High Methoxyl Pectin–Tomato Paste Edible Films Formed Under Different Drying Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Film-Forming Solutions and Films
2.3. Film Characterisation
2.3.1. Physicochemical and Optical Properties
2.3.2. Antioxidant Activity (AA)
2.3.3. Mechanical Properties and Dynamic Mechanical Analysis
2.3.4. Surface pH
2.3.5. Folding Endurance
2.3.6. Water Vapour Permeability (WVP)
2.3.7. Antimicrobial (AM) Activity
2.3.8. Oxygen Barrier Capacity
2.3.9. Water Contact Angle
2.3.10. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4. Statistical Analysis
3. Results and Discussion
3.1. Films Formed in the Presence and Absence of Glycerol After Drying at 40 °C for 40 h
3.2. Films Formed in the Presence of Glycerol Under Different Drying Temperatures
3.3. Films Formed at Different Drying Conditions Sharing the Same Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Morrugares-Carmona, R.; Wellner, N.; Cross, K.; Bajka, B.; Waldron, K.W. Development of pectin films with pomegranate juice and citric acid. Food Chem. 2016, 198, 101–106. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed]
- Mercadal, P.A.; Picchio, M.L.; González, A. Food-protecting films based on soy protein isolate and natural deep eutectic solvents: Antimicrobial and antioxidant properties. Food Hydrocolloid 2024, 147, 109414. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef]
- Blum, A.; Monir, M.; Wirsansky, I.; Ben-Arzi, S. The beneficial effects of tomatoes. Eur. J. Intern. Med. 2005, 16, 402–404. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Young, J.C.; Zhu, H.; Loewen, S.; Tsao, R. Characterization of phytochemicals and antioxidant activities of a purple tomato (Solanum lycopersicum L.). J. Agric. Food Chem. 2011, 59, 11803–11811. [Google Scholar] [CrossRef]
- Martinez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic Compounds, Lycopene and Antioxidant Activity in Commercial Varieties of Tomato (Lycopersicon esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Zioga, M.; Chroni, A.; Evageliou, V. Utilization of pectins extracted from orange peels by non—Conventional methods in the formation of edible films in the presence of herbal infusions. Polysaccharides 2022, 3, 574–588. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarsh, R.; Lopusiewicz, L.; Biswas, D.; Chandel, V.; Rhim, J.-W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Kalita, P.; Bhattacharje, B.; Pachuau, L.; Roy, S. Recent trends in pectin sources, extraction, and active-edible coating applications. Food Control 2025, 171, 111105. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Hanif Muflih, M.; Izzah, N.; Fadillah, U.; Fadiah Ainani, A.; Dirpan, A. Pectin-based edible films and coatings: From extraction to application on food packaging towards circular economy—A review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100680. [Google Scholar] [CrossRef]
- Zioga, M.; Papantonopoulou, G.; Evageliou, V. High internal phase emulsions and edible films with high methoxyl pectin and pea protein isolate or sodium caseinate. Food Hydrocolloid 2023, 140, 108605. [Google Scholar] [CrossRef]
- Drakos, A.; Pelava, E.; Evageliou, V. Properties of flour films as affected by the flour’s source and particle size. Food Res. Int. 2018, 107, 551–558. [Google Scholar] [CrossRef]
- Lin, L.; Peng, S.; Shi, C.; Li, C.; Hua, Z.; Cui, H. Preparation and characterization of cassava starch/sodium carboxymethyl cellulose edible film incorporating apple polyphenols. Int. J. Biol. Macromol. 2022, 212, 155–164. [Google Scholar] [CrossRef]
- ASTM Standard D5946; Standard Test Method for Corona-Treated Polymer Films Using Water Contact Angle Measurements. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gapiery, Y.; Raghavan, V. Development of Biodegradable Films with Improved Antioxidant Properties Based on the Addition of Carrageenan Containing Olive Leaf Extract for Food Packaging Applications. J. Polym. Envirom 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocolloid 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohyd Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Effects of drying methods and plasticizer concentration on some physical and mechanical properties of edible chitosan films. J. Food Eng. 2010, 99, 216–224. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; HadiNezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohyd Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Tavakkoli Yaraki, M.; Ghorbanpour, M.; Wang, S. Biodegradable κ-carrageenan/nanoclay nanocomposite films containing Rosmarinus officinalis L. extract for improved strength and antibacterial performance. Int. J. Biol. Macromol. 2018, 115, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Pirsa, S. Production of Biodegradable Film Based on Polylactic Acid, Modified with Lycopene Pigment and TiO2 and Studying Its Physicochemical Properties. J. Polym. Envirom 2020, 28, 433–444. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; Miyahira, R.F.; Fai, A.E.C. Biodegradable films based on fruit puree: A brief review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Liu, H.; Li, M.; Ma, Z. Barrier and mechanical properties of carrot puree films. Food Bioprod. Process 2011, 89, 149–156. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Ahmadi, R.; Kalbasi-Ashtari, A.; Oromiehie, A.; Yarmand, M.-S.; Jahandideh, F. Development and characterization of a novel biodegradable edible film obtained from psyllium seed (Plantago ovata Forsk). J. Food Eng. 2012, 109, 745–751. [Google Scholar] [CrossRef]
- Roy, S.; Deshmukh, R.K.; Tripathi, S.; Gaikwad, K.K.; Das, S.S.; Sharma, D. Recent advances in the carotenoids added to food packaging films: A Review. Foods 2023, 12, 4011. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Influence of drying conditions on physical properties of alginate films. Dry. Technol. 2012, 30, 72–79. [Google Scholar] [CrossRef]
- Qin, J.; Xiao, M.; Wang, S.; Peng, C.; Wu, X.; Jiang, F. Effect of drying temperature on microstructural, mechanical, and water barrier properties of konjac glucomannan/agar film produced at industrial scale. LWT—Food Sci. Technol. 2023, 173, 114275. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Tailoring physicochemical properties of chitosan films and their protective effects on meat by varying drying temperature. Carbohyd Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, S.; Lan, P.; Wang, W.; Zhu, J. Topography and physical properties of carboxymethyl cellulose films assembled with calcium and gelatin at different temperature and humidity. Food Chem. 2022, 382, 132391. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocolloid 2019, 90, 162–171. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Oliveira de Moraes, J.; Vicente, A.R.; Laurindo, J.B.; Mauri, A.N. Scale-up of the production of soy (Glycine max L.) protein films using tape casting: Formulation of film-forming suspension and drying conditions. Food Hydrocolloid 2017, 66, 110–117. [Google Scholar] [CrossRef]
- Jomlapeeratikul, P.; Poomsa-Ad, P.; Wiset, L. Effect of drying temperatures and plasticizers on the properties of konjac flour film. J. Food Process Eng. 2017, 40, 12443. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Krochta, J.M. Drying Temperature Effect on Water Vapor Permeability and Mechanical Properties of Whey Protein−Lipid Emulsion Films. J. Agric. Food Chem. 2000, 48, 687–692. [Google Scholar] [CrossRef]
- Assis, R.Q.; Lopes, S.M.; Costa, T.M.H.; Hickmann Flôres, S.; de Oliveira Rios, A. Active biodegradable cassava starch films incorporated lycopene nanocapsules. Ind. Crops Prod. 2017, 109, 818–827. [Google Scholar] [CrossRef]
- Manzo, N.; Santini, A.; Pizzolongo, F.; Aiello, A.; Romano, R. Degradation kinetic (D100) of lycopene during the thermal treatment of concentrated tomato paste. Nat. Prod. Res. 2019, 33, 1835–1841. [Google Scholar] [CrossRef]
- Shi, J.; Dai, Y.; Kakuda, Y.; Mittal, G.; Jun Xue, S. Effect of heating and exposure to light on the stability of lycopene in tomato purée. Food Control 2008, 19, 514–520. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 16–22. [Google Scholar] [CrossRef]
- Çetiner, E.; Bayburt, A.; Acarali, N. A novel aspect on different preservation methods for tomato paste by examining the protective effect of herb and spice oils. Food Chem. 2023, 427, 136715. [Google Scholar] [CrossRef] [PubMed]
- Ochida, C.O.; Itodo, A.U.; Anhwange, B.A.; Nwanganga, P.A.; Abah, C.N. Chemical and microbial quality evaluation of fresh tomato and its processed products using FTIR, SEM and GC-MS. J. Post. Harvest. Technol. 2019, 7, 30–44. Available online: https://acspublisher.com/journals/index.php/jpht/article/view/15414 (accessed on 1 December 2024).
- Espinosa-Andrews, H.; Sandoval-Castilla, O.; Vázquez-Torres, H.; Vernon-Carter, E.J.; Lobato-Calleros, C. Determination of the gum Arabic–chitosan interactions by Fourier Transform Infrared Spectroscopy and characterization of the microstructure and rheological features of their coacervates. Carbohyd Polym. 2010, 79, 541–546. [Google Scholar] [CrossRef]
- Kamil, M.M.; Mohamed, G.F.; Shaheen, M.S. Fourier Transformer Infrared Spectroscopy for Quality Assurance of Tomato Products. J. Am. Sci. 2011, 7, 559–572. [Google Scholar]
- Scibisz, I.; Reich, M.; Bureau, S.; Gouble, B.; Causse, M.; Bertrand, D.; Renard, C.M.G.C. Mid-infrared spectroscopy as a tool for rapid determination of internal quality parameters in tomato. Food Chem. 2011, 125, 1390–1397. [Google Scholar] [CrossRef]
- De Nardo, T.; Shiroma-Kian, C.; Halim, Y.; Francis, D.; Rodriguez-Saona, L.E. Rapid and Simultaneous Determination of Lycopene and Carotene Contents in Tomato Juice by Infrared Spectroscopy. J. Agric. Food Chem. 2009, 57, 1105–1112. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Colina, M.; Rodriguez, J.; Ptitchkina, N.M.; Ignatov, V.V. Comparative spectroscopic characterization of different pectins and their sources. Food Hydrocolloid 1998, 12, 263–271. [Google Scholar] [CrossRef]
- Synytsya, A.; Copikova, J.; Matejka, P.; Machovic, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohyd Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Pérez Zamora, C.M.; Michaluk, A.G.; Chiappetta, D.A.; Nuñez, M.B. Herbal buccal films with in vitro antibacterial and anti-inflammatory effects. J. Herb. Med. 2022, 31, 100527. [Google Scholar] [CrossRef]
- Katırcı, N.; Işık, N.; Güpür, C.; Guler, H.O.; Gursoy, O.; Yilmaz, Y. Differences in antioxidant activity, total phenolic and flavonoid contents of commercial and homemade tomato pastes. J. Saudi Soc. Agric. Sci. 2020, 19, 249–254. [Google Scholar] [CrossRef]
- Athanasopoulou, E.; Bigi, F.; Maurizzi, E.; Karello, E.I.E.; Pappas, C.S.; Quartieri, A.; Tsironi, T. Synthesis and characterization of polysaccharide- and protein-based edible films and application as packaging materials for fresh fish fillets. Sci. Rep. 2024, 14, 517. [Google Scholar] [CrossRef] [PubMed]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Mandrell, R.; Friedman, M. Antibacterial effects of allspice, garlic, and oregano essential oils in tomato films determined by overlay and vapor-phase methods. J. Food Sci. 2009, 74, M390–M397. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, D.; Eichers, M.; Shojaeiarani, J.; Kallmeyer, A. Influence of biobased plasticizers on 3D printed polylactic acid composites filled with sustainable biofiller. Ind. Crops Prod. 2021, 173, 114132. [Google Scholar] [CrossRef]
- Ghernaout, D.; Belaadi, A.; Boumaaza, M.; Chai, B.X.; Jawaid, M.; Abdullah, M.M.S.; Krishnasamy, P.; Al-Khawlani, A. Effects of incorporating cellulose fibers from Yucca treculeana L. on the thermal characteristics of green composites based on high-density poly-ethylene: An eco-friendly material for cleaner production. J. Mater. Res. Technol. 2024, 31, 787–798. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.T. A review on dynamic mechanical properties of natural fibre reinforced polymer composites. Constr. Build. Mater. 2016, 106, 149–159. [Google Scholar] [CrossRef]
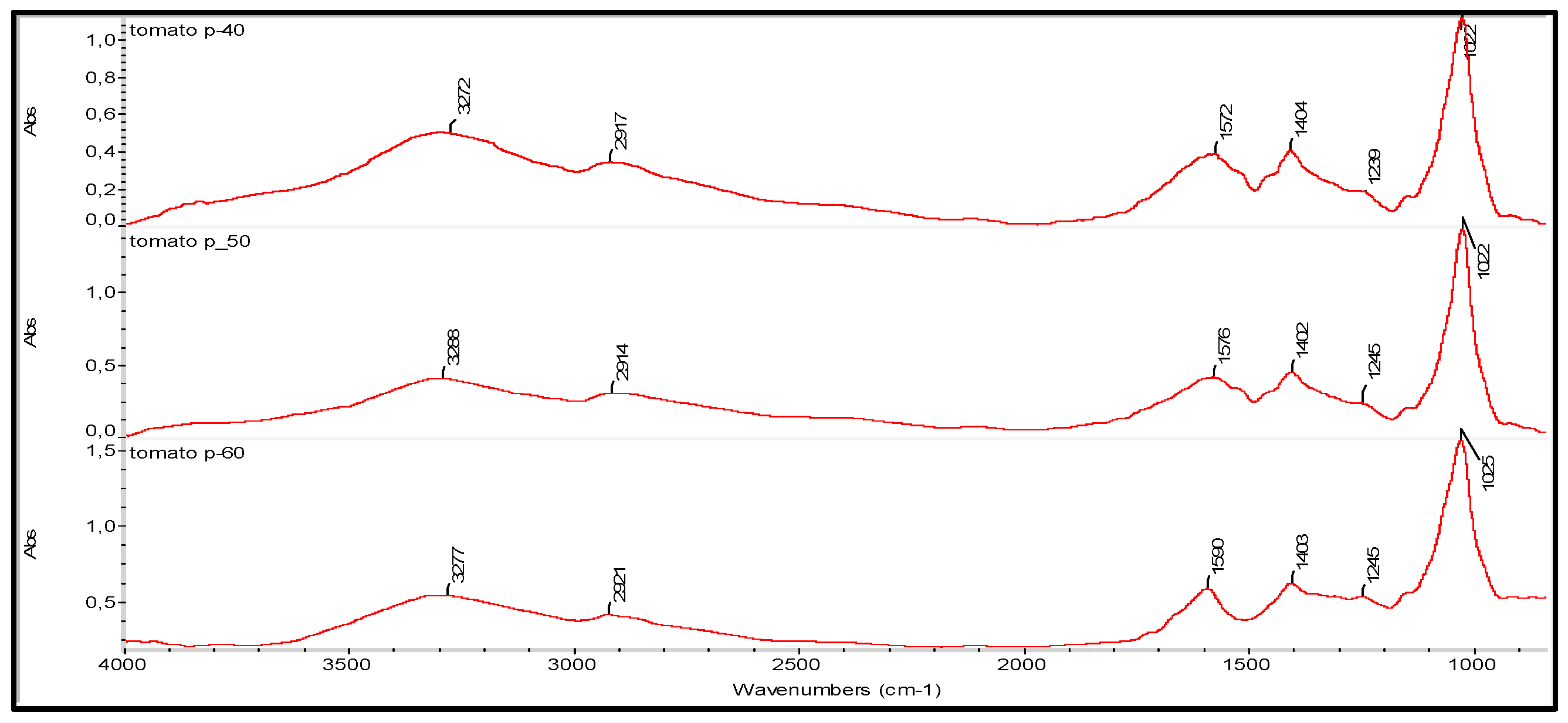
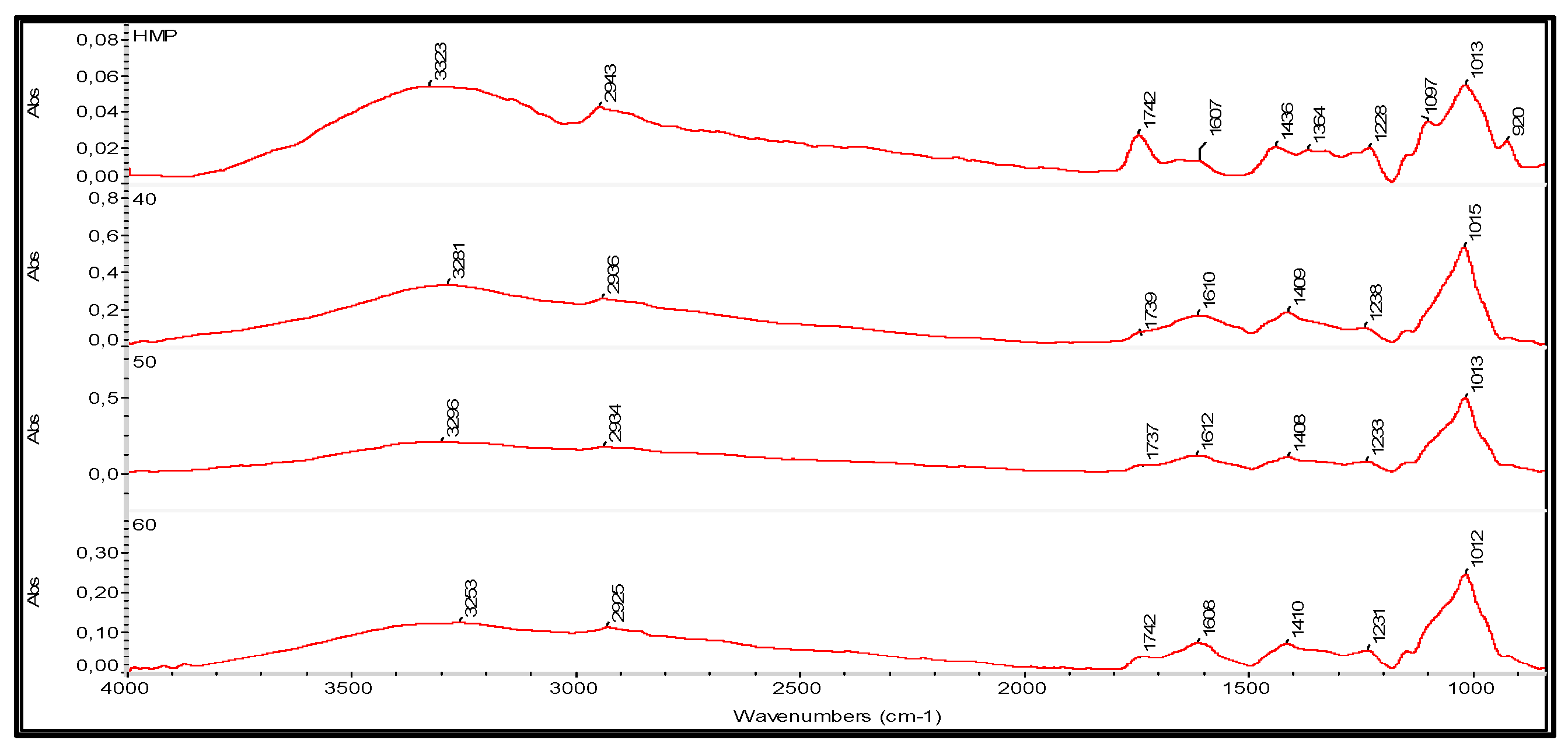
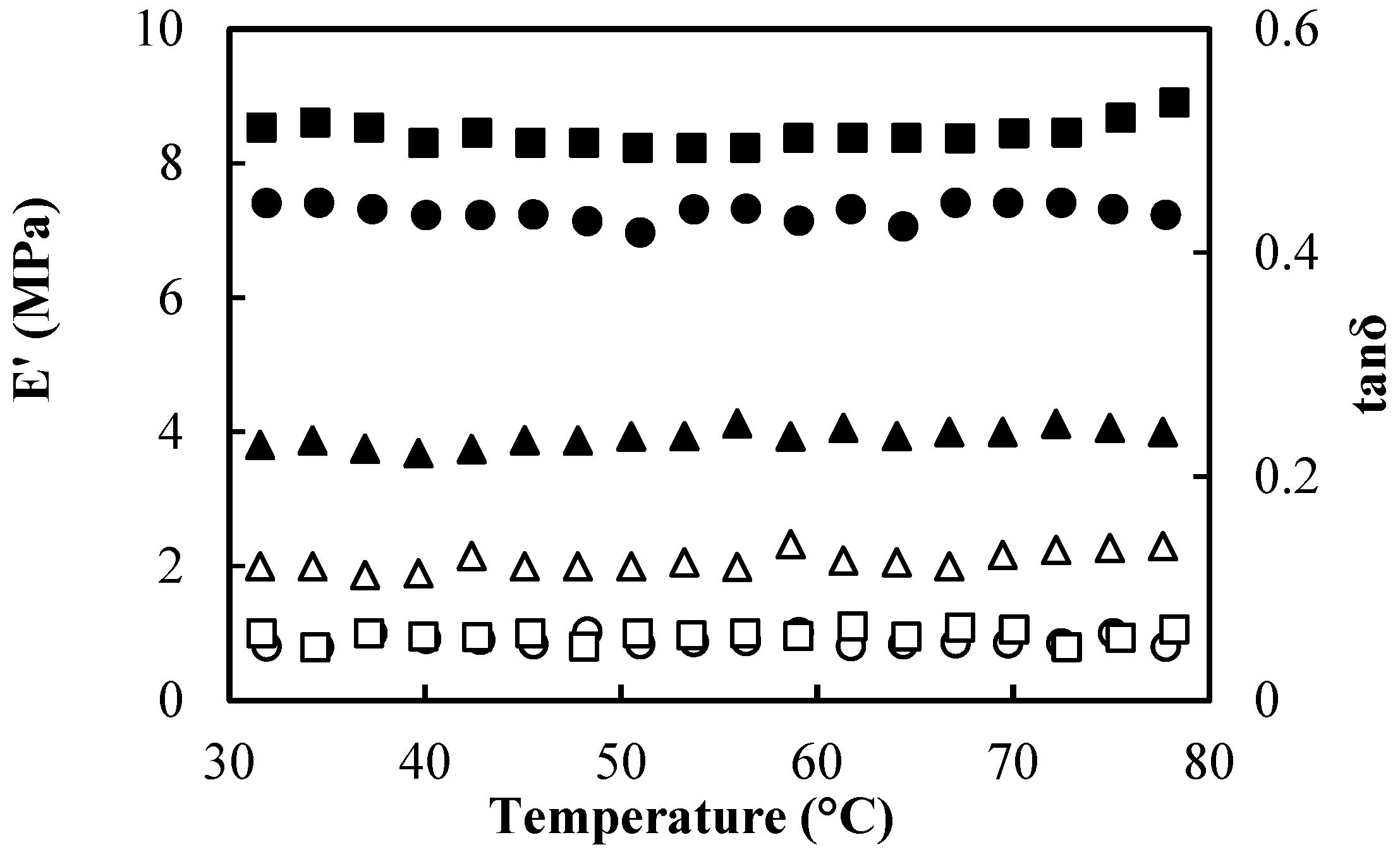
| HΜP (% wt) | Tomato Paste (% wt) | Glycerol (%wt) | Thickness (μm) | Weight (g) | Moisture Content (%) | Stress at Break (kPa) | Young’s Modulus (kPa) | Opacity (Area T%) | [L*] | [a*] | Antioxidant Activity (AA%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 0 | 57.73 a ± 5.18 | 0.27 a ± 0.00 | 56.66 a ± 2.57 | 202.1 a ± 63.6 | 85.94 a ± 5.24 | 25484 a ± 1194 | 81.07 a ± 2.57 | 7.97 a ± 2.33 | 33.65 a ± 1.14 |
| 2 | 0 | 81.15 b ± 9.39 | 0.38 b ± 0.01 | 44.26 b ± 3.12 | 227.7 a ± 55.1 | 114.35 a,b ± 10.19 | 12125 b ± 87 | 72.16 b ± 3.46 | 17.47 b ± 2.19 | 35.18 a,b ± 0.14 | |
| 5 | 0 | 106.00 c ± 7.84 | 0.61 c ± 0.01 | 32.73 c ± 1.55 | 234.2 a ± 46.7 | 123.51 b ± 13.64 | 6273 c ± 127 | 60.34 c ± 2.30 | 27.03 c ± 2.34 | 36.40 b ± 0.27 | |
| 10 | 0 | 154.23 d ± 11.70 | 1.10 d ± 0.01 | 23.39 d ± 3.74 | 908.6 b ± 64.0 | 317.86 c ± 40.10 | 4201 d ± 212 | 48.26 d ± 2.33 | 36.46 d± 1.41 | 53.17 c ± 0.49 | |
| 0.5 | 1 | 0.15 | 50.83 a ± 5.15 | 0.28 a ± 0.01 | 21.21 a ± 1.98 | 397.9 a ± 41.0 | 107.74 a ± 11.02 | 18395 a ± 438 | 81.88 a ± 1.30 | 6.41 a ± 0.81 | 30.81 a ± 0.76 |
| 2 | 0.15 | 71.25 b ± 8.76 | 0.37 b ± 0.00 | 20.84 a ± 0.06 | 525.8 b ± 64.2 | 152.86 b ± 16.50 | 12649 b ± 494 | 74.08 b ± 2.47 | 12.67 b ± 1.72 | 41.97 b ± 1.60 | |
| 5 | 0.15 | 130.83 c ± 16.63 | 0.68 c ± 0.03 | 24.02 b ± 0.06 | 752.6 c ± 43.3 | 192.48 c ± 3.51 | 7563 c ± 165 | 60.41 c ± 2.69 | 26.48 c ± 1.48 | 45.53 c ± 1.37 | |
| 10 | 0.15 | 182.31 d ± 14.95 | 1.24 d ± 0.04 | 25.75 b ± 0.12 | 866.5 d ± 61.0 | 198.27 c ± 7.52 | 4866 d ± 346 | 45.70 d ± 1.47 | 34.56 d ± 1.92 | 48.93 d ± 0.28 |
| HΜ Pectin (%wt) | Tomato Paste (%wt) | Drying Conditions | Thickness (μm) | Weight (g) | Moisture Content (%) | Stress at Break (kPa) | Young’s Modulus (kPa) |
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 40 °C–40 h | 50.83 a ± 5.15 | 0.28 a ± 0.01 | 21.21 a ± 1.98 | 397.9 a ± 41.0 | 107.74 a ± 11.02 |
| 2 | 71.25 b ± 8.76 | 0.37 b ± 0.00 | 20.84 a ± 0.06 | 525.8 b ± 64.2 | 152.86 b ± 16.50 | ||
| 5 | 130.83 c ± 16.63 | 0.68 c ± 0.03 | 24.02 b ± 0.06 | 752.6 c ± 43.3 | 192.48 c ± 3.51 | ||
| 10 | 182.31 d ± 14.95 | 1.24 d ± 0.04 | 25.75 b ± 0.12 | 866.5 d ± 61.0 | 198.27 c ± 7.52 | ||
| 0.5 | 1 | 50 °C–20 h | 53.13 a ± 8.73 | 0.30 a ± 0.01 | 21.15 a ± 0.27 | 189.1 a ± 59.2 | 215.09 a ± 29.04 |
| 2 | 60.63 b ± 7.93 | 0.39 b ± 0.01 | 22.92 b ± 0.18 | 310.8 b ± 68.5 | 289.17 b ± 31.08 | ||
| 5 | 107.35 c ± 9.54 | 0.70 c ± 0.02 | 24.67 c ± 0.27 | 417.6 c ± 35.3 | 357.47 c ± 25.89 | ||
| 10 | 144.69 d ± 10.87 | 1.18 d ± 0.01 | 24.71 c ± 0.90 | 659.4 d ± 58.5 | 382.09 c ± 42.60 | ||
| 0.5 | 1 | 60 °C–10 h | 45.00 a ± 8.42 | 0.31 a ± 0.04 | 20.87 a ± 0.62 | 441.2 a ± 80.5 | 154.82 a ± 22.74 |
| 2 | 75.45 a ± 9.61 | 0.36 a ± 0.01 | 20.74 a ± 1.03 | 563.8 a ± 67.7 | 182.36 a ± 32.70 | ||
| 5 | 108.85 b ± 7.12 | 0.64 b ± 0.02 | 21.02 a ± 0.08 | 725.1 b ± 57.5 | 222.92 b ± 22.58 | ||
| 10 | 166.82 c ± 7.83 | 1.13 c ± 0.04 | 20.87 a ± 0.62 | 959.2 c ± 59.1 | 267.39 c ± 32.06 |
| HΜ Pectin (%wt) | Tomato Paste (%wt) | Glycerol (%wt) | Drying Conditions | Opacity (Area T%) | [L*] | [a*] | Antioxidant Activity (AA%) |
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 0.15 | 40 °C–40 h | 18,395 a ± 438 | 81.88 a ± 1.30 | 6.41 a ± 0.81 | 30.81 a ± 0.76 |
| 2 | 0.15 | 12,649 b ± 494 | 74.08 b ± 2.47 | 12.67 b ± 1.72 | 41.97 b ± 1.60 | ||
| 5 | 0.15 | 7563 c ± 165 | 60.41 c ± 2.69 | 26.48 c ± 1.48 | 45.53 c ± 1.37 | ||
| 10 | 0.15 | 4866 d ± 346 | 45.70 d ± 1.47 | 34.56 d ± 1.92 | 48.93 d ± 0.28 | ||
| 0.5 | 1 | 0.15 | 50 °C–20 h | 23,950 a ± 556 | 81.06 a ± 2.08 | 6.83 a ± 1.16 | 27.53 a ± 1.72 |
| 2 | 0.15 | 15,258 b ± 210 | 74.03 b ± 2.06 | 12.84 b ± 2.01 | 34.44 b ± 0.17 | ||
| 5 | 0.15 | 9643 c ± 192 | 59.51 c ± 1.53 | 25.71 c ± 1.41 | 44.51 c ± 0.20 | ||
| 10 | 0.15 | 6226 d ± 321 | 49.44 d ± 1.19 | 33.27 d ± 0.63 | 52.06 d ± 0.81 | ||
| 0.5 | 1 | 0.15 | 60 °C–10 h | 19,968 a ± 978 | 81.40 a ± 2.40 | 7.01 a ± 1.76 | 16.93 a ± 0.90 |
| 2 | 0.15 | 12,220 b ± 947 | 73.89 b ± 2.22 | 13.37 b ± 1.86 | 24.14 a ± 0.35 | ||
| 5 | 0.15 | 9018 c ± 288 | 59.16 c ± 1.66 | 26.48 c ± 1.64 | 38.10 b ± 0.45 | ||
| 10 | 0.15 | 4648 d ± 109 | 43.69 d ± 2.12 | 35.57 d ± 0.65 | 44.28 c ± 0.46 |
| Sample Name | Tomato Paste (%wt) | Drying Conditions | Density (g/cm3) | Folding Endurance | pH | Contact Angle | Oxygen Barrier Properties (POV; meq/kg) | WVTR (10−4 g/h cm2) | WVP (10−8 g mm/h cm2 Pa) |
|---|---|---|---|---|---|---|---|---|---|
| F40 | 5 | 40 °C–40 h | 1.60 a ± 0.18 | No break | 3.5 a ± 0.4 | 25.65 a ± 1.99 | 3.05 a ± 0.11 | 8.12 a ± 0.49 | 4.00 a ± 0.24 |
| F50 | 5 | 50 °C–20 h | 1.54 a ± 0.08 | No break | 3.5 a ± 0.4 | 29.93 b ± 2.61 | 3.86 b ± 0.10 | 11.29 b ± 0.57 | 6.03 a ± 0.30 |
| F60 | 10 | 60 °C–10 h | 2.02 b ± 0.19 | No break | 3.5 a ± 0.4 | 34.54 c ± 1.63 | 1.82 c ± 0.10 | 8.04 a ± 0.41 | 5.01 a ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palavouzi, G.; Oikonomidis, C.; Zioga, M.; Pappas, C.; Evageliou, V. High Methoxyl Pectin–Tomato Paste Edible Films Formed Under Different Drying Temperatures. Polysaccharides 2025, 6, 55. https://doi.org/10.3390/polysaccharides6030055
Palavouzi G, Oikonomidis C, Zioga M, Pappas C, Evageliou V. High Methoxyl Pectin–Tomato Paste Edible Films Formed Under Different Drying Temperatures. Polysaccharides. 2025; 6(3):55. https://doi.org/10.3390/polysaccharides6030055
Chicago/Turabian StylePalavouzi, Georgia, Charalampos Oikonomidis, Marianthi Zioga, Christos Pappas, and Vasiliki Evageliou. 2025. "High Methoxyl Pectin–Tomato Paste Edible Films Formed Under Different Drying Temperatures" Polysaccharides 6, no. 3: 55. https://doi.org/10.3390/polysaccharides6030055
APA StylePalavouzi, G., Oikonomidis, C., Zioga, M., Pappas, C., & Evageliou, V. (2025). High Methoxyl Pectin–Tomato Paste Edible Films Formed Under Different Drying Temperatures. Polysaccharides, 6(3), 55. https://doi.org/10.3390/polysaccharides6030055








