Abstract
Adsorption has been found to be highly effective for removing heavy metals from polluted industrial wastewater. Adsorbents of biological origin, such as negatively charged polysaccharides, e.g., alginate and carrageenan, have attracted a lot of attention recently. In this study, these three polysaccharides were used to adsorb different heavy metal ions from aqueous solutions. The results showed that the sorption yields of various lanthanides with the kappa and iota carrageenan were similar, though the sorption yields of the iota beads were higher. Also, the iota and the kappa beads had higher sorption yields for Ru3+ and Rh3+ than they did for the lanthanides. In general, the presence of light metal ions in the solution affected the sorption yields of the heavy metal ions, depending on the type and concentration of the light metal ions. All three polysaccharides were also capable of adsorbing mixtures of lanthanides and heavy metal ions. In binary solutions that contained both lanthanide ions (Ce3+ or Eu3+) and transition heavy metal ions (Ru3+ or Rh3+), differences in sorption yields were observed, with all polysaccharides exhibiting higher selectivity for Ru3+ and Rh3+. Finally, FTIR, SEM/EDS, and TGA analyses confirmed that all metal ions were adsorbed onto both types of carrageenan.
1. Introduction
The adsorption of heavy metal ions from wastewater is one of the simplest and cheapest ways to separate them from industrial effluents [1,2]. Over the years, various organic and inorganic adsorbents have been proposed for heavy metal ion adsorption, such as activated carbon [3], zeolites [4], biopolymers [5], and a variety of different kinds of biomass [6]. Among the benefits of bio-based adsorbents is that they are renewable, non-toxic, and biodegradable [7,8].
One class of bio-adsorbents for heavy metal ion removal comprises polysaccharide hydrogels [9,10]. Different polysaccharides have been used in this capacity, each possessing different functional groups, such as starch with only hydroxyl groups [11], alginate (A) with hydroxyl and carboxylic groups [12], chitosan with hydroxyl and amine groups [13], and carrageenan forms with hydroxyl and sulfate ester groups [14].
Aqueous industrial effluents contain a range of different salts and other pollutants, all of which may mutually and significantly affect the adsorption of others [15]. In addition, most industrial wastewaters contain both heavy metal ions and light metal ions. The presence of light metal ions may significantly affect the heavy metal adsorption performance of the chosen absorbent due to the competition between the different metal ions over the binding sites and depending on the variety of interactions involved and on the conditions under which the interactions take place [16,17]. Some studies have reported an inhibitory effect by light metal ions on the adsorption of heavy metals by a biological adsorbent [18,19], whereas others reported that no effect was observed [20,21]. Moreover, the combined effects on adsorption of the different components in the wastewater stream are complex and may also be a function of one or several of the following parameters: ionic radius, electronegativity, hydration capacity, solution pH, and availability of the active sites on the adsorbent [22]. The ionic strength of the solution also plays an important role in the biological adsorption of metal ions [23].
Another important issue in the recovery of heavy metal ions from wastewaters is whether the adsorbent has the ability to remove several metal ions simultaneously and, in contrast, whether it can selectively remove certain metal ions from solutions that contain multiple metal ions [24,25,26]. Furthermore, some wastewaters contain additional contaminants other than metal ions, such as dyes, which could also affect absorbent performance [27,28].
We recently used different polysaccharides from the carrageenan family—iota (I), kappa (K) and lambda (λ)—to remove ions of europium (Eu3+) [29,30,31,32,33], samarium (Sm3+), erbium (Er3+), and uranyl (UO22+) [33]. Our studies showed that the various carrageenan were able to remove the different metal ions. Relatively similar sorption yields were obtained for all of the tested lanthanides and the actinide. Furthermore, although sorption from a binary or ternary mixture of Sm3+, Er3+, and UO22+ with A showed no selectivity, employing both the I and λ carrageenan forms to adsorb a mixture of binary and ternary metal ions that comprised one or two lanthanides together with uranyl showed higher selectivity for the trivalent and smaller lanthanides [34]. It was, therefore, concluded that the lanthanide ions have higher affinities for the sulfate ester groups in the carrageenan than for the carboxylic group in A.
In this paper, we compared the sorption yields and the selectivity of representative heavy metal ions (Ce3+, Eu+3, Nd3+, Dy3+, Ru3+ and Rh3+), with or without the addition of representative light metal ions (Na+, K+, Ca2+ and Mg2+), by using hydrogel beads of K, I and A that were prepared with CaCl2 (Figure 1). After we completed the sorption analysis, we characterized I and K-based hydrogel beads after metal sorption.
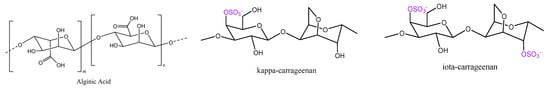
Figure 1.
Basic polysaccharides that were used for metal sorption.
2. Materials and Methods
2.1. Materials and Chemicals
All the materials were purchased from Sigma Aldrich, Israel. The average molecular weights of I and K were 380 and 400 kDa, and the sulfur contents were 16 and 28%wt, respectively. The molecular weight of A was 250–350 kDa, which comprised 60–70% mannuronic acid and 30–40% glucuronic acid, respectively. All salts were analytical grade: CeCl3·7H20, EuCl3·6H2O, NdCl3·6H20, DyCl3·6H20, RhCl3, NaCl, KCl, CaCl2, and MgCl2·6H20.
2.2. Sorption Experiments
Polysaccharide stock solutions were prepared by dissolving 1 g of each polysaccharide in 100 mL double-distilled water (DDW) to yield a 1 wt/v% solution. Then, 3 mL of the polysaccharide solution (0.03 g polysaccharide) was added dropwise to a 30 mL solution of CaCl2 (0.5 M) and stirred at 100 rpm for 5 min to yield hydrogel beads that were filtered and washed with DDW. The beads were added to metal ion solutions of 500 ppm that were prepared by dissolution of the various salts in DDW. The mixture was then stirred for 1 h at 100 rpm, and samples were withdrawn to measure the metal ion concentrations in the solution using a UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). For the lanthanide ion, the spectrophotometric determination of its concentration in the solution required the use of xylenol orange [35]. The concentrations of the heavy metal ions (Rh3+ and Ru3+) in the solution were determined by measuring the UV-Vis absorbance at 525 and 450 cm−1, respectively. When a mixture of lanthanides was tested, inductively coupled plasma optical emission spectroscopy (ICP-OES) (SPECTRO, model ARCOS) was used to determine both the sorption yield and selectivity.
The sorption yield (Y(%)) was calculated using a calibration curve according to Equation (1), where Cin (mg/L) is the initial metal ion concentration, and Cf (mg/L) is the final metal ion concentration (after sorption) in the solution.
Y(%) = (Cin − Cf)/Cin × 100
The sorption selectivity (S(%)) was defined as the ratio between the concentration of a specific metal (designated as M) that was adsorbed to the concentration of all metals adsorbed (designated as total) according to Equation (2).
where Cin,M (mg/L) is the initial concentration of a specific metal, and Cf,M (mg/L) is the final concentration of a specific metal in the solution.
S(%) = (Cin,M − Cf,M)/(Cin,total − Cf,total) × 100
2.3. Desorption Experiments
In a typical procedure, after sorption for 1 h, I (1 wt/v%) hydrogel beads were separated from the metal ions solutions and added to 10 mL of an aqueous solution of various salts or acids (0.5 M), and stirred at room temperature with a magnetic stirrer at 100 rpm for 0.5 h, whereas the metal ions concentration in the solutions was determined as detailed Section 2.2. Then, the desorption yield (D-Y) was calculated using Equation (3), where C*in (mg/L) is the metal ions concentration in the hydrogel beads after sorption and C*f (mg/L) is the metal ions concentration in the beads at the end of the desorption.
D-Y = (C*in − C*f)/C*in
2.4. Characterization of Hydrogel Beads
I and K beads were separated from the solution after sorption and were frozen at −20 °C for 24 h and then freeze-dried using a lyophilizer (Lyovac GT 2, Leybold, Köln, Germany). The freeze-dried beads and the pristine polysaccharide were subjected to Fourier transform infrared spectroscopy (FTIR) with the Nicolet 6700 FTIR (Thermo Scientific, Waltham, MA, USA), which contained an attenuated total reflectance (ATR) device outfitted with a diamond crystal plate. The recorded spectra were the means of 36 spectra taken in the wavelength range of 650–4000 cm−1 with a 0.5 cm−1 resolution and atmospheric correction switched on at room temperature (25 °C). In addition, the freeze-dried beads, after sorption, and the pristine polysaccharides were subjected to thermal gravimetric analysis (TGA, Q500 V20.13-Instrument, TA Instrument, New Castle, DE, USA) to record the thermal changes in the adsorbents. The beads were heated at a heating rate of 10 °C/min over a temperature range of 25–1000 °C under a continuous nitrogen flow of 90 mL/min. The freeze-dried beads after adsorption were also analyzed using SEM by the Phenom XL scanning electron microscope (SEM) with a backscatter electron detector equipped with an energy-dispersive X-ray spectroscopy detector (ThermoFisher, Waltham, MA, USA). The acceleration voltage was 15 kV, and the sample pressure was 60 Pa.
3. Results and Discussion
3.1. Sorption Yields of Hydrogel Beads
The investigation began by studying the sorption yields of the four representative heavy metal ions (HM): Ce3+, Eu+3, Ru3+, and Rh3+ (500 mg/L) alone and in the presence of different light metal ions (LM): Na+, K+, Ca2+, and Mg2+ (with Cl− as the counter anion for all ions). The sorption yields of the different metal ion species were measured at different molar ratios of heavy metal ions to light metal ions (HM/LM), i.e., 1:1, 1:5 and 1:10. As detailed in Section 2.2, UV-Vis analysis was used to detect sorption yields while using K, I and A. The results of the experiments are presented in Figure 2.
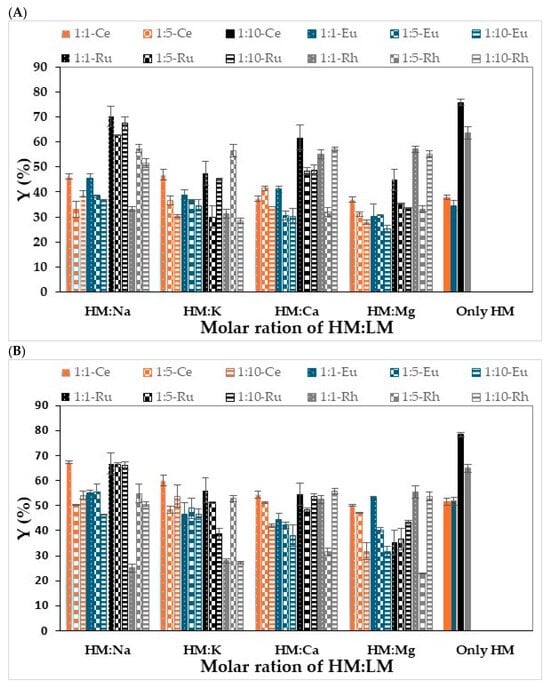
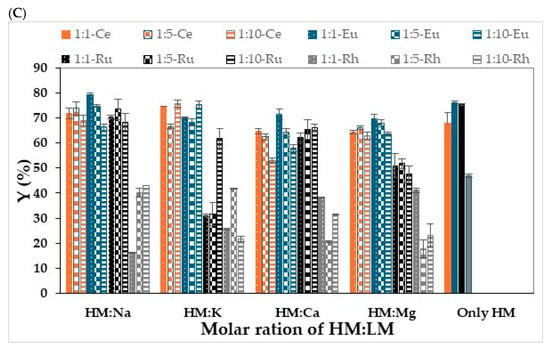
Figure 2.
Sorption yields of heavy metal ions on hydrogel-based polysaccharides in the presence of light metal ions and only with Heavy metals. (A) K (B) I (C) A; the color of the bars represents the HM that was used in each experiment. Solid fill of the bars colored with orange, blue, black, and gray represents the Ce, Eu, Ru, and Rh, respectively. Hydrogel bead preparation conditions: 3 mL of 1 wt/v% solution of polysaccharide, 30 mL solution of CaCl2 0.5 M. Sorption conditions: 10 mL solution of 500 ppm metal ions, 100 rpm, 1 h. The results are each an average of five independent experiments ± STDEV.
As can be seen from the results in Figure 2, the sorption yields for the four heavy metal ions depended on the type of polysaccharide and the type of metal, as well as the ratio between the heavy metal and the light metal. In general, the sorption yield of Ce3+ or Eu3+ with K (Figure 2A) or I (Figure 2B) when each metal was the only metal in solution was similar due to the similar ionic radius and valence of the two metal cations. The sorption yields observed for the I-beads were higher than those for the K-beads, probably due to the higher sulfate ester groups content of the former [29,30,31,32,33]. In addition, the sorption yields of Ru3+ and Rh3+ with I (Figure 2B) or K beads (Figure 2A) were higher than those of Ce3+ and Eu3+, probably as they adsorb mainly via the hydroxyl groups. In the case of A, the sorption yields of Ce3+, Eu3+, and Ru3+ were comparable, though it adsorbed the Eu3+ and Ru3+ ions better than it did the Ce3+ ions. Notably, the sorption yield of Rh3+ with A was much lower than the values obtained for the rest of the tested metals. This finding may be attributable to the different coordination of Rh3+ and Ru3+ with the functional groups on the polysaccharide [36]. In addition, the capacity of the A beads to adsorb the lanthanides from the metal solution was higher than that of the carrageenan forms. Nonetheless, the carrageenan forms were much more effective adsorbents of the Rh3+ than was A.
When LM ions were added to the solution of the four representative heavy metal ions, the sorption yields were affected by the types of light and heavy metal ions, by the type of polysaccharide, and by the concentrations of the light metal ions. This finding may be related to the differences in the electronegativities of the different light metal ions, because the higher the electronegativity and ionization energy of the cation, the greater its sorption to the polysaccharide, thus resulting in the reduced sorption yield of the heavy metal ions. Among the light metals examined, Mg2+ has the highest ionization energy and electronegativity and the smallest ionic radius, and therefore, its concentration in the solution should exhibit the strongest effect on sorption yields. Indeed, in general, this effect is notable even though there are also other effects. For example, in the adsorption of Ce3+ ions with I-beads, increasing the Mg2+ concentration reduced the sorption yield. In addition, the sorption yields of all the cations with the three polysaccharides in a weight ratio of 1:10 heavy metal: light divalent metal ions exhibited much lower sorption capacity than those obtained without the light divalent ions. This phenomenon may be explained by several factors, e.g., the competition for binding sites, the formation of precipitates, and complexation via hydroxides. Furthermore, the presence of light metal cations might reduce the charge density of the anionic sites on the polysaccharide bead surfaces, which will then be less available for metal binding.
The findings in this study were also supported by the results from other studies. Young et al., who examined the effects of light metal ions on the sorption process of heavy metal ions with natural polysaccharides, also found that as the concentration of light metal ions increased the extent of their interference in sorption increased [37]. Furthermore, the adsorption of Pb2+ and Cu2+ ions was not significantly affected by increasing the concentration of the light metals, but Cd2+ and Zn2+ ions exhibited higher sensitivity to the presence of the light metal ions. Increasing the concentration of Na+ ions had the smallest effect on sorption process efficiency compared to the other light metals that were examined, e.g., Ca2+ and Mg2+. This result can be explained by the fact that multivalent cations have a direct influence on the adsorption process due to the competition for binding sites and the formation of complexes by carbonates or hydroxides of Ca2+ and Mg2+ [18].
In contrast to the observed effects of the light divalent metal cations on sorption, the addition of monovalent cations, like Na+ and K+, apparently does not have a significant effect on the sorption process, meaning that there is no direct competition for binding between the light metal and the adsorbent, and in some cases, they have no effect at all [17,38]. It is important to note, however, that other studies have shown that high concentrations of monovalent cations such as Na+ and K+ increase the ionic strength and may sometimes lead to a decrease in adsorption efficiency [39,40]. In addition, no trend was seen for the sorption of lanthanide in the presence of light monovalent cations (Figure 2). Interestingly, in some cases, the addition of light monovalent cations caused elevated sorption yield compared to those obtained in experiments on the sorption of lanthanides alone. For example, the yield of Ce3+ sorption using I-beads in a solution that contained Na+ in a weight ratio of 1:1 (67.4%) was higher than the yield in a solution that contained only Ce3+ cations (51.7%) (Figure 2B).
3.2. Solution Acidity Effect
Metal adsorption onto polysaccharides is often influenced by the pH of the solution as changes in acidity result in the protonation or deprotonation of acidic functional groups on the polysaccharide [41]. This, in turn, leads to competition between H3O+ ions and metal ions for binding sites [42,43,44]. As shown in Figure 3, the sorption yield of Ce3+ ions on the carrageenan forms increases with rising pH from 3 or 5 to 7 (Figure 3A), whereas an opposite trend is observed for Rh3+ ions (Figure 3B). In the case of Ru3+, increasing the pH above 3 led to the formation of a solid precipitate.
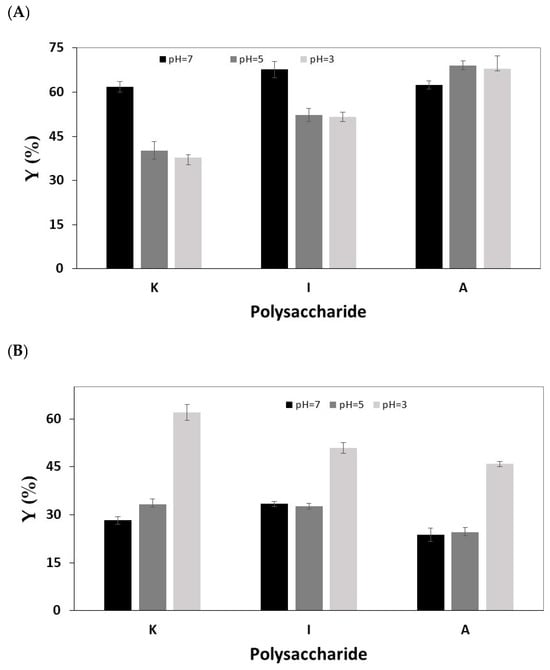
Figure 3.
The effect of solution pH on the sorption yield of (A) Ce3+; (B) Rh3+. Hydrogel bead preparation conditions: 3 mL of 1 wt/v% solution of I, 30 mL solution of CaCl2 (0.5 M). Sorption conditions: 10 mL solution of 500 mg/L metal ion, 100 rpm, 1 h. The results are each an average of five independent experiments ± STDEV.
3.3. Metal Desorption and Adsorbent Reuse
The desorption yields (D-Y) of Ce3+ and Rh3+ ions from the I hydrogel beads, using representative acids and salts: HCl, HNO3, NaNO3, and KNO3, at a concentration of 0.5 M [45,46] was also performed, following by hydrogel beads reuse in second sorption stage (Y-2) (Figure 4).
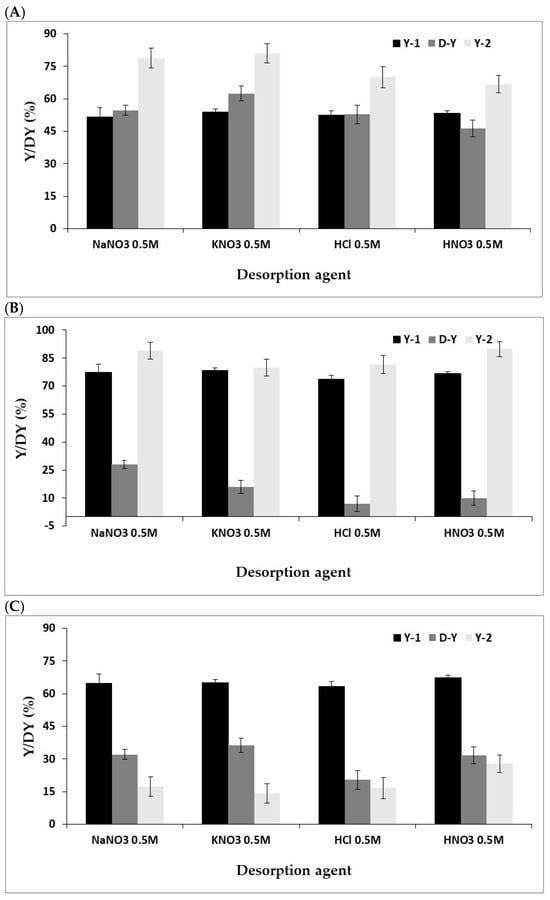
Figure 4.
Sorption and desorption yields with different removal agents: (A) Ce3+, (B) Ru3+, and (C) Rh3+. Hydrogel bead preparation conditions: 3 mL of 1 wt/v% solution of I, 30 mL solution of CaCl2 (0.5 M). Sorption conditions: 10 mL solution of 500 mg/L metal ion, 100 rpm, 1 h. Desorption conditions: 10 mL solution of salt or aqueous acid (0.5 M), 100 rpm, 0.5 h. The results are each an average of five independent experiments ± STDEV.
As shown in Figure 4, the type of desorption agent significantly affects the desorption yield (D-Y), as expected. Generally, the two tested salts were more effective than their corresponding parent acids. This can be attributed to the fact that acids increase the concentration of hydronium ions, which protonate the active sites of the hydrogels, facilitating the release of metal ions into the solution. In contrast, salt solutions disrupt the interactions between the adsorbent and the metal ions, promoting their release via an ion-exchange mechanism [45]. Generally, the I hydrogel beads demonstrated successful reusability. Notably, in the case of Ce3+, the sorption yield in the second stage (Y-2) was even higher than in the first stage (Y-1). This observation may be explained by the partial structural modification of the polysaccharide by the removal of salts, which rendered it more permeable and enhanced the accessibility of sulfate ester groups on the polysaccharide. Also, the D-Y of Ce3+ was higher than that of Rh3+ and Ru3+, likely due to differences in their binding mechanisms to the polysaccharide.
3.4. Comparison of Adsorption Using Different Polysaccharides
Table 1 compares the experimental maximum sorption capacities of Ce3+—chosen as a representative lanthanide—on various polysaccharide-based adsorbents, including our system. While varying experimental conditions can make direct comparisons challenging, the data still reveal meaningful trends. It can be observed that sorption capacities varied in a wide range, where carrageenan-based hydrogel beads prepared in this study demonstrate a relatively high sorption capacity.

Table 1.
Comparison of Ce3+ with different adsorbents.
Table 1.
Comparison of Ce3+ with different adsorbents.
| Adsorbent | Maximum Capacity (mg/g) | Reference |
|---|---|---|
| sulfonic-functionalized cellulose | 84.8 | [47] |
| Sodium alginate-coated magnetite (Alg-Fe3O4) nanoparticles | 31.8 | [48] |
| Chitosan-functionalized magnetite-pectin | 9.7 | [49] |
| Pectin | 180 | [50] |
| Iota-carrageenan | 86 | This work |
3.5. Sorption Yields and Selectivities with Binary and Quaternary Lanthanide Ion Solutions
The next step was to test the sorption yields and selectivity with binary and quaternary lanthanide ion solutions using different mixtures. The sorption of a 500 ppm binary solution of 250 ppm Ce3+ with 250 ppm Eu3+, 250 ppm Ce+3 with 250 ppm Nd3+ or 250 ppm Ce3+ with 250 ppm Dy3+ was tested using K, I and A. The quaternary mixture, which comprised 125 ppm Ce3+, 125 ppm Eu3+, 125 ppm Nd3+ and 125 ppm Dy3+, was similarly tested for metal sorption. The sorption yields and selectivity, which were calculated with ICP-OES, were similar for all three polysaccharides, probably because all lanthanides have similar valence and ionic radii.
Because the sorption yields of both lanthanide ions (Ce3+ and Eu+3) differed from those of both heavy metal ions (Ru3+ and Rh3+), it was suggested that a test of their mixtures might yield selective sorption (Table 2).

Table 2.
Sorption yields (Y) and selectivity (S) with polysaccharide-based beads in solutions of different metal ions a.
The data listed in Table 2 show the differences in the sorption yields and the selectivity of the tested polysaccharides in a binary solution of lanthanide ions and transition metal ions. In general, as previously illustrated, the sorption yield of Ce3+ ions resembled that of Eu3+ ions because the two are similar in terms of size and charge (Figure 1). On the other hand, as previously measured, the adsorption of Ru3+ or Rh3+ ions differed because the ionic radius of Ru3+ is larger than that of Rh3+. Accordingly, in a mixture of Ru3+ ions with Ce3+ or Eu3+, the adsorption efficiency of Ru3+ was higher than that of the lanthanide ions. Yet, in a mixture that contained Rh3+ ions with Ce3+ or Eu3+, the sorption yields of Rh3+ on K and A were higher than those of the lanthanide ions and lower with I.
A comparison of the sorption yields in a single-metal system to those in a binary-metal system shows that for Ce3+ ions, the sorption yields are not significantly different. This is likely due to the fact that, in contrast to Ce3+ and Eu3+ ions, Ru3+ and Rh3+ ions do not bind to the sulfate ester group; rather, they interact with the hydroxyl group on the polysaccharide backbone. As a result, they do not compete with the binding of metal ions, which adhere to the polysaccharide’s functional groups. The data in Table 2 also show that the two lanthanide ions exhibited similar behavior in mixtures with heavy metals, and the adsorption efficiency in the mixture was similar to that of a single-metal system. According to Texier et al., significant competition for binding sites occurs in solutions that contain metals from the same group, such as lanthanides [51].
3.6. Kinetics
The kinetic of Ce3+, Ru3+ and a binary solution of Ce3++Ru3+ ions sorption using I hydrogel beads as a representative system were studied using three models: the pseudo first order (Equation (4)), the pseudo second order (Equation (5)), and an intraparticle diffusion model (Equation (6)), where qt and qe are the metal ion adsorption capacity (mg/g) at any time t (min) and at equilibrium, correspondingly, k1 (min−1) is the pseudo first-order rate constant and k2 (g mg−1 min−1) is the pseudo-second order rate constant [51,52,53], and k3 (g × mg−1 × min−0.5) is the rate constant an intraparticle diffusion model, and C (mg/g) is a constant shown to be correlated to the boundary layer thickness [54].
As illustrated in Figure 5, the pseudo second-order model is the most suitable for representing the sorption kinetics, indicating chemisorption processes characterized by strong interactions between the adsorbate and the surface, and this trend was observed for all metal ions.
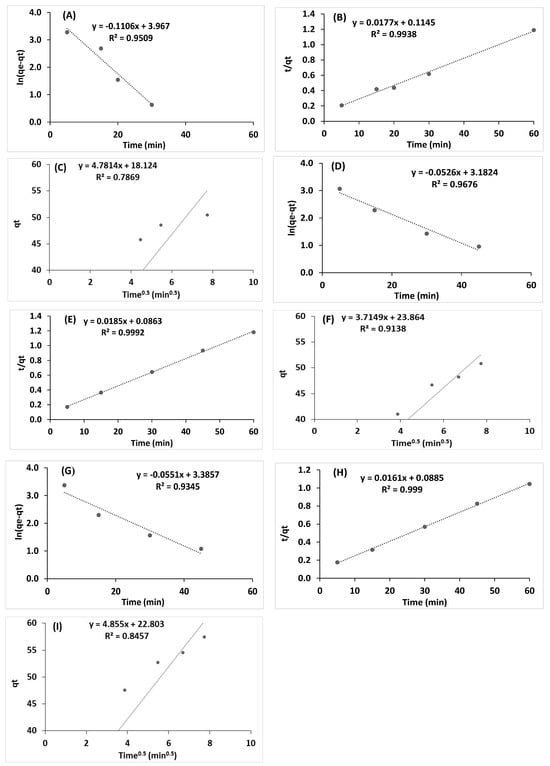
Figure 5.
Kinetic model for sorption of 250 ppm Ce3+: (A) pseudo first order, (B) pseudo second order, and (C) an intraparticle diffusion model; for sorption of 250 ppm Ru3+ (D) pseudo first order, (E) pseudo second order, and (F) an intraparticle diffusion model; for sorption of 250 ppm Ce3+ + 250 ppm Ru3+ (G) pseudo first order, (H) pseudo second order, and (I) an intraparticle diffusion model; Hydrogel bead preparation conditions: 3 mL of 1 wt/v% solution of I, 30 mL solution of CaCl2 (0.5 M). Sorption conditions: 10 mL solution of 500 mg/L metal ion, 100 rpm, 1 h. The results are each an average of five independent experiments.
3.7. Hydrogel-Based Beads Characterization
In the next step, we used FTIR-spectrometry to study the structural changes that occurred due to heavy metal adsorption to the carrageenan forms, which were selected as representative polysaccharides for this study because they demonstrated higher selectivity than was observed for the alginate. The FTIR spectrum of I or K before and after metal sorption in the spectral range of 800–3750 cm−1 is presented in Figure 2. Most of the characteristic peaks that correspond to the basic chemical bonds present in both pristine polysaccharides were identified following the adsorption and according to the literature [55,56] from the following bands: 924 cm−1 (Figure 2A) and 922 cm−1 (Figure 2B) were assigned to C-O 4-Linked 3,6-anhydro-α-D-galactopyranose, and 849 cm−1 and 844 cm−1 assigned to C-O-SO4 on 3-Linked β-Dgaactopyranose 4-sulfate specific for I and K, respectively; also, the band at 805 cm−1 (Figure 2A) was assigned to C-O-SO4 on 4-Linked 3,6-anhydroα-D-galactopyranose 2-sulfate for I; lastly, the stretching peaks of the C-O bond at 1069 cm−1 and 1160 cm−1 for I (Figure 2A) and at 1065 cm−1 and 1159 cm−1 for K (Figure 2B) were present. The consistency of these peaks in all the samples before and after the metal sorption indicates that there was no direct interaction between these functional groups and the cations. However, the characteristic peak of the ester sulfate stretching vibration (-OSO3−), i.e., O=S=O, which appears at 1209 cm−1 (Figure 2A) and 1232 cm−1 (Figure 2B) in the spectra for pristine I and K, respectively, was missing in all the spectra that were involved in Ce3+ sorption. These results are in agreement with our former findings, which demonstrate that lanthanide cations, i.e., Ce3+ or Eu3+, interact directly with the ester sulfate, which demonstrated the formation of a complex between the metal ions and both carrageenan through ester sulfate groups [29,30,31]. In addition, all the spectra contain the characteristically large shoulder peak of the OH stretching vibration at 3050–3700 cm−1. Furthermore, the peak intensities in all the beads obtained after sorption with the different metal combinations were much lower compared to the corresponding peaks in the spectra of pristine I and K. These findings indicate that the interaction with the metal reduced the hydrophilicity of the pristine polysaccharide (Figure 6).
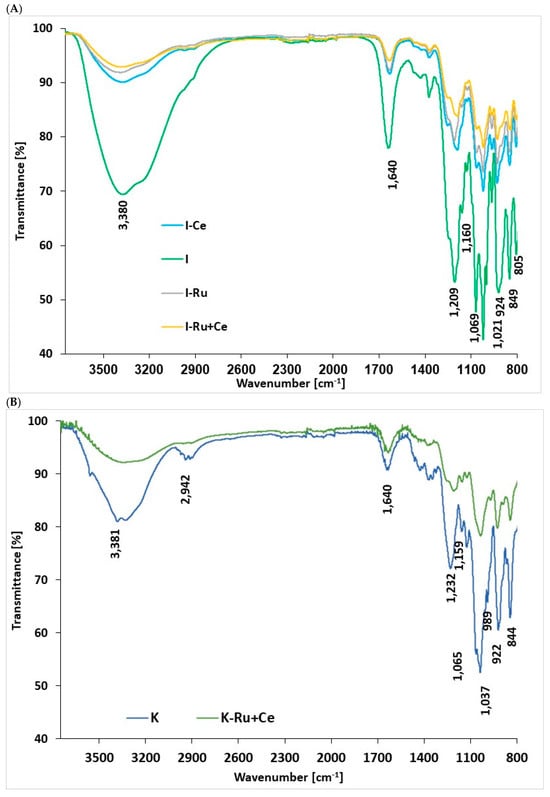
Figure 6.
ATR-FTIR spectra of carrageenan beads in the wavenumber range of 800–37,501 cm−1 (A) I-beads and pristine I (B) K-beads and pristine K Hydrogel bead preparation conditions: 3 mL of 1 wt/v% solution of carrageenan, 30 mL solution of 0.5 M CaCl2. Sorption conditions: 10 mL solution of metal ions (total metal cation concentration 500 ppm), at 100 rpm, 1 h.
Insofar as the beads can be reused in repeated adsorption cycles, the thermal stability of the polysaccharides following metal adsorption was also studied. Table 3 and Figure S1 in the Supplementary Data present the TGA thermogram profile of I- and K-beads before and after ion metal sorption, including the percentage of mass loss for each stage (%Wt loss), the onset temperature, and the differential temperature (DTG).

Table 3.
Thermal properties of pristine I or K and of I or K beads after metal ion adsorption a.
As expected, the thermograms of all the samples show weight losses in three major stages with small differences as follows: (1) The first stage occurred at temperatures below 150 °C and was due mainly to the interactions between the water molecules and the hydroxyl groups present on the polymer backbone, confirming the hydrophilic nature of the various polysaccharides [57]. The total weight losses in this step for all beads after metal adsorption were in the same range (13.3–15.3%). Notably, the beads, after metal adsorption, exhibited a slightly higher mass loss in comparison to pristine polysaccharides. For example, the pristine polysaccharide exhibited mass loss of 11.5 and 9.5% in the first stage for I and K, respectively, in comparison to 14.3 and 14.0% mass loss for I-Ce + Ru and K-Ce + Ru, respectively. We assume that the interactions between the metal ions and the binding sites on the polysaccharide formed a cross-linked network that enabled the polysaccharides to retain a substantial amount of water after drying. (2) The second degradation stage observed in the TGA spectrum was due to the thermal degradation of the polysaccharides associated with the removal of sulfur dioxide and the fragmentation of the carbohydrate backbone [57]. The total weight losses during this stage for I-Ru + Ce at 150–525 °C and for K-Ru + Ce at 125–500 °C were 35.2% and 36.1%, respectively. This wide temperature range may have been due to the decomposition of the hydrogel network. However, the DTG of I-Ru in the temperature range of (150.0–550.0 °C) differed from that of I-Ce in the temperature range of (139.1–528.8 °C), indicating that the matrix formed by the hydrogel with Ce+3 ions differed from that of the I-Ru matrix. After the sorption of both metal ions, we observed hybrid thermal behavior that was probably due to the rearrangement of the polysaccharide chains as a result of the metal adsorption. (3) The third degradation stage, observed at temperatures above 500 °C, may have been due to the decomposition of the inorganic salts in the carrageenan [58]. The weight losses for I-Ru + Ce and K-Ru + Ce in the second step of the third degradation stage were almost similar (4.5%, 5.7%, respectively).
In order to confirm metal deposition and to characterize the morphological structure of the lyophilized I and K- based beads after metal sorption SEM/EDS analysis were conducted (Figure 7). As expected, based on sorption experiments and FTIR data, representative EDS spectrum of the sample has revealed that all the metals adsorb within the carrageenan beads (Figure 7). Comparing the microphotographs of the beads surfaces indicates that the network structure considerably changes after Ce sorption, characterized by “needle-like” structure in I-beads and more ordered structure in K-beads. However, the beads that were prepared after Ru sorption yielded a sealed and smooth surface. In accordance with TGA results, the morphological surface of the beads that were obtained after sorption of the metal mixture was hybrid in accordance with the morphology that was obtained following the sorption of only one metal.
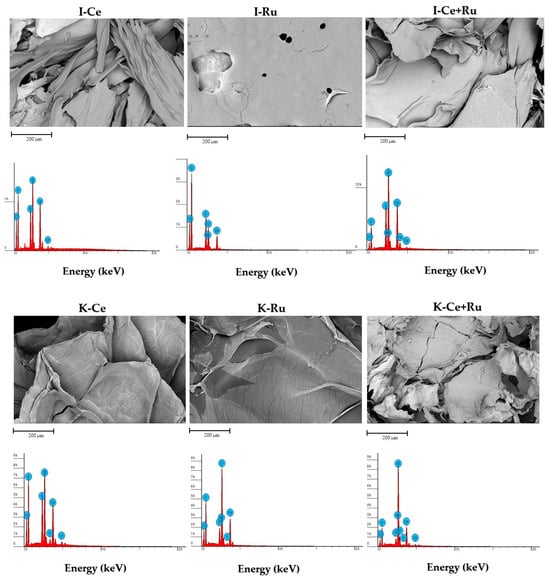
Figure 7.
SEM/EDS analysis (microphotographs at magnitude X600 and EDS spectra) of lyophilized carrageenan-based beads after metal sorption. Hydrogel bead preparation conditions: 3 mL of 1 wt/v% solution of carrageenan, 30 mL solution of 0.5 M CaCl2. Sorption conditions: 10 mL solution of metal ions (total metal cation concentration 500 ppm), at 100 rpm, 1 h.
4. Conclusions
Polysaccharide-based adsorbents offer a sustainable and effective solution for the removal of heavy metal ions from industrial wastewater. In this study, three representative polysaccharides—I- and K-carrageenan, which contain sulfate ester and hydroxyl groups, and A, which contains carboxyl and hydroxyl groups—were successfully used to adsorb six representative metal ions (Ce3+, Eu3+, Nd3+, Dy3+, Ru3+, and Rh3+), either individually or in various mixtures.
All three polysaccharides effectively adsorbed the metal ions. The sorption yields for the four lanthanide ions were similar across all polysaccharides, likely due to their comparable ionic radii and valency. The I-carrageenan, however, which has a higher sulfate ester content than the K-carrageenan, exhibited greater sorption yields for all metal ions. A demonstrated the highest sorption yields for Ce3+ and Eu3+, but its sorption capacities for Ru3+ and Rh3+ were similar to or lower than those of the carrageenan. These findings suggest that the sorption mechanisms of the lanthanide ions differ from those of the platinum-group metal ions.
In some cases, the addition of light metal ions (Na+, K+, Ca2+ and Mg2+) to the heavy metal ion solutions led to a reduction in heavy metal sorption due to competition for binding sites. This reduction was dependent on both the type and concentration of the light metal ions. Sorption experiments using binary and quaternary mixtures of lanthanides revealed no selectivity among the three polysaccharides. However, when a binary mixture containing both lanthanide ions (Ce3+ and Eu3+) and heavy metal ions (Ru3+ and Rh3+) was tested, all three polysaccharides exhibited higher selectivity for the heavy metal ions.
FTIR analysis of I- and K-carrageenan beads confirmed that the metal ions interacted with the hydroxyl and sulfate ester groups, leading to a reduction in the hydrophilic nature of the pristine polysaccharides. Additionally, TGA thermographs indicated that chemical interactions occurred between the metal ions and the functional groups in both carrageenan. After sorption of Ce3+ and Ru3+, the hydrogels exhibited hybrid thermal stability in accordance with their performances that were obtained following the sorption of only one metal, further confirming these interactions. In addition, SEM and EDS-SEM also proved the metal deposition of the metal after sorption.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polysaccharides6020042/s1, Figure S1: TGA thermograms of I/K-based beads after metal adsorption Vs. I/K pristine carrageenan.
Author Contributions
Conceived and designed the experiments, A.W. and O.L.-O.; investigation, A.W. and O.L.-O.; resources, A.W. and O.L.-O.; data curation, A.W. and O.L.-O.; writing—original draft preparation, A.W., O.P.-T. and O.L.-O.; writing—review and editing, A.W. and O.L.-O.; supervision, A.W., O.P.-T. and O.L.-O.; formal analysis, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A | Alginate |
| DDW | Double-distilled water |
| DTG | Differential temperature |
| D-Y | Desorption yield |
| FTIR | Fourier transform infrared spectroscopy |
| HM | Heavy metal |
| I | Iota carrageenan |
| K | Kappa carrageenan |
| LM | Light metal |
| S | Sorption selectivity |
| SEM | Scanning electron microscope |
| TGA | Thermal gravimetric analysis |
| Y | Sorption yields |
References
- Hussain, A.; Madan, S.; Madan, R. Removal of heavy metals from wastewater by adsorption. In Heavy Metals—Their Environmental Impacts and Mitigation; IntechOpen: London, UK, 2021. [Google Scholar]
- Arora, R. Adsorption of heavy metals—A review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Zamzow, M.J.; Eichbaum, B.R.; Sandgren, K.R.; Shanks, D.E. Removal of heavy metals and other cations from wastewater using zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, D. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Mathew, B.B.; Jaishankar, M.; Biju, V.G.; Beeregowda, K.N. Role of bioadsorbents in reducing toxic metals. J. Toxicol. 2016, 2016, 4369604. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, R.; Pullela, R.T.; Pundir, V. Green adsorbents for the removal of heavy metals from Wastewater: A review. Mater. Today Proc. 2022, 57, 1468–1472. [Google Scholar] [CrossRef]
- Minamisawa, M.; Minamisawa, H.; Yoshida, S.; Takai, N. Adsorption behavior of heavy metals on biomaterials. J. Agri. food chem. 2004, 52, 5606–5611. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of heavy metals by polysaccharide: A review. Polym. Plast. Technol. Mater. 2020, 59, 1770–1790. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rawat, K.P.; Bhadauria, V.; Singh, H. Recent trends in the application of modified starch in the adsorption of heavy metals from water: A review. Carbohydr. Polym. 2021, 269, 117763. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent: A review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef] [PubMed]
- Sewvandi, G.A.; Adikary, S.U. Removal of heavy metals from wastewater using chitosan. Soc. Soc. Manag. Syst. Internet J. 2011, 38, 1–6. [Google Scholar]
- Abdellatif, M.M.; Soliman, S.M.A.; El-Sayed, N.H.; Abdellatif, F.H.H. Iota-carrageenan based magnetic aerogels as an efficient adsorbent for heavy metals from aqueous solutions. J. Porous Mater. 2020, 27, 277–284. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Correlative biosorption equilibria model for a binary batch system. Chem. Eng. Sci. 2000, 55, 817–825. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Characterization and optimization of Ni and Cu sorption from aqueous solution by Chlorella vulgaris. Ecol. Eng. 2001, 18, 1–13. [Google Scholar] [CrossRef]
- Mehta, S.K.; Singh, A.; Gaur, J.P. Kinetics of adsorption and uptake of Cu2+ by Chlorella vulgaris: Influence of pH, temperature, culture age, and cations. J. Environ. Sci. Health Part A 2002, 37, 399–414. [Google Scholar] [CrossRef]
- Rai, L.C.; Gaur, J.P.; Kumar, H.D. Phycology and heavy-metal pollution. Biol. Rev. Phil. Soc. 1981, 56, 99–151. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Concurrent sorption of Ni2+ and Cu2+ by Chlorella vulgaris from a binary metal solution. Appl. Microbiol. Biotechnol. 2001, 55, 379–382. [Google Scholar] [CrossRef]
- Axtell, N.R.; Sternberg, S.P.; Claussen, K. Lead and nickel removal using Microspora and Lemna minor. Bioresour. Technol. 2003, 89, 8–41. [Google Scholar] [CrossRef]
- Adhya, J.; Cai, X.; Sayre, R.T.; Traina, S.J. Binding of aqueous cadmium by the lyophilized biomass of Chlamydomonas reinhardtti. Colloids Surf. A Physicochem. Eng. Asp. 2002, 210, 1–11. [Google Scholar] [CrossRef]
- Allen, S.J.; Brown, P.A. Isotherm analyses for single component and multi-component metal sorption onto lignite. J. Chem. Technol. Biotechnol. 1995, 62, 17–24. [Google Scholar] [CrossRef]
- Chang, J.; Hong, J. Estimation of kinetics of mercury detoxification from low-inoculum batch cultures of Pseudomonas aeruginosa PU21 (Rip64). Biotechnol. Bioeng. 1994, 42, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Chiban, M.; Soudani, A.; Sinan, F.; Persin, M. Single, binary and multi-component adsorption of some anions and heavy metals on environmentally friendly Carpobrotus edulis plant. Colloids Surf. B Biointerfaces 2011, 82, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Albayati, T.M.; Sabri, A.A.; Abed, D.B. Adsorption of binary and multi heavy metals ions from aqueous solution by amine functionalized SBA-15 mesoporous adsorbent in a batch system. Desalination Water Treat. 2009, 151, 315–321. [Google Scholar] [CrossRef]
- Visa, M.; Bogatu, C.; Duta, A. Simultaneous adsorption of dyes and heavy metals from multicomponent solutions using fly ash. Appl. Surf. Sci. 2010, 256, 5486–5491. [Google Scholar] [CrossRef]
- Huang, S.; Jin, S.; Wang, Y.; Liu, J.; Yu, J.; Liu, D.; Chi, R. Selective adsorption of heavy metal ions from aqueous solution by modified bagasse. Chem. Ecol. 2020, 36, 839–854. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, F.; Pang, Z.; Yang, G. Efficient and selective adsorption of multi-metal ions using sulfonated cellulose as adsorbent. Carbohydr. Polym. 2016, 151, 230–236. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Yanay, C.; Paz-Tal, O.; Wolfson, A. Iota-carrageenan as sustainable bio-adsorbent for the removal of europium ions from aqueous solutions. Mater. Today Commun. 2022, 32, 104111. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Yanay, C.; Paz-Tal, O.; Wolfson, A. Red Algae Sulfur-Based Polysaccharides as Bioadsorbents for Europium Removal from Aqueous Solutions. J. Polym. Environ. 2023, 31, 2321–2333. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Nagar, S.; Paz-Tal, O.; Wolfson, A. Cation Effect in Polysaccharide-Based Hydrogel Beads Produced for Europium Adsorption from Aqueous Solutions. J. Polym. Environ. 2024, 32, 4017–4034. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Nagar, S.; Paz-Tal, O.; Wolfson, A. Iota-carrageenan as a regenerating system for Eu3+ recovery: Adsorption/desorption cycles. Environ. Technol. 2024, 46, 1730–1743. [Google Scholar] [CrossRef] [PubMed]
- Levy-Ontman, O.; Paz-Tal, O.; Alfi, Y.; Wolfson, A. Biosorption of uranyl ions from aqueous solutions by soluble renewable polysaccharides. RSC Adv. 2023, 13, 35831–35840. [Google Scholar] [CrossRef] [PubMed]
- Levy-Ontman, O.; Yanay, C.; Alfi, Y.; Paz-Tal, O.; Wolfson, A. Selective Sorption of Heavy Metals by Renewable Polysaccharides. Polymers 2023, 15, 4457. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Park, C.M.; Kim, I.K.; Lee, J.M. Studies on the Spectrophotometric Determination, Electrochemical Behavior of Heavy Lanthanide ions in Nonaqueous System and Heavy Chelates Complexes with Bidendate Ligands (II) Electrochemical Behavior of Heavy Lanthanide Ions in Acetonitrile. J. Korean Chem. Soc. 1993, 37, 483–490. [Google Scholar]
- Negrea, A.; Gabor, A.; Davidescu, C.M.; Ciopec, M.; Negrea, P.; Duteanu, N.; Barbulescu, A. Rare earth elements removal from water using natural polymers. Sci. Rep. 2018, 8, 316. [Google Scholar] [CrossRef]
- Young, R.N.; McDonald, G.; Randall, J.M. Effect of light metal ions on the sorption of heavy metal ions on natural polymers. J. Appl. Polym. Sci. 1979, 23, 1027–1035. [Google Scholar] [CrossRef]
- Pawlik, B.; Skowroński, T. Transport and toxicity of cadmium: Its regulation in the cyanobacterium Synechocystis aquatilis. Environ. Exp. Bot. 1994, 34, 225–233. [Google Scholar] [CrossRef]
- Greene, B.; McPherson, R.; Darnall, D. Algal sorbents for selective metal ion recovery. In Metals Speciation, Separation, and Recovery; Lewis Publishers: Chelsea, MI, USA, 1987; pp. 315–332. [Google Scholar]
- Ramelow, G.J.; Fralick, D.; Zhao, Y.F. Factors affecting the uptake of aqueous metal ions by dried seaweed biomass. Microbios 1992, 72, 81–93. [Google Scholar]
- Mehta, S.K.; Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Fuks, L.; Herdzik-Koniecko, I.; Polkowska-Motrenko, H.; Oszczak, A. Novel procedure for removal of the radioactive metals from aqueous wastes by the magnetic calcium alginate. Int. J. Environ. Sci. Technol. 2018, 15, 2657–2668. [Google Scholar] [CrossRef]
- Al-Wakeel, K.Z.; Abd El Monem, H.; Khalil, M.M. Removal of divalent manganese from aqueous solution using glycine modified chitosan resin. J. Environ. Chem. Eng. 2015, 3, 179–186. [Google Scholar] [CrossRef]
- Esposito, A.; Reverberi, A.P. Copper adsorption on calcium alginate beads: Equilibrium pH-related models. Hydrometallurgy 2002, 65, 43–57. [Google Scholar]
- Chatterjee, A.; Abraham, J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef]
- Tan, L.; Bai, X.; Yao, R.; Fu, Z.; Wang, J.; Wang, Y.; Lin, T.; Hao, Y.; Yang, H.; Sai, H. A sulfonic-functionalized cellulose adsorbent for the rapid removal of cerium (III) from aqueous solutions. Fibers Polym. 2024, 25, 1713–1725. [Google Scholar] [CrossRef]
- Serunting, M.A.; Rusnadi, R.; Setyorini, D.A.; Ramadan, B.S. An effective cerium (III) ions removal method using sodium alginate-coated magnetite (Alg-Fe3O4) nanoparticles. J. Water Supply Res. Technol. AQUA 2018, 67, 754–765. [Google Scholar] [CrossRef]
- Chaibou Yacouba, A.R.; Oral, A.E.; Sert, S.; Kaptanoglu, I.G.; Natatou, I.; Yusan, S.; Aytas, S. Removal of lanthanum and cerium from aqueous solution using chitosan-functionalized magnetite-pectin. Discov. Water 2024, 4, 1. [Google Scholar] [CrossRef]
- Khotimchenko, M.Y.; Kolenchenko, E.A.; Khotimchenko, Y.S.; Khozhaenko, E.V.; Kovalev, V.V. Cerium binding activity of different pectin compounds in aqueous solutions. Colloids Surf. B Biointerfaces 2010, 77, 104–110. [Google Scholar] [CrossRef]
- Texier, A.C.; Andrès, Y.; Le Cloirec, P. Selective biosorption of lanthanide (La, Eu, Yb) ions by Pseudomonas aeruginosa. Environ. Sci. Technol. 1999, 33, 489–495. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, Y.; Zhu, Z. Biosorption Characteristic and Cytoprotective Effect of Pb2+, Cu2+ and Cd2+ by a Novel Polysaccharide from Zingiber strioatum. Molecules 2022, 27, 8036. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Brezuleanu, C.O.; Ungureanu, V.I.; Chiruță, C.; Rotaru, R.; Tofanica, B.M.; Popa, V.I.; Jităreanu, D.C. Comparison Adsorption of Cd (II) onto Lignin and Polysaccharide-Based Polymers. Polymers 2023, 15, 3794. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater. 2002, 93, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef] [PubMed]
- Chitra, R.; Sathya, P.; Selvasekarapandian, S.; Meyvel, S. Synthesis and characterization of iota-carrageenan biopolymer electrolyte with lithium perchlorate and succinonitrile (plasticizer). Polym. Bull. 2020, 77, 1555–1579. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Vincekovic, M.; Pustak, A.; Tusek-Bozic, L.; Liu, F.; Ungar, G.; Bujan, M.; Smit, I.; Filipovic-Vincekovic, N. Structural and thermal study of mesomorphic dodecylammonium carrageenates. J. Colloid Interf. Sci. 2010, 341, 117–123. [Google Scholar] [CrossRef]
- Ma, S.; Chen, L.; Liu, X.; Li, D.; Ye, N.; Wang, L. Thermal behavior of carrageenan: Kinetic and characteristic studies. Int. J. Green Energy 2012, 9, 13–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).