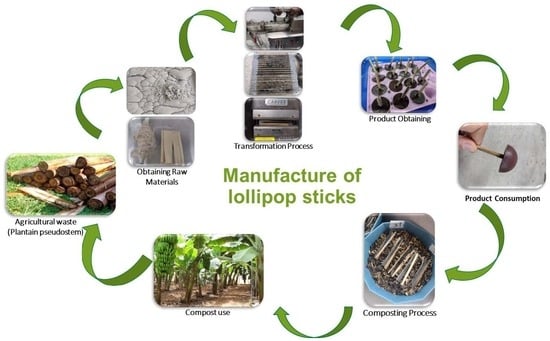
Fully Biobased Composite from Lignocellulosic Plantain Waste with Potential Use in the Manufacture of Lollipop Sticks
Abstract
1. Introduction
2. Materials and Methods
2.1. Plantain Pulp Flour and Starch
2.2. Plantain Peel Cake (PPC)
2.3. Obtaining Yarn from the Lignocellulosic Fibers of Plantain Pseudostems
2.4. Plantain Pseudostem Treated Sheaths
2.5. Glycerin
2.6. Additional Materials
2.7. Development of Biobased Composite Material
2.8. Preparation of Confectionery and Incorporation of the Biobased Lollipop Stick
2.9. Physicochemical and Structural Characterization
2.9.1. Migration
2.9.2. Contact Angle
2.9.3. FT-IR Spectrophotometry with ATR
2.9.4. Scanning Electron Microscopy (SEM)
2.9.5. Mechanical Properties
Tensile Test
Flexural Test
2.9.6. Dynamic–Mechanical Analysis of the Lollipop Stick
3. Results and Discussions
3.1. Global Migration
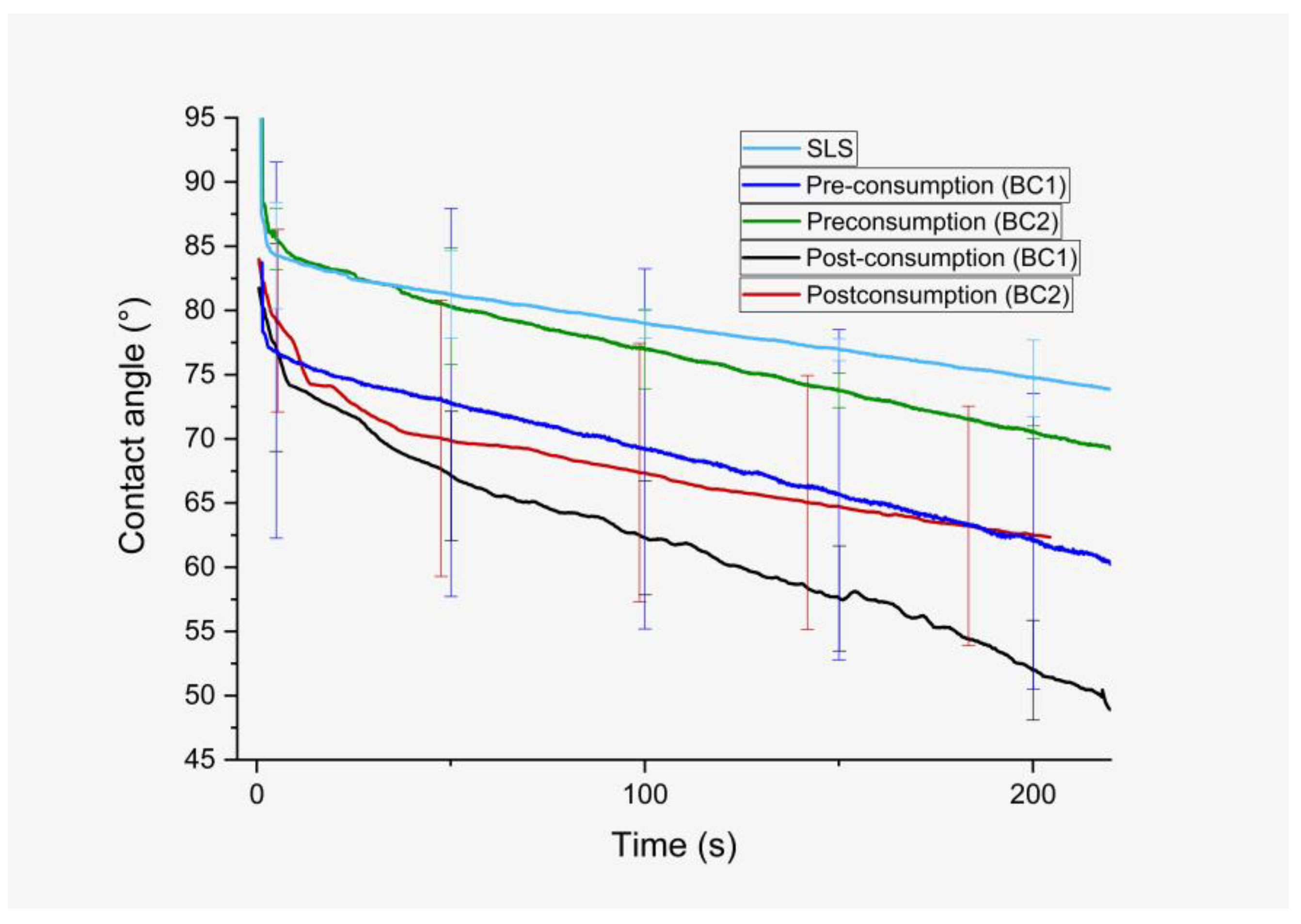
3.2. Contact Angle
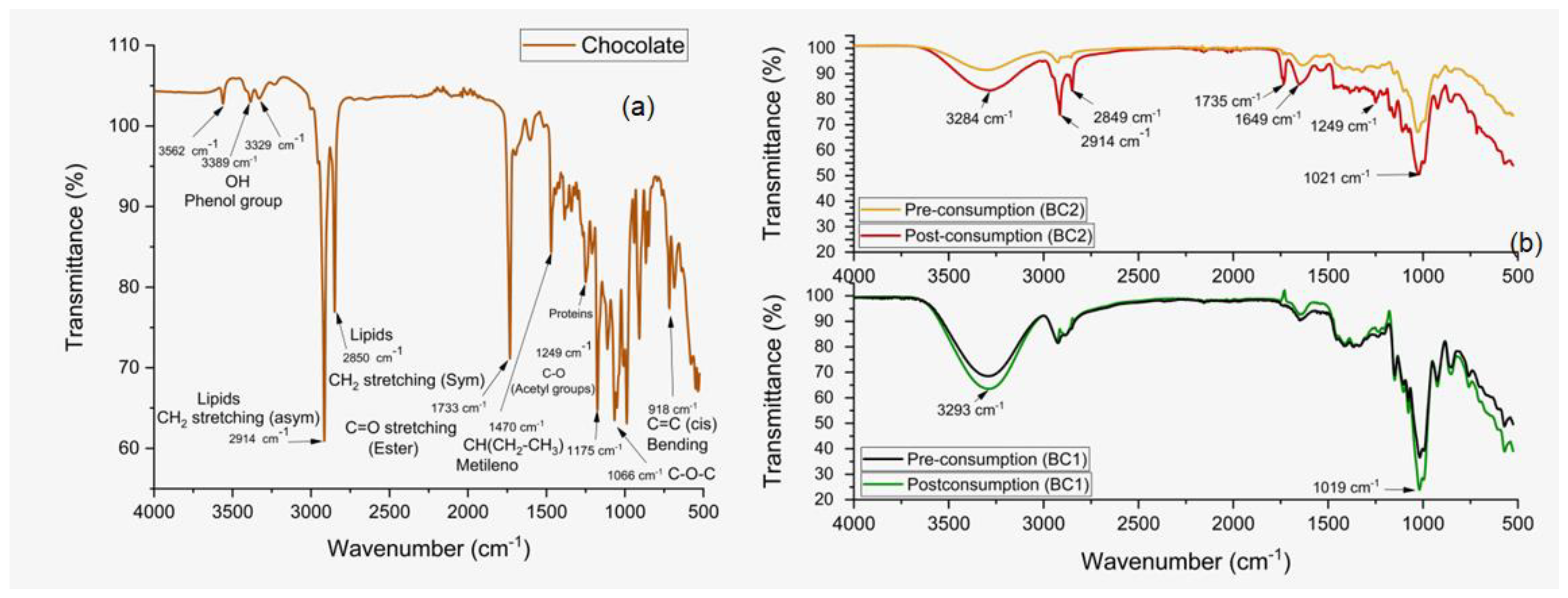
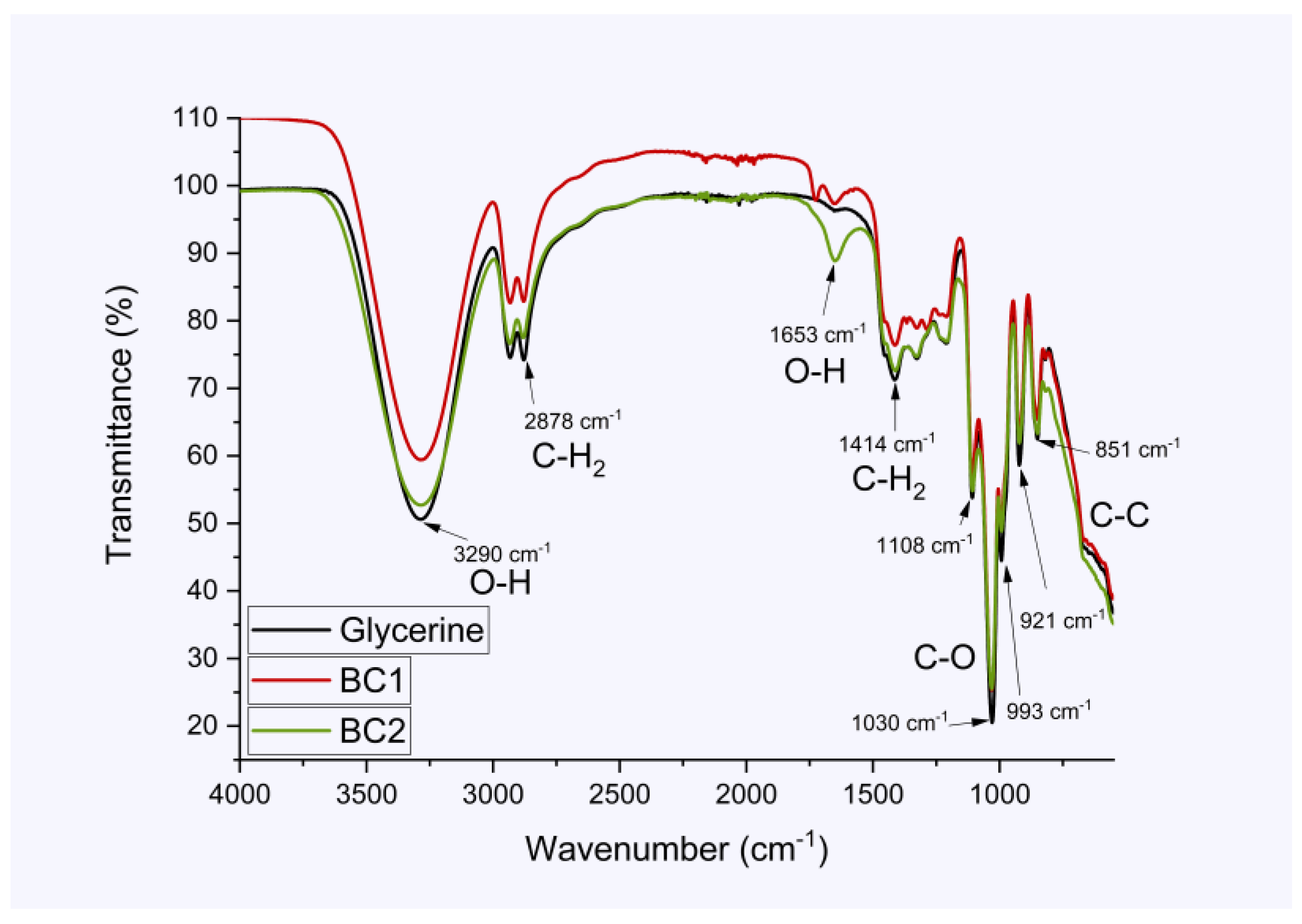
3.3. Fourier Transform Infrared Spectroscopy (FT-IR-ATR)
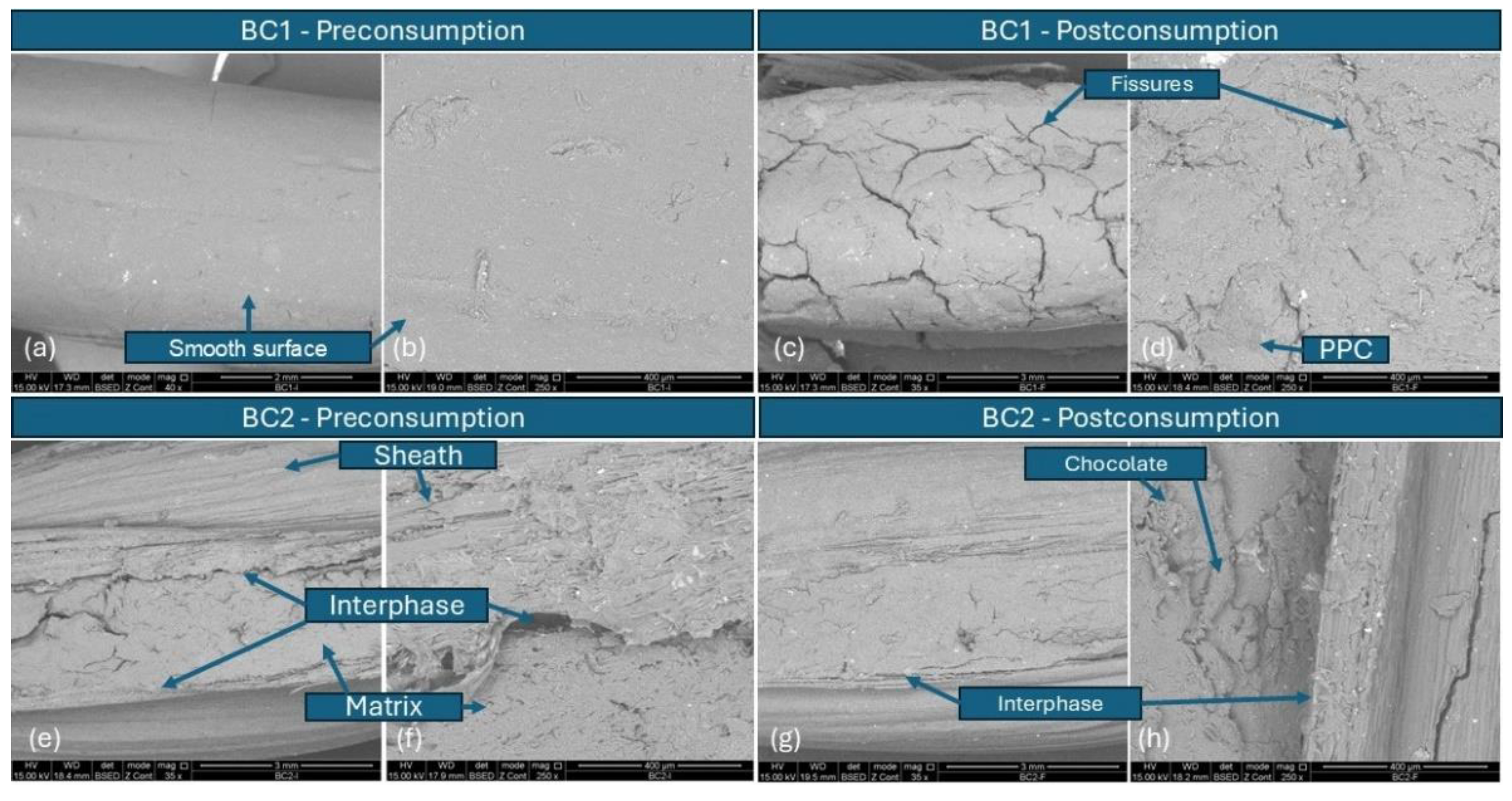
3.4. Scanning Electron Microscopy (SEM)
3.5. Tensile Test
3.6. Flexural Test
3.7. Dynamic Mechanical Analysis (DMA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Montesinos, F.; Oliva Ramírez, M.; González-Leal, J.M.; Carrizo, D.; Anfuso, G. Characterization of plastic beach litter by Raman spectroscopy in South-western Spain. Sci. Total Environ. 2020, 744, 140890. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mousania, Z.; Angulo, A.V.; Poon, J.P.H.; Atkinson, J.D. Quantifying the environmental impact of transportation for plastic film packaging end-of-life: Landfill, incineration, physical recycling, or chemical recycling. Resour. Conserv. Recycl. 2024, 207, 107681. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, A.; Ozbakkaloglu, T. A Review of Natural Fiber Composites: Properties, Modification and Processing Techniques, Characterization, Applications. J. Mater. Sci. 2020, 55, 829–892. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Chang, B.P.; Misra, M. Studies on durability of sustainable biobased composites: A review. RSC Adv. 2020, 10, 17955–17999. [Google Scholar] [CrossRef]
- Wagner, T.P. Reducing single-use plastic shopping bags in the USA. Waste Manag. 2017, 70, 3–12. [Google Scholar] [CrossRef]
- Lightfoot, S.J.; Grant, T.; Boyden, A.; McAlister, S. Single-Use Synthetic Plastic and Natural Fibre Anaesthetic Drug Trays: A Comparative Life Cycle Assessment of Environmental Impacts. Br. J. Anaesth. 2024, 133, 1465–1477. [Google Scholar] [CrossRef]
- Consoli, P.; Costa, V.; Sciutteri, V.; Malara, D.; Pedà, C.; Figurella, F.; Campbell, I.; Deery, E.; Romeo, T.; Andaloro, F. Synthetic polymers: A global threat to aquatic benthic environments. J. Hazard. Mater. 2024, 474, 134848. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Luthra, P.; Kapur, G.S.; Ramakumar, S.S.V. Biodegradable/Bio-plastics: Myths and Realities. J. Polym. Environ. 2021, 29, 3079–3104. [Google Scholar] [CrossRef]
- Holguín-Posso, A.M.; Macías-Silva, J.C.; Castañeda-Niño, J.P.; Mina-Hernandez, J.H.; Fajardo Cabrera de Lima, L.D.P. Characterization and Implementation of Cocoa Pod Husk as a Reinforcing Agent to Obtain Thermoplastic Starches and Bio-Based Composite Materials. Polymers 2024, 16, 1608. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural fibers, biopolymers, and biocomposites. In Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Mo, X.; Qi, X.; Zhong, Y.; Li, R.; Mo, C. Preparation and Properties of Tapioca Starch-Banana Fiber Composites Modified with Magnesium Hydroxide. Adv. Mat. Res. 2011, 194–196, 1707–1710. [Google Scholar] [CrossRef]
- Rodríguez-Soto, K.; Piñeros-Castro, N.; Ortega-Toro, R. Laminated Composites Reinforced with Chemically Modified Sheets-Stalk of Musa Cavendish. Rev. Mex. Ing. Quim. 2019, 18, 749–758. [Google Scholar] [CrossRef]
- Migneault, S.; Koubaa, A.; Erchiqui, F.; Chaala, A.; Englund, K.; Wolcott, M.P. Effects of Processing Method and Fiber Size on the Structure and Properties of Wood-Plastic Composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 80–85. [Google Scholar] [CrossRef]
- De La Torre-Gutierrez, L.; Torruco-Uco, J.G.; Castellanos-Ruelas, A.; Chel-Guerrero, L.A.; Betancur-Ancona, D. Isolation and structure investigations of square banana (Musa balbisiana) starch. Starch/Staerke 2007, 59, 326–333. [Google Scholar] [CrossRef]
- Ferreira-Villadiego, J.; García-Echeverri, J.; Vidal, M.V.; Pasqualino, J.; Meza-Castellar, P.; Lambis-Miranda, H.A. Chemical modification and characterization of starch derived from plantain (Musa paradisiaca) peel waste, as a source of biodegradable material. Chem. Eng. Trans. 2018, 65, 763–768. [Google Scholar]
- Castañeda-Niño, J.P.; Mina-Hernandez, J.H.; Solanilla-Duque, J.F. Potential of Plantain Pseudostems (Musa AAB Simmonds) for Developing Biobased Composite Materials. Polymers 2024, 16, 1357. [Google Scholar] [CrossRef]
- Sato, S.; Hondoh, H.; Ueno, S. Fat Bloom Caused by Partial De-Oiling on Chocolate Surfaces after High-Temperature Exposure. J. Am. Oil Chem. Soc. 2021, 98, 269–280. [Google Scholar] [CrossRef]
- European Commission. Comission Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Chongcharoenyanon, B.; Sane, A. Effect of overall migration on tensile properties of biobased plastics containing polylactic acid, thermoplastic starch and zeolite. Agric. Nat. Resour. 2021, 55, 611–617. [Google Scholar]
- Tsochatzis, E.D.; Vidal, N.P.; Bai, W.; Diamantidou, D.; Theodoridis, G.; Martinez, M.M. Untargeted screening and in silico toxicity assessment of semi- and non-volatile compounds migrating from polysaccharide-based food contact materials. Food Chem. 2023, 425, 136499. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Migration of odorous compounds from adhesives used in market samples of food packaging materials by chromatography olfactometry and mass spectrometry (GC-O-MS). Food Chem. 2014, 145, 237–244. [Google Scholar] [CrossRef]
- Zhou, R.; Geng, J.; Jiang, J.; Lin, L.; Zhang, J.; Yang, Y.; Wang, X.; Niu, Y.; Shao, B. Occurrence and migration of organophosphite and organophosphate esters into food simulants from single-use food packaging in China. Environ. Pollut. 2023, 330, 121782. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, X.; Du, Y.; Wang, D. Effect of water-soluble polymers on the performance of dust-suppression foams: Wettability, surface viscosity and stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 92–98. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). In Standard Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis (E1252); ASTM International: West Conshohocken, PA, USA, 2021; pp. 1–13. [CrossRef]
- Lipsa, R.; Tudorachi, N.; Darie-Nita, R.N.; Oprică, L.; Vasile, C.; Chiriac, A. Biodegradation of poly(lactic acid) and some of its based systems with Trichoderma viride. Int. J. Biol. Macromol. 2016, 88, 515–526. [Google Scholar] [CrossRef]
- Dorigato, A.; Fredi, G.; Pegoretti, A. Thermo-mechanical behavior of novel wood laminae-thermoplastic starch biodegradable composites with thermal energy storage/release capability. Front. Mater. 2019, 6, 76. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2014.
- Mina-Hernandez, J.H. Effect of the incorporation of polycaprolactone (PCL) on the retrogradation of binary blends with cassava thermoplastic starch (TPS). Polymers 2021, 13, 38. [Google Scholar] [CrossRef]
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM: West Conshohocken, PA, USA, 2017.
- Heckadka, S.S.; Kini, M.V.; Ballambat, R.P.; Beloor, S.S.; Udupi, S.R.; Kini, U.A. Flexural Strength Analysis of Starch Based Biodegradable Composite Using Areca Frond Fibre Reinforcement. Int. J. Manuf. Eng. 2014, 2014, 769012. [Google Scholar] [CrossRef]
- Muñoz-Velez, M.F.; Hidalgo-Salazar, M.A.; Mina-Hernandez, J.H. Effect of content and surface modification of fique fibers on the properties of a low-density polyethylene (LDPE)-Al/fique composite. Polymers 2018, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, J.; Markowski, M.; Barczewski, M. The Use of Nanoscale Montmorillonite (MMT) as Reinforcement for Polylactide Acid (PLA) Prepared by Fused Deposition Modeling (FDM)—Comparative Study with Biocarbon and Talc Fillers. Materials 2022, 15, 5205. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Misra, M.; Mohanty, A.K.; Williams, K.; Mielewski, D.F. A study on biocomposites from recycled newspaper fiber and poly(lactic acid). Ind. Eng. Chem. Res. 2005, 44, 5593–5601. [Google Scholar] [CrossRef]
- Velásquez, E.; López-de-Dicastillo, C.; Patiño Vidal, C.; Copello, G.; Pérez, C.J.; Guarda, A.; José Galotto, M. Upgrading properties and circularity of the recycled flexible polypropylene by developing composites with an optimal combination of a fumed silica and maleated polypropylene copolymer: Influence of the addition of copolymer, type of fumed silica and the silica/copolymer ratio on packaging properties. Polym. Test. 2024, 138, 108556. [Google Scholar]
- Chen, W.; Wang, H.; Lan, W.; Zhang, A.; Liu, C. Fabrication of sugarcane bagasse ester-based porous nanofiber membrane by electrospinning for efficient oil-water separation. Ind. Crops Prod. 2022, 187, 115480. [Google Scholar] [CrossRef]
- Karatay, S.E.; Demiray, E.; Dönmez, G. Usage potential of apple and carrot pomaces as raw materials for newly isolated yeast lipid-based biodiesel production. Biomass Convers. Biorefinery 2022, 12, 4773–4783. [Google Scholar] [CrossRef]
- Meile, K.; Dobele, G.; Iljina, N.; Zhurinsh, A.; Jurkjane, V. Lignocellulose pyrolysis by-products as an underestimated source of chemicals: Separation and characterisation. Biomass Convers. Biorefinery 2023, 13, 5709–5720. [Google Scholar] [CrossRef]
- Solá-Pérez, J.E.; Saldarriaga-Noreña, H.; Murillo-Tovar, M.; Ronderos-Lara, G.; López-Martínez, V.G. Chemical Characterization of N-Hexane Extract Obtained from Lignocellulose Residual Contained in Agroindustrial Wastes. J. Agric. Chem. Environ. 2018, 7, 94–104. [Google Scholar] [CrossRef][Green Version]
- Pandiselvam, R.; Harikrishnan, M.P.; Khanashyam, A.C.; Basil, M.; Anirudh, M.; Manikantan, M.R.; Kothakota, A. Development and characterization of gelatinized starch doped microcellulose paper from tender coconut (Cocos nucifera L) husk. Process Saf. Environ. Prot. 2024, 184, 615–623. [Google Scholar] [CrossRef]
- Stepczyńska, M.; Rytlewski, P.; Moraczewski, K.; Pawłowska, A.; Karasiewicz, T. Novel Biocomposite of Starch and Flax Fiber Modified with Tannic Acid with Biocidal Properties. Polymers 2024, 16, 1108. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.G.; Stasiak, D.M.; Mleko, S.; Terpiłowski, K.; Łyszczek, R.J.; Tomasevic, I.B.; Tomczyńska-Mleko, M. Development and physicochemical properties of reformulated, high-protein, untempered sugar-free dark chocolates with addition of whey protein isolate and erythritol. Int. Dairy J. 2022, 134, 105450. [Google Scholar] [CrossRef]
- Averous, L.; Moro, L.; Dole, P.; Fringant, C. Properties of thermoplastic blends: Starch-polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Batista, N.N.; de Andrade, D.P.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res. Int. 2016, 90, 313–319. [Google Scholar] [CrossRef]
- Cerit, İ.; Demirkol, O.; Avcı, A.; Arkan, B.S. Phenolic content and oxidative stability of chocolates produced with roasted and unroasted cocoa beans. Food Sci. Technol. Int. 2024, 30, 450–461. [Google Scholar] [CrossRef]
- Che Man, Y.B.; Syahariza, Z.A.; Mirghani, M.E.S.; Jinap, S.; Bakar, J. Analysis of potential lard adulteration in chocolate and chocolate products using Fourier transform infrared spectroscopy. Food Chem. 2005, 90, 815–819. [Google Scholar] [CrossRef]
- Deus, V.L.; Resende, L.M.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. FTIR and PLS-regression in the evaluation of bioactive amines, total phenolic compounds and antioxidant potential of dark chocolates. Food Chem. 2021, 357, 129754. [Google Scholar] [CrossRef]
- Quispe-Chambilla, L.; Pumacahua-Ramos, A.; Choque-Quispe, D.; Curro-Pérez, F.; Carrión-Sánchez, H.M.; Peralta-Guevara, D.E.; Masco-Arriola, M.L.; Palomino-Rincón, H.; Ligarda-Samanez, C.A. Rheological and Functional Properties of Dark Chocolate with Partial Substitution of Peanuts and Sacha Inchi. Foods 2022, 11, 1142. [Google Scholar] [CrossRef]
- Gáspár, M.; Benko, Z.; Dogossy, G.; Réczey, K.; Czigány, T. Reducing water absorption in compostable starch-based plastics. Polym. Degrad. Stab. 2005, 90, 563–569. [Google Scholar] [CrossRef]
- Bodirlau, R.; Teaca, C.A.; Spiridon, I. Influence of natural fillers on the properties of starch-based biocomposite films. Compos. Part B Eng. 2013, 44, 575–583. [Google Scholar] [CrossRef]
- Villanueva, E.; Glorio-Paulet, P.; Giusti, M.M.; Sigurdson, G.T.; Yao, S.; Rodríguez-Saona, L.E. Screening for pesticide residues in cocoa (Theobroma cacao L.) by portable infrared spectroscopy. Talanta 2023, 257, 124386. [Google Scholar] [CrossRef]
- Arofai, T.; Gunawan, S.; Purwanto, M. Separation and Purification Residual Salt from Glycerine Pitch from Waste of Refined Glycerine Production. BIO Web Conf. 2024, 98, 06008. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Suniaga, J.; Monsalve, A.; García, N.L. Influence of beet flour on the relationship surface-properties of edible and intelligent films made from native and modified plantain flour. Food Hydrocoll. 2016, 54, 234–244. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccolli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Development of quaternary nanocomposites made up of cassava starch, cocoa butter, lemongrass essential oil nanoemulsion, and brewery spent grain fibers. J. Food Sci. 2021, 86, 1979–1996. [Google Scholar] [CrossRef]
- Do, T.A.L.; Vieira, J.; Hargreaves, J.M.; Mitchell, J.R.; Wolf, B. Structural characteristics of cocoa particles and their effect on the viscosity of reduced fat chocolate. LWT 2011, 44, 1207–1211. [Google Scholar] [CrossRef]
- James, B.J.; Smith, B.G. Surface structure and composition of fresh and bloomed chocolate analysed using X-ray photoelectron spectroscopy, cryo-scanning electron microscopy and environmental scanning electron microscopy. LWT 2009, 42, 929–937. [Google Scholar] [CrossRef]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. Improving heat and fat bloom stabilities of “dark chocolates” by addition of mango kernel fat-based chocolate fats. J. Food Eng. 2019, 246, 33–41. [Google Scholar] [CrossRef]
- Kinta, Y.; Hatta, T. Composition, structure, and color of fat bloom due to the partial liquefaction of fat in dark chocolate. J. Am. Oil Chem. Soc. 2007, 84, 107–115. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Shan, L.; Jin, Q.; Wang, X.; Li, L. Blooming in cocoa butter substitutes based compound chocolate: Investigations on composition, morphology and melting behavior. J. Am. Oil Chem. Soc. 2010, 87, 1137–1143. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef]
- Wongpajan, R.; Mathurosemontri, S.; Takematsu, R.; Xu, H.Y.; Uawongsuwan, P.; Thumsorn, S.; Hamada, H. Interfacial Shear Strength of Glass Fiber Reinforced Polymer Composites by the Modified Rule of Mixture and Kelly-Tyson Model. Energy Procedia 2016, 89, 328–334. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Banana and plantain fiber-reinforced polymer composites. J. Polym. Eng. 2019, 39, 597–611. [Google Scholar] [CrossRef]
- Yee-Chang, S.; Ismail, H.; Ahsan, Q. MAH on kenaf-filled composites. BioResources 2012, 7. [Google Scholar]
- Venegas, R.; Torres, A.; Rueda, A.M.; Morales, M.A.; Arias, M.J.; Porras, A. Development and Characterization of Plantain (Musa paradisiaca) Flour-Based Biopolymer Films Reinforced with Plantain Fibers. Polymers 2022, 14, 748. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W. Preparation and properties of lignocellulosic fiber/CaCO3/thermoplastic starch composites. Carbohydr. Polym. 2019, 211, 204–208. [Google Scholar] [CrossRef]
- Müssig, J. Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 9780470660324. [Google Scholar]
- Sepe, M. Dynamic Mechanical Analysis for Plastic Engineering; Plastic Design Library; PDL Handbook Series; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Bashir, M.A. Use of Dynamic Mechanical Analysis (DMA) for Characterizing Interfacial Interactions in Filled Polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K.; Drzal, L.T.; Pourboghrat, F.; Misra, M. Renewable resource-based green composites from recycled cellulose fiber and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic. Biomacromolecules 2006, 7, 2044–2051. [Google Scholar] [CrossRef]
- Biswal, M.; Mohanty, S.; Nayak, S.K. Mechanical, thermal and dynamic-mechanical behavior of banana fiber reinforced polypropylene nanocomposites. Polym. Compos. 2011, 32, 1190–1201. [Google Scholar] [CrossRef]
- Babaee, M.; Jonoobi, M.; Hamzeh, Y.; Ashori, A. Biodegradability and mechanical properties of reinforced starch nanocomposites using cellulose nanofibers. Carbohydr. Polym. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- De Freitas, R.R.M.; Do Carmo, K.P.; De Souza Rodrigues, J.; De Lima, V.H.; Osmari da Silva, J.; Botaro, V.R. Influence of alkaline treatment on sisal fibre applied as reinforcement agent in composites of corn starch and cellulose acetate matrices. Plast. Rubber Compos. 2021, 50, 9–17. [Google Scholar] [CrossRef]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Preparation and characterization of thermoplastic starch and cellulose nanofibers as green nanocomposites: Extrusion processing. Int. J. Biol. Macromol. 2018, 112, 442–447. [Google Scholar] [CrossRef]
- Shanks, R.A.; Hodzic, A.; Wong, S. Thermoplastic biopolyester natural fiber composites. J. Appl. Polym. Sci. 2003, 91, 2114–2121. [Google Scholar] [CrossRef]
- Nomadolo, N.; Mtibe, A.; Ofosu, O.; Mekoa, C.; Letwaba, J.; Muniyasamy, S. The Effect of Mechanical Recycling on the Thermal, Mechanical, and Chemical Properties of Poly (Butylene Adipate-Co-Terephthalate) (PBAT), Poly (Butylene Succinate) (PBS), Poly (Lactic Acid) (PLA), PBAT-PBS Blend and PBAT-TPS Biocomposite. J. Polym. Environ. 2024, 32, 2644–2659. [Google Scholar] [CrossRef]
- Lila, M.K.; Shukla, K.; Komal, U.K.; Singh, I. Accelerated thermal ageing behaviour of bagasse fibers reinforced Poly (Lactic Acid) based biocomposites. Compos. Part B Eng. 2019, 156, 121–127. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Åkesson, D.; Rajan, R.; Periyasamy, A.P.; Skrifvars, M. Mechanical performance of biofibers and their corresponding composites. Mech. Phys. Test. Biocomposites Fibre-Reinf. Compos. Hybrid. Compos. 2019, 259–292. [Google Scholar]
- Castañeda-Niño, J.P.; Mina-Hernandez, J.H.; Valadez-Gonzalez, A. Potential uses of musaceae wastes: Case of application in the development of bio-based composites. Polymers 2021, 13, 1844. [Google Scholar] [CrossRef]






| BCM | Plantain Peel Cake (%) | Glycerin (%) | Native Yarn (%) | Treated Sheath (%) | Polypropylene (%) |
|---|---|---|---|---|---|
| BM | 7.5 | 35.0 | 0 | 0 | 0 |
| BC1 | 6.8 | 34.4 | 1.7 | 0 | 0 |
| BC2 | 4.4 | 23.6 | 0 | 32.58 | 0 |
| SLS | 0 | 0 | 0 | 0 | 100 |
| Treatment | Simulants | Global Migration (µg/dm2) |
|---|---|---|
| SLS | Water | 0 |
| Hexane | 120.97 ± 25.18 | |
| Ehtanol—50% | 86.51 ± 65.77 | |
| BC1 | Water | N/A |
| Hexane | 52.45 ± 41.17 | |
| Ethanol—50% | 4344.96 ±1640.15 | |
| BC2 | Water | N/A |
| Hexane | 92.55 ± 66.65 | |
| Ethanol—50% | 2369.50 ± 1347.34 |
| Treatment | Chocolate Candy Consumption | Initial Contact Angle (°) | Slope |
|---|---|---|---|
| SLS | Before | 83.86 ± 4.23 | –0.055 |
| BC1 | Before | 75.21 ± 15.40 | –0.074 |
| After | 74.14 ± 7.80 | –0.109 | |
| BC2 | Before | 83.20 ± 4.04 | –0.066 |
| After | 74.43 ± 10.41 | –0.065 |
| Treatment | Candy Consumption | σmax (MPa) | E (MPa) | ε (%) | σFmax (MPa) | εF (%) |
|---|---|---|---|---|---|---|
| SLS | Before | 20.61 ± 1.91 | 116.42 ± 20.22 | 1001.60 ± 314.19 | 32.66 ± 7.11 | 7.83 ± 1.32 |
| BC1 | Before | 7.34 ± 3.59 | 122.34 ± 55.12 | 21.41 ± 12.54 | 10.16 ± 2.04 | 27.78 ± 8.05 |
| After | 3.25 ± 1.29 | 23.50 ± 8.11 | 43.70 ± 9.17 | 6.01 ± 3.97 | 22.09 ± 2.96 | |
| BC2 | Before | 12.62 ± 1.42 | 110.28 ± 19,93 | 26.11 ± 4.63 | 19.07 ± 7.30 | 5.47 ± 2.62 |
| After | 11.76 ± 2.70 | 37.21 ± 16.64 | 39.68 ± 12.10 | 10.11 ± 3.26 | 23.20 ± 7.10 |
| MCB | Candy Consumption | E′ (GPa) | Tan Delta | |||
|---|---|---|---|---|---|---|
| 30 °C | 50 °C | 100 °C | T° | Intensity | ||
| BM | Pre | 0.70 | 0.20 | 0.07 | 51.33 | 0.41 |
| Post | 0.24 | 0.14 | 0.07 | N.R. | N.R. | |
| BC1 | Pre | 2.75 | 0.90 | 0.12 | 61.71 | 0.37 |
| Post | 0.51 | 0.21 | 0.06 | 44.80 | 0.35 | |
| BC2 | Pre | 4.97 | 3.03 | 0.91 | 66.90 | 0.29 |
| Post | 1.65 | 1.08 | 0.55 | 52.64 | 0.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Niño, J.P.; Ospina-Aguilar, L.G.; Zapata-Diaz, Y.C.; Zuluaga-Gallego, R.O.; Serna-Jiménez, J.A.; Solanilla-Duque, J.F.; Pérez-Pacheco, E.; Mina-Hernandez, J.H. Fully Biobased Composite from Lignocellulosic Plantain Waste with Potential Use in the Manufacture of Lollipop Sticks. Polysaccharides 2025, 6, 41. https://doi.org/10.3390/polysaccharides6020041
Castañeda-Niño JP, Ospina-Aguilar LG, Zapata-Diaz YC, Zuluaga-Gallego RO, Serna-Jiménez JA, Solanilla-Duque JF, Pérez-Pacheco E, Mina-Hernandez JH. Fully Biobased Composite from Lignocellulosic Plantain Waste with Potential Use in the Manufacture of Lollipop Sticks. Polysaccharides. 2025; 6(2):41. https://doi.org/10.3390/polysaccharides6020041
Chicago/Turabian StyleCastañeda-Niño, Juan Pablo, Lina Gisselth Ospina-Aguilar, Yean Carlos Zapata-Diaz, Robin Octavio Zuluaga-Gallego, Johanna Andrea Serna-Jiménez, José Fernando Solanilla-Duque, Emilio Pérez-Pacheco, and Jose Herminsul Mina-Hernandez. 2025. "Fully Biobased Composite from Lignocellulosic Plantain Waste with Potential Use in the Manufacture of Lollipop Sticks" Polysaccharides 6, no. 2: 41. https://doi.org/10.3390/polysaccharides6020041
APA StyleCastañeda-Niño, J. P., Ospina-Aguilar, L. G., Zapata-Diaz, Y. C., Zuluaga-Gallego, R. O., Serna-Jiménez, J. A., Solanilla-Duque, J. F., Pérez-Pacheco, E., & Mina-Hernandez, J. H. (2025). Fully Biobased Composite from Lignocellulosic Plantain Waste with Potential Use in the Manufacture of Lollipop Sticks. Polysaccharides, 6(2), 41. https://doi.org/10.3390/polysaccharides6020041