Improving the Corrosion Resistance of Titanium by PAA/Chitosan Bilayer Architecture Through the Layer-by-Layer Method
Abstract
1. Introduction
2. Materials and Methods
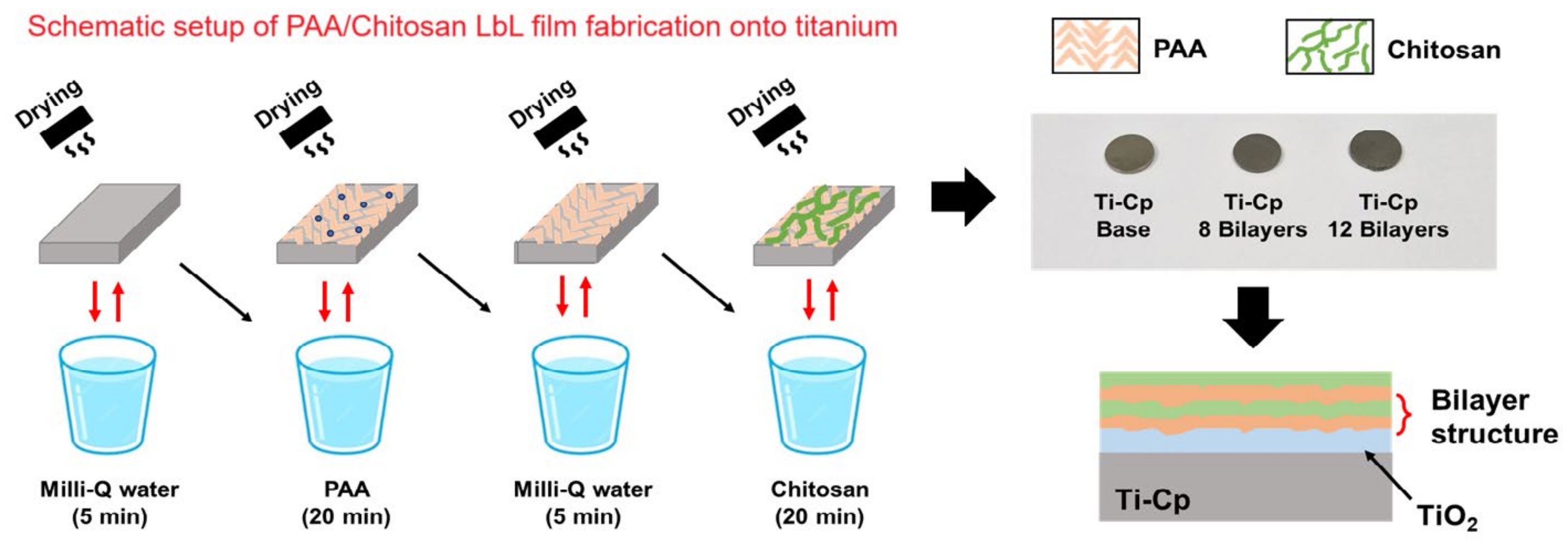
2.1. Specimen Preparation
2.2. Deposition of the Chitosan/PAA Coating on Ti-Cp
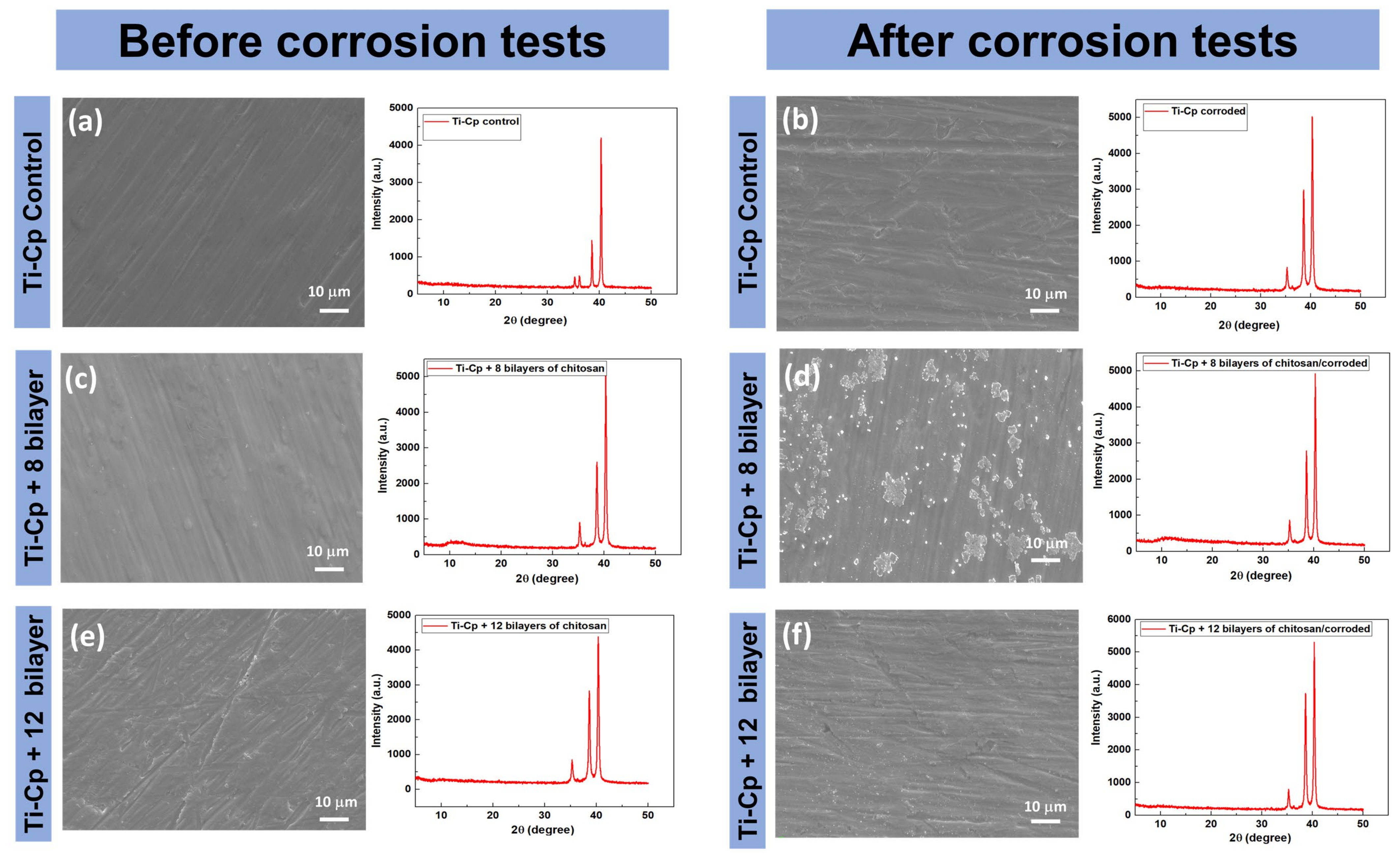
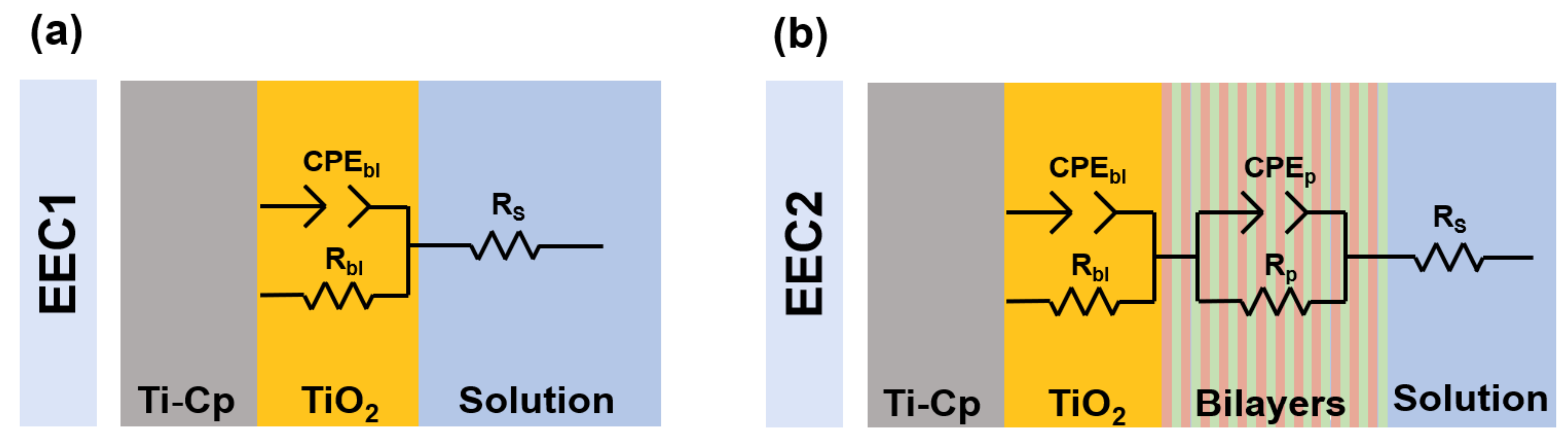
2.3. Corrosion Tests
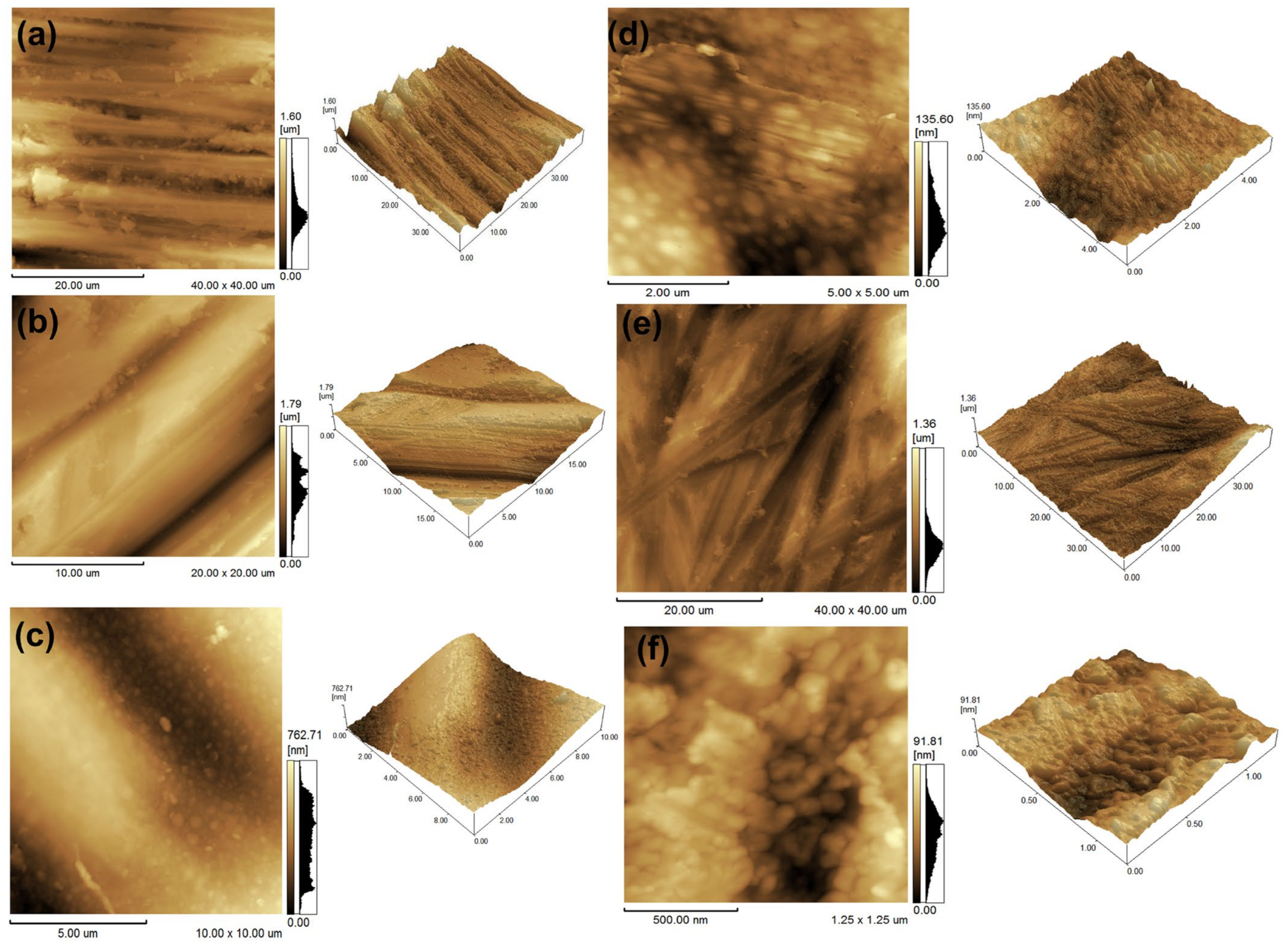
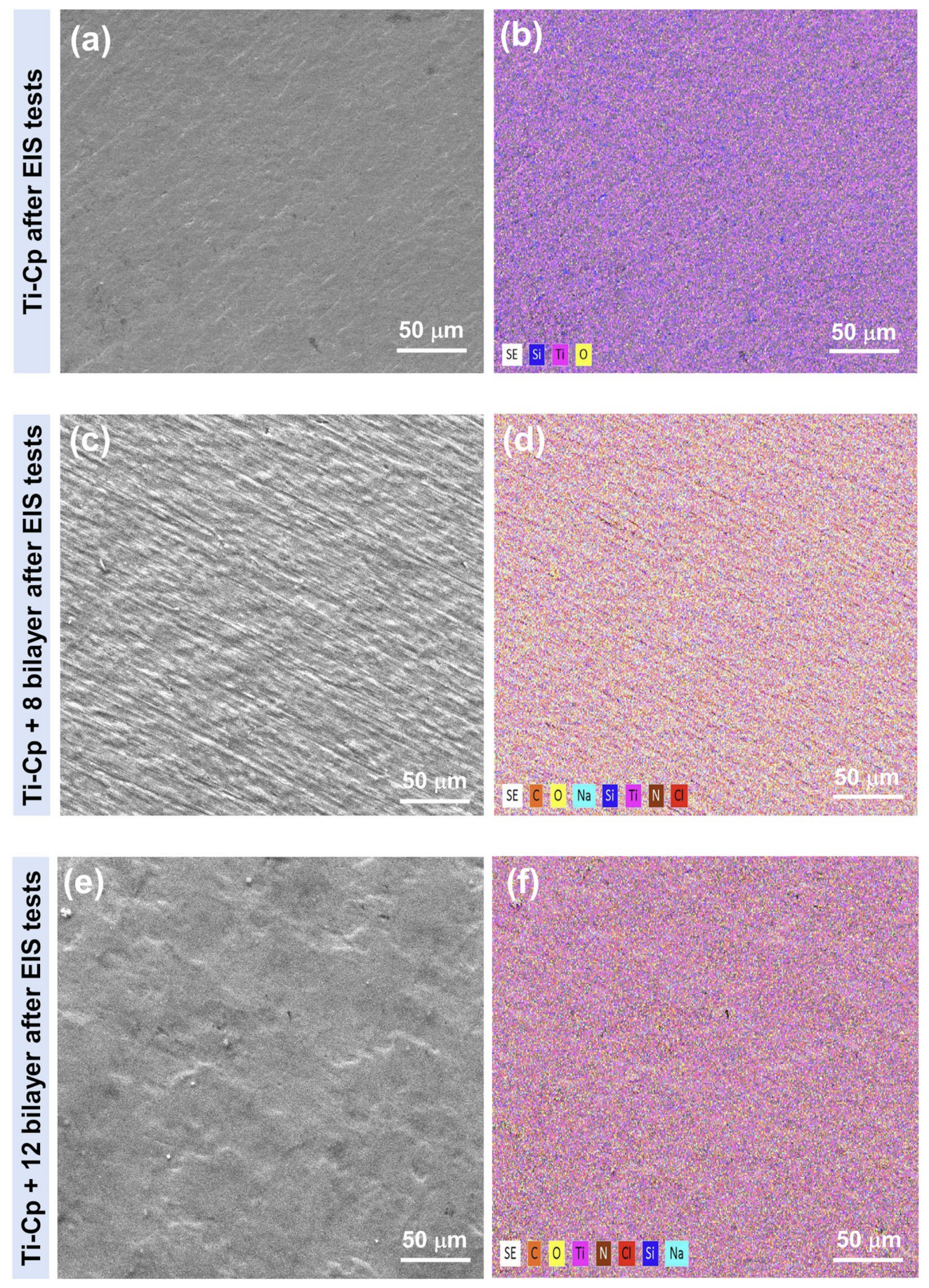
2.4. Morphological and Structural Characterization
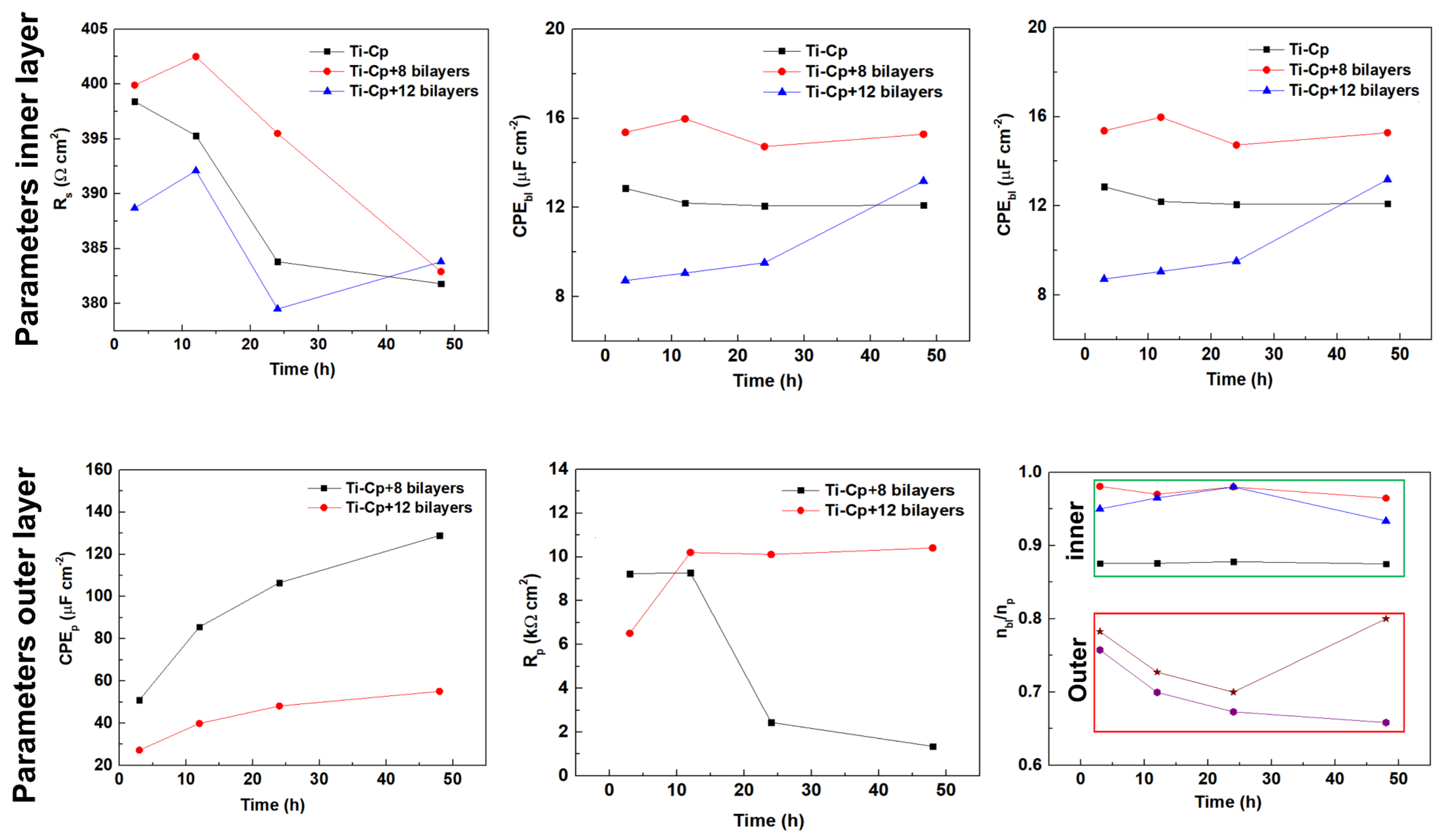
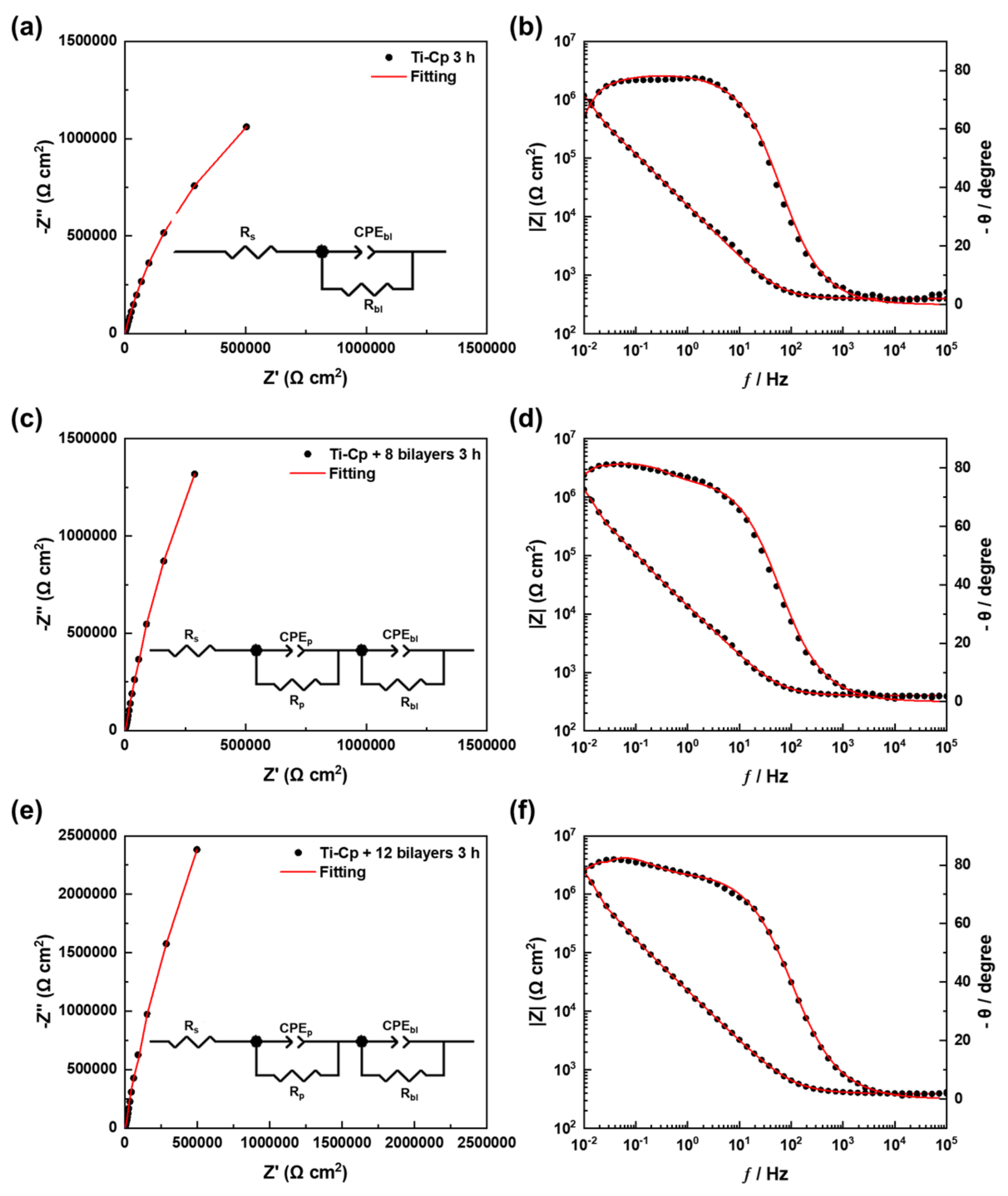
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashtiani, R.E.; Alam, M.; Tavakolizadeh, S.; Abbasi, K. The Role of Biomaterials and Biocompatible Materials in Implant-Supported Dental Prosthesis. Evid.-Based Complement. Altern. Med. 2021, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis–a review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Oliveira, F.; Boldrini, L.C.; Leite, P.E.; Falagan-Lotcsch, P.; Linhares, A.B.R.; Zambuzzi, W.F.; Fragneaud, B.; Campos, P.C.; Gouvêa, C.P.; et al. Micro-arc oxidation as a tool to develop multifunctional calcium-rich surfaces for dental implant applications. Mater. Sci. Eng. 2015, 54, 196–206. [Google Scholar] [CrossRef]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration– communication of cells. Clin. Oral Implant. research 2012, 23, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.; Ribeiro, A.R.L.; Rocha, L.A.; Celis, J.P. Influence of pH and corrosion inhibitors on the tribocorrosion of titanium in artificial saliva. Wear 2006, 261, 994–1001. [Google Scholar] [CrossRef]
- Elias, C.N.; Fernandes, D.J.; Resende, C.R.S.; Roestel, J. Mechanical properties, surface morphology and stability of a modified commercially pure high-strength titanium alloy for dental implants. Dent. Mater. 2015, 31, e1–e13. [Google Scholar] [CrossRef]
- Kulkarni, M.; Flasker, A.; Lokar, M.; Polisak, K.M.; Mazare, A.; Artenjak, A.; Cucnik, S.; Kralj, S.; Velikonja, A.; Iglic, V.K.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar]
- Shibata, Y.; Tanimoto, Y. A review of improved fixation methods for dental implants. Part I: Surface optimization for rapid osseointegration. J. Prosthodont. Res. 2015, 59, 20–33. [Google Scholar] [CrossRef]
- Bronze-Uhle, E.S.; Dias, L.F.G.; Trino, L.D.; Matos, A.A.; De Oliveira, R.C.; Lisboa-Filho, P.N. Physicochemical characterization of albumin immobilized on different TiO2 surfaces for use in implant materials. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 39–50. [Google Scholar] [CrossRef]
- López-Valverde, N.; Aragoneses, J.; López-Valverde, A.; Rodríguez, C.; Macedo de Sousa, B.; Aragoneses, J.M. Role of chitosan in titanium coatings trends and new generations of coatings. Front. Bioeng. Biotechnol. 2022, 10, 907589. [Google Scholar] [CrossRef]
- Trino, L.D.; Bronze-Uhle, E.S.; Ramachandran, A.; Lisboa-Filho, P.N.; Mathew, M.T.; George, A. Titanium surface bio-functionalization using osteogenic peptides: Surface chemistry, biocompatibility, corrosion and tribocorrosion aspects. J. Mech. Behav. Biomed. Mater. 2018, 81, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef]
- Mathew, M.T.; Barao, V.A.; Yuan, J.C.; Assunção, W.G.; Sukotjo, C.; Wimmer, M.A. In Situ Corrosion Mapping of Dental Implants. J. Dent. Res. 2022, 101, 245–253. [Google Scholar]
- Fernandes, A.C.; Vaz, F.; Ariza, E.; Rocha, L.A.; Ribeiro, A.R.L.; Vieira, C.; Riviére, J.P.; Pichon, L. Tribocorrosion behaviour of plasma nitrided and plasma nitrided+ oxidised Ti6Al4V alloy. Surf. Coat. Technol. 2006, 200, 6218–6224. [Google Scholar] [CrossRef]
- Mathew, M.T.; Barao, V.A.; Yuan, J.C.; Assunção, W.G.; Sukotjo, C.; Wimmer, M.A. What is the role of lipopolysaccharide on the tribocorrosive behavior of titanium? J. Mech. Behav. Biomed. Mater. 2012, 8, 71–85. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Bayón, R.; Igartua, A.; Gonzalez, J.J.; De Gopegui, U.R. Influence of the carbon content on the corrosion and tribocorrosion performance of Ti-DLC coatings for biomedical alloys. Tribol. Int. 2015, 88, 115–125. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 2—Review focusing on clinical knowledge of different surfaces. Int. J. Prosthodont. 2004, 17, 544–564. [Google Scholar]
- Wang, Z.; Zhang, X.; Gu, J.; Yang, H.; Nie, J.; Ma, G. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carbohydr. Polym. 2014, 103, 38–45. [Google Scholar] [CrossRef]
- El-Banna, A.; Bissa, M.W.; Khurshid, Z.; Zohaib, S.; Asiri, F.Y.I.; Zafar, M.S. Surface modification techniques of dental implants. In Dental Implants; Woodhead Publishing: Sawston, UK, 2020; pp. 49–58. [Google Scholar]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qiu, K.J.; Wang, B.L.; Li, L.; Zheng, Y.F. Surface characterization and cell response of binary Ti-Ag alloys with CP Ti as material control. J. Mater. Sci. Technol. 2012, 28, 779–784. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Wolf, E.; Richter, G.; Meissner, H.; Boening, K. New concept of polymethyl methacrylate (PMMA) and polyethylene terephthalate (PET) surface coating by chitosan. Polymers 2016, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef]
- Husain, A.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaid, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Hongbin, L.V.; chen, Z.; Yang, X.; Cen, L.; Zhang, X.; Gao, P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014, 42, 1464–1472. [Google Scholar]
- Renoud, P.; Toury, B.; Benayoun, S.; Attik, G.; Brigitte, G.B. Functionalization of titanium with chitosan via silanation: Evaluation of biological and mechanical performances. PLoS ONE 2012, 7, e39367. [Google Scholar] [CrossRef]
- Lin, M.H.; Wang, Y.H.; Kuo, C.H.; Ou, S.F.; Huang, P.Z.; Song, T.Y.; Chen, Y.C.; Chen, S.T.; Wu, C.H.; Hsueh, Y.H.; et al. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Wang, D.; Chen, C.; Liu, X.; Sheng, X.; Lee, H.-P.; Li, Y. Layer-by-Layer Chitosan/Poly(acrylic acid) Multilayer Coatings for Enhanced Bioactivity of Titanium. Carbohydr. Polym. 2014, 111, 134–142. [Google Scholar]
- Eliaz, N.; Eliyahu, M. Electrochemical Processes of Biomaterials in Human Body Fluids. Corros. Sci. 2019, 165, 108395. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. On Osseointegration in Relation to Implant Surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Caio, Z.; Nakajima, H.; Woldu, M.; Berglund, A.; Bergman, M.; Okabe, T. In vitro corrosion resistance of titanium made using different fabrication methods. Biomaterials 1999, 20, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.P.; Pavarina, A.C.; Dovigo, L.N.; Sanitá, P.V.; Vergani, C.E.; Jorge, J.H.; Machado, A.L. Controlling Growth of Bioactive Films on Titanium Surfaces. J. Mater. Chem. B 2015, 3, 2455–2464. [Google Scholar]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH-Dependent Thickness Behavior of Sequentially Adsorbed Layers of Weak Polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Moreto, J.A.; Gelamo, R.V.; Nascimento, J.P.L.; Taryba, M.; Fernandes, J.C.S. Improving the corrosion protection of 2524-T3-Al alloy through reactive sputtering Nb2O5 coatings. Appl. Surf. Sci. 2021, 556, 149750. [Google Scholar] [CrossRef]
- Freitas, L.R.; Gelamo, R.V.; Marino, C.E.B.; Nascimento, J.P.L.; Figueiredo, J.A.M.; Fernandes, J.C.S.; Moreto, J.A. Corrosion behaviour of reactive sputtering deposition niobium oxide-based coating on the 2198-T851 aluminium alloy. Surf. Coat Technol. 2022, 434, 128197. [Google Scholar] [CrossRef]
- Ferreira, M.O.A.; Teixeira, G.T.L.; Leite, N.B.; Gelamo, R.V.; Pinto, H.C.; Aoki, I.V.; Moreto, J.A. Effect of Nb2O5 coating on the corrosion resistance of the 7050-T7451 aluminium alloy. Emergent. Mater. 2023, 6, 2001–2017. [Google Scholar] [CrossRef]
- Ferreira, M.O.A.; Mariani, F.E.; Leite, N.B.; Gelamo, R.V.; Aoki, I.V.; de Siervo, A.; Pinto, H.C.; Moreto, J.A. Niobium and carbon nanostructured coatings for corrosion protection of the 316L stainless steel. Mater. Chem. Phys. 2024, 312, 128610. [Google Scholar] [CrossRef]
- Picart, C.; Lavalle, P.; Hubert, P.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.C. Buildup Mechanism for Poly(L-lysine)/Hyaluronic Acid Films onto a Solid Surface. Langmuir 2001, 17, 7419–7425. [Google Scholar] [CrossRef]
- Ramos, A.P.; Pavarina, A.C.; Dovigo, L.N.; Sanitá, P.V.; Vergani, C.E.; Jorge, J.H.; Machado, A.L. Nanoscale Architecture of Layer-by-Layer Films Containing Bioactive Glass Nanoparticles. Langmuir 2013, 29, 2746–2753. [Google Scholar]
- Borges, J.; Mano, J.F. Layer-by-Layer Self-Assembly: A Powerful Tool for the Nanoengineering of Biomaterials. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- do Nascimento, J.P.L.; Ferreira, M.O.A.; Teixeira, G.T.L.; Slade, N.B.L.; Aoki, I.V.; Moreto, J.A. The Use of POPE Phospholipid as a First-Layer Coating of the Ti-6Al-4V Alloy: Preliminary Studies. Orbital Electron. J. Chem. 2024, 16, 10–16. [Google Scholar] [CrossRef]
- Moreto, J.A.; Marino, C.E.B.; Bose Filho, W.W.; Rocha, L.A.; Fernandes, J.C.S. SVET, SKP and EIS study of the corrosion behaviour of high strength Al and Al–Li alloys used in aircraft fabrication. Corros. Sci. 2014, 84, 30–41. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, D.M.; Ferreira, M.O.A.; Ramos, A.P.; Wolf, W.; Moreto, J.A.; Galo, R. Improving the Corrosion Resistance of Titanium by PAA/Chitosan Bilayer Architecture Through the Layer-by-Layer Method. Polysaccharides 2025, 6, 57. https://doi.org/10.3390/polysaccharides6030057
Dias DM, Ferreira MOA, Ramos AP, Wolf W, Moreto JA, Galo R. Improving the Corrosion Resistance of Titanium by PAA/Chitosan Bilayer Architecture Through the Layer-by-Layer Method. Polysaccharides. 2025; 6(3):57. https://doi.org/10.3390/polysaccharides6030057
Chicago/Turabian StyleDias, Daniele Morais, Murilo Oliveira Alves Ferreira, Ana Paula Ramos, Witor Wolf, Jéferson Aparecido Moreto, and Rodrigo Galo. 2025. "Improving the Corrosion Resistance of Titanium by PAA/Chitosan Bilayer Architecture Through the Layer-by-Layer Method" Polysaccharides 6, no. 3: 57. https://doi.org/10.3390/polysaccharides6030057
APA StyleDias, D. M., Ferreira, M. O. A., Ramos, A. P., Wolf, W., Moreto, J. A., & Galo, R. (2025). Improving the Corrosion Resistance of Titanium by PAA/Chitosan Bilayer Architecture Through the Layer-by-Layer Method. Polysaccharides, 6(3), 57. https://doi.org/10.3390/polysaccharides6030057





