Composite Based on Biomineralized Oxidized Bacterial Cellulose with Strontium Apatite for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Oxidation of Bacterial Cellulose
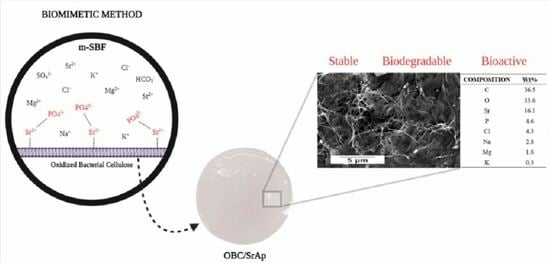
2.2. SrAp Biomineralization
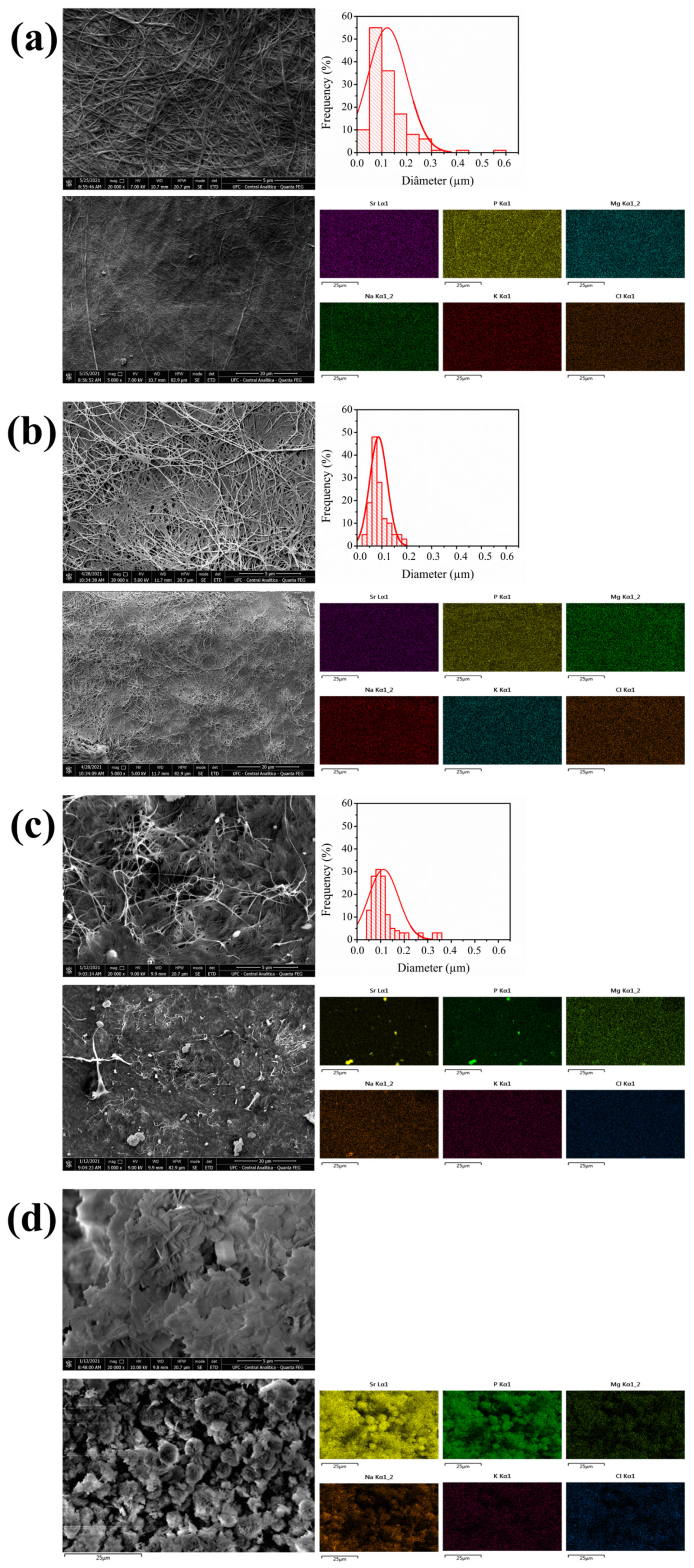
2.3. Scanning Electron Microscopy–Energy Dispersive Spectroscopy (SEM-EDS)
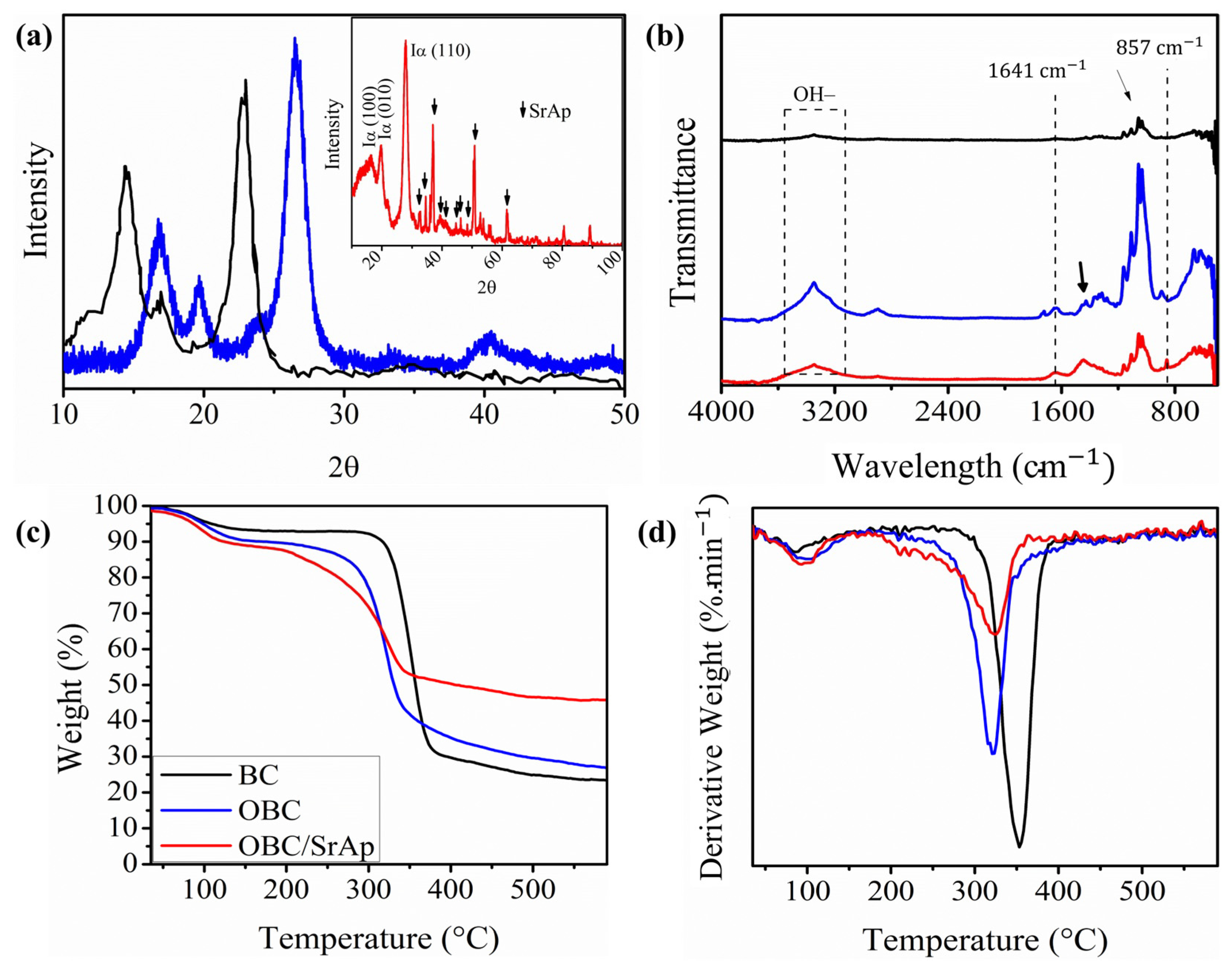
2.4. X-Ray Diffraction (XRD)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Thermal Analysis
2.7. In Vitro Degradability Test
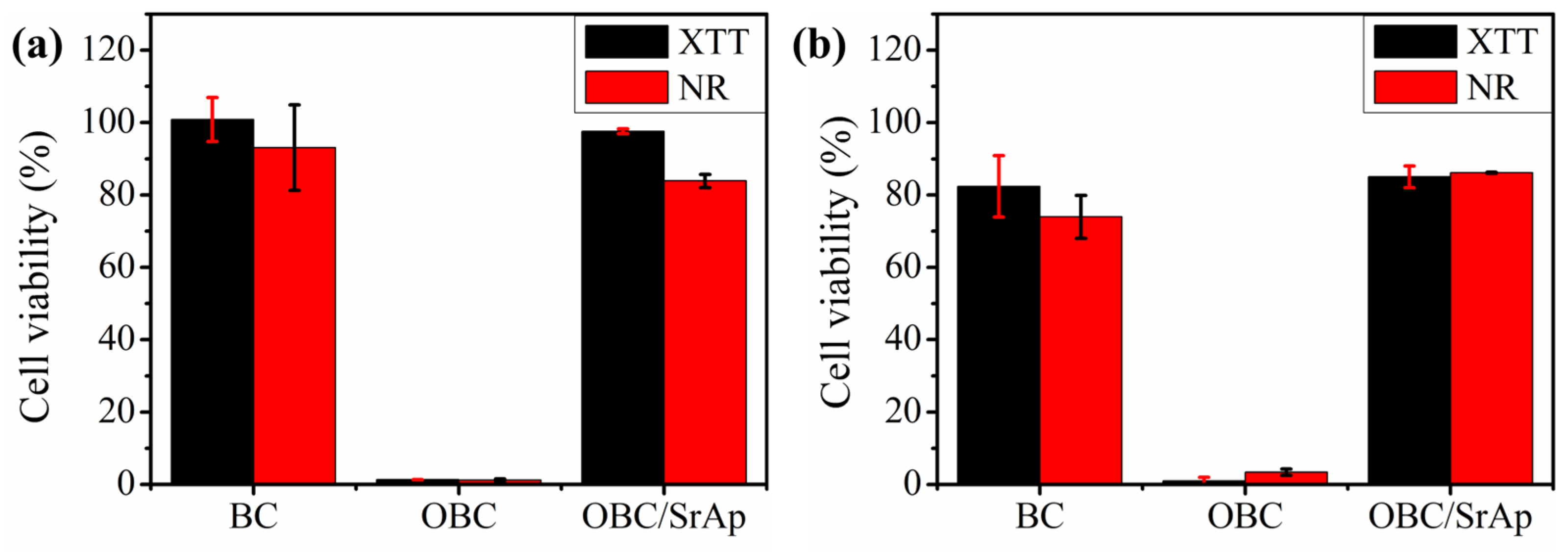
2.8. In Vitro Viability Assay
2.9. Artemia Salina Lethality Assay
3. Results and Discussion
3.1. Obtaining Oxidized Bacterial Cellulose
3.2. SrAp Biomineralization
3.3. Scanning Electron Microscopy–Energy Dispersive Spectroscopy (SEM-EDS)
3.4. X-Ray Diffraction (XRD)
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.6. Thermal Analysis
3.7. In Vitro Degradability Test
3.8. In Vitro Viability Assay
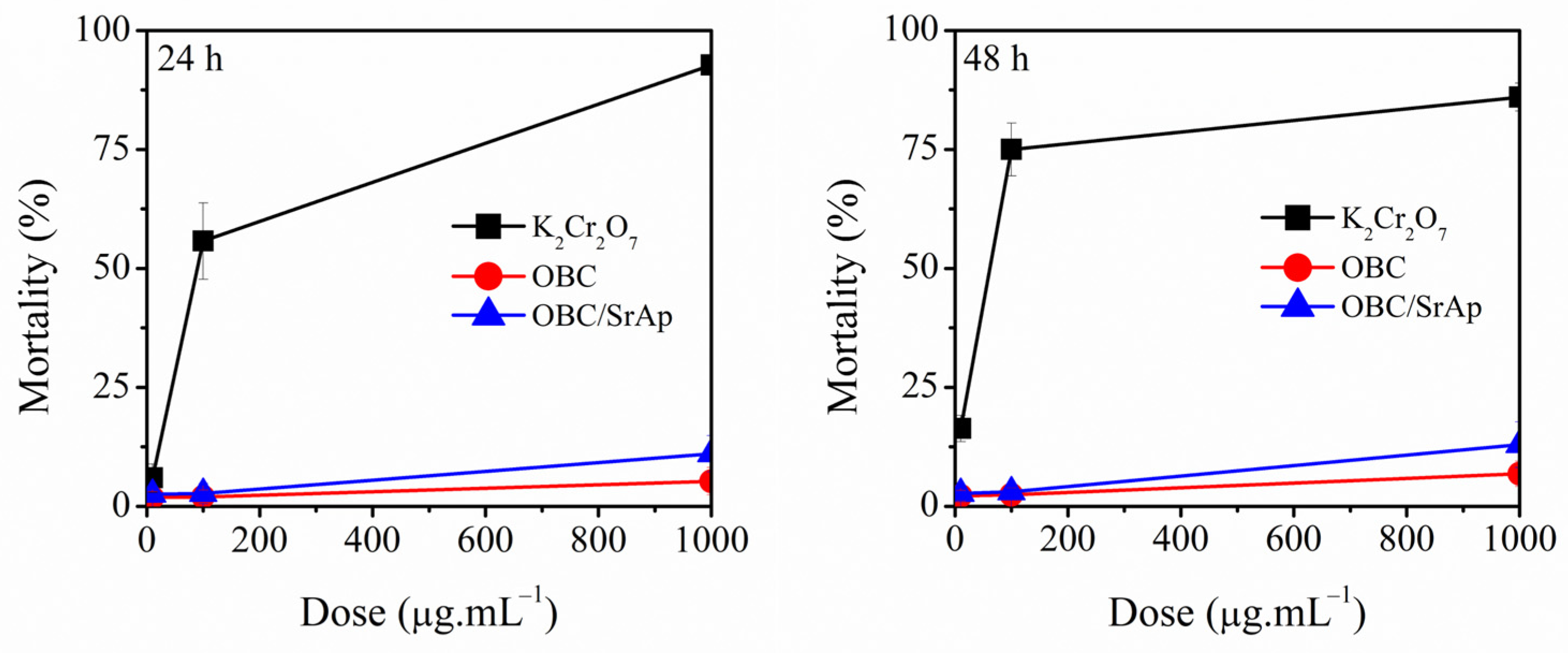
3.9. Artemia Salina Lethality Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, C.R.M.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O.C. Bone Tissue Engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Windhager, R.; Hobusch, G.M.; Matzner, M. Allogene Transplantate Für Biologische Rekonstruktionen von Knochendefekten. Orthopade 2017, 46, 656–664. [Google Scholar] [CrossRef]
- Yang, M.; Zhen, W.; Chen, H.; Shan, Z. Biomimetic Design of Oxidized Bacterial Cellulose-Gelatin-Hydroxyapatite Nanocomposites. J. Bionic Eng. 2016, 13, 631–640. [Google Scholar] [CrossRef]
- Fragal, E.H.; Cellet, T.S.P.; Fragal, V.H.; Witt, M.A.; Companhoni, M.V.P.; Ueda-Nakamura, T.; Silva, R.; Rubira, A.F. Biomimetic Nanocomposite Based on Hydroxyapatite Mineralization over Chemically Modified Cellulose Nanowhiskers: An Active Platform for Osteoblast Proliferation. Int. J. Biol. Macromol. 2019, 125, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Barud, H.G.; Barud, H.d.S.; Cavicchioli, M.; do Amaral, T.S.; Junior, O.B.d.O.; Santos, D.M.; Petersen, A.L.d.O.A.; Celes, F.; Borges, V.M.; de Oliveira, C.I.; et al. Preparation and Characterization of a Bacterial Cellulose/Silk Fibroin Sponge Scaffold for Tissue Regeneration. Carbohydr. Polym. 2015, 128, 41–51. [Google Scholar] [CrossRef]
- Andrade, F.K.; Silva, J.P.; Carvalho, M.; Castanheira, E.M.S.; Soares, R.; Gama, M. Studies on the Hemocompatibility of Bacterial Cellulose. J. Biomed. Mater. Res. A 2011, 98A, 554–566. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and Characterization of Bacterial Cellulose and Gelatin-Based Hydrogel Composites for Drug-Delivery Systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef]
- Weyell, P.; Beekmann, U.; Küpper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-Made Material Characteristics of Bacterial Cellulose for Drug Delivery Applications in Dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In Situ and Ex Situ Modifications of Bacterial Cellulose for Applications in Tissue Engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of Bacterial Cellulose in Skin and Bone Tissue Engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing Cellulose (in)Solubility: Reviewing Basic Physicochemical Aspects and Role of Hydrophobic Interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Rodríguez, K.; Gatenholm, P.; Renneckar, S. Electrospinning Cellulosic Nanofibers for Biomedical Applications: Structure and in Vitro Biocompatibility. Cellulose 2012, 19, 1583–1598. [Google Scholar] [CrossRef]
- Chavan, Y.V.; Panesar, P.S.; Kumar, V. Application of Bacterial Cellulose in the Food Industry. In Bacterial Cellulose; CRC Press: Boca Raton, FL, USA, 2023; pp. 123–140. [Google Scholar] [CrossRef]
- Faria, M.; Cunha, C.; Gomes, M.; Mendonça, I.; Kaufmann, M.; Ferreira, A.; Cordeiro, N. Bacterial Cellulose Biopolymers: The Sustainable Solution to Water-Polluting Microplastics. Water Res. 2022, 222, 118952. [Google Scholar] [CrossRef]
- Mathew, A.; Poulose, A.; Gopakumar, D.A.; Pasquini, D.; Grohens, Y.; George, J.J. Nanocellulose-Based Ultralight Porous Material for Various Environmental Applications. In Nanomaterials for Air- and Water Purification; Wiley: Hoboken, NJ, USA, 2024; pp. 373–397. [Google Scholar] [CrossRef]
- Bianchet, R.T.; Vieira Cubas, A.L.; Machado, M.M.; Siegel Moecke, E.H. Applicability of Bacterial Cellulose in Cosmetics—Bibliometric Review. Biotechnol. Rep. 2020, 27, e00502. [Google Scholar] [CrossRef]
- Lima, N.F.; Maciel, G.M.; Lima, N.P.; Fernandes, I.d.A.A.; Haminiuk, C.W.I. Bacterial Cellulose in Cosmetic Innovation: A Review. Int. J. Biol. Macromol. 2024, 275, 133396. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Li, Q.; Zhang, S.; Liu, K.; Yang, Y.; Chen, J. Oxidized Bacterial Cellulose Reinforced Nanocomposite Scaffolds for Bone Repair. Colloids Surf. B Biointerfaces 2022, 211, 112316. [Google Scholar] [CrossRef]
- Luz, E.P.C.G.; das Chagas, B.S.; de Almeida, N.T.; de Fátima Borges, M.; Andrade, F.K.; Muniz, C.R.; Castro-Silva, I.I.; Teixeira, E.H.; Popat, K.; de Freitas Rosa, M.; et al. Resorbable Bacterial Cellulose Membranes with Strontium Release for Guided Bone Regeneration. Mater. Sci. Eng. C 2020, 116, 111175. [Google Scholar] [CrossRef]
- Jun, S.-H.; Park, S.-G.; Kang, N.-G. One-Pot Method of Synthesizing TEMPO-Oxidized Bacterial Cellulose Nanofibers Using Immobilized TEMPO for Skincare Applications. Polymers 2019, 11, 1044. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Andrade, F.K.; Vieira, L.d.A.P.; Vieira, R.S.; Vaz, J.M.; Chevallier, P.; Mantovani, D.; Borges, M.d.F.; Rosa, M.d.F. Oxidized Bacterial Cellulose Membrane as Support for Enzyme Immobilization: Properties and Morphological Features. Cellulose 2020, 27, 3055–3083. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Hu, D.; Ren, K.; Yao, F.; Zhu, Y.; Gao, C.; Wan, Y. Characterization of TEMPO-Oxidized Bacterial Cellulose Scaffolds for Tissue Engineering Applications. Mater. Chem. Phys. 2013, 143, 373–379. [Google Scholar] [CrossRef]
- Hori, S.; Hirano, M.; Ohta, R. Electrodeposition of Strontium Apatite Nanorod Arrays and Their Cell Compatibility. Ceram. Int. 2017, 43, 9047–9052. [Google Scholar] [CrossRef]
- Jiménez, M.; Abradelo, C.; San Román, J.; Rojo, L. Bibliographic Review on the State of the Art of Strontium and Zinc Based Regenerative Therapies. Recent Developments and Clinical Applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, A.D.; Nerantzaki, M.; Gounari, E.; Duggal, M.S.; Giannoudis, P.V.; Jha, A.; Bikiaris, D. Antibacterial Properties and Regenerative Potential of Sr2+ and Ce3+ Doped Fluorapatites; a Potential Solution for Peri-Implantitis. Sci. Rep. 2019, 9, 14469. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Barberi, J.; Ferraris, S.; Miola, M.; Rimondini, L.; Vernè, E.; Yamaguchi, S.; Spriano, S. Competitive Surface Colonization of Antibacterial and Bioactive Materials Doped with Strontium and/or Silver Ions. Nanomaterials 2020, 10, 120. [Google Scholar] [CrossRef]
- Ullah, I.; Li, W.; Lei, S.; Zhang, Y.; Zhang, W.; Farooq, U.; Ullah, S.; Ullah, M.W.; Zhang, X. Simultaneous Co-Substitution of Sr2+/Fe3+ in Hydroxyapatite Nanoparticles for Potential Biomedical Applications. Ceram. Int. 2018, 44, 21338–21348. [Google Scholar] [CrossRef]
- Ullah, I.; Gloria, A.; Zhang, W.; Ullah, M.W.; Wu, B.; Li, W.; Domingos, M.; Zhang, X. Synthesis and Characterization of Sintered Sr/Fe-Modified Hydroxyapatite Bioceramics for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Zhang, W.; Yang, L.; Ullah, M.W.; Atta, O.M.; Khan, S.; Wu, B.; Wu, T.; Zhang, X. Impact of Structural Features of Sr/Fe Co-Doped HAp on the Osteoblast Proliferation and Osteogenic Differentiation for Its Application as a Bone Substitute. Mater. Sci. Eng. C 2020, 110, 110633. [Google Scholar] [CrossRef]
- Luz, E.P.C.G.; Borges, M.d.F.; Andrade, F.K.; Rosa, M.d.F.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Vieira, R.S. Strontium Delivery Systems Based on Bacterial Cellulose and Hydroxyapatite for Guided Bone Regeneration. Cellulose 2018, 25, 6661–6679. [Google Scholar] [CrossRef]
- Luz, E.P.C.G.; Chaves, P.H.S.; Vieira, L.d.A.P.; Ribeiro, S.F.; Borges, M.d.F.; Andrade, F.K.; Muniz, C.R.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Rosa, M.d.F.; et al. In Vitro Degradability and Bioactivity of Oxidized Bacterial Cellulose-Hydroxyapatite Composites. Carbohydr. Polym. 2020, 237, 116174. [Google Scholar] [CrossRef]
- Luz, E.P.C.G.; Soares, A.L.d.B.; Souza, F.F.P.d.; Andrade, F.K.; Castro-Silva, I.I.; Rosa, M.d.F.; Vieira, R.S. Implantable Matrixes of Bacterial Cellulose and Strontium Apatite: Preclinical Analysis of Cytotoxicity and Osteoconductivity. Mater. Today Commun. 2022, 33, 104871. [Google Scholar] [CrossRef]
- Soares, A.L.d.B.; Luz, E.P.C.G.; Vieira, R.S. Adsorption Processes for Forming Biomaterials of Cellulose and Hydroxyapatite for Applications in Bone Tissue Regeneration. Adsorption 2024, 30, 595–607. [Google Scholar] [CrossRef]
- Maia, M.T.; De Brito Soares, A.L.; Caetano, M.A.; Andrade, F.K.; Rodríguez-Castellón, E.; Vieira, R.S. Biomimetic Strontium Substituted Calcium Phosphate Coating for Bone Regeneration. Coatings 2021, 11, 908. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; He, J.; Shu, Y.; Yang, H.; Liu, J.; Zhang, C.; Xiao, W.; Liu, Z.; Liao, X. Silk Fibroin/Methacrylated Gelatine/Hydroxyapatite Biomimetic Nanofibrous Membranes for Guided Bone Regeneration. Int. J. Biol. Macromol. 2024, 263, 130380. [Google Scholar] [CrossRef] [PubMed]
- Gaulke, E.; Garcia, M.C.F.; Segat, B.; Apati, G.P.; Schneider, A.L.d.S.; Pezzin, A.P.T.; Cesca, K.; Porto, L.M. Evaluation of Potential Biomaterials for Application in Guide Bone Regeneration from Bacterial Nanocellulose/Hydroxyapatite. Polímeros 2023, 33, e20230034. [Google Scholar] [CrossRef]
- Kaya, Y.; Jodati, H.; Evis, Z. Effects of Biomimetic Synthesis Route and Sintering Temperature on Physicochemical, Microstructural, and Mechanical Properties of Hydroxyapatite. J. Aust. Ceram. Soc. 2021, 57, 1117–1129. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines 2021, 12, 1447. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of Cellulose by Acetobacter Xylinum. 2. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can Bioactivity Be Tested in Vitro with SBF Solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. European Committee for Standardization, Europe: Brussels, Belgium, 2012.
- XENOMETRIX AG. Instructions for Use In Cytotox—LDH-XTT-NR 3—Parameter Cytotoxicity Kit; Roche: Basel, Switzerland, 2015. [Google Scholar]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- ISO/TS 20787:2017; Nanotechnologies—Aquatic Toxicity Assessment of Manufactured Nanomaterials in Saltwater Lakes Using Artemia Sp. Nauplii. European Committee for Standardization, Europe: Brussels, Belgium, 2017.
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Fu, R.; Ren, Y.; Fang, K.; Sun, Y.; Zhang, Z.; Luo, A. Preparation, Characterization and Biocompatibility of Chitosan/TEMPO-Oxidized Bacterial Cellulose Composite Film for Potential Wound Dressing Applications. Fibers Polym. 2021, 22, 1790–1799. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Ozeki, K.; Hoshino, T.; Aoki, H.; Masuzawa, T. Phase Composition of Sputtered Film from a Mixture Target of Hydroxyapatite and Strontium-Apatite. J. Mater. Sci. Technol. 2013, 29, 1–6. [Google Scholar] [CrossRef]
- AL-Wafi, R.; Jafer, R.; Yahia, I.S.; Al-Ghamdi, A.A.; Al-ghamdi, M.A.; El-Naggar, A.M. Fast and Easy Synthesis of Novel Strontium Apatite Nanostructured Phase: Structure, Spectroscopy, and Dielectric Analysis. Ceram. Int. 2017, 43, 17153–17159. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; Andrade, F.K.; Miranda, M.A.R.; Sasaki, J.M.; Morais, J.P.S.; e Silva, L.M.A.; Canuto, K.M.; Rosa, M.d.F. Chemically Modified Cellulose Nanocrystals as Polyanion for Preparation of Polyelectrolyte Complex. Cellulose 2019, 26, 1725–1746. [Google Scholar] [CrossRef]
- Tureck, B.C.; Hackbarth, H.G.; Neves, E.Z.; Garcia, M.C.F.; Apati, G.P.; Recouvreux, D.d.O.S.; Pezzin, A.P.T.; Schneider, A.L.d.S. Obtaining and Characterization of Bacterial Cellulose Synthesized by Komagataeibacter Hansenii from Alternative Sources of Nitrogen and Carbon. Matéria 2021, 26. [Google Scholar] [CrossRef]
- Motalebi Moghanjougi, Z.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M.; Almasi, H.; Amiri, S. Bio-Preservation of White Brined Cheese (Feta) by Using Probiotic Bacteria Immobilized in Bacterial Cellulose: Optimization by Response Surface Method and Characterization. LWT 2020, 117, 108603. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; El-Damrawi, G.; Oraby, A.H.; Madshal, M.A. Optical and FTIR Structural Studies on CoO-Doped Strontium Phosphate Glasses. J. Non Cryst. Solids 2018, 499, 153–158. [Google Scholar] [CrossRef]
- Solomevich, S.O.; Dmitruk, E.I.; Aharodnikau, U.E.; Salamevich, D.A.; Bychkovsky, P.M.; Golub, N.V.; Yurkshtovich, T.L. Characterization of H3PO4/HNO3–NANO2 Oxidized Bacterial Cellulose and Its Usage as a Carrier for the Controlled Release of Cephalexin. Cellulose 2021, 28, 9425–9439. [Google Scholar] [CrossRef]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; Rawn, C.J.; O’Neill, H. A Resorbable Calcium-Deficient Hydroxyapatite Hydrogel Composite for Osseous Regeneration. Cellulose 2009, 16, 887–898. [Google Scholar] [CrossRef]
- Scelza, M.Z.; Linhares, A.B.; da Silva, L.E.; Granjeiro, J.M.; Alves, G.G. A Multiparametric Assay to Compare the Cytotoxicity of Endodontic Sealers with Primary Human Osteoblasts. Int. Endod. J. 2012, 45, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity Tests of Cellulose Nanofibril-Based Structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Kiani, F.A.; Shamraiz, U.; Badshah, A.; Tabassum, S.; Ambreen, M.; Patujo, J.A. Optimization of Ag2O Nanostructures with Strontium for Biological and Therapeutic Potential. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), 1083–1091. [Google Scholar] [CrossRef]

| Scaffold | Ions | Aggregate Properties | Ref. |
|---|---|---|---|
| Cellulose acetate | Sr2+ | Delayed long-term degradation, increased crystallinity, increased biocompatibility, and high osteoblast viability. | [34] |
| Silk fibroin/methacrylated gelatine | Ca2+ | Increased mechanical strength and hydrophilicity, lower degradation rates. In vivo studies demonstrated almost complete repair of most bone defect areas studied after 12 weeks. | [35] |
| Bacterial nanocellulose | Mg2+ | High cell viability of mouse fibroblasts (line- L929). | [36] |
| Cu2+ | Antimicrobial action against E. coli and S. aureus. | ||
| - | Ca2+ | It achieves high purity, with mechanical properties and density comparable to natural human femur bone when sintered at 900 °C. | [37] |
| Titanium alloys | Ca2+ | It presented biocompatibility, nanometric crystals and microindentation with Young’s modulus between 55.35 ± 0.3 GPa and 56.45 ± 0.3 GPa, showing similarity to human bone. | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, A.L.d.B.; Luz, E.P.C.G.; Castro-Silva, I.I.; Monteiro, R.R.d.C.; Andrade, F.K.; Vieira, R.S. Composite Based on Biomineralized Oxidized Bacterial Cellulose with Strontium Apatite for Bone Regeneration. Polysaccharides 2025, 6, 23. https://doi.org/10.3390/polysaccharides6010023
Soares ALdB, Luz EPCG, Castro-Silva II, Monteiro RRdC, Andrade FK, Vieira RS. Composite Based on Biomineralized Oxidized Bacterial Cellulose with Strontium Apatite for Bone Regeneration. Polysaccharides. 2025; 6(1):23. https://doi.org/10.3390/polysaccharides6010023
Chicago/Turabian StyleSoares, Ana Lorena de Brito, Erika Patrícia Chagas Gomes Luz, Igor Iuco Castro-Silva, Rodolpho Ramilton de Castro Monteiro, Fábia Karine Andrade, and Rodrigo Silveira Vieira. 2025. "Composite Based on Biomineralized Oxidized Bacterial Cellulose with Strontium Apatite for Bone Regeneration" Polysaccharides 6, no. 1: 23. https://doi.org/10.3390/polysaccharides6010023
APA StyleSoares, A. L. d. B., Luz, E. P. C. G., Castro-Silva, I. I., Monteiro, R. R. d. C., Andrade, F. K., & Vieira, R. S. (2025). Composite Based on Biomineralized Oxidized Bacterial Cellulose with Strontium Apatite for Bone Regeneration. Polysaccharides, 6(1), 23. https://doi.org/10.3390/polysaccharides6010023