Wild Yam (Dioscorea remotiflora) Tubers: An Alternative Source for Obtaining Starch Particles Chemically Modified After Extraction by Acid Hydrolysis and Ultrasound
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Starch Extraction
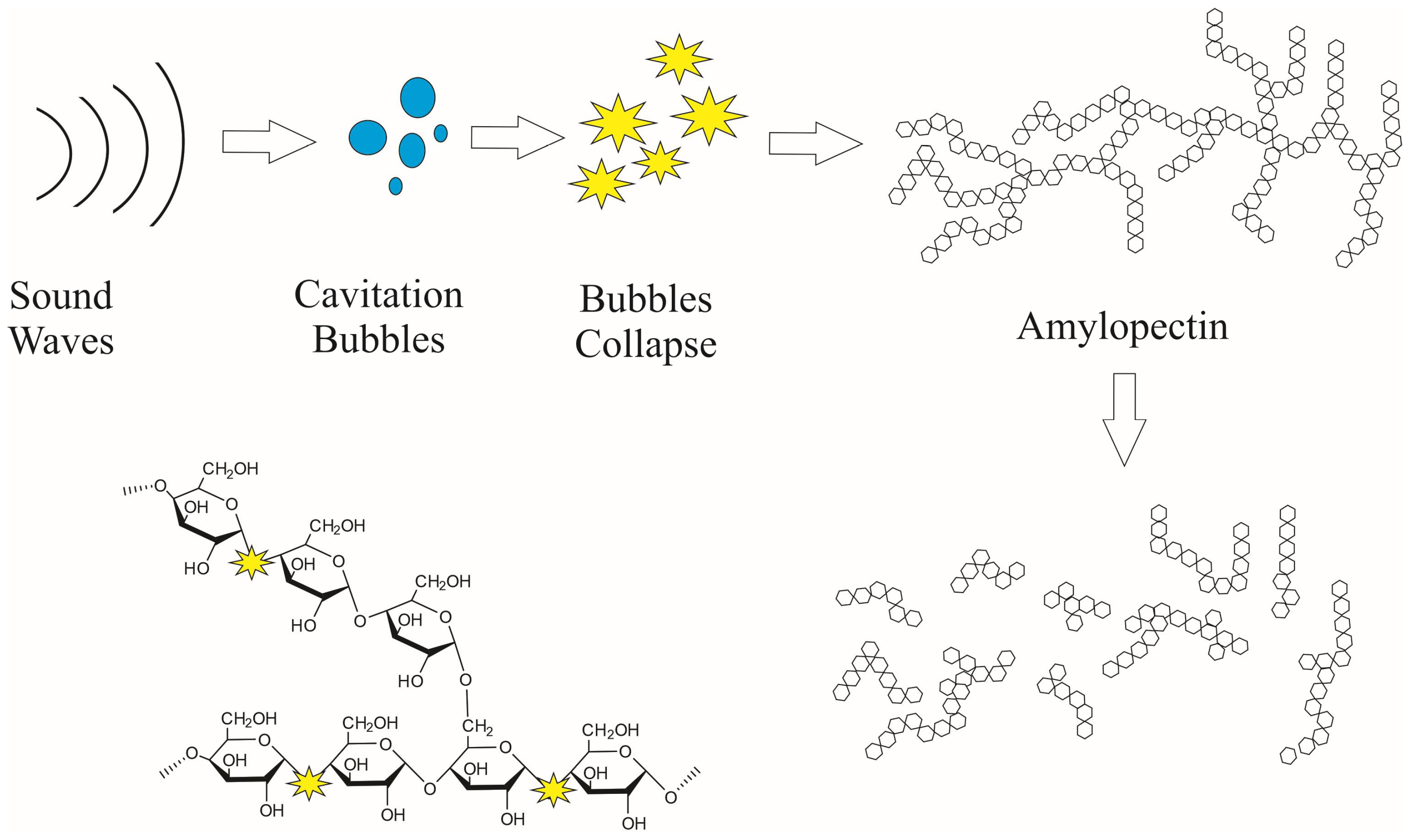
2.3. Acid Hydrolysis (AH) and Acid Hydrolysis-Ultrasound (AH-US) Treatments
2.4. Chemical Modification of Starch
2.4.1. Acetic Anhydride
2.4.2. Lauroyl Chloride
2.4.3. Citric Acid
2.4.4. Sodium Hexametaphosphate
2.5. SPs Yield, Amylose, and Native Starch Residual Moisture Content
2.6. Characterization of Modified SPs
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. Dynamic Light Scattering (DLS)
2.6.3. Fourier Transform Infrared Spectroscopy (FT-IR)-Attenuated Total Reflectance
2.6.4. X-Ray Diffraction Pattern (XDR)
2.6.5. Thermal Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yield and Amylose Content
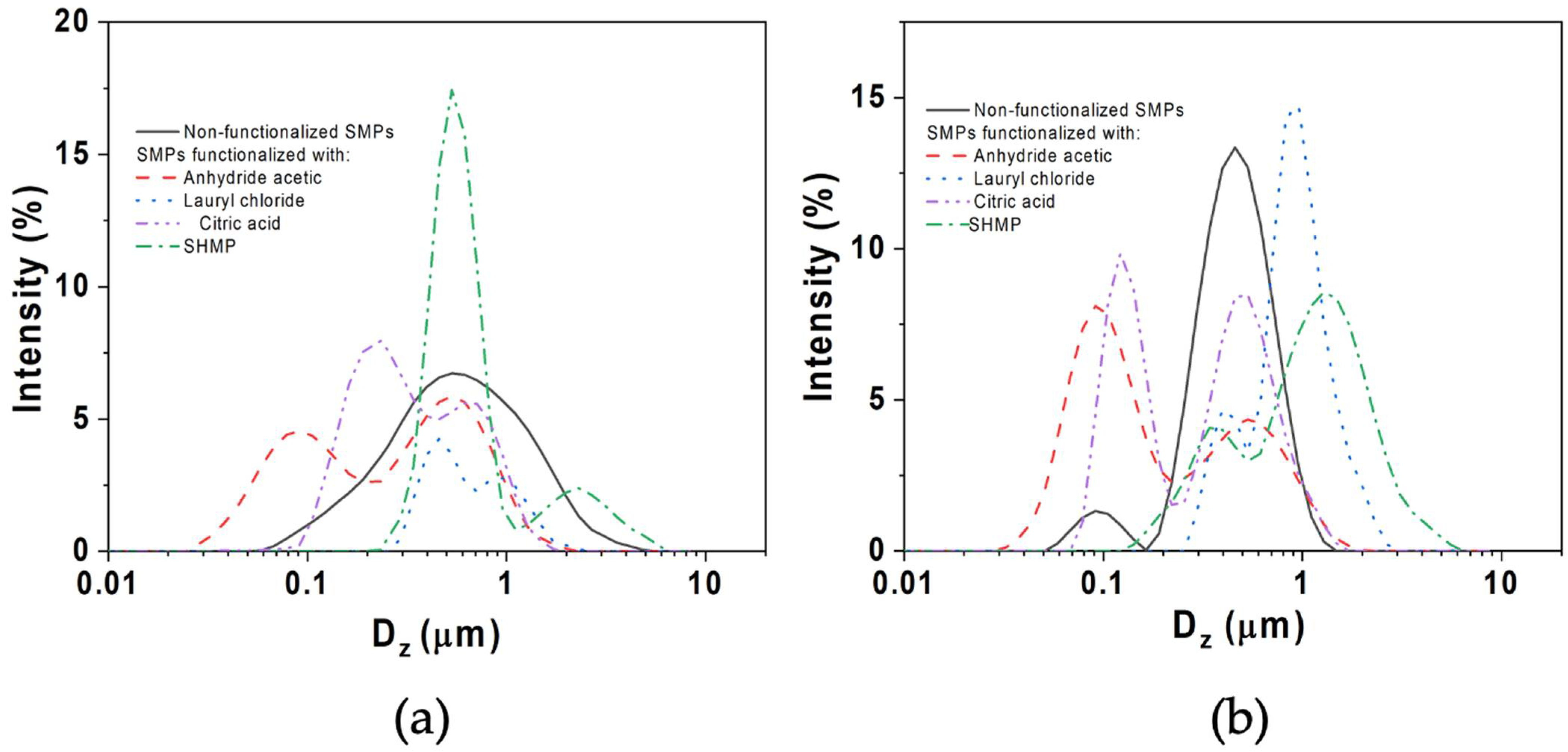
3.2. Particle-Size Distribution
3.3. Morphology
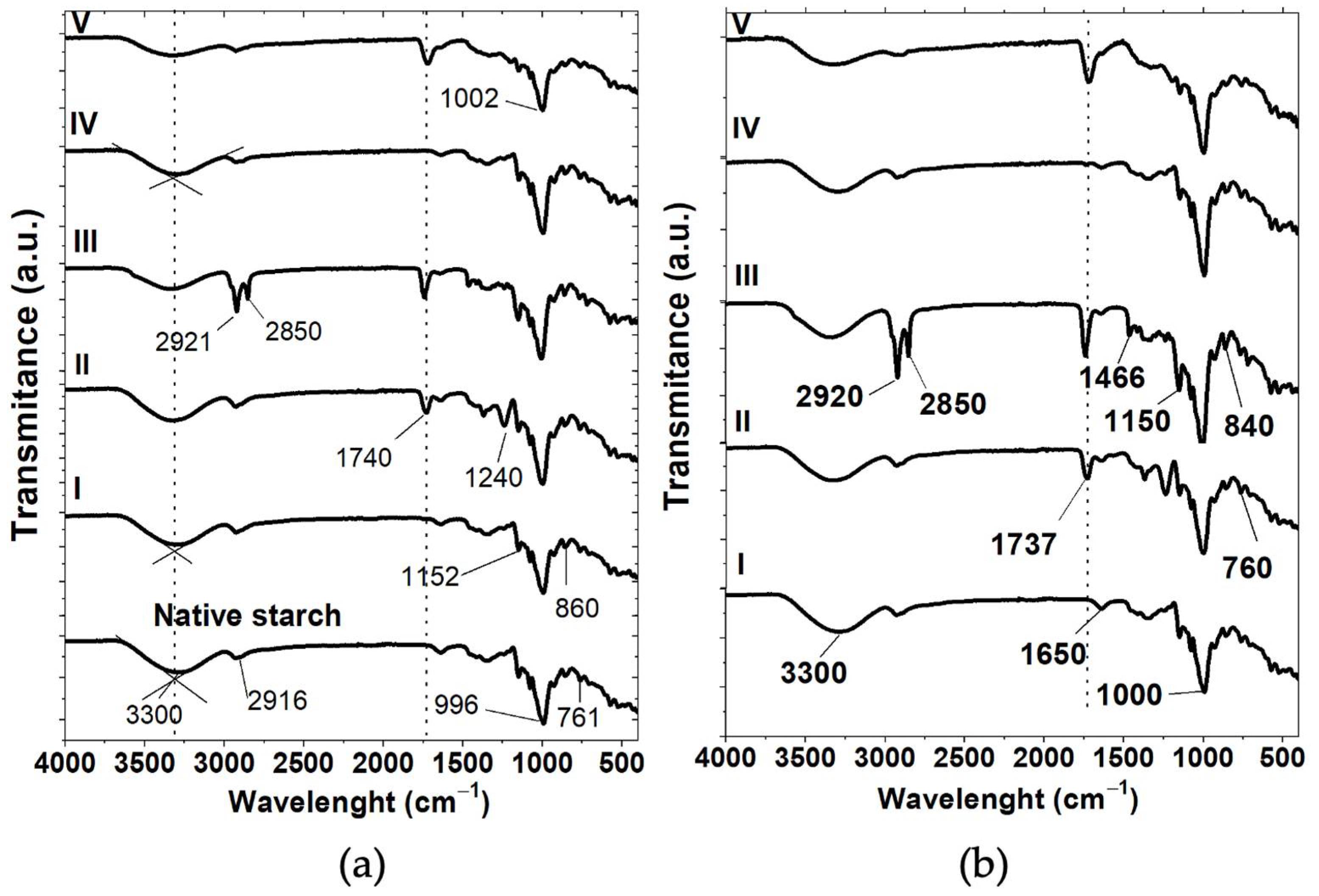
3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
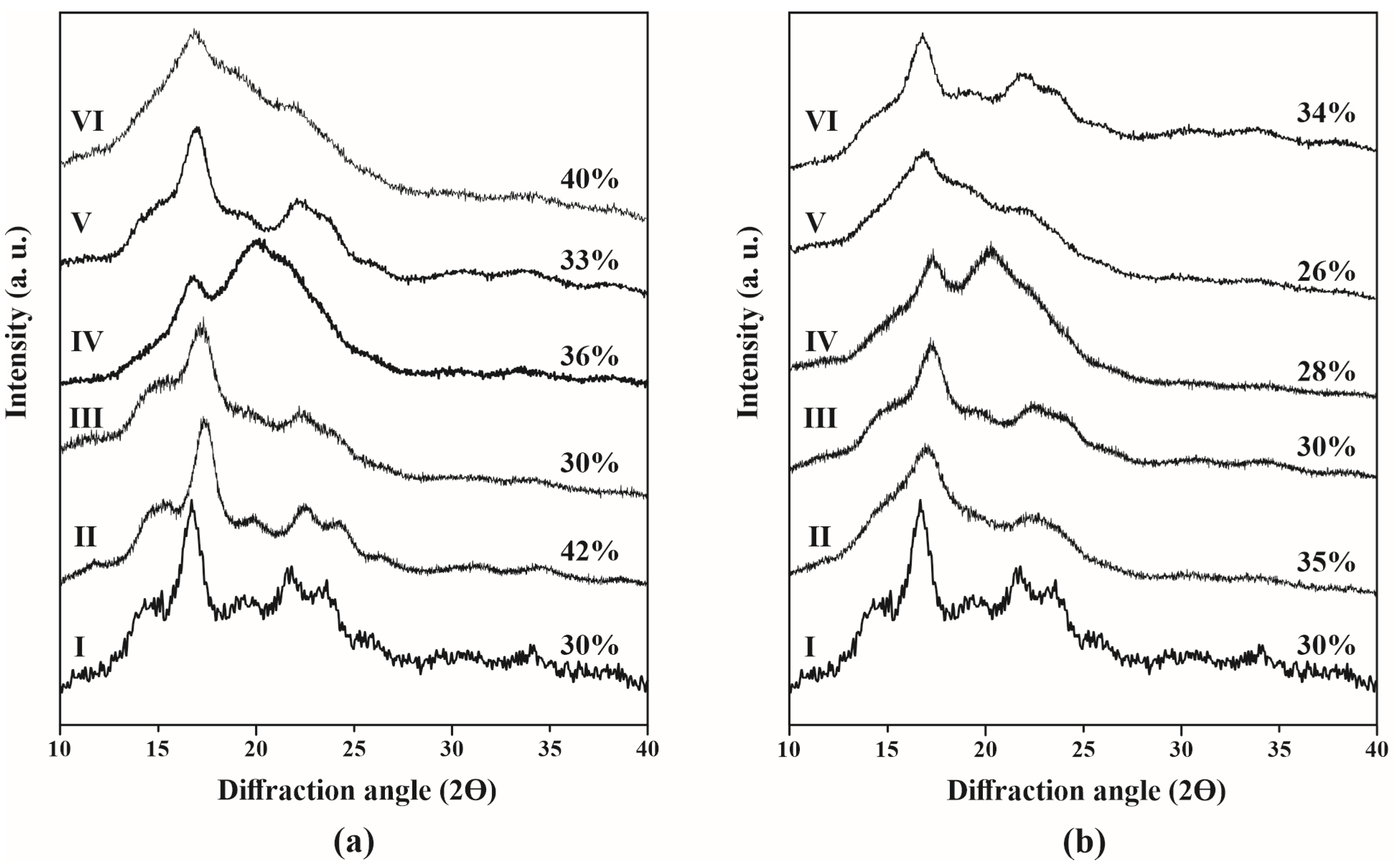
3.5. X-Ray Diffraction
3.6. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Horn, M.M.; Martins, V.C.A.; Plepis, A.M.G. Effect of Amylopectin Content on Mechanical, Barrier and Thermal Properties of Plasticized Starch/Chitosan Films. Polysaccharides 2023, 4, 208–218. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef]
- Chin, S.F.; Pang, S.C.; Tay, S.H. Size controlled synthesis of starch nanoparticles by a simple nanoprecipitation method. Carbohydr. Polym. 2011, 86, 1817–1819. [Google Scholar] [CrossRef]
- Hore, R.; Rashid, H.; Syrowatka, F.; Kressler, J. Synthesis and Characterization of Self-Assembled Highly Stearate-Grafted Hydroxyethyl Starch Conjugates. Polysaccharides 2024, 5, 142–157. [Google Scholar] [CrossRef]
- Hasanvand, E.; Rafe, A.; Emadzadeh, B. Phase separation behavior of flaxseed gum and rice bran protein complex coacervates. Food Hydrocoll. 2018, 82, 412–423. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, B.S.; Yadav, R.B. Synthesis and modification approaches for starch nanoparticles for their emerging food industrial applications: A review. Food Res. Int. 2020, 128, 108765. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; El-Rafie, M.H.; El-sheikh, M.A.; El-Feky, G.S.; Hebeish, A. Synthesis, characterization, release kinetics and toxicity profile of drug-loaded starch nanoparticles. Int. J. Biol. Macromol. 2015, 81, 718–729. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Villa, C.C.; Sanchez, L.T.; Rodriguez-Marin, N.D. Starch Nanoparticles and Nanocrystals as Bioactive Molecule Carriers. In Polymers for Agri-Food Applications; Springer Cham: Cham, Switzerland, 2019; pp. 91–98. [Google Scholar] [CrossRef]
- Yang, J.; Li, F.; Li, M.; Zhang, S.; Liu, J.; Liang, C.; Sun, Q.; Xiong, L. Fabrication and characterization of hollow starch nanoparticles by gelation process for drug delivery application. Carbohydr. Polym. 2017, 173, 223–232. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple ultrasound method to obtain starch micro- and nanoparticles from cassava, corn and yam starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef]
- Yang, F.; Wei, Y.; Xiao, H.; Zhang, Q.; Li, J.; Lin, Q.; Zhu, D.; Huang, Z.; Liu, G.Q. Acetylated rice starch nanocrystals improved the physical, mechanical, and structural properties of native rice starch based films. Int. J. Biol. Macromol. 2023, 253, 127271. [Google Scholar] [CrossRef]
- Mahmoudi Najafi, S.H.; Baghaie, M.; Ashori, A. Preparation and characterization of acetylated starch nanoparticles as drug carrier: Ciprofloxacin as a model. Int. J. Biol. Macromol. 2016, 87, 48–54. [Google Scholar] [CrossRef]
- Forouzandehdel, S.; Forouzandehdel, S.; Rezghi Rami, M. Synthesis of a novel magnetic starch-alginic acid-based biomaterial for drug delivery. Carbohydr. Res. 2020, 487, 107889. [Google Scholar] [CrossRef]
- Cazotti, J.C.; Fritz, A.T.; Garcia-Valdez, O.; Smeets, N.M.B.; Dubé, M.A.; Cunningham, M.F. Graft modification of starch nanoparticles using nitroxide-mediated polymerization and the grafting from approach. Carbohydr. Polym. 2020, 228, 115384. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wang, Q.; Ren, L.; Tong, J.; Zhou, J. High efficiency and low cost preparation of size controlled starch nanoparticles through ultrasonic treatment and precipitation. Food Chem. 2017, 227, 369–375. [Google Scholar] [CrossRef]
- Jain, A.K.; Khar, R.K.; Ahmed, F.J.; Diwan, P.V. Effective insulin delivery using starch nanoparticles as a potential trans-nasal mucoadhesive carrier. Eur. J. Pharm. Biopharm. 2008, 69, 426–435. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, T.; Lin, Q.; Liu, G.Q.; Zhang, L.; Yu, F.; Chen, Y. Acetylated starch nanocrystals: Preparation and antitumor drug delivery study. Int. J. Biol. Macromol. 2016, 89, 456–464. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- Fuentes, C.; Kang, I.; Lee, J.; Song, D.; Sjöö, M.; Choi, J.; Lee, S.; Nilsson, L. Fractionation and characterization of starch granules using field-flow fractionation (FFF) and differential scanning calorimetry (DSC). Anal. Bioanal. Chem. 2019, 411, 3665–3674. [Google Scholar] [CrossRef]
- Dularia, C.; Sinhmar, A.; Thory, R.; Pathera, A.K.; Nain, V. Development of starch nanoparticles based composite films from non-conventional source-Water chestnut (Trapa bispinosa). Int. J. Biol. Macromol. 2019, 136, 1161–1168. [Google Scholar] [CrossRef]
- Lamanna, M.; Morales, N.J.; Garcia, N.L.; Goyanes, S. Development and characterization of starch nanoparticles by gamma radiation: Potential application as starch matrix filler. Carbohydr. Polym. 2013, 97, 90–97. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Anderson, D.P.; Yu, J. Preparation, modification, and application of starch nanocrystals in nanomaterials: A review. J. Nanomater. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Q.; Chang, P.R.; Yu, J. Chemical Modification of Starch. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 283–321. [Google Scholar]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and characterization of citric acid-modified starch nanoparticles/plasticized-starch composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Miranda Sechi, N.; Marques, P.T. Preparation and physicochemical, structural and morphological characterization of phosphorylated starch. Mater. Res. 2017, 20, 174–180. [Google Scholar] [CrossRef]
- Adewale Adetunji, O. Chemically Modified Starches as Excipients in Pharmaceutical Dosage Forms. In Chemical Properties of Starch; Intech Open Source: Rijeka, Croatia, 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Guízar-Miranda, A.; Montañéz-Soto, J.L.; García- Ruiz, I. Parcial caracterización de nuevos almidones obtenidos del tubérculo de camote del cerro (Dioscorea spp.). Rev. Iberoam. Tecnol. Postcosecha 2008, 9, 81–88. [Google Scholar]
- Perea, D.; Buitrago, H.G. Aplicación de la biotecnología agrícola al cultivo de ñame. In Ñame: Producción de Semillas por Biotecnología; Guzmán-Barney, M., Buitrago-Hurtado, G., Eds.; Universidad Nacional de Colombia, Unibiblos: Bogotá, Colombia, 2000; pp. 17–19. [Google Scholar]
- Estrada-Girón, Y.; Fernández-Escamilla, V.V.A.; Martín-del-Campo, A.; González-Nuñez, R.; Canché-Escamilla, G.; Uribe-Calderón, J.; Tepale, N.; Aguilar, J.; Moscoso-Sánchez, F.J. Characterization of Polylactic Acid Biocomposites Filled with Native Starch Granules from Dioscorea remotiflora Tubers. Polymers 2024, 16, 899. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Mariano, M.; Dufresne, A.; Mellem, J.J.; Amonsou, E.O. Microstructure, thermal properties and crystallinity of amadumbe starch nanocrystals. Int. J. Biol. Macromol. 2017, 102, 241–247. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, D.J.; Kim, J.Y.; Lim, S.T. Preparation of crystalline starch nanoparticles using cold acid hydrolysis and ultrasonication. Carbohydr. Polym. 2013, 98, 295–301. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, M.; Wang, L.; Zhou, J.; Tong, J. A method for improving dispersion of starch nanocrystals in water through crosslinking modification with sodium hexametaphosphate. Carbohydr. Polym. 2012, 87, 1874–1876. [Google Scholar] [CrossRef]
- Kim, H.Y.; Han, J.A.; Kweon, D.K.; Park, J.D.; Lim, S.T. Effect of ultrasonic treatments on nanoparticle preparation of acid-hydrolyzed waxy maize starch. Carbohydr. Polym. 2013, 93, 582–588. [Google Scholar] [CrossRef]
- Horwitz, W. Method 925.09: Solids (Total) and Loss on Drying (Moisture) in Flour. Vacuum Oven Method. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Angellier, H.; Molina-Boisseau, S.; Belgacem, M.N.; Dufresne, A. Surface chemical modification of waxy maize starch nanocrystals. Langmuir 2005, 21, 2425–2433. [Google Scholar] [CrossRef]
- Shujun, W.; Jinglin, Y.; Jiugao, Y.; Haixia, C.; Jiping, P. The effect of acid hydrolysis on morphological and crystalline properties of Rhizoma Dioscorea starch. Food Hydrocoll. 2007, 21, 1217–1222. [Google Scholar] [CrossRef]
- Jivan, M.J.; Madadlou, A.; Yarmand, M. An attempt to cast light into starch nanocrystals preparation and cross-linking. Food Chem. 2013, 141, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, G.; Zhu, F. Impact of long-term ultrasound treatment on structural and physicochemical properties of starches differing in granule size. Carbohydr. Polym. 2023, 320, 121195. [Google Scholar] [CrossRef]
- Zhu, F. Impact of ultrasound on structure, physicochemical properties, modifications, and applications of starch. Trends Food Sci. Technol. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Wei, B.; Qi, H.; Zou, J.; Li, H.; Wang, J.; Xu, B.; Ma, H. Degradation mechanism of amylopectin under ultrasonic irradiation. Food Hydrocoll. 2021, 111, 106371. [Google Scholar] [CrossRef]
- Sanchez de la Concha, B.B.; Agama-Acevedo, E.; Nuñez-Santiago, M.C.; Bello-Perez, L.A.; Garcia, H.S.; Alvarez-Ramirez, J. Acid hydrolysis of waxy starches with different granule size for nanocrystal production. J. Cereal Sci. 2018, 79, 193–200. [Google Scholar] [CrossRef]
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA-J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Truong, V.-D.; Wang, L. Structures and rheological properties of corn starch as affected by acid hydrolysis. Carbohydr. Polym. 2003, 52, 327–333. [Google Scholar] [CrossRef]
- Hoover, R. Acid-Treated Starches. Food Rev. Int. 2000, 16, 369–392. [Google Scholar] [CrossRef]
- Perez Herrera, M.; Vasanthan, T.; Hoover, R. Characterization of Maize Starch Nanoparticles Prepared by Acid Hydrolysis. Cereal Chem. 2016, 93, 323–330. [Google Scholar] [CrossRef]
- García-Gurrola, A.; Rincón, S.; Escobar-Puentes, A.A.; Zepeda, A.; Pérez-Robles, J.F.; Martínez-Bustos, F. Synthesis and succinylation of starch nanoparticles by means of a single step using sonochemical energy. Ultrason. Sonochem. 2019, 56, 458–465. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Dynamic Light Scattering. B. J. Berne and R. Pecora, John Wiley, New York, 1976, pp. 376. Price £16.50. Br. Polym. J. 1977, 9, 177. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: Sydney, NSW, Australia, 2003; 1086p. [Google Scholar]
- Ren, L.; Wang, Q.; Yan, X.; Tong, J.; Zhou, J.; Su, X. Dual modification of starch nanocrystals via crosslinking and esterification for enhancing their hydrophobicity. Food Res. Int. 2016, 87, 180–188. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, W.; Liu, J.; Li, Y.; Kennedy, J.F.; Gu, Q.; Shao, S. Preparation and characterization of organic-soluble acetylated starch nanocrystals. Carbohydr. Polym. 2010, 80, 1078–1084. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Meng, L.; Liu, H.; Ali, A.; Chen, L. Poly(lactic acid)/starch composites: Effect of microstructure and morphology of starch granules on performance. J. Appl. Polym. Sci. 2017, 134, 45504. [Google Scholar] [CrossRef]
- Sjöö, M.; Nilsson, L. (Eds.) Starch in Food: Structure, Function and Applications; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Thorston, UK, 2017; pp. 1–893. [Google Scholar]
- Sha, X.S.; Xiang, Z.J.; Bin, L.; Jing, L.; Bin, Z.; Jiao, Y.J.; Kun, S.R. Preparation and physical characteristics of resistant starch (type 4) in acetylated indica rice. Food Chem. 2012, 134, 149–154. [Google Scholar] [CrossRef]
- Colussi, R.; El Halal, S.L.M.; Pinto, V.Z.; Bartz, J.; Gutkoski, L.C.; da Rosa Zavareze, E.; Dias, A.R.G. Acetylation of rice starch in an aqueous medium for use in food. LWT-Food Sci. Technol. 2015, 62, 1076–1082. [Google Scholar] [CrossRef]
- Thitisomboon, W.; Opaprakasit, P.; Jaikaew, N.; Boonyarattanakalin, S. Characterizations of modified cassava starch with long chain fatty acid chlorides obtained from esterification under low reaction temperature and its PLA blending. J. Macromol. Sci. Part A 2018, 55, 253–259. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch-Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Imre, B.; Vilaplana, F. Organocatalytic esterification of corn starches towards enhanced thermal stability and moisture resistance. Green Chem. 2020, 22, 5017–5031. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; Xia, H.; Gao, Q. Surface chemical functionalization of starch nanocrystals modified by 3-aminopropyl triethoxysilane. Int. J. Biol. Macromol. 2019, 126, 987–993. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, B.; Wu, P.; Wang, Y.; Gao, W. Ultrasound effects on the acetylation of dioscorea starch isolated from Dioscorea zingiberensis C.H. Wright. Chem. Eng. Process. Process Intensif. 2012, 54, 29–36. [Google Scholar] [CrossRef]
- Spiridon, I.; Teaca, C.-A.; Bodirlau, R. Preparation and characterization of adipic acid-modified starch microparticles/plasticized starch composite films reinforced by lignin. J. Mater. Sci. 2011, 46, 3241–3251. [Google Scholar] [CrossRef]
- Seguchi, M.; Higasa, T.; Mori, T. Study of wheat starch structures by sonication treatment. Cereal Chem. 1994, 71, 636–639. [Google Scholar]
- Huang, Q.; Li, L.; Fu, X. Ultrasound Effects on the Structure and Chemical Reactivity of Cornstarch Granules. Starch-Stärke 2007, 59, 371–378. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, X.; He, X.; Luo, F.; Gao, Q.; Yu, S. Effect of Ultrasonic Treatment on the Physicochemical Properties of Maize Starches Differing in Amylose Content. Starch-Stärke 2008, 60, 646–653. [Google Scholar] [CrossRef]
- Huang, J.; Schols, H.A.; Jin, Z.; Sulmann, E.; Voragen, A.G.J. Pasting properties and (chemical) fine structure of acetylated yellow pea starch is affected by acetylation reagent type and granule size. Carbohydr. Polym. 2007, 68, 397–406. [Google Scholar] [CrossRef]
- Huber, K.C.; BeMiller, J.N. Channels of maize and sorghum starch granules. Carbohydr. Polym. 2000, 41, 269–276. [Google Scholar] [CrossRef]
- Mahesh, B.; Kathyayani, D.; Channe Gowda, D.; Mrutunjaya, K. Blends of synthetic plastic-derived polypeptide with Hydroxypropylmethylcellulose and polyvinyl alcohol: Unraveling the specific interaction parameters, morphology and thermal stability of the polymers couple. J. Polym. Res. 2020, 27, 278. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Li, X.; Gao, W. Physicochemical properties of starches from two different yam (Dioscorea opposita Thunb.) residues. Brazilian Arch. Biol. Technol. 2011, 54, 243–251. [Google Scholar] [CrossRef]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers. Polymers 2021, 13, 584. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-Toxic Crosslinking of Starch Using Polycarboxylic Acids: Kinetic Study and Quantitative Correlation of Mechanical Properties and Crosslinking Degrees. J. Polym. Environ. 2015, 23, 588–594. [Google Scholar] [CrossRef]
- Ren, L.; Dong, Z.; Jiang, M.; Tong, J.; Zhou, J. Hydrophobization of starch nanocrystals through esterification in green media. Ind. Crops Prod. 2014, 59, 115–118. [Google Scholar] [CrossRef]
- Cui, S.; Liu, Q.; Xie, S. Starch Modifications and Applications. In Food Carbohydrates; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Colonna, P.; Buleon, A. Thermal transitions of starches. In Starches; CRC Press: Boca Raton, FL, USA, 2009; pp. 71–102. [Google Scholar]
- Slade, L.; Levine, H. Non-equilibrium melting of native granular starch: Part I. Temperature location of the glass transition associated with gelatinization of A-type cereal starches. Carbohydr. Polym. 1988, 8, 183–208. [Google Scholar] [CrossRef]
- Boufi, S.; Bel Haaj, S.; Magnin, A.; Pignon, F.; Impéror-Clerc, M.; Mortha, G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason. Sonochem. 2018, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Xiaofan, L.; Chen, Y.; Zhou, W. Effect of cross-linking with sodium trimetaphosphate on structural and physicochemical properties of tigernut starch. Food Sci. Technol. 2022, 42, e76422. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, Y. Extraction of flavonoids from flavonoid-rich parts in tartary buckwheat and identification of the main flavonoids. J. Food Eng. 2007, 78, 584–587. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Z.; Wang, Y.; Yuan, S.; Jiang, Y.; Li, Z.; Li, Y.; Yan, Z.; Meng, L.; Qiu, L. Recent progress of starch modification assisted by ultrasonic wave. Food Sci. Technol. 2023, 43, e107522. [Google Scholar] [CrossRef]
- Rahaman, A.; Kumari, A.; Zeng, X.-A.; Adil Farooq, M.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Faisal Manzoor, M. Ultrasound based modification and structural-functional analysis of corn and cassava starch. Ultrason. Sonochem. 2021, 80, 105795. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Afolabi, T.A.; Olu-Owolabi, B.I. Functional, physicochemical and retrogradation properties of sword bean (Canavalia gladiata) acetylated and oxidized starches. Carbohydr. Polym. 2006, 65, 93–101. [Google Scholar] [CrossRef]
- Andrade, I.H.P.; Otoni, C.G.; Amorim, T.S.; Camilloto, G.P.; Cruz, R.S. Ultrasound-assisted extraction of starch nanoparticles from breadfruit (Artocarpus altilis (Parkinson) Fosberg). Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124277. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Liu, H.; Chen, L. Starch Modification Using Reactive Extrusion. Starch-Stärke 2006, 58, 131–139. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, D.J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Koo, S.H.; Lee, K.Y.; Lee, H.G. Effect of cross-linking on the physicochemical and physiological properties of corn starch. Food Hydrocoll. 2010, 24, 619–625. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.H.; Kim, J.; Lim, W.; Lim, S. Characterization of nanoparticles prepared by acid hydrolysis of various starches. Starch-Stärke 2012, 64, 367–373. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Hernández-Jaimes, C.; Carrillo-Navas, H.; González, F.; Rodríguez, E.; Bello-Pérez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Acid hydrolysis of native corn starch: Morphology, crystallinity, rheological and thermal properties. Carbohydr. Polym. 2014, 103, 596–602. [Google Scholar] [CrossRef]
- Xu, Y.; Miladinov, V.; Hanna, M.A. Synthesis and Characterization of Starch Acetates with High Substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef]
- Karim, A. Methods for the study of starch retrogradation. Food Chem. 2000, 71, 9–36. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Q.; Cui, S.W. Studies on the granular structure of resistant starches (type 4) from normal, high amylose and waxy corn starch citrates. Food Res. Int. 2006, 39, 332–341. [Google Scholar] [CrossRef]
- Li, E.; Cao, P.; Cao, W.; Li, C. Relations between starch fine molecular structures with gelatinization property under different moisture content. Carbohydr. Polym. 2022, 278, 118955. [Google Scholar] [CrossRef]
- EL Halal, S.L.M.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Carreño, N.L.V.; Dias, A.R.G.; da Rosa Zavareze, E. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef]
- Eisenberg, A. The glassy state and glass transition. In Physical Properties of Polymers; Mark, J.E., Ed.; American Chemical Society: Washington, DC, USA, 1984; p. 55. [Google Scholar]
- Liu, P.; Yu, L.; Liu, H.; Chen, L.; Li, L. Glass transition temperature of starch studied by a high-speed DSC. Carbohydr. Polym. 2009, 77, 250–253. [Google Scholar] [CrossRef]
- Lourdin, D.; Coignard, L.; Bizot, H.; Colonna, P. Influence of equilibrium relative humidity and plasticizer concentration on the water content and glass transition of starch materials. Polymer 1997, 38, 5401–5406. [Google Scholar] [CrossRef]
- Rindlava, Å.; Hulleman, S.H.D.; Gatenholma, P. Formation of starch films with varying crystallinity. Carbohydr. Polym. 1997, 34, 25–30. [Google Scholar] [CrossRef]
- Liu, P.; Yu, L.; Wang, X.; Li, D.; Chen, L.; Li, X. Glass transition temperature of starches with different amylose/amylopectin ratios. J. Cereal Sci. 2010, 51, 388–391. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R. Starch: Chemistry and Technology, 3rd ed.; Academic Press: Boston, MA, USA, 2009. [Google Scholar]



| Starch Particles (SPs) | Yield (%) | Residual Amylose (%) |
|---|---|---|
| AH | ||
| Unmodified SPs | 19.4 ± 2.3 a | 3.2 ± 0.2 c |
| Acetic anhydride | 22.0 ± 0.8 ab | 2.1 ± 0.2 a |
| Lauroyl chloride | 33.6 ± 6.6 cd | 2.1 ± 0.1 a |
| SHMP | 27.6 ± 0.6 c | 2.1 ± 0.1 a |
| Citric acid | 38.7 ± 3.3 de | 2.8 ± 0.2 b |
| AH-US | ||
| Unmodified SPs | 17.5 ± 0.9 a | 2.8 ± 0.3 c |
| Acetic anhydride | 22.9 ± 2.7 b | 1.9 ± 0.2 a |
| Lauroyl chloride | 36.5 ± 1.3 d | 1.8 ± 0.2 a |
| SHMP | 16.5 ± 1.8 a | 2.2 ± 0.1 b |
| Citric acid | 32.1 ± 2.6 c | 2.7 ± 0.2 c |
| Starch Particles (SPs) | Peak (1) Dz (μm) | Peak (2) Dz (μm) | PDI |
|---|---|---|---|
| AH | |||
| Unmodified SPs | 0.55 ± 0.02 | -- | 0.45 ± 0.14 |
| Acetic anhydride | 0.09 ± 0.01 | 0.53 ± 0.00 | 0.69 ± 0.16 |
| Lauroyl chloride | 0.46 ± 0.31 | 0.93 ± 0.05 | 0.84 ± 0.14 |
| SHMP | 0.53 ± 0.05 | 2.31 ± 0.03 | 0.31 ± 0.10 |
| Citric acid | 0.22 ± 0.03 | 0.69 ± 0.01 | 0.71 ± 0.05 |
| AH-US | |||
| Unmodified SPs | 0.09 ± 0.02 | 0.46 ± 0.01 | 0.36 ± 0.07 |
| Acetic anhydride | 0.10 ± 0.01 | 0.55 ± 0.04 | 0.52 ± 0.04 |
| Lauroyl chloride | 0.41 ± 0.01 | 0.91 ± 0.32 | 0.82 ± 0.09 |
| SHMP | 0.34 ± 0.02 | 1.32 ± 0.07 | 0.41 ± 0.10 |
| Citric acid | 0.12 ± 0.01 | 0.50 ± 0.01 | 0.54 ± 0.04 |
| Starch Particles (SPs) | A(-OH) (%Tcm−1) | A(-C=O) (%Tcm−1) | DS | Diffraction Peak at 17° | Diffraction Peak at 22° | RCI (%) |
|---|---|---|---|---|---|---|
| Native starch | 723 | -- | -- | 16.8 | 21.7 | 30 |
| AH | ||||||
| Unmodified SPs | 707 | -- | -- | 17.3 | 22.5 | 42 |
| Acetic anhydride | 587 | 71 | 0.12 | 17.3 | 22.3 | 30 |
| Lauroyl chloride | 600 | 91 | 0.15 | 17.5 | 22.6 | 36 |
| SHMP | 683 | -- | -- | 16.9 | 22.3 | 33 |
| Citric acid | 608 | 265 | 0.43 | 16.7 | 22.0 | 40 |
| AH-US | ||||||
| Unmodified SPs | 619 | -- | -- | 17.2 | 22.8 | 35 |
| Acetic anhydride | 560 | 81 | 0.14 | 17.3 | 22.5 | 30 |
| Lauroyl chloride | 577 | 101 | 0.17 | 17.3 | 22.3 | 28 |
| SHMP | 596 | -- | -- | 16.8 | 21.8 | 26 |
| Citric acid | 535 | 242 | 0.45 | 17.0 | 22.1 | 34 |
| Modified Starch Particles * | a Tg (°C) | b T0 (°C) | c Tp (°C) | d Tc (°C) | e ∆Hf (J/g) |
|---|---|---|---|---|---|
| Native starch | 68.7 ± 0.2 | 42.8 ± 0.2 | 93.8 ± 0.2 | 148.8 ± 0.2 | 339.0 ± 0.2 |
| AH | |||||
| Unmodified SPs | 60.8 ± 0.2 | 43.1 ± 0.2 | 88.6 ± 0.1 | 136.8 ± 0.2 | 187.6 ± 0.1 |
| Acetic anhydride | 73.9 ± 0.1 | 42.0 ± 0.1 | 90.9 ± 0.2 | 144.0 ± 0.2 | 246.0 ± 0.2 |
| Lauroyl chloride | 67.7 ± 0.2 | 40.5 ± 0.2 | 94.8 ± 0.3 | 148.5 ± 0.4 | 159.0 ± 0.2 |
| SHMP | 65.3± 0.2 | 28.0 ± 0.2 | 91.1 ± 0.2 | 161.0 ± 0.2 | 286.0 ± 0.3 |
| Citric acid | 68.9 ± 0.1 | 35.0 ± 0.4 | 83.5 ± 0.1 | 142.3 ± 0.4 | 215.0 ± 0.4 |
| AH-US | |||||
| Unmodified SPs | 59.3 ± 0.2 | 35.0 ± 0.1 | 88.7 ± 0.2 | 132.5 ± 0.1 | 234.0 ± 0.4 |
| Acetic anhydride | 76.7 ± 0.3 | 42.5 ± 0.2 | 92.8 ± 0.3 | 157.0 ± 0.2 | 278.0 ± 0.1 |
| Lauroyl chloride | 68.0 ± 0.1 | 39.0 ± 0.2 | 88.1 ± 0.3 | 139.5 ± 0.1 | 124.7 ± 0.4 |
| SHMP | 84.1 ± 0.4 | 54.5 ± 0.3 | 97.6 ± 0.3 | 152.0 ± 0.2 | 189.0 ± 0.2 |
| Citric acid | 70.5 ± 0.3 | 41.0 ± 0.1 | 91.2 ± 0.2 | 162.0 ± 0.2 | 343.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esparza-Merino, R.M.; Estrada-Girón, Y.; Puebla-Pérez, A.M.; Fernández-Escamilla, V.V.A.; Martín-del-Campo, A.; Uribe-Calderón, J.A.; Tepale, N.; Ceja, I. Wild Yam (Dioscorea remotiflora) Tubers: An Alternative Source for Obtaining Starch Particles Chemically Modified After Extraction by Acid Hydrolysis and Ultrasound. Polysaccharides 2025, 6, 69. https://doi.org/10.3390/polysaccharides6030069
Esparza-Merino RM, Estrada-Girón Y, Puebla-Pérez AM, Fernández-Escamilla VVA, Martín-del-Campo A, Uribe-Calderón JA, Tepale N, Ceja I. Wild Yam (Dioscorea remotiflora) Tubers: An Alternative Source for Obtaining Starch Particles Chemically Modified After Extraction by Acid Hydrolysis and Ultrasound. Polysaccharides. 2025; 6(3):69. https://doi.org/10.3390/polysaccharides6030069
Chicago/Turabian StyleEsparza-Merino, Rosa María, Yokiushirdhilgilmara Estrada-Girón, Ana María Puebla-Pérez, Víctor Vladimir Amílcar Fernández-Escamilla, Angelina Martín-del-Campo, Jorge Alonso Uribe-Calderón, Nancy Tepale, and Israel Ceja. 2025. "Wild Yam (Dioscorea remotiflora) Tubers: An Alternative Source for Obtaining Starch Particles Chemically Modified After Extraction by Acid Hydrolysis and Ultrasound" Polysaccharides 6, no. 3: 69. https://doi.org/10.3390/polysaccharides6030069
APA StyleEsparza-Merino, R. M., Estrada-Girón, Y., Puebla-Pérez, A. M., Fernández-Escamilla, V. V. A., Martín-del-Campo, A., Uribe-Calderón, J. A., Tepale, N., & Ceja, I. (2025). Wild Yam (Dioscorea remotiflora) Tubers: An Alternative Source for Obtaining Starch Particles Chemically Modified After Extraction by Acid Hydrolysis and Ultrasound. Polysaccharides, 6(3), 69. https://doi.org/10.3390/polysaccharides6030069







