Abstract
In times of increasing demand for resources, processing various waste materials is becoming more economically and ecologically viable. Red mud is a waste material that originates from the bauxite process, also known as the Bayer process. Red mud, due to its high alkalinity and heavy metal content, is often stored in landfills, which can lead to accidents such as those in Brazil or Hungary, especially if the storage takes place above ground. Red mud contains not only iron and aluminum residues but also other economically valuable metals such as manganese, titanium, cadmium, or cobalt. Currently, only 4 million tons of the annual production of 150 million tons are utilized in various industries, which is a relatively small amount. Typically, only the iron content is further processed, leaving other potential resources untapped. Chitin has a high binding capacity for various trivalent and divalent metal ions, making it a suitable material for separating red mud into its components. It has been demonstrated that chitin can effectively remove aluminum, barium, cadmium, cobalt, copper, manganese, iron, nickel, lead, strontium, and various lanthanides from a red mud-like sludge. The elements bound to chitin can be easily removed using wet chemistry. Biologically compatible substances are predominantly used in this process, with few exceptions. The removal of elements from red sludge or other mining wastewater using chitin is a viable alternative to traditional mining methods.
1. Introduction
Modern society’s increasing reliance on technology has resulted in a greater demand for heavy metals and rare earth elements. The mining of these elements can have various negative effects on ecosystems and people, particularly in third-world countries. These negative effects can be partly avoided by recycling not only old technology products, but also waste products like red mud from chemical industries. Red mud is a waste product from the Bayer process that contains various metals, such as iron oxides, titanium dioxide, and silica. Due to its high sodium hydroxide (NaOH) content, red mud is often highly toxic and cannot be treated as normal waste. As a result, it must be stored. Currently, red mud is often stored in underground caves or above ground in pools. Both methods have benefits, but they also have negative ecological effects. If the stored red mud is not handled correctly, it can contaminate groundwater or surface water, as has happened before in Kolontár (Hungary 2010) or Proto Trombetas (Brazil 2015) [1,2]. It is estimated that up to 150,000,000 tons of red mud are produced annually from the bauxite process. Of this, only up to 3,900,000 tons (i.e., <3%) are reused, mostly as an additive in various industries, as shown below [3,4,5].
| Additive in cement: | 500,000 up to 1,500,000 tons |
| Raw material for iron/steel manufacturing: | 400,000 up to 1,500,000 tons |
| Additive in landside construction: | 200,000 up to 500,000 tons |
| Additive in construction industry: | 100,000 up to 300,000 tons |
| Different proposes in chemical industries: | 100,000 tons |
Red mud, or sludge like it, can occur naturally in soils containing iron and other metal salts. Figure 1 shows an example of natural red mud which occurs near a small pond in Zittau, Saxony, Germany.

Figure 1.
Natural red mud-like slugged in a small pound near Zittau, Saxony, Germany. Left: Spring area. Right: Part of a stream from the spring to a small lake. (S. Fränzle 2024©).
Certain partly mineralized Fe-rich phases also containing S and partly alkali metals, which exist on both Earth and Mars [6], do rapidly form, like schwertmannite and (natro-)jarosite. Their redox chemistry deals with reduction in S rather than that in Fe(III); there are no data concerning the activity of Fe ions over these phases at the local pH of about 7.5. Nevertheless, chitin does adsorb metal ions, including Fe(III), quite effectively at [Mn+] < 1 nMol/L, even though the presence of citrate (but not of malate, hydroxamates, caffeinate, or SCN− which also form rather stable to very stable Fe(III) complexes) does somewhat delay iron adsorption to chitin, yet not to an extent that it might compromise Fe uptake in the chosen conditions. The fact that only around 20% of the binding capacity is used (mainly by Fe(III)) in this setting thus is unlikely to be attributed to very low “free” Fe activity. Both man-made and natural red mud can contain high levels of various elements and rare earth elements [6,7], making them a potential resource for these elements. Extracting these elements could have several positive benefits, such as avoiding the need for new mining sites and, most importantly, reducing the threat of toxic waste by replacing it with less toxic products. In general, the extraction of elements from complex sources can be difficult to accomplish due to its different element composition. Consequently, extracting elements from red mud with traditional methods might not be the best option in economical terms. Established mining reagents and procedures are often distinguished by the use of toxic or otherwise potentially damaging ions or reagents (e.g., HSO4−) for the retrieval of either primary or secondary (urban mining) metal resources. Therefore, a cost-effective and user-friendly method is required to eliminate these components from red mud. Biomining using biopolymers such as chitin (ß-1→4 N-acetyl-D-glucosamine) (in general, the degree of acetylation of the amino group is used to distinguish between chitin and chitosan. Chitin is distinguished by an acetyl group content above 50%, while chitosan has a lower content below this threshold. It is important to note, however, that native chitin is not typically completely acetylated. This implies that chitin may be a copolymer of D-glucosamine and acetyl-D-glucosamine, as illustrated in Figure 2), chitosan (ß-1→4 D-glucosamine), or ‘modified’ cellulose (ß-1→4 D-glucose) is a promising alternative to traditional mining processes. The utilization of biopolymers in biomining has been widely discussed in recent decades [8,9,10,11,12,13,14,15,16]. Chitin, for example, has been shown to bind a wide range of metals, mostly as Me2+-Ions [9,13,14,17,18,19,20,21]. As illustrated in Figure 2, chitin is known to contain a variety of potential binding sites for metal ions like Fe2+. Fränzle et al. and others [9,17,18,19] have mainly used chitin for biomonitoring as an alternative to moss monitoring. In this context, the authors have already demonstrated the high binding capability of chitin to various Me2+-ions [7,8,9,11,12,17,18,19,22].
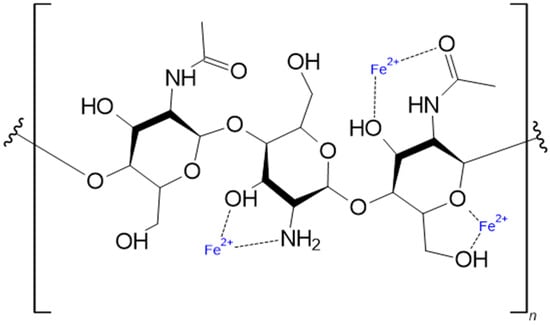
Figure 2.
A section of a chitin molecule is presented, along with the potential binding sites for divalent metal ions here using Fe2+-Ions as an example.
Therefore, the application of chitin in biopolymers for different mining options or recycling metals from aqueous sources has been widely discussed over the past decade [7,8,11,12,20,22,23,24]. To the best of our knowledge, there are currently no projects attempting to use chitin as an extraction material for elements from red mud, although there are a few projects exploring the use of red mud as a source for different elements [1,25,26,27,28,29]. As should be evident, the extraction of various metals from red mud is possible using chemical processes involving water. However, the utilization of such processes necessitates the employment of specific chemicals, several of which have been identified as toxic. The objective of the current study is to perform an initial feasibility assessment at the laboratory scale to determine whether it is feasible to utilize chitin to remove various metals from red mud.
2. Materials and Methods
2.1. Red Mud Sample Collection
To collect red mud samples from the small stream near a fishpond in Zittau, Germany, a standard method described before by Budelmann [17] is employed. In contrast to the approach proposed by Budelmann, we have adapted the sampling technique to align with local circumstances. The methodology is illustrated below. A glass beaker was submerged in the water near the red mud to obtain samples from the water surface, ensuring that the samples accurately represented the composition of the red mud present on the surface of the stream. To collect samples from the bottom, a beaker was skillfully driven through the upper layer of the riverbed. By using both methods, we collected a comprehensive and representative set of red mud samples, which were carefully stored in a 1 L sampling glass bottle to preserve their integrity for further analysis.
2.2. Cleaning of the Red Mud Samples
Both red mud samples contained unwanted plant material (surface sample) or small stones (riverbed sample); therefore, the samples needed to be cleaned. To clean the samples, they were first shaken and then allowed to sediment. After sedimentation occurred, the plant material was collected from the water surface of both samples. Following this, both samples were filtered. Small stones and remaining plant material were then removed from the filter cake using tweezers. Finally, both samples were washed twice with 100 mL of distilled water and once with 100 mL of methanol. After the methanol wash, both samples were left to dry overnight at room temperature.
2.3. Adsorption of ‘Heavy’ Metals and Lanthanides on Chitin
The dried samples were suspended in either 150 mL DMF (Dimethylformamide) (VWRChemicals, Darmstadt, Germany; P-Code 83634.320) or distilled water, as described in Table 1. After stirring all samples at 300 rpm for 30 min, 12 g chitin (Sigma-Aldrich, Darmstadt, Germany; P-Code: 1001295340) were added to the red mud samples, and the reaction flasks were sealed with thin foil. To ensure that the metal contained in the red mud was adsorbed to the equilibrium level of chitin, the samples were mixed for 120 h at 300 rpm, following the procedure described by Pinto et al. [12]. Afterwards, the liquid was removed from the chitin samples, and the chitin was left to dry for about one hour at room temperature. Sampling was performed twice at this location for accuracy.

Table 1.
Sample description for adsorption of heavy metals and lanthanides on chitin.
2.4. Desorption of ‘Heavy’ Metals and Lanthanides from Chitin
Based on previous research by the working group, it has been found that metals can be desorbed from chitin (Sigma-Aldrich) by using LiClO4-DMF solutions [9,19]. To achieve this, chitin samples were mixed at 300 rpm with 100 mL of a 1.0 M LiClO4-DMF solution for 24 h. The LiClO4 solution was then decanted. The chitin still appeared brown and discolored, indicating that metals may still be bound to the outer chitin layer. To extract those metals, chitin was mixed with 150 mL of 75% formic acid at 300 rpm, just because formic acid can dissolve chitin [10]. After 24 h, the formic acid solution turned brownish, while the chitin phase turned white again. Both the LiClO4 solution and the formic acid solution were stored in 250 mL sampling flasks. Due to the high sample volume, the samples were concentrated by vacuum distillation (60 °C, 20 mbar) with a rotary evaporator to a final volume of 50–75 mL.
2.5. Preparing Solutions for ICP-OES-Analysis
The method for preparing the test solution for OES-Analysis was described in more detail before [9,17,18,19].
2.6. ICP-OES-Analysis of ‘Heavy’ Metals and Lanthanides
The procedure for measuring heavy metal and lanthanide levels using OES has been previously described [9,17,18,19].
3. Results and Discussion
3.1. Comparison of Element Concentrations in Water with Those in the Sampled Red Mud-like Sludges
The element concentrations of the red mud-like sludge samples are given in Figure 3, showing huge differences in element concentrations for the tested elements. The tested red mud-like sludge contains mostly iron, manganese, and copper, with minor concentrations of aluminum, barium, cobalt, chromium, and strontium, as well as traces of cobalt, nickel, and lead. According to the literature, red mud mainly consists of aluminum, iron, and titanium (which is not tested in this publication), and different rear earth elements [26,27]. When comparing the background data from the surrounding water, as previously described by Budelmann [17], it is evident that both samples enriched the test elements by a factor of up to 1806 times. The comprehension of the enrichment capability of both test sludges revealed some interesting differences. In this context, the surface sludge enriched Fe, Mn, Al, Ba, and Cr up to a factor of 1.8, which is nearly twice as much as the riverbed sludge. The riverbed sludge only enriched Co by a factor of 1.45. The concentrations of Cd, Ni and Pb in both samples were below the detection limit, which is not necessarily an indication of their abundance. As demonstrated in the following chapter, Cd, Ni, and Pb can be extracted from the red mud-like sludges using chitin, which means that these metals must be in sample. This assumption is supported by the findings from Ghorbani et al., who used red mud to remove Pb from different water samples [25]. Red mud can also remove cadmium by complexing Cd ions on Fe/Mn oxide-bound [28,29]. This suggests that most of the tested elements in the red mud-like sludge are complexed by Fe/Mn oxide-bound. This could explain why the tested sludge enriches the tested elements from the surrounding water body.
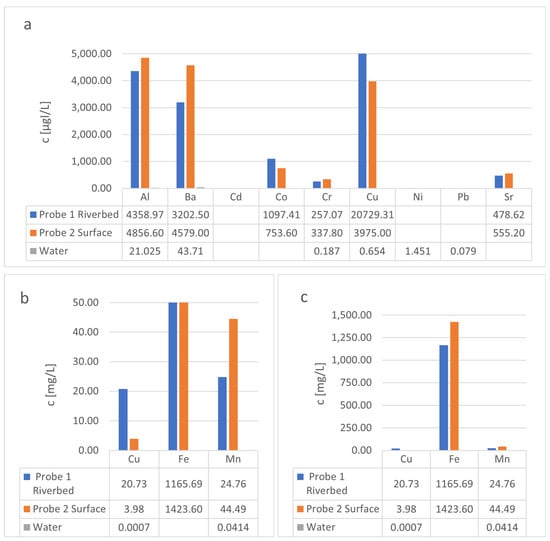
Figure 3.
(a–c): Measured concentration of the tested elements in red mud-like sludges from the riverbed and the water surface in relation to the concentration of these elements in the water body. (Blanks meaning the measured concentration was lower than the detection limit).
3.2. Biomining of Different Elements from Red Mud-like Sludges with Chitin
As shown in Figure 4, it is possible to extract the test elements from two different red mud-like sludges. In contrast to the findings from Budelmann [17], we did not observe any enrichment of the test elements on chitin. However, this is due to the experimental setup we used. Our setup only allowed us to extract the amount we put in the test with the sludge samples. This means that the concentration in the riverbed sludge of Cd, Ni, and Pb must be greater than 32.33 μg/L (Cd), 262.486 μg/L (Ni), and 117.173 μg/L (Pb). This represents a concentration factor for these elements on the red mud-like sludge of at least 261 (Ni) and 1483.20 (Pb) compared to the concentration mentioned by Budelmann for the waterbody from the same spring. This matches the data given in the literature for Pb enrichment by red mud [25].
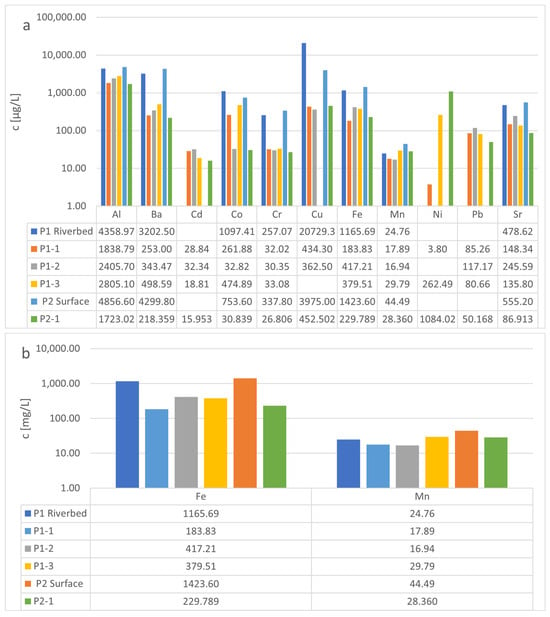
Figure 4.
(a,b): Comparison of the concentration for the tested elements in both red mud-like sludges with the concentration in the desorption media after desorption from chitin. (Blanks meaning the measured concentration was lower than the detection limit).
As mentioned above, Cd could be complexed by Fe/Mn oxides [22,23]. Due to the high concentration of Fe (up to 417 mg/L) and Me (up to 30 mg/L), it is clear that the tested red mud-like sludge is able to bind Cd ions from different sources. Depending on the composition of the extraction media, red mud could enrich Ni, as described by Smičiklas et al., and is highly influenced by citrate anion in the extraction media [30]. They considered that the high alkalinity is responsible for this trend [30]. On the basis of this, it is clear that the red mud-like sludge that was tested is able to bind Ni from the surrounding body of water at the test site. Although the alkalinity of the media affects the extraction by chitin in the test system, as seen before by Erler, higher pHs in the alkaline range up to pH 10 benefit the uptake of Me-ions such as Fe by chitin. This could explain why the concentration extracted by chitin varies depending on the extraction media we used [31]. As shown, Cd, Fe, Pb and Sr concentrations were slightly higher when water was used as the extraction medium for extraction with chitin from the red mud-like sludge. In contrast, Al, Ba, Co, Mn, Cu and Ni concentrations are slightly higher when extraction by chitin takes place in dimethylformamide (DMF).
As previously described in the literature, the binding capacity of chitin for different ions is not only affected by the pH and extraction media, but also by the exact elemental composition itself [18,25,30,31,32]. Bauer demonstrated that the binding capacity for different elements are affected by each other [32]. For example, the binding capacity for Al is positively affected by Cd, Co, Cu, and Mn, but negatively affected by Cr, Fe, and Pb [32]. Due to the varying element concentrations in the tested red mud-like sludges, their binding capabilities onto chitin may differ slightly. As the binding capacity of chitin is influenced by numerous factors, it is not possible to make a uniform prediction about the binding behavior of individual elements. Therefore, a different approach is necessary to compare the different extraction media used in this research. In this case, the best approach is to compare them by measuring the total bound ions and comparing them with the maximum binding capacity of chitin. According to Bauer, chitin has a maximum binding capacity of approximately 45 μmol/g for most elements [32]. In our case, the maximum binding for 12 g chitin was 540 μmol. The total amount of Mex+ is obtained by adding together the amount bound to chitin from each tested element. The results of this simple equation and the saturation factor on chitin are given in Table 2.

Table 2.
Determined total concentration of ions bound to chitin and the resulting saturation rates.
A comparison of the total amount of ions bound shows that, as expected, adsorption to chitin depends on the initial sample concentration. A higher sample volume leads to an increased binding of different Mex+-ions to chitin. At the same time, the data indicate that the binding of the test elements to chitin is significantly better in water than in DMF. As shown, the binding rate in water was almost twice as high as the binding rate in DMF. A similar trend has already been described by several authors [18,19,31].
The reasons for this could be the different solvation of chitin and the corresponding ions. Also, different complexation rates of the investigated ions on chitin are conceivable. Furthermore, it cannot be excluded that the red mud-like sludges used here contained certain amounts of Li salts, especially lithium chloride. However, these would lead to the formation of a solution in DMF capable of partially dissolving chitin. This would significantly reduce the binding capacity of chitin for the elements studied. The possibility of lithium’s influence on the extraction with chitin in DMF cannot be excluded, as it occurs in soils at an average of 40 ppm [33].
According to the saturation rates in Table 2, the recovery rates for the investigated elements shown in Figure 5 were expected to be around 100%. However, they were found to be in the range of 30–60%, significantly lower than anticipated. The reason for this may be due to the binding behavior of the elements to chitin, as described by Bauer [32]. However, the significant surplus of 12 g chitin suggests that the binding behavior of the studied elements is not the primary factor. Instead, the results indicate two different phenomena. Firstly, the low saturation rates suggest that not all possible binding sites on the chitin are occupied by a Mex+-ion. This assumption is in line with previously reported findings, where only one of our approximately 100–1000 binding sites are occupied by an ion. The data presented in Table 2 and Figure 3, though, suggest that certain ions found in the examined sludges are insoluble in water or DMF, and therefore cannot bind to chitin. However, this may have a negative impact on the concept of biomining, as it may not be possible to recover all metals from actual red mud.
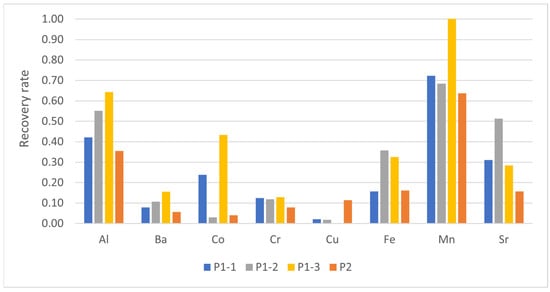
Figure 5.
Recovery rate for the tested elements after desorption from chitin by DMF and formic acid. (For Cd, Ni and Pb, no value could be given due to the fact that the concentration of these elements in both red mud-like sludges were lower than the detection limit).
3.3. Biomining of Rare Earth Elements from Red Mud-like Sludges
In the literature, it is well-known that chitin is highly capable of binding different rare earth elements such as europium (Eu), lanthanum (La), cerium (Ce), samarium (Sm), or ytterbium (Yb). Additionally, the literature has described that red mud often contains not just trace amounts of rare earth elements [27]. This has been described previously [7,9,17,31]. Budelmann conducted a study on the distribution of crayfish in Upper Lusatia [17]. In this research, Budelmann describes the absorption of various rare earth elements on different parts of crayfish bodies, specifically legs, claws, and carapace [17]. Crayfish accumulate different elements from various sources, such as the riverbed (legs) or the water body (carapace). Therefore, these body parts should have different concentrations of rare earth elements. Budelmann demonstrated that crayfish accumulate rare earth elements, among others, in varying concentrations depending on the body part [17]. This research paper focuses solely on the concentration of rare earth elements in the legs of crayfish, as they come into contact with the red mud-like sludges found on the riverbed [17]. As shown in Table 3, crayfish do accumulate rare earth elements from the riverbed sludge. Budelmann’s findings reveal two key points. The red mud-like sludge contains rare earth elements such as europium. These elements can be adsorbed on chitin, making the biomining of rare earth elements with chitin possible. The only limitation is the adsorption capability of chitin.

Table 3.
Rear earth concentration on chitin of legs from crayfish which live in the testd red mud-like sludge [17].
4. Conclusions
The method described has limitations. The pH of the red mud must not exceed 10, as it would interfere with the binding of various elements to chitin [31]. The red mud is treated before storage to recover some of the sodium hydroxide used in the Bayer process. Environmental influences, such as rain or CO2 uptake from the atmosphere, will cause the pH of stored red mud to be well below 14 [34]. Over time the pH decreases due to these effects, from 13 to 10 and below [34]. However, if necessary, the pH can be further reduced by adding appropriate acids to compensate for the negative effects of a pH that may be too high. Figure 6 presents a potential mining configuration using chitin, which offers several advantages. This design enables in situ treatment of existing red mud heaps, and various extraction agents can be used to remove the metal ions bound to chitin. The elements detached from chitin can be easily separated from the respective extraction agents, for example, by electrochemical methods. However, for the cyclic process shown in Figure 5, it is essential that the chitin used passes through the process several times.
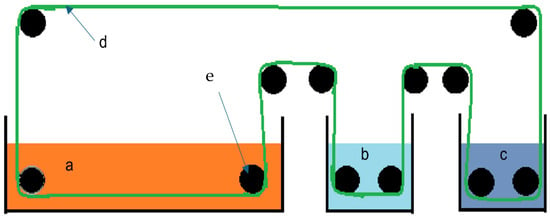
Figure 6.
Schematic representation of a possible design of a plant for biomining various elements from red mud with chitin. a: red sludge heap; b: first desorption basin for removing the elements bound to chitin (e.g., LiCl in DMF); c: second desorption basin for removing the elements bound to chitin (e.g., formic acid); d: conveyor belt coated with chitin; e deflection rollers.
This is entirely possible, as previously described in the literature. Although the binding capacity of chitin deteriorates somewhat over time, it is still feasible to use it for multiple cycles. The negative process can be counteracted by reducing the reaction time of the chitin ribbon in the respective tanks. This is possible by following the method described in the literature, as most chitin exchange processes reach equilibrium after a maximum of 10 min [18,19,31]. Therefore, the authors suggest that chitin can be used to remove various elements from red mud, making them suitable for use as raw materials. Additionally, degrading existing red mud dumps over time could significantly reduce their environmental risk.
Author Contributions
Data curation, F.B.; Formal analysis, F.B.; Writing manuscript, F.B.; Supervision, S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We would like to thank B. Bittner for her help with ICP-OES-Analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marschall, L. Aluminium: Metall der Moderne; 1., Aufl.; Oekom Verlag: München, Germany, 2008; ISBN 9783865816566. [Google Scholar]
- Kovács, T.; Sas, Z.; Jobbágy, V.; Csordás, A.; Szeiler, G.; Somlai, J. Radiological aspects of red mud disaster in Hungary. Acta Geophys. 2013, 61, 1026–1037. [Google Scholar] [CrossRef]
- Pontikes, Y.; Angelopoulos, G.N. Bauxite residue in cement and cementitious applications: Current status and a possible way forward. Resour. Conserv. Recycl. 2013, 73, 53–63. [Google Scholar] [CrossRef]
- Biswas, W.K.; Cooling, D. Sustainability Assessment of Red Sand as a Substitute for Virgin Sand and Crushed Limestone. J. Ind. Ecol. 2013, 17, 756–762. [Google Scholar] [CrossRef]
- Genç-Fuhrman, H.; Tjell, J.C.; McConchie, D. Adsorption of arsenic from water using activated neutralized red mud. Environ. Sci. Technol. 2004, 38, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Das, P.; Pandey, J.K.; Mishra, K.K.; Cardinale, M. Understanding redox processes during iron precipitation in standing water: Implications in formation of iron oxides minerals in the terrestrial planetary environment (especially Mars). Proc. Indian Natl. Sci. Acad. 2022, 88, 729–741. [Google Scholar] [CrossRef]
- Boulaiche, W.; Hamdi, B.; Trari, M. Removal of heavy metals by chitin: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2019, 9, 39. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin Adsorbents for Toxic Metals: A Review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Blind, F.; Fränzle, S. Chitin as a Sorbent Superior to Other Biopolymers: Features and Applications in Environmental Research, Energy Conversion, and Understanding Evolution of Animals. Polysaccharides 2021, 2, 773–794. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, N. Formic acid-mediated liquefaction of chitin. Green Chem. 2016, 18, 5050–5058. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Zhou, J.; Guo, S. Cellulose/chitin beads for adsorption of heavy metals in aqueous solution. Water Res. 2004, 38, 2643–2650. [Google Scholar] [CrossRef]
- Pinto, P.X.; Al-Abed, S.R.; Reisman, D.J. Biosorption of heavy metals from mining influenced water onto chitin products. Chem. Eng. J. 2011, 166, 1002–1009. [Google Scholar] [CrossRef]
- Göksungur, Y.; Uren, S.; Güvenç, U. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour. Technol. 2005, 96, 103–109. [Google Scholar] [CrossRef]
- de Rossi, A.; Rigon, M.R.; Zaparoli, M.; Braido, R.D.; Colla, L.M.; Dotto, G.L.; Piccin, J.S. Chromium (VI) biosorption by Saccharomyces cerevisiae subjected to chemical and thermal treatments. Environ. Sci. Pollut. Res. Int. 2018, 25, 19179–19186. [Google Scholar] [CrossRef] [PubMed]
- Bustard, M.; McHale, A.P. Biosorption of heavy metals by distillery-derived biomass. Bioprocess Eng. 1998, 19, 351. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. Biosorption of cobalt(II) and nickel(II) by seaweeds: Batch and column studies. Sep. Purif. Technol. 2005, 44, 53–59. [Google Scholar] [CrossRef]
- Budelmann, P. Verbreitung der Flusskrebse (Decapoda) in der Südlichen Oberlausitz und die Eignung des Incasiven Kamberkrebses (Orconectes limosus) für Chitin-Basiertes Monitoring von Schwemetallen in Limnischen Ökosystemen. Master’s Thesis, TU Dresden, Zittau, Germany, 2021. [Google Scholar]
- Gebauer, T. Methodische Optimierung des Übertrags von Metallionen aus Umweltprobenmodellen auf Chitinoberflächen und von Diesen zu Zwecken Analytischem Biomonitroings sowie Untersuchungen zur Diffusion/Ausbereitung von Analyten in Chitinproben. Master’s Thesis, TU Dresden, Zittau, Germany, 2016. [Google Scholar]
- Fraenzle, S.; Erler, M.; Blind, F.; Ariuntsetseg, L.; Narangarvuu, D. Chitin Adsorption in Environmental Monitoring: Not an Alternative to Moss Monitoring but a Method Providing (Lots of) Bonus Information. J. Sci. Arts 2019, 19, 659–674. [Google Scholar]
- Machado, M.D.; Santos, M.S.F.; Gouveia, C.; Soares, H.M.V.M.; Soares, E.V. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: The flocculation as a separation process. Bioresour. Technol. 2008, 99, 2107–2115. [Google Scholar] [CrossRef]
- Vasudevan, P.; Padmavathy, V.; Dhingra, S.C. Kinetics of biosorption of cadmium on Baker’s yeast. Bioresour. Technol. 2003, 89, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Ojima, Y.; Kosako, S.; Kihara, M.; Miyoshi, N.; Igarashi, K.; Azuma, M. Recovering metals from aqueous solutions by biosorption onto phosphorylated dry baker’s yeast. Sci. Rep. 2019, 9, 225. [Google Scholar] [CrossRef]
- Amini, M.; Younesi, H.; Bahramifar, N. Biosorption of U(VI) from Aqueous Solution by Chlorella vulgaris: Equilibrium, Kinetic, and Thermodynamic Studies. J. Environ. Eng. 2013, 139, 410–421. [Google Scholar] [CrossRef]
- Ghorbani, A.; Nazarfakhari, M.; Pourasad, Y.; Mesgari Abbasi, S. Removal of Pb ion from water samples using red mud (bauxite ore processing waste). E3S Web Conf. 2013, 1, 41019. [Google Scholar] [CrossRef]
- Jovičević-Klug, M.; Souza Filho, I.R.; Springer, H.; Adam, C.; Raabe, D. Green steel from red mud through climate-neutral hydrogen plasma reduction. Nature 2024, 625, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, J.; Zheng, G.; Xia, T. Effects of Red Mud on Cadmium Uptake and Accumulation by Rice and Chemical Changes in Rhizospheres by Rhizobox Method. Minerals 2022, 12, 929. [Google Scholar] [CrossRef]
- Yang, T.; Sheng, L.; Wang, Y.; Wyckoff, K.N.; He, C.; He, Q. Characteristics of Cadmium Sorption by Heat-Activated Red Mud in Aqueous Solution. Sci. Rep. 2018, 8, 13558. [Google Scholar] [CrossRef]
- Smičiklas, I.; Smiljanić, S.; Perić-Grujić, A.; Šljivić-Ivanović, M.; Antonović, D. The influence of citrate anion on Ni(II) removal by raw red mud from aluminum industry. Chem. Eng. J. 2013, 214, 327–335. [Google Scholar] [CrossRef]
- Erler, M. Untersuchung des Bindungsverhaltens Ausgewählter Elemente und Ihrer Bodenrelavanter Komplexe an Chitin. Master’s Thesis, TU Dresden, Zittau, Germany, 2020. [Google Scholar]
- Bauer, A. Orientierende Untersuchung zur Bindung von Metallionen an Chitin und zur Davon Abhängigen Eignung von Arthropoden zur Bestimmung von Metallionenkonzentrationen in der Umwelt. Master’s Thesis, TU Dresden, Zittau, Germany, 2014. [Google Scholar]
- Emsley, J. The Oxford Book of the Elements; Oxford University Press: Oxford, UK, 2001; ISBN 9780198503415. [Google Scholar]
- Liu, Y.; Lin, C.; Wu, Y. Characterization of red mud derived from a combined Bayer Process and bauxite calcination method. J. Hazard. Mater. 2007, 146, 255–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).