Abstract
Starch particles (SPs) were extracted from underutilized wild yam (Dioscorea remotiflora) tubers using two methods: (1) acid hydrolysis (AH) alone and (2) acid hydrolysis assisted by ultrasound (AH-US). The SPs were chemically modified through esterification (using acetic anhydride [AA] and lauroyl chloride [LC]) and crosslinking (with citric acid [CA] and sodium hexametaphosphate [SHMP]). They were subsequently characterized by their yield, amylose content, and structural and physical properties. The yield of particles was 17.5–19.7%, and the residual amylose content was 2.8–3.2%. Particle sizes ranged from 0.46 to 0.55 µm, which exhibited mono-modal and bi-modal distributions for AH and AH-US treatments, respectively. Following chemical modification, yield notably increased, especially with substitution by LC (33.6–36.5%) and CA (32.6–38.7%). Modified SPs exhibited bi-modal particle distributions with micro- and nanoparticles and variable peak intensities depending on the chemical compound used. Unmodified SPs displayed irregular morphologies, showing disruptions (AH) or aggregation (AH-US). Chemical substitutions altered morphologies, leading to amorphous surfaces (CA: AH), clustering (LC), or fragmentation into smaller particles (SHMP) under AH-US treatment. FT-IR analysis indicated a decrease in hydroxyl groups’ peak area (A(-OH)), confirming the substitution of these groups in the starch structure. Crosslinking with CA resulted in the highest degree of substitution (AH: 0.43; AH-US: 0.44) and melting enthalpy (ΔHf: 343.0 J/g for AH-US), revealing stronger interactions between SPs from both methods. These findings demonstrate that the extraction treatment of D. remotiflora SPs and the type of chemical modifier significantly influence the properties of SPs, underscoring their potential applications as natural biocarriers.
1. Introduction
Naturally derived polysaccharides have earned significant attention due to their remarkable versatility and extensive applications in various fields. These materials, also known as biopolymers, are essential for their biocompatibility, biodegradability, and ability to form structured networks, usually referred to as biocarriers, capable of encapsulating and releasing bioactive molecules in a controlled manner [1]. Polysaccharides used for this purpose include cellulose derivatives, chitosan, alginate, pectin, gums, and starch [2,3]. These materials can be tailored to release active compounds at specific rates and sites within the body [4].
Although synthetic polymers are extensively used as carriers, biopolymers derived from starch have gained significance for many advantages, such as abundance, non-toxicity, biodegradability, and cost-effectiveness. Starch, abundant in cereals (rice, corn, wheat) and tubers (tapioca, potato, sweet potato, taro), is of particular interest in the food industry for its multiple applications as fat substitutes, thickening agents, stabilizers, or reinforcements of biodegradable food packaging to improve food nutritional value [5,6]. Moreover, starch derivatives have been extensively used for encapsulating bioactive compounds, ensuring their stability and targeted release, particularly in pharmaceutical and biomedical applications [7,8,9,10]. Its capacity to form biodegradable films and nanoparticles positions starch as an excellent vehicle or biocarrier for controlled drug delivery and bioactive compounds [11,12,13,14,15].
Under specific physical and chemical procedures, starch derivatives may result in small-sized molecules capable of maintaining their stability, absorption, and bioavailability at specific delivery sites [7,11,16,17]. Further, their hydrophilic nature enables efficient interaction with biological environments, making them suitable for use in controlled drug-release systems. As drug delivery systems, potato starch NPs cross-linked with epichlorohydrin have been developed for the controlled release of insulin [18]. Likewise, hollow nanoparticles (HNPs) of chickpea starch have been used to encapsulate and release doxorubicin hydrochloride (DOX HCl), a chemotherapeutic agent [10]. Another approach includes the use of acetylated starch nanocrystals obtained from broken rice, also evaluated for their DOX loading and release capacity [19]. Furthermore, banana starch nanoparticles have been developed as vehicles for curcumin, a compound with anti-inflammatory and antioxidant properties [20], while acetylated corn starch nanoparticles have been characterized as carriers of ciprofloxacin, a broad-spectrum antibiotic [14]. These systems represent a promising alternative for the targeted and efficient delivery of drugs in various therapeutic applications.
Starch granules possess variable sizes depending on the botanical source [rice, 6 μm; maize, 35 μm; wheat, 14 μm; potato, 100 μm] [21], but particles larger than 1 µm are known to be too large to diffuse across epithelial membranes passively; hence, they remain at the administration site [7]. Therefore, the use of micromaterials could aid in increasing diffusion. For this purpose, starch treated under acid hydrolysis, nanoprecipitation, sonication, gamma irradiation, or microfluidization [11,17,22,23] causes the breakdown of starch chains into smaller particles.
Furthermore, starch microparticles are modified using chemical modification for a more effective target as carriers of bioactive molecules [8]. Chemical modification is carried out by chemical reactions with small molecules and the polymerization of monomers on the microparticle surface through esterification reactions with anhydrides, acyl chlorides, or phosphorylating using organic and inorganic acids, sodium trimetaphosphate, among others [16,24,25]. However, depending on the chemical molecule, the formation of specific types of bonds will determine the functionality and, consequently, the applications of the particles. For instance, anhydride-modified starch microparticles exhibit lower surface energy, enhanced solubility in organic solvents, and more stable drug-release rates than native starch microparticles [19,24]. With citric acid, the modification creates intermolecular bonds to form networks for more stable molecules [26,27], and these acquire a hydrophobic character with greater affinity for solvents of low polarity [28]. While modification with phosphate groups exerts an effect on the solubility and morphology of starch [29], phosphorylated starches are useful as a drug-delivery system and for other technological applications [30].
In this context, alternative and less-exploited starch sources, such as wild yam or “camote de cerro” (Dioscorea remotiflora) tubers, native to the western regions of Mexico, could serve as promising resources for starch extraction. This tuber species is composed of 85% d.b. carbohydrates [31], 11% proteins, and vitamins such as thiamine, riboflavin, niacin, and ascorbic acid [32]. Notably, starch yield reaches up to 21.8% [33], exceeding other species like D. esculenta or D. alata, which yield 19.6% and 11.5%, respectively [31]. It is worth mentioning that D. remotiflora tubers are mainly consumed by the local population as boiled snacks and are scarcely used for industrial purposes, positioning them as an excellent botanical source of starch without impacting staple crops like wheat or corn.
To the best of our knowledge, there is limited information on the chemical modification of starch from wild yam (D. remotiflora); therefore, this research aimed to produce particles from this novel starch source using acid hydrolysis alone or in combination with ultrasound. The study evaluated the effects of esterification and crosslinking with various chemical reagents (acetic anhydride, lauroyl chloride, citric acid, and sodium hexametaphosphate) on the yield, residual amylose, particle size distribution, morphology, functional groups, XRD patterns, and thermal properties of the starch particles as potential carriers of bioactive molecules.
2. Materials and Methods
2.1. Materials and Reagents
Tubers of D. remotiflora were purchased at a local market in Guadalajara, Jalisco, Mexico. Anhydrous citric acid (AC) (C6H7O7; Golden Bell, Mexico City, Mexico), sulfuric acid, (H2SO4, 98%; Golden Bell, Mexico City, Mexico), sodium hydroxide (NaOH, 99%; Golden Bell, Mexico City, Mexico), deionized water (H2O; Meyer, Mexico City, Mexico), acetic anhydride ((CH3CO)2O; Merck, Mexico City, Mexico), hydrochloric acid (HCl, 38%; Golden Bell, Mexico City, Mexico), ethanol (EtOH, 95°; Golden Bell, Mexico City, Mexico), acetic acid (CH3COOH, 99%; Golden Bell, Mexico City, Mexico), potassium iodide (KI, 99%; Sigma Aldrich, St. Louis, MO, USA), iodine (I2, 99.8%; Sigma Aldrich, St. Louis, MO, USA), lauroyl chloride (LC) (CH3(CH2)10COCl, 98%; Sigma Aldrich, St. Louis, MO, USA), 4-dimethylaminopyridine (DMAP) (C7H10N2, 99%; Sigma Aldrich, St. Louis, MO, USA), triethylamine (TEA) (C6H15N, >99.5%; Sigma Aldrich, St. Louis, MO, USA), methanol (CH3OH, 99%; Golden Bell, Mexico City, Mexico), sodium hexametaphosphate (SHMP) ((NaPO3)6, 95%; Meyer, Mexico City, Mexico), sodium chloride (NaCl), 95%; Golden Bell, Mexico City, Mexico), and distilled water from Selectrum (Guadalajara, Mexico) were used.
2.2. Starch Extraction
Tubers (D. remotiflora) were washed to remove dirtiness and peeled with a knife for starch extraction. Following the procedure reported by Estrada-Girón et al. [33] with some modifications, the tubers, cut into slices of approximately 1 cm thick, were soaked in a 1.5% (w/v) citric acid solution for 48 h under refrigeration for complete mucilage removal. The slices were ground with purified water at a 1:1.5 ratio in a food processor (Moulinex, Mod. LM0400C6, México City, Mexico), with 5 s pulses, to a coarse size. This paste was maintained at 4 °C for 24 h, after which it was filtered through plastic cloth strainers to remove the fiber; then, the suspension was transferred into several containers and allowed to stand at 4 °C for several hours. Most of the supernatant liquid was decanted off, and the starch suspension was washed several times until a starch paste was obtained. To recover the starch, the wet material was centrifuged at 2000 rpm for 10 min in a Premiere XC-2450 centrifuge (Premiere, Shanghai, China). Subsequently, it was dried in an air oven (FRELAB Mod. ITAM-45, Guadalajara, Jal, Mexico) at 40 °C for 24 h and ground in a blade mill until a fine powder was obtained, which was sieved through U.S. 100 mesh (Daigger Scientific, Vernon Hills, IL, USA) with a mesh size of 149 µm in diameter and stored in resealable polystyrene bags for later use.
2.3. Acid Hydrolysis (AH) and Acid Hydrolysis-Ultrasound (AH-US) Treatments
Starch microparticles (SPs) were obtained using the method reported by Mukurumbira et al. [34]. For AH treatment, 20 g of starch was added with 140 mL of 3.16 M sulfuric acid. The suspension was stirred at 100 rpm and 40 °C for 5 days. Subsequently, the hydrolyzed material was neutralized with a solution of NaOH 1 M until neutral pH. Then, it was centrifuged at 10,000 rpm for 10 min and washed several times with distilled water to remove the resulting sodium sulfate. The precipitated SPs were redispersed in distilled water using a homogenizer (Ultra Turrax, IKA, Wilmington, NC, USA) at 9000 rpm for 2 min to break up the aggregates.
The same procedure was applied to hydrolyze and neutralize the starch suspension for AH-US treatment. Then, the redispersed precipitate was placed in an ultrasonic bath (M1800; Branson, CT, USA) for 20 min [35]. Finally, SPs from both methods were deep-frozen, lyophilized (IlTFD 5503, Ilshin, Houston, TX, USA), ground, and sieved through a mesh size of 100 mm.
2.4. Chemical Modification of Starch
2.4.1. Acetic Anhydride
The acetylation reaction of the SPs was performed by dispersing 2 g of SPs with 20 mL of deionized water in an ultrasonic bath (M1800, Branson), with continuous stirring for 30 min at 25 °C to achieve a suspension [19]. Then, this was stirred for one hour at 25 °C, and the pH was adjusted to 8.0 with a 3% (w/v) NaOH solution; subsequently, AA (9% w/v) was added dropwise, and as the reaction proceeded, the pH was maintained between 8.0 and 8.5 with a 3% (w/v) NaOH solution. The reaction was prolonged for 30 min after the AA addition was complete, and the pH was adjusted to 6.5 with a solution of 0.5 M HCL. The unreacted anhydride was removed by washing the precipitate thoroughly with ethanol and distilled water several times. The modified SPs were dried in an oven at 40 °C until reaching a constant weight.
2.4.2. Lauroyl Chloride
The esterification reaction was carried out in two stages [36]. In the first stage, 2 g of SPs were added to a solution of DMAP (10 mL, 0.25 M) at 25 °C and stirred for one hour. Then, 1.5 mL of TEA was added as a catalyst under constant stirring for 10 min to obtain a gel. In the second stage, 3 mL of LC was added to the gel, with continuous stirring at 300 rpm for 20 min at 25 °C until the formation of a precipitate. The precipitate sample was extracted with methanol in a Soxhlet-type extractor to remove non-esterified LC for 24 h. Esterified SPs were washed thoroughly with distilled water and ethanol several times and dried in an oven at 40 °C until constant weight.
2.4.3. Citric Acid
The crosslinking reaction with CA was carried out with samples of 2 g of SPs dissolved in 20 mL of an ethanol CA solution (3 g of CA in 30 mL of ethanol) on Petri dishes, which were conditioned for 12 h at 25 °C to allow the complete absorption of CA by the SPs [28]. The Petri dishes were dried in an oven at 50 °C to remove the ethanol; then, to end the reaction, the samples were heated in a Benchmark hotplate (Benchmark Scientific, Sayreville, NJ, USA) at 130 °C for 6 h. The modified SPs were washed three times with distilled water and once with ethanol to remove unreacted CA. The SPs were dried in an oven at 40 °C until constant weight.
2.4.4. Sodium Hexametaphosphate
For crosslinking with SHMP, 2 g of SPs were dispersed in 200 mL of deionized water with the aid of the ultrasonic bath at 690 W, with continuous stirring for 30 min at 40 °C [37]. Subsequently, 2 g of NaCl and 8% (w/w) of SHMP were added; the pH of the suspension was adjusted to 10 with a 0.1 M NaOH solution, and this was left to react for 4 h under continuous stirring at 40 °C. After the reaction was completed, the pH was adjusted to 6.8 with a 0.1 M HCl solution. To remove the unreacted SHMP, the suspension was washed five times with distilled water and dried in an oven at 40 °C until constant weight.
2.5. SPs Yield, Amylose, and Native Starch Residual Moisture Content
The yield of the modified SPs was determined as follows [38]:
where mSPs(f), mSPs(Tot), and m[AA,LC,CA,SHMP] represent the weight of the SPs recovered after the chemical modification, the weight of total SPs added, and the weight of each chemical reagent used, respectively.
The amylose content was calculated for both treatments, as were the chemical modifications of the starch, according to the method reported by Estrada-Girón et al. [33]. A total of 0.1 g of SPs was placed into a volumetric flask of 100 mL, and a solution of 1 mL of EtOH and 9 mL of 1 N NaOH was added. The mixture was heated for 15 min and then cooled at 25 °C to adjust the volume to 100 mL with distilled water. Then, 2.5 mL of the solution and 25 mL of distilled water were transferred into volumetric flasks of 50 mL, and 0.5 mL of 1 M CH3COOH, and 1 mL of iodine solution composed of 0.2% of I2 and 2% of KI were added. Absorbance was read in an ultraviolet (UV)–visible UNICO Spectroquest 2800 spectrometer (UNICO, Dayton, NJ, USA) at a wavelength of 620 nm, and distilled water was used as a blank. The amylose content was determined based on a calibration curve at different amylose concentrations. The residual moisture content of the extracted dried native starch was measured according to AOAC Official Method 925.10 for Total Solids and Moisture in Flour [39]. Two grams of the extracted, dried native starch was placed on a weighed plate and heated in an oven at 130 ± 3 °C for 1 h. The covered plate was then transferred to a desiccator and weighed when the sample reached room temperature.
2.6. Characterization of Modified SPs
2.6.1. Scanning Electron Microscopy (SEM)
The morphology of the native starch and the SPs was observed using a TESCAN MIRA 3 LMU scanning electron microscope (TESCAN, Brünn, Czech Republic). The samples were covered with gold prior to imaging using a coating machine (SPi, West Chester, PA, USA). Then, these were placed in the equipment and observed at magnifications of 9000×, with an acceleration potential of 20 kV.
2.6.2. Dynamic Light Scattering (DLS)
The z-average particle sizes (Dz) and the particle-size distribution (PSD) of the SPs were determined by dynamic light scattering (DLS) utilizing a ZEN 3600 Nano Zetasizer Malvern equipment (ZEN 3600; Worcestershire, UK). The DLS, equipped with a standard operating procedure subroutine, automatically calculates PSD. The SPs were diluted in deionized water to a concentration of 0.1 mg/mL, which showed a good response to the DLS intensity. Subsequently, 1 mL of the diluted solution was transferred into a disposable polystyrene cuvette, which was then placed in the cell area on top of the instrument for measurement [22].
2.6.3. Fourier Transform Infrared Spectroscopy (FT-IR)-Attenuated Total Reflectance
The functional groups of the SPs were determined by a Fourier transform infrared spectrophotometer using a Bruker Corp-ALPHA spectrophotometer (Billerica, MA, USA) with attenuated total reflectance (ATR). The measurements were performed within a wavenumber range of 400–4000 cm−1 [40]. The powder samples were dried in a vacuum oven (Precision Scientific, Chicago, IL, USA) at 50 °C and 15 kg/cm2 for 24 h before testing; then, they were placed directly onto the sample holder for analysis. The areas under the curve for hydroxyl A(-OH) and carbonyl A(-C=O) groups were calculated from the area under the curve of the FTIR spectra. The degree of substitution (DS) was calculated from FTIR spectra.
2.6.4. X-Ray Diffraction Pattern (XDR)
The crystalline structure of the SPs was analyzed in a Siemens D500 Theta/2 Theta Bragg-Bretano X-ray diffractometer (Siemens, Munich, Germany). Diffraction patterns were obtained using a copper source at 40 Kv and 30 mA with a Kα radiation of 1.5406 Å. The diffraction angle was scanned from 4° to 40° (2ϴ) at a rate of 1 °C/min [38]. The powder samples were directly mounted on the specimen holder. The relative crystallinity index (RCI) was calculated as the ratio of the sum of the crystalline diffraction peak areas to the total diffraction area of the diffractograms [41].
2.6.5. Thermal Properties
Differential scanning calorimeter (DSC) measurements of the native starch and the SPs were examined using a PerkinElmer Pyris 6 (Norwalk, CT, USA) DSC. The instrument was calibrated with indium, and an empty pan was used as a reference. The Tg was taken at the midpoint temperature of the heat-capacity change of the thermal transition detected in the thermograms using the Pyris Instrument Managing Software version 7.0 (Norwalk, CT, USA). The powder samples were dried in an oven at 40 °C for 12 h prior to testing and then placed inside a desiccator to protect them from humidity [34]. Then, approximately 5.0 mg of the sample was transferred into a hermetically aluminum-sealed pan. The samples were scanned from 20 to 200 °C and held for 1 min before being cooled to 20 °C at a heating rate of 2 °C/min. A nitrogen purge gas flow of 50 mL/min was used [42]. The measurements were performed three times, with the average values reported.
2.7. Statistical Analysis
The analyses were performed in triplicate, and the results were expressed as mean ± standard deviation (SD). The data were subjected to analysis of variance (ANOVA) using PASW Statistics version 18 software (SPSS, Inc., 2009, Chicago, IL, USA) with a 95% confidence level (p < 0.05). To assess significant differences between treatments, Duncan’s multiple range test was applied with a 5% significance level (p < 0.05).
3. Results and Discussion
3.1. Yield and Amylose Content
The yield of SPs obtained by acid hydrolysis (AH) was 19.4%, slightly higher than that of ultrasound-assisted (AH-US) with 17.5% (Table 1). Statistically, the use of ultrasound had no significant effect on the yield of SPs; however, the slight differences between methods suggest that ultrasound may potentially degrade starch chains, depending on the intensity and duration [43]. Ultrasonic treatment of starch is known to cause molecular scission of starch chains and erosion of crystals within the granules, as starch is susceptible to ultrasonic cavitation [44], influencing yield. The formation of bubbles during cavitation generates localized high temperatures and pressures, which promote the cleavage of glycosidic bonds, particularly the α-1,4 linkages, which are more susceptible than α-1,6 bonds [45]. This degradation is attributed to both physical and chemical effects, including mechanical shear forces from bubble collapse and the generation of free radicals, which further contribute to the breakdown of starch structure. Although moderate ultrasound intensity may result in limited depolymerization, increasing both the intensity and exposure time can enhance the extent of molecular scission. Previous studies have shown that starch is highly susceptible to ultrasonic cavitation, which can lead to chain degradation and structural alterations within granules, ultimately influencing functional properties [44] (Appendix A).

Table 1.
Yield and residual amylose of D. remotiflora starch particles.
Although few investigations report the yield of micro- or nanoparticles, botanical sources such as waxy rice starch treated with ultrasound or amaranth by acid hydrolysis yield approximately 10% [38,46], which is lower than that obtained in this study. This could be related to the size of D. remotiflora starch granules that reach up to 35 µm, as is further shown in the SEM images below. Furthermore, the studied starch source is typically regarded by local consumers as a hard-cooking yam. In contrast, other Dioscorea varieties have been reported to exhibit greater resistance to shear stresses attributable to dipole-dipole interactions between the hydrogen bonds of starch constituents [33]. On the contrary, smaller granules, such as those found in rice or amaranth (1–3 µm), are potentially more susceptible to acidic conditions and tend to agglomerate, thereby affecting yield [46].
After modification, the yield significantly (p < 0.05) increased with all chemical compounds (Table 1), except for SPs obtained by AH-US and crosslinked with SHMP. Crosslinking with CA and esterification with LC resulted in the highest percentages of 38.7 and 36.5% for SPs from AH and AH-US treatments, respectively, compared to the other compounds. Limited information is reported about the yield of SPs; however, for citric acid, it is known that the application of heat in the first step of the reaction [28], followed by additional heating, favors the efficiency of crosslinking, thus increasing yield. Moreover, crosslinking with CA from SPs of AH treatment, the low area for hydroxyl groups (A(-OH)), and the high degree of substitution confirmed the efficiency of the reaction (Table 3). In contrast, the low yield (16.51%) of crosslinked SPs with SHMP (AH-US) was attributed to the agglomeration of particles, as also observed in the SEM images. This may have caused the alteration of surface binding sites in the amorphous material, limiting their interaction during the crosslinking reaction. Thus, it is important to optimize the reaction conditions for the production of SPs (pH, temperature, time, and reactant concentration) to achieve good yields of SPs; overall, the scaling up for industrial production.
Native starch from D. remotiflora tubers has been reported to contain about 48% amylose [33], like other types of tubers [47]. According to the literature, starches with amylose content >30% are classified as high amylose starches [48]. In this study, after applying AH and AH-US treatments to native starch, the amylose content of SPs was significantly reduced to 2.8–3.2% for the unmodified SPs (Table 1). With modification, the amylose content further decreased to 1.9–2.8% (Table 1). It has been documented that acid hydrolysis disrupts the amorphous starch lamellae, reducing amylose content, whereas crystalline lamella is more resistant to hydrolysis [49,50]. Nonetheless, both treatments, AH and AH-US, reduced the amylose content, indicating that SPs consist mainly of the crystalline regions of starch. This provides an advantage for developing starch matrices with more stable structures and improved accessibility to binding sites for chemical reactants.
3.2. Particle-Size Distribution
Figure 1 depicts the particle-size distribution (PSD) of SPs from AH (Figure 1a) and AH-US (Figure 1b) treatments and their chemical modifications. AH treatment resulted in SPs exhibiting a broad mono-modal distribution with a single intensity peak at a diameter (Dz) of 0.55 µm. Upon application of ultrasound (AH-US), the particle distribution transitioned to bi-modal, characterized by a minor peak at Dz = 0.09 μm and a predominant peak at Dz = 0.53 μm, with the latter displaying higher intensity and narrow amplitude. Consequently, the application of ultrasound resulted in a more uniform particle size distribution of SPs, increasing the intensity peak to 13%, compared to the 7% achieved by the AH treatment.
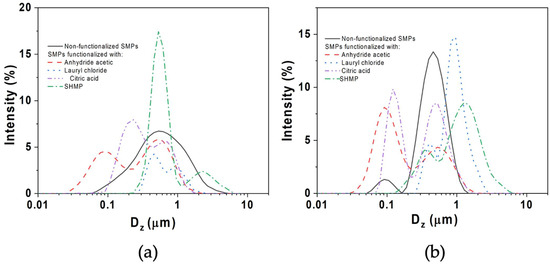
Figure 1.
Intensity of particle size distribution of unmodified and modified starch particles from D. remotiflora: (a) AH: acid hydrolysis and (b) AH-US: acid hydrolysis-ultrasound.
The Dz values obtained from the AH treatment were comparable to those reported for SPs derived from high amylose (47%) corn starch, which exhibited sizes of 50–100 nm. Whereas regular maize starch with an amylose content of 21.6% was reported with particle sizes ranging from 50 to 200 nm [51]. Therefore, the starch granules’ amylose content influences the resulting SPs’ Dz. This occurs because amylose modifies the morphology and crystallinity of starch nanoparticles, resulting in the formation of amylopectin blocks and retrograded crystalline amylose [51], which in turn influences particle size. In addition, other research has demonstrated that waxy maize starch hydrolyzed in aqueous H2SO4 solutions formed starch nanoparticles with sizes smaller than 0.2 µm. However, those nanoparticles exhibited a tendency to aggregate and sediment, resulting in the formation of microparticles exceeding 2 µm in size [35].
With modification, the type of chemical compound strongly influenced the particle distribution behavior. For instance, SHMP and LC exhibited the highest intensity peaks (close to or exceeding 15%) for AH and US-AH treatments, respectively (Table 2). The crosslinked SPs with SHMP displayed a narrow bi-modal distribution with Dz values of 0.53 ± 0.05 and 2.31 ± 0.03 µm. Notably, SMHP was the compound that produced a second peak with larger Dz values, even when ultrasound (AH-US) was used. This indicated the formation of SP aggregates, likely due to agglomeration, as confirmed in Figure 2e.

Table 2.
DLS average particle sizes (Dz) and polydispersity index (PDI) of D. remotiflora starch particles.
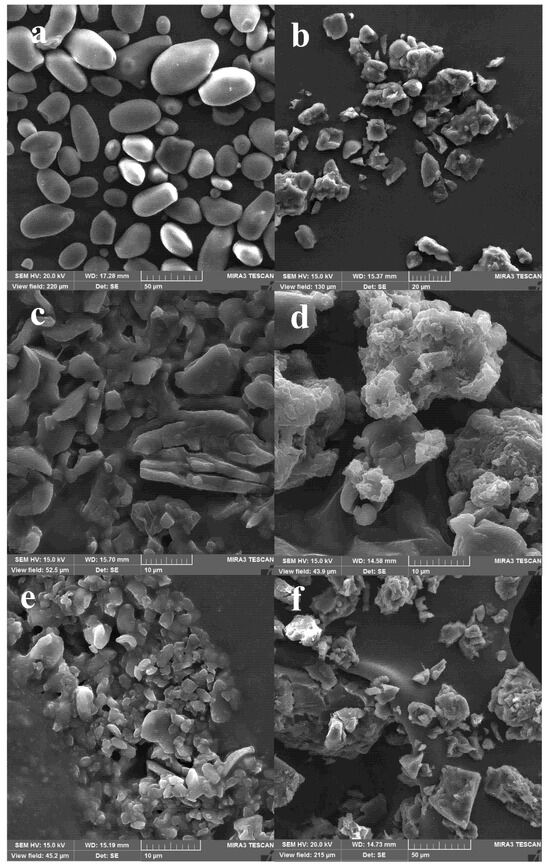
Figure 2.
SEM images of (a) native starch and acid hydrolysis starch particles from D. remotiflora: (b) unmodified; and modified with: (c) acetic anhydride, (d) lauroyl chloride, (e) sodium hexametaphosphate, and (f) citric acid.
The acetylation of SPs from both treatments also resulted in intensity peaks within ranges for micro (0.53 ± 0.00 and 0.55 ± 0.04 μm) and nanoparticles (0.09 ± 0.01 and 0.10 ± 0.01 μm). The lower values corresponded to SPs obtained by AH-US. Thus, ultrasonication effectively broke up the modified starch particles, reducing their size and polydispersity (Table 2). Other studies have reported similar results [34,52]. In addition, US increases the peak intensities (%) due to the reduction of agglomerates or large particles in the colloidal solution [53]. Diffraction light scattering detects the intensity fluctuations over the time of particles in movement; large particles scatter light more strongly than smaller particles. Moreover, Rayleigh scattering intensity is determined by the sixth power of the molecular size. This means that high-molecular-weight aggregates scatter light more effectively than smaller particles, making them detectable [54].
In general, smaller particle sizes of SPs were detected by modification with AA and CA. The variability in SPs’ particle sizes may be associated with the production method and the properties of the chemical reagents, such as reactivity, carbon-chain length, degree of substitution, number of substituent groups, bond energy, polarity, and molecular weight, among others [55].
Furthermore, the PDI index values of the modified SPs with both treatments varied as a function of the treatment and the chemical agent. Although the SHMP showed the highest Dz values compared to those of the other compounds, its PDI value was the lowest, indicating a narrow particle-size distribution. However, those modified with LC of both treatments and CA for AH-US showed values of 0.71–0.84, suggesting that there are large aggregates and a broad particle-size distribution. The PDI index is a parameter that defines the size distribution of particles obtained from a DLS. A value of 0.1–0.5 is considered adequate, while values higher than 0.7 indicate a broad particle-size distribution that can contain large particles or aggregates and that are characteristic of colloidal suspensions [28].
The low PDI index values of 0.36–0.54 obtained for the AH-US/SPs indicate that the US energy dissociates the particle aggregates [38], except for the PDI index value (0.82 ± 0.09) of SPs modified with LC, which is comparable to that of the AH treatment. Similar results were reported for esterified and cross-linked waxy corn starch nanocrystals with AA and SHMP, with PDI index values of 0.60 and 0.59, respectively [56]. In contrast, other investigations reported that cross-linked waxy corn starch nanocrystals with CA produce particle sizes of 0.503 μm and a PDI index value of 0.45 [28].
To acquire insights on the solubility of SPs, a qualitative assessment was performed on samples modified with LC and AA, dissolved in water, ethanol, acetone, and chloroform (ChCl3) (Appendix A). Initially, both unmodified and modified SPs from the AH treatment exhibited solubility; however, precipitation occurred after 24 h. Except for those modified with AA dissolved in water, which remained relatively soluble with minimal particle sedimentation, all other samples showed a similar trend. Comparable behavior was observed in SPs subjected to AH-US treatment when dissolved in the tested solvents. Moreover, SPs modified with AA demonstrated enhanced stability in water and complete dissolution with ChCl3 with no visible particle sedimentation. This indicates improved solubility, likely attributed to their low PDI of 0.52, which was significantly lower than that of SPs modified with LC under both AH and AH-US treatments. These findings align with those reported for corn starch modified with acetic anhydride, which was dispersed in acetone, carbon tetrachloride, and toluene, demonstrating increased solubility [57]. Accordingly, D. remotiflora SPs showed improvement through acetylation, underscoring the need for further research to evaluate their compatibility with hydrophobic compounds for potential applications.
3.3. Morphology
Native starch from D. remotiflora presented well-defined granules of ellipsoidal and spherical shapes and smooth edges, with particle sizes between 5 and 30 μm (Figure 2a). Note that the larger microparticles appear to comprise clusters of irregular smaller microparticles. Similar morphologies of hydrolyzed starch microparticles have been reported elsewhere [22,58]. The morphology of D. remotiflora starch was similar to that observed for other tubers and legumes such as potatoes, peas, green beans [58], and water chestnuts [22]. These types of cultivars contain large starch granules (ca. 35 μm) with similar oval or spherical shapes. Although the variation in the size of the starch granules is characteristic of the botanical source, the shear forces during milling can cause the rupture of the granules; therefore, this could also explain the wide range in particle size.
After hydrolysis, the starch granules break down, forming irregular and amorphous particles with rough surfaces, the majority with sizes of approximately 5 and 20 µm (Figure 2b). While the larger microparticles appear to comprise clusters of irregular smaller microparticles. Comparable morphologies of hydrolyzed starch microparticles are reported in other studies for water chestnut [22], and potato starches [59]. The esterification of AH/SPs with anhydrate acetic (Figure 2c) promoted the formation of particles ranging from 1–30 µm. The starchy material of large and irregular shapes had the tendency to form lamellar aggregates, with little material exhibiting fracture on its surface. Furthermore, the SPs appear to be stuck together and have a plasticized appearance. The breakage of the particles is related to the degree of acetylation because it causes the destabilization of the intermolecular hydrogen bonds, and hence, the breakage of the starch particles takes place [60]. The plasticized appearance may be due to the leaching of the remaining amylose and the incorporation of acetate groups [61]. In comparison, the esterification of AH/SPs with lauroyl chloride (Figure 2d) tended to form structures of different sizes (10–40 µm) with undefined and rounded shapes. The structures compacted and exhibited rough surfaces, causing agglomeration of particles that formed larger assemblies [62].
On the other hand, for the crosslinked AH/SPs with SHMP (Figure 2e), the particle sizes decreased considerably (to 2–8 µm). SPs exhibited irregular shapes, with few of them being well-defined and separated from each other, but others strongly agglomerated. As explained above for particle size, aggregation of nanoparticles induces the formation of bigger-sized particles. Unlike crosslinking with citric acid (Figure 2f), the SPs exhibit an irregular structure with random particle sizes between 5 and 70 μm, the biggest forming clusters. The surface of intact starch AH/SPs appeared to be smooth. In contrast to the surface of intact AH/SPs, the modified SPs using citric acid exhibited roughness and interactions between particles that confirm the crosslinking of SPs. Zhou et al. [28] reported similar structures for waxy maize-starch nanoparticles modified with citric acid, with the tendency to decrease the aggregation of starch nanocrystals with the increase of reaction time, and the large particles of starch nanocrystals were also reduced.
When acid hydrolysis was combined with US (Figure 3a), the SPs showed dense or bulging forms of different sizes (<5 µm). Also, they appear coated by a plasticized surface, probably by hydrolyzed amylose, which is observed as a gelled layer [63]. The micrograph showing the esterification of AH-US/SPs with acetic anhydride (Figure 3b) revealed polygonal microparticles of different shapes and sizes (1–30 µm). Nonetheless, the flat and smooth surfaces were related to the chemical process of acetylation. In addition, the abundant presence of particles sized <1 µm is notable, induced by a mechanical breakdown due to the cavitation waves of ultrasound that dissociated the SPs [38].
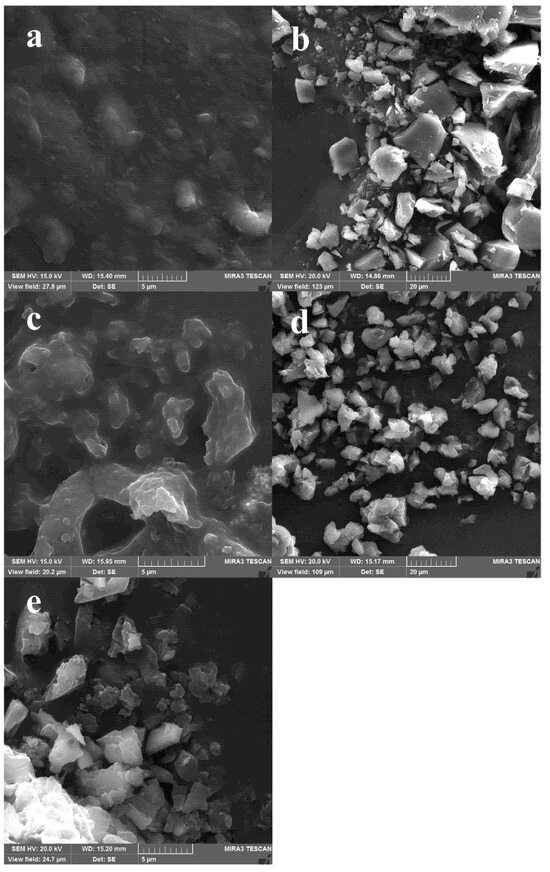
Figure 3.
SEM images of acid hydrolysis-ultrasound starch particles from D. remotiflora: (a) unmodified; and modified with: (b) acetic anhydride, (c) lauroyl chloride, (d) sodium hexametaphosphate, and (e) citric acid.
On the other hand, the esterification of AH-US/SPs with lauroyl chloride (Figure 3c) exhibited irregular and fragmented microparticles of different sizes (1–10 μm) with smooth and plasticized surfaces. The ultrasonication treatment breaks down the particles into separated and independent SPs, unlike esterified AH/SPs, which form bigger clusters of microparticles.
Crosslinking of AH-US/SPs with SHMP (Figure 3d) formed particles with irregular edges, heterogeneous sizes (10–50 mm), and rough surfaces. Also, agglomerations of 3–5 particles were detected because of the reaction between the chemical agent and OH groups of starch particles. Whereas the crosslinking of AH-US/SPs with citric acid (Figure 3e) resulted in amorphous and elongated particles fused with one another and yet forming fibrous-like structures [64]. These changes were attributed to the effect produced by the gelatinization of starch that occurs at high temperatures during the crosslinking reaction and the reassociation of starch particles.
The particle size and the morphology of SPs strongly depend on the treatment selected to extract starch particles and also on the type of chemical reaction that occurs with each molecule used to functionalize the starch. As particles tend to agglomerate with compounds like SHMP, the enhanced interactions of -OH groups form covalent bonds (C–O–P), which increased the thermostability (386 J/g) of SPs, mainly under AH treatment. Therefore, diversifying the applications of crosslinked starch could be beneficial as food stabilizers or for composite materials processed at high temperatures [33]. Moreover, to obtain particles for use as biocarriers, optimization conditions must be tailored to their intended application, complemented by additional technical approaches. For instance, using an ultrasound probe instead of a water bath ultrasound could help reduce particle aggregation and decrease particle size by adjusting the ultrasound frequency and exposure time.
3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
Figure 4 shows the infrared spectra of the native starch (Figure 4a), unmodified and modified SPs obtained by AH (Figure 4a) and AH-US (Figure 4b) treatments. All spectra exhibited the standard bands that correlate with the molecular structure of starch, such as the broadband from 3500 to 3200 cm−1, characteristic of hydroxyl groups (-OH), and the band at 2916 cm−1 corresponding to the stretching of the CH2- and CH3- groups. The peak at 1300–1000 cm−1 corresponded to the carbon skeleton of the glucose molecule [65]. Additional vibration bands between 860 and 1000 cm−1 are associated with the C-O groups, while those at 761 and 992 cm−1 correspond to the C-O-C ring of starch.
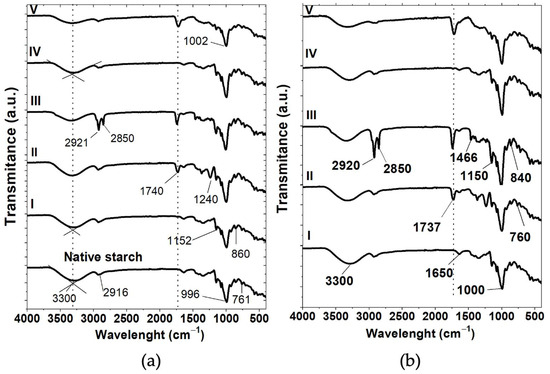
Figure 4.
Infrared spectra of native starch and starch particles from D. remotiflora obtained by (a) acid hydrolysis: (I) unmodified and modified with (II) acetic anhydride, (III) lauroyl chloride, (IV) sodium hexametaphosphate, and (V) citric acid; and (b) acid hydrolysis-ultrasound: (I) unmodified and modified with (II) acetic anhydride, (III) lauroyl chloride, (IV) sodium hexametaphosphate, and (V) citric acid.
FTIR analysis confirmed the modification of the SPs with different chemical agents. For both treatments, AH and AH-US, the intensity peak areas (A(-OH)) of the -OH group located in the region from 3500 to 3200 cm−1 decreased compared to the A(-OH) of unmodified SPs. The decrease in the peak area is attributed to the introduction of new functional groups that react with the hydroxyl groups of starch (see Table 3). In addition, the A(-OH) of unmodified AH/SPs (Figure 4a, I) and AH-US/SPs (Figure 4b, I) was lower than that of native starch due to the solubilization of amorphous regions of starch granules and consequently, the reduction of hydroxyl groups [40]. This occurs because hydrolysis causes the degradation and erosion of starch’s crystalline regions, leading to the formation of hydrolysate [38]. These observations may be correlated with the decrease in amylose content determined in the hydrolyzed SPs (Table 1).

Table 3.
FTIR intensity peak areas of the hydroxyl (A(-OH)) and carbonyl (A(-C=O)) groups, degree of substitution (DS), and relative crystallinity index (RCI) of starch particles.
For AH/SPs and those acetylated with AA (Figure 4a, II), the new peak observed at 1740 cm−1 corresponded to the carbonyl group (-C=O), which is related to the insertion of the acetyl group into the starch molecule. This confirms the acetylation process, where the esterification of the -OH groups occurs by binding with the anhydroglucose units of starch [61]. Moreover, the peak at 1240 cm−1 was associated with the stretching vibration of the carbonyl group (-C-O). The absence of unreacted acetic anhydride and acetic acid is evidenced by the lack of a band at 1850–1760 cm−1. A similar result was reported elsewhere for another variety of Dioscorea starch (D. zingiberensis C.H. Wright) acetylated with acetic anhydride [66].
In the esterification process with LC (Figure 4a, III), the peak corresponding to the -C=O group at 1740 cm−1 was also identified, indicating the esterification reaction and the formation of starch laurate ester. Also note that A(-OH) was lower than the corresponding peak area of unmodified SPs (Figure 4a, I), indicating that the esterification reaction occurred due to the substitution of the -OH groups of starch with laurate chains (Table 3). Moreover, the intense peaks observed at 2920 and 2850 cm−1 corresponded to the C-H stretching of the aliphatic chain of the substituted laureate.
On the other hand, for the crosslinking reaction with SHMP (Figure 4a, IV), the specific bands of the phosphate groups were not observed, suggesting that a low number of phosphate groups were introduced. The stretching vibration peak of the P=O group is usually located in the range of 1400–1150 cm−1. Some authors indicate that the absorbance peak of the P-O group, located at 1050–995 cm−1, could be undetected because it overlaps with the CH- bonds of starch [37]. Therefore, as the A(-OH) decreased with respect to the unmodified SPs, and the -OH peak shifted to higher wavenumbers (3325 cm−1), this suggests that a chemical reaction is occurring, albeit with the -OH groups of starch [37]. Similar results to those obtained for D. remotiflora have been reported elsewhere in cross-linked starch with sodium trimetaphosphate and sodium tripolyphosphate [29,37].
From the spectrum of AH/SPs crosslinked with CA (Figure 4a, V), the band at 1740 cm−1 corresponded to the carbonyl stretching in the ester groups, confirming the crosslinking between starch and CA. Moreover, note that the peak attributed to the C-O bonds at 996 cm−1 shifted to higher wavenumbers (1002 cm−1), which suggests a new interaction between the starch and CA after modification [67]. Crosslinking promotes the formation of random intra- and intermolecular bonds in the starch molecule by creating ether or ester bonds with the chemical agent and the -OH groups in the starch molecule [37]; thus, the degree of substitution causes a wavelength displacement.
DS values of the modified SPs treated with AH increased and were further enhanced with AH-US, as follows: AA < LC < CA. As confirmed, the US reduces the particle size and polydispersity of SPs, thereby increasing the reaction area and the availability of specific sites, which improves the efficiency of chemical reactions [68,69]. These results were in accordance with those reported for acetylated Dioscorea zingiberensis starch combined with ultrasound [66], and comparable results have been reported elsewhere with other starch varieties [38,69,70]. Moreover, it is important to consider that other factors also influence DS, such as the reactivity of the chemical reagent, its ionic charge, polarity, concentration, pH, reaction time, and the characteristics of the size and structure of the SPs [71,72].
3.5. X-Ray Diffraction
Native starch exhibited a type B diffraction pattern at 2ϴ with peaks at 16.7°, 21.8°, and 23.6° (Figure 5a,b, I). The low-intensity peak observed at 20° in the native starch suggests the presence of V-type amylose-lipid complexes related to the semi-crystalline structure of starch [73]. The RCI value of native starch (30%) was higher compared to other Dioscorea species, such as Dioscorea opposita Thunb [74] and Dioscorea hispida tuber [75], which were 23.7% and 27.5%, respectively.
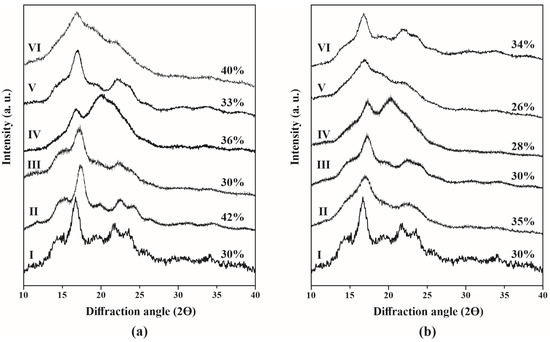
Figure 5.
XRD patterns of starch particles from D. remotiflora obtained by (a) acid hydrolysis and (b) acid hydrolysis-ultrasound. (I) Native starch: (II) Unmodified and modified with: (III) acetic anhydride, (IV) lauroyl chloride, (V) sodium hexametaphosphate, and (VI) citric acid.
For unmodified SPs under the AH treatment (Figure 5a, II), the peak at 2θ = 22.3° broadened, and an additional peak appeared at 24.3°, characteristic of a B-type pattern [76]. Consequently, the relative crystallinity index (RCI) decreased, indicating that acid modification disrupted the structural organization of starch [77].
On the other hand, for chemically modified SPs, the crystalline structure of the starch remained intact. However, the maximum intensity varied depending on the specific treatment, the chemical modification applied, and the degree of substitution achieved. The acetylation with AA (Figure 5a, III) notably reduced the intensity peaks at 17° and 22°. Consequently, the decrease in the RCI values indicates a partial loss of starch’s crystalline patterns compared to unmodified SPs (Figure 5a, II). Acetyl groups disrupt hydrogen bonding, resulting in a less ordered structure [19]. This was reflected in the RCI, which decreased from 42% in unmodified SPs to 30% in acetylated samples (Table 3). This reduction is consistent with findings by Ren et al. [78], who reported RCI values between 17.4% and 23.5% for waxy maize nanocrystals modified with acetic anhydride, indicating partial disruption of crystalline domains while preserving the general structure.
The diffraction patterns of SPs esterified with LC (Figure 5a, IV) indicated that the peak at around 22.6° became less defined, likely due to structural modifications in the amorphous regions of starch induced by esterification [79]. Previous studies have reported that this type of modification primarily impacts the amorphous phase [76]. Additionally, the characteristic diffraction peaks of V-type starch-lipid complexes (7°, 13°, and 20°) were absent, probably due to the low amylose content (2–3%), which restricts the formation of helical inclusion complexes [80]. Instead, a single broad peak replaced the distinct peaks near 22° and 24°, suggesting a rearrangement of the crystalline structure [81]. Similar results have been reported for esterified corn and potato starch nanocrystals, which retained only A- and B-type diffraction patterns without forming V-type complexes [36,65].
Cross-linking with SHMP shows two distinct peaks at 2θ ≈ 17° and 23°, clearly separated, indicating enhanced organization. The RCI value increased compared to native starch, suggesting the formation of a more stable crystalline structure [65]. However, the RCI value is lower than that of the modified SPs. It has been reported elsewhere that the degree of cross-linking is moderate, sufficient to induce some organization without forming an extensive crystal lattice [37]. Similar increases in crystallinity after phosphate crosslinking were reported by Ren et al. [56] for waxy maize starch nanocrystals, which reached higher RCI values (51.1%).
In contrast, cross-linking with CA resulted in diffraction patterns displaying a broad main peak at 2θ ≈ 17° and a second, less intense peak at 2θ ≈ 20°, typical of a type B structure. The RCI value was highest for the chemically modified SPs (40%), indicating an enhanced crystalline phase of starch that resulted in changes to its structural order. The chemical modification of starch with CA involves an esterification reaction, leading to the formation of cross-linking bonds and partial depolymerization of the starch chains. This modification introduces ester bridges between amylose and amylopectin chains, which restrict chain mobility and impact structural reorganization, consequently increasing crystallinity.
On the other hand, for the unmodified SPs, the peak at 22.83° was further smoothed due to ultrasound treatment. Cavitation waves generated by ultrasound can induce physical impacts on starch granules, resulting in changes to their crystalline structure [11]. This aligns with our observation of a decrease from 42% (AH) to 35% (AH-US) in unmodified SPs. For the AH-US/SPs chemically modified samples, the RCI values decreased compared to the AH/SPs, attributed to the increase in reaction sites where polar substituents were incorporated, which in turn elevated the DS values, thereby reducing their remaining structural order. For example, RCI values were found to decrease as DS values increased. This result is expected, since as the DS value rises, the starch structure is modified by the incorporation of bulk substituent groups, which alters the bonding of hydrogen atoms, particularly affecting the crystalline lamellae of starch [82].
The diffractogram of the AH-US/SPs modified with AA (Figure 5b, III) exhibited broad diffraction peaks at approximately 15.5°, 17.3°, and 22.3°, corresponding to an RCI value of 30%, which is a decrease compared to the unmodified AH-US/SPs (35%). Although acetylation disrupts hydrogen bonding, ultrasound-assisted acetylation increased peak intensity, particularly at 17.3°, suggesting that under specific conditions, ultrasound treatment can promote starch chain rearrangement and enhance crystallinity. Meanwhile, for AH-US/SPs esterified with LC (Figure 5b, IV), slightly sharper peaks appeared at similar angles to those of AA, with an RCI value of 28%. Crystallinity remains low due to the US, as ultrasonic cavitation generates microjets and shear forces that break hydrogen bonds and weaken the crystalline lamellae, especially in amylopectin [11]. This results in the partial disruption of long-range order, leading to a broader and less intense set of peaks, especially around 2θ ≈ 17° and 23°, which are characteristic of B-type starches.
For SPs modified with SHMP, diffraction peak intensities at 16.2°and 21.8° increased, indicating that the crystalline structure remained intact after modification. This behavior was similarly observed in Rhizoma Dioscorea starch under acid hydrolysis, even with prolonged extraction times [83]. However, in AH-US, peak intensities were lower and more diffuse compared to AH, suggesting partial disruption of the crystalline organization due to the mechanical effects of ultrasound [84,85]. This phenomenon has been previously linked to the fragmentation and rearrangement of starch structure, resulting in a reduction of overall crystallinity [86]. Despite this disruption, phosphate crosslinking contributed to structural stabilization, preserving crystallinity to some extent [87]. Similar findings have been reported in studies where phosphate crosslinking improved starch crystallinity and protected its structural integrity [88]. Conversely, Andrade et al. [89] observed the complete disappearance of diffraction peaks after 75 min of ultrasound treatment, confirming the disruptive effect of sonication on starch crystallinity.
Crystallinity changes in CA-modified SPs were evident for the AH-US treatment. However, the characteristic diffraction peaks at approximately 16.7° and 22° for AH-CA and 17.0° and 22.1° for AH-US-CA remained prominent, indicating that crosslinking altered, but did not eliminate, the crystalline structure [90]. The crystallinity percentage of 34% suggests that CA crosslinking restricted polymer chain mobility, forming a three-dimensional network that limited gelatinization and preserved structural order [91]. These findings are consistent with those reported by Reyma et al. [27], who observed only a slight reduction in crystallinity following citric acid modification of root starches, such as potato (from 73.0% to 70.6%) and sweet potato (from 78.3% to 72.3%). Similarly, previous studies have shown that crosslinking with polyfunctional acids preserves crystalline regions and prevents a complete transition to an amorphous state [28]. Furthermore, this observation reinforces earlier reports indicating that non-toxic polycarboxylic acids, such as citric acid, can improve the mechanical properties of starch while maintaining its semi-crystalline structure [77].
Although certain chemicals disrupt starch crystallinity, crosslinking agents such as SHMP and CA can enhance the structural stability [92]. As demonstrated for the crosslinking of D. remotiflora SPs with CA, which appears to have modified the starch structure while partially maintaining its crystalline arrangement. While ultrasound treatment induced partial disorder in the starch structure, under specific conditions, it could also facilitate starch chain rearrangements, thereby influencing crystallinity [34].
3.6. Thermal Properties
Figure 6 shows DSC thermograms of the native starch (Figure 6a) and the SPs obtained with the AH (Figure 6a) and AH-US (Figure 6b) treatments for the unmodified and modified SPs. The DSC curve of native starch presented a single endothermic transition peak at 93.8 °C (Table 4). The transition peak corresponds to the melting of crystallites, indicating the order and arrangement of starch’s crystalline regions [34]. This value was lower than the melting temperatures of dry crystallites (160 and 205 °C) from rice starch, as reported elsewhere [80]. Nonetheless, the properties of starch nanocrystals (mainly composed of the crystalline region of starch) are different from those of starch particles, for which there is limited information regarding their behavior. In addition, the ΔHf of native starch was 339.0 J/g, comparable to other high-amylose starches, such as amadumbe (Colocasia esculenta), as reported elsewhere [34].
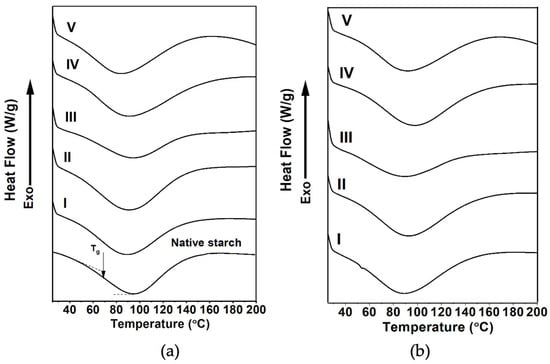
Figure 6.
DSC thermograms of native starch and starch particles from (a) acid hydrolysis and (b) acid hydrolysis-ultrasound. (I) unmodified; and modified with (II) acetic anhydride, (III) lauroyl chloride, (IV) sodium hexametaphosphate, and (V) citric acid. The arrow in Figure (a) points out the Tg’s location of native starch.

Table 4.
Thermal properties of native starch and particles from D. remotiflora.
Regarding unmodified SPs, the Tp decreased to 88.6–88.7 °C, with values nearly identical for both AH and AH-US treatments; thus, the extraction method does not significantly influence the melting temperature. However, ΔHf values exhibited a significant decrease between treatments, with 187.6 and 234.0 J/g for AH and AH-US, respectively, with respect to that for native starch. As hydrolysis first degrades amylose and subsequently unfolds the double helices of the amylopectin chains, the crystallinity of SPs decreases [93]. This behavior was revealed in SPs of D. remotiflora, which contained low percentages of residual amylose (2.8–3.6%) and a reduction in the crystallinity index (RCI: 15.8–26.3%). Similar results have been reported for corn starch with 32% amylose [94]. Additionally, under ultrasound, there was lower residual amylose and a higher RCI, resulting in a reordering of the hydrolyzed chains that promoted a more crystalline structure.
After acetylation, Tp and ΔHf increased compared to the unmodified SPs for both treatments. The substitution with acetyl groups promoted the intermolecular bonding of the crystalline part of SPs, thereby increasing their thermal stability [95]. Some studies report that acetylation enhances the thermal properties of starch, even in high-amylose maize starch containing up to 70% amylose [95]. Meanwhile, esterification with LC from the AH treatment provided higher thermal stability (Tp = 94.8 °C) than acetylation, indicating the formation of stronger interactions. Nonetheless, less energy was required to break the bonds between molecules, as evidenced by the decrease in ΔHf. This parameter provides information on the energy changes that occur during the fusion of amylopectin crystals [96]. Thus, the reduced ΔHf values are associated with the decreased crystallinity observed after modification with LC, as shown in Table 4.
Moreover, the Tp of SPs crosslinked with CA decreased in those obtained by AH but increased in those of AH-US treatment. A similar behavior was observed for ΔHf of both treatments (AH and AH-US). This is because with AH-US treatment, ultrasound increases the specific area of reaction of SPs, and CA penetrates through channels and cavities, thereby favoring the crosslinking reaction [97]. Thus, more reagent reacts at the surface of SPs, forming covalent bonds that reinforce SPs [28] and improve thermal stability. In fact, crosslinked SPs with CA had the highest ΔHf (343 ± 0.4 J/g) and DS value (Table 3) compared to the other modification reactions (Table 3). confirming the formation of strongly organized crosslinked structures. Conversely, SPs crosslinked with SHMP exhibited an increased Tp value for both treatments (AH and AH-US). Notably, the AH-US treatment resulted in the highest Tp value, shifting from 88.7 °C (unmodified SPs) to 97.6 ± 0.3 °C (Table 4). Therefore, higher energy was required to fuse the crosslinked crystallites [34].
Furthermore, the thermal properties of the particles are correlated with the RCI and DS values, which depend on their chemical modification and ultrasonic treatment. In general, as mentioned above in Section 3.5, as the DS values increase due to the substituent group and ultrasound, crystallinity decreases due to a decrease in structural order, and in turn, ΔHf decreases due to the reduction in crystalline regions [98]. For example, ΔHf increased for chemical cross-linking treatments and decreased for the esterification reaction (see Table 4). Additionally, T0 values related to thermal resilience increased due to the effect of ultrasound and the chemical esterification reaction but decreased during the cross-linking process. It has been reported that the thermal resilience effect depends on the polarity of the substituent group, with hydrophobic groups such as LC increasing their thermal stability [99].
Starch is a semi-crystalline polymer; therefore, its glass transition temperature (Tg) is a crucial parameter that influences its mechanical properties, processing, and stability [100]. When thermal energy is applied, the Tg is the temperature at which a glassy polymer transitions from a rigid/glassy state to a rubbery or viscous state. Glass transition is associated with a second-order transition reflected by an increase in segmental motion of 20–50 carbons on the polymer backbone, detected by a discontinuous change in heat capacity. For D. remotiflora, the Tg was 68.7 °C (Table 4) with a residual moisture of 10.64 ± 0.3%, which is close to that reported for corn starch with a moisture content of 8.7–13.3% (Tg = 67.3–59.2 °C) [101]. Other authors have reported potato starch Tg values of 90–100 °C [102] and 75–95 °C [103] with 13–15% moisture content. The differences between the Tg values found in this study and those reported in the literature, despite variations in starch sources, are mainly attributed to differences in moisture and amylose content, as Tg decreases linearly with an increase in moisture [103]. Still, it increases with the rise in amylose [104]. In this respect, the Tg is known to increase from 52 to 60 °C when the amylose content increases from 0 to 80% for different corn starches with 13% moisture [104].
The Tg of unmodified SPs from both AH and AH-US treatments decreased (59.3–60.8 °C) compared to that of native starch, as the amylose content and crystallinity dropped to 2.85–3.21% and 15.4–26.3%. Similar results were obtained for native corn starch after 3 days of acid hydrolysis at 35 °C, with amylose content dropping and then remaining constant (approximately 2.94%) [94]. Such a decrease in Tg is attributed to the hydrolyzed amylose, which disrupts the structural order within the amylopectin crystallites, reducing the branching of the α-(1→6) glycoside linkages. These types of linkages increase the total free volume, requiring less energy input to initiate the glass transition, thereby lowering the Tg [105]. On the other hand, after chemical modification, the Tg of SPs shifted to higher values (65.3–84.1 °C) for all compounds, compared to those of the unmodified SPs from both treatments (Table 4). Primarily, esterification with AA is one of the compounds that causes a significant increase in Tg, indicating an increase in rigidity induced by strong intramolecular interactions. However, crosslinking SPs with CA also shifted to higher temperatures than those that were unmodified, adding rigidity through a different mechanism [105]. In general, for both treatments (AH and AH-US), the Tg of the SPs increased as follows: SHMP > AA > CA > LC. These results are relevant because functionalizing SPs with, for example, AA acid or CA results in smaller particle sizes with larger surface areas and Tg values higher than those of unmodified SPs. This suggests that the esterification with AA or crosslinking with CA may reinforce SPs by enhancing their stability and reorganizing them into more structured materials.
Additionally, the Tg increment (∆Tg) is defined as ∆Tg = Tg(F) − Tg(N), where Tg(F) and Tg(N) are the glass transition temperatures of the modified and unmodified SPs, respectively. The ∆Tg values of SPs esterified with AA and LC were 13.1 °C and 6.9 °C for the AH treatment and 17.4 °C and 8.7 °C for the AH-US treatment, respectively. Intermolecular interactions caused by the esterification improve and restrict the chain mobility of SPs. While ∆Tg values of SPs crosslinked with CA and SHMP were 8.1 and 4.5 °C for the AH and AH-US treatment and 8.7 and 24.8 °C for the AH-US treatment, respectively. Notably, ∆Tg was greater for SPs treated by AH-US than for those treated by AH alone. As previously demonstrated, the DS values of the sonicated SPs were higher, indicating that sonication increases the surface area of SPs, thereby increasing the degree of esterification and crosslinking.
4. Conclusions
Starch particles (SPs) produced from wild yam (D. remotiflora) tubers exhibited versatile characteristics depending on the treatments, such as acid hydrolysis (AH) alone or ultrasound-assisted (AH-US) and chemical modification with four different compounds (AA: acetic anhydride, LC: lauroyl chloride, CA: citric acid, and SHMP: sodium hexametaphosphate). Both treatments promoted a decrease in amylose content; therefore, SPs mainly comprise the crystalline region of starch. The yield of SPs exceeded that reported for other botanical sources and further increased after chemical modification. Hence, selecting the appropriate treatment may help maintain yield in scaled-up production. The SPs exhibited mono- and bi-modal distributions for AH and AH-US treatments, respectively, and lower polydispersity indices, indicating a narrow particle-size distribution. The chemical modification resulted in bi-modal distributions and particle sizes of micro- and nanoparticles. Compounds such as AA promoted the formation of nanoparticles, while others, like LC or SHMP, resulted in large particles, which increased the polydispersity index, indicating a broader distribution. Moreover, AH-US treatment induced low polydispersity indexes, demonstrating the benefits of using US to prepare micro- and nanomaterials. From a morphological perspective, SPs exhibited surface alterations, including disruption, clustering, and particle aggregation, which confirmed the large sizes of LC and SHMP particles. The surface changes were driven by the nature of the chemical reaction and the intramolecular bonding that occurred at specific sites within the starch structure. These changes were confirmed by the peak areas in the FT-IR spectra, where the -OH and -C=O groups decreased significantly after chemical substitution. Meanwhile, the higher degree of substitution obtained by crosslinking with CA indicated the formation of stronger interactions between the starch-hydrolyzed chains compared to AA or LC. Although SPs esterified with AA showed high glass transition temperature and melting enthalpy, for the AH treatment, the use of ultrasound (AH-US) increased both parameters. Furthermore, ultrasound extraction promoted the highest melting enthalpy of those SPs crosslinked with CA, demonstrating that higher amounts of energy are needed to overcome the rigidity attained after their modification. This behavior suggests that SPs exhibit enhanced stability, making them suitable for applications that require performance under critical pH or temperature conditions. Among the chemical compounds evaluated, modifications with AA and CA strengthened SPs by enhancing their stability and structural organization. But the modification of SPs with LC and SHMP warrants further exploration under varying concentrations and reaction conditions to optimize their properties.
In this context, the development of starch particles from D. remotiflora tubers presents a viable and cost-effective botanical source for obtaining SPs with significant potential in applications that require stable, well-organized micro- and nanoparticles. With chemical modifications, optimized conditions are necessary to minimize aggregation and improve dispersibility. Although the incorporation of ultrasound-assisted techniques improved the dispersibility of modified SPs with AA, compounds like SHMP responded differently; thus, other methods using an ultrasound probe may be more efficient for disrupting aggregation. Moreover, further investigations to comprehend the interactions between SPs and target molecules will be essential for establishing specific conditions to assess their efficiency as biocarriers.
Author Contributions
Conceptualization, Y.E.-G. and V.V.A.F.-E.; methodology, R.M.E.-M., V.V.A.F.-E., A.M.-d.-C., A.M.P.-P., J.A.U.-C., and I.C.; investigation, Y.E.-G.; resources, Y.E.-G.; data curation, V.V.A.F.-E., R.M.E.-M., A.M.-d.-C., and N.T.; writing—original draft preparation, Y.E.-G., V.V.A.F.-E., and R.M.E.-M.; writing—review and editing, R.M.E.-M., N.T., A.M.P.-P., V.V.A.F.-E., J.A.U.-C., and I.C.; supervision, Y.E.-G., V.V.A.F.-E., and A.M.P.-P. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research of this article: The authors are thankful to the Universidad de Guadalajara for their support and institutional funding (reference number II/2021/105) in the development of this research.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author, R.M.E.-M., wants to thank the Mexican National Council of Humanities, Science and Technology (CONAHCyT, now transitioned to SECIHTY) for the scholarship she received to pursue her doctoral degree.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
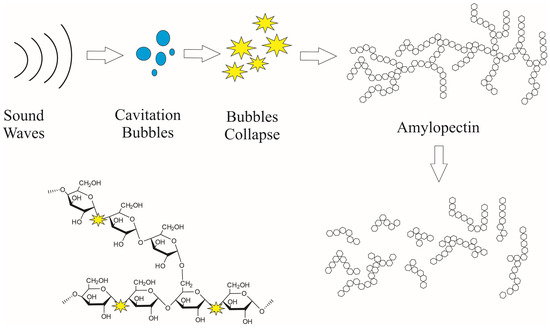
Figure A1.
Mechanism of amylopectin fragmentation induced by ultrasonic cavitation.
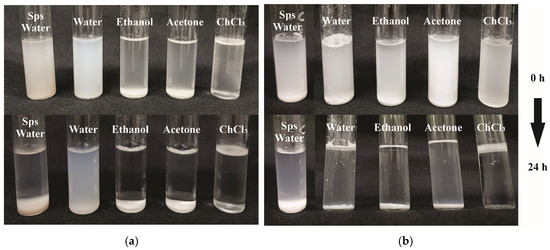
Figure A2.
Dispersion stability of D. remotiflora starch particles (SPs) modified by acid hydrolysis (AH) with (a) acetic anhydride and (b) lauroyl chloride, evaluated at 0 and 24 h at room temperature.
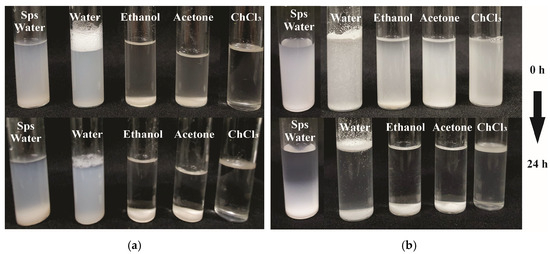
Figure A3.
Dispersion stability of D. remotiflora starch particles obtained by acid hydrolysis-ultrasound (AH-US), modified with (a) acetic anhydride and (b) lauroyl chloride, evaluated at 0 and 24 h at room temperature.
References
- Horn, M.M.; Martins, V.C.A.; Plepis, A.M.G. Effect of Amylopectin Content on Mechanical, Barrier and Thermal Properties of Plasticized Starch/Chitosan Films. Polysaccharides 2023, 4, 208–218. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef]
- Chin, S.F.; Pang, S.C.; Tay, S.H. Size controlled synthesis of starch nanoparticles by a simple nanoprecipitation method. Carbohydr. Polym. 2011, 86, 1817–1819. [Google Scholar] [CrossRef]
- Hore, R.; Rashid, H.; Syrowatka, F.; Kressler, J. Synthesis and Characterization of Self-Assembled Highly Stearate-Grafted Hydroxyethyl Starch Conjugates. Polysaccharides 2024, 5, 142–157. [Google Scholar] [CrossRef]
- Hasanvand, E.; Rafe, A.; Emadzadeh, B. Phase separation behavior of flaxseed gum and rice bran protein complex coacervates. Food Hydrocoll. 2018, 82, 412–423. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, B.S.; Yadav, R.B. Synthesis and modification approaches for starch nanoparticles for their emerging food industrial applications: A review. Food Res. Int. 2020, 128, 108765. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; El-Rafie, M.H.; El-sheikh, M.A.; El-Feky, G.S.; Hebeish, A. Synthesis, characterization, release kinetics and toxicity profile of drug-loaded starch nanoparticles. Int. J. Biol. Macromol. 2015, 81, 718–729. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Villa, C.C.; Sanchez, L.T.; Rodriguez-Marin, N.D. Starch Nanoparticles and Nanocrystals as Bioactive Molecule Carriers. In Polymers for Agri-Food Applications; Springer Cham: Cham, Switzerland, 2019; pp. 91–98. [Google Scholar] [CrossRef]
- Yang, J.; Li, F.; Li, M.; Zhang, S.; Liu, J.; Liang, C.; Sun, Q.; Xiong, L. Fabrication and characterization of hollow starch nanoparticles by gelation process for drug delivery application. Carbohydr. Polym. 2017, 173, 223–232. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple ultrasound method to obtain starch micro- and nanoparticles from cassava, corn and yam starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef]
- Yang, F.; Wei, Y.; Xiao, H.; Zhang, Q.; Li, J.; Lin, Q.; Zhu, D.; Huang, Z.; Liu, G.Q. Acetylated rice starch nanocrystals improved the physical, mechanical, and structural properties of native rice starch based films. Int. J. Biol. Macromol. 2023, 253, 127271. [Google Scholar] [CrossRef]
- Mahmoudi Najafi, S.H.; Baghaie, M.; Ashori, A. Preparation and characterization of acetylated starch nanoparticles as drug carrier: Ciprofloxacin as a model. Int. J. Biol. Macromol. 2016, 87, 48–54. [Google Scholar] [CrossRef]
- Forouzandehdel, S.; Forouzandehdel, S.; Rezghi Rami, M. Synthesis of a novel magnetic starch-alginic acid-based biomaterial for drug delivery. Carbohydr. Res. 2020, 487, 107889. [Google Scholar] [CrossRef]
- Cazotti, J.C.; Fritz, A.T.; Garcia-Valdez, O.; Smeets, N.M.B.; Dubé, M.A.; Cunningham, M.F. Graft modification of starch nanoparticles using nitroxide-mediated polymerization and the grafting from approach. Carbohydr. Polym. 2020, 228, 115384. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wang, Q.; Ren, L.; Tong, J.; Zhou, J. High efficiency and low cost preparation of size controlled starch nanoparticles through ultrasonic treatment and precipitation. Food Chem. 2017, 227, 369–375. [Google Scholar] [CrossRef]
- Jain, A.K.; Khar, R.K.; Ahmed, F.J.; Diwan, P.V. Effective insulin delivery using starch nanoparticles as a potential trans-nasal mucoadhesive carrier. Eur. J. Pharm. Biopharm. 2008, 69, 426–435. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, T.; Lin, Q.; Liu, G.Q.; Zhang, L.; Yu, F.; Chen, Y. Acetylated starch nanocrystals: Preparation and antitumor drug delivery study. Int. J. Biol. Macromol. 2016, 89, 456–464. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- Fuentes, C.; Kang, I.; Lee, J.; Song, D.; Sjöö, M.; Choi, J.; Lee, S.; Nilsson, L. Fractionation and characterization of starch granules using field-flow fractionation (FFF) and differential scanning calorimetry (DSC). Anal. Bioanal. Chem. 2019, 411, 3665–3674. [Google Scholar] [CrossRef]
- Dularia, C.; Sinhmar, A.; Thory, R.; Pathera, A.K.; Nain, V. Development of starch nanoparticles based composite films from non-conventional source-Water chestnut (Trapa bispinosa). Int. J. Biol. Macromol. 2019, 136, 1161–1168. [Google Scholar] [CrossRef]
- Lamanna, M.; Morales, N.J.; Garcia, N.L.; Goyanes, S. Development and characterization of starch nanoparticles by gamma radiation: Potential application as starch matrix filler. Carbohydr. Polym. 2013, 97, 90–97. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Anderson, D.P.; Yu, J. Preparation, modification, and application of starch nanocrystals in nanomaterials: A review. J. Nanomater. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Q.; Chang, P.R.; Yu, J. Chemical Modification of Starch. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 283–321. [Google Scholar]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and characterization of citric acid-modified starch nanoparticles/plasticized-starch composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Miranda Sechi, N.; Marques, P.T. Preparation and physicochemical, structural and morphological characterization of phosphorylated starch. Mater. Res. 2017, 20, 174–180. [Google Scholar] [CrossRef]
- Adewale Adetunji, O. Chemically Modified Starches as Excipients in Pharmaceutical Dosage Forms. In Chemical Properties of Starch; Intech Open Source: Rijeka, Croatia, 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Guízar-Miranda, A.; Montañéz-Soto, J.L.; García- Ruiz, I. Parcial caracterización de nuevos almidones obtenidos del tubérculo de camote del cerro (Dioscorea spp.). Rev. Iberoam. Tecnol. Postcosecha 2008, 9, 81–88. [Google Scholar]
- Perea, D.; Buitrago, H.G. Aplicación de la biotecnología agrícola al cultivo de ñame. In Ñame: Producción de Semillas por Biotecnología; Guzmán-Barney, M., Buitrago-Hurtado, G., Eds.; Universidad Nacional de Colombia, Unibiblos: Bogotá, Colombia, 2000; pp. 17–19. [Google Scholar]
- Estrada-Girón, Y.; Fernández-Escamilla, V.V.A.; Martín-del-Campo, A.; González-Nuñez, R.; Canché-Escamilla, G.; Uribe-Calderón, J.; Tepale, N.; Aguilar, J.; Moscoso-Sánchez, F.J. Characterization of Polylactic Acid Biocomposites Filled with Native Starch Granules from Dioscorea remotiflora Tubers. Polymers 2024, 16, 899. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Mariano, M.; Dufresne, A.; Mellem, J.J.; Amonsou, E.O. Microstructure, thermal properties and crystallinity of amadumbe starch nanocrystals. Int. J. Biol. Macromol. 2017, 102, 241–247. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, D.J.; Kim, J.Y.; Lim, S.T. Preparation of crystalline starch nanoparticles using cold acid hydrolysis and ultrasonication. Carbohydr. Polym. 2013, 98, 295–301. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, M.; Wang, L.; Zhou, J.; Tong, J. A method for improving dispersion of starch nanocrystals in water through crosslinking modification with sodium hexametaphosphate. Carbohydr. Polym. 2012, 87, 1874–1876. [Google Scholar] [CrossRef]
- Kim, H.Y.; Han, J.A.; Kweon, D.K.; Park, J.D.; Lim, S.T. Effect of ultrasonic treatments on nanoparticle preparation of acid-hydrolyzed waxy maize starch. Carbohydr. Polym. 2013, 93, 582–588. [Google Scholar] [CrossRef]
- Horwitz, W. Method 925.09: Solids (Total) and Loss on Drying (Moisture) in Flour. Vacuum Oven Method. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Angellier, H.; Molina-Boisseau, S.; Belgacem, M.N.; Dufresne, A. Surface chemical modification of waxy maize starch nanocrystals. Langmuir 2005, 21, 2425–2433. [Google Scholar] [CrossRef]
- Shujun, W.; Jinglin, Y.; Jiugao, Y.; Haixia, C.; Jiping, P. The effect of acid hydrolysis on morphological and crystalline properties of Rhizoma Dioscorea starch. Food Hydrocoll. 2007, 21, 1217–1222. [Google Scholar] [CrossRef]
- Jivan, M.J.; Madadlou, A.; Yarmand, M. An attempt to cast light into starch nanocrystals preparation and cross-linking. Food Chem. 2013, 141, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, G.; Zhu, F. Impact of long-term ultrasound treatment on structural and physicochemical properties of starches differing in granule size. Carbohydr. Polym. 2023, 320, 121195. [Google Scholar] [CrossRef]
- Zhu, F. Impact of ultrasound on structure, physicochemical properties, modifications, and applications of starch. Trends Food Sci. Technol. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Wei, B.; Qi, H.; Zou, J.; Li, H.; Wang, J.; Xu, B.; Ma, H. Degradation mechanism of amylopectin under ultrasonic irradiation. Food Hydrocoll. 2021, 111, 106371. [Google Scholar] [CrossRef]
- Sanchez de la Concha, B.B.; Agama-Acevedo, E.; Nuñez-Santiago, M.C.; Bello-Perez, L.A.; Garcia, H.S.; Alvarez-Ramirez, J. Acid hydrolysis of waxy starches with different granule size for nanocrystal production. J. Cereal Sci. 2018, 79, 193–200. [Google Scholar] [CrossRef]
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA-J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Truong, V.-D.; Wang, L. Structures and rheological properties of corn starch as affected by acid hydrolysis. Carbohydr. Polym. 2003, 52, 327–333. [Google Scholar] [CrossRef]
- Hoover, R. Acid-Treated Starches. Food Rev. Int. 2000, 16, 369–392. [Google Scholar] [CrossRef]
- Perez Herrera, M.; Vasanthan, T.; Hoover, R. Characterization of Maize Starch Nanoparticles Prepared by Acid Hydrolysis. Cereal Chem. 2016, 93, 323–330. [Google Scholar] [CrossRef]
- García-Gurrola, A.; Rincón, S.; Escobar-Puentes, A.A.; Zepeda, A.; Pérez-Robles, J.F.; Martínez-Bustos, F. Synthesis and succinylation of starch nanoparticles by means of a single step using sonochemical energy. Ultrason. Sonochem. 2019, 56, 458–465. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Dynamic Light Scattering. B. J. Berne and R. Pecora, John Wiley, New York, 1976, pp. 376. Price £16.50. Br. Polym. J. 1977, 9, 177. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: Sydney, NSW, Australia, 2003; 1086p. [Google Scholar]
- Ren, L.; Wang, Q.; Yan, X.; Tong, J.; Zhou, J.; Su, X. Dual modification of starch nanocrystals via crosslinking and esterification for enhancing their hydrophobicity. Food Res. Int. 2016, 87, 180–188. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, W.; Liu, J.; Li, Y.; Kennedy, J.F.; Gu, Q.; Shao, S. Preparation and characterization of organic-soluble acetylated starch nanocrystals. Carbohydr. Polym. 2010, 80, 1078–1084. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Meng, L.; Liu, H.; Ali, A.; Chen, L. Poly(lactic acid)/starch composites: Effect of microstructure and morphology of starch granules on performance. J. Appl. Polym. Sci. 2017, 134, 45504. [Google Scholar] [CrossRef]
- Sjöö, M.; Nilsson, L. (Eds.) Starch in Food: Structure, Function and Applications; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Thorston, UK, 2017; pp. 1–893. [Google Scholar]
- Sha, X.S.; Xiang, Z.J.; Bin, L.; Jing, L.; Bin, Z.; Jiao, Y.J.; Kun, S.R. Preparation and physical characteristics of resistant starch (type 4) in acetylated indica rice. Food Chem. 2012, 134, 149–154. [Google Scholar] [CrossRef]
- Colussi, R.; El Halal, S.L.M.; Pinto, V.Z.; Bartz, J.; Gutkoski, L.C.; da Rosa Zavareze, E.; Dias, A.R.G. Acetylation of rice starch in an aqueous medium for use in food. LWT-Food Sci. Technol. 2015, 62, 1076–1082. [Google Scholar] [CrossRef]
- Thitisomboon, W.; Opaprakasit, P.; Jaikaew, N.; Boonyarattanakalin, S. Characterizations of modified cassava starch with long chain fatty acid chlorides obtained from esterification under low reaction temperature and its PLA blending. J. Macromol. Sci. Part A 2018, 55, 253–259. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch-Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Imre, B.; Vilaplana, F. Organocatalytic esterification of corn starches towards enhanced thermal stability and moisture resistance. Green Chem. 2020, 22, 5017–5031. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; Xia, H.; Gao, Q. Surface chemical functionalization of starch nanocrystals modified by 3-aminopropyl triethoxysilane. Int. J. Biol. Macromol. 2019, 126, 987–993. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, B.; Wu, P.; Wang, Y.; Gao, W. Ultrasound effects on the acetylation of dioscorea starch isolated from Dioscorea zingiberensis C.H. Wright. Chem. Eng. Process. Process Intensif. 2012, 54, 29–36. [Google Scholar] [CrossRef]
- Spiridon, I.; Teaca, C.-A.; Bodirlau, R. Preparation and characterization of adipic acid-modified starch microparticles/plasticized starch composite films reinforced by lignin. J. Mater. Sci. 2011, 46, 3241–3251. [Google Scholar] [CrossRef]
- Seguchi, M.; Higasa, T.; Mori, T. Study of wheat starch structures by sonication treatment. Cereal Chem. 1994, 71, 636–639. [Google Scholar]
- Huang, Q.; Li, L.; Fu, X. Ultrasound Effects on the Structure and Chemical Reactivity of Cornstarch Granules. Starch-Stärke 2007, 59, 371–378. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, X.; He, X.; Luo, F.; Gao, Q.; Yu, S. Effect of Ultrasonic Treatment on the Physicochemical Properties of Maize Starches Differing in Amylose Content. Starch-Stärke 2008, 60, 646–653. [Google Scholar] [CrossRef]
- Huang, J.; Schols, H.A.; Jin, Z.; Sulmann, E.; Voragen, A.G.J. Pasting properties and (chemical) fine structure of acetylated yellow pea starch is affected by acetylation reagent type and granule size. Carbohydr. Polym. 2007, 68, 397–406. [Google Scholar] [CrossRef]
- Huber, K.C.; BeMiller, J.N. Channels of maize and sorghum starch granules. Carbohydr. Polym. 2000, 41, 269–276. [Google Scholar] [CrossRef]
- Mahesh, B.; Kathyayani, D.; Channe Gowda, D.; Mrutunjaya, K. Blends of synthetic plastic-derived polypeptide with Hydroxypropylmethylcellulose and polyvinyl alcohol: Unraveling the specific interaction parameters, morphology and thermal stability of the polymers couple. J. Polym. Res. 2020, 27, 278. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Li, X.; Gao, W. Physicochemical properties of starches from two different yam (Dioscorea opposita Thunb.) residues. Brazilian Arch. Biol. Technol. 2011, 54, 243–251. [Google Scholar] [CrossRef]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers. Polymers 2021, 13, 584. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-Toxic Crosslinking of Starch Using Polycarboxylic Acids: Kinetic Study and Quantitative Correlation of Mechanical Properties and Crosslinking Degrees. J. Polym. Environ. 2015, 23, 588–594. [Google Scholar] [CrossRef]
- Ren, L.; Dong, Z.; Jiang, M.; Tong, J.; Zhou, J. Hydrophobization of starch nanocrystals through esterification in green media. Ind. Crops Prod. 2014, 59, 115–118. [Google Scholar] [CrossRef]
- Cui, S.; Liu, Q.; Xie, S. Starch Modifications and Applications. In Food Carbohydrates; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Colonna, P.; Buleon, A. Thermal transitions of starches. In Starches; CRC Press: Boca Raton, FL, USA, 2009; pp. 71–102. [Google Scholar]
- Slade, L.; Levine, H. Non-equilibrium melting of native granular starch: Part I. Temperature location of the glass transition associated with gelatinization of A-type cereal starches. Carbohydr. Polym. 1988, 8, 183–208. [Google Scholar] [CrossRef]
- Boufi, S.; Bel Haaj, S.; Magnin, A.; Pignon, F.; Impéror-Clerc, M.; Mortha, G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason. Sonochem. 2018, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Xiaofan, L.; Chen, Y.; Zhou, W. Effect of cross-linking with sodium trimetaphosphate on structural and physicochemical properties of tigernut starch. Food Sci. Technol. 2022, 42, e76422. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, Y. Extraction of flavonoids from flavonoid-rich parts in tartary buckwheat and identification of the main flavonoids. J. Food Eng. 2007, 78, 584–587. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Z.; Wang, Y.; Yuan, S.; Jiang, Y.; Li, Z.; Li, Y.; Yan, Z.; Meng, L.; Qiu, L. Recent progress of starch modification assisted by ultrasonic wave. Food Sci. Technol. 2023, 43, e107522. [Google Scholar] [CrossRef]
- Rahaman, A.; Kumari, A.; Zeng, X.-A.; Adil Farooq, M.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Faisal Manzoor, M. Ultrasound based modification and structural-functional analysis of corn and cassava starch. Ultrason. Sonochem. 2021, 80, 105795. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Afolabi, T.A.; Olu-Owolabi, B.I. Functional, physicochemical and retrogradation properties of sword bean (Canavalia gladiata) acetylated and oxidized starches. Carbohydr. Polym. 2006, 65, 93–101. [Google Scholar] [CrossRef]
- Andrade, I.H.P.; Otoni, C.G.; Amorim, T.S.; Camilloto, G.P.; Cruz, R.S. Ultrasound-assisted extraction of starch nanoparticles from breadfruit (Artocarpus altilis (Parkinson) Fosberg). Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124277. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Liu, H.; Chen, L. Starch Modification Using Reactive Extrusion. Starch-Stärke 2006, 58, 131–139. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, D.J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Koo, S.H.; Lee, K.Y.; Lee, H.G. Effect of cross-linking on the physicochemical and physiological properties of corn starch. Food Hydrocoll. 2010, 24, 619–625. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.H.; Kim, J.; Lim, W.; Lim, S. Characterization of nanoparticles prepared by acid hydrolysis of various starches. Starch-Stärke 2012, 64, 367–373. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Hernández-Jaimes, C.; Carrillo-Navas, H.; González, F.; Rodríguez, E.; Bello-Pérez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Acid hydrolysis of native corn starch: Morphology, crystallinity, rheological and thermal properties. Carbohydr. Polym. 2014, 103, 596–602. [Google Scholar] [CrossRef]
- Xu, Y.; Miladinov, V.; Hanna, M.A. Synthesis and Characterization of Starch Acetates with High Substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef]
- Karim, A. Methods for the study of starch retrogradation. Food Chem. 2000, 71, 9–36. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Q.; Cui, S.W. Studies on the granular structure of resistant starches (type 4) from normal, high amylose and waxy corn starch citrates. Food Res. Int. 2006, 39, 332–341. [Google Scholar] [CrossRef]
- Li, E.; Cao, P.; Cao, W.; Li, C. Relations between starch fine molecular structures with gelatinization property under different moisture content. Carbohydr. Polym. 2022, 278, 118955. [Google Scholar] [CrossRef]
- EL Halal, S.L.M.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Carreño, N.L.V.; Dias, A.R.G.; da Rosa Zavareze, E. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef]
- Eisenberg, A. The glassy state and glass transition. In Physical Properties of Polymers; Mark, J.E., Ed.; American Chemical Society: Washington, DC, USA, 1984; p. 55. [Google Scholar]
- Liu, P.; Yu, L.; Liu, H.; Chen, L.; Li, L. Glass transition temperature of starch studied by a high-speed DSC. Carbohydr. Polym. 2009, 77, 250–253. [Google Scholar] [CrossRef]
- Lourdin, D.; Coignard, L.; Bizot, H.; Colonna, P. Influence of equilibrium relative humidity and plasticizer concentration on the water content and glass transition of starch materials. Polymer 1997, 38, 5401–5406. [Google Scholar] [CrossRef]
- Rindlava, Å.; Hulleman, S.H.D.; Gatenholma, P. Formation of starch films with varying crystallinity. Carbohydr. Polym. 1997, 34, 25–30. [Google Scholar] [CrossRef]
- Liu, P.; Yu, L.; Wang, X.; Li, D.; Chen, L.; Li, X. Glass transition temperature of starches with different amylose/amylopectin ratios. J. Cereal Sci. 2010, 51, 388–391. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R. Starch: Chemistry and Technology, 3rd ed.; Academic Press: Boston, MA, USA, 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).