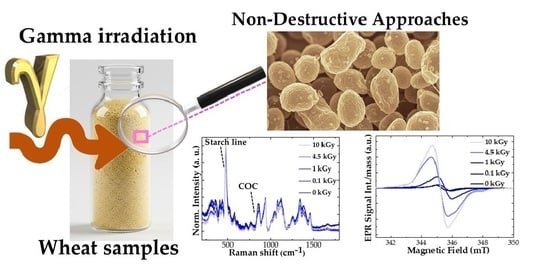
Gamma Irradiation for Agrifood: Non-Destructive Approaches to Study the Secondary Effects Produced in Italian Wheat Matrices
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
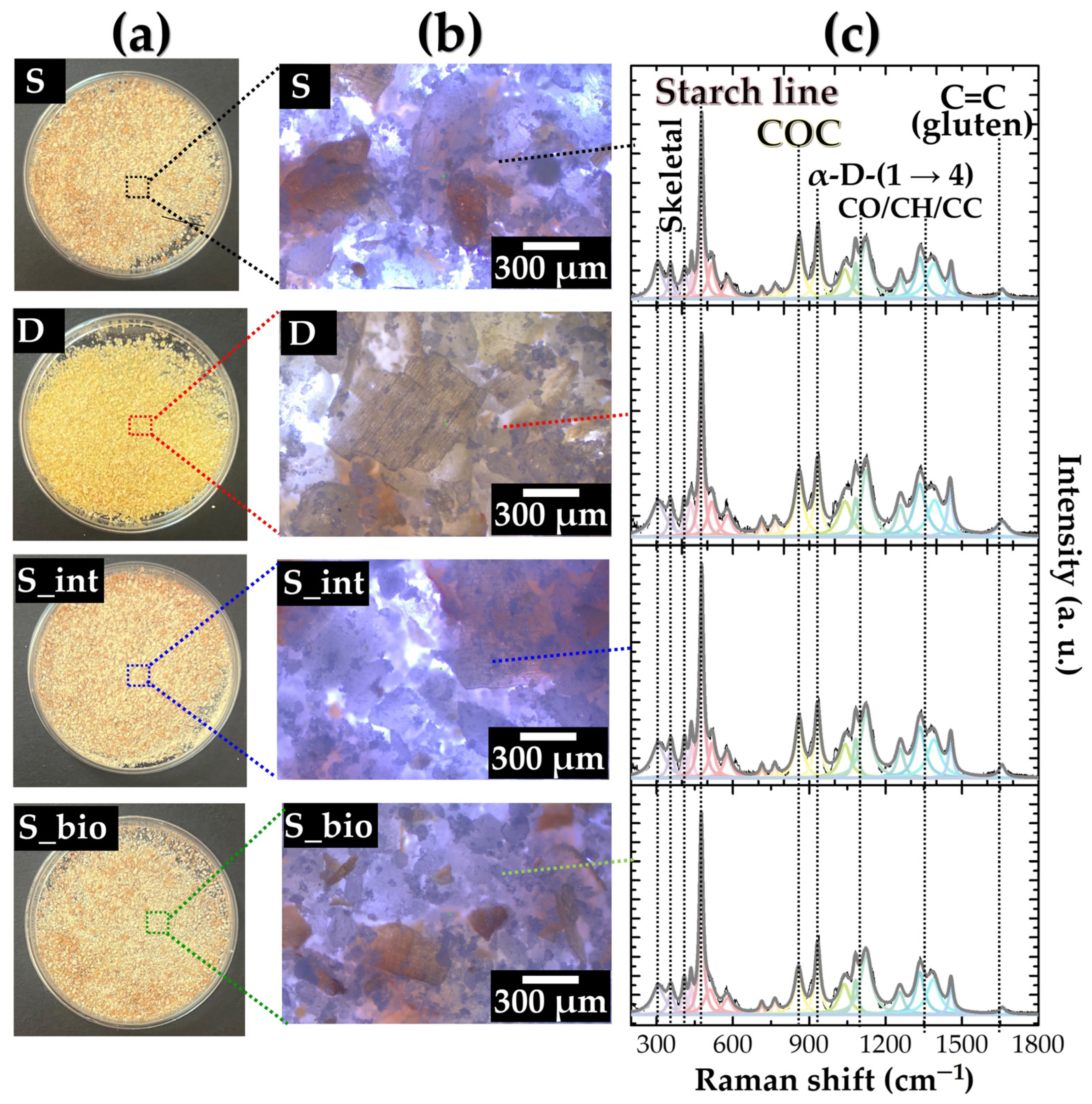
3.1. Wheat Samples Before Irradiation
3.2. Wheat Samples After Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Svanidze, M.; Ðuri’c, I.Ð.; Samoggia, A.; Ólafsdóttir, G. Global Wheat Market Dynamics: What Is the Role of the EU and the Black Sea Wheat Exporters? Agriculture 2021, 11, 799. [Google Scholar] [CrossRef]
- Dohlman, E.; Hansen, J.; Boussios, D. USDA Agricultural Projections to 2031; United States Department of Agriculture: Washington, DC, USA, 2022. [CrossRef]
- Rosicka-Kaczmarek, J.; Kwaśniewska-Karolak, I.; Nebesny, E.; Komisarczyk, A. The Functionality of Wheat Starch. In Starch in Food Structure, Function and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–352. [Google Scholar] [CrossRef]
- Cornell, H.J.; Hoveling, A.W. Wheat: Chemistry and Utilization; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Finney, K.F.; Yamazaki, W.T.; Youngs, V.L.; Rubenthaler, G.L. Quality of Hard, Soft, and Durum Wheats. Wheat Wheat Improv. 2015, 13, 677–748. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Borrelli, G.M.; Giovanniello, V.; Platani, C.; De Vita, P. Production of Anthocyanin-Enriched Flours of Durum and Soft Pigmented Wheats by Air-Classification, as a Potential Ingredient for Functional Bread. J. Cereal Sci. 2018, 79, 118–126. [Google Scholar] [CrossRef]
- Sharma, R.C.; Akramkhanov, A.; Amanov, S.; Boboev, H. Integrated Wheat Crop Management Manual for Cold Winter Deserts; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Abdul Mottaleb, K.; Sonder, K.; Donovan, J.; Braun, H.J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement Food Security in a Changing Climate; Springer: Berlin/Heidelberg, Germany, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- Hashim, M.S.; Yusop, S.M.; Rahman, I.A. The Impact of Gamma Irradiation on the Quality of Meat and Poultry: A Review on Its Immediate and Storage Effects. Appl. Food Res. 2024, 4, 100444. [Google Scholar] [CrossRef]
- Mshelia, R.D.-z.; Dibal, N.I.; Chiroma, S.M. Food Irradiation: An Effective but under-Utilized Technique for Food Preservations. J. Food Sci. Technol. 2023, 60, 2517–2525. [Google Scholar] [CrossRef]
- Handayani, M.; Permawati, H. Gamma Irradiation Technology to Preservation of Foodstuffs as an Effort to Maintain Quality and Acquaint the Significant Role of Nuclear on Food Production to Indonesia Society: A Review. Energy Procedia 2017, 127, 302–309. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Cingolani, M.C.; Li, L.; Chazot, G.; Salmieri, S.; Horak, C.; Lacroix, M. Effect of γ-Irradiation and the Use of Combined Treatments with Edible Bioactive Coating on Carrot Preservation. Food Packag. Shelf Life 2021, 28, 100635. [Google Scholar] [CrossRef]
- D’Orsi, B.; Carcione, R.; Di Sarcina, I.; Ferrara, G.; Oliviero, M.; Rinaldi, T.; Scifo, J.; Verna, A.; Cemmi, A. Gamma Irradiation for Cultural Heritage Conservation: Comparison of the Side Effects on New and Old Paper. J. Cult. Herit. 2024, 70, 335–344. [Google Scholar] [CrossRef]
- Sunder, M.; Mumbrekar, K.D.; Mazumder, N. Gamma Radiation as a Modifier of Starch—Physicochemical Perspective. Curr. Res. Food Sci. 2022, 5, 141–149. [Google Scholar] [CrossRef]
- Atrous, H.; Benbettaieb, N.; Hosni, F.; Danthine, S.; Blecker, C.; Attia, H.; Ghorbel, D. Effect of γ-Radiation on Free Radicals Formation, Structural Changes and Functional Properties of Wheat Starch. Int. J. Biol. Macromol. 2015, 80, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Zhiguang, C.; Rui, Z.; Qi, Y.; Haixia, Z. The Effects of Gamma Irradiation Treatment on Starch Structure and Properties: A Review. Int. J. Food Sci. Technol. 2023, 58, 4519–4528. [Google Scholar] [CrossRef]
- Kang, I.J.; Byun, M.W.; Yook, H.S.; Bae, C.H.; Lee, H.S.; Kwon, J.H.; Chung, C.K. Production of Modified Starches by Gamma Irradiation. Radiat. Phys. Chem. 1999, 54, 425–430. [Google Scholar] [CrossRef]
- Zhu, F. Impact of γ-Irradiation on Structure, Physicochemical Properties, and Applications of Starch. Food Hydrocoll. 2016, 52, 201–212. [Google Scholar] [CrossRef]
- Raffi, J.J.; Agnel, J.P.; Thiery, C.J.; Frejaville, C.M.; Saint-Lébe, L.R. Study of Gamma-Irradiated Starches Derived from Different Foodstuffs: A Way for Extrapolating Wholesomeness Data. J. Agric. Food Chem. 1981, 29, 1227–1232. [Google Scholar] [CrossRef]
- Bertolini, A.C.; Mestres, C.; Colonna, P.; Raffi, J. Free Radical Formation in UV- and Gamma-Irradiated Cassava Starch. Carbohydr. Polym. 2001, 44, 269–271. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; George, J.; Kumar, S.; Vikas; Sajeevkumar, V.A. Impact of Γ− Irradiation on the Physico-Chemical, Rheological Properties and in Vitro Digestibility of Kithul (Caryota urens) Starch; a New Source of Nonconventional Stem Starch. Radiat. Phys. Chem. 2019, 162, 54–65. [Google Scholar] [CrossRef]
- Labelle, M.A.; Ispas-Szabo, P.; Mateescu, M.A. Structure-Functions Relationship of Modified Starches for Pharmaceutical and Biomedical Applications. Starch-Stärke 2020, 72, 2000002. [Google Scholar] [CrossRef]
- Bhat, N.A.; Wani, I.A.; Hamdani, A.M.; Masoodi, F.A. Effect of Gamma-Irradiation on the Thermal, Rheological and Antioxidant Properties of Three Wheat Cultivars Grown in Temperate Indian Climate. Radiat. Phys. Chem. 2020, 176, 108953. [Google Scholar] [CrossRef]
- Rigas, N.; Maharjan, P.; Partington, D.; McDonald, L.; Walker, C.; Bekes, F.; Florides, C.; Panozzo, J. Adverse Effects of High-Dose Gamma Irradiation on Wheat Quality and Processing Traits. Cereal Chem. 2023, 100, 945–953. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mahapatra, S.; Hooi, A.; Ali, M.N.; Satdive, R. Determination of Median Lethal (LD50) and Growth Reduction (GR50) Dose of Gamma Irradiation for Induced Mutation in Wheat. Brazilian Arch. Biol. Technol. 2023, 66, e23220294. [Google Scholar] [CrossRef]
- Kiani, D.; Borzouei, A.; Ramezanpour, S.; Soltanloo, H.; Saadati, S. Application of Gamma Irradiation on Morphological, Biochemical, and Molecular Aspects of Wheat (Triticum aestivum L.) under Different Seed Moisture Contents. Sci. Rep. 2022, 12, 11082. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Awika, J.M. Effect of Protein–Starch Interactions on Starch Retrogradation and Implications for Food Product Quality. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2081–2111. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, P.; Zhou, Q.; Zhong, Y.; Wang, X.; Cai, J.; Huang, M.; Jiang, D. Effects of Different Gluten Proteins on Starch’s Structural and Physicochemical Properties during Heating and Their Molecular Interactions. Int. J. Mol. Sci. 2022, 23, 8523. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; He, H.; Zhang, L.; Hu, X.; Wu, C. Fungicidal Effect of Strong Oxidative Free Radicals Against Fusarium Graminearum and Their Impact on Wheat Growth and Yield. Agriculture 2025, 15, 404. [Google Scholar] [CrossRef]
- Abdi, R.; Cao, W.; Zogheib, A.; Pukazhendhi, K.M.K.; Espinal-Ruiz, M.; Gammage, S.; Warriner, K.; Joye, I.J. Surface Disinfection of Wheat Kernels Using Gas Phase Hydroxyl-Radical Processes: Effect on Germination Characteristics, Microbial Load, and Functional Properties. J. Food Sci. 2024, 89, 1154–1166. [Google Scholar] [CrossRef]
- Boshevska, M.; Sandeva, I.; Cabo Verde, S.; Spasevska, H.; Jankuloski, Z. Effects of Different Irradiation Doses and Storage Period on Microbiological Characteristics of Wheat (Triticum aestivum L.). Food Control 2024, 158, 110201. [Google Scholar] [CrossRef]
- Melios, S.; Stramarkou, M.; Grasso, S. Innovations in Food: A Review on the Consumer Perception of Non-Thermal Processing Technologies. LWT 2025, 223, 117688. [Google Scholar] [CrossRef]
- Junaedi, I.; McNeill, L.S.; Hamlin, R.P. Developing Food Consumer Attitudes towards Ionizing Radiation and Genetic Modification. Nutrients 2024, 16, 3427. [Google Scholar] [CrossRef]
- Osman, A.M.A.; Hassan, A.B.; Osman, G.A.M.; Mohammed, N.; Rushdi, M.A.H.; Diab, E.E.; Babiker, E.E. Effects of Gamma Irradiation and/or Cooking on Nutritional Quality of Faba Bean (Vicia faba L.) Cultivars Seeds. J. Food Sci. Technol. 2014, 51, 1554–1560. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singla, M.; Goraya, R.K.; Kumar, Y.; Jan, K.; Bashir, K. Dose-Dependent Effects of Gamma Irradiation on Microbiological, Antioxidant, and Functional Properties of Buckwheat, Cowpea, Oat, and Brown Rice Flour. J. Food Process. Preserv. 2024, 2024, 1196594. [Google Scholar] [CrossRef]
- Pagliarello, R.; Bennici, E.; Cemmi, A.; Di Sarcina, I.; Spelt, C.; Nardi, L.; Del Fiore, A.; De Rossi, P.; Paolini, F.; Koes, R.; et al. Designing a Novel Tomato Ideotype for Future Cultivation in Space Manned Missions. Front. Astron. Space Sci. 2023, 9, 1040633. [Google Scholar] [CrossRef]
- Massa, S.; Pagliarello, R.; Cemmi, A.; Di Sarcina, I.; Bombarely, A.; Demurtas, O.C.; Diretto, G.; Paolini, F.; Petzold, H.E.; Bliek, M.; et al. Modifying Anthocyanins Biosynthesis in Tomato Hairy Roots: A Test Bed for Plant Resistance to Ionizing Radiation and Antioxidant Properties in Space. Front. Plant Sci. 2022, 13, 830931. [Google Scholar] [CrossRef] [PubMed]
- Cemmi, A.; Di Sarcina, I.; D’Orsi, B. Gamma Radiation-Induced Effects on Paper Irradiated at Absorbed Doses Common for Cultural Heritage Preservation. Radiat. Phys. Chem. 2023, 202, 110452. [Google Scholar] [CrossRef]
- Baccaro, S.; Casieri, C.; Cemmi, A.; Chiarini, M.; D’Aiuto, V.; Tortora, M. Characterization of γ-Radiation Induced Polymerization in Ethyl Methacrylate and Methyl Acrylate Monomers Solutions. Radiat. Phys. Chem. 2017, 141, 131–137. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Wheat-Based Food and Feed. In Wheat—An Exceptional Crop; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–102. [Google Scholar] [CrossRef]
- Boggini, G.; Namoune, H.; Abecassis, J.; Cuq, B. Other Traditional Durum-Derived Products. In Durum Wheat Chem. Chemistry and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 177–199. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Yang, S.; Wang, X.; Li, L.; Sun, B.; Ma, S.; Zheng, S. Microstructure Observation of Multilayers Separated from Wheat Bran. Grain Oil Sci. Technol. 2021, 4, 165–173. [Google Scholar] [CrossRef]
- Dronzek, B.L.; Dexter, J.E.; Matsuo, R.R. A Scanning Electron Microscopy Study of Wheat Gluten. Can. Inst. Food Sci. Technol. J. 1980, 13, 162–166. [Google Scholar] [CrossRef]
- Zafar, T.A.; Aldughpassi, A.; Al-Mussallam, A.; Al-Othman, A. Microstructure of Whole Wheat versus White Flour and Wheat-Chickpea Flour Blends and Dough: Impact on the Glycemic Response of Pan Bread. Int. J. Food Sci. 2020, 2020, 8834960. [Google Scholar] [CrossRef]
- Hammami, R.; Barbar, R.; Laurent, M.; Cuq, B. Durum Wheat Couscous Grains: An Ethnic Mediterranean Food at the Interface of Traditional Domestic Preparation and Industrial Manufacturing. Foods 2022, 11, 902. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wu, Y.; Liu, L.; Ouyang, J. Physicochemical Properties and in Vitro Digestibility of Starch from Naturally Air-Dried Chestnut. Int. J. Biol. Macromol. 2018, 117, 1074–1080. [Google Scholar] [CrossRef]
- Cael, S.J.; Koenig, J.L.; Blackwell, J. Infrared and Raman Spectroscopy of Carbohydrates: Part III: Raman Spectra of the Polymorphic Forms of Amylose. Carbohydr. Res. 1973, 29, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fechner, P.M.; Wartewig, S.; Kleinebudde, P.; Neubert, R.H.H. Studies of the Retrogradation Process for Various Starch Gels Using Raman Spectroscopy. Carbohydr. Res. 2005, 340, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Xing, J.; Liu, H.; Pan, D.H.; Corke, H. Potential Use of Raman Spectroscopy for Determination of Amylose Content in Maize Starch. Cereal Chem. 1999, 76, 821–823. [Google Scholar] [CrossRef]
- Wellner, N.; Georget, D.M.R.; Parker, M.L.; Morris, V.J. In Situ Raman Microscopy of Starch Granule Structures in Wild Type and Ae Mutant Maize Kernels. Starch-Stärke 2011, 63, 128–138. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, H.; Kim, M.S.; Qin, J.; Cho, B.K. Raman Hyperspectral Imaging and Spectral Similarity Analysis for Quantitative Detection of Multiple Adulterants in Wheat Flour. Biosyst. Eng. 2019, 181, 103–113. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Chikaguchi, H.; Marin, E.; Masumura, T.; Sato, Y.-i.; Nakazaki, T. Raman Spectroscopic Analysis of Polysaccharides in Popular Japanese Rice Cultivars. Food Chem. 2021, 354, 129434. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhang, W.; Li, C.; Yu, J.; Wang, S. Molecular Order and Functional Properties of Starches from Three Waxy Wheat Varieties Grown in China. Food Chem. 2015, 181, 43–50. [Google Scholar] [CrossRef]
- Gautam, S.; Morey, R.; Rau, N.; Scheuring, D.C.; Kurouski, D.; Vales, M.I. Raman Spectroscopy Detects Chemical Differences between Potato Tubers Produced under Normal and Heat Stress Growing Conditions. Front. Plant Sci. 2023, 14, 1105603. [Google Scholar] [CrossRef]
- Egging, V.; Nguyen, J.; Kurouski, D. Detection and Identification of Fungal Infections in Intact Wheat and Sorghum Grain Using a Hand-Held Raman Spectrometer. Anal. Chem. 2018, 90, 8616–8621. [Google Scholar] [CrossRef]
- Ispas-Szabo, P.; Ravenelle, F.; Hassan, I.; Preda, M.; Mateescu, M.A. Structure-Properties Relationship in Cross-Linked High-Amylose Starch for Use in Controlled Drug Release. Carbohydr. Res. 2000, 323, 163–175. [Google Scholar] [CrossRef]
- Lin, H.; Bean, S.R.; Tilley, M.; Peiris, K.H.S.; Brabec, D. Qualitative and Quantitative Analysis of Sorghum Grain Composition Including Protein and Tannins Using ATR-FTIR Spectroscopy. Food Anal. Methods 2021, 14, 268–279. [Google Scholar] [CrossRef]
- Bajer, D.; Kaczmarek, H.; Bajer, K. The Structure and Properties of Different Types of Starch Exposed to UV Radiation: A Comparative Study. Carbohydr. Polym. 2013, 98, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, R.; Marikkar, N.; Yalegama, C.; Mendis, E. FTIR Spectral Analysis Combined with Chemometrics in Evaluation of Composite Mixtures of Coconut Testa Flour and Wheat Flour. J. Food Meas. Charact. 2022, 16, 1796–1806. [Google Scholar] [CrossRef]
- Bajer, D.; Janczak, K.; Bajer, K. Novel Starch/Chitosan/Aloe Vera Composites as Promising Biopackaging Materials. J. Polym. Environ. 2020, 28, 1021–1039. [Google Scholar] [CrossRef]
- Tew, W.Y.; Ying, C.; Wujun, Z.; Baocai, L.; Yoon, T.L.; Yam, M.F.; Jingying, C. Application of FT-IR Spectroscopy and Chemometric Technique for the Identification of Three Different Parts of Camellia Nitidissima and Discrimination of Its Authenticated Product. Front. Pharmacol. 2022, 13, 931203. [Google Scholar] [CrossRef]
- Demidov, S.V.; Rudneva, T.N.; Allayarova, U.Y.; Klimanova, E.N.; Allayarova, A.S.; Shitikova, A.V.; Chekalina, S.D.; Allayarov, S.R. Fourier Transform IR Spectroscopic Study of the Influence of a High Dose of Gamma Radiation on the Composition of Functional Groups in Potato Tubers. High Energy Chem. 2023, 57, 356–363. [Google Scholar] [CrossRef]
- Allayarov, S.R.; Rudneva, T.N.; Demidov, S.V.; Allayarova, U.Y.; Klimanova, E.N. A Study of the Influence of γ-Irradiation on the Composition of Functional Groups on the Surfaces of Oat Grain Shell, Kernel, and Seed Powder. High Energy Chem. 2022, 56, 429–436. [Google Scholar] [CrossRef]
- Raffi, J.J.; Agnel, J.P.L. Influence of the Physical Structure of Irradiated Starches on Their Electron Spin Resonance Spectra Kinetics. J. Phys. Chem. 1983, 87, 2369–2373. [Google Scholar] [CrossRef]
- Raffi, J.; Agnel, J.-P.; Boizot, C.; Thiéry, C.; Vincent, P. Glucose Oligomers as Models to Elucidate the Starch Radiolysis Mechanism. Starch-Stärke 1985, 37, 228–231. [Google Scholar] [CrossRef]
- Ukai, M.; Shimoyama, Y. Free Radicals in Irradiated Wheat Flour Detected by Electron Spin Resonance. Appl. Magn. Reson. 2005, 29, 315–324. [Google Scholar] [CrossRef]
- Vazirov, R.A.; Narkhova, A.A.; Vazirova, E.N.; Sokovnin, S.Y. Electron Paramagnetic Resonance Signal in Wheat Seeds Irradiated with Low-Energy Electron Beam. Radiat. Phys. Chem. 2023, 208, 110934. [Google Scholar] [CrossRef]
- Polat, M.; Korkmaz, M. The ESR Spectroscopic Features and Kinetics of the Radiation-Induced Free Radicals in Maize (Zea mays L.). Food Res. Int. 2004, 37, 293–300. [Google Scholar] [CrossRef]







| Sample Name | Crop | Typology | Variety | Origin | Region |
|---|---|---|---|---|---|
| S | M02 | Soft | Taylor | Italy | Piemonte |
| D | M05 | Durum | Colombo | Italy | Piemonte |
| S_int | M26 | Soft integrated | Italy | Toscana | |
| S_bio | M31 | Soft bio | Italy | Toscana |
| Sample | Slope | Intercept | R2 |
|---|---|---|---|
| S | −12.1 | −17.3 | 0.929 |
| D | −13.7 | −17.8 | 0.994 |
| S_int | −10.5 | −16.3 | 0.954 |
| S_bio | −9.0 | −15.1 | 0.948 |
| Sample | Slope | Intercept | R2 |
|---|---|---|---|
| S | 21 | 50 | 0.926 |
| D | 56 | 88 | 0.978 |
| S_int | 30 | 87 | 0.990 |
| S_bio | 27 | 73 | 0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carcione, R.; Lanzetta, L.; D’Orsi, B.; Di Sarcina, I.; Mansi, E.; Scifo, J.; Cemmi, A. Gamma Irradiation for Agrifood: Non-Destructive Approaches to Study the Secondary Effects Produced in Italian Wheat Matrices. Polysaccharides 2025, 6, 39. https://doi.org/10.3390/polysaccharides6020039
Carcione R, Lanzetta L, D’Orsi B, Di Sarcina I, Mansi E, Scifo J, Cemmi A. Gamma Irradiation for Agrifood: Non-Destructive Approaches to Study the Secondary Effects Produced in Italian Wheat Matrices. Polysaccharides. 2025; 6(2):39. https://doi.org/10.3390/polysaccharides6020039
Chicago/Turabian StyleCarcione, Rocco, Leonardo Lanzetta, Beatrice D’Orsi, Ilaria Di Sarcina, Emiliana Mansi, Jessica Scifo, and Alessia Cemmi. 2025. "Gamma Irradiation for Agrifood: Non-Destructive Approaches to Study the Secondary Effects Produced in Italian Wheat Matrices" Polysaccharides 6, no. 2: 39. https://doi.org/10.3390/polysaccharides6020039
APA StyleCarcione, R., Lanzetta, L., D’Orsi, B., Di Sarcina, I., Mansi, E., Scifo, J., & Cemmi, A. (2025). Gamma Irradiation for Agrifood: Non-Destructive Approaches to Study the Secondary Effects Produced in Italian Wheat Matrices. Polysaccharides, 6(2), 39. https://doi.org/10.3390/polysaccharides6020039