Abstract
Starch films were obtained by solvent casting and thermoprocessing using glycerol as a plasticiser from a new starch source: tiger nut waste from horchata production. The tiger nut starch (TNS) films showed a barrier capacity to water vapour and gases in the typical range of other starch films, such as corn starch (CS) films, with a high barrier capacity to oxygen. The tensile properties of the films were affected by the processing method, exhibiting higher stiffness and resistance to break and lower stretchability than the more common CS films. Thermoprocessed TNS films were less water soluble than CS films, and their solubility was higher than that of cast TNS films. However, all films exhibited similar swelling power. Thermal stability was also similar for all TNS and CS films, showing the typical thermal degradation pattern of starch–glycerol films. Therefore, TNS obtained from horchata production waste can be used to obtain thermoplastic starch films for packaging applications, with characteristics comparable to the most common corn starch films.
1. Introduction
Increasing awareness of the environmental impact caused by conventional petroleum-based plastics, particularly their persistence in ecosystems and poor recyclability [1,2], has intensified research efforts toward sustainable, biodegradable substitutes. Global production capacity for bioplastics is expected to rise from around 1.1 million tons in 2023 to approximately 4.6 million tons by 2028 [3]. Stricter environmental regulations and growing consumer consciousness of the need for eco-packaging solutions in the food industry and other sectors promote this evolution. Packaging remains the largest market segment for bioplastics, comprising 43% (0.934 million tons) of the total bioplastics market in 2023 [3].
Starch, a naturally abundant and low-cost polysaccharide obtained from various plant sources, is known for its biodegradability and ability to form films. With the aid of plasticisers such as glycerol, it can be easily converted into thermoplastic material, positioning it as a strong candidate for the creation of eco-friendly packaging materials [4] for food packaging applications [2,5]. In 2023, bioplastic starch-based blends comprised 6.4% of the global bioplastics production capacity, constituting the world’s second-leading bioplastic material after polylactic acid blends, which represented about 31% [3]. Despite its potential, the large-scale adoption of starch-based bioplastics faces some challenges, particularly regarding raw material availability and competition with food supply chains. The predominant sources of industrial starch (corn, wheat, potatoes, and rice) are staple food crops, essential for an increasing global food demand (by 35% to 56% between 2010 and 2050) [6]. Furthermore, fluctuations in agricultural yields further threaten the stability of starch supply for non-food applications [7]. To address these concerns, exploring alternative starch sources, such as starchy waste generated by the agri-food industry, could represent an opportunity to develop more starch-based bioplastics, alleviating competition with food resources and enhancing the economic viability and environmental sustainability of bioplastic packaging solutions. Some studies have reported the use of starch from unexplored sources to obtain coatings or films [8], such as mango kernel [9], arrowroot starch [10], and sago starch [11]. This strategy aligns with circular economy principles by reducing waste, adding value, and enhancing sustainability [12,13].
One such underutilised source is the tiger nut, which is considered an emerging source of edible oil and is also rich in starch, containing 14–37% starch on a dry basis [14,15]. In Spain, this tuber is used to obtain horchata, a traditional and healthy drink that has gained popularity in several countries, including France, Portugal, the United Kingdom, China, and Argentina [14]. The solid waste resulting from the extraction represents up to 60% of the harvested plant material [16] and contains about 23% (dry basis) of starch [17]. It is usually used as organic mass for combustion, composting, or animal feeding [18], but recently, it has been studied as a potential source of starch and bioactive compounds [17,19], which could be used in active food packaging manufacturing. A starch recovery process from this waste, using water homogenisation and starch separation by density, has been described with adequate starch yield (70%) and purity (92%) and low environmental impact [17]. Tiger nut starch presents similar characteristics to corn starch in terms of amylose content [17], degree of crystallinity (about 39%) [17,20], molecular mass (7–10 × 107 g/mol) [14,21], and average chain length of amylopectin (around 20) [14,21,22]. Although the X-R diffraction pattern of tiger nut starch is also classified as A-type [14,17], some authors have established that it is C-type based on the small diffraction peak at 5.6° [23,24]. Likewise, there are some differences in granule morphology and average size, as well as the entanglements between amylose and amylopectin inside the granule, which affect the starch water binding capacity and swelling power, starch gelatinisation behaviour, gel strength, and tendency to retrograde [22,24,25]. These structural differences may also affect the film-forming ability and the properties of tiger nut starch films, which have only been previously investigated by Li et al. [26], who produced tiger nut starch films by solvent casting. To the best of our knowledge, no previous studies have analysed the potential of tiger nut byproduct to obtain biodegradable, low-cost, starch-based films for food packaging applications.
Starch-based films can be obtained using different wet and dry processes, such as solution casting, compression moulding, extrusion process, reactive extrusion, coextrusion, injection moulding, and film blowing [27,28]. Solvent casting is a widely used approach at laboratory scale that involves starch gelatinisation in water, followed by casting and solvent evaporation, resulting in thin and flexible films [2,5]. The advantage of the casting method is that the films are highly transparent and flexible, but it requires high energy and time consumption for water evaporation and low production efficiency [29]. The thermoprocessing of starch using extrusion or compression moulding techniques allows the production of thermoplastic starch films, improving scalability and process efficiency [30]; these are the most commonly used methods for producing plastic materials due to their fast temperature rise and simple operation [29].
This study analyses the properties of starch films obtained from a new starch source, tiger nut solid waste from horchata production, compared with those of corn starch films, one of the most used sources for producing starch-based thermoplastic starch. Tiger nut starch films were obtained by casting solvent and thermoprocessing using glycerol as a plasticiser. The films obtained were characterised at different storage times as to their equilibrium moisture content; water solubility and swelling capacity; mechanical, optical, and barrier properties; and thermal stability. The results obtained make it possible to evaluate the potential of tiger nut residue for obtaining thermoplastic starch for packaging applications.
2. Materials and Methods
2.1. Raw Materials
Horchata solid residue supplied by Horchatería Rin (Alboraya, Spain) was used to extract starch, following the methodology described in a previous study [17]. The obtained tiger nut starch (TNS) had 92% purity; contained 0.5% total fibre, 0.15% protein, 1% lipids, 0.4% ash, and 9% moisture content; and had an amylose ratio of 23% [17].
Corn starch was obtained from Roquette (Roquette Laisa, Spain). Glycerol (C3H8O3, 99%), methanol (HPLC grade), sodium chloride (NaCl, 99%), calcium chloride dihydrate (CaCl2, 99.5%), sodium azide (NaN3, 99%), magnesium nitrate hexahydrate (Mg(NO3)2 6H2O, 99%) and sodium carbonate (Na2CO3, 99.5%) were supplied by PanReac Quimica S.L.U. (Castellar del Vallés, Spain). Folin–Ciocalteu reagent (2 N), gallic acid, D-limonene, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were supplied by Sigma-Aldrich (USA). Phosphorus pentoxide (P2O5, 98.2%) was obtained from VWR Chemicals (Leuven, Belgium).
2.2. Film Preparation
Glycerol was used as a plasticiser in every film formulation at a starch-to-glycerol ratio of 1:0.3. TNS films were obtained from both solvent-casting and thermoprocessing methods. Thermoprocessed corn starch films (CST) were also obtained for comparison purposes, as they contain similar (22–26%) amylose content [31,32], and, as noted above, they are one of the most commonly used sources for producing starch-based thermoplastic starch.
2.2.1. Casting Process
The TNS was dispersed in water under magnetic stirring at room temperature and 700 rpm for 10 min and subsequently heated in a water bath at 95 °C and 150 rpm for 3 h. Then, the dispersion was left to cool to around 50 °C, stirring at 700 rpm, and then glycerol was added, and the mixture was stirred for another 5 min.
The films were prepared by weighing the film-forming dispersion to provide 1.48 g of total solids on Teflon casting plates (150 mm diameter). Casting plates containing the dispersion were levelled and dried under controlled conditions (55% RH and 25–30 °C) for 48 h in a germination chamber with moisture control (model GERHRT-1000, Radiber, Barcelona, Spain). Dry films were peeled from the plates and conditioned at 25 °C and 53% RH or 0% RH in desiccators containing an oversaturated solution of Mg(NO3)2, or P2O5, respectively, before further analysis. These films from tiger nut starch obtained by casting were named TNSC.
2.2.2. Melt Blending and Compression Moulding
The starch was pre-conditioned at 53% RH and then melt-blended with glycerol at 50 rpm and 130 °C for 10 min using an internal mixer (HAAKETM PolyLabTM QC, Thermo Fisher Scientific, Karlsruhe, Germany) to obtain thermoplastic starch. The blends were cold powdered with liquid N2 using a Thermomix TM-5 (Vorwerk Spain M.S.L., S.C., Madrid, Spain) at 10,200 rpm and conditioned at 53% RH for a week. Subsequently, films were produced by thermocompressing 4 g of the powder using a hot plate hydraulic press (Model LP20, Labtech Engineering, Thailand). The compression cycle included a preheating step at 160 °C for 3 min, a first compression at 50 bar and 160 °C for 2 min, a second compression at 100 bar and 160 °C for 6 min, and finally a cooling step to 70 °C. The obtained films were conditioned at 25 °C and 53% RH or 0% RH in desiccators containing an oversaturated solution of Mg(NO3)2, or P2O5, respectively, before further analysis. The thermoprocessed films were named TNST for tiger nut starch and CST for corn starch. All films were characterised after 1 and 5 weeks of conditioned storage in order to analyse changes associated with starch retrogradation.
2.3. Film Characterisation
The equilibrium moisture content of the films at 53% RH equilibrium and 25 °C was determined gravimetrically, in triplicate, by drying in a vacuum oven at 60 °C and subsequent conditioning in P2O5. The film thickness was measured at six random positions, using a Palmer digital micrometre (Comecta, Barcelona, Spain) to the nearest 0.001 mm.
2.3.1. Microstructural Analysis
The film microstructure in both the cross-section and the surface was analysed by using a high-resolution field emission scanning electron microscope (GeminiSEM 500, Zeiis, Oxford Instruments, Oxford, UK). For this analysis, samples were mounted on stubs and gold-coated before the microscopic observations at an accelerating voltage of 2 kV. For the cross-section observations, samples were previously cryo-fractured using slush N2. Three films of each formulation, conditioned at 0% RH, were considered for this analysis.
2.3.2. Optical Properties
The colour coordinates and transparency of the films, conditioned at 53% RH, were evaluated using a Spectrocolourimeter CM-3600d (Minolta CO., Tokyo, Japan). Measurements were taken at 3 random points of 3 different samples for each film formulation. Measurements were carried out with an 8 mm mask, using both black (R0) and white (Rg) backgrounds to obtain the reflectance spectra from 400 to 700 nm (R). Kubelka–Munk theory (Equations (1)–(4)) was applied to determine the reflectance of an infinite thickness film (R∞) and the internal transmittance (Ti) [33]. The CIEL*a*b* colour coordinates (L*, a* and b*), were obtained from the R∞, considering illuminant D65, and observer 10°. The chroma (C*ab) and hue angle (h*ab) were calculated using Equations (5) and (6).
2.3.3. Mechanical Properties
The ASTM D882 method [34] was applied to determine the tensile properties of the films (25 × 100 mm strips), conditioned at 53% RH, using a universal testing machine (TA-XT plus (Stable Micro Systems, Haslemere, UK). The two grips were initially separated by 50 mm, and the sample was stretched at 50 mm/min. Tensile strength at break (TS), elastic modulus (EM), and elongation at break (%E) were obtained from the stress–strain curves. At least 8 replicates were carried out for each film formulation.
2.3.4. Barrier Properties
Water vapour permeability (WVP) was measured following the ASTM E96–95 method [35]. Payne permeability cups (Elcometer SPRL, Hermelle/s Argenteau, Belgium) with a 3.5 cm diameter were filled with 5 mL of distilled water (100% RH), and circular film samples whose thickness had been previously measured were placed on them and properly sealed. The cups were introduced in desiccators containing an oversaturated solution of Mg(NO3)2 (53% RH) at 25 °C. The water transmission rate was determined from the slope of the weight loss–time curves once the stationary state was reached, and the WVP values were obtained considering the film thickness and the vapour pressure gradient. Three replicates were obtained for each sample.
Aroma permeability using D-limonene (LP) was measured by a gravimetric method, similar to that described for WVP, but introducing D-limonene (5 mL) inside the Payne permeability cups and proceeding under the same controlled conditions (25 °C and 53% RH). The rate of weight loss over time was used to estimate the L transmission rate, and LP was obtained considering the film thickness and the limonene pressure gradient. Measurements were carried out in triplicate for each sample.
Oxygen permeability (OP) was determined following the ASTM Standard Method D3985-05 [36] using an oxygen permeation analyser (Model 8101e, Systech Illinois, Thame, UK). The tests were performed in duplicate for each formulation at 53% RH, and the film exposure area was 50 cm2. OP was calculated by dividing the oxygen transmission rate by the difference in the oxygen partial pressure between the two sides of the film and multiplying by the film thickness.
2.3.5. Solubility and Swelling Power
The solubility and swelling power of the films were determined following the method described by Moreno et al. [37], with some modifications. Film samples were cut into 2 cm2 strips and conditioned at 0% RH for 2 weeks. Approximately 0.1 g of each sample (W1) was weighed in glass tubes, where 10 mL of distilled water was added and kept under constant magnetic stirring for 24 h at room temperature. Then, the tubes were centrifuged at 2000× g for 5 min, the supernatant was deeply separated, and the swollen films were weighed (W3). Finally, the swollen films were dried at 105 °C for 24 h in a natural convection oven (Model Conterm J.P. Selecta, Barcelona, Spain) and placed in a desiccator containing P2O2 until reaching constant weight (W2). Solubility (S) and swelling power (SP) were calculated using Equations (7) and (8), respectively. This procedure was carried out in triplicate for each film formulation.
2.3.6. Thermal Analysis
The thermal stability of the films previously conditioned at 0% RH was analysed in triplicate with a thermogravimetric analyser (TGA/SDTA 851e, Mettler Toledo, Schwarzenbach, Switzerland) by heating the samples (about 4 mg) in alumina crucibles from 25 to 900 °C at 10 °C·min−1 under nitrogen flow (30 mL·min−1). The derivative curves (DTGA) were obtained using STARe Evaluation Software version 12.00b (Mettler-Toledo, Switzerland). The initial degradation temperature, the temperature at maximum degradation rate, and the mass loss percentage in each thermal event were determined.
2.3.7. Antioxidant Capacity and Total Phenolic Content (TPC)
Antioxidant capacity was measured in triplicate for each film formulation using a 2,2-diphenyl-1-pikryl-hydrazyl (DPPH) reduction method [38]. To this end, films (0.5 g) were extracted with methanol (10 mL) for 24 h at 20 °C. A gradient from 0.2 to 1 mL of the different film extracts was mixed with a methanol solution of DPPH 0.07 mM to a final volume of 4 mL. The decrease in absorbance at 25 °C was determined using a spectrophotometer (ThermoScientific Evolution 201 UV–vis, Waltham, MA, USA) at 515 nm. Measurements were taken every hour until the reaction reached a steady state. The DPPH concentration (mM) in the reaction medium was calculated from the calibration curve (R2 = 0.999). The percentage of remaining DPPH (%[DPPH]r) after the reaction was calculated using Equation (9):
where [DPPH]t=n is the concentration of DPPH at the steady state and [DPPH]t=0 is the initial value. The parameter EC50 was calculated as the amount of the extract solids per mass unit of DPPH necessary to reduce the initial DPPH concentration by 50%. The results were expressed in g dry film per g DPPH.
The total phenolic content (TPC) was determined in triplicate for each sample, according to the modified Folin–Ciocalteu methodology described by Menzel et al. [39]. To this end, 0.4 mL of the film extract, 6 mL of distilled water, and 0.5 mL of Folin–Ciocalteu reagent were placed into a volumetric flask. After 1 min, 1.5 mL of a 20% Na2CO3 solution was added and flushed up to 10 mL with distilled water. After 2 h of reaction in darkness, TPC was determined from the absorbance measured at 725 nm in a spectrophotometer (ThermoScientific Evolution 201 UV-vis, Waltham, MA, USA). The results were expressed as mg gallic acid equivalent per g of dry film.
2.3.8. Residual α-Amylase Activity
The residual α-amylase activity was determined in the starch films using the spectrophotometric assay (Ceralpha method K-CERA, Neogen Co., Lansing, MI, USA, 2022) previously described for cereal and microbial α-amylase [40]. The obtained results were expressed as International Units on a starch substrate.
2.4. Statistical Analysis
Statgraphics Plus for Windows 5.1 (Manugistics Corp., Rockville, MD, USA) was used to conduct statistical analyses of data through an analysis of variance (ANOVA). Fisher’s least significant difference (LSD) was used at the 95% level of confidence.
3. Results
3.1. Microstructure Observations
The analysis of the film microstructure resulting from the interactions between the film components and the processing conditions provides information about the surface morphology and internal microstructure of the films, allowing for a deeper understanding of their macroscopic properties, such as mechanical and optical characteristics. Figure 1 shows the micrographs corresponding to the surface (left) and cross-section (right) of the films produced by casting (TNSC) and by thermoprocessing (TNST and CST). Films produced by casting exhibited a significantly lower thickness (80 and 90 μm) due to the lower surface density of solids in the films. Thermally processed samples (TNST and CST) had similar thicknesses (150–160 μm) regardless of the starch source. Then, different magnification levels were used to reflect the film cross-section.
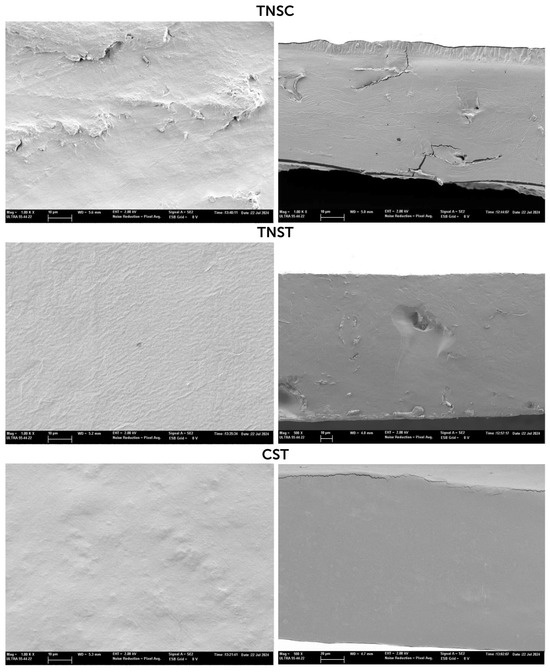
Figure 1.
FESEM micrographs at 1000× of the surface (left) and at 1000× (TNST) or 500× (CST) of the cross-section (right) of the different films based on starch from horchata solid residue (TNSC: casting and TNST: thermal processing), as well as corn starch films made by thermal processing (CST).
The surface micrographs reveal the homogeneous structure and smooth appearance of the corn starch-based film, reflecting the total gelatinisation of starch granules, with a similar appearance to that reported in other studies for thermoprocessed glycerol–corn starch films [41,42] or cast pea starch films [43]. No starch granules were observed in the TNS films obtained by casting or thermoprocessing, which indicates the complete gelatinisation of this starch, both during heating in water and during hot blending with glycerol. In contrast, the surface micrographs of the TNSC films revealed the presence of dispersed particles, which could be attributed to the presence of starch impurities, especially lipids (1% of the starch powder), which could migrate to the top of the cast films during the slow drying step and accumulate on the film surface. This did not occur on the surface of TNST films, as the impurity particles remained dispersed in the viscous melt blended mass and did not separate during the film thermoforming. However, some dispersed particles could also be observed in the cross-sections of both TNSC and TNST films.
3.2. Optical Properties
Figure 2 displays the visual appearance and internal transmittance spectra (Ti) of the different films within the visible light spectrum (400–700 nm) at 1 and 5 weeks (1W and 5W) of storage at 25 °C and 53% RH. Ti values are an indicator of the transparency of the films and the homogeneity of their internal structure. Corn starch-based films (CST) exhibited higher transmittance values across the entire tested wavelength range, indicating a high degree of homogeneity and transparency in this polymeric matrix. In contrast, films from TNS exhibited light absorption at low wavelengths, mainly when these were thermoprocessed. This must be attributed to the original darker colour of the starch powder due to the coloured impurities present in the separated starch from the horchata solid residue [17]. The final purity of TNS was 92%, including small amounts of coloured cellular fragments of the tiger nut skin, which are difficult to remove by density separation in the applied purification process [17]. The darker appearance and lower transparency at wavelengths below 600 nm of thermoprocessed TNS films also revealed the progress of browning reactions during the heat treatment through Maillard reactions or caramelization of sugars present in the starch, which absorb light in the 400–500 nm range [44]. High processing temperatures, such as those used in melt blending and compression moulding, enhance the formation of these compounds [37]. The transmittance spectra of the films did not change during the storage time, which suggests their stability against browning reactions. Therefore, structural changes resulting from starch retrogradation did not affect how light was absorbed and/or scattered by the films.
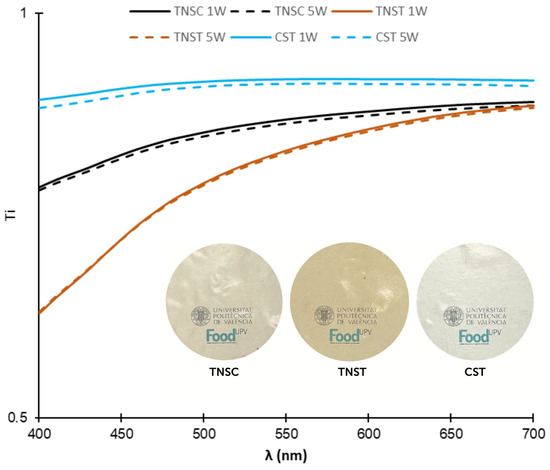
Figure 2.
Internal transmittance spectra and visual appearance of the different films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) and corn starch films made by thermal processing (CST) at one (1W) and five (5W) weeks of storage at 53% RH and 25 °C.
Table 1 shows the values of colour coordinates, lightness (L*), chroma (C*ab), and hue angle (h*ab) for each film sample. The CST films exhibited a significantly (p < 0.05) more yellowish hue, lower colour saturation (Cab* values), and higher lightness (L*) values compared to cast and thermoprocessed TNS films. As also deduced from the transmittance spectra, the initial presence and/or neoformation of coloured compounds affected the final colour of TNS films. Thermoprocessing of TNS (TNST films) resulted in darker (lower L*), reddish-brown films with greater colour saturation compared to films produced by casting (TNSC). This observation confirms the neoformation of brown compounds through Maillard reactions or caramelization, which are promoted by the high temperature of thermoprocessing. As observed for the Ti spectra, no significant differences in colour coordinates were observed due to the storage time (p > 0.05). This suggests that the components responsible for the colour in TNS films remain stable over time.

Table 1.
Lightness (L*), chrome (C*ab), and hue angle (h*ab) of films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) and thermoprocessed corn starch films (CST) at 1 week (1W) and 5 weeks (5W) of storage (mean ± standard deviation).
3.3. Tensile Properties
Figure 3 shows the typical stress–strain curves for the different films stored for 1 and 5 weeks at 53% RH, where the different tensile behaviours of the matrices and the effects of ageing can be observed. These curves highlight the more plastic behaviour of corn starch-based films, which fractured under lower stress and exhibited higher strain compared to tiger nut starch-based films, despite the similar amylose/amylopectin ratios of both starch types (27 and 23% amylose, respectively CS and TNS). The structural differences in the starch chains and crystallinity degree in the films may explain the observed differences. A high crystallinity degree greatly promotes the film’s stiffness and resistance to break while reducing the film’s extensibility. The crystallisation of amylose and amylopectin is greatly affected by the molecular mobility in the system, which in turn depends on the molecular weight and presence of plasticisers, especially water, as described by other authors for amylose and amylopectin films and their mixtures [45,46]. Compared with common starch from wheat, corn, potato, or cassava, tiger nut starch shows differences in fine molecular structures, swelling power, water solubility, gelatinisation and pasting properties, and in vitro digestibility [14]. These differences were also reflected in the tensile behaviour of the obtained TNS films, which were stiffer and more resistant to break but more brittle than CS films. Therefore, the molecular size of amylopectin and amylose and their respective molecular mobility affected their overall crystallisation in the films and their potential reinforcing effect on the amorphous phase.
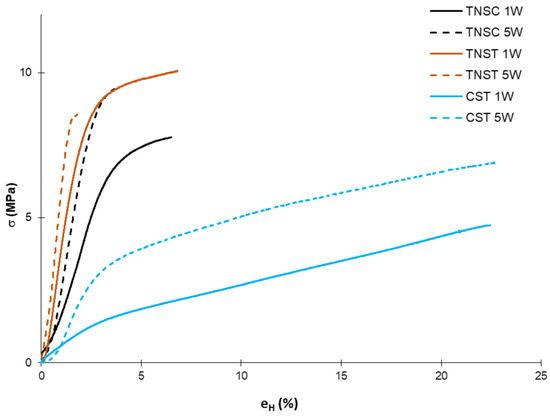
Figure 3.
Typical stress–strain curves of the different films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) methods and thermoprocessed corn starch films (CST) at 1 week (1W) and 5 weeks (5W) of storage.
Table 2 shows the values of tensile parameters, elastic modulus (EM), tensile strength at break (TS), and percentage of elongation at break (eH, %), which represent, respectively, the stiffness of the material, the resistance to break, and the film stretchability. At the initial storage time, the thermally processed corn starch films (CST) showed values in the previously reported range for films obtained and stored under similar conditions [42]. Both cast and thermoprocessed TNS-based films were stiffer, more resistant, and less extensible than CS films, which, as noted above, can be attributed to the structural differences in the starch chains that would produce different structural arrangement and crystallisation in the film matrix. Nevertheless, the tensile parameters obtained for TNS films are in the reported range for starch films in a great number of previous studies [8]. Considering the high variability in these values, which is greatly affected not only by the starch source but also by the processing method, the type and amount of plasticiser, the equilibrium moisture content, and the ageing time. In fact, differences in the tensile properties of TNS films could be observed due to the kind of processing and storage time.

Table 2.
Elastic modulus (EM), tensile strength at break (TS), and percentage of elongation at break (εH) of films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) and thermoprocessed corn starch films (CST) at 1 week (1W) and 5 weeks (5W) of storage (mean ± standard deviation).
At the initial storage time (1W), thermally processed TNS films exhibited superior mechanical strength and stiffness compared to those obtained by casting (TNSC 1W) while showing similar stretchability. Therefore, the processing method affected the properties of the TNS films, as previously reported by other authors for many polymeric films [47,48]. Differences must be attributed to the different structural arrangement of the amylose and amylopectin chains in the films; in cast films, the chain aggregation occurs slowly as the solvent evaporates, while in the thermoprocessed ones, the chain arrangement occurs in the more viscous polymer melt as the temperature decreases. Mechanical behaviour is strongly affected by the chain arrangement in the film, and this depends on their capacity to interact with each other. In the casting method, the chain unfolding in the good solvent favours the exposure of interacting groups and the formation of hydrogen bonds and hydrophobic interactions along the chains, in line with the solvent evaporation. In contrast, melt blending implies the formation of more viscous systems, where the chain interactions and the film structure achieved in the solid state imply other molecular order and chain organisation.
After 5 weeks of storage at 53% RH and 25 °C, all the films exhibited an increase in rigidity (EM) and a decrease in stretchability as a result of the starch retrogradation phenomenon, associated with the recrystallisation and gradual aggregation of the polymer chains over time [49]. The change in stiffness was more marked in CS films (128% increase) than in cast and thermoprocessed TNS films (42% increase). In contrast, the highest reduction in the film flexibility occurred in thermoprocessed films of TNS (71%), followed by cast films of TNS (36% reduction) and CS films (20% reduction). Therefore, structural differences in the starch chains and processing method affected the retrogradation of starch during storage under intermediate relative humidity conditions, as observed for other starch films [42].
Alfa amylase activity could also be responsible for changes in film properties throughout the storage time. However, it was hardly detected in corn starch (0.2 CU/g starch), while it was found in greater amounts in tiger nut starch (23.8 CU/g starch). During the gelatinisation step of cast film preparation, the enzyme was largely inactivated, remaining at 1.5 CU/g dry film, while it was completely inactivated in thermoformed films, which, as expected, showed no residual alpha-amylase activity. Therefore, no changes in film properties associated with the enzyme activity were expected in any case.
3.4. Water-Related Properties: Water Content, Solubility, and Swelling Power
Starch-based films are limited for food preservation due to their hygroscopicity and water-sensitive properties, which result in water solubility and poor water vapour barrier properties. Table 3 shows the equilibrium moisture of the films conditioned at 53% RH, the water solubility, and the swelling power of the different films. At the beginning of storage, cast films (TNSC) had significantly higher equilibrium moisture levels (p < 0.05) than thermoprocessed films (TNST and CST), likely due to a better chain organization during the slower drying step in the casting process, which enhances water retention in the three-dimensional network, as reported by Gerçekaslan [50]. After 5 weeks, the moisture content of all three formulations increased, which occurred to a greater extent in the thermoformed films. This suggests the lack of complete equilibrium of the samples with the ambient relative humidity after 1 week of conditioning and their progressive water gain throughout storage time. Cast films were nearer the equilibrium conditions after 1 week of storage, whereas water sorption was slower in thermoprocessed films, reaching values in the range previously reported for starch films [42].

Table 3.
Moisture at equilibrium (xW), solubility (S), and swelling power (SP) of films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) methods or corn starch films made by thermal processing (CST) at 1 week (1W) and 5 weeks (5W) of storage (mean ± standard deviation).
The water solubility of all films was relatively high, consistent with the hydrophilic properties of the polymer [51]. Comparing thermoprocessed films, TNS films were less water soluble than CS films, while thermoprocessing promoted the solubility of TNS films. This could be attributed to the partial hydrolysis of starch provoked by the thermal processing due to high shear and temperature, which reduces the molecular weight of the starch chains while increasing the number of active sites for water binding [39]. The differences in solubility of the thermoprocessed CS and TNS films can be attributed to the tighter packing of polymer chains in TNS films, as deduced from the tensile behaviour. After 5 weeks of storage (5W), the solubility of all three films was reduced, probably due to the starch retrogradation phenomenon, which promotes recrystallisation and chain aggregation, reducing the chain availability for water binding. This was hardly observed in cast films, whereas it was significant (p < 0.05) in thermoprocessed films. This suggests that more structural changes occurred in the thermoprocessed films, in which the chain arrangement happens faster during cooling, leaving a more disordered structure. Throughout storage, amylose and amylopectin chains progressively reorganize into more ordered crystalline structures, making water penetration more difficult after five weeks of storage.
Swelling power was similar for the different films at the beginning of storage, and it was significantly reduced after 5 weeks of storage, mainly for thermoprocessed films, which lost swelling capacity. This also agrees with the starch retrogradation process, since crystalline domains exhibited lower water sorption/retention capacity than amorphous regions. These results also point to the greater progress of starch retrogradation in thermoprocessed films, with a similar extent in TNS and CS starch films.
3.5. Barrier Properties
The barrier properties partially define the final application of the films for food contact, as they must be as low as possible to prevent gas exchange with the outside environment [51]. Oxygen transmission rate is a key parameter of food packaging materials, as it affects food stability, for instance, lipid oxidation. The oxygen permeability (OP), water vapour permeability (WVP), and limonene permeability (LP) of the films after 1 and 5 weeks of storage are shown in Table 4. All films exhibited high values of WVP but low OP values and moderate aroma barrier capacity, which aligns with the expectations for hydrophilic materials. As previously reported, starch films have excellent oxygen barrier properties, with OP values within the range of the corn starch films: 8.6–10.4 × 10−14 cm3·m−1·s−1·Pa−1 [52,53,54]. In contrast, the high values of WVP make the starch films inadequate for packaging water-sensitive foods, where control of water transfer is essential. Likewise, these values are strongly affected by the content of plasticiser (glycerol) and the equilibrium moisture content of the films [55]. The WVP values reported for cast TNS films, with a 5:1 starch–glycerol ratio, conditioned at 50% RH, were 34 × 10−11 g·Pa−1·s−1·m−1 [26], very similar to the values obtained in the present study.

Table 4.
Oxygen permeability (OP), water vapour permeability (WVP), and aroma permeability measured with D-limonene (LP) of films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) methods or corn starch films made by thermal processing (CST) at 1 (1W) and 5 weeks (5W) of storage (mean ± standard deviation).
The differences obtained in the respective permeability values can be attributed to the starch structural differences in the films, which result in varying transport rates of water, aroma, or oxygen molecules through the matrix [49]. The mass transport properties of films are primarily influenced by the degree of packing of the polymer chains and their free volume, which determines the effective molecular diffusion [56], as well as by the solubility of the permeant molecules in the matrix.
At the beginning of storage, differences in the permeability values were observed due to the film processing method, as TNS cast films were less permeable to water vapour and limonene and more permeable to oxygen than TNST films. Berti et al. [57] reported similar results regarding the barrier capacity of cassava starch films obtained via casting and thermal processing. Comparing thermoprocessed TNS and CS films, the latter were more permeable to oxygen, with similar WVP and LP values. The differences in the OP values could be attributed to the more tightly packed chains in TNS films, also deduced from the tensile behaviour. After 5 weeks of storage, no significant changes in the WVP and LP values were observed, whereas a small increase in OP values of thermoprocessed TNS and CS films was observed, while OP decreased in TNS cast film. Therefore, as previously observed [42], the starch retrogradation, which notably affected the mechanical properties of the films, did not markedly modify their barrier capacity or their ability to protect and maintain the quality of packaged food throughout storage.
3.6. Thermal Stability
Figure 4 shows the TGA and DGTA curves of the different films based on CS and TNS after 1 week and 5 weeks of storage, conditioned at 0% RH. The observed thermodegradation patterns were typical of glycerol-plasticised starch films [58], with three main thermal events, whose thermal degradation temperatures (onset and peak) and mass losses are summarised in Table 5.
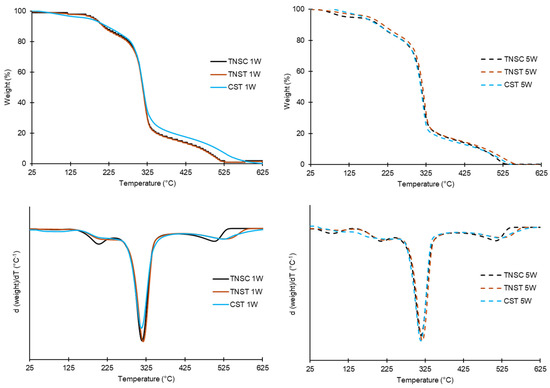
Figure 4.
DTGA curves of films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) methods or corn starch films made by thermal processing (CST) at 1 week (1W) and 5 weeks (5W) of storage.

Table 5.
Thermal parameters of the different films based on starch from horchata solid residue obtained by casting (TNSC) and thermal processing (TNST) methods or corn starch films made by thermal processing (CST) at 1 (1W) and 5 weeks (5W) of storage (mean ± standard deviation).
The first progressive mass loss occurred between 30–130 °C, corresponding to the loss of bound and structural water, which varied from 0 to 5% depending on the film and conditioning time at 0% RH. The second mass loss step (130–240 °C) can be attributed to the evaporation or decomposition of glycerol [59,60]. The greatest weight loss step (about 65%) occurred between 245 and 370 °C, with the maximum degradation rate around 315 °C, corresponding to starch degradation. This temperature range agrees with those reported by other authors for corn starch-glycerol films [58,59]. In this stage, starch chain decomposition, depolymerisation, and degradation occur [21,31]. The last degradation step, extending to approximately 700 °C, corresponds to the degradation of secondary products formed during earlier decomposition stages. Residual masses at 700 °C were negligible, indicating nearly complete decomposition of organic material.
No significant differences in the thermal degradation behaviour of starch were observed for the different films. These results agree with those reported by other authors for wheat, corn, potato, and rice starch films [59,61], revealing that neither the type of starch nor the film processing method significantly affects the thermal stability of the films. However, a higher degradation peak of glycerol in the TNS cast film was deduced from the higher weight loss values associated with the second thermal event. This could be attributed to the partial degradation of the incorporated glycerol during the thermal processing or to its stronger binding to the starch chains, which limits its thermal degradation as a free component in the film. As these differences disappeared at longer storage times, the second hypothesis seems more probable, and the results suggest the progressive release/exudation of glycerol from the thermoprocessed starch matrix, thus degrading independently.
3.7. Antioxidant Properties
Usually, starch films with no added antioxidant compounds did not exhibit antioxidant capacity. Nevertheless, starch obtained from horchata solid byproduct retains small amounts of other plant components, such as phenolic compounds linked to the cellular walls of the plant tissue, which could exhibit antioxidant activity [17,62]. Furthermore, film-forming conditions, especially concerning the temperatures reached during the casting or the thermoforming process, could affect the antioxidant activity of the obtained films. However, no detectable content of phenolic compounds was quantified by the Folin–Ciocalteu method. Furthermore, the film’s effective concentration to reduce the initial DPPH concentration by 50% (EC50) had very high values for every sample (1200–1900 g dry film/g DPPH), which indicates a lack of notable radical scavenging capacity of the films.
4. Conclusions
The starch obtained by homogenisation and density separation of the tiger nut residue from horchata production exhibited good filmogenic properties with both solvent casting and thermoplastic processing using glycerol as a plasticiser. The films obtained showed a barrier capacity to water vapour and gases in the typical range of starch films, with a high barrier capacity to oxygen. The tensile properties of the films were affected by the processing method, exhibiting higher stiffness and resistance to break and lower stretchability than the more common corn starch films. Likewise, the solubility of the thermoprocessed TNS films was lower than that of the CS films and higher than that of cast films. However, all films exhibited similar swelling power. Thermal stability was also similar for all TNS and CS films, showing the typical thermal degradation pattern of starch. Therefore, TNS obtained from horchata production waste can be used to obtain thermoplastic starch films for packaging applications, with tuned characteristics compared to the most common starch films. Thus, the horchata production waste can be considered a potential source of thermoplastic starch, which in turn constitutes an interesting method for its valorisation.
Author Contributions
A.P.-E.: conceptualization, methodology, formal analysis, investigation, writing—original draft preparation, and writing—review and editing; M.E.M.-E.: conceptualization, methodology, investigation, writing—original draft preparation, writing—review and editing, and supervision; A.C.: conceptualization, methodology, investigation, writing—review and editing, supervision and project administration; C.G.-M.: conceptualization, methodology, investigation, writing—review and editing, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Generalitat Valenciana for funding the project CIPROM/2021/071.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Menzel, C. Improvement of Starch Films for Food Packaging through a Three-Principle Approach: Antioxidants, Cross-Linking and Reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef] [PubMed]
- European Bioplastics Bioplastics Market Development Update 2023. Available online: https://docs.european-bioplastics.org/publications/market_data/2023/EUBP_Market_Data_Report_2023.pdf (accessed on 23 September 2024).
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and Applicability of Starch-Based Films: An Eco-Friendly Approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Palanisamy, C.P.; Srinivasan, G.P.; Panagal, M.; Kumar, S.S.D.; Mironescu, M. A Comprehensive Review on Starch-Based Sustainable Edible Films Loaded with Bioactive Components for Food Packaging. Int. J. Biol. Macromol. 2024, 274, 133332. [Google Scholar] [CrossRef]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Guo, B.; Zhu, C.; Huang, Z.; Yang, R.; Liu, C. Microcapsules with Slow-Release Characteristics Prepared by Soluble Small Molecular Starch Fractions through the Spray Drying Method. Int. J. Biol. Macromol. 2022, 200, 34–41. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q. V Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Hasnain, A. Mango Kernel Starch as a Novel Edible Coating for Enhancing Shelf-Life of Tomato (Solanum Lycopersicum) Fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; de Oliveira, R.A. Bioactive Films of Arrowroot Starch and Blackberry Pulp: Physical, Mechanical and Barrier Properties and Stability to PH and Sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch–Gelatin Edible Films: Water Vapor Permeability and Mechanical Properties as Affected by Plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sowjanya, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process–a Sustainable Symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S. Agro-Waste for Renewable and Sustainable Green Production: A Review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, Q.; Zhao, G.; Ye, F. Tiger Nut (Cyperus esculentus) Starch: Extraction, Composition, Structure, Properties, Modification and Uses. Sustain. Food Technol. 2024, 2, 635–651. [Google Scholar] [CrossRef]
- Abu, L.M. Tiger Nut (Cyperus esculentus) Tuber: A Sustainable Resource for Industrial Starch: A Review. Commun. Phys. Sci. 2024, 11. [Google Scholar]
- Sánchez-Zapata, E.; Fernández-López, J.; Angel Pérez-Alvarez, J. Tiger Nut (Cyperus esculentus) Commercialization: Health Aspects, Composition, Properties, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Patrón, A.; Martin-Esparza, M.E.; González-Martínez, C.; Chiralt, A. Starch Recovery Process from the Tiger Nut Horchata Processing Waste. Food Bioprocess Technol. 2025, 18, 1042. [Google Scholar] [CrossRef]
- Pelegrín, C.J.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Chemical Composition and Bioactive Antioxidants Obtained by Microwave-Assisted Extraction of Cyperus esculentus L. by-Products: A Valorization Approach. Front. Nutr. 2022, 9, 944830. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Lorenzo, J.M.; Cantavella-Ferrero, Y. Enhancing Bioactive Antioxidants’ Extraction from “Horchata de Chufa” by-Products. Foods 2018, 7, 161. [Google Scholar] [CrossRef]
- Manek, R.V.; Builders, P.F.; Kolling, W.M.; Emeje, M.; Kunle, O.O. Physicochemical and Binder Properties of Starch Obtained from Cyperus esculentus. AAPS PharmSciTech 2012, 13, 379–388. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q. Impact of Molecular Structure of Amylopectin and Amylose on Amylose Chain Association during Cooling. Carbohydr. Polym. 2009, 77, 807–815. [Google Scholar] [CrossRef]
- Zhang, R.-Y.; Chen, P.-X.; Liu, A.-B.; Zhu, W.-X.; Jiang, M.-M.; Wang, X.-D.; Liu, H.-M. Effects of Different Isolation Methods on the Structure and Functional Properties of Starch from Tiger Nut (Cyperus esculentus L.) Meal. LWT 2024, 196, 115853. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Ma, C.; Zhang, Y. Thermal Behavior of Sweet Potato Starch by Non-Isothermal Thermogravimetric Analysis. Materials 2019, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, S.; Zhen, S.; Shi, Y.; Liu, B. Physicochemical Properties of Tigernut (Cyperus esculentus) Tuber Starch and Its Application in Steamed Bread. J. Food Process Preserv. 2022, 46, e16792. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Li, F.; Mao, S.; Zhou, X.; Li, S.; Lu, C.; Zhang, T. The Cyperus esculentus Starch-Based Bioactive Films: Characterisation, UV-Shielding and Antioxidant Capacity. Int. J. Food Sci. Technol. 2023, 58, 4446–4454. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A Review on Present Status and Future Challenges of Starch Based Polymer Films and Their Composites in Food Packaging Applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and Intelligent Starch-Based Films: A Review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Onyeaka, H.; Ghosh, S.; Obileke, K.; Miri, T.; Odeyemi, O.A.; Nwaiwu, O.; Tamasiga, P. Preventing Chemical Contaminants in Food: Challenges and Prospects for Safe and Sustainable Food Production. Food Control 2024, 155, 110040. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Xie, F.; Chen, L. Gelatinization of Cornstarch with Different Amylose/Amylopectin Content. Carbohydr. Polym. 2006, 65, 357–363. [Google Scholar] [CrossRef]
- Altayan, M.M.; Al Darouich, T.; Karabet, F. Thermoplastic Starch from Corn and Wheat: A Comparative Study Based on Amylose Content. Polym. Bull. 2021, 78, 3131–3147. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food and Colour Appearance (Chapman & Hall Food Science Book), 2nd ed.; Aspen Publications, Springer: Gaithersburg, MD, USA, 1999. [Google Scholar]
- ASTM D-882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 2001; pp. 162–170.
- ASTM 96-95; Standard Test Method for Water Vapor Transmission of Materials. Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 1995.
- ASTM D-3985; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM: West Conshohocken, PA, USA, 1995.
- Moreno, O.; Cárdenas, J.; Atarés, L.; Chiralt, A. Influence of Starch Oxidation on the Functionality of Starch-Gelatin Based Active Films. Carbohydr. Polym. 2017, 178, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Chiralt, A.; Vilaplana, F. Antioxidant Starch Films Containing Sunflower Hull Extracts. Carbohydr. Polym. 2019, 214, 142–151. [Google Scholar] [CrossRef]
- McCleary, B.V.; Sheehan, H. Measurement of Cereal α-Amylase: A New Assay Procedure. J. Cereal Sci. 1987, 6, 237–251. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, Mechanical and Barrier Properties of Corn Starch Films Incorporated with Plant Essential Oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Thermoprocessed Starch-Polyester Bilayer Films as Affected by the Addition of Gellan or Xanthan Gum. Food Hydrocoll. 2021, 113, 106509. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Mechanical and Physical Properties of Pea Starch Edible Films in the Presence of Glycerol. J. Food Process Preserv. 2016, 40, 1339–1351. [Google Scholar] [CrossRef]
- Wong, C.W.; Wijayanti, H.B.; Bhandari, B.R. Maillard Reaction in Limited Moisture and Low Water Activity Environment. Water Stress Biol. Chem. Pharm. Food Syst. 2015, 41–63. [Google Scholar] [CrossRef]
- Stading, M.; Hermansson, A.-M.; Gatenholm, P. Structure, Mechanical and Barrier Properties of Amylose and Amylopectin Films. Carbohydr. Polym. 1998, 36, 217–224. [Google Scholar]
- Rindlav-Westling, Å.; Stading, M.; Gatenholm, P. Crystallinity and Morphology in Films of Starch, Amylose and Amylopectin Blends. Biomacromolecules 2002, 3, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Moreno, O.; Díaz, R.; Atarés, L.; Chiralt, A. Influence of the Processing Method and Antimicrobial Agents on Properties of Starch-gelatin Biodegradable Films. Polym. Int. 2016, 65, 905–914. [Google Scholar] [CrossRef]
- Mohan, C.C.; Harini, K.; Karthikeyan, S.; Sudharsan, K.; Sukumar, M. Effect of Film Constituents and Different Processing Conditions on the Properties of Starch Based Thermoplastic Films. Int. J. Biol. Macromol. 2018, 120, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Fortunati, E.; Cháfer, M.; Kenny, J.M.; Chiralt, A.; González-Martínez, C. Properties and Ageing Behaviour of Pea Starch Films as Affected by Blend with Poly(Vinyl Alcohol). Food Hydrocoll. 2015, 48, 84–93. [Google Scholar] [CrossRef]
- Gerçekaslan, K.E. Hydration Level Significantly Impacts the Freezable-and Unfreezable-Water Contents of Native and Modified Starches. Food Sci. Technol. 2021, 41, 426–431. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Microstructural Properties of Biodegradable Films Based on Pea Starch and PVA. J. Food Eng. 2015, 167, 59–64. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karim, R.; Rahman, R.A.; Sultan, M.T.; Johnson, S.K.; Paykary, M. Effect of Glycerol on the Physicochemical Properties of Cereal Starch Films. Czech J. Food Sci. 2018, 36, 403–409. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Gil, N.J.B.; González-Martínez, C.; Chiralt, A. Antioxidant Poly (Lactic Acid) Films with Rice Straw Extract for Food Packaging Applications. Food Packag. Shelf Life 2022, 34, 101003. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving Properties of Thermoplastic Starch Films by Incorporating Active Extracts and Cellulose Fibres Isolated from Rice or Coffee Husk. Food Packag. Shelf Life 2019, 22, 100383. [Google Scholar] [CrossRef]
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and Aroma Barrier Properties of Edible Films: A Review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Berti, S.; Jagus, R.J.; Flores, S.K.; González-Martínez, C. Antimicrobial Edible Starch Films Obtained By Casting and Thermo-compression Techniques. Food Bioprocess Technol. 2024, 17, 904–916. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of Glycerol and Thymol on Physical, Mechanical, and Thermal Properties of Corn Starch Films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of Starch Type on the Physico-Chemical Properties of Edible Films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal Properties of Thermoplastic Starch/Synthetic Polymer Blends with Potential Biomedical Applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef]
- Piyada, K.; Waranyou, S.; Thawien, W. Mechanical, Thermal and Structural Properties of Rice Starch Films Reinforced with Rice Starch Nanocrystals. Int. Food Res. J. 2013, 20, 439. [Google Scholar]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and PH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).