Abstract
The growing demand for sustainable materials has led to innovation in the development of natural compound-based solutions for industrial applications. This study introduces composite nanoparticles (NP-CsYBE) synthesized from chitosan (Cs) and saponin-rich yucca extract (YBE), highlighting their application in Pickering emulsions (PE). Characterization via DLS and AFM revealed NP-CsYBE as spherical particles with a hydrodynamic diameter of 230 nm and a ζ-potential of +36.9 mV, showing a non-aggregated morphology. Comparative analyses of emulsions formulated with Cs nanoparticles (Cs-NP) and YBE were conducted to assess the individual contributions of each component. Functional evaluations revealed that PE based on NP-CsYBE exhibited superior stability over time compared to those with Cs-NP or YBE alone. Additionally, the rheological properties of NP-CsYBE PE were influenced by pH: liquid-viscous behavior dominated at pH 4, while at pH 6.5, solid-elastic properties prevailed. Notably, increased temperature enhanced its mechanical properties. This innovative approach provides a framework for applying natural nanoparticles in PE formation, offering potential applications in the pharmaceutical, food, medical, and cosmetic industries, as well as biomaterials for protecting lipophilic substances. By leveraging natural resources, this work advances the understanding of natural nanoparticle-based systems and their role in developing sustainable and functional materials for industrial use.
1. Introduction
The type of emulsions that are stabilized by solid particles are known as Pickering emulsions (PEs). The particles are adsorbed onto the liquid–liquid interface and hinder the coalescence of the droplets of the emulsified colloidal system. This provides high stability over time, mainly due to their reduced Ostwald ripening [1]. The particles used to produce PEs can be of different natures. The first developed were inorganic (e.g., silica, clay, metal oxides, or hydroxyapatite), while later particles made of synthetic polymers, natural polymers, and diverse composites have been effectively used to produce PEs [2]. In this regard, there is a notable effort to identify natural sources to produce particles suitable for PEs. This aligns with the development of more sustainable systems that respond to the growing demand for “clean label” and synthetic surfactant-free products [3,4]. The use of natural compounds offers additional advantages, such as biocompatibility, biodegradability, cost-effectiveness, and availability from renewable sources. Consequently, the development of efficient natural particles for Pickering emulsion stabilization has become an area of growing interest and research [3].
Chitosan is a natural polymer obtained through the partial deacetylation of chitin, a structural polysaccharide that is abundant in nature as an essential component of arthropod exoskeletons. Due to its chemical characteristics, it possesses several functional properties, including structuring properties for the development of biomaterials [5]. On the other hand, saponins are natural compounds synthesized by plants in response to biotic stress [6]. Several investigations have successfully isolated and identified predominantly steroidal saponins from various Yucca species [7,8]. Saponin-rich yucca extracts exhibit remarkable functionalities as emulsifying and foaming agents [9]. From these natural compounds, composite nanoparticles can be developed by combining the structural properties of Cs with the surface interaction properties of yucca extracts. These nanoparticles hold significant potential as effective Pickering emulsifying agents, offering a natural and sustainable solution for stabilizing emulsions.
The emulsifying properties of Cs have been studied. Chitosan in solution is considered a poor emulsifier with almost no activity at the oil–water interface; its emulsifying properties are related to the viscosity of the solution. However, Cs particles in the micro- and nanometer range can produce Pickering emulsions. For example, efficient emulsifying activity has been reported for self-aggregated particles prepared by pH modification in the range of 6 to 9 in Cs acidic solution [10]. This type of self-aggregated chitosan nanoparticles was used to protect curcumin in emulsions [11]. The report indicates that a higher molecular weight of Cs, higher pH, and lower degree of acetylation resulted in stronger emulsions with gel-like properties that effectively protected curcumin. Several studies have evaluated the preparation of PE with composite particles of chitosan and proteins of different sources (e.g., soybean, moringa, spirulina), peptides, or other polysaccharides [12,13,14]. However, the available information on chitosan nanoparticles combined with saponin or similar plant extracts for Pickering emulsions is scarce.
The emulsifying properties of saponin-rich plant extracts are known. These properties are related to the saponin content and its molecular surfactant activity. Most of the reported uses of saponins or saponin-rich extracts are for the formulation of traditional emulsions. Recently, the use of quillaja (Quillaja saponaria) saponins to improve the stability of a PE formed with zein nanoparticles was reported [15]. There are also research reports on the use of saponins as emulsifiers in nano- and microcarriers of lipophilic substances [16]. However, the design and characterization of composite nanoparticles containing saponins intended to prepare Pickering emulsions have not been previously reported. Herein is reported the preparation of novel composite nanoparticles (NP-CsYBE) by combining chitosan (Cs) and a saponin-rich extract of Yucca baccata (YBE). A comprehensive analysis of the physicochemical characteristics of NP-CsYBE was conducted, along with an assessment of their functionality in the production of Pickering emulsions. Furthermore, the primary characteristics, stability, and rheological behavior of the obtained PE were evaluated.
The proposed composite nanoparticles (NP-CsYBE) incorporate natural, biocompatible, and biodegradable substances, which can be considered safe for consumption or direct application. Both are obtained from abundant renewable natural resources; chitosan is derived from the byproducts of crustacean fisheries, while YBE is an aqueous extract of an arid zone plant. The procedure for the production of NP-CsYBE is straightforward and does not utilize volatile solvents, following the guidelines of green chemistry. This study presents a framework for producing natural composite nanoparticles (NP-CsYBE) that are appropriate for preparing Picking emulsions. Such emulsions could be applied to carry and possibly protect labile lipophilic substances for their use in various fields, including nutrition, food technology, drug delivery, biomedicine, and others.
2. Materials and Methods
2.1. Reagents and Materials
Chitosan, Yucca baccata extract, Na tripolyphosphate (TPP), and deionized water were used for NP-CsYBE production. Chitosan was provided by Primex ehf (Oskarsgata 7, 580 Siglufjordur, Iceland); it has a 20% degree of acetylation (DA) by direct titration and 250 kg mol−1 of weight average molecular mass (Mw) using the Mark–Houwink equation based on measurements of the polymer’s viscosity (supplier data). Yucca baccata was harvested in the Cananea region (Sonora, México) during the summer. The collected plant samples were identified using reference specimen 25075 at the herbarium of the Universidad de Sonora (Hermosillo, Sonora, Mexico). Subsequently, YBE was obtained following a previously reported methodology [9]. The proximal composition of the extract was moisture, 5.39 ± 0.20%, and ash, 0.72 ± 0.01%, both measured using thermogravimetric methods; protein, 4.08 ± 0.19%, carbohydrates, 63.03 ± 1.64%, and saponins, 22.07 ± 0.09%, which were determined using respective colorimetric methods [17,18,19]. The fat content (1.10 ± 0.002%) was estimated using low-field NMR [20,21] with a Minispec mq20 tdNMR spectrometer (Bruker Optics, Billerica, MA, USA). Practical grade TPP was obtained from Sigma-Aldrich (St. Louis, Missouri, USA). Deionized type I water (18.2 MΩ·cm resistivity at 25 °C) was used. The emulsions were prepared with food-grade canola oil purchased from a local market. All analytical chemicals and solvents were reagent grade and acquired from recognized commercial distributors.
2.2. Preparation of the Composite Nanoparticles
Chitosan was dissolved (0.004 g mL−1) in 0.2 M CH3COOH (w/w) under magnetic stirring for 24 h. Simultaneously, an aqueous YBE solution was prepared by adding 0.4 g of YBE powder to 100 mL of water until completely dissolved. Both solutions were mixed 1:1 (v/v) and stirred for 10 min to obtain a homogeneous solution (Sol 1). TPP aqueous solution (Sol 2) was prepared at 0.02% (w/v). Sol 1 and Sol 2 were separately subjected to ultrasonic atomizing, and the resulting mists were conducted with an air stream to a mixing chamber containing 20 mL of water. Composite NPs formed within this chamber, and the resulting NP-CsYBE were collected in the water (Figure S1). The yield of composite NPs was gravimetrically estimated by mass balance. A sample of the collected nanoparticles was dried, and its final mass was related to the initial mass of the components used to produce it, namely Cs, YBE, and TPP.
2.3. Determination of Nanoparticle Concentration
After production, NP-CsYBE were concentrated up to 2% (w/v) in a rotary vacuum evaporator (BÜCHI Labortechnik AG, Meierseggstrasse, Switzerland). The NP-CsYBE concentration was estimated using a five-point calibration curve (λ600 Abs = 16.4 [NP-CsYBE] + 0.043, R2 = 0.972) of optical density (OD) at λ = 600 nm, and the NP-CsYBE mass in a known volume was determined gravimetrically. The composite nanoparticles were maintained in aqueous suspension to preserve their integrity and prevent the excessive aggregation that occurs during drying (evaporation or lyophilization). The NP-CsYBE samples were produced immediately before each analytical procedure. When storage was necessary, they were kept refrigerated (~4 °C) for no more than three days to ensure that they were always used fresh.
2.4. Saponin Content
The YBE content of NP-CsYBE was estimated indirectly by quantifying the saponins. For this purpose, the nanoparticles were freeze-dried and then subjected to acid hydrolysis using 50% sulfuric acid in ethyl acetate (v/v). The saponins were then quantified according to the method described by Baccou et al. (1977) [17]. Subsequently, the immobilization efficiency (IE) and loading capacity (LC) were calculated. IE was determined using the following Equation (1):
where M represents the amount of saponin measured in NP-CsYBE, and M0 represents the initial amount of saponin in YBE.
IE (%) = M/M0 × 100
Afterward, loading capacity (LC) was calculated to determine the amount of saponins per 100 g of NP-CsYBE using the following Equation (2):
where M represents the amount of saponin measured in NP-CsYBE, and Mf represents the total mass of NP-CsYBE.
LC (%) = M/Mf × 100
2.5. Physicochemical Characterization of the Composite Nanoparticles
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
The physicochemical characteristics of NP-CsYBE, Cs, and YBE were determined using spectroscopic techniques. Their chemical features were analyzed using an FTIR spectrometer (Nicolet iS50, Thermo Scientific, Madison, WI, USA) in attenuated total reflection (ATR) mode. The measurements were performed at room temperature, collecting 32 scans at 4 cm−1 resolution in the wavelength range of 4000 to 500 cm−1.
2.5.2. Dynamic Light Scattering (DLS)
The average hydrodynamic diameter (DH), polydispersity index (PDI), and ζ-potential measurements of NP-CsYBE were simultaneously measured using a Möbiuζ instrument (Wyatt Technology, Santa Barbara, CA, USA) at a controlled temperature (25 °C). The composite NP aqueous dispersion (1 mg/mL) was loaded in a quartz cuvette with the electrode assembly. The Möbiuζ system uses a 45 mW single longitudinal-mode laser operating at a wavelength of 532 nm. The measurements were made at a scattering angle of θ = 163.5. All experiments were carried out in quadruplicate. Data collection and analysis were conducted using DYNAMICS 7.10.1.21 software (Wyatt Technology).
2.5.3. Atomic Force Microscopy (AFM)
The morphology of the NP-CsYBE samples was examined using atomic force microscopy (AFM). Imaging was conducted utilizing the XE-Bio system (KANC 15F, Gwanggyo-ro 109, Suwon 16229, Republic of Korea) operating in non-contact mode under atmospheric conditions, employing an NCHR cantilever. Data were collected and analyzed using XEI 1.8 software (KANC 15F, Gwanggyo-ro 109, Suwon 16229, Republic of Korea). Before imaging, samples were prepared by depositing a single drop of the respective aqueous dispersion (1 mg/mL) onto small glass plates (12 mm in diameter and 1 mm in thickness), followed by natural evaporation at room temperature. Morphological features and height measurements were acquired in triplicate across a 2 μm × 2 μm area.
2.6. Preparation of Pickering Emulsions
The emulsions were prepared by mixing the oil phase (canola oil) in varying proportions with the NP-CsYBE suspension (aqueous phase) at different concentrations. These mixtures were dispersed (20,000 rpm) for 2 min using an Ultra Turrax instrument (model T25, IKA Labortechnic, Staufen, Germany). Once the emulsions were prepared, they were kept at 4 °C until analysis.
Two distinct emulsions were prepared as benchmarks for comparison. One was a Pickering emulsion prepared only with chitosan nanoparticles (NP-Cs). The NP-Cs were obtained using the same process as composite NP-CsYBE without YBE. The second comparison emulsion was a conventional emulsion made with the saponin-rich yucca extract. The emulsions prepared with nanoparticles, NP-CsYBE, or NP-Cs, using 1.7% (w/v) nanoparticles in the aqueous phase. The emulsion with YBE was prepared with 3.33% (w/v) extract in the aqueous phase, which was equivalent to the estimated content of saponin contained in the composite NP-CsYBE. The emulsions were prepared with a fixed oil fraction of φ = 0.5 (v/v), and stability was evaluated for one month, measuring on days 1, 7, and 30.
2.7. Pickering Emulsions with Different Oil Fractions and Nanoparticle Concentrations
To study the effect of the oil volume fraction on emulsion properties, emulsions of different oil volume fractions (φ = 0.2, 0.3, 0.4, 0.5, and 0.6 v/v) were prepared with NP-CsYBE at a fixed concentration of 1.7% (w/v). Additionally, the effect of the concentration of nanoparticles on emulsion preparation and stability was investigated. Aqueous suspensions of NP-CsYBE at different concentrations (0.1%, 0.5%, 1%, 1.5%, and 2% w/v), and a fixed oil volume fraction of φ = 0.5 (v/v) were used to produce PE.
2.8. Characterization of the Pickering Emulsions
2.8.1. Emulsification Index
The emulsification index (EI) was determined to evaluate the functionality of the nanoparticles in the preparation of PE and their stability over time according to previously reported procedures [22,23]. The total height (TH) of the liquid mixture and the height of the emulsified layer (HEL) were measured, and the emulsification index (EI) was calculated using Equation (3):
IE (%) = HEL/TH × 100
2.8.2. Microscopy
The assembly and morphology of micelles in the different emulsions were observed using an inverted microscope model XSB 1A equipped with a 9 MP digital camera model MU900 (14370 Myford Rd #150, Irvine, CA 92606, USA). A drop of each emulsion was deposited on a microscope slide, which was then covered with a cover slip. All of the measurements were performed at 25 °C. A 10× image magnification was used to capture and process images using AmLite software version 20180326 (14370 Myford Rd #150, Irvine, CA 92606, USA). The Fiji program version 1.54i was used to analyze and quantify the micelles in the selected quadrant (ImageJ2, ver. 2.14.0/1.54f, 2023).
2.8.3. Dynamic Light Scattering
The mean hydrodynamic diameter (DH) and polydispersity index (PDI) of the micelles in the emulsions were measured using the Möbiuζ instrument. For this, the emulsion samples were diluted 1:5 with water. The parameters and measurement conditions were the same as previously described for NP-CsYBE analysis.
2.8.4. Rheological Measurements
The rheological behavior of the Pickering emulsion with NP-CsYBE with a higher emulsification index and stability over time was studied. A dynamic shear AR-G2 rheometer (TA Instruments, New Castle, DE, USA) equipped with a stainless steel cone-plate geometry (diameter: 60 mm; gap: 65 µm) and a Peltier system for temperature control was used. After loading the emulsion, the samples were coated around their periphery with light silicone oil to minimize evaporation. All experiments were performed within a frequency range of ω = 0.1–100 rad·s−1, with 1% strain, to measure the storage and loss moduli (G′ and G″, respectively). According to preliminary strain sweep tests (Figure S2), the linear viscoelastic region spanned up to 2% strain, so a strain of 1% was selected. During the viscoelastic measurements, the emulsion was heated and cooled between 25 and 85 °C at 0.3 °C min−1. Mechanical spectra (ω = 0.1–100 rad s−1; γ = 1%) were recorded at the beginning, after heating, and after cooling. Additionally, the effect of pH on the obtained Pickering emulsions was studied. The pH of the PE, measured with a Starter 3100 (Ohaus, Parsippany, NJ, USA) potentiometer, was 4. Two separate PE samples were prepared similarly, and their pH was adjusted to 2 using 0.5 M HCl and to 6.5 by adding 0.5 M NaOH, respectively. The temperature rheological experiments were repeated on these samples.
2.9. Statistical Analysis
All quantitative data were statistically analyzed and expressed as the mean ± standard deviation (SD) based on triplicate measurements. The statistical significance of mean values among different treatment groups was determined by a one-way analysis of variance (ANOVA) using Tukey’s test. A value of p ≤ 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Physicochemical Characterization of Composite Nanoparticles
Composite nanoparticles comprising chitosan and saponin-rich yucca extract were effectively produced. The obtained nanoparticles were kept in aqueous suspension to avoid aggregation as much as possible. The amount of saponin incorporated into the nanoparticles relative to the amount of saponin of the YBE was IE = 34 ± 2%. Each gram of NP-CsYBE contained 325 ± 17 mg of saponin. This loading capacity of NP-CsYBE indicated the integration of both compounds during NP formation. This indicated that the implemented process combining chitosan and YBE effectively achieved the formation of composite nanoparticles. However, some research opportunity areas remain because some aspects of the process, like control of the YBE quantity incorporated, were not optimized. Previous studies using different compounds have reported that the processes used to obtain composite nanoparticles can be affected by the characteristics of the molecules present in each component and their interfacial relationship. Specifically for chitosan, the significant influences of molecular weight and degree of acetylation have been observed. For instance, at higher Cs Mw and DA, higher LC values have been reported, which has been explained by an increase in the hydrophobicity of the particle-forming composite [11,16].
The FT-IR spectrum of the composite nanoparticles and the spectra of their pristine components, Cs and YBE, are shown in Figure 1. In the three spectra, the absorption bands at 3300 cm−1 (associated with the stretching vibration of the hydroxyl group), 2900 and 2800 cm−1 (attributed to the symmetric and asymmetric stretching of C H, respectively), and the carbohydrate fingerprint region (complex absorption bands from 800 to 1200 cm−1) can be observed. The appearance of late vibrations in the spectrum of the composite NPs indicated the presence of carbohydrates from Cs and YBE in the NPs.
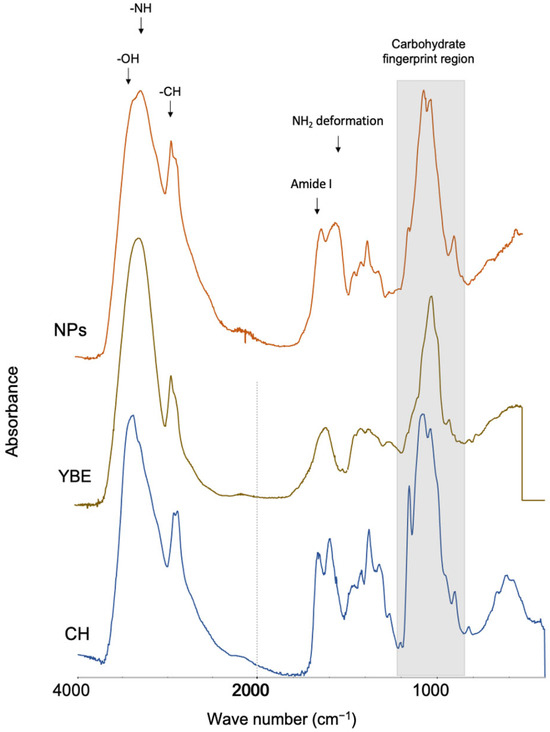
Figure 1.
FT-IR spectra of chitosan (Cs), Yucca baccata saponin-rich extract (YBE), and composite NPs of Yucca baccata saponin-rich extract and chitosan (NPs). The arrows indicate the main absorption bands. The shaded area indicates the “fingerprint” region of the carbohydrates.
Both the NP-CsYBE and Cs spectra showed a characteristic absorption pattern corresponding to chitosan: 1652–1630 cm−1 (amide I, C=O stretching), 1593–1557 cm−1 (amide II, NH2 deformation) and 1373 cm−1 (amide III, C–N stretching) [24]. These results confirmed the presence of chitosan (Cs) in the composite NPs. On the other hand, the band signals detected in the Yucca baccata extract (YBE) extract were consistent with previous reports and suggested the presence of steroidal saponin [8]. In addition, it could be observed that due to the occurrence of various compounds, the intensity of these bands was altered. Indeed, due to the interaction of Cs and YBE, the intensity of the absorption peaks in the spectrum of composite NPs changed, as could be appreciated in several bands, such as those at 3300 cm−1, 2800 cm−1, and those in the fingerprint region. A similar phenomenon was reported for chitosan nanoparticles with pea proteins, where the presence of other compounds led to a modification of the intensity of the signals, probably due to the presence of other molecules [5]. These results suggested that both compounds (Cs and YBE) coexisted in the resulting composite NPs.
The size distribution of NP-CsYBE, given by the hydrodynamic diameter (DH), was analyzed using DLS. Figure 2 shows the size distribution histogram of the composite NPs, where a broad size distribution can be observed with three populations centered at 18 nm, 235 nm, and 2069 nm. The insert in Figure 2 summarizes the main parameters of the three populations. The second population has the highest mass (78.6%), representing the predominant population in the obtained NP-CsYBE.
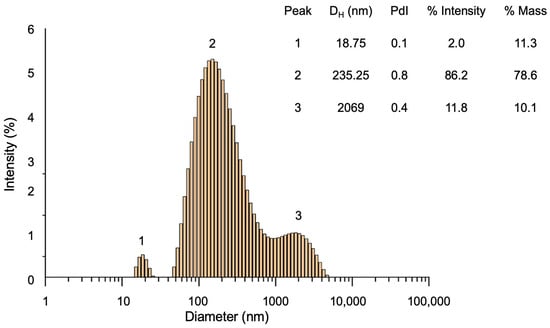
Figure 2.
Histogram of size distribution of NP-CsYBE obtained by DLS. DH: hydrodynamic diameter, PdI: polydispersity index.
The DH and ζ-potential of NP-CsYBE were compared with those of the nanoparticles prepared from chitosan alone using the same preparation process. The chitosan nanoparticles were slightly smaller, with a DH of 201.03 nm (97% intensity percentage) and ζ-potential of +22.79 mV, corresponding to the charge of chitosan. The formation of nanoparticles based on biopolymers is challenging because they tend to produce larger particles than those from inorganic compounds. They also tend to increase in size when mixed with other compounds. The size of nanoparticles is a fundamental property because it affects their application and use. In this sense, it has been reported that nanoparticles could be used as drug carriers and, more recently, as emulsifiers for the preparation of PE due to their nanometric dimension [25].
NP-CsYBE had a ζ-potential value of +36.9 mV, indicating that the surface of the particles was positively charged, possibly due to the presence of protonated amino groups from chitosan [26]. It has been reported that ζ-potential values higher than 30 mV are associated with better stability of nanoparticle colloidal suspensions [27]. Furthermore, positive ζ-potential values indicate the presence of cationic surface charges that could interact with negatively charged biological membranes [26].
AFM was used to analyze the morphology of the composite nanoparticles (Figure 3). NP-CsYBE appeared as quasi-spheres in a non-aggregated state. The particles were observed consistently within a range of size smaller than 300 nm. These observations of the dried nanoparticles were in agreement with the DLS measurements of the NP-CsYBE aqueous suspension. Several studies have produced composite nanoparticles using chitosan due to its structural properties. For example, chitosan-alginate nanoparticles for controlled release of vitamin B2 were obtained with an average size of about 264 ± 32 nm [28].
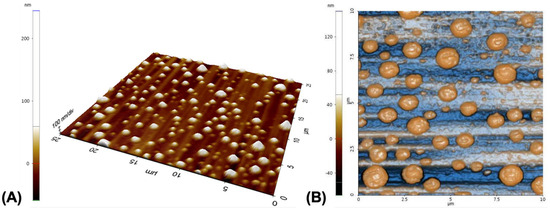
Figure 3.
Atomic force microscopy (AFM) images of NP-CsYBE: (A) 3D AFM image, and (B) 2D AFM image.
In another study, zein/chitosan complex particles for Pickering emulsions reported size ranges of 1–2 μm with a zein concentration of 10% [29]. In a biomedical application for ocular treatment, chitosan-coated PLGA nanoparticles were developed with a size range of 334 ± 67.95 to 386 ± 15.14 nm [30]. It has been noted that when working with Cs in the elaboration of materials, the size of the resulting material is strongly influenced by the physicochemical characteristics of Cs, its concentration in solution, and the physicochemical characteristics of other compounds present in the material. NP-CsYBE, prepared from natural materials, had dimensions within the previously reported range, making them ideal for potential applications as vehicles of bioactive substances, biomedicine, and especially as emulsifiers for Pickering emulsions.
PE stabilization is achieved through the adsorption of nanoparticles onto emulsion droplets. This process decreases the energy at the interface, thereby enhancing colloidal stability [31]. The properties of the particles, such as size, concentration, and hydrophobicity, as well as the properties of the oil phase, including the amount (volume) and type of oil, can influence the performance of the system [32]. For NP-CsYBE, Cs provides the structural features and positive electrostatic charges that improve their suspension stability, and the YBE content can enhance the emulsifying effect during PE formation. Furthermore, NP-CsYBE production does not use organic solvents that could alter their interfacial activity. In addition, the observed size and morphology of NP-CsYBE appeared adequate to produce Pickering emulsions.
3.2. Composite Nanoparticles in Pickering Emulsions
3.2.1. Characterization of the Composite Nanoparticles
The emulsification index (EI) and micelle size were used to characterize and evaluate the functionality of NP-CsYBE to produce and stabilize Pickering emulsions. The Pickering emulsions obtained with NP-CsYBE were compared with PE produced with chitosan NPs (NP-Cs) and conventional emulsions made with YBE. The emulsions were measured after 1, 7, and 30 days of preparation to compare their stability over time.
NP-CsYBE effectively produced Pickering emulsions. NP-Cs and YBE also produced emulsions. In Figure 4A–C, it is possible to observe the different emulsions and their storage stability. Only the PE made with NP-Cs shows the formation of a top layer formed by oil coalescence over the emulsified phase, which is more evident after 30 days. The EI values of all emulsions were significantly different. The YBE emulsion (non-PE) exhibited the highest EI value, followed by the PE of NP-CsYBE and the PE of NP-Cs, which exhibited the lowest EI values across all days evaluated (Figure 4D). The stability of the YBE emulsion exhibited a notable decline, with a significant reduction in the emulsified phase from 70.29 ± 1.16% at day 1 to 64.81 ± 1.18% at day 30. Conversely, the PE of NP-CsYBE demonstrated stability of the emulsified phase, with minimal fluctuations over time, from day 1 (61.22 ± 1.07%) to day 30 (61.58 ± 0.66%). The emulsified phase of the PE made with NP-Cs also showed a significant decline from 40.03 ± 1.35% on day 1 to 35.58 ± 1.52% on day 30.
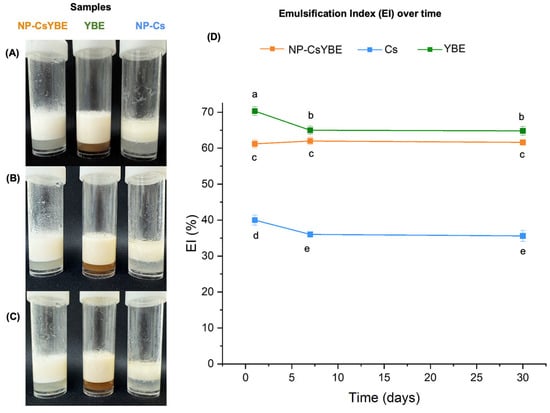
Figure 4.
Photographs and emulsification index (in percentage) of emulsions at oil fraction φ = 0.5 over time: (A) day 1; (B) day 7, and (C) day 30 at 4 °C. Values of all PEs over time plotted (D). Values are presented as mean ± standard deviation; means that do not share a letter are significantly different.
The favorable emulsifying and foaming properties of Yucca schidigera extract have been well documented. These properties have been attributed to the presence of saponins in the extract. Emulsions with an EI of over 70% have been reported [9,33]. Due to their molecular structure, saponins are considered amphiphilic molecules with high interfacial activity [34]. However, like other emulsions based on amphiphilic molecules, they are considered thermodynamically unstable systems with micelle coalescence and Ostwald ripening occurring [32]. This phenomenon can be observed in the EI results obtained. Despite the YBE emulsion achieving a higher EI, this emulsion did not maintain its stability over time.
Chitosan in solution has been used as an emulsifier in traditional emulsions mainly because of the viscosity that it adds to the system and its hydrophilic–lipophilic balance. However, its activity is less efficient than that of other surfactant molecules [31]. By contrast, chitosan particles, particularly those obtained by self-aggregation, show good emulsifying properties for Pickering emulsions. It has been observed that chitosan particle PEs have better properties when the system is at pH 6 or higher and low emulsifying activity at pH 4.5 or lower [20,35]. In this work, the PE with NP-Cs did not undergo any modification, maintaining the natural pH of the emulsion as obtained according to the methodology (pH 4). Accordingly, the EI of the PE made with NP-Cs was low. Apparently there were not enough chitosan nanoparticles, and their interfacial activity was insufficient to completely emulsify the oil fraction, favoring the formation of the upper oil layer.
The composite nanoparticle NP-CsYBE formed a PE with an EI value close to that observed for an equivalent amount of YBE. By contrast, the stability of the emulsions seems to be improved since the EI values did not show significant differences in 30 days. The difference between the PE formed with the composite nanoparticles and chitosan nanoparticles could indicate the presence of saponins in the surface of NP-CsYBE. In this way, the saponins enhanced the interfacial activity of NP-CsYBE.
The stability of the emulsions was also evaluated by measuring the micelle size (DH) over time using DLS. The results were plotted for each sample (Figure 5). It can be observed in Figure 5B that the emulsion with the smallest DH at day 1 was YBE (1.87 ± 0.19 µm), followed by the Pickering emulsion with NP-Cs (4.88 ± 0.12 µm) in Figure 5C, and finally the PE with NP-CsYBE (5.23 ± 0.19 µm) in Figure 5A. However, an increase in size can be observed, with the most significant increase of 90.69% for YBE related to day 1, followed by NP-Cs, which presented a DH size increase of 50.36%, and finally, NP-CsYBE, which presented an increase of 24.32%.
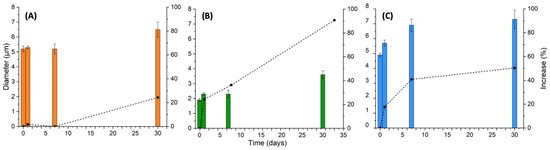
Figure 5.
Diameters of the micelles (bars, left axes) and their relative size increases (black dots, right axes) for the samples (A) NP-CsYBE, (B) YBE, and (C) NP-Cs, as a function of time (0, 1, 7, and 30 days) and percentage increase in size relative to day 0. Dotted lines included as guides to the eye.
Emulsion stability is the ability of a system to maintain its physicochemical properties such as size, shape, morphology, and other characteristics over time. An increase in micelle size could indicate a coalescence process, which in turn would indicate thermodynamic instability of the system [36]. The amphiphilic molecular emulsion with YBE showed the greatest increase in micelle size related to day 1, doubling its initial size. Despite the excellent emulsifying activity shown by its EI, the coalescence of micelles was constantly observed over time. The PE with chitosan NPs presented an initial micelle size similar to the PE with NP-CsYBE. Despite the lowest EI, it showed better stability than the emulsion with YBE. The PE prepared with NP-CsYBE showed the best relative stability, as indicated by the overall change in micelle size at day 30. The better relative stability of the PE suggested that the coverage of the micelles with nanoparticles prevented, to some extent, the coalescence process. In addition, the saponins provided by YBE in NP-CsYBE improved the interfacial activity of these composite nanoparticles.
3.2.2. Effect of Oil Volume Fraction and Nanoparticle Concentration
The effects of the oil volume fraction and NP concentration on the properties of the PE with NP-CsYBE were investigated. To this end, a series of oil fractions (φ = 0.2, 0.3, 0.4, 0.5, and 0.6 v/v) were utilized, with a constant NP-CsYBE concentration of 1.7% w/v. In addition, varying concentrations of NP-CsYBE (0.1, 0.5, 1, 1.5, and 2% w/v) with a fixed oil fraction of φ = 0.5 (v/v) were examined to estimate the minimum concentration of nanoparticles required for PE formation. These tests also provided information about the relationship between nanoparticle concentration and EI.
As the oil fraction increased at a constant nanoparticle concentration, the EI values concomitantly rose (Table 1). In the range of oil fraction from φ = 0.2 to φ = 0.5, the emulsification index of the PE was proportional to the oil fraction. This behavior was observed up to the fraction φ = 0.5, since at fraction φ = 0.6, the formation of the PE with NPs was not achieved under the used conditions. The EI values of the PEs of each oil fraction showed no significant differences over time, indicating the stability of the PEs provided by NP-CsYBE. However, there were significant differences between the oil fractions (Tukey’s pairwise comparison, one-way ANOVA, significance level 0.05), with a higher value in the φ = 0.5 oil fraction of 67% and a lower value in the φ = 0.2 oil fraction of 32% at day 1.

Table 1.
Emulsification index % (EI) of all Pickering emulsions at different oil fractions and different NP-CsYBE concentrations over time.
The results for the different concentrations of NPs tested showed an increase in EI proportional to the increase in NP concentration equal to or greater than 1% (Table 1). Below this concentration, PE formation was not achieved. The EI values on day one for NP-CsYBE at concentrations of 1, 1.5, and 2% were 64.17 ± 1.44%, 66.11 ± 1.35%, and 68.38 ± 0.73%, respectively. These EI values did not remain constant over time and showed a decrease at day 21, with significantly different values at concentrations of 1 and 1.5% (Tukey’s pairwise comparison, one-way ANOVA, significance level 0.05). On the contrary, at a concentration of 2%, there were no significant differences in EI values from day 1 to day 21, indicating stability over time.
Figure 6 shows images of the PEs of the different oil fractions (Figure 6A) and NP-CsYBE concentrations (Figure 6B). It is possible to observe the evolution from day 0 to day 21. For the oil fraction, a uniform mix can be seen at day 0, and from day 1, the emulsified phase can be observed in all oil fractions except for the φ = 0.6 oil fraction. This oil fraction (φ = 0.6) does not form a uniform emulsified phase, maybe because the concentration of NPs was insufficient to surround the oil droplets that consequently coalesced. The observed effects of this phenomenon are particularly pronounced on day 7. The images of the PEs prepared with different NP-CsYBE concentrations (Figure 6B) show a phase separation at 0.1 and 0.5% from day 0, which is accentuated on day 1; also, it is possible to appreciate an oil layer without proper PE formation at these concentrations. Conversely, similar emulsified phases and stability behavior are observed for NP-CsYBE concentrations of 1, 1.5, and 2%. It has been noted that higher concentrations of NPs favor the formation of the emulsified phase. This is attributed to an excess of particles relative to the oil/water interfacial area created during emulsification rapidly stabilizing the droplets. Furthermore, the rheological properties of the continuous phase can be modified by the surplus of nanoparticles, improving the stability of the emulsion [26].
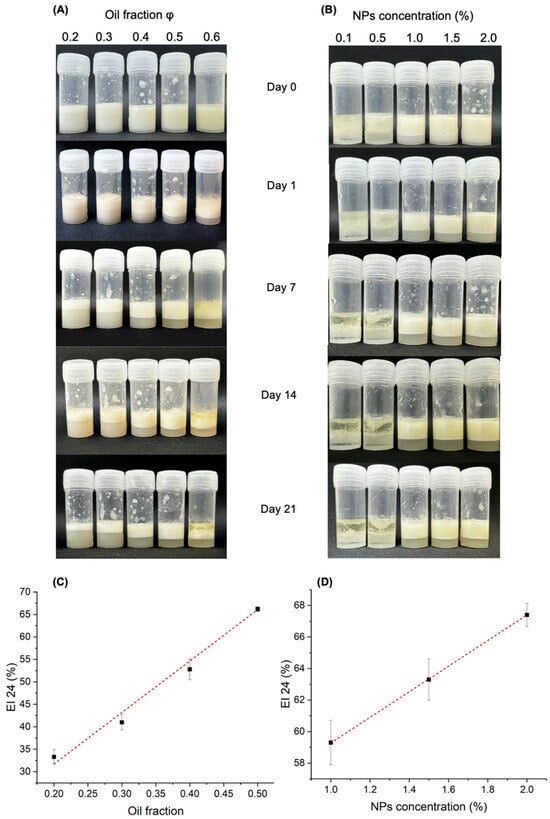
Figure 6.
Appearance and emulsification index (EI) of Pickering emulsions: (A) photographs at different oil volume fractions of φ = 0.2, 0.3, 0.4, 0.5, and 0.6 (v/v) at a fixed concentration of NP-CsYBE (1.7% w/v); (B) photographs at different NP concentrations of 0.1, 0.5, 1, 1.5, and 2% (w/v) at a fixed oil fraction of 0.5 (v/v) over time at 4 °C. (C) Plot of EI values (black circles) for all oil fractions at day 21; (D) plot of EI values (black circles) for all NP-CsYBE concentrations at day 21. Dotted lines included as guides to the eye.
Due to the apparent behaviors of the obtained PEs, the EI values at day 21 of the formed PEs were plotted against their respective oil fractions and NP-CsYBE concentrations (Figure 6C and Figure 6D, respectively). In Figure 6C, a linear trend (y = 110.5x + 9.65, R2 = 0.986) can be observed up to an oil fraction of φ = 0.5. A possible explanation is that as the oil fraction increases, larger droplets are formed and covered by the NPs, and the integration of different liquid phases increases the viscosity of the colloidal suspension and consequently improves the EI [37,38]. Conversely, when the oil fraction exceeds φ = 0.6, the amount of nanoparticles is apparently insufficient to cover the oil droplets and overcome their coalescence. A similar behavior of PEs was reported previously. A PE stabilized with zein and Arabic gum composite nanoparticles had a higher emulsification index with a higher oil fraction; however, flocculation was observed up to φ = 0.7 [37]. This behavior was also reported for a PE stabilized by chitosan and Arabic gum composite nanoparticles [38]. Differences among studies about the highest oil fraction that can produce a PE could be related to the properties of the particles used (i.e., size, morphology, composition, chemical characteristics, etc.) and the properties of the oil.
The variation in EI values at day 21 related to the NP-CsYBE concentration also showed linear behavior (y = 8.1x + 51.18, R2 = 0.999). In this case, only three nanoparticle concentrations were able to produce PE. From 1% NP-CsYBE onward, the EI value rose proportionally with the nanoparticle concentration. NP-CsYBE concentrations lower than 1% did not achieve PE formation. Apparently the quantity of nanoparticles in the aqueous phase was insufficient to cover the oil droplets and stabilize the emulsion, and phase separation occurred [38].
The morphology of the PE droplets at different oil fractions and the evolution of their diameter over 21 days can be observed in Figure 7. In the micrographs (Figure 7A), the micelles appear smaller at lower oil fractions. Oval and deformed micelles are present at the highest oil fraction (φ = 0.6). The statistical mean hydrodynamic diameters, determined using DLS, of the higher oil fraction PE were consistently larger, over 6 μm for φ = 0.6. For the PE with φ = 0.5, the droplet diameter increased on the first day up to 4 μm and to over 5 μm after 7 days; thereafter, the size remained stable until day 21. The PE with φ ≤ 0.4 had the lowest micelle size, ranging between 2 and 3.5 μm, with relatively good stability over 21 days. Both the microscopy and DLS results were consistent and reinforced the proposition that in emulsions with an oil fraction greater than 0.5, the amount of nanoparticles added was insufficient to stabilize the oil droplets [38,39].
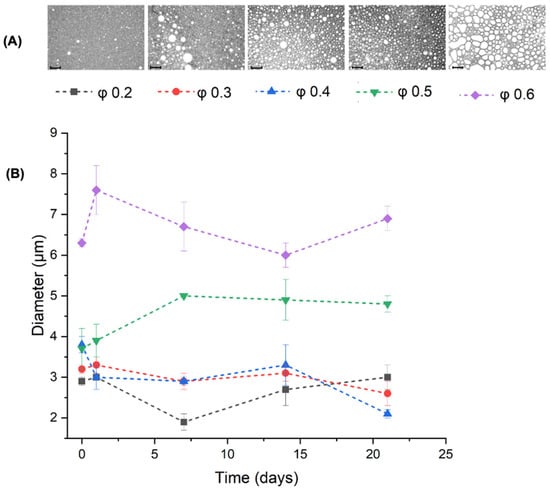
Figure 7.
(A) Optical micrographs of Pickering emulsions prepared with different oil volume fractions at day 21 (scale bar = 0.10 mm). (B) DLS analysis of the diameter of PE droplets at different at different oil ratios (φ) with a fixed NP-CsYBE concentration of 1.7% (w/v).
The effect of the NP-CsYBE concentration on the PEs prepared with a fixed oil fraction can be observed in Figure 8. The micrographs of the PEs prepared with less than 1% NP-CsYBE do not show oil droplets. At higher NP-CsYBE concentrations (≥1%), an increase in the number of spherical shape oil droplets is observed (Figure 8A), which is consistent with the observation that at higher concentrations of NPs, more of them are incorporated into the liquid interfaces created during emulsification, thus forming and stabilizing the PE. Conversely, if the quantity of nanoparticles at the interface is insufficient to form an effective barrier between liquid phases, processes leading to instability, such as coalescence and flocculation, are not hindered [40]. In such cases, the occurrence of irregularly shaped oil droplets is probable, and the formation of PE is unlikely.
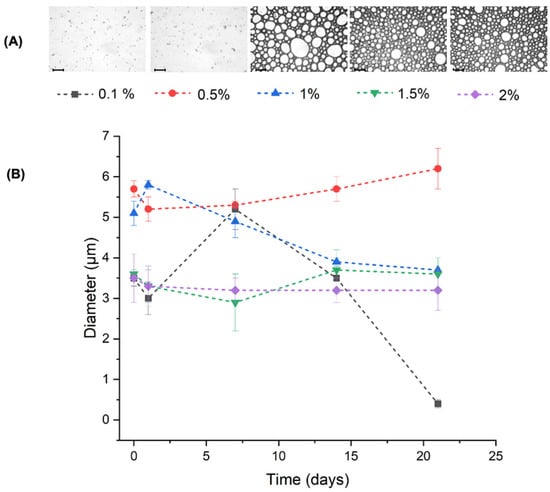
Figure 8.
(A) Optical micrography of Pickering emulsions prepared with different NP concentrations at day 21 (scale bar = 0.10 mm). (B) DLS analysis of the diameter of micelles smaller than 10 μm of Pickering emulsions at different NP-CsYBE (w/v) with a fixed oil fraction of φ = 0.5 (v/v) over time.
The PEs with NP-CsYBE concentrations of 1.5 and 2% exhibited stability over time, with the DH ranging between 3 and 4 μm (Figure 8B). The size variations observed in the PEs with lower NP-CsYBE concentrations (≤1%) could be attributed to instability at the interface of the droplets, leading to their coalescence and the formation of droplet groups of different sizes. Recent findings have demonstrated that the addition of high concentrations of nanoparticles enhances stability at the oil–water interface through spatial impedance, leading to an effective reduction in droplet aggregation [37].
3.2.3. Rheological Properties of Pickering Emulsions
A Pickering emulsion with 2% w/v nanoparticles and φ = 0.5 was prepared, and its rheological behavior was studied. The pH of this sample was 4. This sample was subjected to a temperature ramp from 25 to 85 °C, followed by cooling from 85 to 25 °C, and viscoelastic parameters were measured at five different angular frequencies. These tests were combined with a mechanical spectrum before and after each temperature ramp (at 25, 85, and back at 25 °C). The results of the rheological study are summarized in Figure 9.
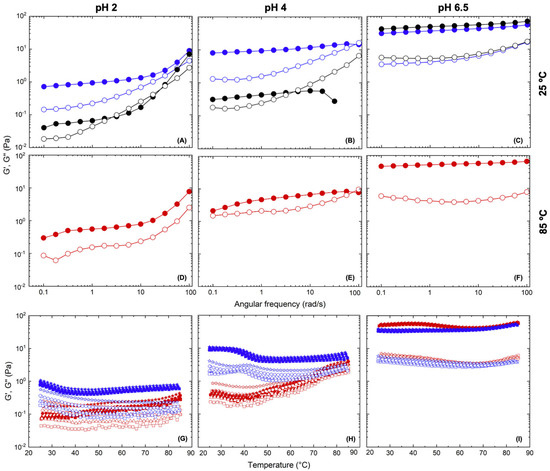
Figure 9.
Rheological spectra. Frequency sweeps of PEs with NP (2% w/v and φ = 0.5 oil fraction v/v) at different pH values (2, 4, and 6.5) at: (A–C) room temperature (black symbols), (D,E) 80 °C (red symbols), and (A–C) 25 °C (blue symbols). All of the measurements were conducted at γ = 1.0%. On the bottom (G–I), dynamic temperature-frequency sweeps of PE at different pH values at ω = 1 (square), 2.15 (triangle), 4.64 (circle), and 10 (star) rad/s; γ 1%. G′, closed symbols, and G″, open symbols.
The mechanical spectrum of this original emulsion (pH 4) looked like that of a very weak gel: G′ had practically no dependence on frequency and predominated over G″ up to 2 rad⸱s−1 (black traces, Figure 9B). After this cross point, G″ (ω) increased as a function of frequency. This behavior is typical of a semi-dilute emulsion (φ ≤ 0.6). It is at this point that the droplets of the dispersed phase begin to become caged by their neighbors and cannot easily flow over each other as they would in the dilute regime. Under these conditions, hydrodynamic interactions between the particles govern the viscoelastic response of the emulsion, which resembles that of a weak gel [41].
When this emulsion was heated to 85 °C (red traces, Figure 9H), a gradual and sustained strengthening of the mechanical properties was noticed (particularly at temperatures above 40 °C), which was also evident from the mechanical spectrum at 85 °C (Figure 9E). There was an increase in G′ values of more than one order of magnitude. Furthermore, it is noteworthy that during cooling back to 25 °C (Figure 9H), this improvement in mechanical properties was preserved, with a slight reinforcement at temperatures below 40 °C. The final mechanical spectrum at 25 °C displayed the behavior of a gel stronger than the initial one, in which the elastic modulus was independent of frequency and higher than the loss modulus (blue traces, Figure 9B). At long deformation times (low frequencies) no terminal flow was observable, while at short times there was a slight increase in G″, as if approaching the glassy region.
Undoubtedly, the results indicated the presence of sui generis behavior, which should be the consequence of changes in the morphology and interactions between droplets of the dispersed phase, probably involving the nanoparticles stabilizing the emulsion and changing the nature of the overall network, as will be discussed below.
Two analogous NP-CsYBE Pickering emulsions were also prepared for evaluation at different pH values (2.0 and 6.5), simulating physiological conditions. This was performed in order to evaluate the behavior of NP-CsYBE Pickering emulsions at the pH present in the digestive system due to their potential use in the transport of lipophilic bioactive substances or in case of being added to some food and later consumed. The emulsions were then subjected to the same test procedures. The initial frequency sweep at 25 °C of the sample at pH 2 (black traces, Figure 9A) showed a certain resemblance to the behavior of the sample at pH 4, but with lower values of both moduli and a marked increase in the storage modulus at high frequencies. However, during the heating/cooling experiment, it was possible to observe a different behavior from that of the original sample. Compared to the sample at pH 4, only a very slight strengthening of the mechanical properties was noticed during the heating of the sample at pH 2 (red traces, Figure 9G). This was more evident in the mechanical spectrum at 85 °C (Figure 9E), which was very similar to the original one at 25 °C. However, during cooling (blue traces, Figure 9G), it was possible to appreciate a strengthening of the mechanical properties below 40 °C—very similar to what was observed in the sample at pH 4. This was confirmed by the mechanical spectrum at 25 °C after thermal treatment (blue traces, Figure 9A), which exhibited the pattern of an extremely weak gel, but stronger than the original one before the heating/cooling processes.
Compared to the samples at pH 2 and 4, the sample at pH 6.5 showed a very different behavior. The mechanical spectrum of the emulsion before heating was typical of a gel that was stronger than the previous ones, with higher G′ values, which were frequency independent, while the loss modulus was almost an order of magnitude lower (black traces, Figure 9C). During heating/cooling, this emulsion did not display significant changes in viscoelastic properties, retaining, in general, the same mechanical properties before, during, and after the heating/cooling processes (Figure 9C,F,I).
A general look at the rheological behavior of the emulsions at different pH values showed a different pattern in each case. In general, as pH increased, the mechanical properties of the emulsion gel became stronger. This was evident by plotting the values of G′ (at 1 rad/s) of the starting emulsion vs. temperature (Figure S3A). To confirm this, a complementary experiment was performed to evaluate the ζ-potential of the nanoparticles at different pH values (2 and 6.5). And it was observed that there was a dependence of the charge of the nanoparticles on pH, with the ζ-potential being higher at pH 2 (~40 mV) and lower at pH 6.5 (~10 mV). Most likely this indicated that a decrease in the surface charge of the nanoparticle-containing chitosan resulted in a strengthening of the emulsion gel. As the pH was lowered below the pK0 of chitosan (6.0), its amino groups began to protonate continuously, increasing electrostatic repulsions between the polysaccharide chains [42]. These Coulombic forces are known to be an important factor in the stability of emulsions, but most likely, in this case, they worked against the associative interactions between the droplets, thus weakening the elastic properties of the gel. Hydrophobic interactions are known to occur in chitosan solutions [43], and these interactions increase with neutralization of the polyelectrolyte charges and with concentration [44]. Particularly for the sample at pH 2, in which almost all of the amino groups of chitosan must be ionized, the weakest mechanical characteristics were observed. Even under these conditions, hydrophobic interactions exist, but very few [45], and therefore have the lowest effect on the rheological properties.
This counterbalance between electrostatic and self-associative hydrophobic interactions between chitosan chains became more evident during the heating/cooling cycle (Figure S3B,C). It is well documented that hydrophobic interactions are enhanced as the temperature increases. Water shields, also known as “icebergs,” are formed at low temperatures. These consist of an outer layer of water molecules around the amide groups (hydrophobic solvation), thus keeping the hydrophobic polymer segments in solution [46]. Upon heating, these “icebergs” begin to break down, resulting in the formation of self-associative chitosan domains due to hydrophobic interactions between segments of the polymer chains. Thus, the entropic increase resulting from the expulsion of the water molecules surrounding the hydrophobic segments becomes the driving force for the strengthening of the elastic characteristics of the emulsion gel.
In the Pickering emulsions with NP-CsYBE, hydrophobic interactions were present at the molecular level, but several singularities of the system should be considered. The chitosan chains were not in solution; they were hydrated on the surface of the nanoparticles that were attached to the oil–water interface. The hydrophobic interactions occurred primarily on the surface of the oil droplets, most likely between lipids and the steroidal moieties of the saponins. Furthermore, hydrophobic interactions could also arise between the composite nanoparticles, participating saponins, and acetylated units of chitosan. With increasing temperature, as hydrophobic interactions are favored, the number of hydrophobic junction points between nanoparticles presumably increased, giving rise to a stronger three-dimensional emulsion network. The hydrated state of the involved molecules before heating was not recovered on cooling. A greater number of interactions between NP-CsYBE, either involved in stabilizing the oil droplets or in the non-adsorbed fraction, resulted in an enhanced solid-elastic behavior observed at pH 6. A similar effect has been observed in coalesced chitosan and chitosan composite nanoparticles to produce Pickering emulsions [35,39,47].
4. Conclusions
Novel natural nanoparticles composed of Cs and YBE were prepared and demonstrated efficacy in PE formation (Figure 10). These composite nanoparticles produced Pickering emulsions with higher stability over time compared to the different emulsifiers tested in this study. The conditions for PE production with NP-CsYBE were also established, requiring an aqueous phase with a concentration of at least 1.5% (w/v) nanoparticles and an oil fraction up to φ = 0.5 (v/v). The rheological properties of the Pickering emulsions were shown to be affected by pH and temperature. The emulsions exhibited viscous-liquid behavior at pH below 4, while they exhibited solid-elastic behavior at pH above 6.5. These results indicated that combining these two natural compounds enabled the formation of a new nanobiomaterial with emulsifying properties, overcoming the challenges associated with the high viscosity of chitosan and the complexity of natural source yucca extract. This information provides the fundamental basis for future research and offers potential for the application of NP-CsYBE in various industries, including food, cosmetics, and pharmaceuticals, as a substitute for synthetic emulsifying compounds.
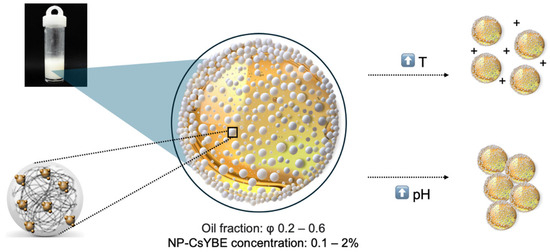
Figure 10.
Pickering emulsion production and interaction of NP-CsYBE at the interface, the conditions tested, and their effects of pH and temperature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polysaccharides6030056/s1, Figure S1: Production scheme of nanoparticles composed of chitosan and Yucca baccata extract; Figure S2: Strain sweep of Pickering emulsion with NP (2% w/v and φ = 0.5 oil fraction v/v) at pH 4. G′, closed symbols, and G″, open symbols; Figure S3: Rheological spectra of G′ (at 1 rad/s) of the starting Pickering emulsion with NP (2% w/v and φ = 0.5 oil fraction v/v) at different pH values (2, 4, and 6.5) against temperature where: (A) environmental temperature, (B) heating to 85 °C, and (C) cooling to 25 °C. G′, closed symbols, and G″, open symbols.
Author Contributions
G.J.G.-C.: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Visualization, Writing—original draft, Writing—review & editing. L.Q.-C.: Validation, Data curation, Investigation, Writing—Review & Editing. Y.L.L.-F.: Validation, Data curation, Investigation, Writing—Review & Editing. W.M.A.-M.: Validation, Data curation, Investigation, Formal analysis, Visualization, Writing—Review & Editing. M.A.L.-M.: Validation, Data curation, Investigation, Writing—Review & Editing. J.L.-M.: Conceptualization, Validation, Supervision, Project administration, Resources, Visualization, Data curation, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONAHCYT, GJGC grant number 803985, and the APC was partially funded by CIAD (IN: 3545336).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Karla G. Martínez Robinson and Luisa Lorena F. Silva Gutiérrez for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, X.M.; Li, X.; Wu, Z.; Wang, Y.; Cheng, J.-S.; Wang, T.; Zhang, B. Chitosan Hydrochloride/Carboxymethyl Starch Complex Nanogels Stabilized Pickering Emulsions for Oral Delivery of Β-Carotene: Protection Effect and in Vitro Digestion Study. Food Chem. 2020, 315, 126288. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 235054. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Ding, Y.; Li, Y.; Guo, M.; Zhang, Y. A Pickering Emulsion Stabilized by Chitosan-g-Poly(N-Vinylcaprolactam) Microgels: Interface Formation, Stability and Stimuli-Responsiveness. Carbohydr. Polym. 2024, 332, 121948. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cao, Y.; Deng, Y.; Li, F.; Kong, B.; Liu, Q.; Wang, H. Preparation and Characterization of Pickering Emulsions Stabilized by Zein/Quercetin/Quaternary Ammonium Chitosan Composite Nanoparticles with Antibacterial Effect. J. Food Eng. 2025, 387, 112305. [Google Scholar] [CrossRef]
- Hadidi, M.; Motamedzadegan, A.; Jelyani, A.Z.; Khashadeh, S. Nanoencapsulation of Hyssop Essential Oil in Chitosan-Pea Protein Isolate Nano-Complex. LWT 2021, 144, 111254. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-Based, Biological-Active Surfactants from Plants. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3325-4. [Google Scholar]
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition. In Saponins in Food, Feedstuffs and Medicinal Plants; Oleszek, W., Marston, A., Eds.; Proceedings of the Phythochemical Society of Europe; Springer: Dordrecht, The Netherlands, 2000; Volume 45, pp. 241–254. ISBN 978-94-015-9339-7. [Google Scholar]
- Morales-Figueroa, G.-G.; Pereo-Vega, G.D.; Reyna-Murrieta, M.E.; Pérez-Morales, R.; López-Mata, M.A.; Sánchez-Escalante, J.J.; Tapia-Rodriguez, M.R.; Ayala-Zavala, J.F.; Juárez, J.; Quihui-Cota, L. Antibacterial and Antioxidant Properties of Extracts of Yucca Baccata, a Plant of Northwestern Mexico, Against Pathogenic Bacteria. BioMed Res. Int. 2022, 2022, e9158836. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Chi, G.J.; Lizardi-Mendoza, J.; Quihui-Cota, L.; López-Franco, Y.L.; López-Mata, M.A.; Pérez-Morales, R. Yucca Schidigera Saponin Rich Extracts: Evaluation of Extraction Methods and Functional Properties. Sustain. Chem. Pharm. 2024, 38, 101470. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Chitosan-Based Pickering Emulsion: A Comprehensive Review on Their Stabilizers, Bioavailability, Applications and Regulations. Carbohydr. Polym. 2023, 304, 120491. [Google Scholar] [CrossRef]
- Bhutto, R.A.; Wang, M.; Iqbal, S.; Yi, J. Curcumin-Loaded Pickering Emulsion Stabilized by pH-Induced Self-Aggregated Chitosan Particles: Effects of Degree of Deacetylation and Molecular Weight. Food Hydrocoll. 2024, 147, 109422. [Google Scholar] [CrossRef]
- Deng, F.; Wang, Z.; Wang, X.; He, R. Pickering Emulsions Stabilized by Moringa Seed Protein: Regulating the Emulsion Properties by Adjusting the Maillard Reaction. Ind. Crops Prod. 2023, 205, 117574. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Zhou, C.; Tan, M. Pickering Emulsions Stabilized with a Spirulina Protein–Chitosan Complex for Astaxanthin Delivery. Food Funct. 2023, 14, 4254. [Google Scholar] [CrossRef]
- Zhao, P.; Ji, Y.; Yang, H.; Meng, X.; Liu, B. Soy Protein Isolate–Chitosan Nanoparticle-Stabilized Pickering Emulsions: Stability and In Vitro Digestion for DHA. Mar. Drugs 2023, 21, 546. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; McClements, D.J.; He, X.; Xu, X.; Tan, F.; Yang, D.; Sun, Q.; Dai, L. Interfacial Properties and Structure of Pickering Emulsions Co-Stabilized by Different Charge Emulsifiers and Zein Nanoparticles. Food Hydrocoll. 2024, 146, 109285. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Sun, C.; Wei, X.; Liu, S.; Jiang, Y. Gastrointestinal pH-Sensitive Pickering Emulsions Stabilized by Zein Nanoparticles Coated with Bioactive Glycyrrhizic Acid for Improving Oral Bioaccessibility of Curcumin. ACS Appl. Mater. Interfaces 2023, 15, 14678–14689. [Google Scholar] [CrossRef]
- Baccou, J.C.; Lambert, F.; Sauvaire, Y. Spectrophotometric Method for the Determination of Total Steroidal Sapogenin. Analyst 1977, 102, 458. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Schtscherbakov, V.G.; Kuptschenko, E.I.; Aspiotis, E.X. Determination of the Oil Content in Different Seeds by the Spin-Ech Method (Original in Russian). Pist. Technol. 1973, 2, 122–124. [Google Scholar]
- Tiwari, P.N.; Gambhi, R.P.N.; Rajan, T.S. Rapid and Nondestructive Determination of Seed Oil by Pulsed Nuclear Magnetic Resonance Technique. J. Am. Oil Chem. Soc. 1974, 51, 104–109. [Google Scholar] [CrossRef]
- Basu, A.; Basu, S.; Bandyopadhyay, S.; Chowdhury, R. Optimization of Evaporative Extraction of Natural Emulsifier Cum Surfactant from Sapindus mukorossi—Characterization and Cost Analysis. Ind. Crops Prod. 2015, 77, 920–931. [Google Scholar] [CrossRef]
- Ralla, T.; Herz, E.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Emulsifying Properties of Natural Extracts from Panax ginseng L. Food Biophys. 2017, 12, 479–490. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An Infrared Investigation in Relation with Chitin and Chitosan Characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering Emulsions: Preparation Processes, Key Parameters Governing Their Properties and Potential for Pharmaceutical Applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Monal, W.M.A.; Valencia, F.M.G. Chapter 1—Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 3–31. ISBN 978-0-12-802735-6. [Google Scholar]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/Chitosan Nanoparticles for Encapsulation and Controlled Release of Vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Yin, S.-W.; Wu, L.-Y.; Qi, J.-R.; Guo, J.; Yang, X.-Q. Fabrication and Characterization of Pickering Emulsions and Oil Gels Stabilized by Highly Charged Zein/Chitosan Complex Particles (ZCCPs). Food Chem. 2016, 213, 462. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated Plga Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-Based Pickering Emulsions and Their Applications: A Review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar] [CrossRef]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 864943. [Google Scholar] [CrossRef]
- Sucharzewska, D.; Stochmal, A.; Oleszek, W. The Effect of Yucca Schidigera Extract on the Physical Structure and on the Oxidative Stability of Sugar-Candy Foam Products. LWT—Food Sci. Technol. 2003, 36, 347–351. [Google Scholar] [CrossRef]
- Góngora-Chi, G.J.; Lizardi-Mendoza, J.; López-Franco, Y.L.; López, M.A.; Quihui-Cota, L. Métodos de extracción, funcionalidad y bioactividad de saponinas de Yucca: Una revisión. Biotecnia 2023, 25, 147–155. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Zou, S.; Wei, Z.; Tong, Z. Simple, Reversible Emulsion System Switched by pH on the Basis of Chitosan without Any Hydrophobic Modification. Langmuir 2012, 28, 11017. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering Emulsions: Versatility of Colloidal Particles and Recent Applications. Curr. Opin. Colloid. Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Lu, Z.; Hu, W.; Wang, L. Zein/Gum Arabic Nanoparticle-Stabilized Pickering Emulsion with Thymol as an Antibacterial Delivery System. Carbohydr. Polym. 2018, 200, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Preparation of Chitosan/Gum Arabic Nanoparticles and Their Use as Novel Stabilizers in Oil/Water Pickering Emulsions. Carbohydr. Polym. 2019, 224, 115190. [Google Scholar] [CrossRef] [PubMed]
- Atarian, M.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A.; Bodaghi, H. Formulation of Pickering Sunflower Oil-in-Water Emulsion Stabilized by Chitosan-Stearic Acid Nanogel and Studying Its Oxidative Stability. Carbohydr. Polym. 2019, 210, 47–55. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.-H. Soy Glycinin as Food-Grade Pickering Stabilizers: Part. I. Structural Characteristics, Emulsifying Properties and Adsorption/Arrangement at Interface. Food Hydrocoll. 2016, 60, 606–619. [Google Scholar] [CrossRef]
- Tatar, B.C.; Sumnu, G.; Sahin, S. Chapter 17—Rheology of Emulsions. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Cambridgeshire, UK, 2017; pp. 437–457. ISBN 978-0-08-100431-9. [Google Scholar]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603. [Google Scholar] [CrossRef]
- Amiji, M.M. Pyrene Fluorescence Study of Chitosan Self-Association in Aqueous Solution. Carbohydr. Polym. 1995, 26, 211–213. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical Physicochemical Behaviors of Chitosan in Aqueous Solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef]
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic Derivatives of Chitosan: Characterization and Rheological Behaviour. Int. J. Biol. Macromol. 1996, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Ben-Naim, A. Hydrophobic Interactions; Springer: Boston, MA, USA, 1980; ISBN 978-1-4684-3547-4. [Google Scholar]
- Mwangi, W.W.; Ho, K.-W.; Tey, B.-T.; Chan, E.-S. Effects of Environmental Factors on the Physical Stability of Pickering-Emulsions Stabilized by Chitosan Particles. Food Hydrocoll. 2016, 60, 543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).