ATP-Binding Cassette (ABC) Transporters and Antibiotic Resistance: Specialized Systems for Capsular Polysaccharide Export in Gram-Negative Pathogens
Abstract
1. Antibiotic-Resistant Bacteria and Their Virulence Factors
| Importance | Bacterium | Gram | Presence of a Capsule | Capsular Transporter |
|---|---|---|---|---|
| Critical | Acinetobacter baumannii | − | Yes [11] | Wzx/Wzy-dependent system |
| Enterobacteria | − | Species dependent | Wzx/Wzy-dependent system + ABC transporter-dependent system | |
| Mycobacterium tuberculosis | + | Yes [12] | N/A | |
| High | Salmonella enterica serovar Typhi | − | Yes [13] | ABC transporter-dependent system |
| Shigella spp. | − | Species dependent | Wzx/Wzy-dependent system | |
| Enterococcus faecium | + | No | N/A | |
| Pseudomonas aeruginosa | − | No | N/A | |
| Non-typhoidal Salmonella | − | Yes [14] | Wzx/Wzy-dependent system | |
| Neisseria gonorrhoeae | − | No | N/A | |
| Staphylococcus aureus | + | Yes [15] | N/A | |
| Medium | Group A Streptococci | + | Yes [16] | N/A |
| Streptococcus pneumoniae | + | Yes [17] | N/A | |
| Haemophilus influenzae | − | Yes [9] | ABC transporter-dependent system | |
| Group B Streptococci | + | Yes [18] | N/A |
2. Gram-Negative Bacterial Capsule
2.1. Gram-Negative Pathogenic Bacteria with Capsular Polysaccharides as a Virulence Factor
2.2. Different Types of Capsular Polysaccharides in Gram-Negative Bacteria
2.3. Why Target the Capsular Polysaccharides in the Fight Against Antibiotic-Resistant Gram-Negative Pathogenic Bacteria?
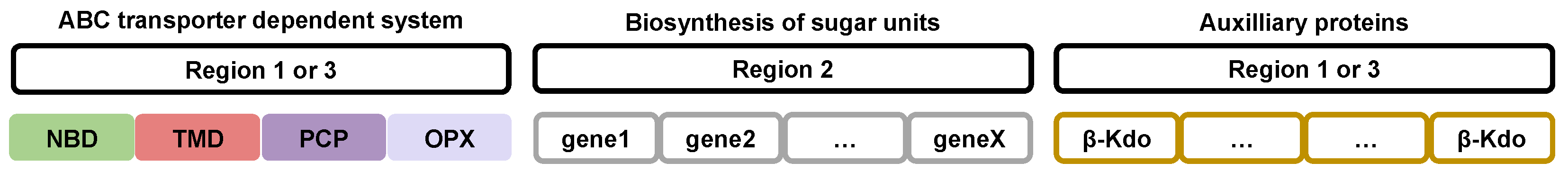
2.4. Gene Organization, Expression, and Regulation of Groups 2 and 3 Capsular Polysaccharides in Gram-Negative Bacteria
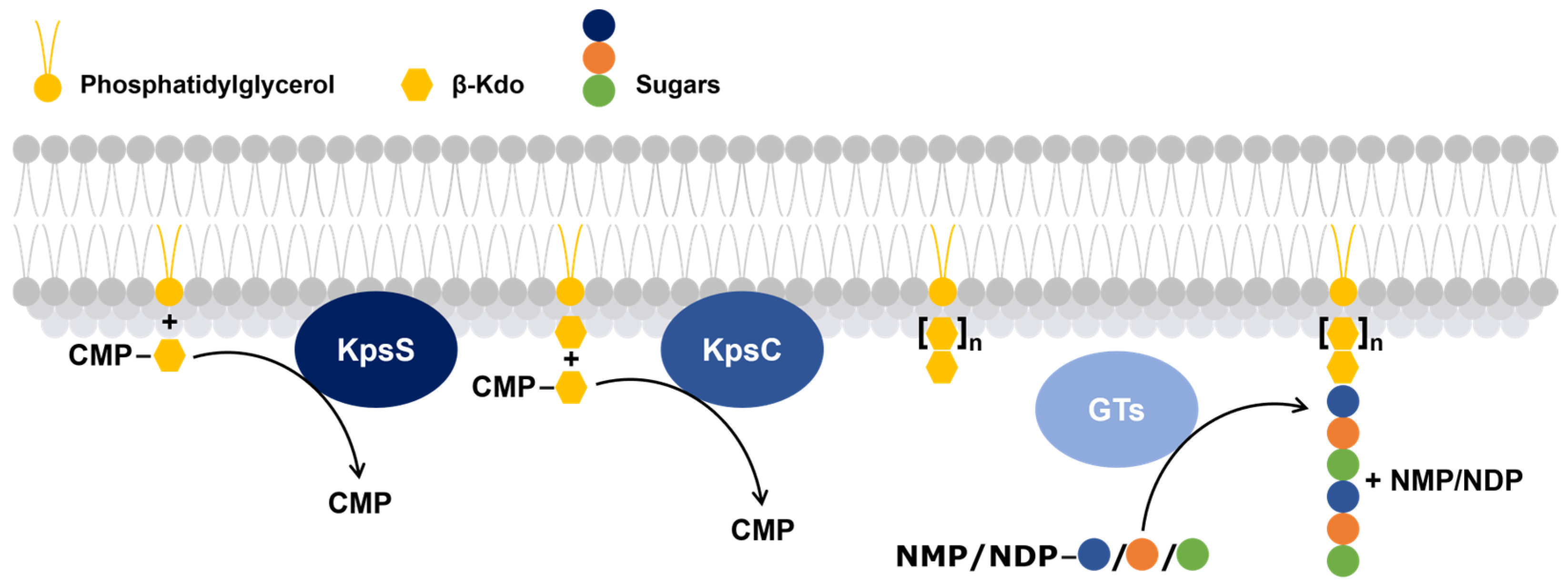
2.5. Polymerization Steps of Groups 2 and 3 Capsular Polysaccharides in Gram-Negative Bacteria
- The first, KpsS, transfers a β-Kdo residue to a phosphatidylglycerol molecule to form a primer.
- The second, KpsC, extends this primer, using one or more molecules of cytidine 5′-monophosphate-Kdo, which serves as the glycolipid anchor of the capsular polysaccharide [61].
3. Export Systems for Capsular Polysaccharides in Gram-Negative Bacteria
3.1. Wzx/Wzy-Dependent System
3.2. Synthase-Dependent System
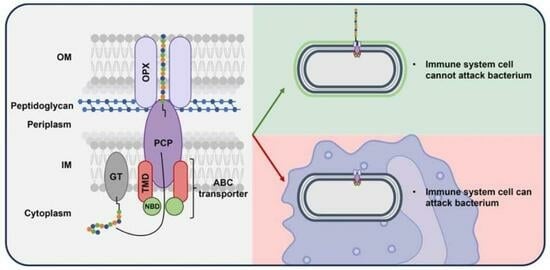
3.3. ABC Transporter-Dependent System
4. Export of Groups 2 and 3 Capsular Polysaccharides by ABC Transporter-Dependent Systems in Gram-Negative Bacteria
4.1. The ABC Transporter and Its Dependent System
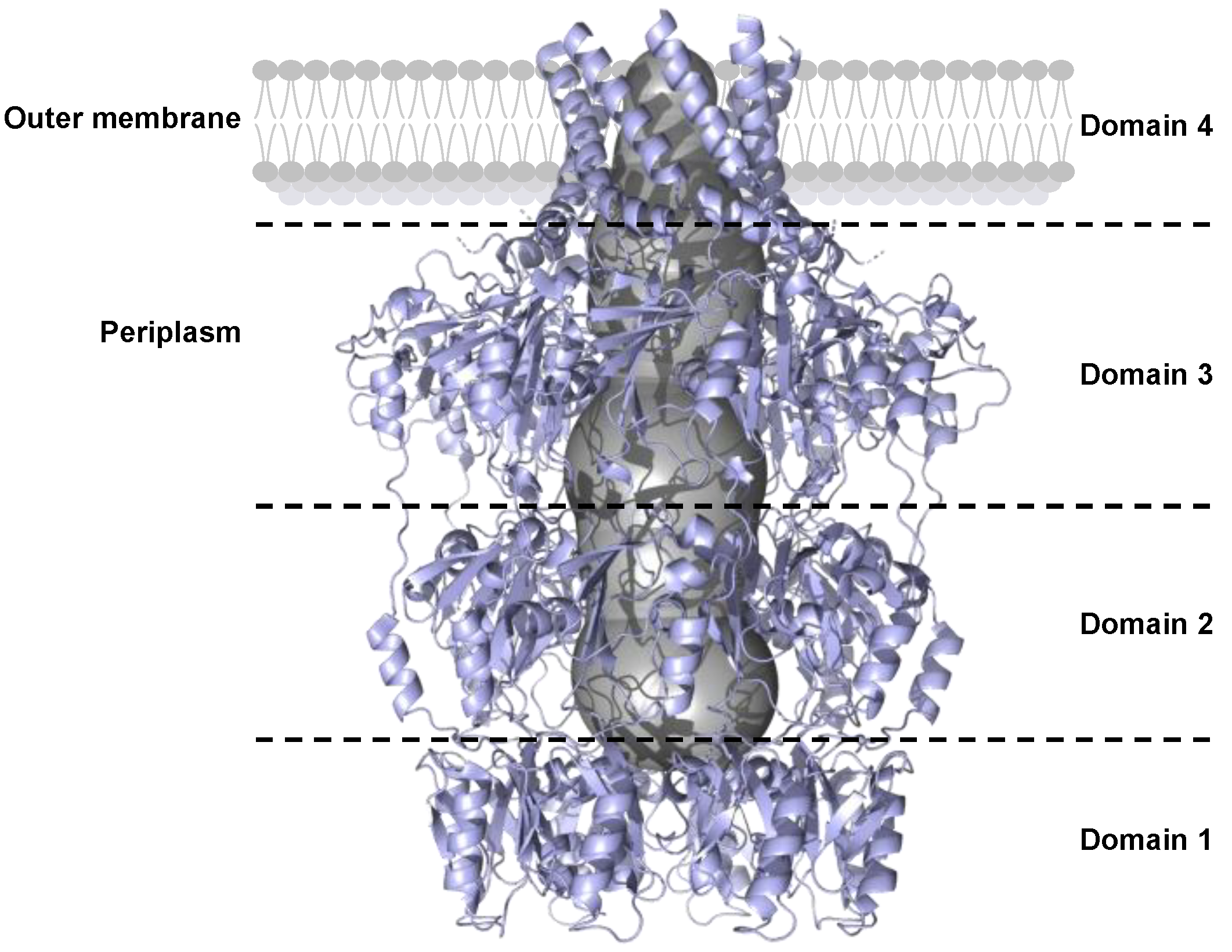
4.2. Structural Description of the ABC Transporter-Dependent System Proteins
4.2.1. NBD and TMD, the Two ABC Transporter Proteins
4.2.2. Polysaccharide Co-Polymerase Protein (PCP)
4.2.3. Outer Membrane Polysaccharide Export Protein (OPX)
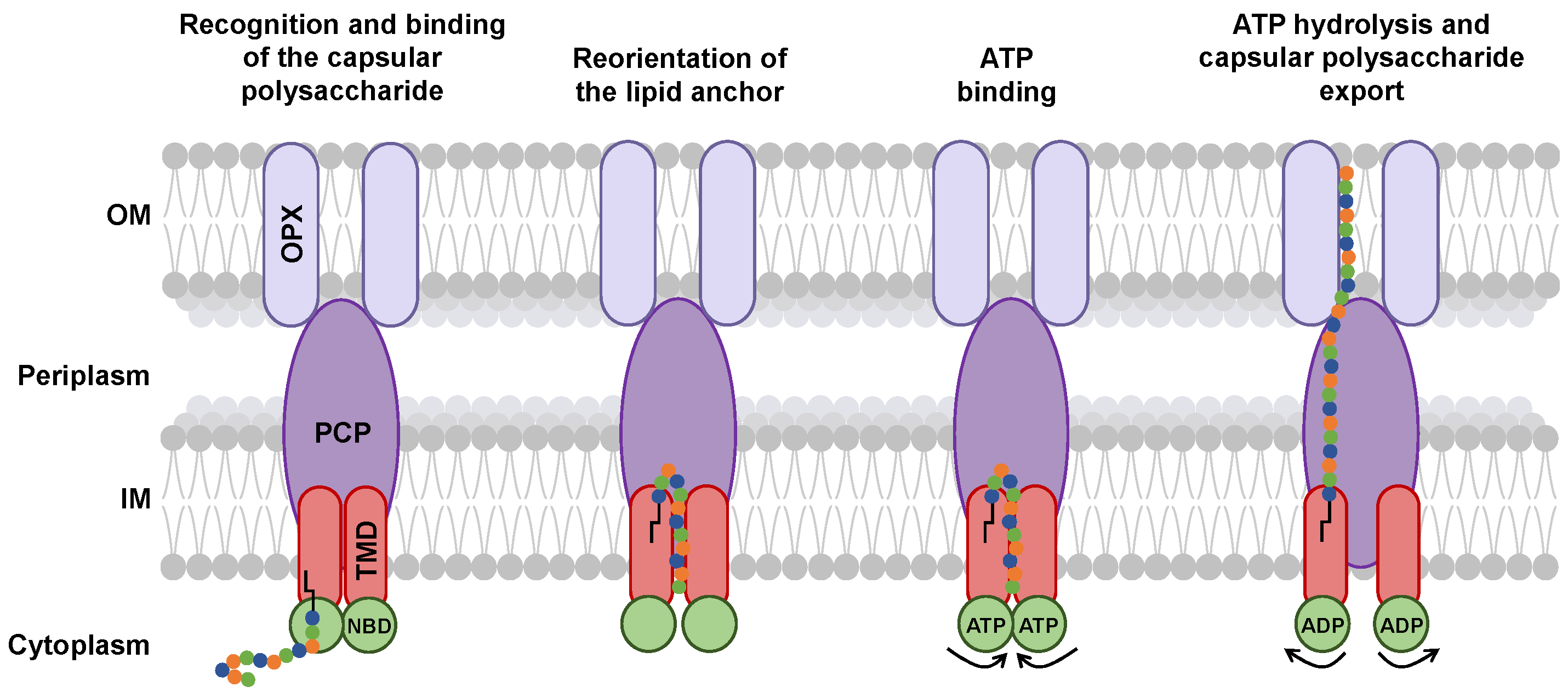
5. Potential Mechanism of Capsular Polysaccharide Transport by the ABC Transporter-Dependent System
6. Discussion
- Is the glycolipid anchor exported with the capsular polysaccharide? Kuklewicz and Zimmer hypothesize that the glycolipid anchor could be cleaved from the capsular polysaccharides when the capsular polysaccharide is engaged in the tunnel [75]. Indeed, there is currently no evidence for lipid transport through the ABC transporter-dependent system. This also implies that another glycolipid anchor would be available in the outer membrane to bind with a capsular polysaccharide.
- How is the glycolipid anchor bound to the polysaccharide chain? The mechanism by which the glycolipid anchor is covalently linked to the polysaccharide chain has remained unclear in the context of capsule biosynthesis. A recent study by Litschko et al. [95] begins to shed light on this process by identifying two transition transferases (TTs; CpsA and CpsC) in Actinobacillus pleuropneumoniae that generate a poly(glycerol-3-phosphate) linker between the conserved glycolipid anchor and the polysaccharide chain. These TTs were also found to stimulate downstream polymerization, suggesting a coordinated mechanism together with GTs. These findings support the idea that the glycolipid anchor could be an export signal and might be already associated with the ABC transporter-dependent system before the capsular polysaccharide chain is extended. While homologs of TTs have been identified in other bacterial genomes, it is still unclear whether this mechanism can be generalized.
- Do PCP and OPX have functions other than that of exporter? Depending on PCP and OPX, this could be possible. Indeed, the C-terminal helix of PCP-3 does not interact in the same way with the inner membrane in different bacterial species [84,87]. This interaction could have an effect on the function of PCP-3. In addition, some OPXs of the ABC transporter-dependent system may have a transmembrane domain that replaces the α-helix [80]. Interaction of this domain with capsular polysaccharides could modify the export process.
7. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Bacterial Priority Pathogens List 2024 Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1.
- Hill, D.J.; Griffiths, N.J.; Borodina, E.; Virji, M. Cellular and Molecular Biology of Neisseria Meningitidis Colonization and Invasive Disease. Clin. Sci. 2010, 118, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Blokesch, M. Natural Competence for Transformation. Curr. Biol. 2016, 26, R1126–R1130. [Google Scholar] [CrossRef]
- Hsiao, A.; Zhu, J. Pathogenicity and Virulence Regulation of Vibrio Cholerae at the Interface of Host-Gut Microbiome Interactions. Virulence 2020, 11, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Bartley, S.N.; Tzeng, Y.L.; Heel, K.; Lee, C.W.; Mowlaboccus, S.; Seemann, T.; Lu, W.; Lin, Y.H.; Ryan, C.S.; Peacock, C.; et al. Attachment and Invasion of Neisseria Meningitidis to Host Cells Is Related to Surface Hydrophobicity, Bacterial Cell Size and Capsule. PLoS ONE 2013, 8, e55798. [Google Scholar] [CrossRef]
- Livorsi, D.J.; Stenehjem, E.; Stephens, D.S. Virulence Factors of Gram-Negative Bacteria in Sepsis with a Focus on Neisseria Meningitidis. Contrib. Microbiol. 2011, 17, 31–47. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L. Virulence Factors and Their Mechanisms of Action: The View from a Damage–Response Framework. J. Water Health 2009, 7, S2–S18. [Google Scholar] [CrossRef]
- Silale, A.; Zhu, Y.; Witwinowski, J.; Smith, R.E.; Newman, K.E.; Bhamidimarri, S.P.; Baslé, A.; Khalid, S.; Beloin, C.; Gribaldo, S.; et al. Dual Function of OmpM as Outer Membrane Tether and Nutrient Uptake Channel in Diderm Firmicutes. Nat. Commun. 2023, 14, 7152. [Google Scholar] [CrossRef] [PubMed]
- Cifuente, J.O.; Schulze, J.; Bethe, A.; Di Domenico, V.; Litschko, C.; Budde, I.; Eidenberger, L.; Thiesler, H.; Ramón Roth, I.; Berger, M.; et al. A Multi-Enzyme Machine Polymerizes the Haemophilus Influenzae Type b Capsule. Nat. Chem. Biol. 2023, 19, 865–877. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Thomas, V.C.; Hancock, L.E. Capsular Polysaccharide Production in Enterococcus Faecalis and Contribution of CpsF to Capsule Serospecificity. J. Bacteriol. 2009, 191, 6203–6210. [Google Scholar] [CrossRef]
- Akoolo, L.; Pires, S.; Kim, J.; Parker, D. The Capsule of Acinetobacter Baumannii Protects against the Innate Immune Response. J. Innate Immun. 2022, 14, 543–554. [Google Scholar] [CrossRef]
- Kalscheuer, R.; Palacios, A.; Anso, I.; Cifuente, J.; Anguita, J.; Jacobs, W.R., Jr.; Guerin, M.E.; Prados-Rosales, R. The Mycobacterium Tuberculosis Capsule: A Cell Structure with Key Implications in Pathogenesis. Biochem. J. 2019, 476, 1995–2016. [Google Scholar] [CrossRef]
- Lee, G.Y.; Song, J. Single Missense Mutations in Vi Capsule Synthesis Genes Confer Hypervirulence to Salmonella Typhi. Nat. Commun. 2024, 15, 5258. [Google Scholar] [CrossRef]
- Perera, S.R.; Sokaribo, A.S.; White, A.P. Polysaccharide Vaccines: A Perspective on Non-Typhoidal Salmonella. Polysaccharides 2021, 2, 691–714. [Google Scholar] [CrossRef]
- O’Riordan, K.; Lee, J.C. Staphylococcus Aureus Capsular Polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef]
- Wessels, M.R. Capsular Polysaccharide of Group A Streptococcus. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Paton, J.C.; Trappetti, C. Streptococcus Pneumoniae Capsular Polysaccharide. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.J.; Dongas, S.; Ratner, A.J. Capsule Production Promotes Group B Streptococcus Intestinal Colonization. Microbiol. Spectr. 2023, 11, e02349-23. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, O.; Garcia-Garcerà, M.; Néron, B.; Touchon, M.; Rocha, E.P.C. Abundance and Co-Occurrence of Extracellular Capsules Increase Environmental Breadth: Implications for the Emergence of Pathogens. PLoS Pathog. 2017, 13, e1006525. [Google Scholar] [CrossRef]
- Willis, L.M.; Stupak, J.; Richards, M.R.; Lowary, T.L.; Li, J.; Whitfield, C. Conserved Glycolipid Termini in Capsular Polysaccharides Synthesized by ATP-Binding Cassette Transporter-Dependent Pathways in Gram-Negative Pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 7868–7873. [Google Scholar] [CrossRef]
- Silver, R.P.; Prior, K.; Nsahlai, C.; Wright, L.F. ABC Transporters and the Export of Capsular Polysaccharides from Gram-Negative Bacteria. Res. Microbiol. 2001, 152, 357–364. [Google Scholar] [CrossRef]
- Pelkonen, S.; Häyrinen, J.; Finne, J. Polyacrylamide Gel Electrophoresis of the Capsular Polysaccharides of Escherichia coli K1 and Other Bacteria. J. Bacteriol. 1988, 170, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, K.D.; Golecki, J.R.; Jann, K. Further Electron Microscopic Studies on the Expression of Escherichia coli Group II Capsules. J. Bacteriol. 1990, 172, 3469–3472. [Google Scholar] [CrossRef]
- Stephens, D.S. Neisseria Meningitidis. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E.M.D., Dolin, R.M.D., Blaser, M.J.M.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 2585–2607.e7. ISBN 978-0-323-48255-4. [Google Scholar]
- Tzeng, Y.-L.; Thomas, J.; Stephens, D.S. Regulation of Capsule in Neisseria Meningitidis. Crit. Rev. Microbiol. 2015, 42, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, W.; Quan, Y.; Li, Y.; Shen, Y.; Yuan, S.; Yi, L.; Wang, Y.; Wang, Y. Bacterial Capsules: Occurrence, Mechanism, and Function. npj Biofilms Microbiomes 2024, 10, 21. [Google Scholar] [CrossRef]
- Sukupolvi-Petty, S.; Grass, S.; StGeme, J.W. The Haemophilus Influenzae Type b hcsA and hcsB Gene Products Facilitate Transport of Capsular Polysaccharide across the Outer Membrane and Are Essential for Virulence. J. Bacteriol. 2006, 188, 3870–3877. [Google Scholar] [CrossRef]
- Frosch, M.; Edwards, U.; Bousset, K.; Krausse, B.; Weisgerber, C. Evidence for a Common Molecular Origin of the Capsule Gene Loci in Gram-Negative Bacteria Expressing Group II Capsular Polysaccharides. Mol. Microbiol. 1991, 5, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.S.; Loynds, B.; Brophy, L.N.; Moxon, E.R. The Bex Locus in Encapsulated Haemophilus Influenzae: A Chromosomal Region Involved in Capsule Polysaccharide Export. Mol. Microbiol. 1990, 4, 1853–1862. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Li, N.; Yokoyama, H.; Ezaki, T. Complete Nucleotide Sequence and Molecular Characterization of ViaB Region Encoding Vi Antigen in Salmonella Typhi. J. Bacteriol. 1993, 175, 4456–4465. [Google Scholar] [CrossRef]
- Moxon, E.R.; Vaughn, K.A. The Type b Capsular Polysaccharide as a Virulence Determinant of Haemophilus Influenzae: Studies Using Clinical Isolates and Laboratory Transformants. J. Infect. Dis. 1981, 143, 517–524. [Google Scholar] [CrossRef]
- Keo, T.; Collins, J.; Kunwar, P.; Blaser, M.J.; Iovine, N.M. Campylobacter Capsule and Lipooligosaccharide Confer Resistance to Serum and Cationic Antimicrobials. Virulence 2011, 2, 30–40. [Google Scholar] [CrossRef]
- Arredondo-Alonso, S.; Blundell-Hunter, G.; Fu, Z.; Gladstone, R.A.; Fillol-Salom, A.; Loraine, J.; Cloutman-Green, E.; Johnsen, P.J.; Samuelsen, Ø.; Pöntinen, A.K.; et al. Evolutionary and Functional History of the Escherichia coli K1 Capsule. Nat. Commun. 2023, 14, 3294. [Google Scholar] [CrossRef]
- Guerry, P.; Poly, F.; Riddle, M.; Maue, A.C.; Chen, Y.-H.; Monteiro, M.A. Campylobacter Polysaccharide Capsules: Virulence and Vaccines. Front. Cell. Inf. Microbiol. 2012, 2, 7. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella Multocida: From Zoonosis to Cellular Microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, R.; Maturrano, L.; Azevedo, V.; Aburjaile, F. Pathogenomics Insights for Understanding Pasteurella Multocida Adaptation. Int. J. Med. Microbiol. 2020, 310, 151417. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Roberts, I.S. Structure, Assembly and Regulation of Expression of Capsules in Escherichia Coli. Mol. Microbiol. 1999, 31, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis and Assembly of Capsular Polysaccharides in Escherichia Coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef]
- Diaz Romero, J.; Outschoorn, I.M. Current Status of Meningococcal Group B Vaccine Candidates: Capsular or Noncapsular? Clin. Microbiol. Rev. 1994, 7, 559–575. [Google Scholar] [CrossRef]
- Lin, D.; Fan, J.; Wang, J.; Liu, L.; Xu, L.; Li, F.; Yang, J.; Li, B. The Fructose-Specific Phosphotransferase System of Klebsiella Pneumoniae Is Regulated by Global Regulator CRP and Linked to Virulence and Growth. Infect. Immun. 2018, 86, e00340-18. [Google Scholar] [CrossRef]
- Niu, T.; Guo, L.; Luo, Q.; Zhou, K.; Yu, W.; Chen, Y.; Huang, C.; Xiao, Y. Wza Gene Knockout Decreases Acinetobacter Baumannii Virulence and Affects Wzy-Dependent Capsular Polysaccharide Synthesis. Virulence 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Schembri, M.A.; Dalsgaard, D.; Klemm, P. Capsule Shields the Function of Short Bacterial Adhesins. J. Bacteriol. 2004, 186, 1249–1257. [Google Scholar] [CrossRef]
- Zafar, M.A.; Hamaguchi, S.; Zangari, T.; Cammer, M.; Weiser, J.N. Capsule Type and Amount Affect Shedding and Transmission of Streptococcus Pneumoniae. mBio 2017, 8, e00989-17. [Google Scholar] [CrossRef]
- Stephens, D.S.; Swartley, J.S.; Kathariou, S.; Morse, S.A. Insertion of Tn916 in Neisseria Meningitidis Resulting in Loss of Group B Capsular Polysaccharide. Infect. Immun. 1991, 59, 4097–4102. [Google Scholar] [CrossRef]
- Ophir, T.; Gutnick, D.L. A Role for Exopolysaccharides in the Protection of Microorganisms from Desiccation. Appl. Environ. Microbiol. 1994, 60, 740–745. [Google Scholar] [CrossRef]
- Gao, S.; Lewis, G.D.; Ashokkumar, M.; Hemar, Y. Inactivation of Microorganisms by Low-Frequency High-Power Ultrasound: 1. Effect of Growth Phase and Capsule Properties of the Bacteria. Ultrason. Sonochem. 2014, 21, 446–453. [Google Scholar] [CrossRef]
- Waz, N.T.; Oliveira, S.; Girardello, R.; Lincopan, N.; Barazzone, G.; Parisotto, T.; Hakansson, A.P.; Converso, T.R.; Darrieux, M. Influence of the Polysaccharide Capsule on the Bactericidal Activity of Indolicidin on Streptococcus Pneumoniae. Front. Microbiol. 2022, 13, 898815. [Google Scholar] [CrossRef]
- Llobet, E.; Tomás, J.M.; Bengoechea, J.A. Capsule Polysaccharide Is a Bacterial Decoy for Antimicrobial Peptides. Microbiology 2008, 154, 3877–3886. [Google Scholar] [CrossRef]
- De Smidt, O.; Albertyn, J.; Bragg, R.R.; Van Heerden, E. Genetic Organisation of the Capsule Transport Gene Region from Haemophilus paragallinarum. Onderstepoort J. Vet. Res. 2004, 71, 139–152. [Google Scholar] [CrossRef]
- Van Eldere, J.; Brophy, L.; Loynds, B.; Celis, P.; Hancock, I.; Carman, S.; Kroll, J.S.; Moxon, E.R. Region II of the Haemophilus Influenzae Type Be Capsulation Locus Is Involved in Serotype-Specific Polysaccharide Synthesis. Mol. Microbiol. 1995, 15, 107–118. [Google Scholar] [CrossRef]
- Kroll, J.S.; Zamze, S.; Loynds, B.; Moxon, E.R. Common Organization of Chromosomal Loci for Production of Different Capsular Polysaccharides in Haemophilus Influenzae. J. Bacteriol. 1989, 171, 3343–3347. [Google Scholar] [CrossRef]
- Bliss, J.M.; Garon, C.F.; Silver, R.P. Polysialic Acid Export in Escherichia Coli K1: The Role of KpsT, the ATP-Binding Component of an ABC Transporter, in Chain Translocation. Glycobiology 1996, 6, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S. The Biochemistry and Genetics of Capsular Polysaccharide Production in Bacteria. Annu. Rev. Microbiol. 1996, 50, 285–315. [Google Scholar] [CrossRef]
- Vimr, E.; Steenbergen, S.; Cieslewicz, M. Biosynthesis of the Polysialic Acid Capsule inEscherichia Coli K1. J. Ind. Microbiol. 1995, 15, 352–360. [Google Scholar] [CrossRef]
- Frosch, M.; Weisgerber, C.; Meyer, T.F. Molecular Characterization and Expression in Escherichia Coli of the Gene Complex Encoding the Polysaccharide Capsule of Neisseria Meningitidis Group B. Proc. Natl. Acad. Sci. USA 1989, 86, 1669–1673. [Google Scholar] [CrossRef]
- Karlyshev, A.V.; Champion, O.L.; Churcher, C.; Brisson, J.-R.; Jarrell, H.C.; Gilbert, M.; Brochu, D.; St Michael, F.; Li, J.; Wakarchuk, W.W.; et al. Analysis of Campylobacter Jejuni Capsular Loci Reveals Multiple Mechanisms for the Generation of Structural Diversity and the Ability to Form Complex Heptoses. Mol. Microbiol. 2005, 55, 90–103. [Google Scholar] [CrossRef]
- Clemence, M.E.A.; Maiden, M.C.J.; Harrison, O.B. Characterization of Capsule Genes in Non-Pathogenic Neisseria Species. Microb. Genom. 2018, 4, e000208. [Google Scholar] [CrossRef]
- Cieslewicz, M.; Vimr, E. Thermoregulation of kpsF, the First Region 1 Gene in the Kps Locus for Polysialic Acid Biosynthesis in Escherichia Coli K1. J. Bacteriol. 1996, 178, 3212–3220. [Google Scholar] [CrossRef]
- Doyle, L.; Ovchinnikova, O.G.; Huang, B.-S.; Forrester, T.J.B.; Lowary, T.L.; Kimber, M.S.; Whitfield, C. Mechanism and Linkage Specificities of the Dual Retaining β-Kdo Glycosyltransferase Modules of KpsC from Bacterial Capsule Biosynthesis. J. Biol. Chem. 2023, 299, 104609. [Google Scholar] [CrossRef]
- Willis, L.M.; Whitfield, C. KpsC and KpsS Are Retaining 3-Deoxy-D-Manno-Oct-2-Ulosonic Acid (Kdo) Transferases Involved in Synthesis of Bacterial Capsules. Proc. Natl. Acad. Sci. USA 2013, 110, 20753–20758. [Google Scholar] [CrossRef]
- Doyle, L.; Ovchinnikova, O.G.; Myler, K.; Mallette, E.; Huang, B.S.; Lowary, T.L.; Kimber, M.S.; Whitfield, C. Biosynthesis of a Conserved Glycolipid Anchor for Gram-Negative Bacterial Capsules. Nat. Chem. Biol. 2019, 15, 632–640. [Google Scholar] [CrossRef]
- Willis, L.M.; Whitfield, C. Structure, Biosynthesis, and Function of Bacterial Capsular Polysaccharides Synthesized by ABC Transporter-Dependent Pathways. Carbohydr. Res. 2013, 378, 35–44. [Google Scholar] [CrossRef]
- Liston, S.D.; Ovchinnikova, O.G.; Whitfield, C. Unique Lipid Anchor Attaches Vi Antigen Capsule to the Surface of Salmonella Enterica Serovar Typhi. Proc. Natl. Acad. Sci. USA 2016, 113, 6719–6724. [Google Scholar] [CrossRef]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of Bacterial Capsular Polysaccharides and Exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543. [Google Scholar] [CrossRef]
- Caffalette, C.A.; Kuklewicz, J.; Spellmon, N.; Zimmer, J. Biosynthesis and Export of Bacterial Glycolipids. Annu. Rev. Biochem. 2020, 89, 741–768. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Mainprize, I.L.; Naismith, J.H.; Whitfield, C. Pivotal Roles of the Outer Membrane Polysaccharide Export and Polysaccharide Copolymerase Protein Families in Export of Extracellular Polysaccharides in Gram-Negative Bacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 155–177. [Google Scholar] [CrossRef]
- Standish, A.J.; Morona, R. Capsule Structure, Synthesis, and Regulation. In Streptococcus Pneumoniae; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 169–179. ISBN 978-0-12-410530-0. [Google Scholar]
- Perez-Burgos, M.; Garcia-Romero, I.; Jung, J.; Schander, E.; Valvano, M.A.; Sogaard-Andersen, L. Characterization of the Exopolysaccharide Biosynthesis Pathway in Myxococcus Xanthus. J. Bacteriol. 2020, 202, e00335-20. [Google Scholar] [CrossRef]
- Cross, A.S.; Kim, K.S.; Wright, D.C.; Sadoff, J.C.; Gemski, P. Role of Lipopolysaccharide and Capsule in the Serum Resistance of Bacteremic Strains of Escherichia Coli. J. Infect. Dis. 1986, 154, 497–503. [Google Scholar] [CrossRef]
- Hug, I.; Feldman, M.F. Analogies and Homologies in Lipopolysaccharide and Glycoprotein Biosynthesis in Bacteria. Glycobiology 2011, 21, 138–151. [Google Scholar] [CrossRef]
- Huszczynski, S.M.; Coumoundouros, C.; Pham, P.; Lam, J.S.; Khursigara, C.M. Unique Regions of the Polysaccharide Copolymerase Wzz(2) from Pseudomonas Aeruginosa Are Essential for O-Specific Antigen Chain Length Control. J. Bacteriol. 2019, 201, e00165-19. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial Exopolysaccharides: Biosynthesis Pathways and Engineering Strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef]
- Weigel, P.H.; DeAngelis, P.L. Hyaluronan Synthases: A Decade-plus of Novel Glycosyltransferases. J. Biol. Chem. 2007, 282, 36777–36781. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Kos, V.; Whitfield, C. ABC Transporters Involved in Export of Cell Surface Glycoconjugates. Microbiol. Mol. Biol. Rev. 2010, 74, 341–362. [Google Scholar] [CrossRef]
- Kuklewicz, J.; Zimmer, J. Molecular Insights into Capsular Polysaccharide Secretion. Nature 2024, 628, 901–909. [Google Scholar] [CrossRef]
- George, A.M. ABC Transporters—40 Years On, 1st ed.; George, A.M., Ed.; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-23475-5. [Google Scholar]
- Theodoulou, F.L.; Kerr, I.D. ABC Transporter Research: Going Strong 40 Years On. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Kalynych, S.; Cherney, M.; Bostina, M.; Rouiller, I.; Cygler, M. Quaternary Structure of WzzB and WzzE Polysaccharide Copolymerases. Protein Sci. 2015, 24, 58–69. [Google Scholar] [CrossRef]
- Thomas, C.; Aller, S.G.; Beis, K.; Carpenter, E.P.; Chang, G.; Chen, L.; Dassa, E.; Dean, M.; Duong Van Hoa, F.; Ekiert, D.; et al. Structural and Functional Diversity Calls for a New Classification of ABC Transporters. FEBS Lett. 2020, 594, 3767–3775. [Google Scholar] [CrossRef]
- Sande, C.; Bouwman, C.; Kell, E.; Nickerson, N.N.; Kapadia, S.B.; Whitfield, C. Structural and Functional Variation in Outer Membrane Polysaccharide Export (OPX) Proteins from the Two Major Capsule Assembly Pathways Present in Escherichia Coli. J. Bacteriol. 2019, 201, e00213-19. [Google Scholar] [CrossRef]
- Harrison, O.B.; Claus, H.; Jiang, Y.; Bennett, J.S.; Bratcher, H.B.; Jolley, K.A.; Corton, C.; Care, R.; Poolman, J.T.; Zollinger, W.D.; et al. Description and Nomenclature of Neisseria Meningitidis Capsule Locus. Emerg. Infect. Dis. 2013, 19, 566. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Kalynych, S.; Yao, D.; Magee, J.; Cygler, M. Structural Characterization of Closely Related O-Antigen Lipopolysaccharide (LPS) Chain Length Regulators. J. Biol. Chem. 2012, 287, 15696–15705. [Google Scholar] [CrossRef]
- Larue, K.; Ford, R.C.; Willis, L.M.; Whitfield, C. Functional and Structural Characterization of Polysaccharide Co-Polymerase Proteins Required for Polymer Export in ATP-Binding Cassette Transporter-Dependent Capsule Biosynthesis Pathways. J. Biol. Chem. 2011, 286, 16658–16668. [Google Scholar] [CrossRef]
- Morona, R.; Purins, L.; Tocilj, A.; Matte, A.; Cygler, M. Sequence-Structure Relationships in Polysaccharide Co-Polymerase (PCP) Proteins. Trends Biochem. Sci. 2009, 34, 78–84. [Google Scholar] [CrossRef]
- Chang, C.W.; Tran, E.N.; Ericsson, D.J.; Casey, L.W.; Lonhienne, T.; Benning, F.; Morona, R.; Kobe, B. Structural and Biochemical Analysis of a Single Amino-Acid Mutant of WzzBSF That Alters Lipopolysaccharide O-Antigen Chain Length in Shigella Flexneri. PLoS ONE 2015, 10, e0138266. [Google Scholar] [CrossRef]
- Phoenix, D.A.; Brandenburg, K.; Harris, F.; Seydel, U.; Hammerton, T.; Roberts, I.S. An Investigation into the Membrane-Interactive Potential of the Escherichia Coli KpsE C-Terminus. Biochem. Biophys. Res. Commun. 2001, 285, 976–980. [Google Scholar] [CrossRef]
- Papadopoulos, M.; Morona, R. Mutagenesis and Chemical Cross-Linking Suggest That Wzz Dimer Stability and Oligomerization Affect Lipopolysaccharide O-Antigen Modal Chain Length Control. J. Bacteriol. 2010, 192, 3385–3393. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Clarke, B.R.; Seidel, L.; Bolla, J.R.; Ward, P.N.; Zhang, P.; Robinson, C.V.; Whitfield, C.; Naismith, J.H. The Molecular Basis of Regulation of Bacterial Capsule Assembly by Wzc. Nat. Commun. 2021, 12, 4349. [Google Scholar] [CrossRef]
- Dong, C.; Beis, K.; Nesper, J.; Brunkan-Lamontagne, A.L.; Clarke, B.R.; Whitfield, C.; Naismith, J.H. Wza the Translocon for E. coli Capsular Polysaccharides Defines a New Class of Membrane Protein. Nature 2006, 444, 226–229. [Google Scholar] [CrossRef]
- Beis, K.; Collins, R.F.; Ford, R.C.; Kamis, A.B.; Whitfield, C.; Naismith, J.H. Three-Dimensional Structure of Wza, the Protein Required for Translocation of Group 1 Capsular Polysaccharide across the Outer Membrane of Escherichia coli. J. Biol. Chem. 2004, 279, 28227–28232. [Google Scholar] [CrossRef]
- Stevenson, G.; Andrianopoulos, K.; Hobbs, M.; Reeves, P.R. Organization of the Escherichia coli K-12 Gene Cluster Responsible for Production of the Extracellular Polysaccharide Colanic Acid. J. Bacteriol. 1996, 178, 4885–4893. [Google Scholar] [CrossRef]
- Caffalette, C.A.; Zimmer, J. Cryo-EM Structure of the Full-Length WzmWzt ABC Transporter Required for Lipid-Linked O Antigen Transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2016144118. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Balaji, S.; Iyer, L.M.; Aravind, L. A Novel Superfamily Containing the β-Grasp Fold Involved in Binding Diverse Soluble Ligands. Biol. Direct 2007, 2, 4. [Google Scholar] [CrossRef]
- Litschko, C.; Di Domenico, V.; Schulze, J.; Li, S.; Ovchinnikova, O.G.; Voskuilen, T.; Bethe, A.; Cifuente, J.O.; Marina, A.; Budde, I.; et al. Transition Transferases Prime Bacterial Capsule Polymerization. Nat. Chem. Biol. 2025, 21, 120–130. [Google Scholar] [CrossRef]
- Yan, X.; Li, S.; Huang, W.; Wang, H.; Zhao, T.; Huang, M.; Zhou, N.; Shen, Y.; Li, X. MPicker: Visualizing and Picking Membrane Proteins for Cryo-Electron Tomography. Nat. Commun. 2025, 16, 472. [Google Scholar] [CrossRef]
- Dunstone, M.A.; de Marco, A. Cryo-Electron Tomography: An Ideal Method to Study Membrane-Associated Proteins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160210. [Google Scholar] [CrossRef]
- Keller, J.; Fernández-Busnadiego, R. In Situ Studies of Membrane Biology by Cryo-Electron Tomography. Curr. Opin. Cell Biol. 2024, 88, 102363. [Google Scholar] [CrossRef]
- Phanphak, S.; Georgiades, P.; Li, R.; King, J.; Roberts, I.S.; Waigh, T.A. Super-Resolution Fluorescence Microscopy Study of the Production of K1 Capsules by Escherichia coli: Evidence for the Differential Distribution of the Capsule at the Poles and the Equator of the Cell. Langmuir 2019, 35, 5635–5646. [Google Scholar] [CrossRef]
- Aldawood, E.; Roberts, I.S. Regulation of Escherichia coli Group 2 Capsule Gene Expression: A Mini Review and Update. Front. Microbiol. 2022, 13, 858767. [Google Scholar] [CrossRef]
- Hao, G.; Shu, R.; Ding, L.; Chen, X.; Miao, Y.; Wu, J.; Zhou, H.; Wang, H. Bacteriophage SRD2021 Recognizing Capsular Polysaccharide Shows Therapeutic Potential in Serotype K47 Klebsiella Pneumoniae Infections. Antibiotics 2021, 10, 894. [Google Scholar] [CrossRef]
- Chorev, D.S.; Baker, L.A.; Wu, D.; Beilsten-Edmands, V.; Rouse, S.L.; Zeev-Ben-Mordehai, T.; Jiko, C.; Samsudin, F.; Gerle, C.; Khalid, S.; et al. Protein Assemblies Ejected Directly from Native Membranes Yield Complexes for Mass Spectrometry. Science 2018, 362, 829–834. [Google Scholar] [CrossRef]
- Bolla, J.R.; Agasid, M.T.; Mehmood, S.; Robinson, C.V. Membrane Protein-Lipid Interactions Probed Using Mass Spectrometry. Annu. Rev. Biochem. 2019, 88, 85–111. [Google Scholar] [CrossRef]
- Husada, F.; Bountra, K.; Tassis, K.; de Boer, M.; Romano, M.; Rebuffat, S.; Beis, K.; Cordes, T. Conformational Dynamics of the ABC Transporter McjD Seen by Single-molecule FRET. EMBO J. 2018, 37, e100056. [Google Scholar] [CrossRef]
- Geertsma, E.R.; Nik Mahmood, N.a.B.; Schuurman-Wolters, G.K.; Poolman, B. Membrane Reconstitution of ABC Transporters and Assays of Translocator Function. Nat. Protoc. 2008, 3, 256–266. [Google Scholar] [CrossRef]
- Huber, F.; Lang, H.P.; Heller, S.; Bielicki, J.A.; Gerber, C.; Meyer, E.; Egli, A. Rapid Bacteria Detection from Patients’ Blood Bypassing Classical Bacterial Culturing. Biosensors 2022, 12, 994. [Google Scholar] [CrossRef]
- Grinevich, D.; Harden, L.; Thakur, S.; Callahan, B. Serovar-Level Identification of Bacterial Foodborne Pathogens from Full-Length 16S rRNA Gene Sequencing. mSystems 2024, 9, e00757-23. [Google Scholar] [CrossRef]
- Del Bino, L.; Østerlid, K.E.; Wu, D.-Y.; Nonne, F.; Romano, M.R.; Codée, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, 122, 15672–15716. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Bino, L.D.; Alfini, R.; Carboni, F.; Romano, M.R.; Adamo, R. Glycoconjugate Vaccines: Current Approaches towards Faster Vaccine Design. Expert. Rev. Vaccines 2019, 18, 881–895. [Google Scholar] [CrossRef]
- Jansen, K.U.; Gruber, W.C.; Simon, R.; Wassil, J.; Anderson, A.S. The Impact of Human Vaccines on Bacterial Antimicrobial Resistance. A Review. Environ. Chem. Lett. 2021, 19, 4031–4062. [Google Scholar] [CrossRef] [PubMed]




| Groups | E. coli Serotypes | Polysaccharide Structures | Capsular Transporter |
|---|---|---|---|
| Group 1 | K27 | 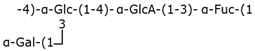 | Wzx/Wzy-dependent system |
| Group 2 | K1 | 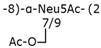 | ABC transporter-dependent system |
| Group 3 | K10 | 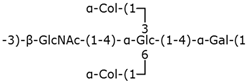 | ABC transporter-dependent system |
| Group 4 | O111 | 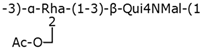 | Wzx/Wzy-dependent system |
| Bacterium | ABC Transporter | PCP | OPX |
|---|---|---|---|
| Escherichia coli | KpsM/KpsT | KpsE | KpsD |
| Neisseria meningitidis | CtrC/CtrD | CtrB | CtrA |
| Haemophilus influenzae | BexB/BexA | BexC | BexD |
| Salmonella enterica serovar Typhi | VexB/VexC | VexD | VexA |
| Campylobacter jejuni | KpsM/KpsT | KpsE | KpsD |
| Pasteurella multocida | KpsM/KpsT | KpsE | KpsD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masselot--Joubert, L.; Di Renzo, M.A. ATP-Binding Cassette (ABC) Transporters and Antibiotic Resistance: Specialized Systems for Capsular Polysaccharide Export in Gram-Negative Pathogens. Polysaccharides 2025, 6, 38. https://doi.org/10.3390/polysaccharides6020038
Masselot--Joubert L, Di Renzo MA. ATP-Binding Cassette (ABC) Transporters and Antibiotic Resistance: Specialized Systems for Capsular Polysaccharide Export in Gram-Negative Pathogens. Polysaccharides. 2025; 6(2):38. https://doi.org/10.3390/polysaccharides6020038
Chicago/Turabian StyleMasselot--Joubert, Loreleï, and María Agostina Di Renzo. 2025. "ATP-Binding Cassette (ABC) Transporters and Antibiotic Resistance: Specialized Systems for Capsular Polysaccharide Export in Gram-Negative Pathogens" Polysaccharides 6, no. 2: 38. https://doi.org/10.3390/polysaccharides6020038
APA StyleMasselot--Joubert, L., & Di Renzo, M. A. (2025). ATP-Binding Cassette (ABC) Transporters and Antibiotic Resistance: Specialized Systems for Capsular Polysaccharide Export in Gram-Negative Pathogens. Polysaccharides, 6(2), 38. https://doi.org/10.3390/polysaccharides6020038