Exploring the Potential of Carboxymethyl Chitosan and Oxidized Agarose to Form Self-Healing Injectable Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Carboxymethyl Chitosan
2.3. Synthesis of Oxidized Agarose
2.4. Preparation of OA:CMCh Hydrogel
2.5. Gelation Evaluation
2.6. Injectability
2.7. Syringeability
2.8. Compression Test
2.9. Rheological Characterization
2.10. Morphological Analysis
2.11. Cute-Heal Method
2.12. Swelling Test
2.13. Degradation Test
2.14. Statistical Analysis
3. Results
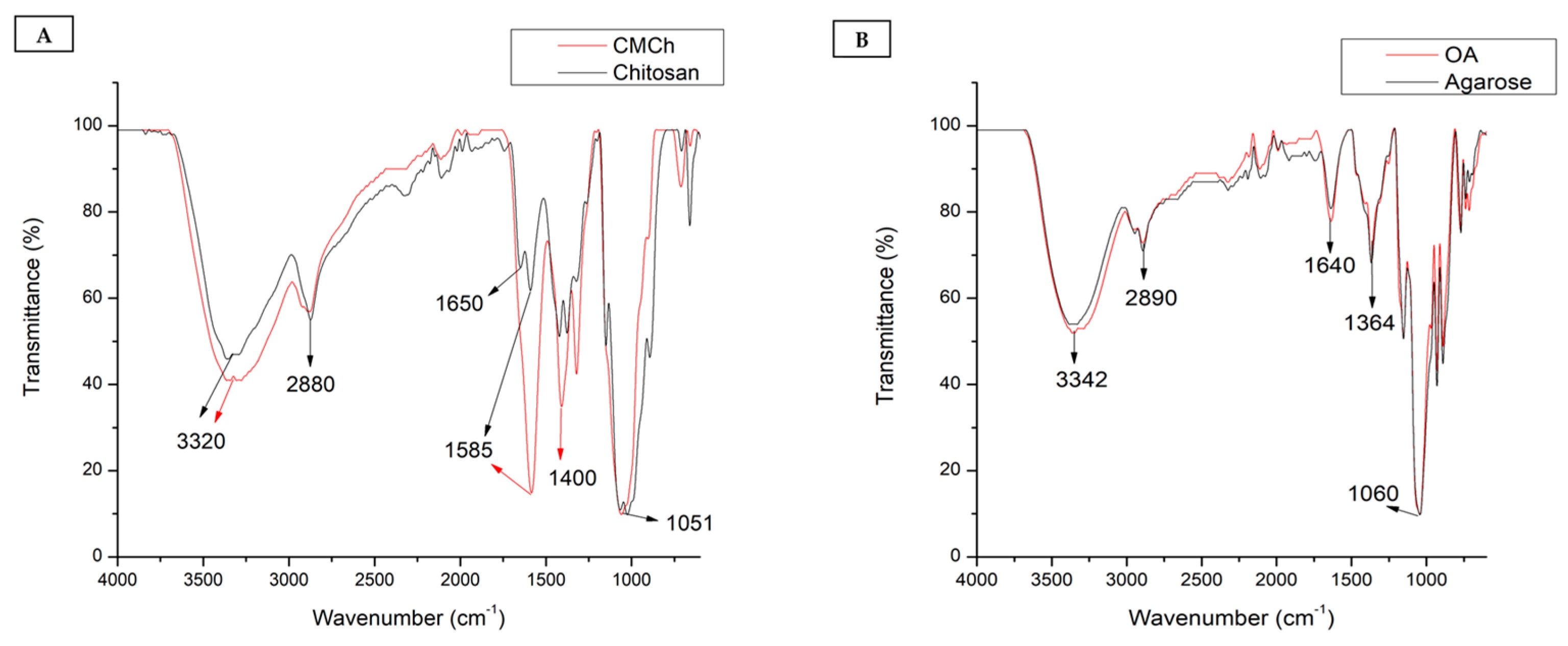
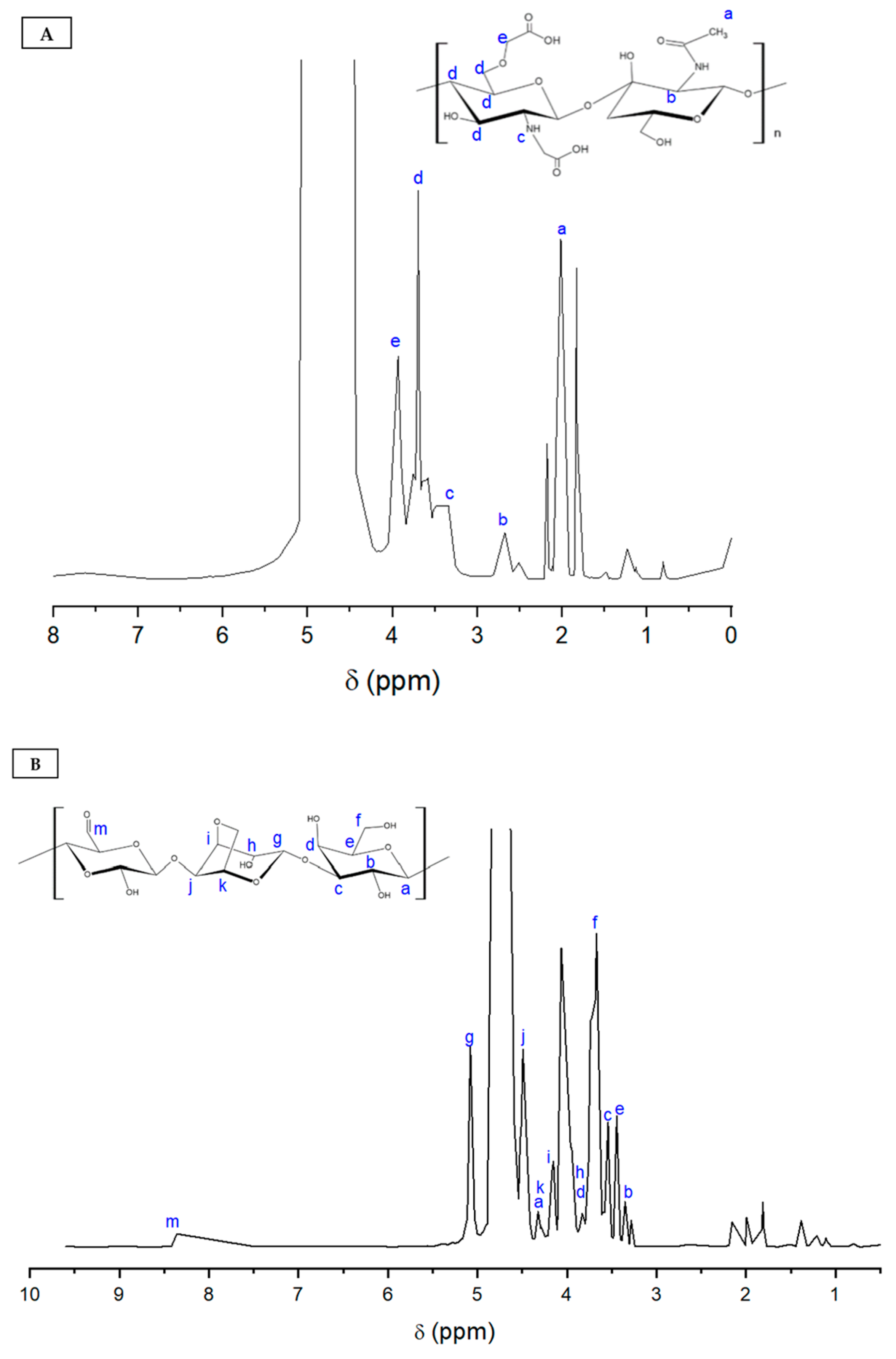
3.1. Characterization of Carboxymethyl Chitosan
3.2. Characterization of Oxidized Agarose
3.3. Hydrogel Gelation Time
3.4. Injectability of Hydrogel
3.5. Syringeability
3.6. Mechanical Properties
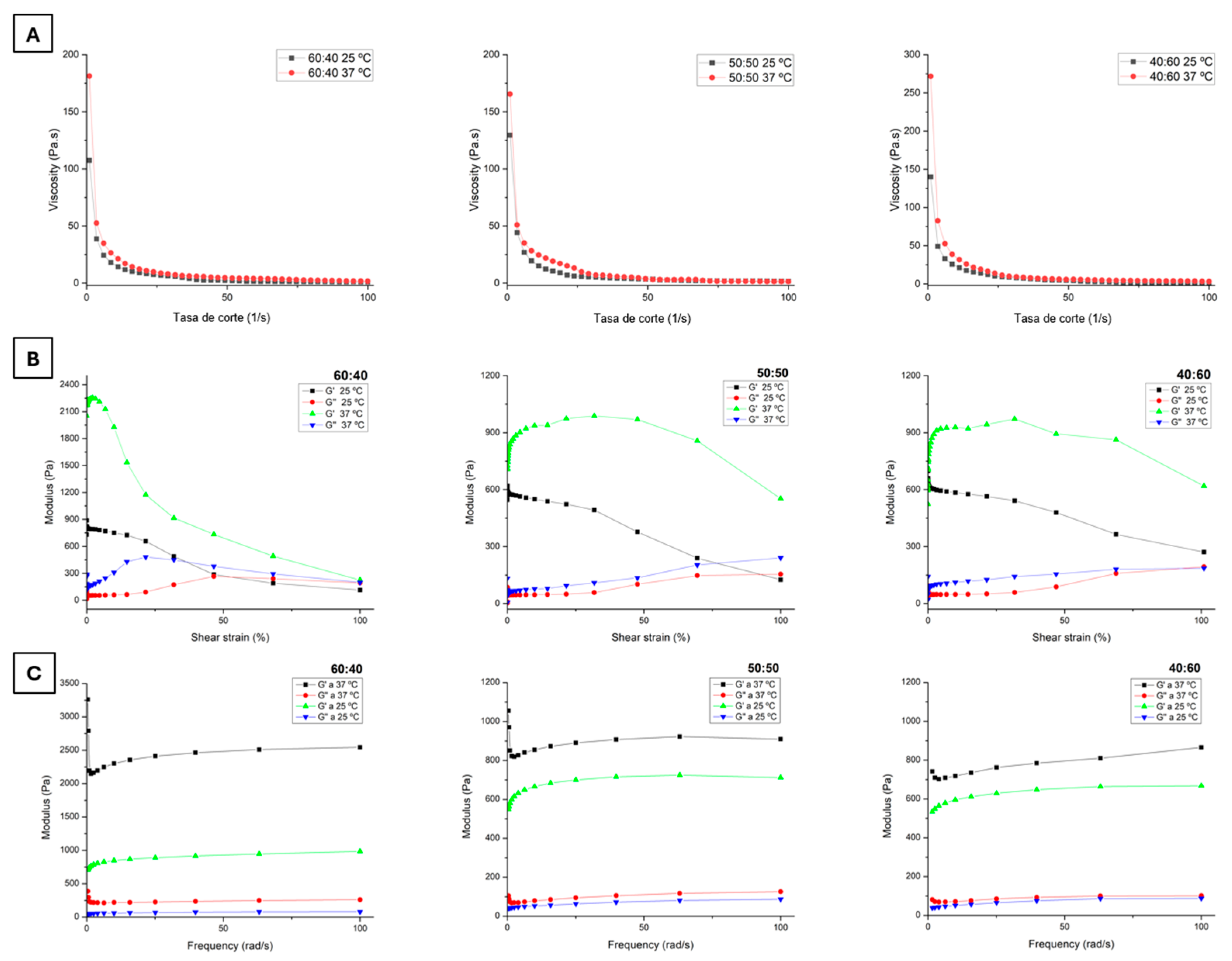
3.7. Rheological Properties
3.8. Scanning Electron Microscopy Analysis
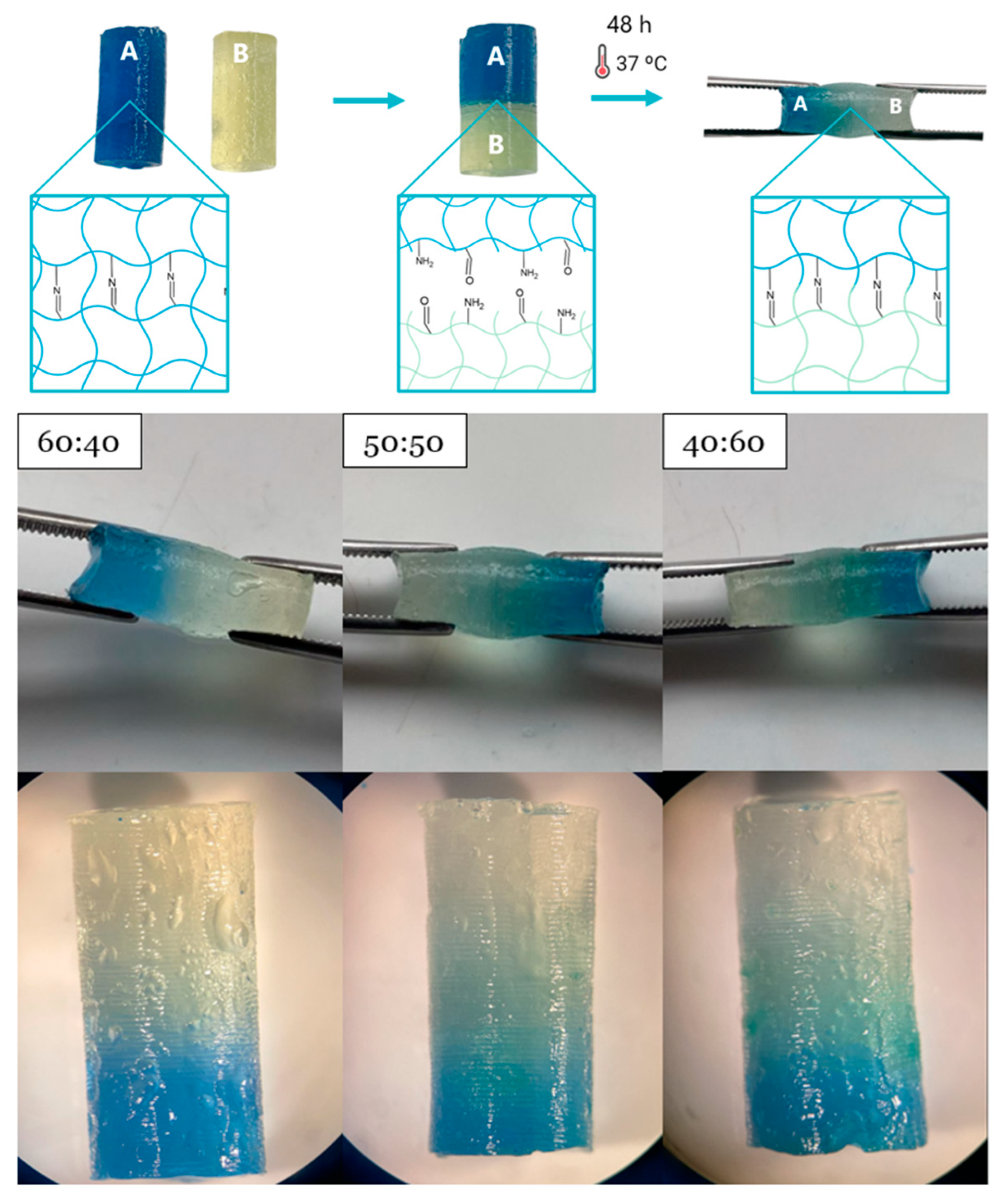
3.9. Self-Healing Evaluation
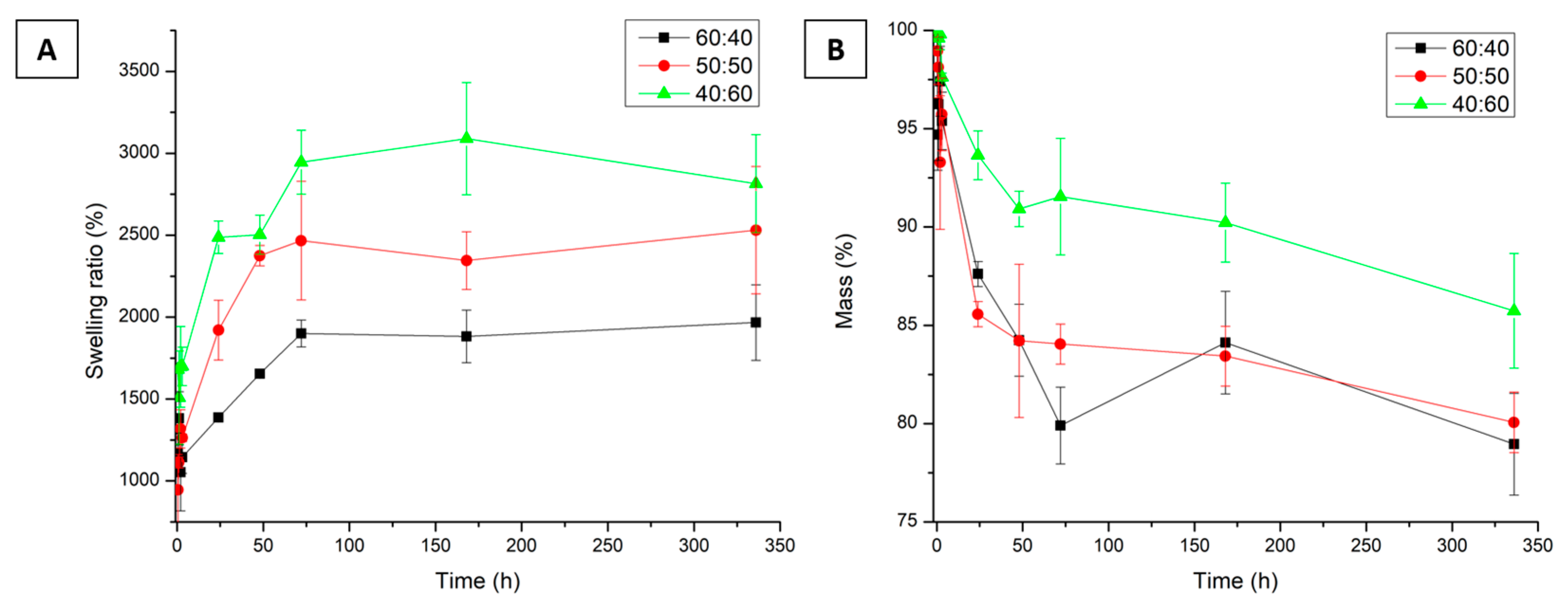
3.10. Swelling Test
3.11. Degradation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMCh | Carboxymethyl chitosan |
| FTIR | Fourier-transform infrared spectroscopy |
| OA | Oxidized agarose |
| PBS | Phosphate-buffered saline |
| 1H NMR | Proton nuclear magnetic resonance |
| SEM | Scanning electron microscopy |
| SR | Swelling ratio |
References
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kousar, M.; Mohsin, S.; Abbasi, M.; Shah, S.A.; et al. Chitosan Based Thermosensitive Injectable Hydrogels for Controlled Delivery of Loxoprofen: Development, Characterization and in-Vivo Evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Singha, I.; Basu, A. Chitosan Based Injectable Hydrogels for Smart Drug Delivery Applications. Sens. Int. 2022, 3, 100168. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In Situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-Based Injectable Hydrogel Composite for PH-Responsive and Controllable Drug Delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef]
- Córdoba, E.A.; Agudelo, N.A.; Echeverri-Cuartas, C.E. Fundamental Concepts of Injectable Hydrogel Based on Natural Polymer for Malignant Solid Tumor: Types and Characterization—Review. J. Drug Deliv. Sci. Technol. 2025, 105, 106587. [Google Scholar] [CrossRef]
- Mellati, A.; Hasanzadeh, E.; Gholipourmalekabadi, M.; Enderami, S.E. Injectable Nanocomposite Hydrogels as an Emerging Platform for Biomedical Applications: A Review. Mater. Sci. Eng. C 2021, 131, 112489. [Google Scholar] [CrossRef]
- Pourbadiei, B.; Adlsadabad, S.Y.; Rahbariasr, N.; Pourjavadi, A. Synthesis and Characterization of Dual Light/Temperature-Responsive Supramolecular Injectable Hydrogel Based on Host-Guest Interaction between Azobenzene and Starch-Grafted β-Cyclodextrin: Melanoma Therapy with Paclitaxel. Carbohydr. Polym. 2023, 313, 120667. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Xie, Z.; Chen, K.; Ma, M.; Huang, Y.; Li, M.; Cai, Z.; Wang, P.; Shen, H. Injectable Hydrogels for Spinal Cord Injury Repair. Eng. Regen. 2022, 3, 407–419. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in Injectable Self-Healing Biomedical Hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Anupama Devi, V.K.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural Polymers-Based Light-Induced Hydrogels: Promising Biomaterials for Biomedical Applications. Coord. Chem. Rev. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent Advances in Natural Polymer-Based Drug Delivery Systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Cirillo, G.; Spizzirri, U.G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics 2019, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, X.; Ren, Y.; Xue, W.; Liu, S.; Wang, P.; Zhao, M.; Xu, H.; Chi, B. Injectable Adaptive Self-Healing Hyaluronic Acid/Poly (γ-Glutamic Acid) Hydrogel for Cutaneous Wound Healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Luan, X.; Pang, Y.; Zhang, K.; Cheng, Y.; Ji, Z.; Pang, J. Adhesive Injectable Cellulose-Based Hydrogels with Rapid Self-Healing and Sustained Drug Release Capability for Promoting Wound Healing. Carbohydr. Polym. 2023, 320, 121235. [Google Scholar] [CrossRef]
- Gao, L.T.; Chen, Y.M.; Aziz, Y.; Wei, W.; Zhao, X.Y.; He, Y.; Li, J.; Li, H.; Miyatake, H.; Ito, Y. Tough, Self-Healing and Injectable Dynamic Nanocomposite Hydrogel Based on Gelatin and Sodium Alginate. Carbohydr. Polym. 2024, 330, 121812. [Google Scholar] [CrossRef]
- Cao, J.; Wu, P.; Cheng, Q.; He, C.; Chen, Y.; Zhou, J. Ultrafast Fabrication of Self-Healing and Injectable Carboxymethyl Chitosan Hydrogel Dressing for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24095–24105. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Farhaj, S.; Agbotui, T.L.; Nirwan, J.S.; Mahmood, Q.; Yousaf, A.M.; Hussain, T.; Shahzad, Y.; Khan, N.; Conway, B.R.; Ghori, M.U. Carbohydrate Polymer-Based Targeted Pharmaceutical Formulations for Colorectal Cancer: Systematic Review of the Literature. Polysaccharides 2022, 3, 692–714. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, Self-Healing, Antibacterial, and Hemostatic N,O-Carboxymethyl Chitosan/Oxidized Chondroitin Sulfate Composite Hydrogel for Wound Dressing. Mater. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.C.; Park, O.O. Self-Gelling Electroactive Hydrogels Based on Chitosan–Aniline Oligomers/Agarose for Neural Tissue Engineering with on-Demand Drug Release. Colloids Surf. B Biointerfaces 2019, 184, 110549. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Witzler, M.; Enkelmann, A.; Schneider, G.; Schulze, M.; Heinze, T. Functional Agarose Hydrogels Obtained by Employing Homogeneous Synthesis Strategies. Polysaccharides 2024, 5, 184–197. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Afraz, S.; Moradi, M.; Hassanpour, S. Antimicrobial Chitosan-Agarose Full Polysaccharide Silver Nanocomposite Films. Int. J. Biol. Macromol. 2021, 179, 532–541. [Google Scholar] [CrossRef]
- Mo, C.; Xiang, L.; Chen, Y. Advances in Injectable and Self-Healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun. 2021, 42, 2100025. [Google Scholar] [CrossRef]
- Priya, M.V.; Kumar, R.A.; Sivashanmugam, A.; Nair, S.V.; Jayakumar, R. Injectable Amorphous Chitin-Agarose Composite Hydrogels for Biomedical Applications. J. Funct. Biomater. 2015, 6, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in Situ Forming Thermo-Responsive Graphene Based Hydrogels for Cancer Chemo-Photothermal Therapy and NIR Light-Enhanced Antibacterial Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111294. [Google Scholar] [CrossRef]
- Karimi, T.; Mottaghitalab, F.; Keshvari, H.; Farokhi, M. Carboxymethyl Chitosan/Sodium Carboxymethyl Cellulose/Agarose Hydrogel Dressings Containing Silk Fibroin/Polydopamine Nanoparticles for Antibiotic Delivery. J. Drug Deliv. Sci. Technol. 2023, 80, 104134. [Google Scholar] [CrossRef]
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Yao, K.D. Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Gu, Q.; Xie, L.; Cai, Y.; Liao, L. Synthesis, Characterization and Potential Applications for Oxidized Agarose. Int. J. Biol. Macromol. 2023, 242, 124643. [Google Scholar] [CrossRef]
- Giraldo, J.C. Hidrogel Inyectable con Posible Aplicación en El Cáncer de Mama. Trabajo de Grado, Universidad EIA, Envigado, 2022. Available online: https://repository.eia.edu.co/bitstream/handle/11190/5359/GiraldoJuan_2022_HidrogelInyectablePosible.pdf?sequence=10&isAllowed=y (accessed on 27 September 2022).
- Taymouri, S.; Amirkhani, S.; Mirian, M. Fabrication and Characterization of Injectable Thermosensitive Hydrogel Containing Dipyridamole Loaded Polycaprolactone Nanoparticles for Bone Tissue Engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102659. [Google Scholar] [CrossRef]
- Li, X.; Fan, D.; Ma, X.; Zhu, C.; Luo, Y.; Liu, B.; Chen, L. A Novel Injectable PH/Temperature Sensitive CS-HLC/β-GP Hydrogel: The Gelation Mechanism and Its Properties. Soft Mater. 2014, 12, 1–11. [Google Scholar] [CrossRef]
- Moreira, C.D.F.; Carvalho, S.M.; Sousa, R.G.; Mansur, H.S.; Pereira, M.M. Nanostructured Chitosan/Gelatin/Bioactive Glass in Situ Forming Hydrogel Composites as a Potential Injectable Matrix for Bone Tissue Engineering. Mater. Chem. Phys. 2018, 218, 304–316. [Google Scholar] [CrossRef]
- ASTM D695-23; Standard Test Method for Compressive Properties of Rigid Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023. Available online: https://www.astm.org/d0695-23.html (accessed on 4 September 2024).
- Aranzana, S.P. Modulación Mecánica En Hidrogeles de Polietilenglicol. Master’s Thesis, Universidad de Zaragoza, Zaragoza, Spain, 2016. [Google Scholar]
- Sharma, S.; Kumar, R.; Kumar Rana, N.; Koch, B. The Consequence of Imine Bond Origination: Fabrication of Rapid Self-Healing Chitosan Hydrogel as a Drug Delivery Candidate for Water-Soluble Drug. Eur. Polym. J. 2022, 180, 111605. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Tiwari, A. Carboxymethyl Chitosan and Its Applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Nadira, P.P.; Mujeeb, V.M.A.; Rahman, P.M.; Muraleedharan, K. Effects of Cashew Leaf Extract on Physicochemical, Antioxidant, and Antimicrobial Properties of N, O–Carboxymethyl Chitosan Films. Carbohydr. Polym. Technol. Appl. 2022, 3, 100191. [Google Scholar] [CrossRef]
- Bukzem, A.L.; Signini, R.; dos Santos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of Carboxymethyl Chitosan Synthesis Using Response Surface Methodology and Desirability Function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef]
- Yan, Y.; Guan, S.; Wang, S.; Xu, J.; Sun, C. Synthesis and Characterization of Protocatechuic Acid Grafted Carboxymethyl Chitosan with Oxidized Sodium Alginate Hydrogel through the Schiff’s Base Reaction. Int. J. Biol. Macromol. 2022, 222, 2581–2593. [Google Scholar] [CrossRef]
- Hu, Z.; Hong, P.; Liao, M.; Kong, S.; Huang, N.; Ou, C.; Li, S. Preparation and Characterization of Chitosan—Agarose Composite Films. Materials 2016, 9, 816. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of Flexible Ph-Responsive Agarose/Succinoglycan Hydrogels for Controlled Drug Release. Polymers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Three-Dimensional Porous Scaffolds Based on Agarose/Chitosan/Graphene Oxide Composite for Tissue Engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef]
- Gericke, M.; Heinze, T. Homogeneous Tosylation of Agarose as an Approach toward Novel Functional Polysaccharide Materials. Carbohydr. Polym. 2015, 127, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, J.; Mao, X.; Tang, S. Preparation and Biological Properties of Schiff-Base Hydrogels Crosslinked by Benzaldehyde Substituted Agarose Oligosaccharides. React. Funct. Polym. 2023, 193, 105745. [Google Scholar] [CrossRef]
- Fathi, A.; Mithieux, S.M.; Wei, H.; Chrzanowski, W.; Valtchev, P.; Weiss, A.S.; Dehghani, F. Elastin Based Cell-Laden Injectable Hydrogels with Tunable Gelation, Mechanical and Biodegradation Properties. Biomaterials 2014, 35, 5425–5435. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Lin, R.; Cui, S.; Jing, X.; Coseri, S. Injectable Multifunctional Carboxymethyl Chitosan/Hyaluronic Acid Hydrogel for Drug Delivery Systems. Int. J. Biol. Macromol. 2023, 249, 125801. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, Z.; Karkhaneh, A.; Khojasteh, A. Simultaneous Release of Melatonin and Methylprednisolone from an Injectable in Situ Self-Crosslinked Hydrogel/Microparticle System for Cartilage Tissue Engineering. J. Biomed. Mater. Res. A 2018, 106, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.H.; Atluri, P.; Burdick, J.A. Methods to Assess Shear-Thinning Hydrogels for Application as Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade del Olmo, J.; Pérez Gonzáles, R.; Sáez-Martínez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef]
- Hikmawati, D.; Maulida, H.N.; Putra, A.P.; Budiatin, A.S.; Syahrom, A. Synthesis and Characterization of Nanohydroxyapatite-Gelatin Composite with Streptomycin as Antituberculosis Injectable Bone Substitute. Int. J. Biomater. 2019, 2019, 7179243. [Google Scholar] [CrossRef]
- Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers 2023, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Fang, S.; Xia, K.; Chen, J.; Guo, L.; Guo, W. Enhancing the Mechanical Properties and Cytocompatibility of Magnesium Potassium Phosphate Cement by Incorporating Oxygen-Carboxymethyl Chitosan. Regen. Biomater. 2021, 8, rbaa048. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of Mechanical Properties and Thermal Stability of Biodegradable Rice Starch–Based Films Blended with Carboxymethyl Chitosan. Ind. Crops Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Zheng, T.; Tang, P.; Shen, L.; Bu, H.; Li, G. Rheological Behavior of Collagen/Chitosan Blended Solutions. J. Appl. Polym. Sci. 2021, 138, 50840. [Google Scholar] [CrossRef]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In Vitro Characterization of a Novel Magnetic Fibrin-Agarose Hydrogel for Cartilage Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef]
- Dave, P.N.; Macwan, P.M.; Kamaliya, B. Synthesis and Rheological Investigations of Gum-Ghatti-Cl-Poly(NIPA-Co-AA)-Graphene Oxide Based Hydrogels. Mater. Adv. 2023, 4, 2971–2980. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Malik, U.S.; Niazi, M.B.K.; Jahan, Z.; Zafar, M.I.; Vo, D.V.N.; Sher, F. Nano-Structured Dynamic Schiff Base Cues as Robust Self-Healing Polymers for Biomedical and Tissue Engineering Applications: A Review. Environ. Chem. Lett. 2021, 20, 495–517. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Polysaccharide-Based in Situ Self-Healing Hydrogels for Tissue Engineering Applications. Polymers 2020, 12, 2261. [Google Scholar] [CrossRef]
- Sampath, T.M.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Barkat, K.; Malik, N.S.; Alqahtani, M.S.; Anjum, I.; Khalid, I.; Tulain, U.R.; Gohar, N.; Zafar, H.; Paiva-Santos, A.C.; et al. PH Sensitive Pluronic Acid/Agarose-Hydrogels as Controlled Drug Delivery Carriers: Design, Characterization and Toxicity Evaluation. Pharmaceutics 2022, 14, 1218. [Google Scholar] [CrossRef]
- Azizullah; Al-Rashida, M.; Haider, A.; Kortz, U.; Joshi, S.A.; Iqbal, J. Development and in Vitro Anticancer Evaluation of Self-Assembled Supramolecular PH Responsive Hydrogels of Carboxymethyl Chitosan and Polyoxometalate. ChemistrySelect 2018, 3, 1472–1479. [Google Scholar] [CrossRef]
- Ghosh, T.; Mohammed, Y.; Murahari, M.; Samual, S.E.; Deveswaran, R.; Basavaraj, B.V. Vanillin Based Crosslinked Films of CMCh-PVA for Wound Healing Application. J. Drug Deliv. Sci. Technol. 2023, 83, 104400. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Huang, Q.; Huang, H. A Self-Healing Hydrogel Based on Oxidized Microcrystalline Cellulose and Carboxymethyl Chitosan as Wound Dressing Material. Int. J. Biol. Macromol. 2022, 221, 1606–1617. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable Hydrogels: Design Mechanisms and Versatile Applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Gámiz-González, M.A.; Guldris, P.; Antolinos Turpín, C.M.; Ródenas Rochina, J.; Vidaurre, A.; Gómez Ribelles, J.L. Fast Degrading Polymer Networks Based on Carboxymethyl Chitosan. Mater. Today Commun. 2017, 10, 54–66. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Z.; Cheng, D.; Mao, X. Agarose Degradation for Utilization: Enzymes, Pathways, Metabolic Engineering Methods and Products. Biotechnol. Adv. 2020, 45, 107641. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and Biodegradable Chitosan/Agarose Film Revealing Slightly Acidic PH for Potential Applications in Regenerative Medicine as Artificial Skin Graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, G.; Ao, Q.; Gong, Y.; Zhang, X. Preparation of Cross-Linked Carboxymethyl Chitosan for Repairing Sciatic Nerve Injury in Rats. Biotechnol. Lett. 2010, 32, 59–66. [Google Scholar] [CrossRef] [PubMed]
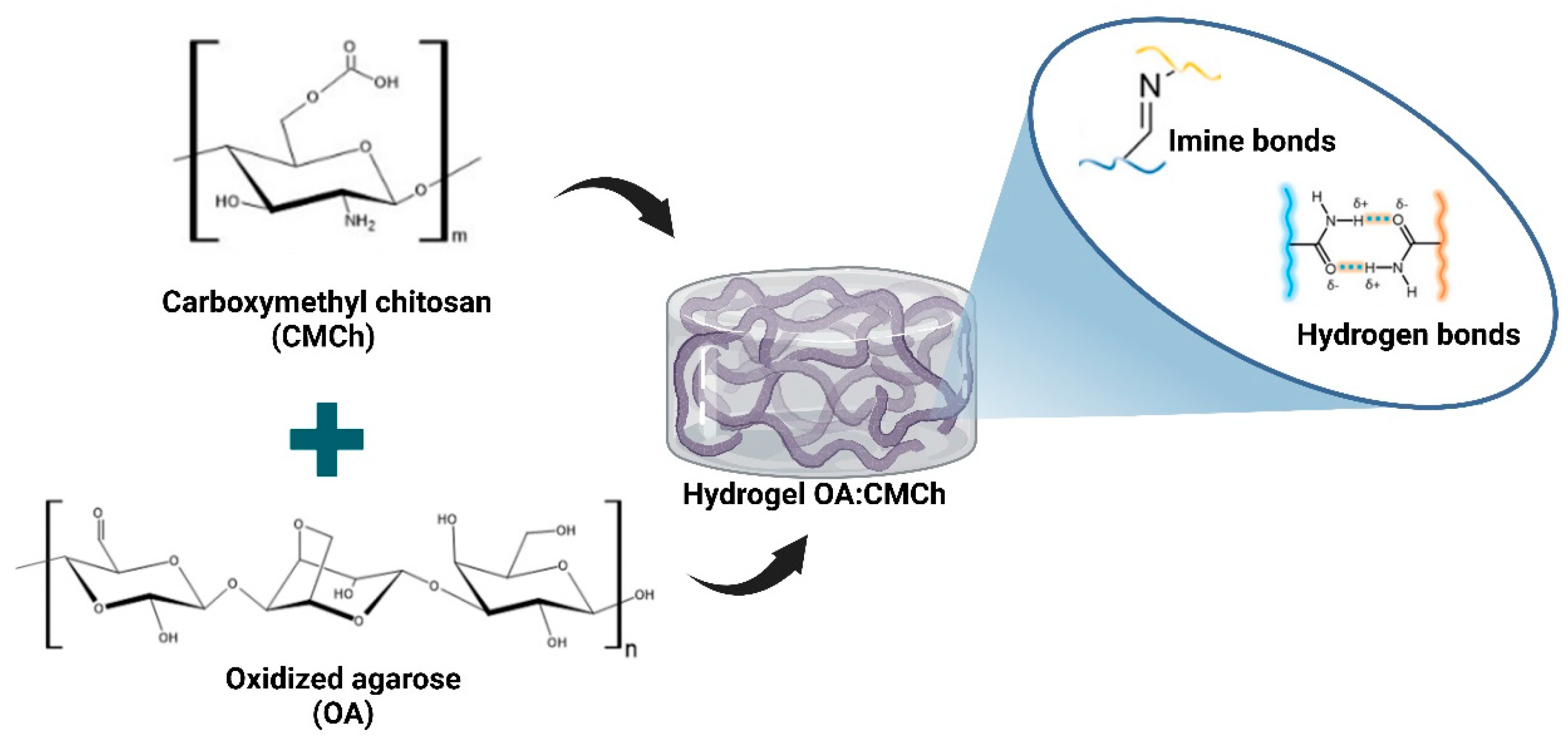


| Concentration 20 mg/mL | Concentration 30 mg/mL | |||
|---|---|---|---|---|
| Ratio (OA:CMCh) | Amount OA (mg) | Amount CMCh (mg) | Amount OA (mg) | Amount CMCh (mg) |
| 80:20 | 16 | 4 | 24 | 6 |
| 60:40 | 12 | 8 | 18 | 12 |
| 50:50 | 10 | 10 | 15 | 15 |
| 40:60 | 8 | 12 | 12 | 18 |
| 20:80 | 4 | 16 | 6 | 24 |
| Maximum Compression Load (N) | Shear Stress (kPa) | Viscosity (Pa.s) | ||||
|---|---|---|---|---|---|---|
| OA:CMCh | 25 °C | 4 °C → 25 °C | 25 °C | 4 °C → 25 °C | 25 °C | 4 °C → 25 °C |
| 80:20 | 5.95 ± 0.84 | 8.86 ± 0.86 | 81.64 ± 11.53 | 121.52 ± 11.77 | 21.45 ± 3.03 | 31.94 ± 3.09 |
| 60:40 | 12.85 ± 0.36 | 20.66 ± 0.55 | 176.26 ± 5.00 | 283.41 ± 7.54 | 46.32 ± 1.32 | 74.48 ± 1.98 |
| 50:50 | 18.51 ± 1.29 | 24.50 ± 0.77 | 253.92 ± 17.74 | 336.19 ± 1.56 | 66.73 ± 4.66 | 88.36 ± 2.78 |
| 40:60 | 20.94 ± 0.56 | 19.49 ± 0.59 | 287.30 ± 7.75 | 267.36 ± 8.10 | 75.51 ± 2.04 | 70.27 ± 2.13 |
| 20:80 | 22.12 ± 1.08 | 17.96 ± 0.25 | 303.49 ± 14.75 | 246.42 ± 3.45 | 79.76 ± 3.88 | 64.76 ± 0.91 |
| OA:CMCh | 25 °C | 4 °C → 25 °C |
|---|---|---|
| 60:40 | 89.74 ± 2.83 | 47.30 ± 3.51 |
| 50:50 | 54.08 ± 3.73 | 28.90 ± 4.25 |
| 40:60 | 37.36 ± 2.37 | 14.55 ± 3.50 |
| Compressive Strength (kPa) | Young’s Modulus (kPa) | |||
|---|---|---|---|---|
| OA:CMCh | 25 °C | 4 °C → 25 °C | 25 °C | 4 °C → 25 °C |
| 60:40 | 26.92 ± 6.04 | 49.15 ± 3.72 | 9.63 ± 1.39 | 20.98 ± 1.88 |
| 50:50 | 46.28 ± 7.44 | 71.06 ± 11.62 | 8.67 ± 0.73 | 16.87 ± 3.74 |
| 40:60 | 46.36 ± 10.54 | 70.14 ± 3.23 | 6.60 ± 1.12 | 9.57 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdoba, E.A.; Agudelo, N.A.; Giraldo, L.F.; Echeverri-Cuartas, C.E. Exploring the Potential of Carboxymethyl Chitosan and Oxidized Agarose to Form Self-Healing Injectable Hydrogels. Polysaccharides 2025, 6, 49. https://doi.org/10.3390/polysaccharides6020049
Córdoba EA, Agudelo NA, Giraldo LF, Echeverri-Cuartas CE. Exploring the Potential of Carboxymethyl Chitosan and Oxidized Agarose to Form Self-Healing Injectable Hydrogels. Polysaccharides. 2025; 6(2):49. https://doi.org/10.3390/polysaccharides6020049
Chicago/Turabian StyleCórdoba, Eduard A., Natalia A. Agudelo, Luis F. Giraldo, and Claudia E. Echeverri-Cuartas. 2025. "Exploring the Potential of Carboxymethyl Chitosan and Oxidized Agarose to Form Self-Healing Injectable Hydrogels" Polysaccharides 6, no. 2: 49. https://doi.org/10.3390/polysaccharides6020049
APA StyleCórdoba, E. A., Agudelo, N. A., Giraldo, L. F., & Echeverri-Cuartas, C. E. (2025). Exploring the Potential of Carboxymethyl Chitosan and Oxidized Agarose to Form Self-Healing Injectable Hydrogels. Polysaccharides, 6(2), 49. https://doi.org/10.3390/polysaccharides6020049







