Postharvest Quality of Plums Treated with Chitosan-Based Edible Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing the Coated Sample Series
2.2. Determination of the Physical Quality
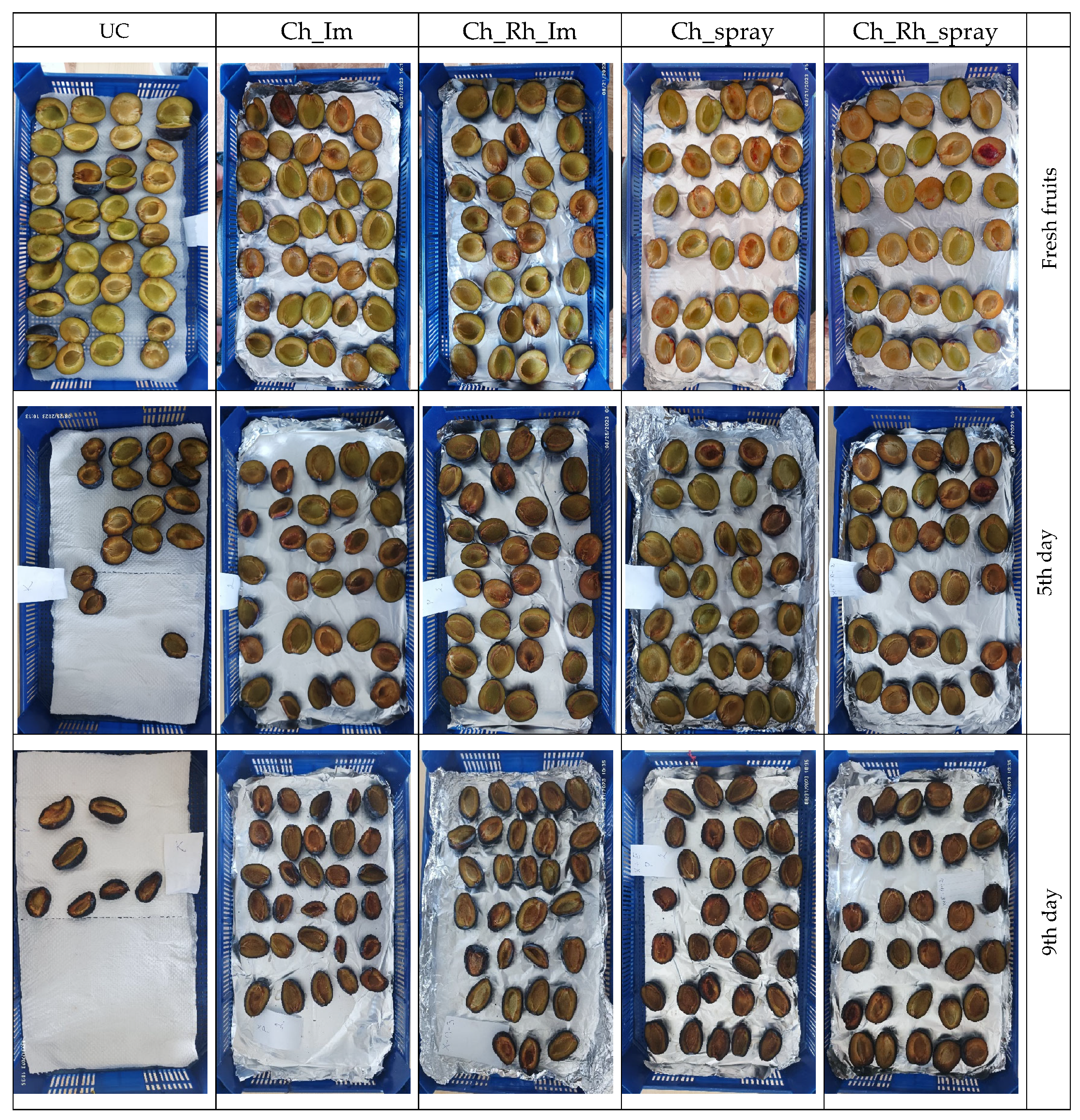
2.2.1. Control of the Visual Disorders
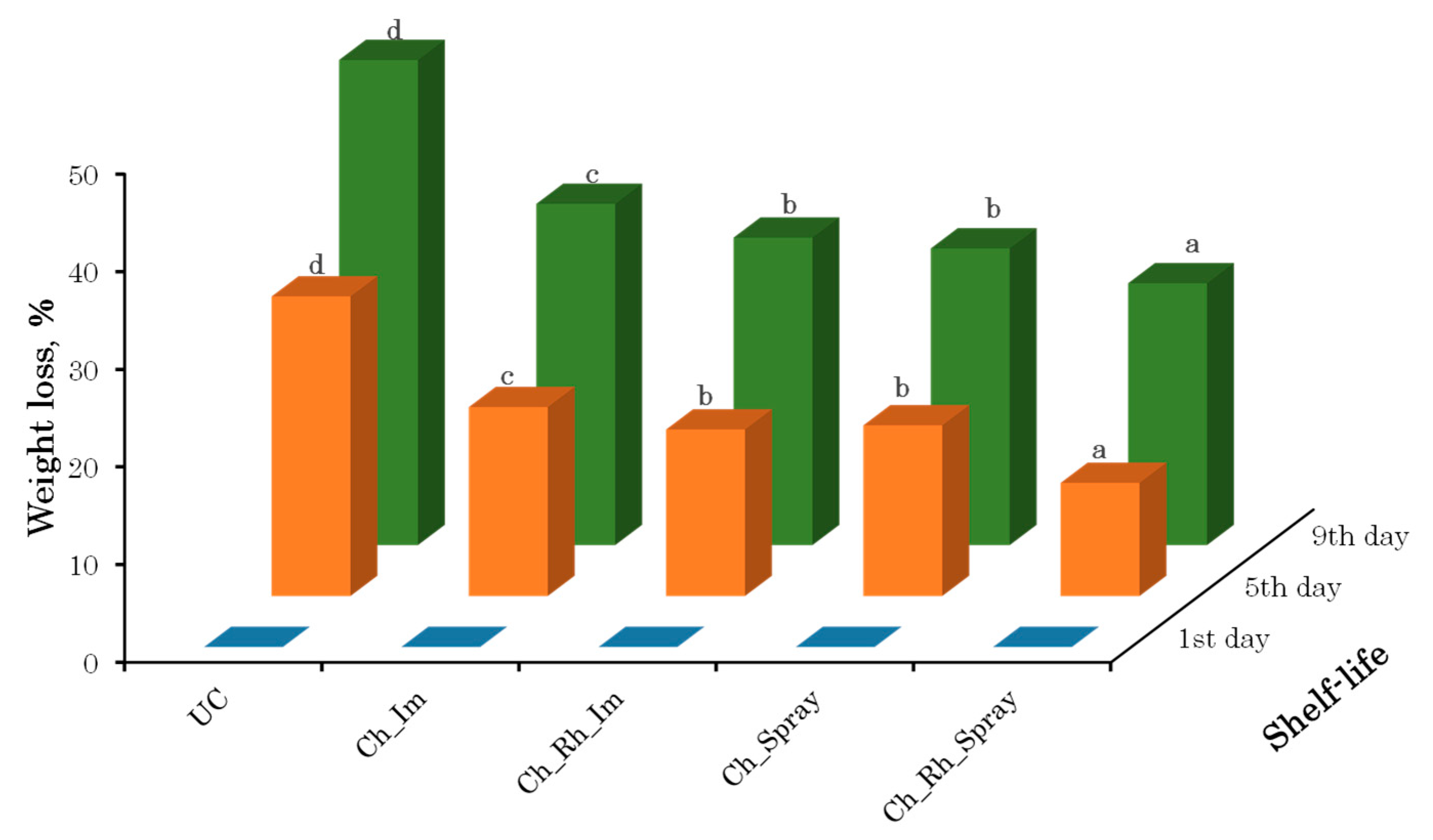
2.2.2. Physiological Weight Loss
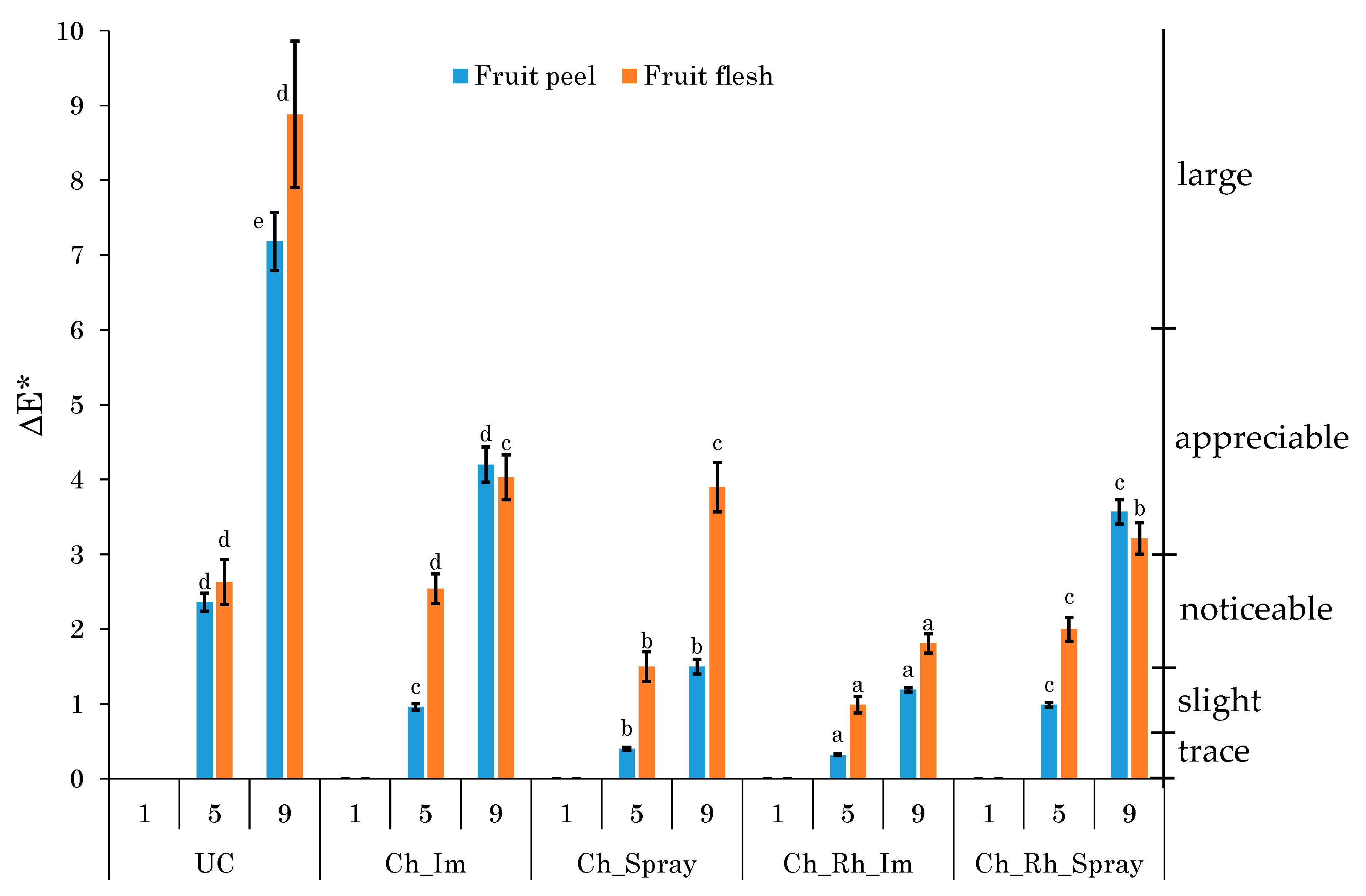
2.2.3. Surface Color Evaluation
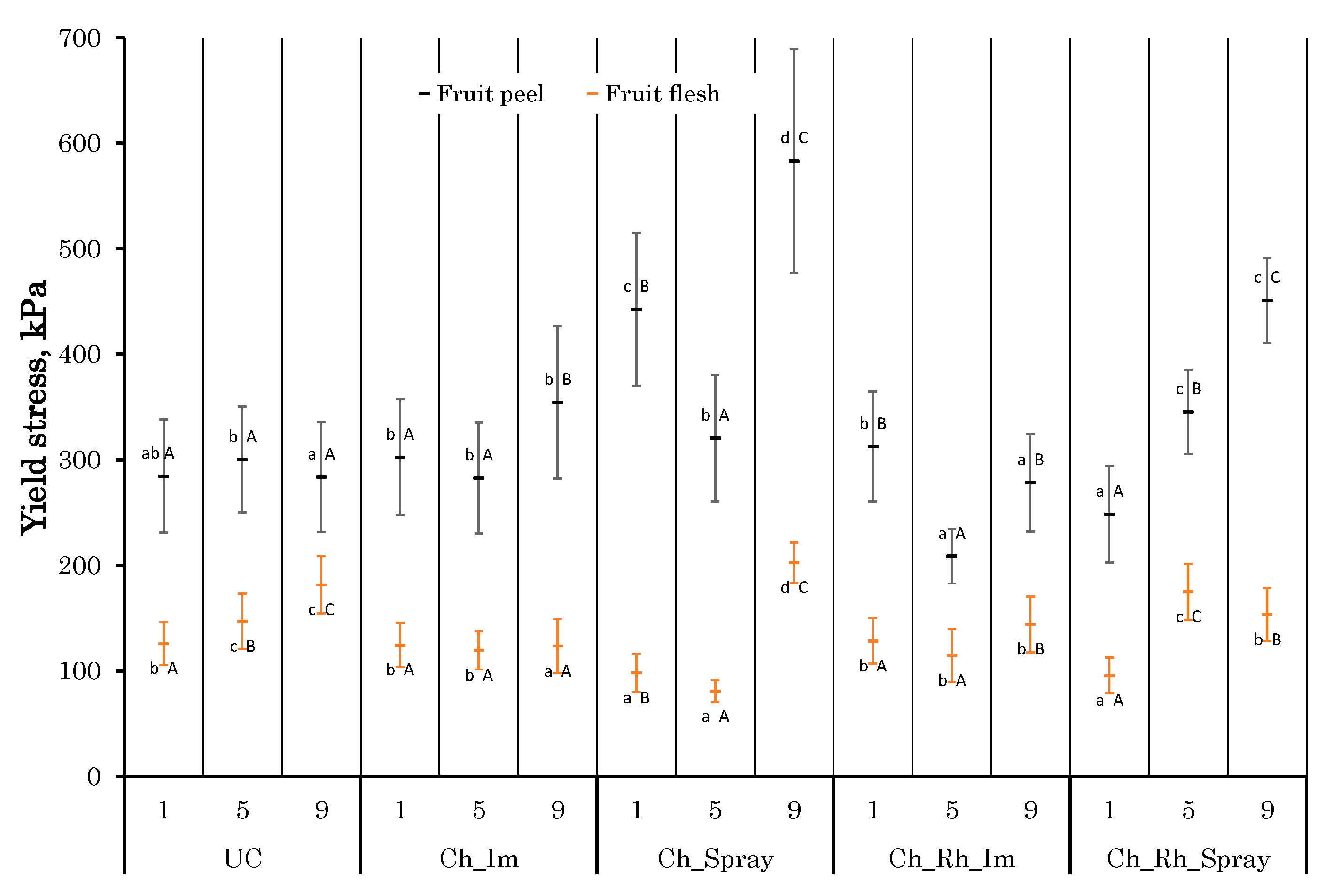
2.2.4. Texture Parameters
2.3. Analysis of the Antioxidant Activity
2.4. Safety Parameters
2.5. Sensory Analyses
2.6. Statistical Analysis of the Data
3. Results and Discussion
3.1. Visual Changes During the Storage
3.2. Weight Loss During Storage
3.3. Changes in the Color Parameters
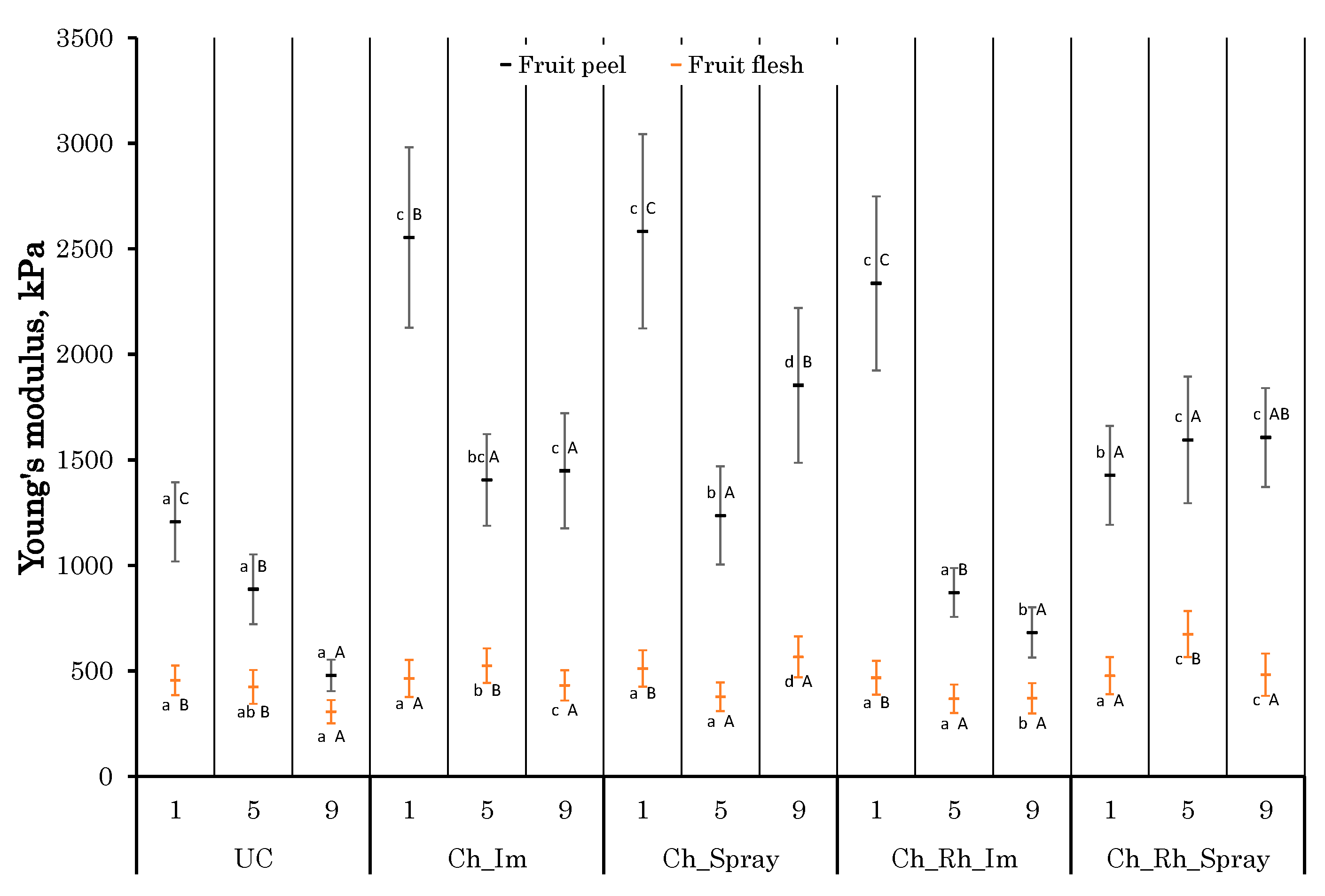
3.4. Changes in the Texture Parameters
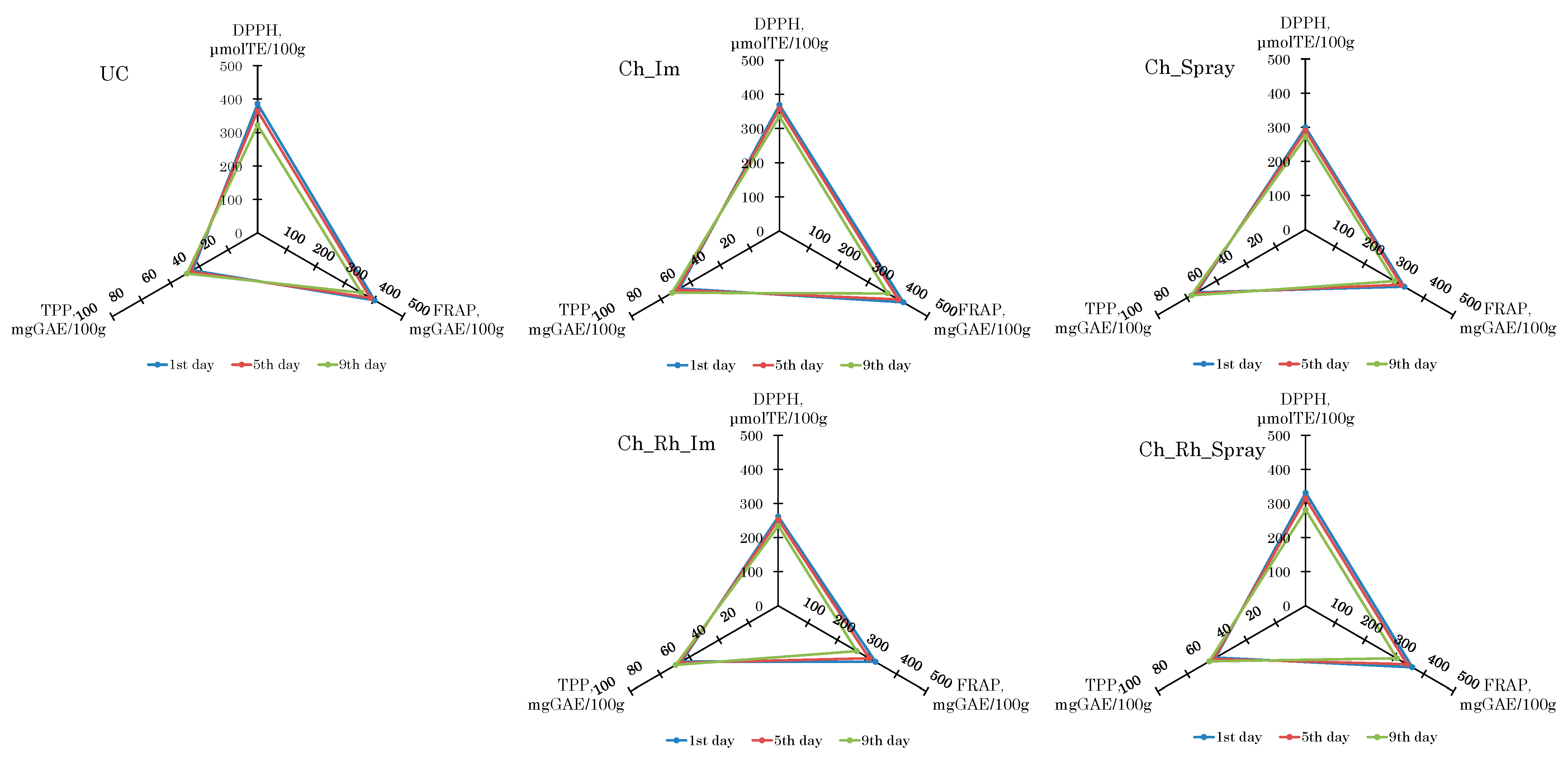
3.5. Changes in the Antioxidant Activity
3.6. Changes in the Safety Parameters
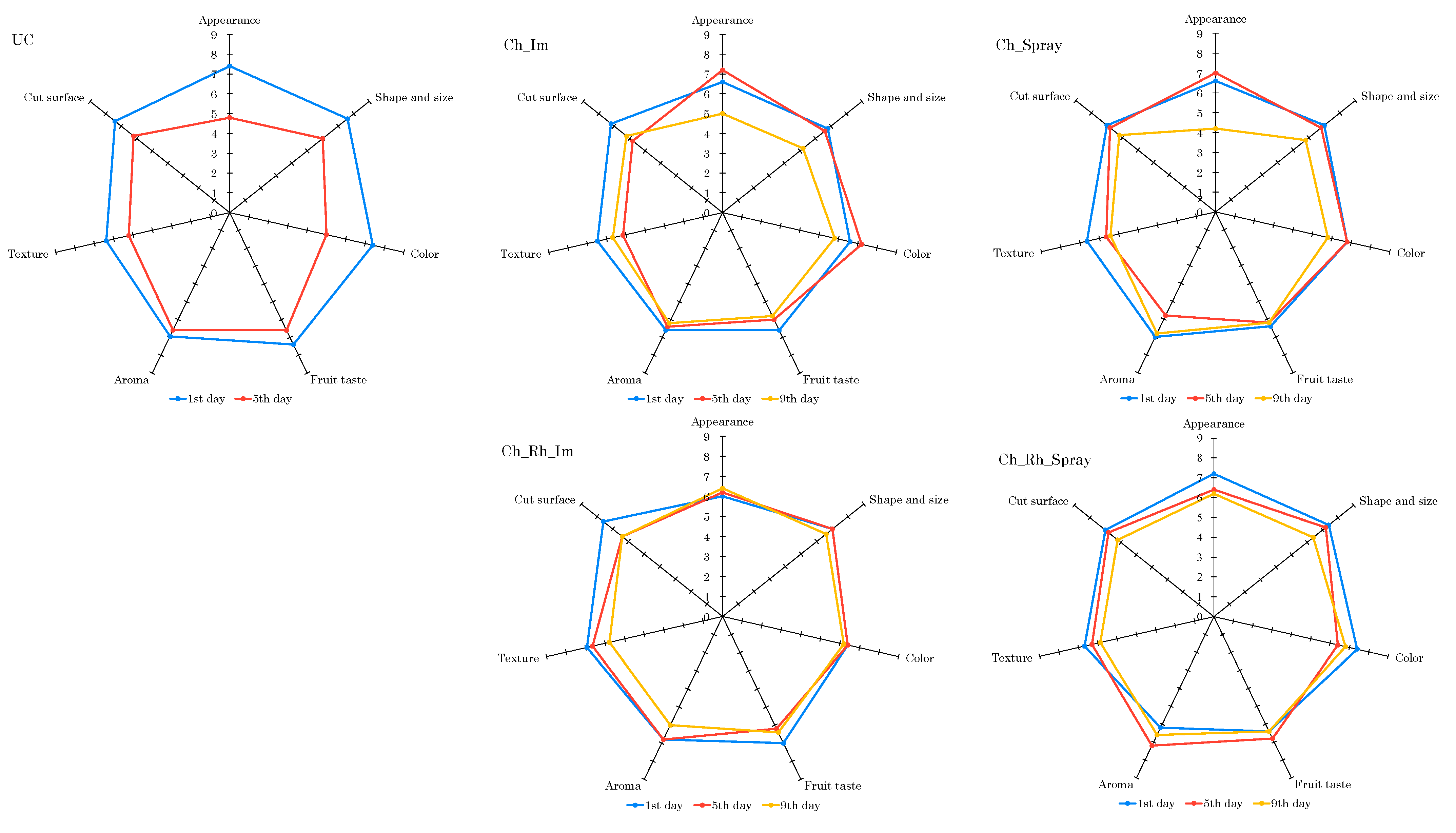
3.7. Sensory Analysis of the Samples with Different Treatment Types
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UC | Uncoated samples |
| Ch | Samples coated with chitosan solution |
| Ch_Im | Samples coated with chitosan solution by immersion method |
| Ch_Rh | Samples coated with chitosan and rosehip oil emulsion |
| Ch_Rh_Im | Samples coated with chitosan and rosehip oil emulsion by immersion method |
| Ch_spray | Samples coated with chitosan solution by spraying method |
| Ch_Rh_Spray | Samples coated with chitosan and rosehip oil emulsion by spraying method |
References
- Romanazzi, G.; Moumni, M. Chitosan and other edible coatings to extend shelf life, manage postharvest decay, and reduce loss and waste of fresh fruits and vegetables. Curr. Opin. Biotechnol. 2022, 78, 102834. [Google Scholar] [CrossRef]
- Andriani, V.; Handayani, N.A. Recent technology of edible coating production: A review. Mater. Today Proc. 2023, 87, 200–206. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Bhowmik, S.; Agyei, D.; Ali, A. Bioactive chitosan and essential oils in sustainable active food packaging: Recent trends, mechanisms, and applications. Food Packag. Shelf Life 2022, 34, 100962. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Sivertsvik, M.; Rosnes, J.T.; Popa, E.E.; Dumitriu, R.P.; Marincaș, O.; Matei, E.; Predescu, C.; Vasile, C. PLA-based materials containing bio-plasticizers and chitosan modified with rosehip seed oil for ecological packaging. Polymers 2021, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. An extensive review of natural polymers used as coatings for postharvest shelf-life extension: Trends and challenges. Polymers 2021, 13, 3271. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of edible coating in extension of fruit shelf life. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Pirozzi, A.; Ferrari, G.; Donsì, F. The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables. Coatings 2021, 11, 990. [Google Scholar] [CrossRef]
- Mushtaq, A.; Wani, S.M.; Malik, A.R.; Gull, A.; Ramniwas, S.; Nayik, G.A.; Ercisli, S.; Marc, R.A.; Ullah, R.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Marudova, M.; Zsivanovits, G.; Viraneva, A.; Gechev, B.; Rusinova-Videva, S. Rosehip Seed Oil-Incorporated Chitosan Films for Potential Fruit Packaging Applications. Appl. Sci. 2024, 14, 7669. [Google Scholar] [CrossRef]
- Bozhkova, V. Chemical composition and sensory evaluation of plum fruits. Trak. Univ. J. Nat. Sci. 2014, 15, 31–35. Available online: https://dergipark.org.tr/en/pub/trkjnat/issue/25382/267874 (accessed on 3 July 2025).
- Iliev, A.; Zsivanovits, G.; Parzhanova, A.; Nenov, N.; Stamenova, E. Comparision Between Subcritical and Supercritical Freon Extraction of Components for Edible Coatings. In Proceedings of the Scientific Technical Conference “Packaging-Tendencies in Development and Application, Plovdiv, Bulgaria; 2022; pp. 22–27. Available online: https://www.researchgate.net/publication/381032886_COMPARISION_BETWEEN_SUBCRITICAL_AND_SUPERCRITICAL_FREON_EXTRACTION_OF_COMPONENTS_FOR_EDIBLE_COATINGS (accessed on 3 July 2025), ISSN 2603-4743 (In Bulgarian).
- Bravi, M.; Spinoglio, F.; Verdone, N.; Adami, M.; Aliboni, A.; Andrea, A.D.; De Santis, A.; Ferri, D. Improving the extraction of α-tocopherol-enriched oil from grape seeds by supercritical CO2. Optimisation of the extraction conditions. J. Food Eng. 2007, 78, 488–493. [Google Scholar] [CrossRef]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.P.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. J. Supercrit. Fluids 2007, 40, 218–226. [Google Scholar] [CrossRef]
- Aguayo, E.; Escalona, V.; Artés, F. Quality of fresh-cut tomato as affected by type of cut, packaging, temperature and storage time. Eur. Food Res. Technol. 2004, 219, 492–499. [Google Scholar] [CrossRef]
- Ke, D.; Rodriguez-Sinobas, L.; Kader, A.A. Physiology and prediction of fruit tolerance to low-oxygen atmospheres. J. Am. Soc. Hortic. Sci. 1991, 116, 253–260. [Google Scholar] [CrossRef]
- Talukder, M.A.H.; Mannaf, M.A.; Alam, M.I.K.; Salam, M.A.; Amin, M.M.U. Influence of Sowing Time, Plant Spacing and Picking Interval on the Growth and Yield of Okra. Pak. J. Biol. Sci. 2003, 6, 1205–1207. [Google Scholar] [CrossRef][Green Version]
- Radi, M.; Firouzi, E.; Akhavan, H.; Amiri, S. Effect of gelatin-based edible coatings incorporated with Aloe vera and black and green tea extracts on the shelf life of fresh-cut oranges. J. Food Qual. 2017, 2017, 9764650. [Google Scholar] [CrossRef]
- Goyeneche, R.; Agüero, M.V.; Roura, S.; Di Scala, K. Application of citric acid and mild heat shock to minimally processed sliced radish: Color evaluation. Postharvest Biol. Technol. 2014, 93, 106–113. [Google Scholar] [CrossRef]
- Cannata, C.; Rutigliano, C.A.C.; Restuccia, C.; Muratore, G.; Sabatino, L.; Geoffriau, E.; Leonardi, C.; Mauro, R.P. Effects of polysaccharide-based edible coatings on the shelf life of fresh-cut carrots with different pigmentations. J. Agric. Food Res. 2025, 20, 101765. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Terashima, M.; Nakatani, I.; Harima, A.; Nakamura, S.; Shiiba, M. New method to evaluate watersoluble antioxidant activity based on protein structural change. J. Agric. Food Chem. 2007, 55, 165–169. [Google Scholar] [CrossRef]
- Petrova, K.; Ivanova, P.; Mihalev, K.; Georgiev, D. Study the influence of pre-treatment of blackberries on the content of polyphenols, anthocyanins and antioxidant capacity in the juices obtained from them. J. Mt. Agric. Balk. 2016, 19, 114–130. Available online: http://www.rimsa.eu/images/Perennial_Plants_vol_19-3_part_1_2016.pdf (accessed on 3 July 2025).
- BS EN ISO 4833-1:2013/Amd 1:2022; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique—Amendment 1: Clarification of Scope. Bulgarian Institute for Standardization, Sofia & International Organization for Standardization: Geneva, Switzerland, 2022.
- BS ISO 21527-2:2011; Part 2 Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0,95. Bulgarian Institute for Standardization, Sofia & International Organization for Standardization: Geneva, Switzerland, 2022.
- BS ISO 16649-2.2014; Part 2, Microbiology of food and animal feeding stuffs—Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli—Part 2: Colony-count technique at 44 degrees C using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. Bulgarian Institute for Standardization, Sofia & International Organization for Standardization: Geneva, Switzerland, 2024.
- BS ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. Bulgarian Institute for Standardization, Sofia & International Organization for Standardization: Geneva, Switzerland, 2021.
- 32. BS EN ISO 6579-1:2017/A1:2020; Part 1 Amendment 1. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.—Amendment 1 Broader Range of Incubation Temperatures, Amendment to the Status of Annex D, and Correction of the Composition of MSRV and SC. Bulgarian Institute for Standardization, Sofia & International Organization for Standardization: Geneva, Switzerland, 2020.
- Asgarian, Z.S.; Palou, L.; Souza, R.F.L.d.; Quintanilla, P.G.; Taberner, V.; Karimi, R.; Pérez-Gago, M.B. Hydroxypropyl methylcellulose and gum Arabic composite edible coatings amended with geraniol to control postharvest brown rot and maintain quality of cold-stored plums. Foods 2023, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.). LWT 2016, 70, 213–222. [Google Scholar] [CrossRef]
- Hussain, P.R.; Dar, M.A.; Wani, A.M. Impact of radiation processing on quality during storage and post-refrigeration decay of plum (Prunus domestica L.) cv. Santaroza. Radiat. Phys. Chem. 2013, 85, 234–242. [Google Scholar] [CrossRef]
- Basaglia, R.R.; Pizato, S.; Santiago, N.G.; de Almeida, M.M.M.; Pinedo, R.A.; Cortez-Vega, W.R. Effect of edible chitosan and cinnamon essential oil coatings on the shelf life of minimally processed pineapple (Smooth cayenne). Food Biosci. 2021, 41, 100966. [Google Scholar] [CrossRef]
- Thumula, P. Studies on Storage Behaviour of Tomatoes Coated with Chitosan-Lysozyme Films. Master’s Thesis, McGill University, Montreal, QC, Canada, 2006. Available online: https://escholarship.mcgill.ca/concern/theses/9g54xh92t (accessed on 3 July 2025).
- Kalita, S.; Kumar, S.; Mukherjee, A. Composite coatings containing silk protein, chitosan, and Aloe vera for prolonging postharvest shelf-life of ghost chilli (Capsicum chinense). Food Humanit. 2024, 2, 100290. [Google Scholar] [CrossRef]
- Pizato, S.; Cortez-Vega, W.R.; de Souza, J.T.A.; Prentice-Hernández, C.; Borges, C.D. Effects of Different Edible Coatings in Physical, Chemical and Microbiological Characteristics of Minimally Processed Peaches (Prunus persica L. Batsch). J. Food Saf. 2013, 33, 30–39. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Shafiei-Hematabad, Z.; Kennedy, J.F. Advancements in coating technologies: Unveiling the potential of chitosan for the preservation of fruits and vegetables. Int. J. Biol. Macromol 2023, 254, 127677. [Google Scholar] [CrossRef]
- Serrano, M.; Martinez-Romero, D.; Guillen, F.; Valero, D. Effects of exogenous putrescine on improving shelf life of four plum cultivars. Postharvest Biol. Technol. 2003, 30, 259–271. [Google Scholar] [CrossRef]
- Sabeva, P.; Zsivanovits, G.; Parzhanova, A.; Iserliyska, D.; Momchilova, M.; Zhelyazkov, S.; Tranenska, P.; Iliev, A. Effect of chitosan/plant oils edible coatings on minimally processed peach quality during storage. Bulg. Chem. Commun. 2024, 56, 100–105. [Google Scholar] [CrossRef]
- Zhelyazkov, S.; Zsivanovits, G.; Marudova-Zsivanovits, M. Investigation of edible coatings on the physical, chemical and microbiological characteristics of processed melons during storage. BIO Web Conf. 2023, 58, 01012. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Liu, R.; Liu, Q.; Xu, J.; Yao, J. Structure and properties of citric acid cross-linked chitosan/poly(vinyl alcohol) composite films for food packaging applications. Carbohydr. Polym. 2023, 312, 120842. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Severino, R.; Vu, K.D.; Donsì, F.; Salmieri, S.; Ferrari, G.; Lacroix, M. Antibacterial and physical effects of modified chitosan based-coating containing nanoemulsion of mandarin essential oil and three non-thermal treatments against Listeria innocua in green beans. Int. J. Food Microbiol. 2014, 191, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Campaniello, D.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Viability of Lactobacillus plantarum on fresh-cut chitosan and alginate-coated apple and melon pieces. Front. Microbiol. 2018, 9, 415039. [Google Scholar] [CrossRef]
- Shalan, A.M. Post-harvest applications by calcium chloride and ascorbic acid enhanced storage ability of peach fruits Cv. floridaprince. J. Plant Prod. 2020, 11, 179–188. [Google Scholar] [CrossRef]
- Alandes, L.; Hernando, I.; Quiles, A.; Pérez-Munuera, I.; Lluch, M.A. Cell wall stability of fresh-cut Fuji apples treated with calcium lactate. J. Food Sci. 2006, 71, S615–S620. [Google Scholar] [CrossRef]
- Kritzinger, I.; Theron, K.I.; Lötze, G.F.A.; Lötze, E. Peel water vapour permeance of Japanese plums as indicator of susceptibility to postharvest shriveling. Sci. Hortic. 2018, 242, 188–194. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Sothornvit, R.; Pérez-Gago, M.B. Effect of plasticizer type and amount on hydroxypropyl methylcellulose-beeswax edible film properties and postharvest quality of coated plums (Cv. Angeleno). J. Agric. Food Chem. 2008, 56, 9502–9509. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.; Rojas-Graü, M.A.; McClements, D.J.; Martín-Belloso, O. Edible nanoemulsions as carriers of active ingredients: A review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring physico-mechanical and antimicrobial /antioxidant properties of biopolymeric films by cinnamaldehydeloaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets. Food Hydrocoll. 2022, 124, 107249. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Bergin, S.; Katam, R.; Mahna, N. Improvement of Postharvest Quality of Plum (Prunus domestica L.) Using Polysaccharide-Based Edible Coatings. Plants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Singh, Z.; Ali, S. Postharvest Biology and Technology of Plum. In Postharvest Biology and Technology of Temperate Fruits; Springer: Cham, Switzerland, 2018; pp. 101–145. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Robledo, N.; López, L.; Bunger, A.; Tapia, C.; Abugoch, L. Effects of antimicrobial edible coating of thymol nanoemulsion/quinoa protein/chitosan on the safety, sensorial properties, and quality of refrigerated strawberries (Fragaria × ananassa) under commercial storage environment. Food Bioprocess Technol. 2018, 11, 1566–1574. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Zsivanovits, G.; Sabeva, P.; Iserliyska, D.; Zhelyazkov, S.; Parzhanova, A.; Nesheva, M. Enhancing postharvest quality of fresh-cut plums with chitosan-grape seed oil edible coatings. BIO Web Conf. 2023, 58, 01011. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Drevinskas, T.; Naujokaitytė, G.; Maruška, A.; Kaya, M.; Sargin, I.; Daubaras, R.; Česonienė, L. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydr. Polym. 2017, 173, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Nguyen, D.H.; Nguyen, H.V. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria × ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Elsayed, M.I.; Al-Qurashi, A.D.; Almasaudi, N.M.; Abo-Elyousr, K.A. Efficacy of essential oils against gray mold and effect on fruit quality during cold storage in table grapes. S. Afr. J. Bot. 2022, 146, 481–490. [Google Scholar] [CrossRef]
- Zhelyazkov, S.; Ivanova, P. Microbiological parameters during storage of minimally processed melons with and without edible coating. Bulg. Chem. Commun. 2021, 53, 91–98. [Google Scholar] [CrossRef]
- Won, J.S.; Lee, S.J.; Park, H.H.; Song, K.B.; Min, S.C. Edible coating using a chitosan-based colloid incorporating grapefruit seed extract for cherry tomato safety and preservation. J. Food Sci. 2018, 83, 138–146. [Google Scholar] [CrossRef] [PubMed]


| Storage Day | Coating Type | Total Number of Microorganisms | Number of Molds and Yeasts |
|---|---|---|---|
| 1st day | UC | 9.0 × 101 | 2.5 × 101 |
| Ch_Im | 1.0 × 101 | <10 | |
| Ch_Rh_Im | 6.5 × 101 | <10 | |
| Ch_spray | 1.0 × 101 | <10 | |
| Ch_Rh_Spray | 1.5 × 101 | <10 | |
| 5th day | UC | 1.2 × 103 | 1.2 × 102 |
| Ch_Im | 2.5 × 102 | 1.0 × 101 | |
| Ch_Rh_Im | 2.2 × 103 | 4.0 × 102 | |
| Ch_spray | 7.0 × 101 | <10 | |
| Ch_Rh_Spray | 6.0 × 101 | <10 | |
| 9th day | UC | 6.0 × 103 | 1.0 × 103 |
| Ch_Im | 9.0 × 103 | 1.2 × 102 | |
| Ch_Rh_Im | 4.7 × 103 | 8.5 × 102 | |
| Ch_spray | 1.0 × 103 | 1.0 × 101 | |
| Ch_Rh_Spray | 1.8 × 103 | 1.0 × 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsivanovits, G.; Zhelyazkov, S.; Sabeva, P. Postharvest Quality of Plums Treated with Chitosan-Based Edible Coatings. Polysaccharides 2025, 6, 68. https://doi.org/10.3390/polysaccharides6030068
Zsivanovits G, Zhelyazkov S, Sabeva P. Postharvest Quality of Plums Treated with Chitosan-Based Edible Coatings. Polysaccharides. 2025; 6(3):68. https://doi.org/10.3390/polysaccharides6030068
Chicago/Turabian StyleZsivanovits, Gabor, Stoil Zhelyazkov, and Petya Sabeva. 2025. "Postharvest Quality of Plums Treated with Chitosan-Based Edible Coatings" Polysaccharides 6, no. 3: 68. https://doi.org/10.3390/polysaccharides6030068
APA StyleZsivanovits, G., Zhelyazkov, S., & Sabeva, P. (2025). Postharvest Quality of Plums Treated with Chitosan-Based Edible Coatings. Polysaccharides, 6(3), 68. https://doi.org/10.3390/polysaccharides6030068






