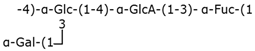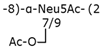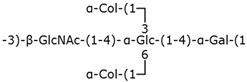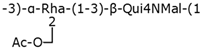Abstract
ABC transporters are a large family of proteins that mediate the export or import of a variety of molecules, including capsular polysaccharides. The capsules are an important virulence factor that protect bacteria from host immune system attacks, antibiotics, and physicochemical changes in their environment. In some Gram-negative pathogenic bacteria, ABC transporter-dependent systems facilitate the export of capsular polysaccharides. These transport systems are composed of three parts: the ABC transporter and the polysaccharide co-polymerase protein in the inner membrane and the outer membrane polysaccharide export protein in the outer membrane. The glycolipid anchor of the capsular polysaccharide binds to a pocket between the two subunits of the ABC transporter transmembrane domain. The three parts of the ABC transporter-dependent system form a tunnel, through which the capsular polysaccharide is exported using energy from ATP hydrolysis. Knowledge of the ABC transporter-dependent system and its function is incomplete, requiring further research to better understand the processes of capsular polysaccharide export. This may also allow, in the future, to develop new molecules that inhibit capsular polysaccharide export, which would help the host immune system fight Gram-negative pathogenic bacteria coated with capsular polysaccharides. This review presents the latest findings on ABC transporter-dependent systems that export capsular polysaccharides in Gram-negative pathogenic bacteria.
1. Antibiotic-Resistant Bacteria and Their Virulence Factors
Antibiotic-resistant bacteria are a major public health problem. The World Health Organization (WHO) estimates that in 2019, six million people died from infections caused by these bacteria [1]. Since 2017, WHO has published a list of priority bacteria to guide the research for new ways to fight these resistant pathogenic bacteria. The list, updated in 2024, includes eight Gram-negative and four Gram-positive bacteria or families of bacteria that cause epidemics, are difficult to treat, and are costly for the global health system [1] (Table 1).
To infect an organism, a bacterium has a toolkit that allows it to enter the host, attach to it, protect itself from the host’s immune system, and spread from one organism to another. These tools are virulence factors that can be transmitted between bacteria in two ways: either by vertical transfer, a binary division of mother bacteria to daughter bacteria, or by horizontal transfer, where genetic material is transferred to another bacterium either by conjugation, transformation, transduction, or released into the environment upon bacterial death and taken up by other naturally competent bacteria, such as those of the Neisseria group or Haemophilus influenzae. This genetic material itself encodes the virulence factors that the bacteria will express to their advantage [2,3].
Virulence factors can be toxins produced by bacteria [4], adhesins that allow the attachment of bacteria to host cells [5], locomotion systems such as flagella [6], or proteins that “steal” nutrients from the host [7,8]. Another virulence factor, and the focus of this review, is the capsule composed of capsular polysaccharides [5,7,9,10].

Table 1.
WHO list of bacteria to be studied as a priority to fight their resistance to antibiotics [1].
Table 1.
WHO list of bacteria to be studied as a priority to fight their resistance to antibiotics [1].
| Importance | Bacterium | Gram | Presence of a Capsule | Capsular Transporter |
|---|---|---|---|---|
| Critical | Acinetobacter baumannii | − | Yes [11] | Wzx/Wzy-dependent system |
| Enterobacteria | − | Species dependent | Wzx/Wzy-dependent system + ABC transporter-dependent system | |
| Mycobacterium tuberculosis | + | Yes [12] | N/A | |
| High | Salmonella enterica serovar Typhi | − | Yes [13] | ABC transporter-dependent system |
| Shigella spp. | − | Species dependent | Wzx/Wzy-dependent system | |
| Enterococcus faecium | + | No | N/A | |
| Pseudomonas aeruginosa | − | No | N/A | |
| Non-typhoidal Salmonella | − | Yes [14] | Wzx/Wzy-dependent system | |
| Neisseria gonorrhoeae | − | No | N/A | |
| Staphylococcus aureus | + | Yes [15] | N/A | |
| Medium | Group A Streptococci | + | Yes [16] | N/A |
| Streptococcus pneumoniae | + | Yes [17] | N/A | |
| Haemophilus influenzae | − | Yes [9] | ABC transporter-dependent system | |
| Group B Streptococci | + | Yes [18] | N/A |
N/A: not applicable.
2. Gram-Negative Bacterial Capsule
A capsule is the external envelope of a bacterium. In 3% of bacteria, such as Bacillus anthracis, it is protein-based; while in 97% of bacteria, such as Escherichia coli (E. coli) and Neisseria meningitidis, it is composed of polysaccharides [19] (Figure 1). In Gram-negative bacteria, capsular polysaccharides are anchored to the outer membrane by a glycolipid anchor.
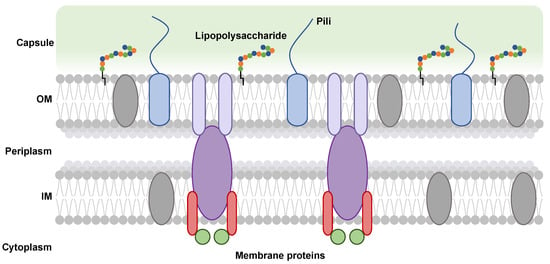
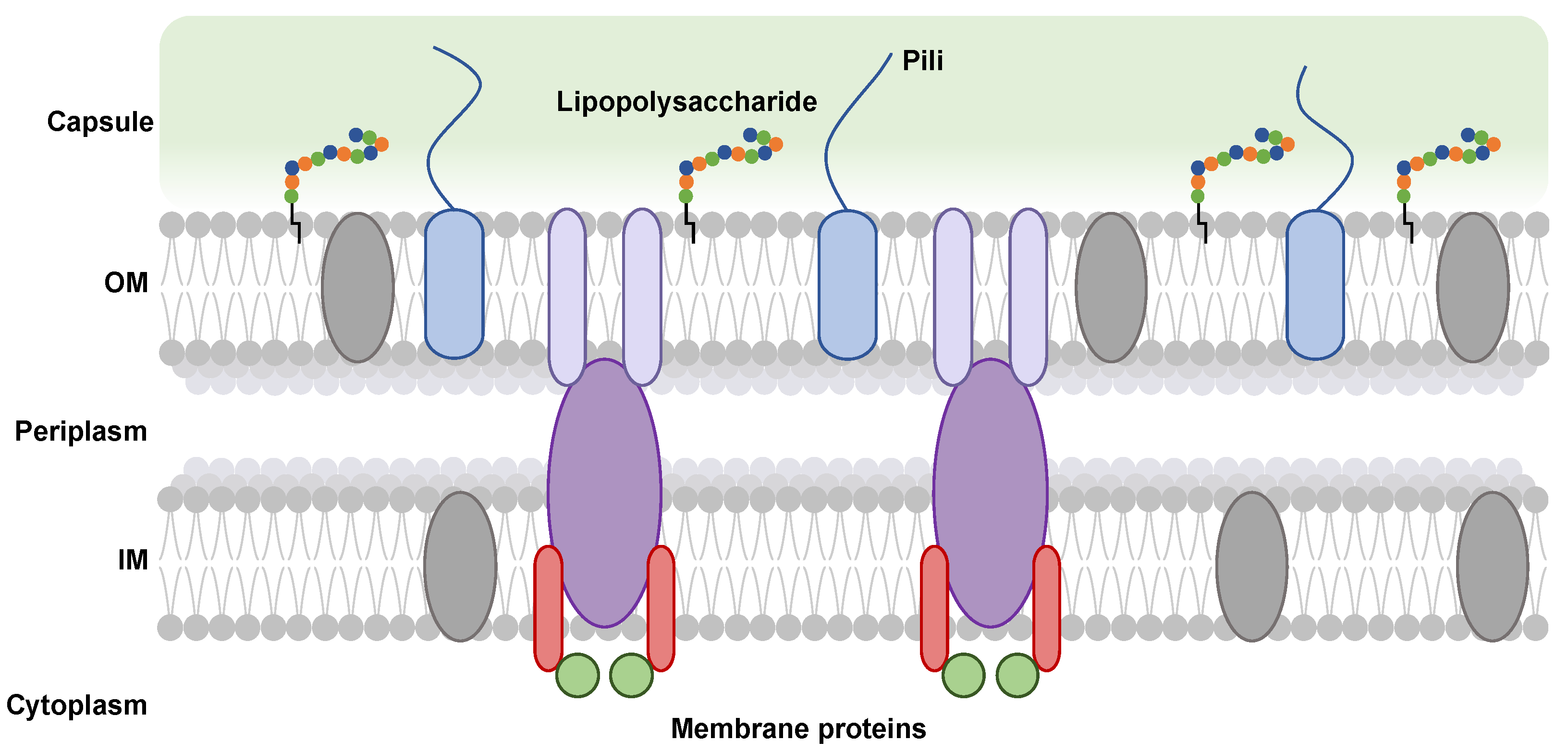
Figure 1.
Schematic representation of the cell envelope of a Gram-negative bacterium. Distinct membrane protein complexes (gray, lilac, purple, and red ovals) are embedded in the outer (OM) and inner (IM) membranes, which play a role in capsular polysaccharide export. These proteins are aided by regulatory and scaffolding soluble periplasmic proteins (green circles). The presence of pili is depicted by blue rounded rectangles terminating in blue lines. In the OM, chains of polysaccharides (multi-colored circles) are anchored by a lipid anchor (black line) forming a lipopolysaccharide.
Capsular polysaccharides are homopolymers or heteropolymers of negatively charged polysaccharides whose export is carried out either by an ABC (ATP-Binding Cassette) transporter-dependent system, a Wzx/Wzy-dependent system, or a synthase-dependent system [20,21]. The composition and structure of capsular polysaccharides are highly diverse. In E. coli, the capsule, visible by electron microscopy, measures between 100 and 400 nm in thickness, with capsular polysaccharides composed of more than 200 saccharide units [22,23]. In pathogenic strains of Neisseria meningitidis, capsular polysaccharides are composed of 130–200 repeating units of amino sugars, sialic acid, glucose, and galactose with an alpha linkage [24,25]. Capsular polysaccharides are exported to the outside of the bacteria to form an additional outer layer of protection for the bacteria. All these capsular polysaccharides are virulence factors that help pathogenic bacteria infect a host and protect themselves from its immune system.
2.1. Gram-Negative Pathogenic Bacteria with Capsular Polysaccharides as a Virulence Factor
Capsular polysaccharides are found in Gram-negative pathogens such as E. coli or Neisseria meningitidis but also in Gram-positive pathogens such as Staphylococcus aureus or Streptococcus pneumoniae [20,26]. They act as virulence factors for several pathogenic bacteria and allow them to survive in different conditions [21,27]. This is the case for Neisseria meningitidis, Haemophilus influenzae, Salmonella enterica serovar Typhi, or Campylobacter jejuni, which can express a capsule exported by the ABC transporter-dependent system (Table 1) [28,29,30,31,32]. These pathogenic bacteria are the most extensively studied capsule bacteria and are responsible for a number of diseases in both humans and livestock. Encapsulated E. coli strains cause blood infections, pyelonephritis, or meningitis [33]. Strains of Neisseria meningitidis can cause meningitis or septicemia [24]. Strains of Haemophilus influenzae cause respiratory diseases such as pneumonia, of which the encapsulated form is the most virulent [31]. Salmonella enterica serovar Typhi strains are enterobacteria that cause typhoid fever [13]. Strains of Campylobacter jejuni cause bacterial diarrhea with sequelae such as Guillain–Barré syndrome, inflammatory bowel disease, reactive arthritis, or irritable bowel syndrome [34]. Pasteurella multocida strains cause many diseases in animals (birds, dogs, cats, cattle, equines) such as chicken cholera, pasteurellosis, pneumonia, atrophic rhinitis, meningitis, and hemorrhagic septicemia. They can be transmitted to humans by bites or scratches [35,36].
2.2. Different Types of Capsular Polysaccharides in Gram-Negative Bacteria
E. coli has over 80 different serogroups [37]. These serogroups have allowed for distinguishing four different categories of capsular polysaccharides according to their chemical composition, their biosynthetic systems, and their export system, which can be the Wzx/Wzy-dependent system or the ABC transporter-dependent system (Table 2). These four groups are also present in other Gram-negative bacteria.

Table 2.
Structure examples for each capsular polysaccharide group.
Group 1 capsular polysaccharides are composed of acidic polysaccharides containing uronic acids and are exported by a Wzx/Wzy-dependent system [38].
Group 2 capsular polysaccharides are linked to the cell via an α-glycerophosphatidic acid residue and, in some bacteria such as E. coli serogroup K1 or Neisseria meningitidis, 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) provides a linker between the lipid and the reducing end of the polysaccharide [37]. Several group 2 capsular polysaccharides resemble vertebrate glycoconjugates, which prevents the vertebrate immune system from recognizing the capsular polysaccharides of bacteria and therefore eliminates them [38]. Group 2 capsular polysaccharides are exported by the ABC transporter-dependent system.
Group 3 capsular polysaccharides are similarly linked to the cell via an α-glycerophosphatidic acid residue, with Kdo as a linker, and are also exported by an ABC transporter-dependent system [37]. However, group 3 capsular polysaccharides differ from group 2 in the regulation of their expression and the organization of the genes involved in their biosynthesis and export [38].
Group 4 capsular polysaccharides are O-antigen capsules not bound to the outer membrane. Its polysaccharides contain acetamido sugars and are exported by a Wzx/Wzy-dependent system [38].
Capsular polysaccharides can be modified, for example, by the incorporation of sialic acids to mimic antigens on the surface of host cells. This chemical modification allows Gram-negative pathogens to become less visible to the host cell’s immune system [2,39].
2.3. Why Target the Capsular Polysaccharides in the Fight Against Antibiotic-Resistant Gram-Negative Pathogenic Bacteria?
Capsular polysaccharides enable bacteria to survive in different environments through bacterial adhesion, resistance to the immune system, adaptation to different physicochemical conditions, and resistance to antibiotics [21,27].
In the case of bacterial adhesion, capsular polysaccharides can have two roles [26]. The first is to allow the formation of biofilms because the excessive production of capsular polysaccharides allows bacteria to attach to each other, which initiates the formation of the biofilm [40,41]. The second is to allow bacteria to leave the biofilm by making capsular polysaccharides that disrupt contacts between bacteria in order to colonize other environments [26,42,43]. Both mechanisms and their regulation are not well understood.
In the case of resistance to the host immune system, capsular polysaccharides can mimic the antigenic composition of some host cells, hide surface antigens that can be recognized by macrophages, or inhibit phagocytosis [24,26,44]. The best-known examples are the capsular polysaccharides of Neisseria meningitidis serogroup B, which are structurally identical to a component of the human NCAM (neuronal cell adhesion molecule), allowing it to evade the host immune system. As a result, the immune response is particularly weak against this type of Neisseria meningitidis, making it difficult to develop vaccines targeting these capsular polysaccharides [2,39].
Capsular polysaccharides can protect bacteria from environmental changes such as lower pH, increased temperatures, lower oxygen concentration, variations in osmotic pressures, or desiccation [26,37]. Indeed, capsular polysaccharides form a hydrated layer around bacteria and maintain it in an aqueous environment [45]. In addition, the capsule coats the bacteria and thus gives them better mechanical resistance [46].
In the case of antibiotic resistance, the negatively charged capsular polysaccharides serve to protect the bacteria from the action of antibiotics by preventing chemical molecules like antimicrobial peptides from coming into contact with the bacterial envelope [47,48].
Since capsular polysaccharides have specific chemical and structural compositions depending on the bacterial strains, it is also interesting to develop vaccines against these Gram-negative pathogenic bacteria to stimulate the immune system of the hosts [49].
2.4. Gene Organization, Expression, and Regulation of Groups 2 and 3 Capsular Polysaccharides in Gram-Negative Bacteria
This section describes how the gene organization of Gram-negative antibiotic-resistant pathogenic bacteria intervenes in the biosynthesis and export of groups 2 and 3 capsular polysaccharides.
The gene organization is identical in all bacteria that express and export group 2 capsular polysaccharides. This gene cluster is divided into three regions corresponding to proteins with specific functions [29,37,38,50,51,52,53,54] (Figure 2).

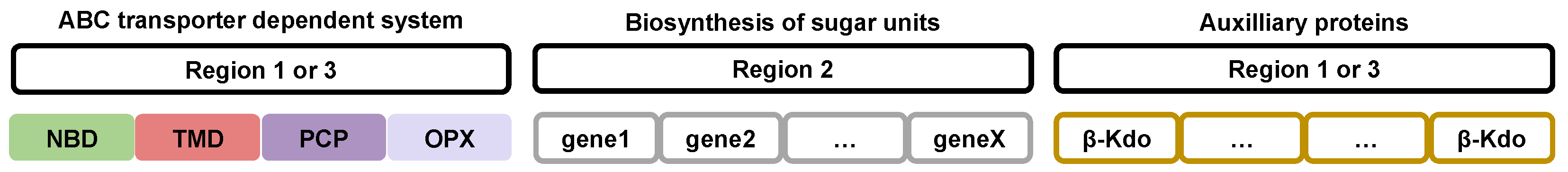
Figure 2.
Gene organization involved in the biosynthesis, polymerization, and export of capsular polysaccharides in Gram-negative pathogenic bacteria. This arrangement is maintained in all bacteria from group 2 capsular polysaccharides. A first region encodes all ABC transporter-related proteins, including the nucleotide-binding domains (NBDs), transmembrane domains (TMDs), polysaccharide co-polymerase protein (PCP), and outer membrane polysaccharide export (OPX) protein. The second cluster encodes proteins that are necessary for the biosynthesis of the saccharide units, while the third region contains β-Kdo-transferases, which catalyze the addition of β-Kdo residues, key for polysaccharide maturation.
Regions 1 and 3 of the gene cluster encode capsular polysaccharide export proteins of the ABC transporter-dependent system and auxiliary proteins useful for this export, such as capsular polysaccharide polymerization proteins [2,21,29,55,56]. These genes are conserved between bacterial species that express group 2 capsular polysaccharides [27,53].
Region 2 groups together a variable number of genes that encode biosynthesizing proteins of the saccharide units. The organization and number of these genes are specific to the type of polysaccharide expressed.
The order of the regions may differ depending on the bacterial species. For example, in species of the genus Neisseria, the regions are named A (capsule synthesis), B (capsule export), and C (capsule translocation), and the order of the three regions appears to be randomly distributed [57].
The organization of genes involved in the biosynthesis and export of group 3 capsular polysaccharides is diverse [38]. Regions 1 and 3 of the gene cluster have a poorly understood organization. Genes encoding the ABC transporter-dependent system and auxiliary proteins have no specific organization and are mixed. Similar to group 2, region 2 comprises the necessary genes for the biosynthesis of saccharic units [38]. The organization and amount of these genes are specific to each polysaccharide type.
Protein expression is thermoregulated for group 2 capsular polysaccharides [33,38]. Indeed, at 20 °C, proteins encoded by genes in regions 1 and 3 of the gene cluster are not expressed in the bacterium [38,58]. Group 3 capsular polysaccharide proteins are expressed regardless of temperature [38].
2.5. Polymerization Steps of Groups 2 and 3 Capsular Polysaccharides in Gram-Negative Bacteria
Capsular polysaccharides of groups 2 and 3 are polymerized in the cytoplasm and then exported across membranes by an ABC transporter-dependent system.
The proteins that polymerize the capsular polysaccharides have retained the same functions; therefore, the polymerization process of capsular polysaccharides has been determined in E. coli. The β-Kdo-transferases are a subfamily of glycosyltransferases (GTs) that play a key role in the polymerization of capsular polysaccharides. These enzymes add a Kdo to a phosphatidylglycerol molecule [59]. For the polymerization, two β-Kdo-transferases are used [59,60,61] (Figure 3):
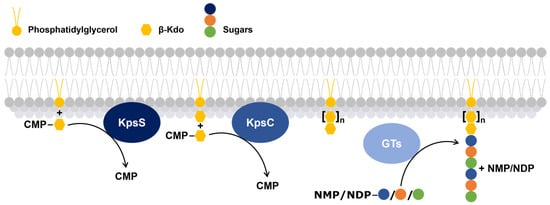
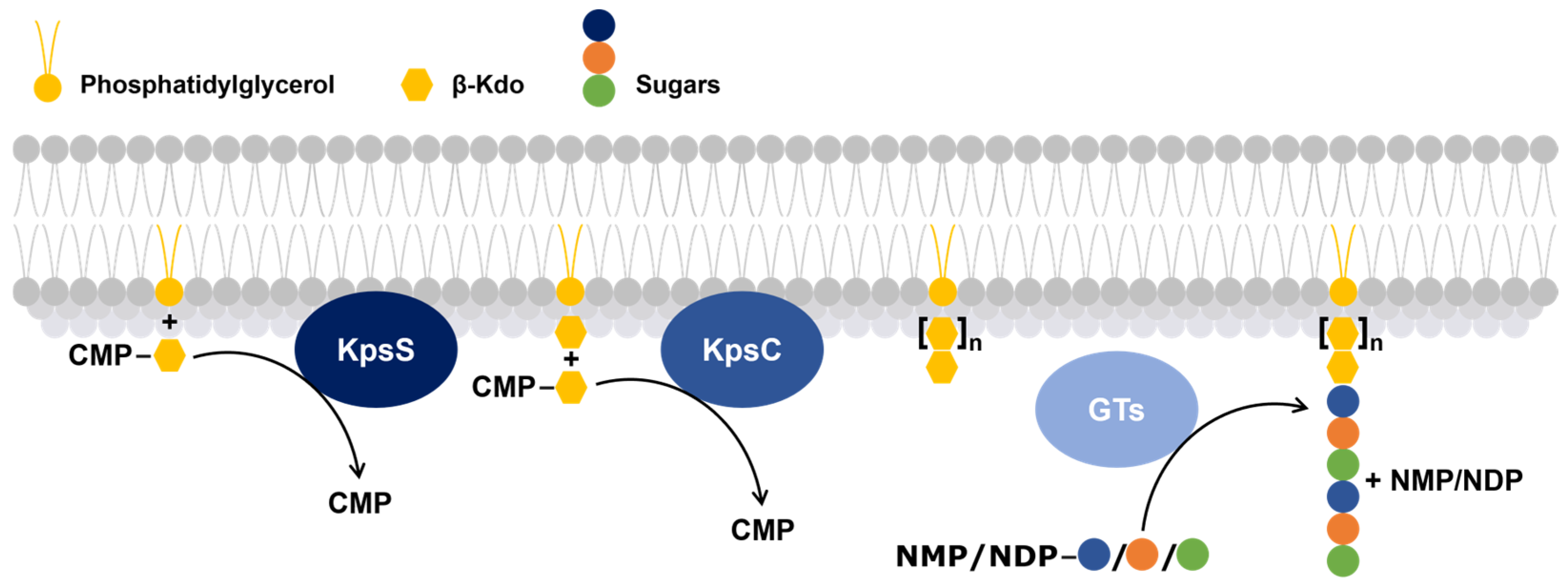
Figure 3.
Schematic overview of the process of glycolipid anchor biosynthesis and subsequent polymerization of a capsular polysaccharide. The process initiates with the KpsS (a β-Kdo-transferase) catalyzed modification of a membrane-embedded phosphatidylglycerol lipid (yellow circle) by the addition of a β-Kdo residue (yellow hexagon), followed by the sequential addition of one or more β-Kdo units by KpsC (a β-Kdo-transferase). This is the core of the glycolipid anchor. Glycosyltransferases (GTs) then catalyze the addition of distinct sugar moieties (blue, orange, and green circles), building the polysaccharide chain. The presence of nucleotide sugar donors—CMP (cytidine 5′-monophosphate), NMP (Nucleoside Monophosphate), and NDP (Nucleoside Diphosphate)—highlights the energy-dependent nature of the process.
- The first, KpsS, transfers a β-Kdo residue to a phosphatidylglycerol molecule to form a primer.
- The second, KpsC, extends this primer, using one or more molecules of cytidine 5′-monophosphate-Kdo, which serves as the glycolipid anchor of the capsular polysaccharide [61].
Subsequently, GTs add sugar residues to the non-reducing end of the capsular polysaccharide [20,60,62].
For Salmonella enterica serovar Typhi, the beginning of the polymerization process, which is partially solved, is different compared to other Gram-negative bacteria due to the absence of KpsS and KpsC, β-Kdo-transferases [63,64]. The glycolipid anchor is composed of a diacyl N-acetylhexosamine molecule instead of a phosphatidylglycerol with Kdos.
3. Export Systems for Capsular Polysaccharides in Gram-Negative Bacteria
Three systems exporting capsular polysaccharides can be found in Gram-negative bacteria: the Wzx/Wzy-dependent system, the synthase-dependent system, and the ABC transporter-dependent system [65] (Figure 4). Some bacteria, such as E. coli, can express different capsular polysaccharide groups and therefore possess different export systems [37,38].
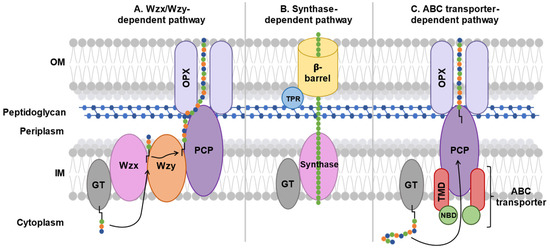
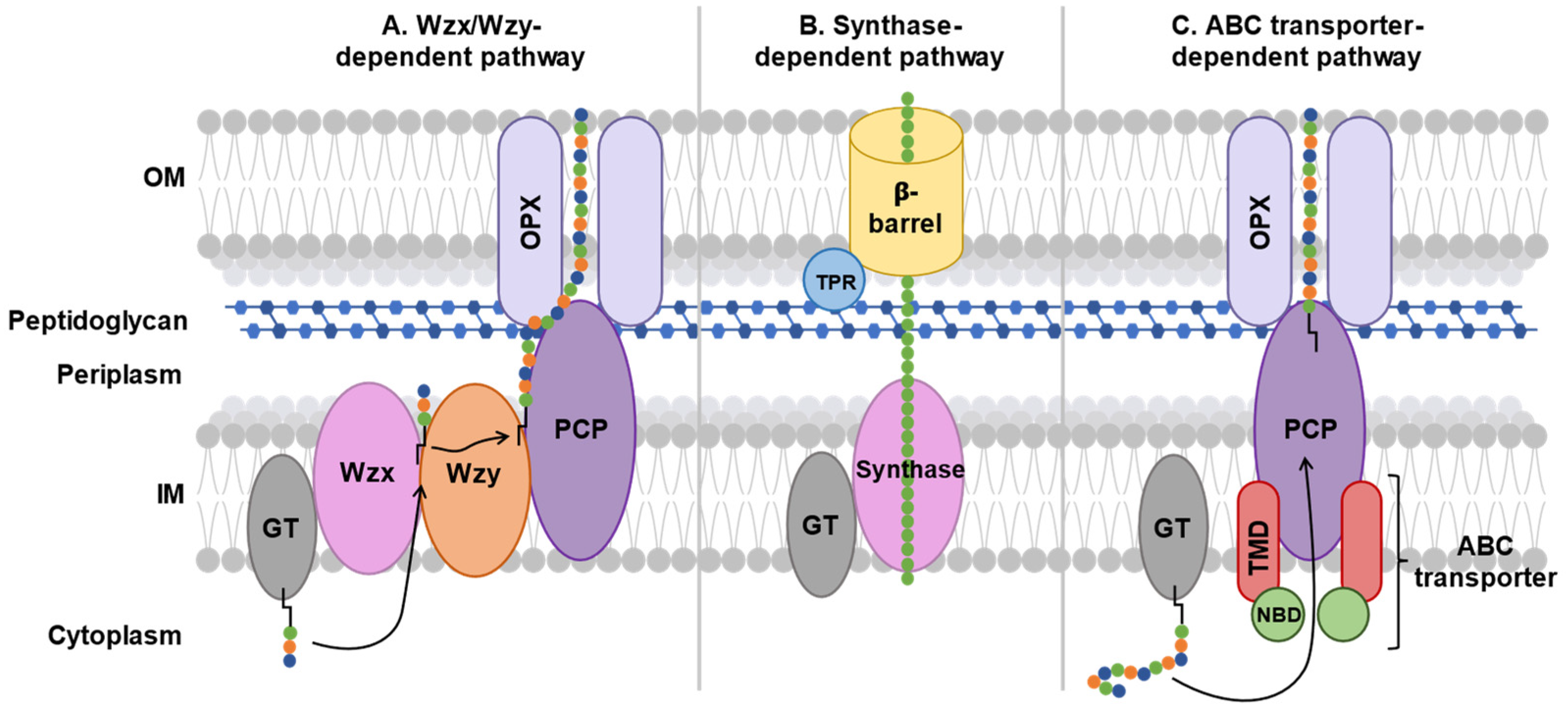
Figure 4.
Schematic comparison of major polysaccharide export pathways in Gram-negative bacteria. In each pathway, sugar residues are depicted as chains of colored circles (A–C) linked to a black glycolipid anchor (A,C). The ABC transporter-dependent pathway (C) features a complex formed by two transmembrane domain (TMD) proteins and two nucleotide-binding domain (NBD) proteins, which actively transports fully assembled polysaccharides by the glycosyltransferase (GT) across the IM. Transport across the periplasm and OM is enabled by a periplasmic polysaccharide co-polymerase (PCP) and outer membrane polysaccharide export (OPX) proteins. In contrast, the Wzx/Wzy-dependent pathway (A) involves a flippase (Wzx) and polymerase (Wzy) for polysaccharide chain assembly followed by export. The synthase-dependent pathway (B) integrates GT and synthase-mediated polymerization with OM export by a β-barrel protein coupled to a tetratricopeptide repeat (TPR) protein.
3.1. Wzx/Wzy-Dependent System
In groups 1 and 4 capsular polysaccharides, export is mediated by the Wzx/Wzy transporter-dependent system. This system is composed of six proteins: a GT, a flippase (Wzx), a polymerase (Wzy), a polysaccharide co-polymerase protein (PCP), and an outer membrane polysaccharide export protein (OPX) (Figure 4A).
Gram-negative bacteria such as E. coli serotypes K27 or O111 use the Wzx/Wzy-dependent system for polymerization of capsular polysaccharides and O-antigens in the periplasm followed by their export from the periplasm to the outer membrane [66,67]. O-antigen polysaccharides will bind to the end of the lipopolysaccharide at the outer membrane to protect bacteria from external attack, increase antibiotic resistance, evade the immune system, and stabilize the outer membrane [64,68]. Capsular polysaccharides will insert themselves into the outer membrane to fulfill their role as virulence factors [21,27].
The polymerization process of capsular polysaccharides and O-antigens occurs in the cytoplasm and begins with a GT that links a sugar residue to a lipid [38]. The flippase Wzx recognizes and transports this activated lipid into the periplasm. Then, the polymerase Wzy adds sugar moieties to the reduced end of the activated lipid, turning it into a polysaccharide. Then, Wzc, a PCP, verifies the length of this polysaccharide and exports it to the outer membrane with the help of Wza, an OPX.
Present in many Gram-negative bacterial pathogens, this Wzx/Wzy transporter-dependent system represents approximately 79% of capsular polysaccharide transporters and is the most studied and best-understood system [19,68,69,70,71].
3.2. Synthase-Dependent System
Gram-negative bacteria, such as Pseudomonas aeruginosa, use the synthase-dependent system to synthesize and export exopolysaccharides, such as cellulose and hyaluronic acid, directly from the cytoplasm [72,73]. These polysaccharide homopolymers form a biofilm that protects bacteria. The synthase-dependent system consists of a GT coupled to a translocase called synthase in the inner membrane and a tetratricopeptide repeat (TPR) protein coupled to a β-barrel exporter protein in the outer membrane (Figure 4B).
This synthase-dependent system represents approximately 10% of capsular polysaccharide transporters in Gram-negative pathogenic bacteria [19]. These are the least known and least studied capsular transporters [64].
3.3. ABC Transporter-Dependent System
In groups 2 and 3 capsular polysaccharides, export is performed using the ABC transporter-dependent system [21]. This system is composed of four proteins: the nucleotide-binding domain (NBD) and the transmembrane domain (TMD), which constitute the ABC transporter, and the PCP and OPX that make up the ABC transporter-dependent system (Figure 4C).
Gram-negative bacteria, such as Neisseria meningitidis or E. coli serotypes K1 or K10, use the ABC transporter-dependent system for the export of capsular polysaccharides to the outer membrane [66,74].
Unlike the Wzx/Wzy transporter-dependent system, polymerization of capsular polysaccharides occurs in the cytoplasm, independently of the ABC transporter-dependent system, with the help of a GT that adds sugar fragments to the glycolipid anchor. Then, the ABC transporter-dependent system recognizes the capsular polysaccharide by a process that is still poorly understood and exports it across the inner membrane [75]. Then, PCP and OPX export the capsular polysaccharide through the periplasm and outer membrane by a little-known process.
Present in Gram-negative pathogenic bacteria, this ABC transporter-dependent system represents approximately 10% of capsular polysaccharide transporters [19].
The rest of the capsule transporters are from unknown systems that allow for the export of proteins forming the protein capsules [19].
4. Export of Groups 2 and 3 Capsular Polysaccharides by ABC Transporter-Dependent Systems in Gram-Negative Bacteria
4.1. The ABC Transporter and Its Dependent System
ABC transporters are a large family of proteins found in all living organisms [76,77]. They use the energy generated by the hydrolysis of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) to import or export molecules. The ABC transporter-dependent system is composed of four proteins for capsular polysaccharides that span both membranes of Gram-negative pathogenic bacteria [21,74]. The ABC transporter itself is composed of two proteins: the NBD in the cytoplasm and the TMD in the inner membrane. The PCP, which is in the periplasm, connects the OPX in the outer membrane to the ABC transporter. The NBD hydrolyzes ATP to ADP while the TMD, PCP, and OPX form a channel that allows capsular polysaccharides to cross the inner and outer bacterial membranes [62]. Then, the capsular polysaccharide is inserted outside the outer membrane.
ABC transporter-dependent systems have been computationally predicted to have similar functions and folds [62,66,78,79,80]. However, since the different proteins of the ABC transporter-dependent systems share little amino acid sequence identity, they could have different functions. For example, Kps (for Kapsel polysaccharide synthesis) proteins were first detected in E. coli, and then homologous proteins were named similarly [54] (Table 3). Others have a different name such as Ctr, for Neisseria meningitidis, which refers to the capsule transporter [55,81].

Table 3.
Names of proteins in ABC transporter-dependent systems across different Gram-negative pathogenic bacteria.
4.2. Structural Description of the ABC Transporter-Dependent System Proteins
4.2.1. NBD and TMD, the Two ABC Transporter Proteins
The ABC transporter superfamily shares a basic architecture that includes two homodimers of NBD and TMD.
NBDs share a common fold and similar functions. The NBD is divisible into two constitutive domains: a central catalytic domain containing a Walker A motif (GXXGXGK(S/T)), a Walker B motif (ϕϕϕϕD, where ϕ is a hydrophobic residue), a Q loop, and an H motif (switch region); and a more structurally diverse α-helical domain containing the ABC transporter signature motif (LSGGQ) [82].
TMDs that export polysaccharides share a similar folding (TM-score between 0.61 and 0.81) and similar functions. TMDs are specific to the exported molecule and form the beginning of the export tunnel in the inner membrane [82].
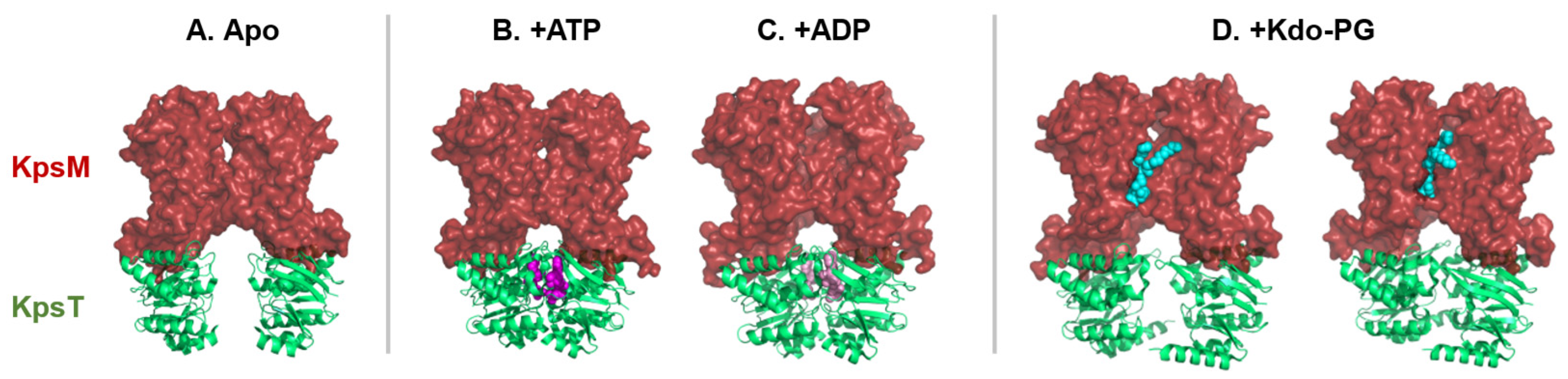
In a recent study, Kuklewicz and Zimmer [75] expressed the ABC transporter-dependent system of Caldimonas thermodepolymerans and the capsule of Pasteurella multocida in an E. coli strain [75]. Then, in the presence of ATP, they were able to obtain three-dimensional structures of the ABC transporter, KpsMT, of Caldimonas thermodepolymerans (Figure 5).
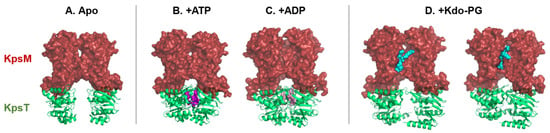
Figure 5.
Structural snapshots of the ABC transporter, KpsMT, in different ligand-bound states reveal dynamic mechanistic transitions during the export cycle. (A) Apo form (PDB: 8TSW), (B) bound to two ATP molecules (PDB: 8TSH), (C) post-hydrolysis with two ADP molecules (PDB: 8TSI) and (D) bound to glycolipid anchor (+Kdo-PG) in two distinct orientations (PDBs: 8TUN and 8TT3). The conformational space between the KpsT subunits (NBD, green cartoon) varies depending on the nucleotide state, ATP (magenta spheres), or ADP (pink spheres), suggesting substrate-dependent modulation of transporter dynamics. The glycolipid anchor (turquoise spheres) adopts distinct orientations within the binding pocket of the KpsM subunit (TMD, red surface), indicating a flexible substrate recognition mechanism.
In the absence of ATP and Pasteurella multocida capsular polysaccharides, the KpsMT transmembrane channel is closed [75] (Figure 5A). Instead, KpsM subunits (TMD) form a lipid-exposed pocket where the glycolipid anchor, a Kdo–phosphatidylglycerol lipid molecule, can bind to and be recognized by KpsM.
In the presence of ATP, the two KpsT subunits (NBD) are closer (Figure 5B). Following ATP hydrolysis in ADP, the space between the KpsM subunits on the periplasmic side increases (Figure 5C). This alters the conformations of the KpsM subunits (TMD), which may cause the polysaccharide to move into the tunnel. Binding of the glycolipid anchor between the two KpsT subunits (NBD) opens the tunnel.
In the presence of capsular polysaccharides from Pasteurella multocida, the glycolipid anchor of capsular polysaccharide is fixed in the pocket of the KpsM subunit (TMD, Figure 5D). Two structures with different orientations of the glycolipid anchor were obtained. The first orientation shows that the glycolipid anchor can be inserted between the KpsM subunits (TMD). The second orientation leads to greater space of the KpsM subunits (TMD), thus forming a tunnel between these two KpsM subunits (TMD).
4.2.2. Polysaccharide Co-Polymerase Protein (PCP)
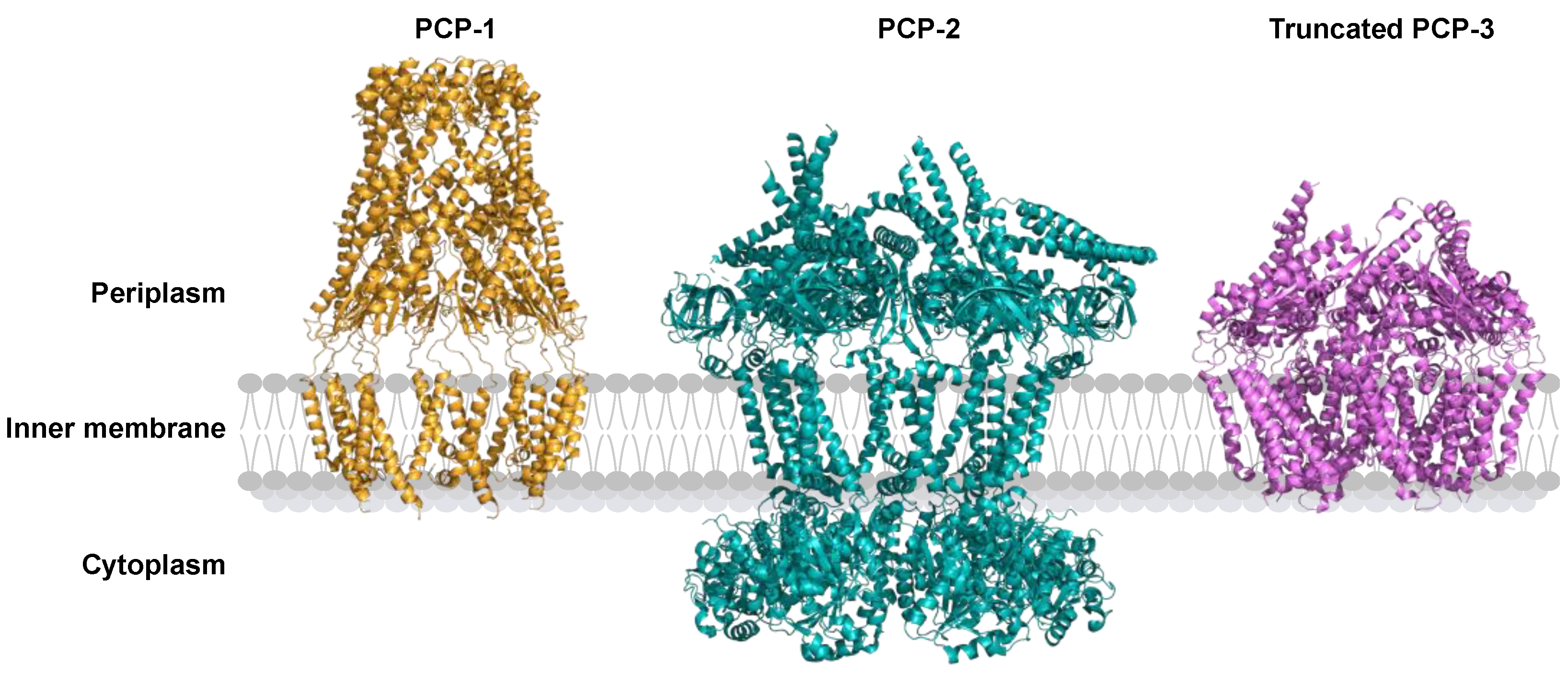
PCPs are a family of proteins that link a transporter in the internal membrane and the OPX in the external membrane [23,38]. Three PCP subfamilies exist. They have a similar folding but can have supplemental functions [66,83,84] (Figure 6).
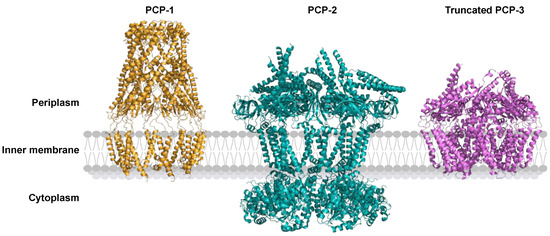
Figure 6.
Cartoon representations of three classes of polysaccharide co-polymerase (PCP) proteins illustrate structural diversity linked to their specific export pathways. PCP-1: WzzE from E. coli (PDB: 6RBG; orange), PCP-2: Wzc from E. coli (PDB: 7NHR; green), and PCP-3: truncated KpsE from Caldimonas thermodepolymerans (PDB: 8TSL; purple).
PCP-1 and PCP-2, both present in the Wzx/Wzy-dependent system, regulate polysaccharide length, with PCP-2 containing a tyrosine kinase domain. PCP-3 is present in the ABC transporter-dependent system, and its function is unknown.
PCPs share less than 10% amino acid sequence identity, but their three-dimensional structures have similarities [66].
The most conserved structural features in the PCP family are two hydrophobic α-helices anchored in the inner membrane, a large periplasmic domain made up of an α/β domain at its base and a helical hairpin at its top (Figure 6). The level of oligomerization depends on experimental conditions and varies between three and twelve polypeptides [66,78,83,85,86].
In the cytoplasmic region, PCP-2 contains a tyrosine kinase domain that PCP-1 and PCP-3 lack [66,78].
In the PCP-3 subfamily, the C-terminal helix can have different contacts with the inner membrane. In KpsE from E. coli, the α-terminal helix lies in the periplasm on the outer part of the inner membrane, whereas the same α-helix is anchored in the inner membrane in CtrB from Neisseria meningitidis [84,87].
In the periplasm, 21 hydrophobic PCP amino acids are conserved in the α/β domain and in the hairpin. The latter consists mainly of residues of leucine, alanine, isoleucine, and glycine, which form a leucine zipper [88]. Figure 6 shows that PCP-1 and PCP-3 share a common fold in the periplasmic domain, and the PCP-2 periplasmic domain is compacted [89].
4.2.3. Outer Membrane Polysaccharide Export Protein (OPX)
OPX is a family of proteins found in the outer membrane of Gram-negative bacteria [80]. These proteins allow capsular polysaccharides export by the ABC transporter-dependent system across the outer membrane.
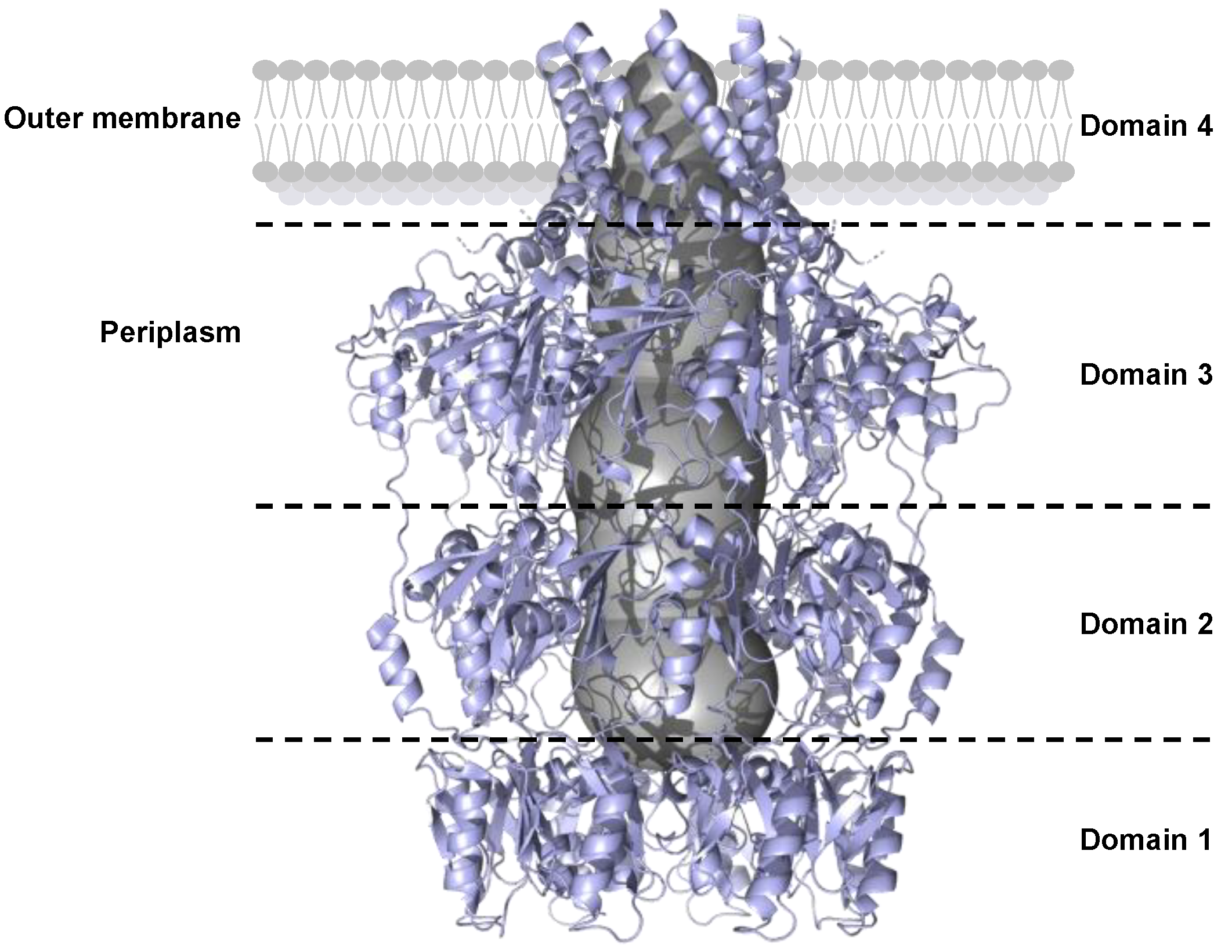
In the Protein Data Bank (PDB), the only OPX structure, including the transmembrane domain, is Wza from E. coli (PDB code: 2J58), which is an acetylated lipoprotein [90] (Figure 7). Wza forms a tunnel with eight polypeptides composed of α-helices and β-sheets. This tunnel is ~18 Å in diameter and can accommodate large molecules such as polysaccharide chains (Figure 7).
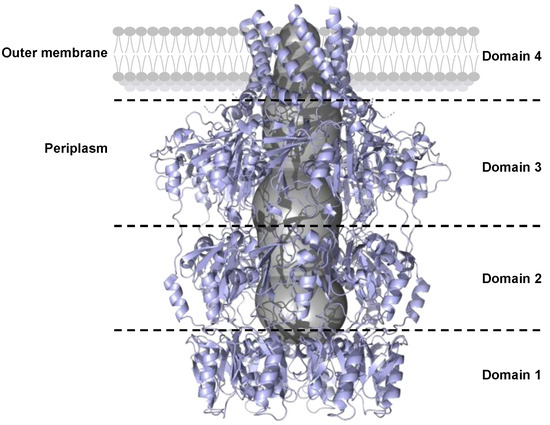
Figure 7.
Side-view cartoon representation of the OPX protein Wza from E. coli (PDB: 2J58). Individual domain organization (separated by black dotted lines) is critical for spanning the outer membrane. The central polysaccharide translocation channel, shown as a gray surface, is essential for capsule export.
Domain 1 of Wza forms a ring that is composed of an antiparallel β-sandwich on the inner part of the tunnel and α-helices on the outer face [91]. The bottom of the domain 1 ring is blocked by eight loops.
Domains 2 and 3 are duplicated and form two rings containing a mixture of β-sheets and α-helices. The ring of domain 3 is larger than that of domain 2.
Domain 4 is anchored to the outer membrane by an amphipathic α-helix barrel [91]. The channel of domain 4 is blocked by eight loops that prevent the passage of unwanted molecules from outside the cell into the periplasm.
In OPXs, very few amino acid residues are identical (approximately 1%). Despite the low level of sequence identity, OPX structure predictions indicate that these proteins fold in a similar manner [21,41,80,90,91]. Domains 1 and 2 of OPX contain the polysaccharide export sequence (PES, Pfam: 02563) that defines the OPX family [92].
OPX proteins of the ABC transporter-dependent system can contain a C-terminal Caps_synth_GfcC module (Pfam: PF06251) [66,80]. They are structurally similar to the GfcC protein belonging to the Wzx/Wzy transporter-dependent system, which exports group 4 capsular polysaccharides [80].
5. Potential Mechanism of Capsular Polysaccharide Transport by the ABC Transporter-Dependent System
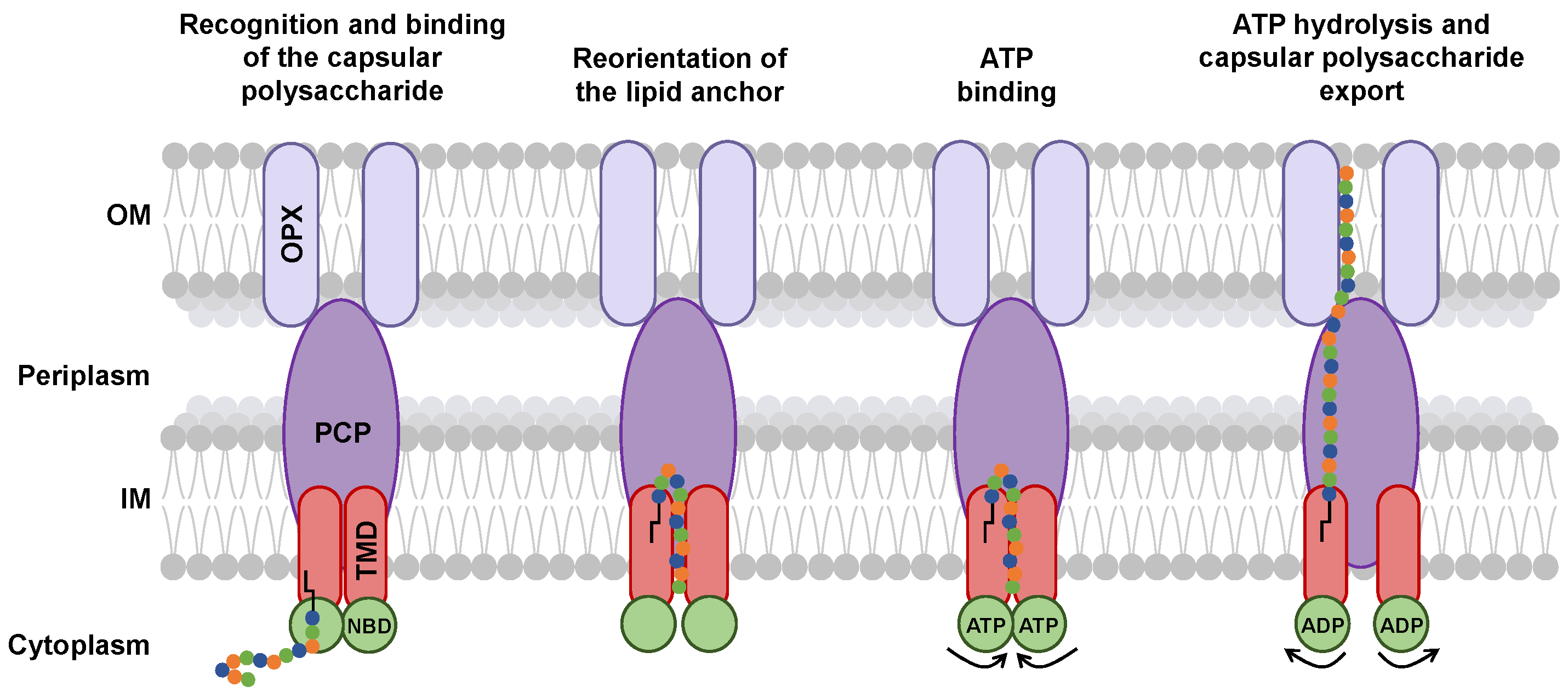
According to the experiments of Kuklewicz and Zimmer [75], the export of capsular polysaccharides by the ABC transporter-dependent system appears to begin with the recognition of the glycolipid anchor by the TMD, followed by spontaneous binding of the glycolipid anchor into the pocket of the TMD [75] (Figure 8). The two structures of the ABC transporter with the glycolipid anchor (Figure 5) show the presence of two distinct binding sites in the TMDs or a reorientation of the glycolipid anchor in the TMD pocket.
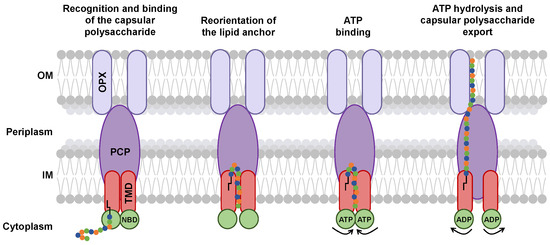
Figure 8.
Proposed mechanism of capsular polysaccharide export via the ABC transporter-dependent system. Capsular polysaccharide secretion begins with the recognition of the glycolipid anchor by the TMD (red ovals) within the IM. Reorientation of the glycolipid anchor could be enhanced by ATP-driven NBD (green circles) closure, initiating proper transport. The adjacent orientation of the TMD subunits forms a transmembrane channel through which the capsular polysaccharide (colored circles with black lipid anchor) is pushed into the transmembrane-embedded region of the PCP (purple oval). In turn, the presence of the capsular polysaccharide induces rearrangements in PCP and OPX (lilac ovals) allowing passage across the periplasm and the OM.
ATP hydrolysis changes the conformation of the KpsM subunits, forming a tunnel [75]. This movement of KpsM subunits could allow the passage of capsular polysaccharide sugars through the tunnel formed by the TMDs, PCP, and OPX [75] (Figure 8).
It is assumed that the glycolipid anchor would undergo translocation within the inner membrane from its inner face to its outer face [75] (Figure 8). There is no information on whether the glycolipid anchor is exported with or without the capsular polysaccharide. However, the hypothesis of a cleavage of the lipid from the glycolipid anchor has been put forward [75]. This would imply the existence of a process allowing the capsular polysaccharide to be linked to a new lipid in the outer membrane.
6. Discussion
Antibiotic-resistant bacteria were responsible for six million deaths in 2019 [1]. To reduce this number of deaths, the WHO published a list of antibiotic-resistant bacteria that should be prioritized for research to develop new antibiotics. In this list, seven bacteria or families of bacteria are Gram-negative [1]. To infect a host, these bacteria use many virulence factors, including capsular polysaccharides [7].
Three transporter-dependent systems (Wzx/Wzy, synthase, and ABC transporter) can export capsular polysaccharides. The Wzx/Wzy-dependent system biosynthesizes capsular polysaccharides in the cytoplasm, exports them to the periplasm, then continues the biosynthesis, and exports them to the outer membrane [64]. The synthase-dependent system biosynthesizes capsular polysaccharides in the cytoplasm and simultaneously exports them to the outer membrane. The ABC transporter-dependent system exports to the outer membrane only those capsular polysaccharides that have been biosynthesized in the cytoplasm by an independent GT.
The ABC transporter-dependent system is composed of four types of proteins: an NBD and a TMD of the ABC transporter, a PCP-3, and an OPX [64]. This complex allows capsular polysaccharides to cross the inner and outer membranes of bacteria using the hydrolysis energy of ATP.
The structures of the inner membrane-anchored ABC transporter have been solved, showing that the glycolipid anchor can spontaneously bind into the TMDs (Figure 5), and the PCP-3 complex forms a cage around the TMDs [75].
Although the capsular polysaccharide export mechanism of the ABC transporter-dependent system is not yet fully understood, Kuklewicz and Zimmer provide insight into this mechanism [75] (Figure 8). For Wzm/Wzt (homologous ABC transporter secreting O-antigen polysaccharides), present in the bacterium Aquifex aeolicus, recognition of O-antigen polysaccharides is achieved by detecting the glycolipid anchor (composed of a phosphatidylglycerol and a Kdo oligosaccharide) [93]. For the ABC transporter, KpsMT, it is possible that this principle may apply. Indeed, Kuklewicz and Zimmer assume that the glycolipid anchor, by binding to a TMD, would initiate the binding of the capsular polysaccharide to the TMD and its export in the ABC transporter, and then, the hydrolysis of ATP would modify the conformation of the ABC transporter to allow the export of the capsular polysaccharide [75]. Furthermore, for this ABC transporter, due to a pseudo-double symmetry, the glycolipid anchor appears to bind in one half of the TMD. Therefore, it seems possible that two glycolipid anchors bind at the same time in both TMD subunits [75]. This could lead to the export of two capsular polysaccharides by an ABC transporter-dependent system.
The truncated structure of octameric PCP-3 shows that it forms a cage around the ABC transporter composed of hydrophobic α-helices embedded in the inner membrane [75]. This arrangement may create a continuous tunnel from the TMD to the OPX. There are three subfamilies of PCP. The structure of PCP-3 is more similar to the structure of PCP-1, but PCP-3 does not control the length of the capsular polysaccharide [66].
In the ABC transporter-dependent system, OPX can have two types of transmembrane domains, either with an α-helix or a GfcC-like domain [80]. GfcC is a soluble ligand-binding β-protein and is expected to interact with the capsular polysaccharide [94]. The difference in transmembrane domains could indicate that OPXs have different functions.
However, some parts of the mechanism of capsular polysaccharide export by the ABC transporter-dependent system are still not understood:
- Is the glycolipid anchor exported with the capsular polysaccharide? Kuklewicz and Zimmer hypothesize that the glycolipid anchor could be cleaved from the capsular polysaccharides when the capsular polysaccharide is engaged in the tunnel [75]. Indeed, there is currently no evidence for lipid transport through the ABC transporter-dependent system. This also implies that another glycolipid anchor would be available in the outer membrane to bind with a capsular polysaccharide.
- How is the glycolipid anchor bound to the polysaccharide chain? The mechanism by which the glycolipid anchor is covalently linked to the polysaccharide chain has remained unclear in the context of capsule biosynthesis. A recent study by Litschko et al. [95] begins to shed light on this process by identifying two transition transferases (TTs; CpsA and CpsC) in Actinobacillus pleuropneumoniae that generate a poly(glycerol-3-phosphate) linker between the conserved glycolipid anchor and the polysaccharide chain. These TTs were also found to stimulate downstream polymerization, suggesting a coordinated mechanism together with GTs. These findings support the idea that the glycolipid anchor could be an export signal and might be already associated with the ABC transporter-dependent system before the capsular polysaccharide chain is extended. While homologs of TTs have been identified in other bacterial genomes, it is still unclear whether this mechanism can be generalized.
- Do PCP and OPX have functions other than that of exporter? Depending on PCP and OPX, this could be possible. Indeed, the C-terminal helix of PCP-3 does not interact in the same way with the inner membrane in different bacterial species [84,87]. This interaction could have an effect on the function of PCP-3. In addition, some OPXs of the ABC transporter-dependent system may have a transmembrane domain that replaces the α-helix [80]. Interaction of this domain with capsular polysaccharides could modify the export process.
A better understanding of the ABC transporter-dependent system would allow the development of capsular polysaccharide export inhibitors to combat Gram-negative bacterial pathogens and to counter antibiotic resistance.
7. Future Perspectives
A deeper understanding of the ABC transporter-dependent system is currently limited by the technical difficulties in expressing and purifying the entire multi-protein complex. Overcoming these challenges requires innovative approaches and a combination of structural, biochemical, and computational tools. To overcome this problem, high-resolution structural information can be directly obtained in cells using cryo-electron tomography, which allows visualization of macromolecular complexes in their native environment as well as dynamic information of the export process [75,96,97,98]. Complementary structural techniques, such as cryo-electron microscopy and X-ray crystallography, particularly in the presence of nucleotides or capsular polysaccharides, can provide static snapshots of distinct conformational states of the ABC transporter-dependent system [75]. Super-Resolution Fluorescence Microscopy offers a powerful addition to the toolkit, enabling real-time tracking of capsular polysaccharide dynamics and localization at a sub-cellular level [99]. These techniques could help fill in the gaps left by static structural data.
Another difficulty in the study of bacterial capsules is related to their ability to express in vitro. Factors such as nutrient availability, temperature, oxygen level, and growth phase can affect the expression of capsular polysaccharides [15,100]. This variability makes it challenging to determine capsular polysaccharides production and test drug candidates or genetic modifications under laboratory conditions, although they can be detected by specific bacteriophages to confirm capsular polysaccharides presence and accessibility [101].
Biophysics methods like native mass spectrometry or single-molecule FRET could be used to study molecular interactions between the exporter and ligands as well as conformational changes in the transporter complex in real time [102,103,104]. Development of functional studies in proteoliposomes will allow a better understanding of mechanisms to export polysaccharides but also to test small molecules developed to inhibit the export of the capsular polysaccharides [105].
As only 10% of Gram-negative bacteria have the ABC transporter-dependent system, detection of strains possessing the ABC transporter-dependent system is necessary to avoid administering unnecessary medication to a patient [19]. On a translational level, quick detection assays could be developed to assess if the host is contaminated with a Gram-negative bacterium with this transporter. A proposed strategy involves a two-step diagnostic pipeline: (1) a 16S ribosomal RNA detection-based assay that allows users to know the bacterial species in less than an hour [106], followed by (2) serotype-specific detection [107].
From a vaccine development perspective, synthetic, enzymatic, and chemo-enzymatic polymer syntheses of capsule antigens, as well as glycoengineering, present promising avenues for glycoconjugate vaccine production, targeting both capsular polysaccharides and OPXs [108,109,110]. In parallel, the availability of computational tools [19] to predict capsule loci and types from genomic data will be a valuable tool for surveillance and typing but also for informing serotype-specific therapeutic and preventive strategies.
In summary, while significant progress has been made in dissecting the structure and function of ABC transporter-dependent systems, many details remain to be elucidated, especially mechanistic. Advancing this field will require the integration of structural, functional, and computational approaches, alongside improved in vitro models and targeted diagnostic tools.
Author Contributions
Individual contributions were as follows: Writing—original draft preparation, L.M.--J.; writing—review and editing, L.M.--J. and M.A.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Kristina Djinovic-Carugo of EMBL Grenoble for her support, encouragement, and valuable advice in the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO Bacterial Priority Pathogens List 2024 Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1.
- Hill, D.J.; Griffiths, N.J.; Borodina, E.; Virji, M. Cellular and Molecular Biology of Neisseria Meningitidis Colonization and Invasive Disease. Clin. Sci. 2010, 118, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Blokesch, M. Natural Competence for Transformation. Curr. Biol. 2016, 26, R1126–R1130. [Google Scholar] [CrossRef]
- Hsiao, A.; Zhu, J. Pathogenicity and Virulence Regulation of Vibrio Cholerae at the Interface of Host-Gut Microbiome Interactions. Virulence 2020, 11, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Bartley, S.N.; Tzeng, Y.L.; Heel, K.; Lee, C.W.; Mowlaboccus, S.; Seemann, T.; Lu, W.; Lin, Y.H.; Ryan, C.S.; Peacock, C.; et al. Attachment and Invasion of Neisseria Meningitidis to Host Cells Is Related to Surface Hydrophobicity, Bacterial Cell Size and Capsule. PLoS ONE 2013, 8, e55798. [Google Scholar] [CrossRef]
- Livorsi, D.J.; Stenehjem, E.; Stephens, D.S. Virulence Factors of Gram-Negative Bacteria in Sepsis with a Focus on Neisseria Meningitidis. Contrib. Microbiol. 2011, 17, 31–47. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L. Virulence Factors and Their Mechanisms of Action: The View from a Damage–Response Framework. J. Water Health 2009, 7, S2–S18. [Google Scholar] [CrossRef]
- Silale, A.; Zhu, Y.; Witwinowski, J.; Smith, R.E.; Newman, K.E.; Bhamidimarri, S.P.; Baslé, A.; Khalid, S.; Beloin, C.; Gribaldo, S.; et al. Dual Function of OmpM as Outer Membrane Tether and Nutrient Uptake Channel in Diderm Firmicutes. Nat. Commun. 2023, 14, 7152. [Google Scholar] [CrossRef] [PubMed]
- Cifuente, J.O.; Schulze, J.; Bethe, A.; Di Domenico, V.; Litschko, C.; Budde, I.; Eidenberger, L.; Thiesler, H.; Ramón Roth, I.; Berger, M.; et al. A Multi-Enzyme Machine Polymerizes the Haemophilus Influenzae Type b Capsule. Nat. Chem. Biol. 2023, 19, 865–877. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Thomas, V.C.; Hancock, L.E. Capsular Polysaccharide Production in Enterococcus Faecalis and Contribution of CpsF to Capsule Serospecificity. J. Bacteriol. 2009, 191, 6203–6210. [Google Scholar] [CrossRef]
- Akoolo, L.; Pires, S.; Kim, J.; Parker, D. The Capsule of Acinetobacter Baumannii Protects against the Innate Immune Response. J. Innate Immun. 2022, 14, 543–554. [Google Scholar] [CrossRef]
- Kalscheuer, R.; Palacios, A.; Anso, I.; Cifuente, J.; Anguita, J.; Jacobs, W.R., Jr.; Guerin, M.E.; Prados-Rosales, R. The Mycobacterium Tuberculosis Capsule: A Cell Structure with Key Implications in Pathogenesis. Biochem. J. 2019, 476, 1995–2016. [Google Scholar] [CrossRef]
- Lee, G.Y.; Song, J. Single Missense Mutations in Vi Capsule Synthesis Genes Confer Hypervirulence to Salmonella Typhi. Nat. Commun. 2024, 15, 5258. [Google Scholar] [CrossRef]
- Perera, S.R.; Sokaribo, A.S.; White, A.P. Polysaccharide Vaccines: A Perspective on Non-Typhoidal Salmonella. Polysaccharides 2021, 2, 691–714. [Google Scholar] [CrossRef]
- O’Riordan, K.; Lee, J.C. Staphylococcus Aureus Capsular Polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef]
- Wessels, M.R. Capsular Polysaccharide of Group A Streptococcus. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Paton, J.C.; Trappetti, C. Streptococcus Pneumoniae Capsular Polysaccharide. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.J.; Dongas, S.; Ratner, A.J. Capsule Production Promotes Group B Streptococcus Intestinal Colonization. Microbiol. Spectr. 2023, 11, e02349-23. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, O.; Garcia-Garcerà, M.; Néron, B.; Touchon, M.; Rocha, E.P.C. Abundance and Co-Occurrence of Extracellular Capsules Increase Environmental Breadth: Implications for the Emergence of Pathogens. PLoS Pathog. 2017, 13, e1006525. [Google Scholar] [CrossRef]
- Willis, L.M.; Stupak, J.; Richards, M.R.; Lowary, T.L.; Li, J.; Whitfield, C. Conserved Glycolipid Termini in Capsular Polysaccharides Synthesized by ATP-Binding Cassette Transporter-Dependent Pathways in Gram-Negative Pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 7868–7873. [Google Scholar] [CrossRef]
- Silver, R.P.; Prior, K.; Nsahlai, C.; Wright, L.F. ABC Transporters and the Export of Capsular Polysaccharides from Gram-Negative Bacteria. Res. Microbiol. 2001, 152, 357–364. [Google Scholar] [CrossRef]
- Pelkonen, S.; Häyrinen, J.; Finne, J. Polyacrylamide Gel Electrophoresis of the Capsular Polysaccharides of Escherichia coli K1 and Other Bacteria. J. Bacteriol. 1988, 170, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, K.D.; Golecki, J.R.; Jann, K. Further Electron Microscopic Studies on the Expression of Escherichia coli Group II Capsules. J. Bacteriol. 1990, 172, 3469–3472. [Google Scholar] [CrossRef]
- Stephens, D.S. Neisseria Meningitidis. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E.M.D., Dolin, R.M.D., Blaser, M.J.M.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 2585–2607.e7. ISBN 978-0-323-48255-4. [Google Scholar]
- Tzeng, Y.-L.; Thomas, J.; Stephens, D.S. Regulation of Capsule in Neisseria Meningitidis. Crit. Rev. Microbiol. 2015, 42, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, W.; Quan, Y.; Li, Y.; Shen, Y.; Yuan, S.; Yi, L.; Wang, Y.; Wang, Y. Bacterial Capsules: Occurrence, Mechanism, and Function. npj Biofilms Microbiomes 2024, 10, 21. [Google Scholar] [CrossRef]
- Sukupolvi-Petty, S.; Grass, S.; StGeme, J.W. The Haemophilus Influenzae Type b hcsA and hcsB Gene Products Facilitate Transport of Capsular Polysaccharide across the Outer Membrane and Are Essential for Virulence. J. Bacteriol. 2006, 188, 3870–3877. [Google Scholar] [CrossRef]
- Frosch, M.; Edwards, U.; Bousset, K.; Krausse, B.; Weisgerber, C. Evidence for a Common Molecular Origin of the Capsule Gene Loci in Gram-Negative Bacteria Expressing Group II Capsular Polysaccharides. Mol. Microbiol. 1991, 5, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.S.; Loynds, B.; Brophy, L.N.; Moxon, E.R. The Bex Locus in Encapsulated Haemophilus Influenzae: A Chromosomal Region Involved in Capsule Polysaccharide Export. Mol. Microbiol. 1990, 4, 1853–1862. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Li, N.; Yokoyama, H.; Ezaki, T. Complete Nucleotide Sequence and Molecular Characterization of ViaB Region Encoding Vi Antigen in Salmonella Typhi. J. Bacteriol. 1993, 175, 4456–4465. [Google Scholar] [CrossRef]
- Moxon, E.R.; Vaughn, K.A. The Type b Capsular Polysaccharide as a Virulence Determinant of Haemophilus Influenzae: Studies Using Clinical Isolates and Laboratory Transformants. J. Infect. Dis. 1981, 143, 517–524. [Google Scholar] [CrossRef]
- Keo, T.; Collins, J.; Kunwar, P.; Blaser, M.J.; Iovine, N.M. Campylobacter Capsule and Lipooligosaccharide Confer Resistance to Serum and Cationic Antimicrobials. Virulence 2011, 2, 30–40. [Google Scholar] [CrossRef]
- Arredondo-Alonso, S.; Blundell-Hunter, G.; Fu, Z.; Gladstone, R.A.; Fillol-Salom, A.; Loraine, J.; Cloutman-Green, E.; Johnsen, P.J.; Samuelsen, Ø.; Pöntinen, A.K.; et al. Evolutionary and Functional History of the Escherichia coli K1 Capsule. Nat. Commun. 2023, 14, 3294. [Google Scholar] [CrossRef]
- Guerry, P.; Poly, F.; Riddle, M.; Maue, A.C.; Chen, Y.-H.; Monteiro, M.A. Campylobacter Polysaccharide Capsules: Virulence and Vaccines. Front. Cell. Inf. Microbiol. 2012, 2, 7. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella Multocida: From Zoonosis to Cellular Microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, R.; Maturrano, L.; Azevedo, V.; Aburjaile, F. Pathogenomics Insights for Understanding Pasteurella Multocida Adaptation. Int. J. Med. Microbiol. 2020, 310, 151417. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Roberts, I.S. Structure, Assembly and Regulation of Expression of Capsules in Escherichia Coli. Mol. Microbiol. 1999, 31, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis and Assembly of Capsular Polysaccharides in Escherichia Coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef]
- Diaz Romero, J.; Outschoorn, I.M. Current Status of Meningococcal Group B Vaccine Candidates: Capsular or Noncapsular? Clin. Microbiol. Rev. 1994, 7, 559–575. [Google Scholar] [CrossRef]
- Lin, D.; Fan, J.; Wang, J.; Liu, L.; Xu, L.; Li, F.; Yang, J.; Li, B. The Fructose-Specific Phosphotransferase System of Klebsiella Pneumoniae Is Regulated by Global Regulator CRP and Linked to Virulence and Growth. Infect. Immun. 2018, 86, e00340-18. [Google Scholar] [CrossRef]
- Niu, T.; Guo, L.; Luo, Q.; Zhou, K.; Yu, W.; Chen, Y.; Huang, C.; Xiao, Y. Wza Gene Knockout Decreases Acinetobacter Baumannii Virulence and Affects Wzy-Dependent Capsular Polysaccharide Synthesis. Virulence 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Schembri, M.A.; Dalsgaard, D.; Klemm, P. Capsule Shields the Function of Short Bacterial Adhesins. J. Bacteriol. 2004, 186, 1249–1257. [Google Scholar] [CrossRef]
- Zafar, M.A.; Hamaguchi, S.; Zangari, T.; Cammer, M.; Weiser, J.N. Capsule Type and Amount Affect Shedding and Transmission of Streptococcus Pneumoniae. mBio 2017, 8, e00989-17. [Google Scholar] [CrossRef]
- Stephens, D.S.; Swartley, J.S.; Kathariou, S.; Morse, S.A. Insertion of Tn916 in Neisseria Meningitidis Resulting in Loss of Group B Capsular Polysaccharide. Infect. Immun. 1991, 59, 4097–4102. [Google Scholar] [CrossRef]
- Ophir, T.; Gutnick, D.L. A Role for Exopolysaccharides in the Protection of Microorganisms from Desiccation. Appl. Environ. Microbiol. 1994, 60, 740–745. [Google Scholar] [CrossRef]
- Gao, S.; Lewis, G.D.; Ashokkumar, M.; Hemar, Y. Inactivation of Microorganisms by Low-Frequency High-Power Ultrasound: 1. Effect of Growth Phase and Capsule Properties of the Bacteria. Ultrason. Sonochem. 2014, 21, 446–453. [Google Scholar] [CrossRef]
- Waz, N.T.; Oliveira, S.; Girardello, R.; Lincopan, N.; Barazzone, G.; Parisotto, T.; Hakansson, A.P.; Converso, T.R.; Darrieux, M. Influence of the Polysaccharide Capsule on the Bactericidal Activity of Indolicidin on Streptococcus Pneumoniae. Front. Microbiol. 2022, 13, 898815. [Google Scholar] [CrossRef]
- Llobet, E.; Tomás, J.M.; Bengoechea, J.A. Capsule Polysaccharide Is a Bacterial Decoy for Antimicrobial Peptides. Microbiology 2008, 154, 3877–3886. [Google Scholar] [CrossRef]
- De Smidt, O.; Albertyn, J.; Bragg, R.R.; Van Heerden, E. Genetic Organisation of the Capsule Transport Gene Region from Haemophilus paragallinarum. Onderstepoort J. Vet. Res. 2004, 71, 139–152. [Google Scholar] [CrossRef]
- Van Eldere, J.; Brophy, L.; Loynds, B.; Celis, P.; Hancock, I.; Carman, S.; Kroll, J.S.; Moxon, E.R. Region II of the Haemophilus Influenzae Type Be Capsulation Locus Is Involved in Serotype-Specific Polysaccharide Synthesis. Mol. Microbiol. 1995, 15, 107–118. [Google Scholar] [CrossRef]
- Kroll, J.S.; Zamze, S.; Loynds, B.; Moxon, E.R. Common Organization of Chromosomal Loci for Production of Different Capsular Polysaccharides in Haemophilus Influenzae. J. Bacteriol. 1989, 171, 3343–3347. [Google Scholar] [CrossRef]
- Bliss, J.M.; Garon, C.F.; Silver, R.P. Polysialic Acid Export in Escherichia Coli K1: The Role of KpsT, the ATP-Binding Component of an ABC Transporter, in Chain Translocation. Glycobiology 1996, 6, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S. The Biochemistry and Genetics of Capsular Polysaccharide Production in Bacteria. Annu. Rev. Microbiol. 1996, 50, 285–315. [Google Scholar] [CrossRef]
- Vimr, E.; Steenbergen, S.; Cieslewicz, M. Biosynthesis of the Polysialic Acid Capsule inEscherichia Coli K1. J. Ind. Microbiol. 1995, 15, 352–360. [Google Scholar] [CrossRef]
- Frosch, M.; Weisgerber, C.; Meyer, T.F. Molecular Characterization and Expression in Escherichia Coli of the Gene Complex Encoding the Polysaccharide Capsule of Neisseria Meningitidis Group B. Proc. Natl. Acad. Sci. USA 1989, 86, 1669–1673. [Google Scholar] [CrossRef]
- Karlyshev, A.V.; Champion, O.L.; Churcher, C.; Brisson, J.-R.; Jarrell, H.C.; Gilbert, M.; Brochu, D.; St Michael, F.; Li, J.; Wakarchuk, W.W.; et al. Analysis of Campylobacter Jejuni Capsular Loci Reveals Multiple Mechanisms for the Generation of Structural Diversity and the Ability to Form Complex Heptoses. Mol. Microbiol. 2005, 55, 90–103. [Google Scholar] [CrossRef]
- Clemence, M.E.A.; Maiden, M.C.J.; Harrison, O.B. Characterization of Capsule Genes in Non-Pathogenic Neisseria Species. Microb. Genom. 2018, 4, e000208. [Google Scholar] [CrossRef]
- Cieslewicz, M.; Vimr, E. Thermoregulation of kpsF, the First Region 1 Gene in the Kps Locus for Polysialic Acid Biosynthesis in Escherichia Coli K1. J. Bacteriol. 1996, 178, 3212–3220. [Google Scholar] [CrossRef]
- Doyle, L.; Ovchinnikova, O.G.; Huang, B.-S.; Forrester, T.J.B.; Lowary, T.L.; Kimber, M.S.; Whitfield, C. Mechanism and Linkage Specificities of the Dual Retaining β-Kdo Glycosyltransferase Modules of KpsC from Bacterial Capsule Biosynthesis. J. Biol. Chem. 2023, 299, 104609. [Google Scholar] [CrossRef]
- Willis, L.M.; Whitfield, C. KpsC and KpsS Are Retaining 3-Deoxy-D-Manno-Oct-2-Ulosonic Acid (Kdo) Transferases Involved in Synthesis of Bacterial Capsules. Proc. Natl. Acad. Sci. USA 2013, 110, 20753–20758. [Google Scholar] [CrossRef]
- Doyle, L.; Ovchinnikova, O.G.; Myler, K.; Mallette, E.; Huang, B.S.; Lowary, T.L.; Kimber, M.S.; Whitfield, C. Biosynthesis of a Conserved Glycolipid Anchor for Gram-Negative Bacterial Capsules. Nat. Chem. Biol. 2019, 15, 632–640. [Google Scholar] [CrossRef]
- Willis, L.M.; Whitfield, C. Structure, Biosynthesis, and Function of Bacterial Capsular Polysaccharides Synthesized by ABC Transporter-Dependent Pathways. Carbohydr. Res. 2013, 378, 35–44. [Google Scholar] [CrossRef]
- Liston, S.D.; Ovchinnikova, O.G.; Whitfield, C. Unique Lipid Anchor Attaches Vi Antigen Capsule to the Surface of Salmonella Enterica Serovar Typhi. Proc. Natl. Acad. Sci. USA 2016, 113, 6719–6724. [Google Scholar] [CrossRef]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of Bacterial Capsular Polysaccharides and Exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543. [Google Scholar] [CrossRef]
- Caffalette, C.A.; Kuklewicz, J.; Spellmon, N.; Zimmer, J. Biosynthesis and Export of Bacterial Glycolipids. Annu. Rev. Biochem. 2020, 89, 741–768. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Mainprize, I.L.; Naismith, J.H.; Whitfield, C. Pivotal Roles of the Outer Membrane Polysaccharide Export and Polysaccharide Copolymerase Protein Families in Export of Extracellular Polysaccharides in Gram-Negative Bacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 155–177. [Google Scholar] [CrossRef]
- Standish, A.J.; Morona, R. Capsule Structure, Synthesis, and Regulation. In Streptococcus Pneumoniae; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 169–179. ISBN 978-0-12-410530-0. [Google Scholar]
- Perez-Burgos, M.; Garcia-Romero, I.; Jung, J.; Schander, E.; Valvano, M.A.; Sogaard-Andersen, L. Characterization of the Exopolysaccharide Biosynthesis Pathway in Myxococcus Xanthus. J. Bacteriol. 2020, 202, e00335-20. [Google Scholar] [CrossRef]
- Cross, A.S.; Kim, K.S.; Wright, D.C.; Sadoff, J.C.; Gemski, P. Role of Lipopolysaccharide and Capsule in the Serum Resistance of Bacteremic Strains of Escherichia Coli. J. Infect. Dis. 1986, 154, 497–503. [Google Scholar] [CrossRef]
- Hug, I.; Feldman, M.F. Analogies and Homologies in Lipopolysaccharide and Glycoprotein Biosynthesis in Bacteria. Glycobiology 2011, 21, 138–151. [Google Scholar] [CrossRef]
- Huszczynski, S.M.; Coumoundouros, C.; Pham, P.; Lam, J.S.; Khursigara, C.M. Unique Regions of the Polysaccharide Copolymerase Wzz(2) from Pseudomonas Aeruginosa Are Essential for O-Specific Antigen Chain Length Control. J. Bacteriol. 2019, 201, e00165-19. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial Exopolysaccharides: Biosynthesis Pathways and Engineering Strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef]
- Weigel, P.H.; DeAngelis, P.L. Hyaluronan Synthases: A Decade-plus of Novel Glycosyltransferases. J. Biol. Chem. 2007, 282, 36777–36781. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Kos, V.; Whitfield, C. ABC Transporters Involved in Export of Cell Surface Glycoconjugates. Microbiol. Mol. Biol. Rev. 2010, 74, 341–362. [Google Scholar] [CrossRef]
- Kuklewicz, J.; Zimmer, J. Molecular Insights into Capsular Polysaccharide Secretion. Nature 2024, 628, 901–909. [Google Scholar] [CrossRef]
- George, A.M. ABC Transporters—40 Years On, 1st ed.; George, A.M., Ed.; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-23475-5. [Google Scholar]
- Theodoulou, F.L.; Kerr, I.D. ABC Transporter Research: Going Strong 40 Years On. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Kalynych, S.; Cherney, M.; Bostina, M.; Rouiller, I.; Cygler, M. Quaternary Structure of WzzB and WzzE Polysaccharide Copolymerases. Protein Sci. 2015, 24, 58–69. [Google Scholar] [CrossRef]
- Thomas, C.; Aller, S.G.; Beis, K.; Carpenter, E.P.; Chang, G.; Chen, L.; Dassa, E.; Dean, M.; Duong Van Hoa, F.; Ekiert, D.; et al. Structural and Functional Diversity Calls for a New Classification of ABC Transporters. FEBS Lett. 2020, 594, 3767–3775. [Google Scholar] [CrossRef]
- Sande, C.; Bouwman, C.; Kell, E.; Nickerson, N.N.; Kapadia, S.B.; Whitfield, C. Structural and Functional Variation in Outer Membrane Polysaccharide Export (OPX) Proteins from the Two Major Capsule Assembly Pathways Present in Escherichia Coli. J. Bacteriol. 2019, 201, e00213-19. [Google Scholar] [CrossRef]
- Harrison, O.B.; Claus, H.; Jiang, Y.; Bennett, J.S.; Bratcher, H.B.; Jolley, K.A.; Corton, C.; Care, R.; Poolman, J.T.; Zollinger, W.D.; et al. Description and Nomenclature of Neisseria Meningitidis Capsule Locus. Emerg. Infect. Dis. 2013, 19, 566. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Kalynych, S.; Yao, D.; Magee, J.; Cygler, M. Structural Characterization of Closely Related O-Antigen Lipopolysaccharide (LPS) Chain Length Regulators. J. Biol. Chem. 2012, 287, 15696–15705. [Google Scholar] [CrossRef]
- Larue, K.; Ford, R.C.; Willis, L.M.; Whitfield, C. Functional and Structural Characterization of Polysaccharide Co-Polymerase Proteins Required for Polymer Export in ATP-Binding Cassette Transporter-Dependent Capsule Biosynthesis Pathways. J. Biol. Chem. 2011, 286, 16658–16668. [Google Scholar] [CrossRef]
- Morona, R.; Purins, L.; Tocilj, A.; Matte, A.; Cygler, M. Sequence-Structure Relationships in Polysaccharide Co-Polymerase (PCP) Proteins. Trends Biochem. Sci. 2009, 34, 78–84. [Google Scholar] [CrossRef]
- Chang, C.W.; Tran, E.N.; Ericsson, D.J.; Casey, L.W.; Lonhienne, T.; Benning, F.; Morona, R.; Kobe, B. Structural and Biochemical Analysis of a Single Amino-Acid Mutant of WzzBSF That Alters Lipopolysaccharide O-Antigen Chain Length in Shigella Flexneri. PLoS ONE 2015, 10, e0138266. [Google Scholar] [CrossRef]
- Phoenix, D.A.; Brandenburg, K.; Harris, F.; Seydel, U.; Hammerton, T.; Roberts, I.S. An Investigation into the Membrane-Interactive Potential of the Escherichia Coli KpsE C-Terminus. Biochem. Biophys. Res. Commun. 2001, 285, 976–980. [Google Scholar] [CrossRef]
- Papadopoulos, M.; Morona, R. Mutagenesis and Chemical Cross-Linking Suggest That Wzz Dimer Stability and Oligomerization Affect Lipopolysaccharide O-Antigen Modal Chain Length Control. J. Bacteriol. 2010, 192, 3385–3393. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Clarke, B.R.; Seidel, L.; Bolla, J.R.; Ward, P.N.; Zhang, P.; Robinson, C.V.; Whitfield, C.; Naismith, J.H. The Molecular Basis of Regulation of Bacterial Capsule Assembly by Wzc. Nat. Commun. 2021, 12, 4349. [Google Scholar] [CrossRef]
- Dong, C.; Beis, K.; Nesper, J.; Brunkan-Lamontagne, A.L.; Clarke, B.R.; Whitfield, C.; Naismith, J.H. Wza the Translocon for E. coli Capsular Polysaccharides Defines a New Class of Membrane Protein. Nature 2006, 444, 226–229. [Google Scholar] [CrossRef]
- Beis, K.; Collins, R.F.; Ford, R.C.; Kamis, A.B.; Whitfield, C.; Naismith, J.H. Three-Dimensional Structure of Wza, the Protein Required for Translocation of Group 1 Capsular Polysaccharide across the Outer Membrane of Escherichia coli. J. Biol. Chem. 2004, 279, 28227–28232. [Google Scholar] [CrossRef]
- Stevenson, G.; Andrianopoulos, K.; Hobbs, M.; Reeves, P.R. Organization of the Escherichia coli K-12 Gene Cluster Responsible for Production of the Extracellular Polysaccharide Colanic Acid. J. Bacteriol. 1996, 178, 4885–4893. [Google Scholar] [CrossRef]
- Caffalette, C.A.; Zimmer, J. Cryo-EM Structure of the Full-Length WzmWzt ABC Transporter Required for Lipid-Linked O Antigen Transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2016144118. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Balaji, S.; Iyer, L.M.; Aravind, L. A Novel Superfamily Containing the β-Grasp Fold Involved in Binding Diverse Soluble Ligands. Biol. Direct 2007, 2, 4. [Google Scholar] [CrossRef]
- Litschko, C.; Di Domenico, V.; Schulze, J.; Li, S.; Ovchinnikova, O.G.; Voskuilen, T.; Bethe, A.; Cifuente, J.O.; Marina, A.; Budde, I.; et al. Transition Transferases Prime Bacterial Capsule Polymerization. Nat. Chem. Biol. 2025, 21, 120–130. [Google Scholar] [CrossRef]
- Yan, X.; Li, S.; Huang, W.; Wang, H.; Zhao, T.; Huang, M.; Zhou, N.; Shen, Y.; Li, X. MPicker: Visualizing and Picking Membrane Proteins for Cryo-Electron Tomography. Nat. Commun. 2025, 16, 472. [Google Scholar] [CrossRef]
- Dunstone, M.A.; de Marco, A. Cryo-Electron Tomography: An Ideal Method to Study Membrane-Associated Proteins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160210. [Google Scholar] [CrossRef]
- Keller, J.; Fernández-Busnadiego, R. In Situ Studies of Membrane Biology by Cryo-Electron Tomography. Curr. Opin. Cell Biol. 2024, 88, 102363. [Google Scholar] [CrossRef]
- Phanphak, S.; Georgiades, P.; Li, R.; King, J.; Roberts, I.S.; Waigh, T.A. Super-Resolution Fluorescence Microscopy Study of the Production of K1 Capsules by Escherichia coli: Evidence for the Differential Distribution of the Capsule at the Poles and the Equator of the Cell. Langmuir 2019, 35, 5635–5646. [Google Scholar] [CrossRef]
- Aldawood, E.; Roberts, I.S. Regulation of Escherichia coli Group 2 Capsule Gene Expression: A Mini Review and Update. Front. Microbiol. 2022, 13, 858767. [Google Scholar] [CrossRef]
- Hao, G.; Shu, R.; Ding, L.; Chen, X.; Miao, Y.; Wu, J.; Zhou, H.; Wang, H. Bacteriophage SRD2021 Recognizing Capsular Polysaccharide Shows Therapeutic Potential in Serotype K47 Klebsiella Pneumoniae Infections. Antibiotics 2021, 10, 894. [Google Scholar] [CrossRef]
- Chorev, D.S.; Baker, L.A.; Wu, D.; Beilsten-Edmands, V.; Rouse, S.L.; Zeev-Ben-Mordehai, T.; Jiko, C.; Samsudin, F.; Gerle, C.; Khalid, S.; et al. Protein Assemblies Ejected Directly from Native Membranes Yield Complexes for Mass Spectrometry. Science 2018, 362, 829–834. [Google Scholar] [CrossRef]
- Bolla, J.R.; Agasid, M.T.; Mehmood, S.; Robinson, C.V. Membrane Protein-Lipid Interactions Probed Using Mass Spectrometry. Annu. Rev. Biochem. 2019, 88, 85–111. [Google Scholar] [CrossRef]
- Husada, F.; Bountra, K.; Tassis, K.; de Boer, M.; Romano, M.; Rebuffat, S.; Beis, K.; Cordes, T. Conformational Dynamics of the ABC Transporter McjD Seen by Single-molecule FRET. EMBO J. 2018, 37, e100056. [Google Scholar] [CrossRef]
- Geertsma, E.R.; Nik Mahmood, N.a.B.; Schuurman-Wolters, G.K.; Poolman, B. Membrane Reconstitution of ABC Transporters and Assays of Translocator Function. Nat. Protoc. 2008, 3, 256–266. [Google Scholar] [CrossRef]
- Huber, F.; Lang, H.P.; Heller, S.; Bielicki, J.A.; Gerber, C.; Meyer, E.; Egli, A. Rapid Bacteria Detection from Patients’ Blood Bypassing Classical Bacterial Culturing. Biosensors 2022, 12, 994. [Google Scholar] [CrossRef]
- Grinevich, D.; Harden, L.; Thakur, S.; Callahan, B. Serovar-Level Identification of Bacterial Foodborne Pathogens from Full-Length 16S rRNA Gene Sequencing. mSystems 2024, 9, e00757-23. [Google Scholar] [CrossRef]
- Del Bino, L.; Østerlid, K.E.; Wu, D.-Y.; Nonne, F.; Romano, M.R.; Codée, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, 122, 15672–15716. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Bino, L.D.; Alfini, R.; Carboni, F.; Romano, M.R.; Adamo, R. Glycoconjugate Vaccines: Current Approaches towards Faster Vaccine Design. Expert. Rev. Vaccines 2019, 18, 881–895. [Google Scholar] [CrossRef]
- Jansen, K.U.; Gruber, W.C.; Simon, R.; Wassil, J.; Anderson, A.S. The Impact of Human Vaccines on Bacterial Antimicrobial Resistance. A Review. Environ. Chem. Lett. 2021, 19, 4031–4062. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).