Sustainable Gels from Polysaccharides in Agriculture
Abstract
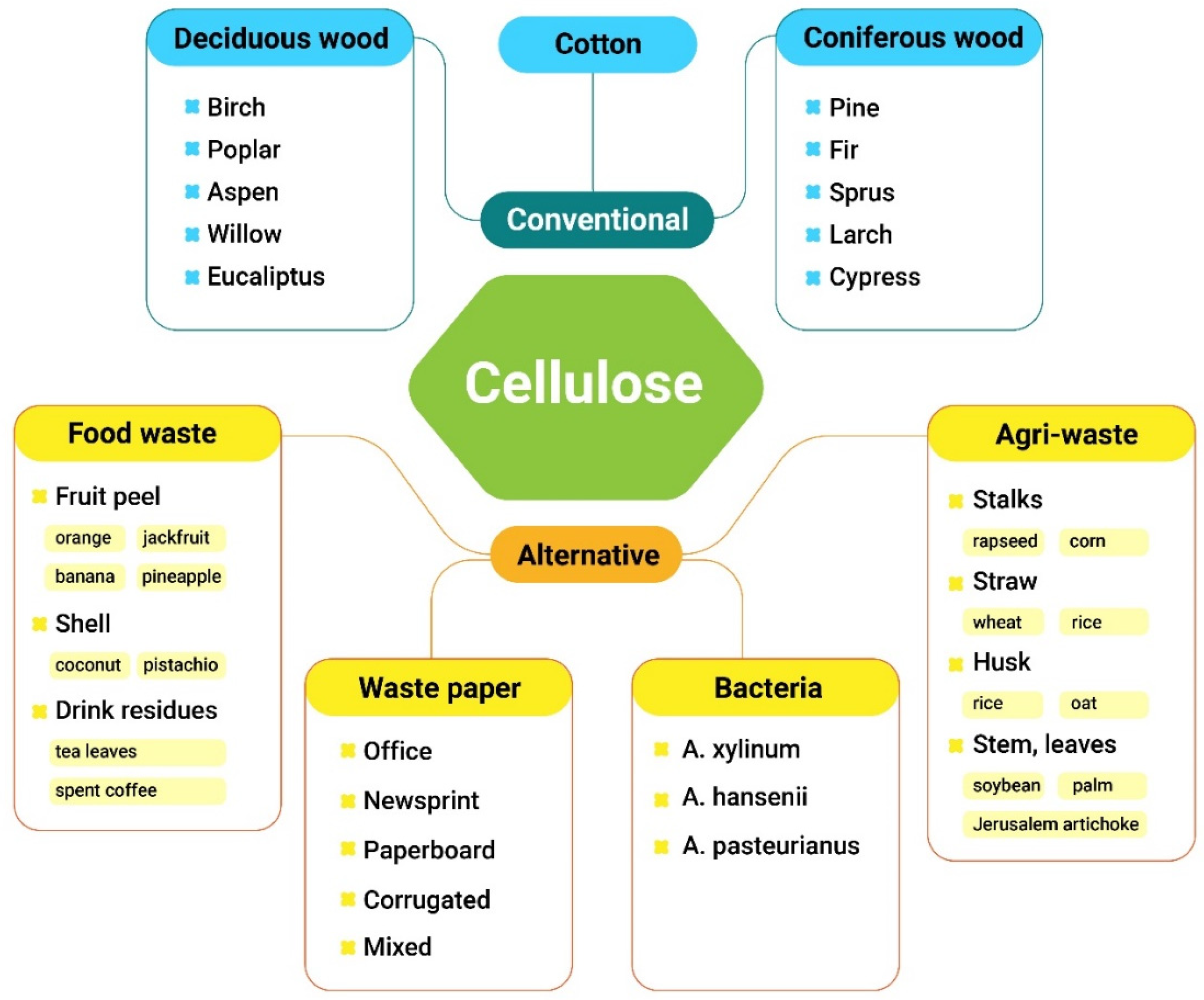
1. Introduction
2. Fundamental Research on Cellulose Gels Used in Agriculture
2.1. Non-Derivatized Cellulose
| Number | Precursors and Additives | Cross-linker | Purpose | Swelling Ability | Critical Agricultural Characteristics | Reference |
|---|---|---|---|---|---|---|
| 1 | Rice straw +AA (HS). | MBA | Water reservoir | 215.0 g/g for HS, 247.6 g/g for HA | Ear yield: 3875, 3747, and 3160 kg/fed; biological yield: 6557, 6335, and 5339 kg/fed; root length: 23.8, 22.0, and 17.3 cm; Nitrogen uptake for soil: 106.0, 101.4, and 82.8 kg/fed. phosphorus uptake: 13.25, 12.98, and 9.04 kg/fed; potassium uptake: 92.11, 82.02, and 68.47 kg/fed, for HA, HS, and control soil without any hydrogel, respectively. All data are mean values related to the factors of irrigation frequency and rate. | [9] |
| 2 | Sago pith (in NaOH/aq./ urea solvent system) | ECH | Water reservoir | 19.7 g/g for 5% hydrogel, 8.1 g/g for 6% hydrogel | Both dry and swollen hydrogel applications facilitated maize seed germination without inhibitory effects | [14] |
| 3 | Wheat straw +AA, PVA. NP fertilizer: urea, potassium dihydrogen phosphate | MBA | Slow-release and controlled-release fertilizer, smart material | 183–243 g/g in water (depending on particle size), 32–124 g/g in NaCl solution (depending on concentration) | Phosphorus fertilizer release in water: 55–70% after 20 min, 70–75% after 60 min. Nitrogen fertilizer release in water: 50–62% after 20 min, 60–65% after 60 min. | [32] |
| 4 | Cotton stalks + AA. Additives: polyvinylpyrrolidone, bentonite. NK fertilizer: urea, potassium persulfate | MBA | Slow-release fertilizer | 1018.4 g/g in distilled water and 71.3 g/g in 0.9 wt.% NaCl solution | 60% of nutrients were released after 30 days. Cotton plant performance: germination rate 80.36 and 58.32%, plant height 22.48 and 19.95 cm, root length 6.15 and 4.75 cm, fresh weight 2.58 and 1.84 g, and dry weight 0.23 and 0.15 g for treatment with the hydrogel and with pure urea, respectively. | [30] |
| 5 | Waste paper cellulose (in NaOH/aq./ urea solvent system): freeze-dried (FD) and oven-dried (OD) hydrogel beads. NPK fertilizer: commercial “Miracle Grow” | None | Slow-release fertilizer | Re-swelling ratio *: 415.62% for FD and 224.16% for OD hydrogel (roughly, it is 4.2 g/g and 2.2 g/g, respectively) | Crop yield: 0.88 kg/plant for FD + 95% AWC, 0.32 kg/plant for OD + 95% AWC, and 0.4–0.53 kg/plant for control. Total biomass: 2.00 kg/plant for FD + 95% AWC, 1.08 kg/plant for OD + 95% AWC, 1.59 kg/plant for control; 1.65 kg/plant for FD + 75% AWC, 1.03 kg/plant for OD + 75% AWC, 1.69 kg/plant for control. Water use efficiency: 3.91, 1.47, 1.51 kg/m-plant for FD + 95% AWC, OD + 95% AWC, and control, respectively | [33,37] |
| 6 | Waste paper cellulose (in NaOH/aq./ urea solvent system). Additive: CMC | ECH | Water reservoir | 3.55, 4.66, 3.20, and 2.10 g/g for the hydrogels with 2, 3, 4, and 6 wt% concentration, respectively | Soil moisture level: 12.3% for the loamy soil, 7.1% for the clayey soil, and ~9% for clay and river sand soils amended with 35% of the hydrogel. The germination rate of the paddy seeds after 30 days was 53%, 35%, 27%, and 18% on the hydrogel, loamy soil, river sand, and clayey soil, respectively | [35] |
| 7 | Waste paper cellulose (in DMAc/LiCl solvent system): cardboard (C) and newsprint paper (NP) | None | Water reservoir | 29.13–45.74 g/g for C hydrogel and 14.35–37.17 g/g for NP. 4.65 g/g—re-swelling capacity of lyophilized C hydrogel | 0.15 g/g—adsorption capacity of C for a mineral fertilizer solution. 52.68%—desorption of fertilizer from the hydrogel into water. Not phytotoxic. Biodegradation: 84–92% of total mass lost in soil within 2 weeks. | [3,36] |
| 8 | Cellulose+ glycerol (in aqueous zinc chloride/glycerol solvent system) | None | Water reservoir | 160% | Germination percentage increased by 21.88%. The average number of leaves on wheat plants increased 100% after 21 days. The water uptake of the plants improved by 94.7%. | [38] |
2.2. Cellulose Derivatives
2.3. Nanocellulose Hydrogels
3. Commercial Hydrogels in Agriculture and Perspectives for Scaling Up Cellulose-Based Hydrogel Production Technologies
4. Conclusions
- −
- Concentration of cellulose: initially increasing concentration has a positive impact, but beyond a threshold value (3–4%), it negatively affects it due to an increased cross-linking degree.
- −
- Formulation of the hydrogel: grafting acrylic acid or additives like silica nanoparticles significantly improves swelling ability, commonly to about 1.000 g/g but, in some reported cases, up to 8.000 g/g, while other additives, such as clays or hydrocarbons, may reduce swelling ability.
- −
- Concentration of chemical cross-linkers: initially improves swelling capacity, but reaching a specific limit becomes a negative factor due to the excessive density of the cross-linked hydrogel. Nanocellulose additives follow this trend as they also act as cross-linkers of the gel.
- −
- pH of the solution, ionic strength, and ionic species: These factors enable the control of the hydrogel swelling state by varying the medium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikhailidi, A.; Volf, I.; Belosinschi, D.; Tofanica, B.-M.; Ungureanu, E. Cellulose-Based Metallogels—Part 1: Raw Materials and Preparation. Gels 2023, 9, 390. [Google Scholar] [CrossRef]
- Abdelaziz, R.M.; El-Maghraby, A.; Sadik, W.A.-A.; El-Demerdash, A.-G.M.; Fadl, E.A. Biodegradable Cellulose Nanocrystals Hydrogels for Removal of Acid Red 8 Dye from Aqueous Solutions. Sci. Rep. 2022, 12, 6424. [Google Scholar] [CrossRef]
- Mikhailidi, A.M.; Kotel’nikova, N.Y. Functional Materials from Paper Wastes: II–Cellulose Hydrogels with High Water Retention Capacity Obtained from Solutions of Waste Paper in DMAc/LiCl. Russ. J. Bioorg. Chem. 2022, 48, 1486–1497. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Agricultural Waste-Derived Superabsorbent Hydrogels: Preparation, Performance, and Socioeconomic Impacts. J. Clean. Prod. 2020, 251, 119669. [Google Scholar] [CrossRef]
- Puițel, A.C.; Tofănică, B.M.; Gavrilescu, D.A. Fibrous Raw Materials from Agricultural Residues. In Pulp Production and Processing: High-Tech Applications; Popa, V., Ed.; De Gruyter: Berlin, Germany, 2020; pp. 49–72. ISBN 978-3-11-065883-5. [Google Scholar]
- Tofanica, B.M. Rapeseed—A Valuable Renewable Bioresource. Cellul. Chem. Technol. 2019, 53, 837–849. [Google Scholar] [CrossRef]
- Gavrilescu, D.; Chesca, A.M.; Tofanica, B.M.; Puitel, A.C.; Nicu, R. Environmentally Friendly Cellulosic Fibers from Corn Stalks. Environ. Eng. Manag. J. 2018, 17, 1765–1771. [Google Scholar] [CrossRef]
- Puițel, A.C.; Moisei, N.; Tofănică, B.M.; Gavrilescu, D. Turning Wheat Straw in a Sustainable Raw Material for Paper Industry. Environ. Eng. Manag. J. 2017, 16, 1027–1032. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Abd-Eladl, M.; Abou-Baker, N.H. Lignocellulosic Biomass for the Preparation of Cellulose-Based Hydrogel and Its Use for Optimizing Water Resources in Agriculture. J. Appl. Polym. Sci. 2015, 132, 42652. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Saurov, S.K.; Anderson, S.; Kotelnikova, N. Lignocellulose Fibers Elaborating Super-Swollen Three-Dimensional Cellulose Hydrogels from Solution in N, N-Dimethylacetamide/Lithium Chloride. TAPPI J. 2018, 17, 81–88. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Wang, Z.; Xia, J.; Su, C. Preparation and Characterization of Lignocellulose Gel from Soybean Stem/LiCl/DMSO Solution. Chem. Ind. For. Prod. 2016, 36, 81–88. [Google Scholar]
- Zhou, X.; Liu, L.; Li, J.; Wang, L.; Song, X. Extraction and Characterization of Cellulose from Jerusalem Artichoke Residue and Its Application in Blueberry Preservation. Foods 2022, 11, 1065. [Google Scholar] [CrossRef]
- Seo, M.; Seo, M.; Choi, S.-E.; Shin, K.; Lee, J.B.; Yang, D.-Y.; Kim, J.W. Cellulose Nanofiber-Multilayered Fruit Peel-Mimetic Gelatin Hydrogel Microcapsules for Micropackaging of Bioactive Ingredients. Carbohydr. Polym. 2020, 229, 115559. [Google Scholar] [CrossRef]
- Jampi, A.L.W.; Chin, S.-F.; Wasli, M.E.; Chia, C.-H. Preparation of Cellulose Hydrogel from Sago Pith Waste as a Medium for Seed Germination. J. Phys. Sci. 2021, 32, 13–26. [Google Scholar] [CrossRef]
- Truong, T.T.C.; Vo, N.T.T.; Nguyen, K.D.; Bui, H.M. Preparation of Cellulose-Based Hydrogel Derived from Tea Residue for the Adsorption of Methylene Blue. Cellul. Chem. Technol. 2019, 53, 573–582. [Google Scholar] [CrossRef]
- Suprabawati, A.; Aisyah, L.S.; Firzatullah, M.R. Crosslinked CMC-Urea Hydrogel Made from Natural Carboxymethyl Cellulose (CMC) as Slow-Release Fertilizer Coating. AIP Conf. Proc. 2020, 2243, 030025. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Volf, I.; Belosinschi, D.; Tofanica, B.-M.; Ungureanu, E. Cellulose-Based Metallogels—Part 2: Physico-Chemical Properties and Biological Stability. Gels 2023, 9, 633. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Ungureanu, E.; Tofanica, B.-M.; Ungureanu, O.C.; Fortună, M.E.; Belosinschi, D.; Volf, I. Agriculture 4.0.: Polymer hydrogels as delivery agents of active ingredients. Gels 2024, 10, 368. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Kotelnikova, N.E.; Mikhailidi, A.M.; Martakova, Y.; Andersson, S. In Vitro Preparation of Self-Assembled Super-Swollen Hydrogels from Solutions of Lignocellulose in N, N-Dimethylacetamide/Lithium Chloride. Cellul. Chem. Technol. 2016, 50, 4. [Google Scholar]
- Hu, S.; Zhi, Y.; Shan, S.; Ni, Y. Research Progress of Smart Response Composite Hydrogels Based on Nanocellulose. Carbohydr. Polym. 2022, 275, 118741. [Google Scholar] [CrossRef]
- Abeer, M.M.; Amin, M.C.I.M.; Martin, C. A Review of Bacterial Cellulose-Based Drug Delivery Systems: Their Biochemistry, Current Approaches and Future Prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Ungureanu, E.; Belosinschi, D.; Tofanica, B.-M.; Volf, I. Cellulose-Based Metallogels—Part 3: Multifunctional Materials. Gels 2023, 9, 878. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Lobiuc, A.; Ungureanu, O.C.; Jităreanu, D.C. Design of Functional Polymer Systems to Optimize the Filler Retention in Obtaining Cellulosic Substrates with Improved Properties. Materials 2023, 16, 1904. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Li, X.F.; Ding, E.Y.; Li, G.K. A method of preparing spherical nano-crystal cellulose with mixed cryslalline forms of cellulose I and II. J. Polym. Sci. 2001, 19, 291–296. [Google Scholar]
- Rotaru, R.; Fortună, M.E.; Ungureanu, E.; Brezuleanu, C.O. Effects of Ultrasonication in Water and Isopropyl Alcohol on High-Crystalline Cellulose: A Fourier Transform Infrared Spectrometry and X-ray Diffraction Investigation. Polymers 2024, 16, 2363. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Robles, E.; Ribeiro, J.S.; Lund, R.G.; Carreño, N.L.V.; Labidi, J. Cellulose nanocrystal membranes as excipients for drug delivery systems. Materials 2016, 9, 1002. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-Release NPK Fertilizer Encapsulated by Carboxymethyl Cellulose-Based Nanocomposite with the Function of Water Retention in Soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef]
- Wen, P.; Wu, Z.; He, Y.; Ye, B.-C.; Han, Y.; Wang, J.; Guan, X. Microwave-Assisted Synthesis of a Semi-Interpenetrating Polymer Network Slow-Release Nitrogen Fertilizer with Water Absorbency from Cotton Stalks. ACS Sustain. Chem. Eng. 2016, 4, 6572–6579. [Google Scholar] [CrossRef]
- Armir, N.A.Z.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Othman, M.H.C.; Zakaria, S. Regenerated Cellulose Products for Agricultural and Their Potential: A Review. Polymers 2021, 13, 3586. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Xu, X.; Su, Y.; Yue, Q.; Gao, B. Characterization, Swelling and Slow-Release Properties of a New Controlled Release Fertilizer Based on Wheat Straw Cellulose Hydrogel. J. Taiwan Inst. Chem. Eng. 2016, 60, 564–572. [Google Scholar] [CrossRef]
- Madramootoo, C.A.; Jain, A.; Oliva, C.; Wang, Y.; Abbasi, N.A. Growth and Yield of Tomato on Soil Amended with Waste Paper Based Hydrogels. Sci. Hortic. 2023, 310, 111752. [Google Scholar] [CrossRef]
- Zainal, S.H.; Nofrianti, R.; Lazim, A.M.; Othaman, R. Cellulose-Based Hydrogel as Halal Agricultural Medium. Malays. Appl. Biol. 2019, 48, 109–113. [Google Scholar]
- Jong, S.-J.; KarunaKaran, K.; Wasli, M.E.; Musa, Z.; Chin, S.-F. Cellulose-Based Hydrogel as a Natural Medium for Paddy Seed Germination. Starch Stärke 2024, 76, 2200234. [Google Scholar] [CrossRef]
- Mikhailidi, A.M.; Gorozhankina, M.A.; Kondratyev, V.M. Hydrogels from recycled waste paper as sources of water and mineral fertilizers for agriculture. In Proceedings of the All-Russian Conference with International Participation “Modern Problems of Polymer Science”, St. Petersburg, Russia, 13 November 2023; p. 282. (In Russian). [Google Scholar]
- Oliva, C. From Wastepaper to Functional Materials: Recycling of Cellulose Through “Green” Aqueous Solvents. Master’s Thesis, McGill University, Montreal, QC, Canada, 2020. [Google Scholar]
- Qin, C.-C.; Abdalkarim, S.Y.H.; Zhou, Y.; Yu, H.-Y.; He, X. Ultrahigh Water-Retention Cellulose Hydrogels as Soil Amendments for Early Seed Germination under Harsh Conditions. J. Clean. Prod. 2022, 370, 133602. [Google Scholar] [CrossRef]
- Messa, L.L.; Hsieh, Y.-L.; Faez, R. Sugarcane Bagasse Derived Phosphorylated Cellulose as Substrates for Potassium Release Induced by Phosphates Surface and Drying Methods. Ind. Crops Prod. 2022, 187, 115350. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M. Progress in the Preparation of Stimulus-Responsive Cellulose Hydrogels and Their Application in Slow-Release Fertilizers. Polymers 2023, 15, 3643. [Google Scholar] [CrossRef]
- Ranganathan, N.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Cellulose-Based Hydrogels for Agricultures. In Cellulose-Based Superabsorbent Hydrogels (Polymers and Polymeric Composites: A Reference Series); Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. ISBN 978-3-319-76573-0. [Google Scholar]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, e435073. [Google Scholar] [CrossRef]
- Kareem, S.A.; Dere, I.; Gungula, D.T.; Andrew, F.P.; Saddiq, A.M.; Adebayo, E.F.; Tame, V.T.; Kefas, H.M.; Joseph, J.; Patrick, D.O. Synthesis and Characterization of Slow-Release Fertilizer Hydrogel Based on Hydroxy Propyl Methyl Cellulose, Polyvinyl Alcohol, Glycerol and Blended Paper. Gels 2021, 7, 262. [Google Scholar] [CrossRef]
- Akalin, G.O.; Pulat, M. Controlled Release Behavior of Zinc-Loaded Carboxymethyl Cellulose and Carrageenan Hydrogels and Their Effects on Wheatgrass Growth. J. Polym. Res. 2019, 27, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Zhang, Q.; Xie, H.; Wang, B.; Feng, Y. Hydrochar-Embedded Carboxymethyl Cellulose-g-Poly(Acrylic Acid) Hydrogel as Stable Soil Water Retention and Nutrient Release Agent for Plant Growth. J. Bioresour. Bioprod. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Dong, H. Controlled Release of Herbicide Acetochlor from Clay/Carboxylmethylcellulose Gel Formulations. J. Agric. Food Chem. 2008, 56, 1336–1342. [Google Scholar] [CrossRef]
- Harada, N.; Mitsukami, Y.; Uyama, H. Preparation and Characterization of Water-Swellable Hydrogel-Forming Porous Cellulose Beads. Polymer 2021, 215, 123381. [Google Scholar] [CrossRef]
- Qi, H.; Ma, R.; Shi, C.; Huang, Z.; Liu, S.; Sun, L.; Hu, T. Novel Low-Cost Carboxymethyl Cellulose Microspheres with Excellent Fertilizer Absorbency and Release Behavior for Saline-Alkali Soil. Int. J. Biol. Macromol. 2019, 131, 412–419. [Google Scholar] [CrossRef]
- Rudich, A.; Sapru, S.; Shoseyov, O. Biocompatible, Resilient, and Tough Nanocellulose Tunable Hydrogels. Nanomaterials 2023, 13, 853. [Google Scholar] [CrossRef]
- Pan, X.; Li, J.; Ma, N.; Ma, X.; Gao, M. Bacterial Cellulose Hydrogel for Sensors. Chem. Eng. J. 2023, 461, 142062. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, P.K.; Pattnaik, R.; Kumar, S.; Ojha, S.K.; Srichandan, H.; Parhi, P.K.; Jyothi, R.K.; Sarangi, P.K. Biochemistry, Synthesis, and Applications of Bacterial Cellulose: A Review. Front. Bioeng. Biotechnol. 2022, 10, 780409. [Google Scholar] [CrossRef]
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, Modification, Biodegradation and Applications in Agriculture as Slow/Controlled Release Fertilizer, Superabsorbent, and Crop Protection: A Review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.; Luan, Q.; Tang, H.; Huang, F.; Xiang, X.; Yang, C.; Bao, Y. Cellulose Anionic Hydrogels Based on Cellulose Nanofibers As Natural Stimulants for Seed Germination and Seedling Growth. J. Agric. Food Chem. 2017, 65, 3785–3791. [Google Scholar] [CrossRef]
- Wang, Y.; Shaghaleh, H.; Hamoud, Y.A.; Zhang, S.; Li, P.; Xu, X.; Liu, H. Synthesis of a PH-Responsive Nano-Cellulose/Sodium Alginate/MOFs Hydrogel and Its Application in the Regulation of Water and N-Fertilizer. Int. J. Biol. Macromol. 2021, 187, 262–271. [Google Scholar] [CrossRef]
- Bauli, C.R.; Lima, G.F.; de Souza, A.G.; Ferreira, R.R.; Rosa, D.S. Eco-Friendly Carboxymethyl Cellulose Hydrogels Filled with Nanocellulose or Nanoclays for Agriculture Applications as Soil Conditioning and Nutrient Carrier and Their Impact on Cucumber Growing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126771. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Zhang, L.; Zhan, H. Superabsorbent Nanocomposite Hydrogels Made of Carboxylated Cellulose Nanofibrils and CMC-g-p(AA-Co-AM). Carbohydr. Polym. 2013, 97, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Grabowska-Polanowska, B.; Garbowski, T.; Bar-Michalczyk, D.; Kowalczyk, A. The Benefits of Synthetic or Natural Hydrogels Application in Agriculture: An Overview Article. J. Water Land Dev. 2021, 51, 208–224. [Google Scholar] [CrossRef]
- Dell’Ambrogio, G.; Wong, J.W.Y.; Ferrari, B.J.D. Ecotoxicological Effects of Polyacrylate, Acrylic Acid, Polyacrylamide and Acrylamide on Soil and Water Organisms. In External Report; Swiss Centre for Applied Ecotoxicology (Ecotox Centre): Lausanne, Switzerland, 2019; p. 50. [Google Scholar]
- Liang, D.; Du, C.; Ma, F.; Shen, Y.; Wu, K.; Zhou, J. Degradation of Polyacrylate in the Outdoor Agricultural Soil Measured by FTIR-PAS and LIBS. Polymers 2018, 10, 1296. [Google Scholar] [CrossRef]
- Pinzon-Moreno, D.D.; Maurate-Fernandez, I.R.; Flores-Valdeon, Y.; Neciosup-Puican, A.A.; Carranza-Oropeza, M.V. Degradation of Hydrogels Based on Potassium and Sodium Polyacrylate by Ionic Interaction and Its Influence on Water. Polymers 2022, 14, 2656. [Google Scholar] [CrossRef]
- Womack, N.C.; Piccoli, I.; Camarotto, C.; Squartini, A.; Guerrini, G.; Gross, S.; Maggini, M.; Cabrera, M.L.; Morari, F. Hydrogel application for improving soil pore network in agroecosystems. Preliminary results on three different soils. CATENA 2022, 208, 105759. [Google Scholar] [CrossRef]
- Turioni, C.; Guerrini, G.; Squartini, A.; Morari, F.; Maggini, M.; Gross, S. Biodegradable Hydrogels: Evaluation of Degradation as a Function of Synthesis Parameters and Environmental Conditions. Soil Syst. 2021, 5, 47. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Zohar, Y.; Hand, B.J.; Ron, E.S. Method for Producing Hydrogels. Patent WO2012170682A1, 13 December 2012. [Google Scholar]
- Boekhout, R. Hydrogel-Based Substrate Shows Promising Results. Available online: https://www.hortidaily.com/article/9535132/hydrogel-based-substrate-shows-promising-results (accessed on 30 March 2025).
- Guerrini, G.; Maggini, M.; Gross, S.; Causin, V. Biodegradable polymer-composite clay - Biodegradable polymer-clay composite. Patent IT201700105979A1, 2019. [Google Scholar]
- Guerrini, G.; Maggini, M.; Gross, S.; Causin, V. Biodegradable Hydrogel. Patent WO2019057935A1, 2019. [Google Scholar]
- Prisa, D.; Guerrini, G. Innovative Hydrogels Use in the Germination and Growth of Tree Species Paulownia Tomentosa and Cupressus Sempervirens. GSC Adv. Res. Rev. 2023, 14, 121–128. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Belosinschi, D.; Volf, I. Gels, Aerogels and Hydrogels: A Challenge for the Cellulose-Based Product Industries. Gels 2022, 8, 497. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Wen, Y.; Song, X.; Tan, W.K.; Ong, C.N.; Li, J. Recent Advances in Superabsorbent Hydrogels Derived from Agro Waste Materials for Sustainable Agriculture: A Review. J. Agric. Food Chem. 2024, 72, 22399–22419. [Google Scholar] [CrossRef]
- Salimi, M.; Idrissi, A.E.; Channab, B.-E.; Essamlali, Y.; Firouzabadi, A.G.; Beygi, M.; Zahouily, M.; Motamedi, E. Cellulose-based controlled release fertilizers for sustainable agriculture: Recent trends and future perspectives. Cellulose 2024, 31, 10679–10726. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Chowdhury, S.D. Advancements in Cellulose-Based Superabsorbent Hydrogels: Sustainable Solutions across Industries. Gels 2024, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Ungureanu, E.; Fortună, M.E.; Țopa, D.C.; Brezuleanu, C.O.; Ungureanu, V.I.; Chiruță, C.; Rotaru, R.; Tofanica, B.M.; Popa, V.I.; Jităreanu, D.C. Comparison adsorption of Cd (II) onto Lignin and Polysaccharide-based polymers. Polymers 2023, 15, 3794. [Google Scholar] [CrossRef]
- Yu, X.; Liao, W.; Wu, Q.; Wei, Z.; Lin, X.; Qiu, R.; Chen, Y. Green remediation of cadmium-contaminated soil by cellulose nanocrystals. J. Hazard. Mater. 2023, 443, 130312. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational applications of hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]



| Derivative | Substituent Groups | Substitution Positions | Key Properties |
|---|---|---|---|
| Carboxymethyl Cellulose (CMC) | -CH2-COOH (carboxymethyl) | C2, C3, and/or C6 | Water-soluble, forms viscous solutions, used in food/pharma as thickener/stabilizer |
| Hydroxyethyl Cellulose (HEC) | -O-CH2-CH2-OH (hydroxyethyl) | C2, C3, and/or C6 | High water solubility, biocompatible, used in drug delivery and biomedical fields |
| Hydroxypropyl Methylcellulose (HPMC) | -O-CH3 (methyl) and -O-CH2-CH(OH)-CH3 (hydroxypropyl) | C2, C3, and/or C6 | Thermogelling, hydrophilic, used in coatings, controlled-release pharmaceuticals |
| Cellulose Acetate | -O-CO-CH3 (acetyl) | C2, C3, and/or C6 | Hydrophobic, thermoplastic (with plasticizers), used in films/fibers/pharma coatings |
| Phosphorylated Cellulose | -PO4H2 (phosphate) | C6 (primary) | Flame-retardant, high charge density (6608 mmol/kg), used in adhesives/adsorbents |
| Number | Precursors and Additives | Cross-Linker | Purpose | Swelling Ability | Critical Agricultural Characteristics | Reference |
|---|---|---|---|---|---|---|
| 1 | CMC + HEC | WSC, CA—catalyst | Water reservoir, smart material | 75 g/g when pH = 7, 95 g/g when pH 10, sensitive to ionic strength variations | 1% of the hydrogel triplicated the time length of the soil humidity; the plant survival time increased to 22 days; 6 months is the degradation time of the hydrogel in soil | [42] |
| 2 | HPMC, PVA, glycerol. Fertilizer: urea. | - | Slow-release fertilizer | 17.2 g/g in distilled water, 14.4 g/g in tap water | Water retention ratio of soil: 54.6 (2nd day), 0.8 (5th day); control 51.9 and 0.04, respectively; Cumulative release of urea in water: 37%, 82.2%, and 85.4% at 1, 6, and 24 h; in soil: 27.1%, 64.5%, and 67.1% at 6, 30, and 44 days. | [43] |
| HPMC, PVA, glycerol. Fertilizer: urea. Coating: HPMC + blended paper. | - | 15.6 g/g in distilled water, 15.2 g/g in tap water | Water retention ratio of soil: 56.2 (2nd day), 1.0 (5th day); with control 51.9 and 0.04, respectively; Cumulative release of urea in water: 28.6%, 59.6%, and 64.4% at 1, 6, and 24 h; in soil: 30.8%, 82.3%, and 87.0% at 6, 30, and 44 days. | |||
| 3 | CMC. Micronutrient: Zn. | FeCl3 | Controlled-release fertilizer | 1.1–4.0 g/g | Water retention of soil: about 85% (10th day), 38% (20th day). Zinc release in soil: 13.5% on the 3rd day, 25.3% on the 5th day, and 65.3% on the 10th day. Improved the plant height, germination rate, fresh weight, and dry weight. | [44] |
| 4 | Sulfonated CMC + AA. Additives: polyvinylpyrrolidone, silica nanoparticles. NPK fertilizer: urea, ammonium phosphate, potassium dihydrogen phosphate | MBA | Slow-release and controlled-release fertilizer, smart material | ~700–850 g/g in distilled water, ~120–120 g/g in saline solution (0.1 M NaCl), ~600–800 g/g when pH = 8, ~100 g/g when pH = 2 | Water retention of soil: ~70% (10th day), 40% (25th day). NKP fertilizer release in water: 11.2% (1st day), 32.1% (7th day), 65.3% (30th day), release in soil: 29% (1st day), 56,4% (7th day), 83.6% (30th day) | [24] |
| 5 | CMC+ AA (HG). Additives: hydrochar (HC-HG). P fertilizer: phosphate solution | MBA | Controlled-release fertilizer | Swelling: ~70 g/g in water and P-solution at 30 °C, Water retention during 5 h of deswelling: 83 and 78% (water-loaded) and 81 and 80% (P-solution loaded) for HG and HC-HG, respectively | Germination vigor: 56.7% for HG, 78.3% for HC-HG with 80% for control; germination ratio: 60.0% for HG, 80.0% for HC-HG, and 83% for control; Length of stem: ~58 cm for both hydrogels, ~60 cm for control when treated with water, ~50, ~60, and ~58 cm for HG, HC-HG, and control, respectively, when treated with P-solution | [45] |
| 6 | CMC. Fertilizer: urea | Mixed CaCl2, FeCl2, FeCl3 | Slow-release fertilizer | - | Urea release in water: coconut husk hydrogel: 0.13% (1st day), 0.60% (5th day), 4.05% (20th day), for palm sugar hydrogel: 2.69% (1st day) with the relatively high release in the following days | [16] |
| 7 | CMC. Additives: different types of clay. Herbicide: acetochlor | Fe(NO3)3 | Controlled-release of herbicide | Swelling ratio: 1.30–2.26 g/g depending on the clay type | 151–522 h—50% of acetochlor was released depending on the clay type, 2.18–14.0 h—50% of acetochlor was released depending on the cross-linking times | [46] |
| 8 | Cellulose acetate + Urea+ NaH2PO4. NP fertilizer: urea, NaH2PO4 | - | Water reservoir, smart material | 17.3–48.1 g/g in water, 7.2–12.4 in saline solution | Germination occurs and at the 7th day the plant length was about 3 cm, in the control experiment the seeds did not germinate | [47] |
| Number | Precursors and Additives | Cross-Linker | Purpose | Swelling Ability | Critical Agricultural Characteristics | Reference |
|---|---|---|---|---|---|---|
| 1 | Cellulose nanofibers (in NaOH/urea aqueous solution), the hydrogels had different carboxylate contents: 0 (CH), 0.7 (CH7), and 1.5 mmol/g (CH15) | ECH | Water reservoir, cultivation media | Swelling ratio: 80%, 174%, 309%, for CH, CH7, and CH15, respectively. | Germination rate on the hydrogel substrate: 100% seeds within 4 days for CH7, 6 days for CH, and 75% for CH15 within 3–7 days; control—100% seeds within 5 days for soil without hydrogels. Root length 5.5, 8, and 1.5 cm, shoot length 3, 2, and 2.5 cm on CH, CH7, and CH15 hydrogels, respectively. Control (in soil)—7 cm for root and 3 cm for shoot. Weight of seeding at fresh (30, 35, and 12 mg for CH, CH7, and soil CH15, respectively) and dry states (about 5 mg for CH, CH7, and soil and 2 mg for CH15). | [53] |
| 3 | Cellulose nanofibrils+ sodium alginate + MOF. Fertilizer: urea. | - | Slow-release and controlled-release fertilizer, smart material | Swelling: 25–55 g/g depending on the amounts of MOF; Moisture content: 95.94–96.28%, | Urea release in water: 80–90% at pH 3, 68–85% at pH 11 after 25 h; Soil water-retention capacity: soil dried in 15–30 days when enhanced with the hydrogels (12 days for unmodified soil); Germination rate: 92.5% and 65.0% for the hydrogel-treated and urea-treated wheat seeds. Number of leaves per plant on the 60th day: 188 and 82 for the hydrogel-treated and urea-treated plants. Not phytotoxic. Improved germination and growth of cucumber. | [54] |
| 4 | CMC. Additive: nanocellulose. NPK fertilizer: commercial | Citric acid | Slow-release and controlled-release fertilizer | ~18.3 g/g—CMC; 25, 29, 19, and 19 g/g for 1, 3, 5, and 10 wt% of NC | 72 h—release of NPK in water from CMC hydrogel; up to 300 h continued the gradual release in water for CMC-NC. Nine days was the duration of release in soil; for CMC-NC, it was slower and more gradual than for CMC. | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, E.; Mikhailidi, A.; Tofanica, B.-M.; Fortună, M.E.; Rotaru, R.; Ungureanu, O.C.; Samuil, C.; Popa, V.I. Sustainable Gels from Polysaccharides in Agriculture. Polysaccharides 2025, 6, 37. https://doi.org/10.3390/polysaccharides6020037
Ungureanu E, Mikhailidi A, Tofanica B-M, Fortună ME, Rotaru R, Ungureanu OC, Samuil C, Popa VI. Sustainable Gels from Polysaccharides in Agriculture. Polysaccharides. 2025; 6(2):37. https://doi.org/10.3390/polysaccharides6020037
Chicago/Turabian StyleUngureanu, Elena, Aleksandra Mikhailidi, Bogdan-Marian Tofanica, Maria E. Fortună, Răzvan Rotaru, Ovidiu C. Ungureanu, Costel Samuil, and Valentin I. Popa. 2025. "Sustainable Gels from Polysaccharides in Agriculture" Polysaccharides 6, no. 2: 37. https://doi.org/10.3390/polysaccharides6020037
APA StyleUngureanu, E., Mikhailidi, A., Tofanica, B.-M., Fortună, M. E., Rotaru, R., Ungureanu, O. C., Samuil, C., & Popa, V. I. (2025). Sustainable Gels from Polysaccharides in Agriculture. Polysaccharides, 6(2), 37. https://doi.org/10.3390/polysaccharides6020037










