo, J.; Zhang, Y.; Shi, N. Resistant starch and the gut microbiome: Exploring beneficial interactions and dietary impacts. Food Chem. X 2024, 21, 101118. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- García-Gurrola, A.; Rincón, S.; Escobar-Puentes, A.A.; Zepeda, A.; Pérez-Robles, J.F.; Martínez-Bustos, F. Synthesis and succinylation of starch nanoparticles by means of a single step using sonochemical energy. Ultrason. Sonochem. 2019, 56, 458–465. [Google Scholar] [CrossRef] [PubMed]
- No, J.; Shin, M. Preparation and characteristics of octenyl succinic anhydride-modified partial waxy rice starches and encapsulated paprika pigment powder. Food Chem. 2019, 295, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Sinhmar, A.; Pathera, A.K.; Sharma, S.; Nehra, M.; Thory, R.; Nain, V. Impact of various modification methods on physicochemical and functional properties of starch: A review. Starch-Stärke 2023, 75, 2200117. [Google Scholar] [CrossRef]
- Fang, S.; Zhao, X.; Liu, Y.; Liang, X.; Yang, Y. Fabricating multilayer emulsions by using OSA starch and chitosan suitable for spray drying: Application in the encapsulation of β-carotene. Food Hydrocoll. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legum. Sci. 2022, 4, e129. [Google Scholar] [CrossRef]
- Santana, Á.L.; Angela, M.; Meireles, A. New Starches are the Trend for Industry Applications: A Review. Food Public Health 2014, 4, 229–241. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, B.; Fan, D.; Zhang, N.; Ma, S.; Wang, L.; Zhang, H. Structural changes of starch subjected to microwave heating: A review from the perspective of dielectric properties. Trends Food Sci. Technol. 2020, 99, 593–607. [Google Scholar] [CrossRef]
- Lewicka, K.; Siemion, P.; Kurcok, P. Chemical modifications of starch: Microwave effect. Int. J. Polym. Sci. 2015, 1, 867697. [Google Scholar] [CrossRef]
- Yi, M.; Tang, X.; Liang, S.; He, R.; Huang, T.; Lin, Q.; Zhang, R. Effect of microwave alone and microwave-assisted modification on the physicochemical properties of starch and its application in food. Food Chem. 2024, 446, 138841. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, W.; Pu, H.; Blennow, A.; Liu, X.; Guo, D. Short-time microwave treatment affects the multi-scale structure and digestive properties of high-amylose maize starch. Int. J. Biol. Macromol. 2019, 137, 870–877. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.; Zhang, Y.; Chen, Y.; Wu, Y.; Ouyang, J. Effect of microwave irradiation-retrogradation treatment on the digestive and physicochemical properties of starches with different crystallinity. Food Chem. 2019, 298, 125015. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, T.; Li, N.; Cheng, Q.; Qiao, D.; Zhang, B.; Lin, Q. Supramolecular structure and pasting/digestion behaviors of rice starches following concurrent microwave and heat moisture treatment. Int. J. Biol. Macromol. 2019, 135, 437–444. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, L.; Sharanagat, V.S.; Patel, A.; Kumar, K. Effect of microwave treatment (low power and varying time) on potato starch: Microstructure, thermo-functional, pasting and rheological properties. Int. J. Biol. Macromol. 2020, 155, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, A.N.; Rajasekharan, K.N.; Moorthy, S.N.; Sreekumar, J. Microwave-assisted synthesis and characterization of succinate derivatives of cassava (Manihot esculenta Crantz) starch. Starch-Stärke 2005, 57, 556–563. [Google Scholar] [CrossRef]
- González, Z.; Pérez, E. Evaluation of lentil starches modified by microwave irradiation and extrusion cooking. Food Res. Int. 2002, 35, 415–420. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Yin, X.; Hu, X.; Boye, J.I. Structural characteristics and physicochemical properties of field pea starch modified by physical, enzymatic, and acid treatments. Food Hydrocoll. 2019, 93, 386–394. [Google Scholar] [CrossRef]
- Tong, F.; Deng, L.; Sun, R.; Zhong, G. Effect of octenyl succinic anhydride starch ester by semi-dry method with vacuum-microwave assistant. Int. J. Biol. Macromol. 2019, 141, 1128–1136. [Google Scholar] [CrossRef]
- González-Mendoza, M.E.; Martínez-Bustos, F.; Castaño-Tostado, E.; Amaya-Llano, S.L. Effect of microwave irradiation on acid hydrolysis of faba bean starch: Physicochemical changes of the starch granules. Molecules 2022, 27, 3528. [Google Scholar] [CrossRef]
- Murúa-Pagola, B.; Beristain-Guevara, C.I.; Martínez-Bustos, F. Preparation of starch derivatives using reactive extrusion and evaluation of modified starches as shell materials for encapsulation of flavoring agents by spray drying. J. Food Eng. 2009, 91, 380–386. [Google Scholar] [CrossRef]
- Kweon, D.K.; Choi, J.K.; Kim, E.K.; Lim, S.T. Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohydr. Polym. 2001, 46, 171–177. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- AACC. Methods of the AACC, 3rd ed.; American Association of Cereal Chemists, Academic Press Inc.: Cambridge, MA, USA, 1999. [Google Scholar]
- Anderson, R.A.; Conway, H.F.; Peplinski, A.J. Gelatinization of corn grits by roll cooking, extrusion cooking and steaming. Starch-Stärke 1970, 22, 130–135. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.L.; Díaz-Vela, J.; Reyes-Menéndez, C.V.; Totosaus, A. Improvement of moisture stability and textural properties of fat and salt reduced cooked sausages by inoculation of thermotolerant lactic acid bacteria. Int. J. Food Prop. 2013, 16, 1789–1808. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.L.; Lara-Labastida, R.; Rodriguez-Huezo, E.; Totosaus, A. Effect of spray drying encapsulation of thermotolerant lactic acid bacteria on meat batters properties. Food Bioprocess Technol. 2013, 6, 1505–1515. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Ambrosio-Martín, J.; Perez-Masiá, R.; Lopez-Rubio, A. Impact of acetic acid on the survival of L. Plantarum upon microencapsulation by coaxial electrospraying. J. Healthc. Eng. 2017, 1, 4698079. [Google Scholar]
- Rajam, R.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT 2015, 60, 773–780. [Google Scholar] [CrossRef]
- Ovando-Martinez, M.; Whitney, K.; Ozsisli, B.; Simsek, S. Physicochemical properties of octenyl succinic esters of cereal, tuber and root starches. J. Food Process. Preserv. 2017, 41, e12872. [Google Scholar] [CrossRef]
- Mehboob, S.; Ali, T.M.; Alam, F.; Hasnain, A. Dual modification of native white sorghum (Sorghum bicolor) starch via acid hydrolysis and succinylation. LWT 2015, 64, 459–467. [Google Scholar] [CrossRef]
- Shaikh, M.; Ali, T.M.; Hasnain, A. Post succinylation effects on morphological, functional and textural characteristics of acid-thinned pearl millet starches. J. Cereal Sci. 2015, 63, 57–63. [Google Scholar] [CrossRef]
- Han, J.A.; Chung, H.J.; Lim, S.T. Physical and emulsifying properties of OSA-corn dextrin with various manufacturing methods. Food Hydrocoll. 2019, 89, 563–569. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martinez, M.; Marefati, A.; Sjӧӧ, M.; Rayner, M. Chemical composition, digestibility and emulsification properties of octenyl succinic esters of various starches. Food Res. Int. 2015, 75, 41–49. [Google Scholar] [CrossRef]
- Zhang, B.; Mei, J.Q.; Chen, B.; Chen, H.Q. Digestibility, physicochemical and structural properties of octenyl succinic anhydride-modified cassava starches with different degree of substitution. Food Chem. 2017, 229, 136–141. [Google Scholar] [CrossRef]
- Dewi, A.M.P.; Santoso, U.; Pranoto, Y.; Marseno, D.W. Dual modification of sago starch via heat moisture treatment and octenyl succinylation to improve starch hydrophobicity. Polymers 2022, 14, 1086. [Google Scholar] [CrossRef]
- Samborska, K.; Poozesh, S.; Barańska, A.; Sobulska, M.; Jedlińska, A.; Arpagaus, C.; Jafari, S.M. Innovations in spray drying process for food and pharma industries. J. Food Eng. 2022, 321, 110960. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Microencapsulation of food bioactive components by spray drying: A review. Dry. Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Altuna, L.; Herrera, M.L.; Foresti, M.L. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocoll. 2018, 80, 97–110. [Google Scholar] [CrossRef]
- Li, J.Z. Starch-based materials for microencapsulation. In Microencapsulation in the Food Industry; Sobel, R., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 225–243. [Google Scholar]
- Vandamme, T.F.; Gbassi, G.K.; Nguyen, T.L.; Li, X. Microencapsulation of probiotics. In Encapsulation and Controlled Release Technologies in Food Systems; Lakkis, J.M., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 97–128. [Google Scholar]
- Rodríguez-Restrepo, Y.A.; Giraldo, G.I.; Rodríguez-Barona, S. Solubility as a fundamental variable in the characterization of wall material by spray drying of food components: Application to microencapsulation of Bifidobacterium animalis subsp. lactis. J. Food Process Eng. 2017, 40, e12557. [Google Scholar] [CrossRef]
- Tao, T.; Ding, Z.; Hou, D.; Prakash, S.; Zhao, Y.; Fan, Z.; Han, J. Influence of polysaccharide as co-encapsulant on powder characteristics, survival and viability of microencapsulated Lactobacillus paracasei Lpc-37 by spray drying. J. Food Eng. 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT 2015, 63, 685–690. [Google Scholar]
- Chen, H.; He, J.; Li, W.; Wang, Z.; Du, M.; Kan, J. Structural and functional characteristics of esterified starch and microencapsulation for urate-lowering probiotic: Effect of hydrophobic side-chain length. Carbohydr. Polym. 2025, 358, 123529. [Google Scholar] [CrossRef]
- Cruz-Benítez, M.M.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Hernández-Hernández, E.E.G.H.; Gómez-Hernández, E.; Fonseca-Florido, H.A. Effect of amylose content and chemical modification of cassava starch on the microencapsulation of Lactobacillus pentosus. LWT 2019, 105, 110–117. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Rubilar, M.; Shene, C. Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linum usitatissimum L.) mucilage and soluble protein by spray drying. Food Chem. 2017, 216, 97–105. [Google Scholar] [CrossRef]
- Ermis, E. A review of drying methods for improving the quality of probiotic powders and characterization. Dry. Technol. 2022, 40, 2199–2216. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Arepally, D.; Goswami, T.K. Effect of inlet air temperature and gum Arabic concentration on encapsulation of probiotics by spray drying. LWT 2019, 99, 583–593. [Google Scholar] [CrossRef]
- Cortés, R.N.F.; Martínez, M.G.; Guzmán, I.V.; Llano, S.L.A.; Grosso, C.R.F.; Bustos, F.M. Evaluation of modified amaranth starch as shell material for encapsulation of probiotics. Cereal Chem. 2014, 91, 300–308. [Google Scholar] [CrossRef]
- Nunes, G.L.; de Araújo Etchepare, M.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; de Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Lee, J.; Kaletunc, G. Evaluation of the heat inactivation of Escherichia coli and Lactobacillus plantarum by differential scanning calorimetry. Appl. Environ. Microbiol. 2002, 68, 5379–5386. [Google Scholar] [CrossRef]


| Sample | Starch | Power Level | Time (s) |
|---|---|---|---|
| MFBS1 | HFBS2 | 2 | 30 |
| MFBS2 | HFBS8 | 2 | 30 |
| MFBS3 | HFBS2 | 2 | 60 |
| MFBS4 | HFBS8 | 2 | 60 |
| MFBS5 | HFBS2 | 10 | 30 |
| MFBS6 | HFBS8 | 10 | 30 |
| MFBS7 | HFBS2 | 10 | 60 |
| MFBS8 | HFBS8 | 10 | 60 |
| Sample | Degree of Substitution | Resistant Starch (%) | Amylose (%) | Reducing Sugars (%) |
|---|---|---|---|---|
| CFBS | 0.020 ± 0.0040 ab | 11.8 ± 1.42 ab | 23.3 ± 1.26 c | 0.05 ± 0.00 b |
| MFBS1 | 0.015 ± 0.0075 abc | 11.8 ± 0.96 ab | 38.4 ± 2.89 b | 0.89 ± 0.23 b |
| MFBS2 | 0.023 ± 0.0005 a | 9.6 ± 1.13 ab | 89.1 ± 4.40 a | 15.43 ± 0.91 a |
| MFBS3 | 0.013 ± 0.0050 abc | 11.6 ± 1.11 ab | 37.8 ± 1.92 b | 1.43 ± 0.39 b |
| MFBS4 | 0.022 ± 0.0017 ab | 9.6 ± 1.50 b | 84.3 ± 4.47 a | 15.11 ± 0.61 a |
| MFBS5 | 0.005 ± 0.0059 c | 13.3 ± 1.01 a | 36.9 ± 2.25 b | 1.07 ± 0.48 b |
| MFBS6 | 0.015 ± 0.0071 abc | 9.5 ± 1.89 b | 84.3 ± 4.21 a | 15.06 ± 0.77 a |
| MFBS7 | 0.003 ± 0.0029 c | 12.2 ± 1.46 ab | 31.6 ± 1.87 bc | 1.25 ± 0.03 b |
| MFBS8 | 0.009 ± 0.0055 bc | 9.5 ± 0.95 b | 84.6 ± 4.84 a | 14.26 ± 0.99 a |
| Inlet (°C) | Outlet (°C) | Flow Rate (mL/min) | Yield (%) | Initial Viable Cells (log UFC) | Final Viable Cells 1 (log UFC) | EE (%) | aw | |
|---|---|---|---|---|---|---|---|---|
| MCLGG1 | 120 | 86 ± 4.0 ab | 7 | 45 ± 2.9 a | 10.5 ± 0.0 a | 9.0 ± 0.3 a | 86 ± 3.7 ab | 0.19 ± 0.03 a |
| MCLGG2 | 140 | 97 ± 6.9 a | 7 | 51 ± 1.7 a | 10.5 ± 0.0 a | 8.5 ± 0.2 a | 81 ± 1.7 b | 0.15 ± 0.02 a |
| MCLGG3 | 120 | 84 ± 3.5 b | 12 | 46 ± 3.7 a | 10.4 ± 0.1 a | 9.3 ± 0.2 a | 90 ± 2.6 a | 0.20 ± 0.02 a |
| MCLGG4 | 140 | 92 ± 2.5 ab | 12 | 48 ± 1.2 a | 10.5 ± 0.0 a | 8.8 ± 0.1 a | 84 ± 1.2 ab | 0.21 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Mendoza, M.E.; Martínez-Bustos, F.; Castaño-Tostado, E.; Cortez-Trejo, M.d.C.; Amaya-Llano, S.L. Microwave–Assisted OSA–Faba Bean Starch Production for Probiotic Microencapsulation. Polysaccharides 2025, 6, 81. https://doi.org/10.3390/polysaccharides6030081
González-Mendoza ME, Martínez-Bustos F, Castaño-Tostado E, Cortez-Trejo MdC, Amaya-Llano SL. Microwave–Assisted OSA–Faba Bean Starch Production for Probiotic Microencapsulation. Polysaccharides. 2025; 6(3):81. https://doi.org/10.3390/polysaccharides6030081
Chicago/Turabian StyleGonzález-Mendoza, Mayra Esthela, Fernando Martínez-Bustos, Eduardo Castaño-Tostado, María del Carmen Cortez-Trejo, and Silvia Lorena Amaya-Llano. 2025. "Microwave–Assisted OSA–Faba Bean Starch Production for Probiotic Microencapsulation" Polysaccharides 6, no. 3: 81. https://doi.org/10.3390/polysaccharides6030081
APA StyleGonzález-Mendoza, M. E., Martínez-Bustos, F., Castaño-Tostado, E., Cortez-Trejo, M. d. C., & Amaya-Llano, S. L. (2025). Microwave–Assisted OSA–Faba Bean Starch Production for Probiotic Microencapsulation. Polysaccharides, 6(3), 81. https://doi.org/10.3390/polysaccharides6030081





