In Situ Silanization of Ligno-Cellulosic Microfibers Derived from Industrial Waste to Enhance Mechanical Properties of Natural Rubber Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Ligno-Cellulosic Microfibers
2.3. Characterization of the Fibers
2.4. Compounding and Vulcanization
2.5. In Situ Silanization
2.6. Compound Characterization
2.6.1. Physical–Mechanical Properties
2.6.2. Thermogravimetric Analysis
2.6.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopic (ATR-FTIR)
2.6.4. Cure Behavior
2.6.5. Crosslink Density
2.6.6. Dynamic Mechanical Analysis
2.6.7. Scanning Electron Microscopy
3. Results and Discussion
3.1. Characterization of the Fibers
3.2. Compound Characterization
3.2.1. Physical–Mechanical Properties
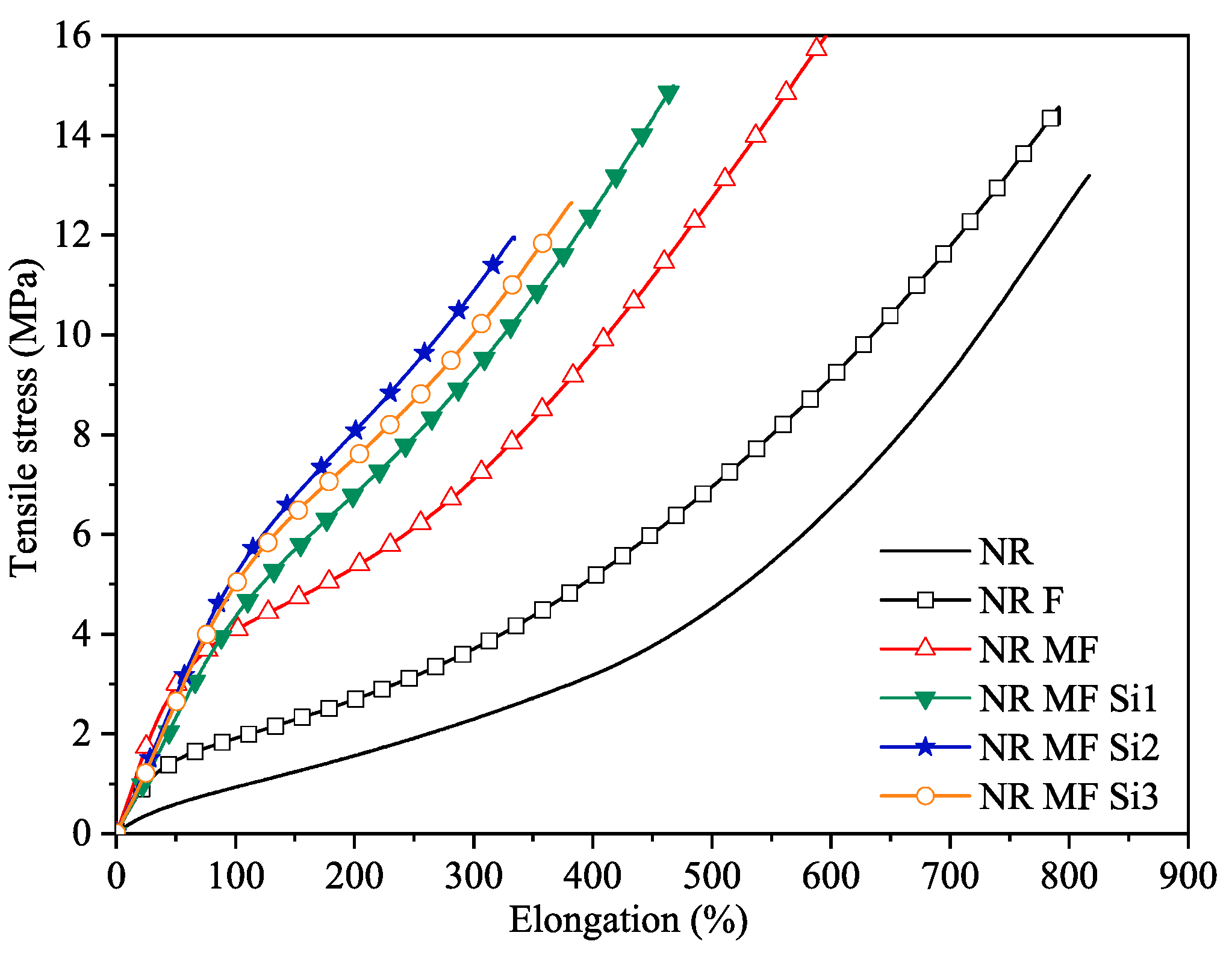
Tensile Properties
Tear Strength Property
Hardness and Abrasion Resistance
3.2.2. Thermogravimetric Analysis
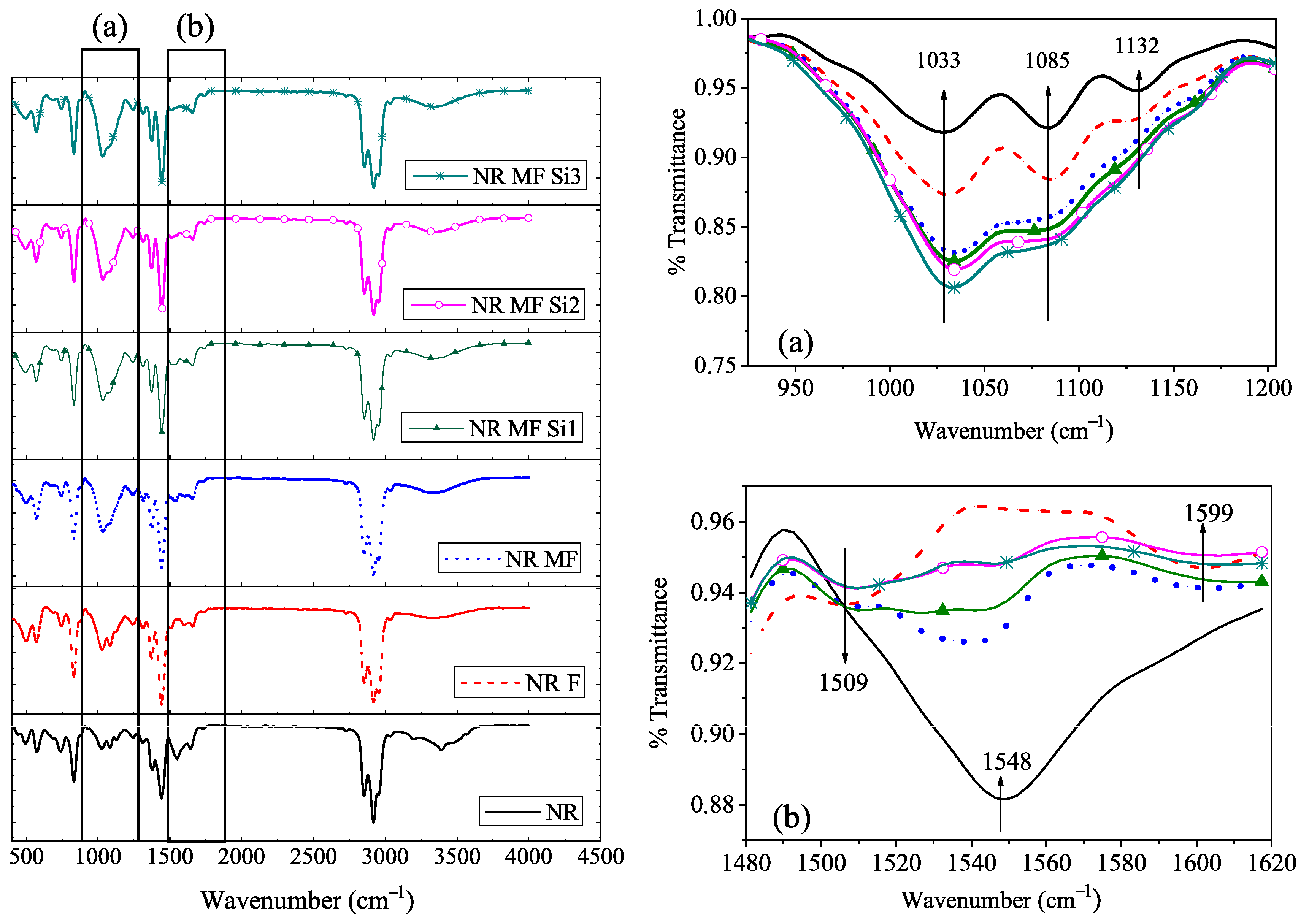
3.2.3. ATR-FTIR Analysis
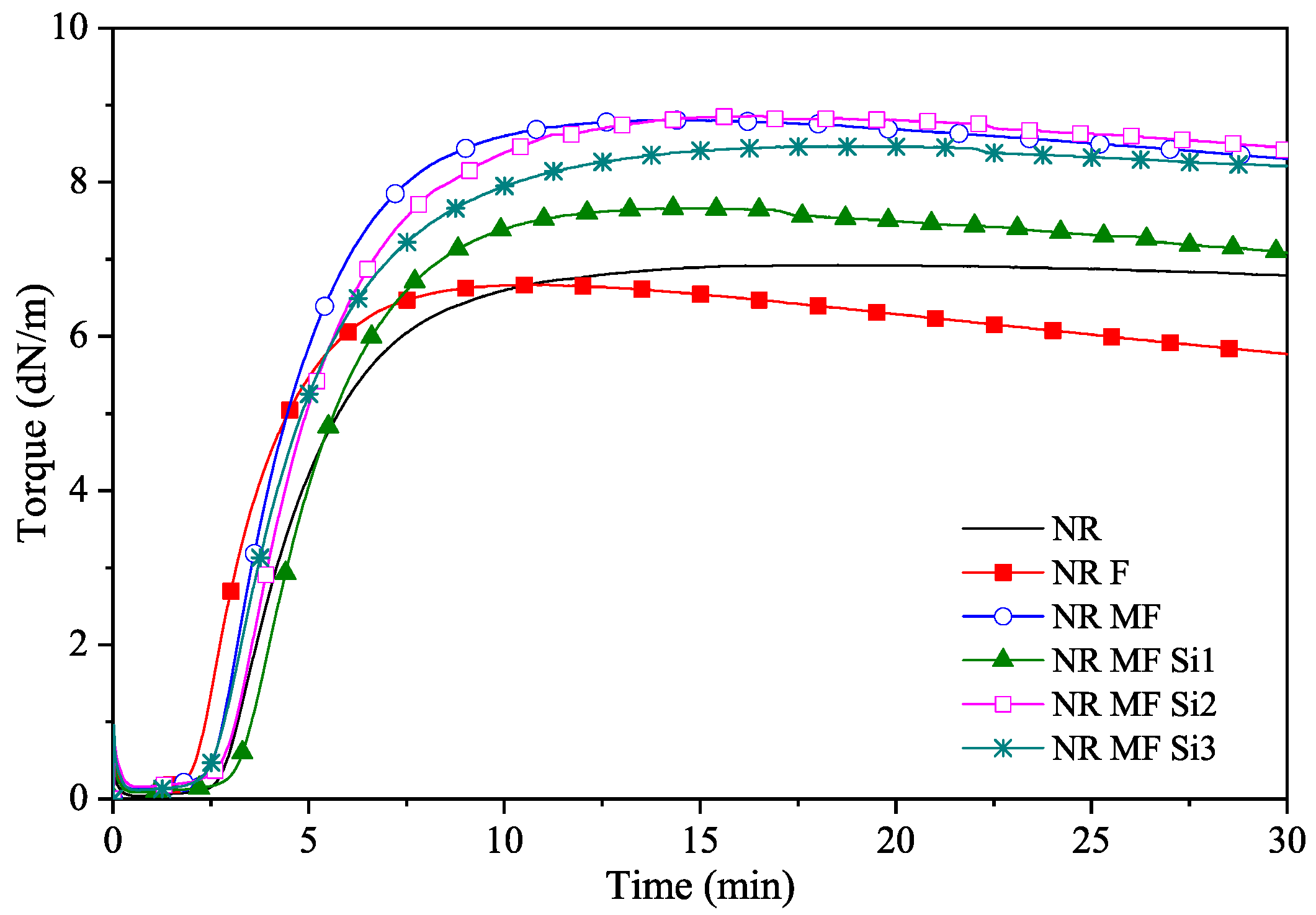
3.2.4. Cure Behavior
3.2.5. Crosslink Density
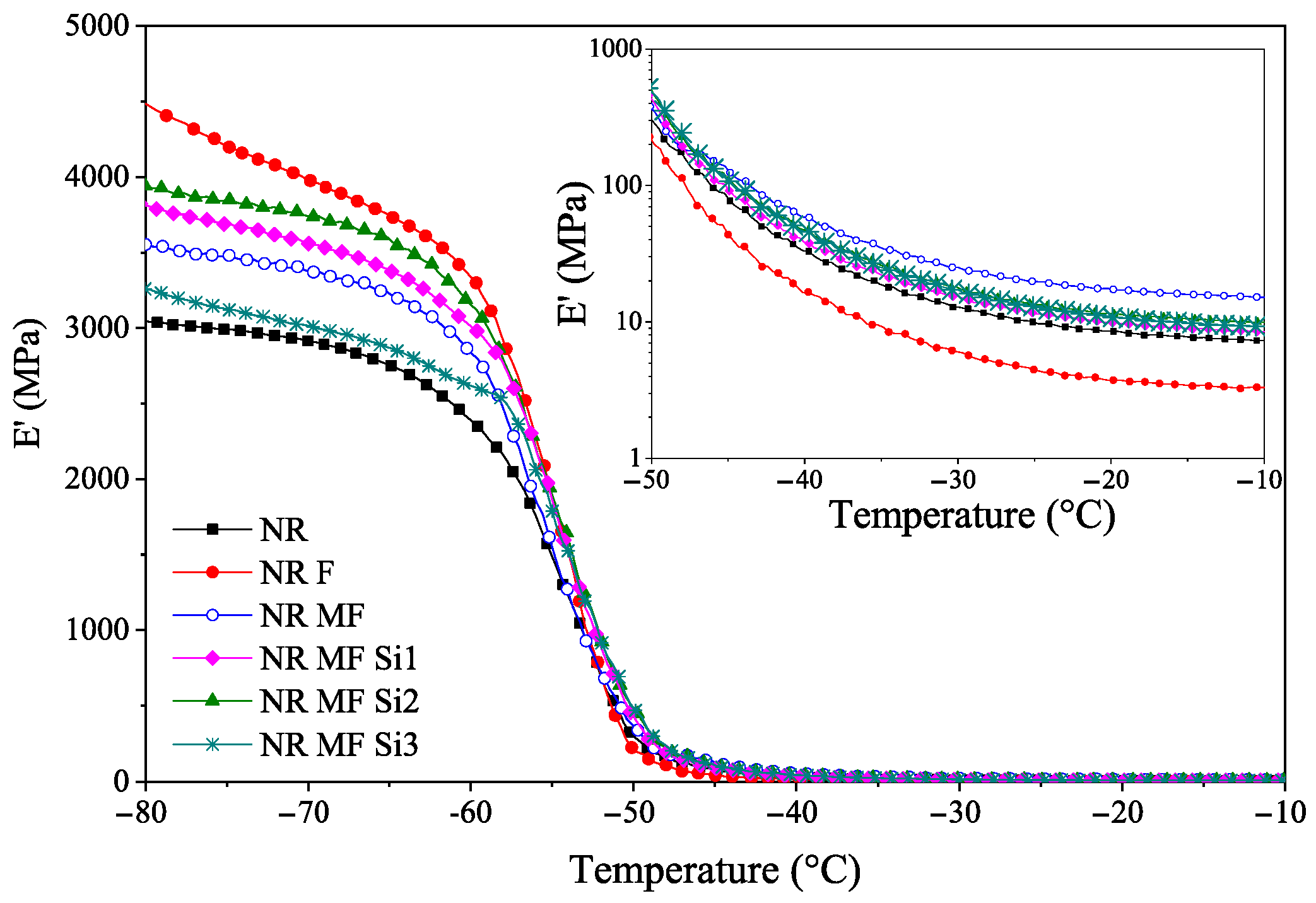
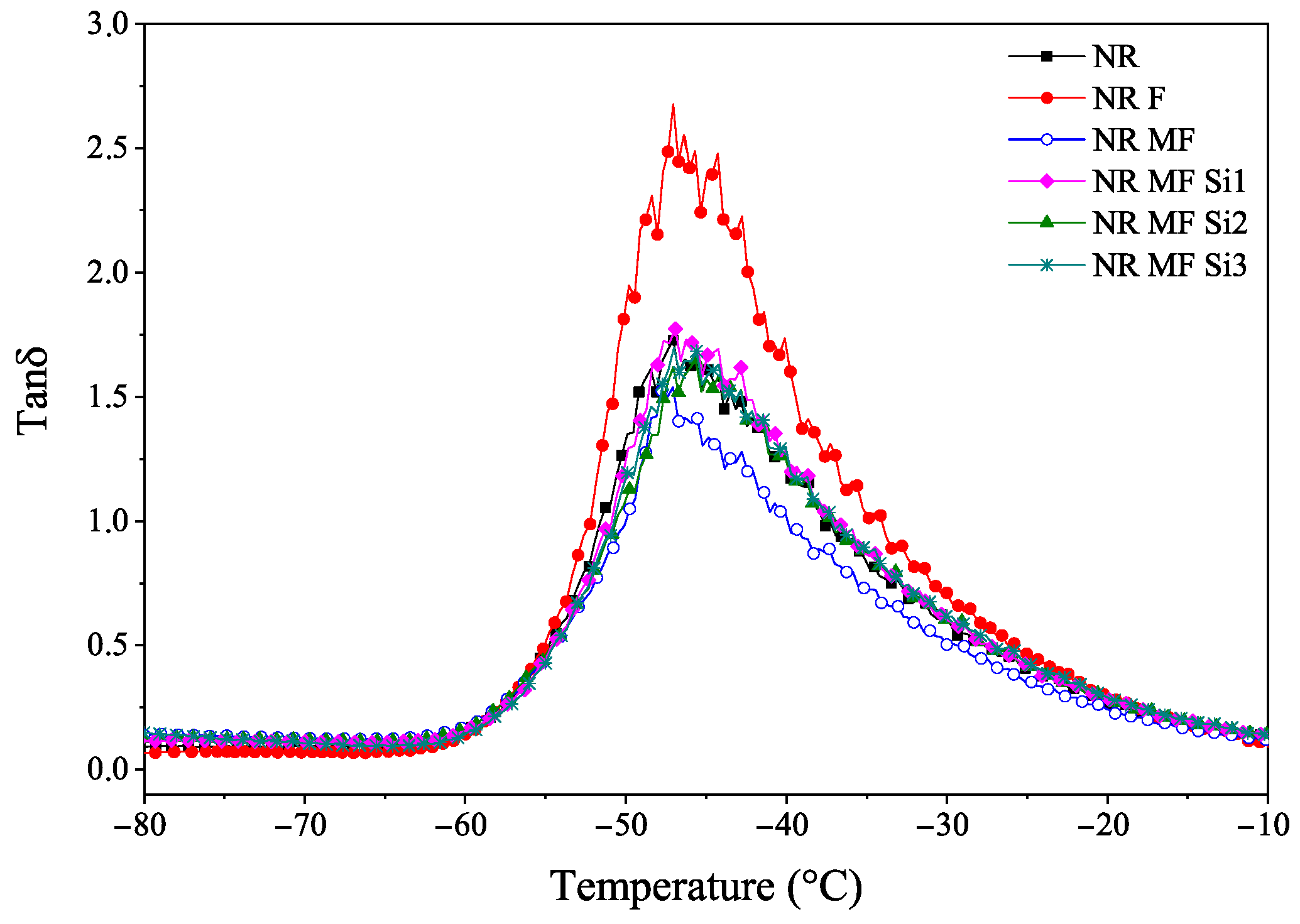
3.2.6. Dynamic Mechanical Analysis
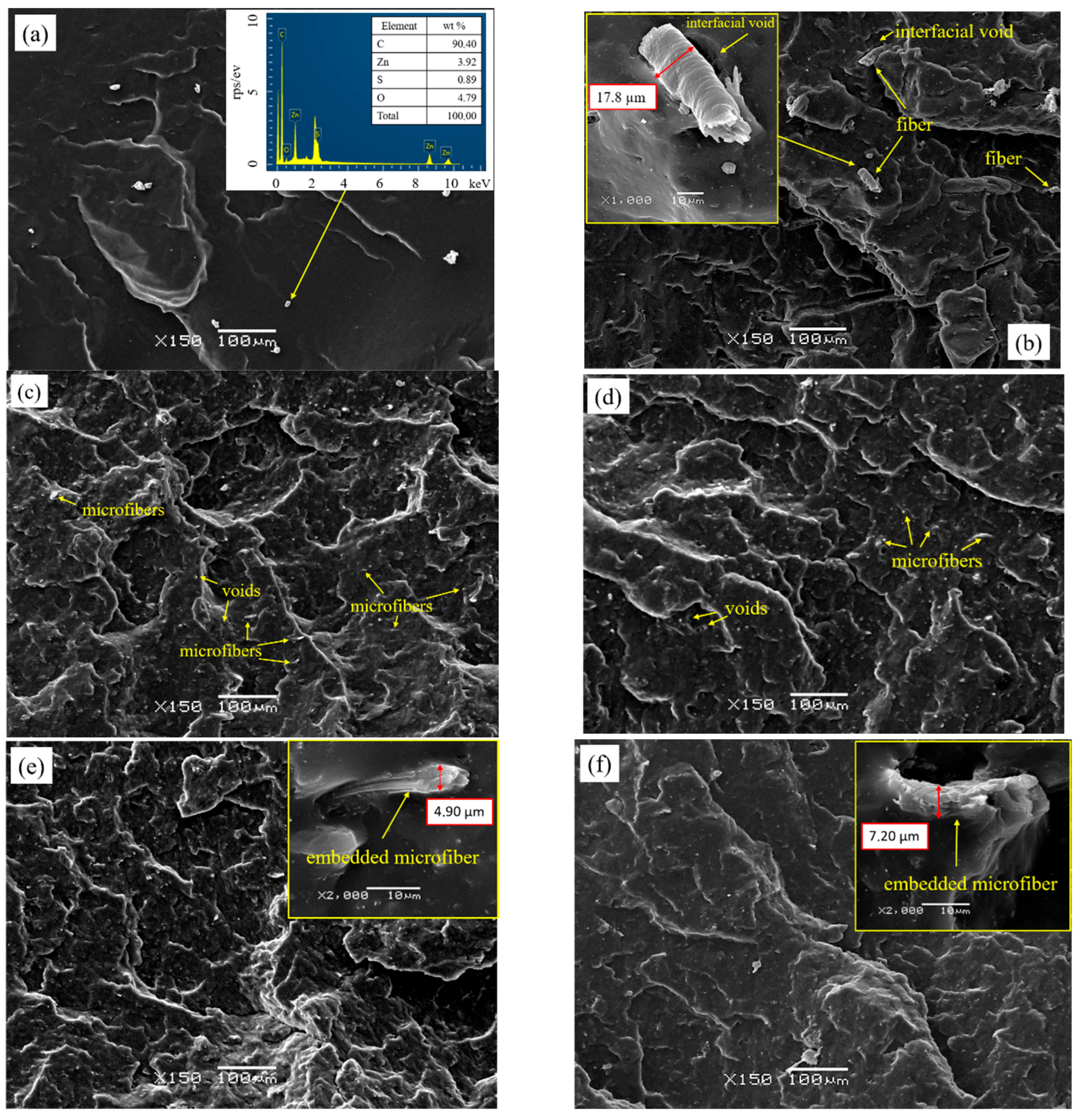
3.2.7. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bajpai, P.K.; Singh, I.; Madaan, J. Development and characterization of PLA-based green composites: A review. J. Thermoplast. Compos. Mater. 2014, 27, 52–81. [Google Scholar] [CrossRef]
- Thomas, S.K.; Parameswaranpillai, J.; Krishnasamy, S.; Begum, P.S.; Nandi, D.; Siengchin, S.; George, J.J.; Hameed, N.; Salim, N.; Sienkiewicz, N. A comprehensive review on cellulose, chitin, and starch as fillers in natural rubber biocomposites. Carbohydr. Polym. Technol. Appl. 2021, 2, 100095. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Flores-Alamo, N.; Tecante, A. Preparation of microcrystalline cellulose from residual Rose stems (Rosa spp.) by successive delignification with alkaline hydrogen peroxide. Int. J. Biol. Macromol. 2020, 155, 324–329. [Google Scholar] [CrossRef]
- Hakimi, N.M.F.; Lee, S.H.; Lum, W.C.; Mohamad, S.F.; Al Edrus, S.S.O.; Park, B.-D.; Azmi, A. Surface modified nanocellulose and its reinforcement in natural rubber matrix nanocomposites: A review. Polymers 2021, 13, 3241. [Google Scholar] [CrossRef] [PubMed]
- Somseemee, O.; Sae-Oui, P.; Siriwong, C. Reinforcement of surface-modified cellulose nanofibrils extracted from Napier grass stem in natural rubber composites. Ind. Crops Prod. 2021, 171, 113881. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Silanized cereal straw as a novel, functional filler of natural rubber biocomposites. Cellulose 2019, 26, 1025–1040. [Google Scholar] [CrossRef]
- Jantachum, P.; Khumpaitool, B.; Utara, S. Effect of silane coupling agent and cellulose nanocrystals loading on the properties of acrylonitrile butadiene rubber/natural rubber nanocomposites. Ind. Crops Prod. 2023, 195, 116407. [Google Scholar] [CrossRef]
- Zhu, G.; Dufresne, A. Synergistic reinforcing and cross-linking effect of thiol-ene-modified cellulose nanofibrils on natural rubber. Carbohydr. Polym. 2022, 278, 118954. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. A critical review on the techniques used for the synthesis and applications of crystalline cellulose derived from agricultural wastes and forest residues. Carbohydr. Polym. 2021, 273, 118537. [Google Scholar] [CrossRef]
- Ehman, N.; Rodríguez-Fabià, S.; Zehner, J.; Chinga-Carrasco, G. Chemical compatibility between poly(ethylene) and cellulose nanofibers from kraft pulps containing varying amounts of lignin: An aqueous acetylation strategy and its effect on biocomposite properties. Compos. Part A Appl. Sci. Manuf. 2024, 184, 108247. [Google Scholar] [CrossRef]
- Yuan, T.; Zeng, J.; Wang, B.; Cheng, Z.; Chen, K. Lignin containing cellulose nanofibers (LCNFs): Lignin content-morphology-rheology relationships. Carbohydr. Polym. 2021, 254, 117441. [Google Scholar] [CrossRef]
- Andrade, A.; Henríquez-Gallegos, S.; Albornoz-Palma, G.; Pereira, M. Effect of the chemical and structural characteristics of pulps of Eucalyptus and Pinus on the deconstruction of the cell wall during the production of cellulose nanofibrils. Cellulose 2021, 28, 5387–5399. [Google Scholar] [CrossRef]
- TAPPI Standard Methods T280 pm-99; Acetone Extractives of Wood and Pulp. Technical Association of the Pulp and Paper Industry: Peachtree Corners, GA, USA, 1999.
- TAPPI Standard Methods T211 om-02; Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C. Technical Association of the Pulp and Paper Industry: Peachtree Corners, GA, USA, 2007.
- Albornoz-Palma, G.; Betancourt, F.; Mendonça, R.T.; Chinga-Carrasco, G.; Pereira, M. Relationship between rheological and morphological characteristics of cellulose nanofibrils in dilute dispersions. Carbohydr. Polym. 2020, 230, 115588. [Google Scholar] [CrossRef] [PubMed]
- ASTM D412-16; Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers-Tension. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D624-00; Standard Test Methods for Tear Strength of Conventional Vulcanized Rubber and Thermoplastic Elastomers. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D2240-15; Standard Test Method for Rubber Property-Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D5963-22; Standard Test Method for Rubber Property-Abrasion Resistance (Rotary Drum Abrader). ASTM International: West Conshohocken, PA, USA, 2022.
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Xue, B.; Wang, X.; Yu, L.; Di, B.; Chen, Z.; Zhu, Y.; Liu, X. Self-assembled lignin-silica hybrid material derived from rice husks as the sustainable reinforcing fillers for natural rubber. Int. J. Biol. Macromol. 2020, 145, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ek, M.; Gellerstedt, G.; Henriksson, G. The Trees. In Pulp and Paper Chemistry and Technology. Volume 1: Wood Chemistry and Wood Biotechnology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter: Berlin, Germany, 2009; pp. 13–44. [Google Scholar]
- Kulshrestha, U.; Gupta, T.; Kumawat, P.; Jaiswal, H.; Ghosh, S.B.; Sharma, N.N. Cellulose nanofibre enabled natural rubber composites: Microstructure, curing behaviour and dynamic mechanical properties. Polym. Test. 2020, 90, 106676. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Amiralian, N.; Martin, D.J.; Annamalai, P.K. Achieving ultra-tear resistant high-performance natural rubber nanocomposite via bio-inspired lignocellulosic compatibilization. Ind. Crops Prod. 2024, 207, 117729. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Rybinski, P.; Strzelec, K. Properties of chemically modified (selected silanes) lignocellulosic filler and its application in natural rubber biocomposites. Materials 2020, 13, 18. [Google Scholar] [CrossRef]
- Moonart, U.; Utara, S. Effect of surface treatments and filler loading on the properties of hemp fiber/natural rubber composites. Cellulose 2019, 26, 7271–7295. [Google Scholar] [CrossRef]
- Srisuwan, L.; Jarukumjorn, K.; Suppakarn, N. Effect of Silane Treatment Methods on Physical Properties of Rice Husk Flour/Natural Rubber Composites. Adv. Mater. Sci. Eng. 2018, 2018, 583974. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Sheltami, R.M.; Ahmad, I.; Abdullah, I.; Dufresne, A. Cellulose nanocrystal reinforced liquid natural rubber toughened unsaturated polyester: Effects of filler content and surface treatment on its morphological, thermal, mechanical, and viscoelastic properties. Polymer 2015, 71, 51–59. [Google Scholar] [CrossRef]
- Dominic, M.C.D.; Joseph, R.; Begum, P.S.; Joseph, M.; Padmanabhan, D.; Morris, L.A.; Kumar, A.S.; Formela, K. Cellulose nanofibers isolated from the Cuscuta Reflexa plant as a green reinforcement of natural rubber. Polymers 2020, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.; Tajima, N.; Iwamoto, S.; Morimoto, T.; Nagatani, A.; Okazaki, T.; Endo, T. Properties of natural rubber reinforced with cellulose nanofibers based on fiber diameter distribution as estimated by differential centrifugal sedimentation. Int. J. Biol. Macromol. 2019, 121, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Somseemee, O.; Saeoui, P.; Schevenels, F.T.; Siriwong, C. Enhanced interfacial interaction between modified cellulose nanocrystals and epoxidized natural rubber via ultraviolet irradiation. Sci. Rep. 2022, 12, 6682. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, M.; Zheng, Q.; Song, Y. Influence of ionic liquid on rheological behaviors of candle soot and cellulose nanocrystal filled natural rubber nanocomposites. Compos. Commun. 2022, 33, 101214. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Ah-Leung, T.; Hojabr, S.; Berry, R. Investigation of the co-coagulation of natural rubber latex and cellulose nanocrystals aqueous dispersion. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 123949. [Google Scholar] [CrossRef]
- Nair, A.R.; Sambhudevan, S.; Shankar, B. Synthesis, characterization and dye removal properties of cellulose nanocrystals embedded natural rubber latex composite. Cellul. Chem. Technol. 2019, 53, 263–270. [Google Scholar] [CrossRef]
- Pandey, K.K. A Study of Chemical Structure of Soft and Hardwood and Wood Polymers by FTIR Spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Mahur, B.K.; Ahuja, A.; Singh, S.; Maji, P.K.; Rastogi, V.K. Different nanocellulose morphologies (cellulose nanofibers, nanocrystals and nanospheres) extracted from Sunn hemp (Crotalaria juncea). Int. J. Biol. Macromol. 2023, 253, 126657. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Annamalai, P.K.; Wang, L.; Martin, D.; Amiralian, N. Reinforcement of natural rubber latex using lignocellulosic nanofibers isolated from spinifex grass. Nanoscale 2017, 9, 9510–9519. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dhakar, G.L.; Kapgate, B.P.; Maji, P.K.; Verma, C.; Chhajed, M.; Rajkumar, K.; Das, C. Synthesis and chemical modification of crystalline nanocellulose to reinforce natural rubber composites. Polym. Adv. Technol. 2020, 31, 3059–3069. [Google Scholar] [CrossRef]
- Kazemi, H.; Mighri, F.; Rodrigue, D. A Review of Rubber Biocomposites Reinforced with Lignocellulosic Fillers. J. Compos. Sci. 2022, 6, 183. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W. Mechanistic Aspects of Silane Coupling Agents with Different Functionalities on Reinforcement of Silica-Filled Natural Rubber Compounds. Polym. Eng. Sci. 2015, 51, 836–842. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Potiyaraj, P. A critical review on the utilization of various reinforcement modifiers in filled rubber composites. J. Elastomers Plast. 2020, 52, 167–193. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Tebal, N. Dynamic Mechanical Behavior of Natural Rubber/Waste Ethylene-Propylene-Diene Rubber Blends. Prüfen Mess. 2014, 7, 33–39. [Google Scholar]
- Koshy, A.T.; Kuriakose, B.; Thomas, S. Studies on the effect of blend ratio and cure system on the degradation of natural rubber-ethylene-vinyl acetate rubber blends. Polym. Degrad. Stab. 1992, 36, 137–147. [Google Scholar] [CrossRef]
- Magnere, S.M.; Toledo, E.A.; Yazdani-Pedram, M.; Fuentealba, P.; Contreras-Soto, A.; Bascuñan-Heredia, A.; Alvarez-Cortes, G.; Zagal, A.; Molina, F.; Hernández-Santana, M.; et al. High performance fluoroelastomer composites filled with graphite and/or bismuth oxide for applications in gamma-ray shielding. Polym. Compos. 2024, 45, 6901–6913. [Google Scholar] [CrossRef]


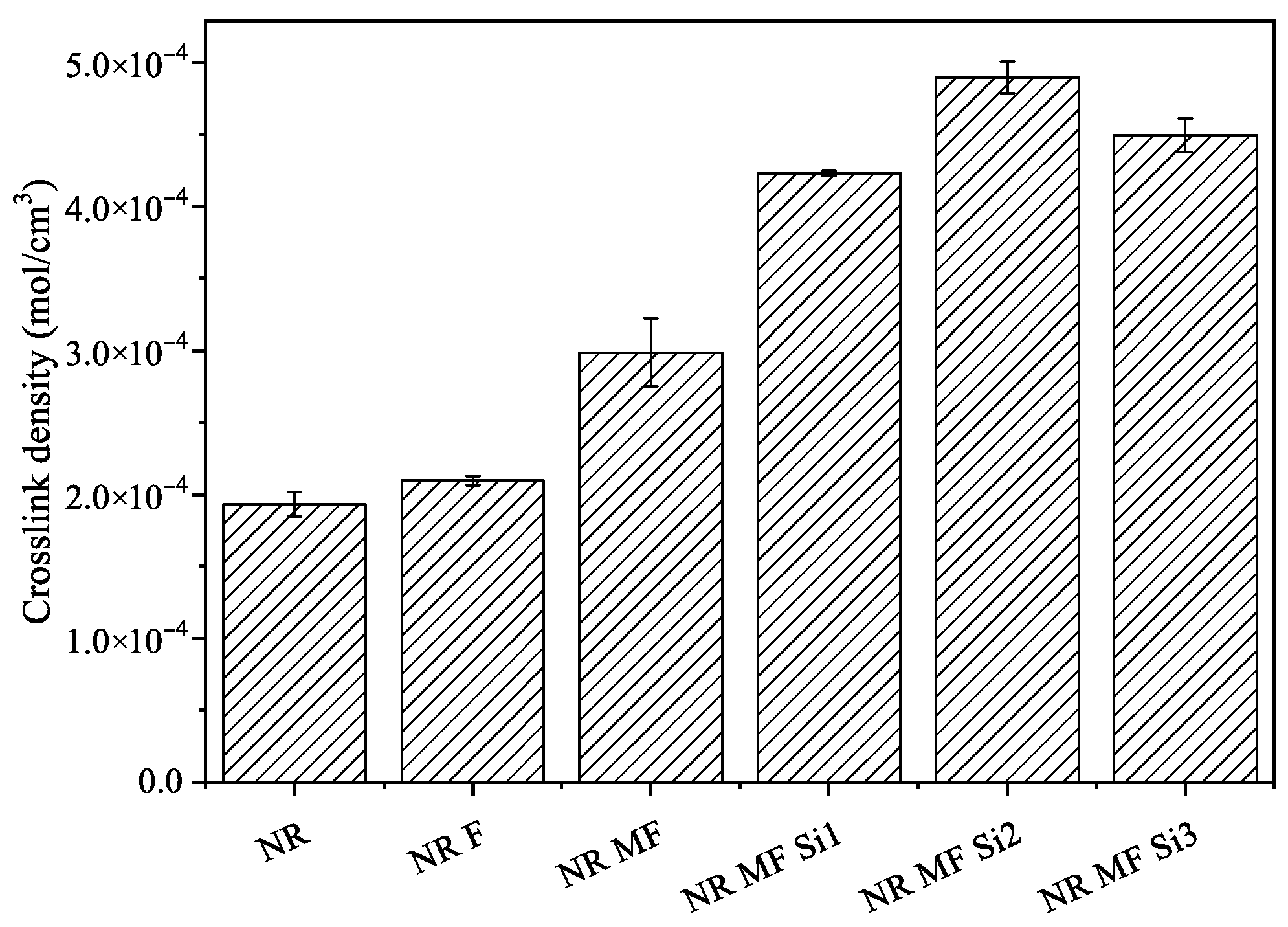

| Compound Codes | ||||||
|---|---|---|---|---|---|---|
| Ingredient | NR | NR F | NR MF | NR MF Si1 | NR MF Si2 | NR MF Si3 |
| NR | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| NTRFs | 0.0 | 15.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| LCMFs | 0.0 | 0.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Si69 | 0.0 | 0.0 | 0.0 | 1.5 | 2.5 | 3.5 |
| Stearic acid | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| IPPD | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| CBS | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| ZnO | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Sulfur | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Cellulose (%) | Hemicellulose (%) | Lignin (%) | Carboxilic Acids (%) | Ash (%) | Extractives (%) |
|---|---|---|---|---|---|
| 40.0 | 9.9 | 22.6 | 1.1 | 16.4 | 7.2 |
| Fiber Type | Average Length (µm) | Average Width (µm) | Intrinsic Viscosity (mL/g) |
|---|---|---|---|
| NTRFs | 142.6 ± 7.3 | 11.5–32.0 | 226 ± 2 |
| LCMFs | 11.1 ± 0.8 | 0.049 ± 0.008 | 638 ± 1 |
| Compound | Tensile Strength at Rupture (MPa) * | Elongation at Break (%) * | Modulus at 300% (MPa) * | Tear Strength (N/mm) * |
|---|---|---|---|---|
| NR | 13.46 ± 0.56 a | 829.24 ± 18.05 a | 2.25 ± 0.05 a | 31.37 ± 2.45 a |
| NR F | 14.67 ± 0.26 a | 793.12 ± 14.35 a | 3.71 ± 0.09 b | 34.19 ± 1.5 a |
| NR MF | 16.56 ± 0.40 b | 601.96 ± 8.70 b | 7.12 ± 0.09 c | 48.20 ± 3.12 b |
| NR MF Si1 | 14.44 ± 0.82 a | 451.85 ± 16.17 c | 9.32 ± 0.19 d | 63.07 ± 4.65 c |
| NR MF Si2 | 11.96 ± 0.46 c | 331.00 ± 18.15 d | 10.97 ± 0.16 e | 60.81 ± 5.02 c |
| NR MF Si3 | 13.65 ± 1.51 a, c | 383.83 ± 25.34 e | 10.99 ± 0.82 e | 61.61 ± 3.91 c |
| Compounds | Hardness (Shore A) * | Volume Loss by Abrasion (mm3) * |
|---|---|---|
| NR | 42.1 ± 0.3 a | 230.31 ± 5.81 a |
| NR F | 50.4 ± 1.3 b | 225.36 ± 7.03 a |
| NR MF | 52.4 ± 0.1 c | 222.40 ± 6.35 a |
| NR MF Si1 | 58.2 ± 1.0 d | 205.75 ± 1.52 b |
| NR MF Si2 | 55.2 ± 0.5 e | 191.97 ± 1.09 c |
| NR MF Si3 | 56.4 ± 0.5 e | 177.89 ± 3.64 d |
| Compound | Temperature (°C) | Mass Loss (%) | ||||
|---|---|---|---|---|---|---|
| T5% | T10% | Tonset | Tpeak | Tend | ||
| NR | 283 | 338 | 183 | 379 | 476 | 93.9 |
| NR F | 281 | 317 | 183 | 376 | 476 | 89.6 |
| NR MF | 269 | 312 | 183 | 379 | 476 | 90.5 |
| NR MF Si1 | 269 | 312 | 183 | 373 | 465 | 85.4 |
| NR MF Si2 | 275 | 315 | 183 | 379 | 476 | 95.6 |
| NR MF Si3 | 281 | 317 | 183 | 380 | 476 | 91.2 |
| Compound | ts2 (min) | t90 (min) | ML (dN.m) | MH (dN.m) |
|---|---|---|---|---|
| NR | 3.67 | 8.08 | 0.04 | 6.93 |
| NR F | 2.75 | 5.88 | 0.09 | 6.67 |
| NR MF | 3.20 | 7.37 | 0.13 | 8.81 |
| NR MF Si 1 | 4.02 | 8.13 | 0.09 | 7.66 |
| NR MF Si 2 | 3.60 | 8.56 | 0.16 | 8.86 |
| NR MF Si 3 | 3.31 | 8.61 | 0.11 | 8.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaño-Rivera, P.; Soto-Arriagada, A.; Ortega, E.T.; Galvez-Garrido, K.; Cabrera-Barjas, G.; Aguilar-Bolados, H.; Castaño, J.; Pereira, M.Á. In Situ Silanization of Ligno-Cellulosic Microfibers Derived from Industrial Waste to Enhance Mechanical Properties of Natural Rubber Compounds. Polysaccharides 2025, 6, 70. https://doi.org/10.3390/polysaccharides6030070
Castaño-Rivera P, Soto-Arriagada A, Ortega ET, Galvez-Garrido K, Cabrera-Barjas G, Aguilar-Bolados H, Castaño J, Pereira MÁ. In Situ Silanization of Ligno-Cellulosic Microfibers Derived from Industrial Waste to Enhance Mechanical Properties of Natural Rubber Compounds. Polysaccharides. 2025; 6(3):70. https://doi.org/10.3390/polysaccharides6030070
Chicago/Turabian StyleCastaño-Rivera, Patricia, Alexandra Soto-Arriagada, Eduardo Troncoso Ortega, Karen Galvez-Garrido, Gustavo Cabrera-Barjas, Héctor Aguilar-Bolados, Johanna Castaño, and Miguel Ángel Pereira. 2025. "In Situ Silanization of Ligno-Cellulosic Microfibers Derived from Industrial Waste to Enhance Mechanical Properties of Natural Rubber Compounds" Polysaccharides 6, no. 3: 70. https://doi.org/10.3390/polysaccharides6030070
APA StyleCastaño-Rivera, P., Soto-Arriagada, A., Ortega, E. T., Galvez-Garrido, K., Cabrera-Barjas, G., Aguilar-Bolados, H., Castaño, J., & Pereira, M. Á. (2025). In Situ Silanization of Ligno-Cellulosic Microfibers Derived from Industrial Waste to Enhance Mechanical Properties of Natural Rubber Compounds. Polysaccharides, 6(3), 70. https://doi.org/10.3390/polysaccharides6030070








