“Revitalizing” Alginate Films: Control of Texture, Hemo- and Cellular Compatibility via Addition of Cellulose Nanocrystals
Abstract
1. Introduction
- Biocompatibility and biodegradability enhance the potential of filled gels for integration with living systems;
- The chemical and functional similarities with the filled alginate gel, along with the presence of a large number of hydroxyl groups, enhance the embedding of particles into the matrix;
- The anisotropic morphology of the most common nanocrystals presents additional opportunities for regulating the rheology of gels, as well as the orientation of particles and agglomerates. This may contribute to the development of materials with anisotropic properties, such as enhanced mechanical characteristics;
- The high intrinsic mechanical strength of nanocrystals.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Biological Assay
3. Results
3.1. CNC Characterization
3.2. Structure and Physicochemical Properties of Composite Films
3.2.1. FTIR and UV–Vis Spectroscopy
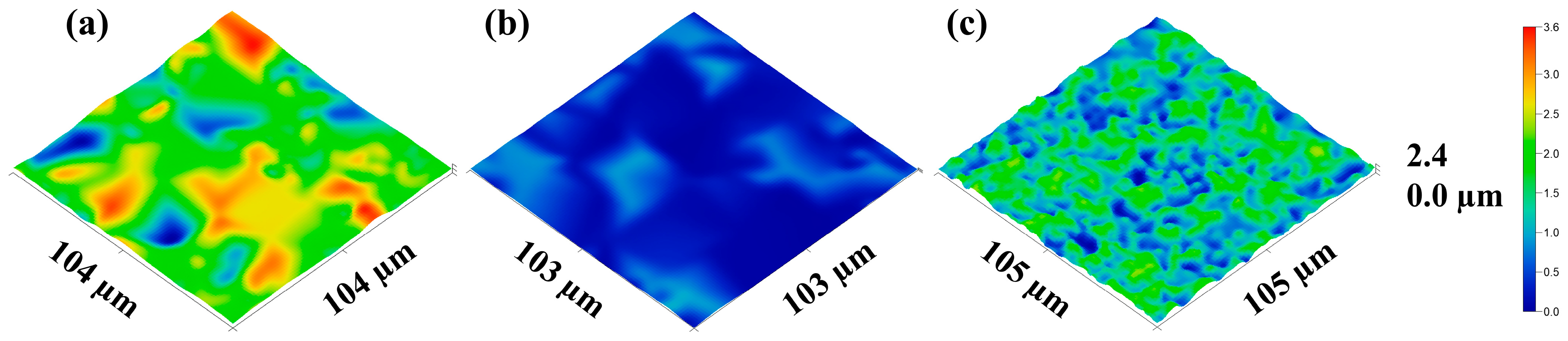
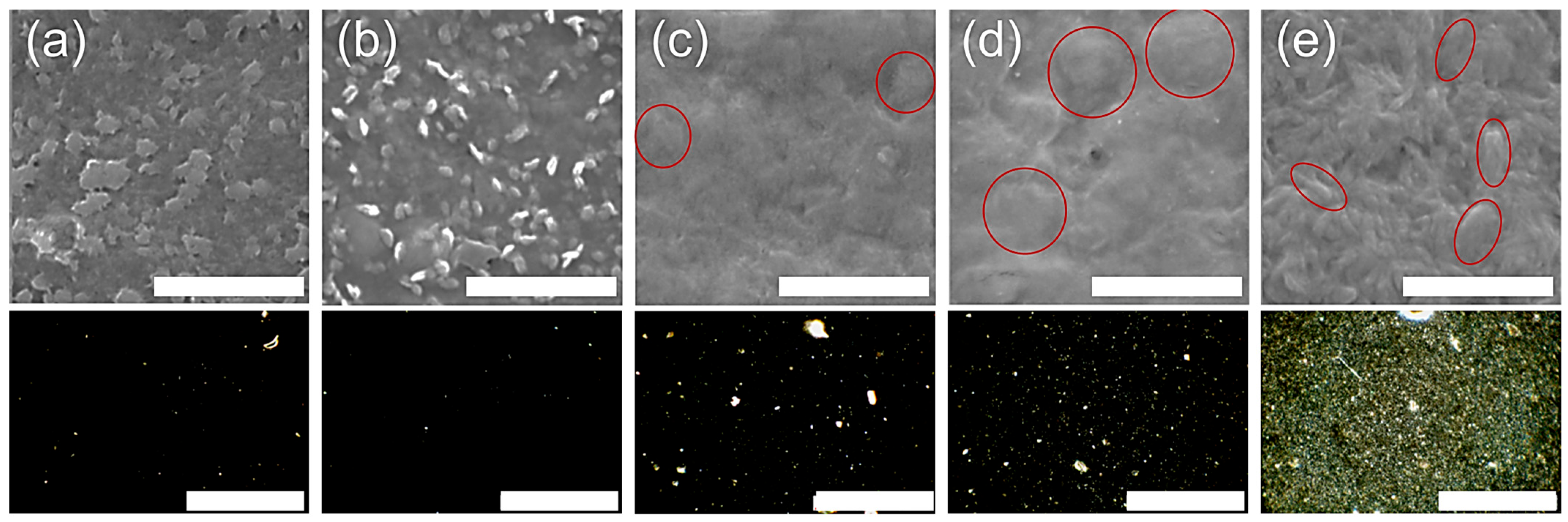
3.2.2. Surface and Microstructure of Films
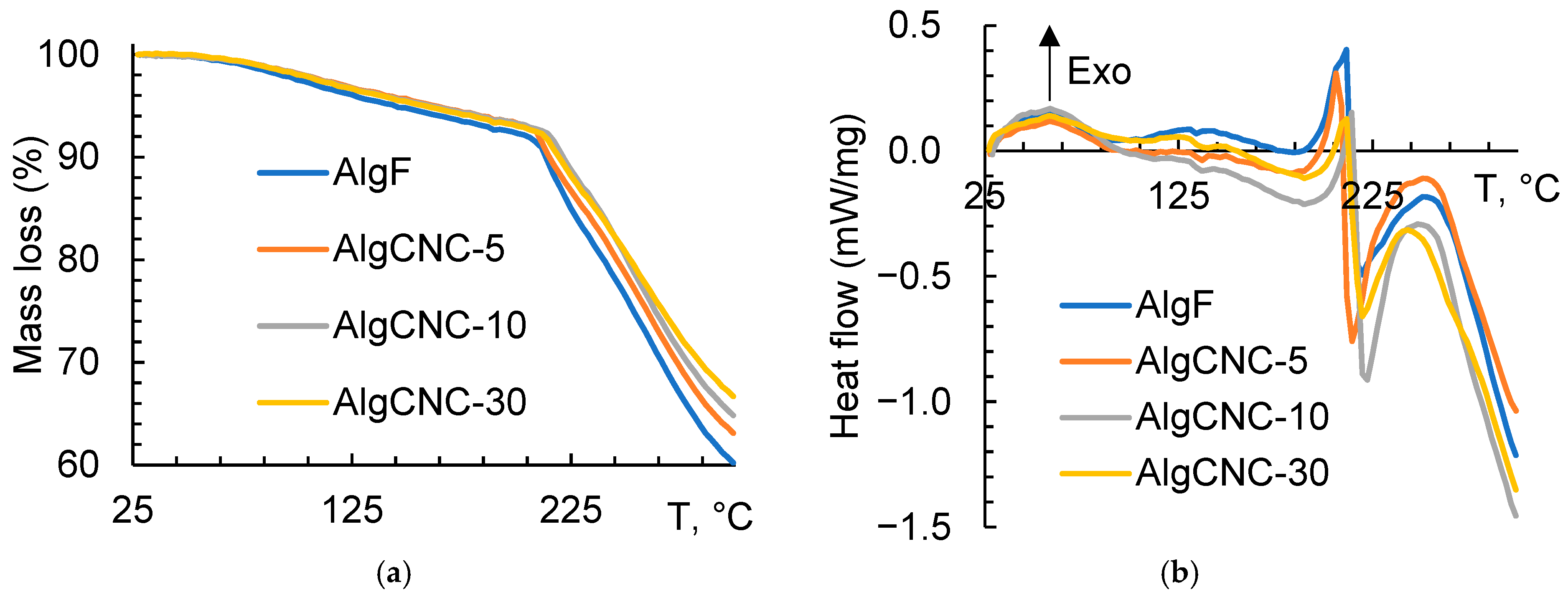
3.2.3. Synchronous Thermal Analysis of Films
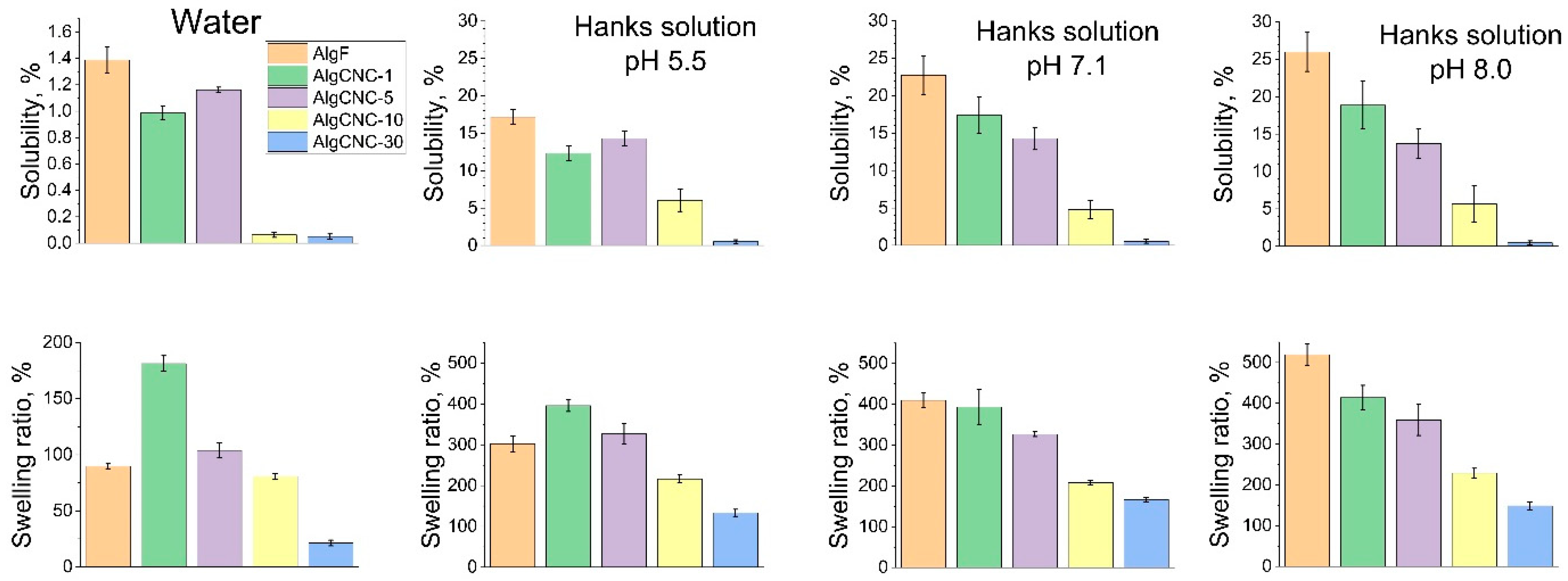
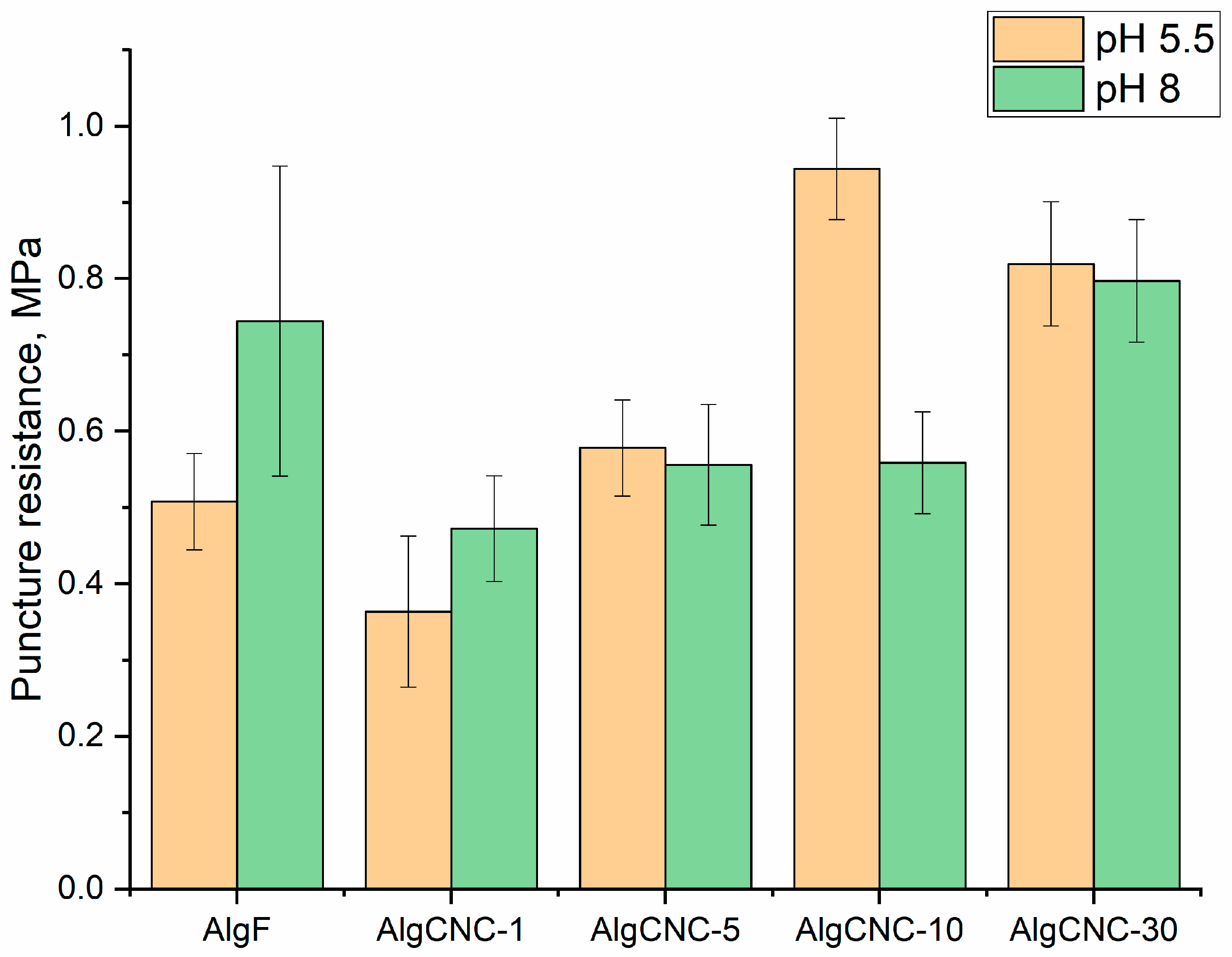
3.2.4. Swelling, Solubility, and Mechanical Properties of Films
Swelling and Solubility of Films at Different pH Values
Mechanical Properties of Films
3.2.5. Contact Angle
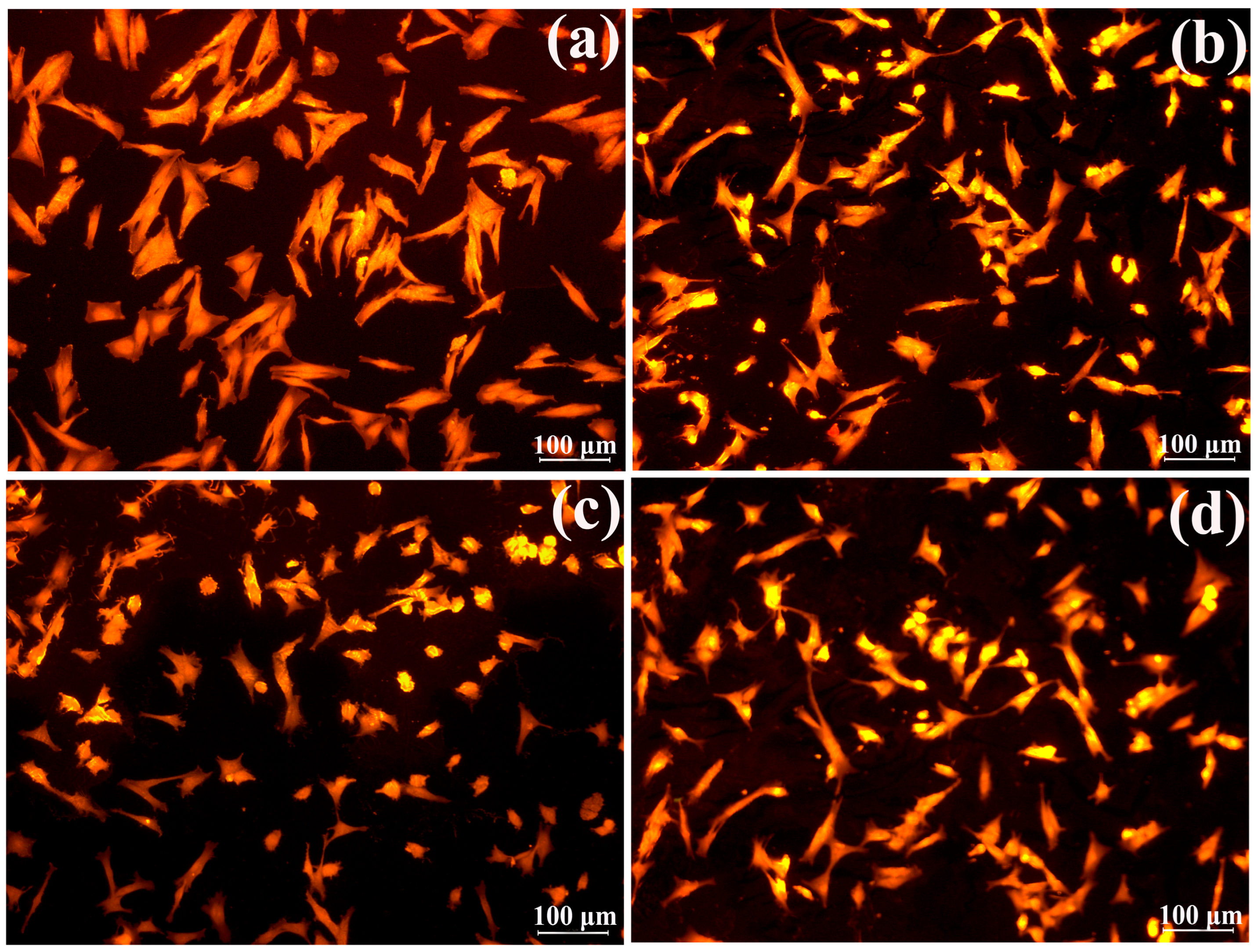
3.2.6. Adhesion of Fibroblasts to Films and Its Components
Morphometric Characteristics and Growth Activity of Fibroblasts
Cell Adhesion to Hydrogel Biomaterials’ Surface
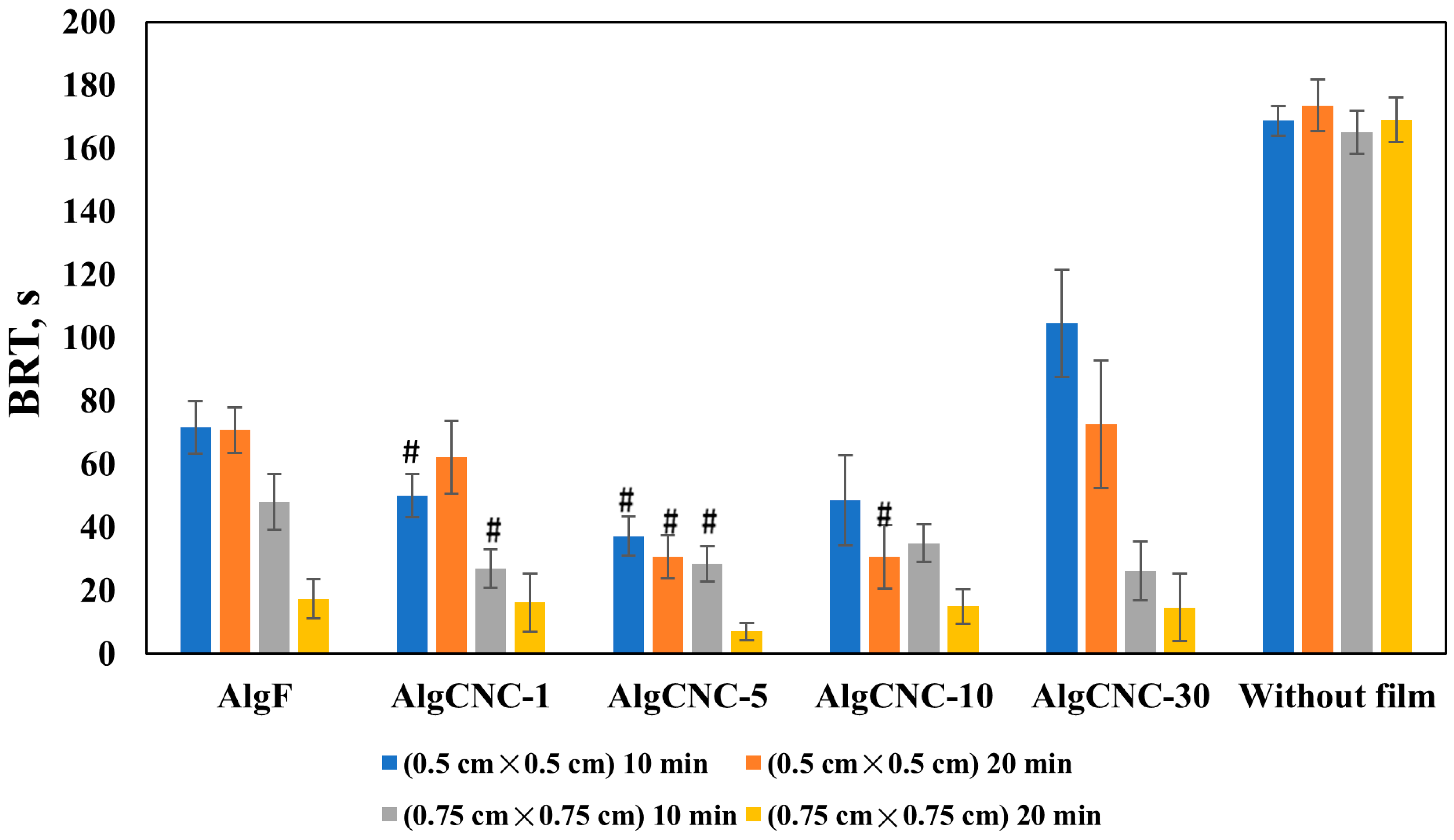
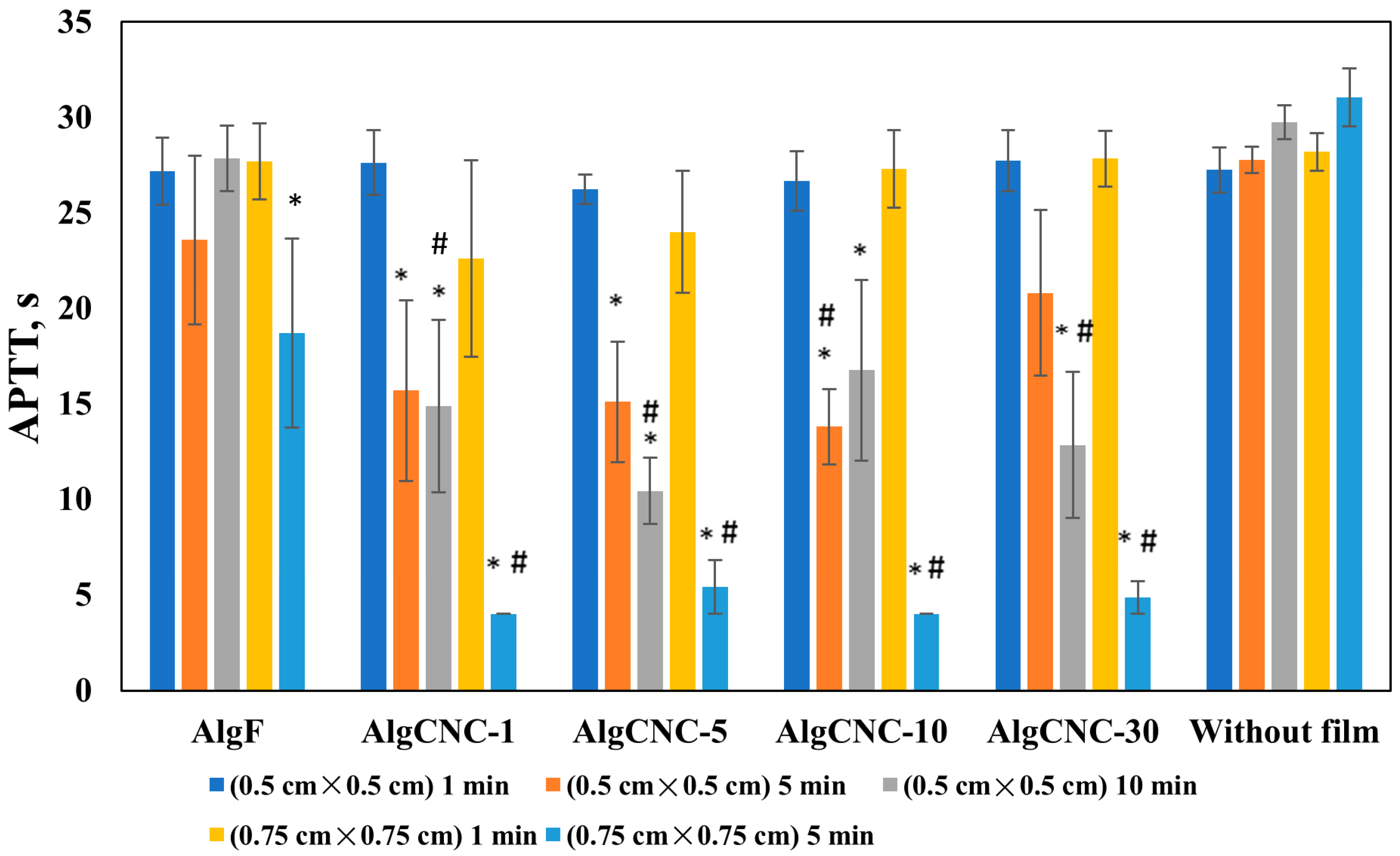
3.2.7. Hemocompatibility of Composite Films
4. Discussion
- (1)
- A decrease in the swelling coefficient and solubility of films at pH levels below 7;
- (2)
- An increase in the mechanical strength of the swollen composite hydrogel matrix.A notable characteristic of these two trends is the presence of extremes in films composed of AlgCNC-1, which contradicts the overall trend. Clearly, these extremes signify a pronounced difference in the materials’ structure, stemming from both the original alginate and composites with a higher content of paCNC. These differences are characterized by a loosening of the structure, resulting in a loss of mechanical strength and an increase in the swelling index;
- (3)
- A decrease in the acceptor component of free surface energy, accompanied by an increase in the donor component, is observed in composites;
- (4)
- An increase in fibroblast adhesion to the hydrogel surface, along with a general tendency to enhance the procoagulant effect of the films, is observed.
- (1)
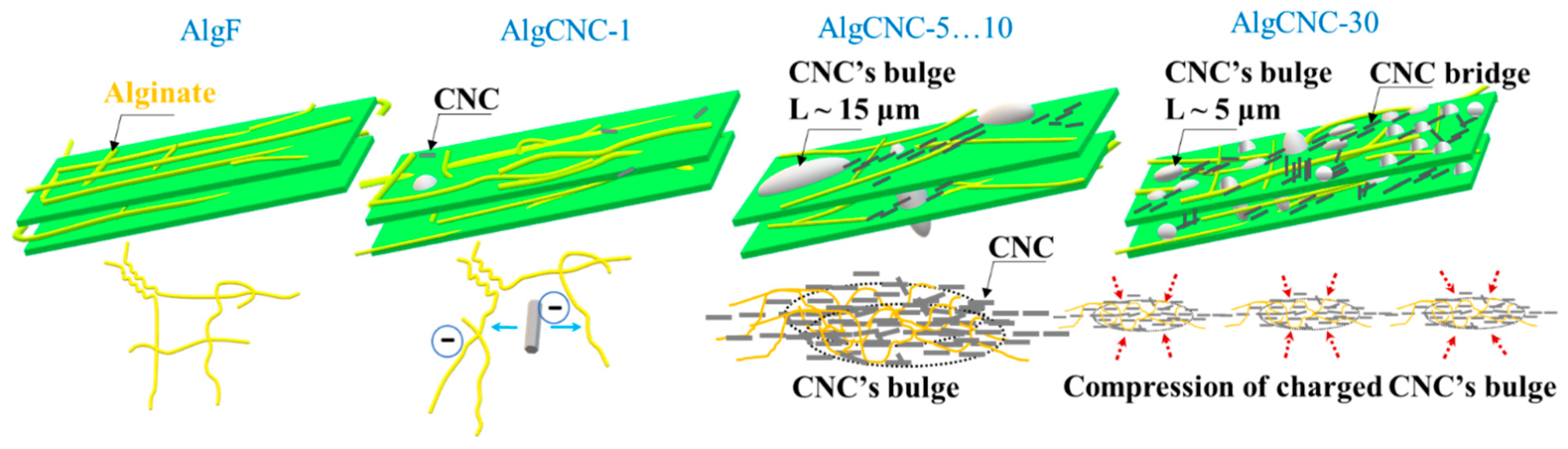
- With a 0 and 1 wt.% paCNC content, the texture of the film in its swollen state is smooth and dense, exhibiting minimal optical inhomogeneities primarily determined by the alginate components;
- (2)
- At a 5 and 10 wt.% paCNC content, the surface texture of the films in the swollen state displays a limited number of relatively large, sparse elements, along with the emergence of relatively rare groups of optical inhomogeneities;
- (3)
- At a 30 wt.% paCNC content, a significant change in surface texture is observed. The surface texture of these films in the swollen state exhibits a higher density of smaller relief elements and a greater number of optical inhomogeneities. The SEM micrographs reveal spindle-shaped structures on the micrometer scale.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurczak, P.; Lach, S. Hydrogels as Scaffolds in Bone-Related Tissue Engineering and Regeneration. Macromol. Biosci. 2023, 23, e2300152. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, F.; Liu, F. Properties of Sodium Alginate-Based Nanocomposite Films: Effects of Aspect Ratio and Surface Charge of Cellulose Nanocrystals. Int. J. Biol. Macromol. 2024, 256, 128420. [Google Scholar] [CrossRef] [PubMed]
- Josyula, A.; Parikh, K.S.; Pitha, I.; Ensign, L.M. Engineering Biomaterials to Prevent Post-Operative Infection and Fibrosis. Drug Deliv. Transl. Res. 2021, 11, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Witherel, C.E.; Abebayehu, D.; Barker, T.H.; Spiller, K.L. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Healthc. Mater. 2019, 8, e1801451. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.P.; Lambert, D.W.; Asencio, I.O. Understanding Fibroblast Behavior in 3D Biomaterials. Tissue Eng. Part B Rev. 2022, 28, 569–578. [Google Scholar] [CrossRef]
- Williams, D. Concepts in Biocompatibility: New Biomaterials, New Paradigms and New Testing Regimes. In Biocompatibility and Performance of Medical Devices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–17. [Google Scholar]
- Maitz, M.F.; Martins, M.C.L.; Grabow, N.; Matschegewski, C.; Huang, N.; Chaikof, E.L.; Barbosa, M.A.; Werner, C.; Sperling, C. The Blood Compatibility Challenge. Part 4: Surface Modification for Hemocompatible Materials: Passive and Active Approaches to Guide Blood-Material Interactions. Acta Biomater. 2019, 94, 33–43. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Shirzadifar, A. A Review on Cellulose Nanocrystals as Promising Biocompounds for the Synthesis of Nanocomposite Hydrogels. Carbohydr. Polym. 2019, 216, 247–259. [Google Scholar] [CrossRef]
- Kumar, V.A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and Its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Drozd, N.N.; Paderin, N.M.; Tarabukin, D.V.; Udoratina, E.V. Hemocompatibility, Biodegradability and Acute Toxicity of Acetylated Cellulose Nanocrystals of Different Types in Comparison. Carbohydr. Polym. 2021, 269, 118307. [Google Scholar] [CrossRef]
- Ventura, C.; Pinto, F.; Lourenço, A.F.; Ferreira, P.J.T.; Louro, H.; Silva, M.J. On the Toxicity of Cellulose Nanocrystals and Nanofibrils in Animal and Cellular Models. Cellulose 2020, 27, 5509–5544. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Mikhaylov, V.I.; Torlopov, M.A.; Vaseneva, I.N.; Legki, P.V.; Paderin, N.M.; Martakov, I.S.; Sitnikov, P.A. Anti-Alzheimer Drug Delivery via Pickering Emulsions Stabilized by Plate-like Cellulose Nanocrystals. Langmuir 2023, 39, 11769–11781. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, M.S.; Tracey, C.; Sidunets, Y.A.; Torlopov, M.A.; Mikhaylov, V.I.; Krivoshapkin, P.V.; Martakov, I.S.; Krivoshapkina, E.F. Environmentally Friendly Au@CNC Hybrid Systems as Prospective Humidity Sensors. RSC Adv. 2020, 10, 35031–35038. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-Alginate Hydrogel for Cell Encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
- Zoia, L.; Binda, A.; Cipolla, L.; Rivolta, I.; La Ferla, B. Binary Biocompatible CNC–Gelatine Hydrogel as 3D Scaffolds Suitable for Cell Culture Adhesion and Growth. Appl. Nano 2021, 2, 118–127. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Leppiniemi, J.; Lipponen, S.; Lombardo, S.; Thielemans, W.; Maloney, T.; Pääkkönen, T.; Kesari, K.K.; Ruokolainen, J.; Hytönen, V.P.; et al. Thermoresponsive and Biocompatible Poly(N-Isopropylacrylamide)–cellulose Nanocrystals Hydrogel for Cell Growth. Mater. Adv. 2024, 5, 570–583. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.C.; Misra, R.D.K. The Functional Response of Alginate-Gelatin-Nanocrystalline Cellulose Injectable Hydrogels toward Delivery of Cells and Bioactive Molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Ikeda, A.; Takemura, A.; Ono, H. Preparation of Low-Molecular Weight Alginic Acid by Acid Hydrolysis. Carbohydr. Polym. 2000, 42, 421–425. [Google Scholar] [CrossRef]
- Torlopov, M.; Shevchenko, O.; Drozd, N.; Udoratina, E. Ethylenediamine-Modified Alginate-A Hemocompatible Platform for Polymer-Drug Conjugates. Int. J. Biol. Macromol. 2025, 287, 138326. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Mikhaylov, V.I.; Udoratina, E.V.; Aleshina, L.A.; Prusskii, A.I.; Tsvetkov, N.V.; Krivoshapkin, P.V. Cellulose Nanocrystals with Different Length-to-Diameter Ratios Extracted from Various Plants Using Novel System Acetic Acid/Phosphotungstic Acid/Octanol-1. Cellulose 2018, 25, 1031–1046. [Google Scholar] [CrossRef]
- Radebaugh, G.W.; Murtha, J.L.; Julian, T.N.; Bondi, J.N. Methods for Evaluating the Puncture and Shear Properties of Pharmaceutical Polymeric Films. Int. J. Pharm. 1988, 45, 39–46. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Vaseneva, I.N.; Mikhaylov, V.I.; Martakov, I.S.; Moskalev, A.A.; Koval, L.A.; Zemskaya, N.V.; Paderin, N.M.; Sitnikov, P.A. Pickering Emulsions Stabilized by Partially Acetylated Cellulose Nanocrystals for Oral Administration: Oils Effect and in Vivo Toxicity. Cellulose 2021, 28, 2365–2385. [Google Scholar] [CrossRef]
- Eyley, S.S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–carboxylate Interactions in Metal–alginate Complexes Studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR Spectra of Alginic Acid Block Fractions in Three Species of Brown Seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Sartori, C.; Finch, D.S.; Ralph, B.; Gilding, K. Determination of the Cation Content of Alginate Thin Films by FTi.r. Spectroscopy. Polymer 1997, 38, 43–51. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Lattice Type. Part II. A New Infrared Ratio for Estimation of Crystallinity in Celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Trejo-O’Reilly, J.-A.; Cavaillé, J.-Y.; Gandini, A. The Surface Chemical Modification of Cellulosic Fibres in View of Their Use in Composite Materials. Cellulose 1997, 4, 305–320. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 9781118311653. [Google Scholar]
- Çetin, N.S.; Tingaut, P.; Özmen, N.; Henry, N.; Harper, D.; Dadmun, M.; Sèbe, G. Acetylation of Cellulose Nanowhiskers with Vinyl Acetate under Moderate Conditions. Macromol. Biosci. 2009, 9, 997–1003. [Google Scholar] [CrossRef]
- Joseph, J.; Jemmis, E.D. Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.; Rezaei, M.; Behrooz, R. Preparation and Characterization Agar-Based Nanocomposite Film Reinforced by Nanocrystalline Cellulose. Int. J. Biol. Macromol. 2014, 70, 537–544. [Google Scholar] [CrossRef]
- Gohil, R.M. Synergistic Blends of Natural Polymers, Pectin and Sodium Alginate. J. Appl. Polym. Sci. 2011, 120, 2324–2336. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of Cross-Linking with Calcium Ions on the Physical Properties of Alginate Films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Bekin, S.; Sarmad, S.; Gürkan, K.; Yenici, G.; Keçeli, G.; Gürdağ, G. Dielectric, Thermal, and Swelling Properties of Calcium Ion-Crosslinked Sodium Alginate Film. Polym. Eng. Sci. 2014, 54, 1372–1382. [Google Scholar] [CrossRef]
- Azzam, F.; Moreau, C.; Cousin, F.; Menelle, A.; Bizot, H.; Cathala, B. Cellulose Nanofibril-Based Multilayered Thin Films: Effect of Ionic Strength on Porosity, Swelling, and Optical Properties. Langmuir 2014, 30, 8091–8100. [Google Scholar] [CrossRef]
- Samanta, T.; Sinha, S.; Mukherjee, M. Effect of Added Salt on Swelling Dynamics of Ultrathin Films of Strong Polyelectrolytes. Polymer 2016, 97, 285–294. [Google Scholar] [CrossRef]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-L.; Lin, Y.-S. Effects of PH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Thomas, D.; Nath, M.S.; Mathew, N.; Reshmy, R.; Philip, E.; Latha, M.S. Alginate Film Modified with Aloevera Gel and Cellulose Nanocrystals for Wound Dressing Application: Preparation, Characterization and in Vitro Evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101894. [Google Scholar] [CrossRef]
- Qi, W.; Yu, J.; Zhang, Z.; Xu, H.-N. Effect of PH on the Aggregation Behavior of Cellulose Nanocrystals in Aqueous Medium. Mater. Res. Express 2019, 6, 125078. [Google Scholar] [CrossRef]
- Yuan, Z.; Ding, J.; Zhang, Y.; Huang, B.; Song, Z.; Meng, X.; Ma, X.; Gong, X.; Huang, Z.; Ma, S.; et al. Components, Mechanisms and Applications of Stimuli-Responsive Polymer Gels. Eur. Polym. J. 2022, 177, 111473. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Drozd, N.N.; Sitnikov, P.A.; Mikhailov, V.I.; Udoratina, E.V. Synthesis, Rheological Properties, and Hemocompatibility of Alginic Acid Modified with Ethylenediamine Fragments. Polym. Sci. Ser. A 2024, 66, 187–201. [Google Scholar] [CrossRef]
- Vleugels, L.F.W.; Ricois, S.; Voets, I.K.; Tuinier, R. Determination of the “Apparent PKa” of Selected Food Hydrocolloids Using Ortho-Toluidine Blue. Food Hydrocoll. 2018, 81, 273–283. [Google Scholar] [CrossRef]
- Maity, C.; Das, N. Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives. Top. Curr. Chem. 2022, 380, 3. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Perea-Carpio, R. Problems of Contact Angle and Solid Surface Free Energy Determination. Adv. Colloid Interface Sci. 2002, 98, 245–264. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of surface energy on dispersion and mechanical properties of polymer/nanocrystalline cellulose nanocomposites. Biomacromolecules 2013, 14, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Dikicier, E.; Şen, M. Effects of Conformation and Molecular Weight on The Surface Energy and Mucoadhesion Properties of Calcium Alginate Gels. Hacettepe J. Biol. Chem. 2011, 39, 371–378. [Google Scholar]
- Lo, I.L.; Kao, C.Y.; Huang, T.H.; Ho, C.T.; Kao, C.T. The cytotoxicity assessment of different clear aligner materials. J. Dent. Sci. 2024, 19, 2065–2073. [Google Scholar] [CrossRef]
- Markov, P.A.; Krachkovsky, N.S.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Mechanical properties, structure, bioadhesion, and biocompatibility of pectin hydrogels. J. Biomed. Mater. Res. A. 2017, 105, 2572–2581. [Google Scholar] [CrossRef]
- Chen, X.; Wu, T.; Bu, Y.; Yan, H.; Lin, Q. Fabrication and Biomedical Application of Alginate Composite Hydrogels in Bone Tissue Engineering: A Review. Int. J. Mol. Sci. 2024, 25, 7810. [Google Scholar] [CrossRef]
- Charbonier, F.; Indana, D.; Chaudhuri, O. Tuning Viscoelasticity in Alginate Hydrogels for 3D Cell Culture Studies. Curr. Protoc. 2021, 1, e124. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Qu, Q.; Pan, S.; Yu, K.; Liu, Y. Graphene Oxide Modified Sodium Alginate/Polyethylene Glycol Phase Change Material Hydrogel Scaffold Composite with Photothermal Temperature Control for Potential Bone Tissue Regeneration. J. Mater. Res. Technol. 2024, 30, 2446–2457. [Google Scholar] [CrossRef]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Katsen-Globa, A.; Huber, E.J.; Mueller, S.C.; Kreiner, A.; Pütz, N.; Gepp, M.M.; Fischer, B.; Stracke, F.; von Briesen, H.; et al. Poly(Amidoamine)-Alginate Hydrogels: Directing the Behavior of Mesenchymal Stem Cells with Charged Hydrogel Surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 105. [Google Scholar] [CrossRef]
- Kang, S.-M.; Lee, J.-H.; Huh, Y.S.; Takayama, S. Alginate Microencapsulation for Three-Dimensional In Vitro Cell Culture. ACS Biomater. Sci. Eng. 2021, 7, 2864–2879. [Google Scholar] [CrossRef]
- Ghaffari Sharaf, M.; Li, S.; Rowe, E.M.; Devine, D.V.; Unsworth, L.D. Characterization and Hemocompatibility of α, β, and γ Cyclodextrin-Modified Magnetic Nano-Adsorbents. Int. J. Mol. Sci. 2024, 25, 10710. [Google Scholar] [CrossRef]
- Pogorielov, M.; Kalinkevich, O.; Deineka, V.; Garbuzova, V.; Solodovnik, A.; Kalinkevich, A.; Kalinichenko, T.; Gapchenko, A.; Sklyar, A.; Danilchenko, S. Haemostatic Chitosan Coated Gauze: In Vitro Interaction with Human Blood and in-Vivo Effectiveness. Biomater. Res. 2015, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Chen, Y.-C.; Lin, T.-H.; Yang, C.-Y.; Kuo, Y.-W.; Lei, U. Hemostatic Enhancement via Chitosan Is Independent of Classical Clotting Pathways—A Quantitative Study. Polymers 2020, 12, 2391. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Weitz, J.I. The Blood Compatibility Challenge. Part 1: Blood-Contacting Medical Devices: The Scope of the Problem. Acta Biomater. 2019, 94, 2–10. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000905. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Y.; Li, W.; Li, D.; Li, H. Preparation of Fully Bio-Based Multilayers Composed of Heparin-like Carboxymethylcellulose Sodium and Chitosan to Functionalize Poly (l-Lactic Acid) Film for Cardiovascular Implant Applications. Int. J. Biol. Macromol. 2023, 231, 123285. [Google Scholar] [CrossRef] [PubMed]
- More, R.V.; Antanitta, S.V.; Khonde, R.; Kandasubramanian, B. Cellulose and Derivatives Serving as Natural, Versatile and Biocompatible Polymers in Biomedical Applications. Int. J. Polym. Mater. Polym. Biomater. 2024, 74, 923–937. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhang, X.; Wu, J.; Zhao, J. Modifications of Polysaccharide-Based Biomaterials under Structure-Property Relationship for Biomedical Applications. Carbohydr. Polym. 2021, 266, 118097. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Savargaonkar, A.V.; Tahir, M.; Sionkowska, A.; Popat, K.C. Surface Modification Strategies for Improved Hemocompatibility of Polymeric Materials: A Comprehensive Review. RSC Adv. 2024, 14, 7440–7458. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.; Sperling, C.; Maitz, M.F.; Siedlecki, C.A.; Werner, C.; Sefton, M.V. The Blood Compatibility Challenge. Part 3: Material Associated Activation of Blood Cascades and Cells. Acta Biomater. 2019, 94, 25–32. [Google Scholar] [CrossRef]
- Spillert, C.R.; Lazaro, E.J. Modified Recalcification Time: A Global Coagulation Screening Test. J. Natl. Med. Assoc. 1993, 85, 611–616. [Google Scholar]
- Sheng, K.; Gao, Y.; Bao, T.; Wang, S. Covalent Coating Strategy for Enhancing the Biocompatibility and Hemocompatibility of Blood-Contacting Medical Materials. Pharm. Sci. Adv. 2023, 1, 100001. [Google Scholar] [CrossRef]
- Saleem, A.; Rehman, R.; Hussain, S.; Salem, M.A.; Ali, F.; Shah, S.A.A.; Younas, U.; El-Bahy, S.M.; El-Bahy, Z.M.; Iqbal, M. Biodegradable and Hemocompatible Alginate/Okra Hydrogel Films with Promising Stability and Biological Attributes. Int. J. Biol. Macromol. 2023, 245, 125532. [Google Scholar] [CrossRef]
- Priya, S.; Choudhari, M.; Tomar, Y.; Desai, V.M.; Innani, S.; Dubey, S.K.; Singhvi, G. Exploring Polysaccharide-Based Bio-Adhesive Topical Film as a Potential Platform for Wound Dressing Application: A Review. Carbohydr. Polym. 2024, 327, 121655. [Google Scholar] [CrossRef]
- Chen, S.; Xia, J.; Hou, Z.; Wu, P.; Yang, Y.; Cui, L.; Xiang, Z.; Sun, S.; Yang, L. Natural Polysaccharides Combined with Mussel-Inspired Adhesion for Multifunctional Hydrogels in Wound Hemostasis and Healing: A Review. Int. J. Biol. Macromol. 2024, 282, 136965. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Tao, K.; Feng, Q.; Li, F.; Mu, X.; Du, C.; Zhao, R.; Wang, D.; Zhou, X.; et al. Blood-Responsive Mussel-Inspired Hydrogels for Hemostasis, Antibacterial Action, and Wound Healing. Int. J. Biol. Macromol. 2024, 278, 135038. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, S.S.; Ehsani, M.; Zandi, M.; Saeed, M.; Sabeti, M. Polysaccharide-Based (Kappa Carrageenan/Carboxymethyl Chitosan) Nanofibrous Membrane Loaded with Antifibrinolytic Drug for Rapid Hemostasis-in Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2023, 247, 125786. [Google Scholar] [CrossRef]
- Brzoska, T.; Vats, R.; Bennewitz, M.F.; Tutuncuoglu, E.; Watkins, S.C.; Ragni, M.V.; Neal, M.D.; Gladwin, M.T.; Sundd, P. Intravascular Hemolysis Triggers ADP-Mediated Generation of Platelet-Rich Thrombi in Precapillary Pulmonary Arterioles. JCI Insight 2020, 5, e139437. [Google Scholar] [CrossRef]
- Warale, D.; Prabhu, A.; Kouser, S.; Shabeena, M.; Manasa, D.J.; Nagaraja, G.K. Incorporation of Sodium Alginate Functionalized Halloysite Nanofillers into Poly (Vinyl Alcohol) to Study Mechanical, Cyto/Heme Compatibility and Wound Healing Application. Int. J. Biol. Macromol. 2023, 232, 123278. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, P.; Siqueira, É.; de Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef]
- Lekkerkerker, H.N.W.; Tuinier, R. Colloids and the Depletion Interaction; Lecture Notes in Physics; Springer: Dordrecht, The Netherlands, 2011; Volume 833, ISBN 978-94-007-1222-5. [Google Scholar]
- Boluk, Y.; Zhao, L.; Incani, V. Dispersions of Nanocrystalline Cellulose in Aqueous Polymer Solutions: Structure Formation of Colloidal Rods. Langmuir 2012, 28, 6114–6123. [Google Scholar] [CrossRef]
- Benselfelt, T.; Kummer, N.; Nordenström, M.; Fall, A.B.; Nyström, G.; Wågberg, L. The Colloidal Properties of Nanocellulose. ChemSusChem 2023, 16, e202201955. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Elkhooly, T.A.; Liu, Y.; Wu, H.; Feng, Q.; Liu, L.; Fang, Y.; Zhu, W.; Hu, T. Effects of titanium surface roughness onthe mediation of osteogenesis via modulating the immune response ofmacrophages. Biomed. Mater 2018, 13, 045013. [Google Scholar] [CrossRef]
- Altankov, G.; Richau, K.; Groth, T. The role of surface zeta potential and substratum chemistry for regulation of dermal fibroblasts interaction. Mater. Sci. Eng. Technol. 2003, 34, 1120–1128. [Google Scholar] [CrossRef]
- Puzas, V.M.V.; Carter, L.N.; Schröder, C.; Colavita, P.E.; Hoey, D.A.; Webber, M.A.; Addison, O.; Shepherd, D.E.T.; Attallah, M.M.; Grover, L.M.; et al. Surface Free Energy Dominates the Biological Interactions of Postprocessed Additively Manufactured Ti-6Al-4V. ACS Biomater. Sci. Eng. 2022, 8, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.-Y.; Li, Q.-J.; Hu, J.-J.; Song, Y.-T.; Zhang, Q.-Y.; Nie, R.; Li-Ling, J.; Xie, H.-Q. Design of Biopolymer-Based Hemostatic Material: Starting from Molecular Structures and Forms. Mater. Today Bio. 2022, 17, 100468. [Google Scholar] [CrossRef] [PubMed]



| Sample | Diiodo-Methane, ° | Water, ° | Glycerol, ° | Ethylene Glycol, ° | Formamide, ° | γsLW | γs+ | γs− |
|---|---|---|---|---|---|---|---|---|
| AlgF | 33.5 ± 0.2 | 51.9 ± 0.4 | 49.6 ± 0.3 | 36.1 ± 0.4 | 36.8 ± 0.5 | 42.94 | 0.45 | 24.64 |
| AlgCNC-1 | 34.2 ± 0.3 | 32.2 ± 0.4 | 56.9 ± 0.4 | 42.1 ± 0.5 | 42.1 ± 0.4 | 42.76 | 0.15 | 31.42 |
| AlgCNC-5 | 33.0 ± 0.3 | 38.5 ± 0.4 | 59.9 ± 0.4 | 39.6 ± 0.4 | 35.7 ± 0.5 | 43.85 | 0.07 | 41.66 |
| AlgCNC-10 | 32.2 ± 0.3 | 29.2 ± 0.4 | 67.2 ± 0.4 | 36.3 ± 0.4 | 28.4 ± 0.5 | 42.17 | 0.05 | 44.13 |
| AlgCNC-30 | 30.1 ± 0.3 | 36.4 ± 0.4 | 50.4 ± 0.4 | 39.7 ± 0.4 | 38.2 ± 0.5 | 41.98 | 0.03 | 47.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torlopov, M.A.; Martakov, I.S.; Mikhaylov, V.I.; Legki, P.V.; Vavrinchuk, K.S.; Markov, P.A.; Drozd, N.N.; Zhuravlev, A.V.; Sitnikov, P.A.; Kutchin, A.V. “Revitalizing” Alginate Films: Control of Texture, Hemo- and Cellular Compatibility via Addition of Cellulose Nanocrystals. Polysaccharides 2025, 6, 43. https://doi.org/10.3390/polysaccharides6020043
Torlopov MA, Martakov IS, Mikhaylov VI, Legki PV, Vavrinchuk KS, Markov PA, Drozd NN, Zhuravlev AV, Sitnikov PA, Kutchin AV. “Revitalizing” Alginate Films: Control of Texture, Hemo- and Cellular Compatibility via Addition of Cellulose Nanocrystals. Polysaccharides. 2025; 6(2):43. https://doi.org/10.3390/polysaccharides6020043
Chicago/Turabian StyleTorlopov, Mikhail A., Ilia S. Martakov, Vasily I. Mikhaylov, Philipp V. Legki, Kirill S. Vavrinchuk, Pavel A. Markov, Natalia N. Drozd, Andrey V. Zhuravlev, Petr A. Sitnikov, and Alexander V. Kutchin. 2025. "“Revitalizing” Alginate Films: Control of Texture, Hemo- and Cellular Compatibility via Addition of Cellulose Nanocrystals" Polysaccharides 6, no. 2: 43. https://doi.org/10.3390/polysaccharides6020043
APA StyleTorlopov, M. A., Martakov, I. S., Mikhaylov, V. I., Legki, P. V., Vavrinchuk, K. S., Markov, P. A., Drozd, N. N., Zhuravlev, A. V., Sitnikov, P. A., & Kutchin, A. V. (2025). “Revitalizing” Alginate Films: Control of Texture, Hemo- and Cellular Compatibility via Addition of Cellulose Nanocrystals. Polysaccharides, 6(2), 43. https://doi.org/10.3390/polysaccharides6020043








