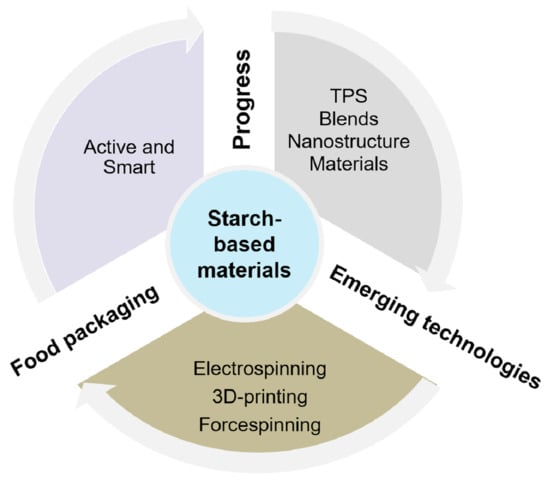
Progress in Starch-Based Materials for Food Packaging Applications
Abstract
1. Introduction
2. Starch
3. Chronology of the Development of Starch-Based Materials for Industrial Applications
3.1. Modification of Native Starches
3.1.1. Thermo-Plasticization
3.1.2. Chemical Modifications
3.1.3. Physical Modifications
3.2. TPS Blends whit Biodegradable Polymers
3.2.1. Poly (Lactic Acid) (PLA)
3.2.2. Poly (Butylene Adipate-Co-Terephthalate) (PBAT)
3.2.3. Polyvinyl Alcohol (PVA)
3.2.4. Chitosan
3.2.5. Polycaprolactone (PCL)
3.2.6. Other
3.3. TPS Blends with Synthetic Polymers
3.3.1. TPS/Polypropylene (PP)
3.3.2. TPS/Natural Rubber (NR)
3.3.3. TPS/Polyethylene (PE)
3.4. Starch Based Composite Materials
3.5. Starch-Based Materials with Filler/Reinforcement
4. Advance in Preparation of Functional Starch-Based Food Packaging
4.1. Incorporating Bioactive
4.2. Starch Nanostructures (SNEs)
5. Processing Techniques
5.1. Traditional Techniques
5.1.1. Extrusion
5.1.2. Foaming Processing
5.1.3. Film Casting
5.2. Emerging Technologies
5.2.1. Electrospinning
5.2.2. Forcespinning
5.2.3. 3D-Printing
5.2.4. Reactive Extrusion
6. Starch Based Materials Application in Food Industry
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe; Conversio Market & Strategy GmbH. Plastics—The Facts 2019; Plastics Europe: Brussels, Belgium, 2019; pp. 14–35. [Google Scholar]
- Waring, R.H.; Harris, R.; Mitchell, S. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Cataldi, P.; Paul, U.C.; Contardi, M.; Cingolani, R.; Bayer, I.S.; Heredia, A.; Athanassiou, A. All-natural sustainable packaging materials inspired by plant cuticles. Adv. Sustain. Syst. 2017, 1, 1600024. [Google Scholar] [CrossRef]
- Leite, L.S.; Bilatto, S.; Paschoalin, R.T.; Soares, A.C.; Moreira, F.K.; Oliveira, O.N., Jr.; Mattoso, L.H.; Bras, J. Eco-friendly gelatin films with rosin-grafted cellulose nanocrystals for antimicrobial packaging. Int. J. Biol. Macromol. 2020, 165, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Panda, A.K.; Kumar, S. Thermal degradation of corn starch based biodegradable plastic plates and determination of kinetic parameters by isoconversional methods using thermogravimetric analyzer. J. Energy Inst. 2020, 93, 1449–1459. [Google Scholar] [CrossRef]
- Marichelvam, M.; Jawaid, M.; Asim, M. Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Liu, J.; Xu, X. Preparation, characterization, physicochemical property and potential application of porous starch: A review. Int. J. Biol. Macromol. 2020, 148, 1169–1181. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Dolci, L.S.; Liccardo, L.; Bigi, A.; Bonvicini, F.; Gentilomi, G.A.; Passerini, N.; Panzavolta, S.; Albertini, B. Cellulose derivatives-snail slime films: New disposable eco-friendly materials for food packaging. Food Hydrocoll. 2021, 111, 106247. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.; Sobral, P.J.; Menegalli, F.C. Development and characterization of biofilms based on Amaranth flour (Amaranthus caudatus). J. Food Eng. 2005, 67, 215–223. [Google Scholar] [CrossRef]
- Parra, D.; Tadini, C.; Ponce, P.; Lugão, A. Mechanical properties and water vapor transmission in some blends of cassava starch edible films. Carbohydr. Polym. 2004, 58, 475–481. [Google Scholar] [CrossRef]
- Shamekh, S.; Myllärinen, P.; Poutanen, K.; Forssell, P. Film formation properties of potato starch hydrolysates. Starch-Stärke 2002, 54, 20–24. [Google Scholar] [CrossRef]
- Yu, H.; Sabato, S.F.; D’Aprano, G.; Lacroix, M. Effect of the addition of CMC on the aggregation behaviour of proteins. Radiat. Phys. Chem. 2004, 71, 131–135. [Google Scholar] [CrossRef]
- Castaño, J.; Rodríguez-Llamazares, S.; Carrasco, C.; Bouza, R. Physical, chemical and mechanical properties of pehuen cellulosic husk and its pehuen-starch based composites. Carbohydr. Polym. 2012, 90, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Miladinov, V.; Hanna, M.A. Synthesis and characterization of starch acetates with high substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef]
- Thuwall, M.; Boldizar, A.; Rigdahl, M. Extrusion processing of high amylose potato starch materials. Carbohydr. Polym. 2006, 65, 441–446. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Dufresne, A. Preparation and properties of cellulose nanomaterials. Pap. Biomater. 2020, 5, 1–13. [Google Scholar]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch nanoparticles: A review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, S.; Lin, L.; Zhao, L.; Liu, A.; Wei, C. Properties of new starches from tubers of Arisaema elephas, yunnanense and erubescens. Food Hydrocoll. 2016, 61, 183–190. [Google Scholar] [CrossRef]
- Silva, A.P.M.; Oliveira, A.V.; Pontes, S.M.; Pereira, A.L.; Rosa, M.F.; Azeredo, H.M. Mango kernel starch films as affected by starch nanocrystals and cellulose nanocrystals. Carbohydr. Polym. 2019, 211, 209–216. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Chang, Q.; Zhang, Y.; Zheng, B.; Zeng, H. Structural and physicochemical properties of ginger (Rhizoma curcumae longae) starch and resistant starch: A comparative study. Int. J. Biol. Macromol. 2020, 144, 67–75. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of native starch’s properties on starch nanocrystals thermal properties. Carbohydr. Polym. 2012, 87, 658–666. [Google Scholar] [CrossRef]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.B.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Freire, A.C.; Fertig, C.C.; Podczeck, F.; Veiga, F.; Sousa, J. Starch-based coatings for colon-specific drug delivery. Part I: The influence of heat treatment on the physico-chemical properties of high amylose maize starches. Eur. J. Pharm. Biopharm. 2009, 72, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Vamadevan, V.; Bertoft, E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Thiré, R.M.; Simão, R.A.; Andrade, C.T. High resolution imaging of the microstructure of maize starch films. Carbohydr. Polym. 2003, 54, 149–158. [Google Scholar] [CrossRef]
- Sindhu, R.; Devi, A.; Khatkar, B. Physicochemical, thermal and structural properties of heat moisture treated common buckwheat starches. J. Food Sci. Technol. 2019, 56, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Condés, M.C.; Añón, M.C.; Dufresne, A.; Mauri, A.N. Composite and nanocomposite films based on amaranth biopolymers. Food Hydrocoll. 2018, 74, 159–167. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Mauri, A.N.; Dufresne, A. Amaranth protein films reinforced with maize starch nanocrystals. Food Hydrocoll. 2015, 47, 146–157. [Google Scholar] [CrossRef]
- da Silva, L.R.; de Carvalho, C.W.P.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rice. Int. J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dhital, S.; Gilbert, R.G.; Gidley, M.J. High-amylose wheat starch: Structural basis for water absorption and pasting properties. Carbohydr. Polym 2020, 245, 116557. [Google Scholar] [CrossRef]
- Irani, M.; Abdel-Aal, E.-S.M.; Razavi, S.M.A.; Hucl, P.; Patterson, C.A. Thermal and Functional Properties of Hairless Canary Seed (Phalaris canariensis L.) Starch in Comparison with Wheat Starch. Cereal Chem. J. 2017, 94, 341–348. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Mariano, M.; Dufresne, A.; Mellem, J.J.; Amonsou, E.O. Microstructure, thermal properties and crystallinity of amadumbe starch nanocrystals. Int. J. Biol. Macromol. 2017, 102, 241–247. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Barros Ferreira, R.S.; Lizárraga, E.; Tapia-Blácido, D.R.; Silva, N.C.C.; Angelats-Silva, L.; Siche, R. Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag. Shelf Life 2020, 23, 100457. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Ferdinand, U.; Gong, X.; Qu, Y.; Gao, W.; Ivanistau, A.; Feng, B.; Liu, M. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef]
- Jiménez-Hernández, J.; Salazar-Montoya, J.A.; Ramos-Ramírez, E.G. Physical, chemical and microscopic characterization of a new starch from chayote (Sechium edule) tuber and its comparison with potato and maize starches. Carbohydr. Polym. 2007, 68, 679–686. [Google Scholar] [CrossRef]
- Jamir, K.; Seshagirirao, K. Isolation, characterization and comparative study of starches from selected Zingiberaceae species, a non-conventional source. Food Hydrocoll 2017, 72, 247–253. [Google Scholar] [CrossRef]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef]
- Yniestra Marure, L.M.; Núñez-Santiago, M.C.; Agama-Acevedo, E.; Bello-Perez, L.A. Starch Characterization of Improved Chickpea Varieties Grown in Mexico. Starch-Stärke 2019, 71, 1800139. [Google Scholar] [CrossRef]
- Saraiva Rodrigues, S.C.; Silva, A.S.d.; Carvalho, L.H.d.; Alves, T.S.; Barbosa, R. Morphological, structural, thermal properties of a native starch obtained from babassu mesocarp for food packaging application. J. Mater. Res. Technol. 2020, 9, 15670–15678. [Google Scholar] [CrossRef]
- Harini, K.; Chandra Mohan, C.; Ramya, K.; Karthikeyan, S.; Sukumar, M. Effect of Punica granatum peel extracts on antimicrobial properties in Walnut shell cellulose reinforced Bio-thermoplastic starch films from cashew nut shells. Carbohydr. Polym. 2018, 184, 231–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Xu, F.; He, S.; Zhang, Y.; Sun, L.; Zhu, K.; Li, S.; Wu, G.; Tan, L. Jackfruit starch: Composition, structure, functional properties, modifications and applications. Trends Food Sci. Technol. 2021, 107, 268–283. [Google Scholar] [CrossRef]
- Turola Barbi, R.C.; Teixeira, G.L.; Hornung, P.S.; Ávila, S.; Hoffmann-Ribani, R. Eriobotrya japonica seed as a new source of starch: Assessment of phenolic compounds, antioxidant activity, thermal, rheological and morphological properties. Food Hydrocoll. 2018, 77, 646–658. [Google Scholar] [CrossRef]
- Macena, J.F.F.; Souza, J.C.A.d.; Camilloto, G.P.; Cruz, R.S. Physico-chemical, morphological and technological properties of the avocado (Persea americana Mill. cv. Hass) seed starch. Ciênc. Agrotecnol. 2020, 44, e001420. [Google Scholar] [CrossRef]
- Shubeena; Wani, I.A.; Gani, A.; Sharma, P.; Wani, T.A.; Masoodi, F.A.; Hamdani, A.; Muzafar, S. Effect of acetylation on the physico-chemical properties of Indian Horse Chestnut (Aesculus indica L.) starch. Starch-Stärke 2015, 67, 311–318. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Preparation and properties of extruded thermoplastic starch/polymer blends. J. Appl. Polym. Sci. 2012, 126, E96–E108. [Google Scholar] [CrossRef]
- Zobel, H.; Young, S.; Rocca, L. Starch gelatinization: An X-ray diffraction study. Cereal Chem. 1988, 65, 443–446. [Google Scholar]
- Wang, T.L.; Bogracheva, T.Y.; Hedley, C.L. Starch: As simple as A, B, C? J. Exp. Bot. 1998, 49, 481–502. [Google Scholar] [CrossRef]
- Pfannemüller, B. Influence of chain length of short monodisperse amyloses on the formation of A- and B-type X-ray diffraction patterns. Int. J. Biol. Macromol. 1987, 9, 105–108. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bulpin, P.V. Crystallisation of malto-oligosaccharides as models of the crystalline forms of starch: Minimum chain-length requirement for the formation of double helices. Carbohydr. Res. 1987, 161, 291–300. [Google Scholar] [CrossRef]
- Srichuwong, S.; Isono, N.; Mishima, T.; Hisamatsu, M. Structure of lintnerized starch is related to X-ray diffraction pattern and susceptibility to acid and enzyme hydrolysis of starch granules. Int. J. Biol. Macromol. 2005, 37, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Frost, K.; Kaminski, D.; Kirwan, G.; Lascaris, E.; Shanks, R. Crystallinity and structure of starch using wide angle X-ray scattering. Carbohydr. Polym. 2009, 78, 543–548. [Google Scholar] [CrossRef]
- Kubo, A.; Yuguchi, Y.; Takemasa, M.; Suzuki, S.; Satoh, H.; Kitamura, S. The use of micro-beam X-ray diffraction for the characterization of starch crystal structure in rice mutant kernels of waxy, amylose extender, and sugary1. J. Cereal Sci. 2008, 48, 92–97. [Google Scholar] [CrossRef]
- Mani, R.; Bhattacharya, M. Properties of injection moulded blends of starch and modified biodegradable polyesters. Eur. Polym. J. 2001, 37, 515–526. [Google Scholar] [CrossRef]
- Rosalina, I.; Bhattacharya, M. Dynamic rheological measurements and analysis of starch gels. Carbohydr. Polym. 2002, 48, 191–202. [Google Scholar] [CrossRef]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956. [Google Scholar] [CrossRef]
- Noivoil, N.; Yoksan, R. Compatibility improvement of poly (lactic acid)/thermoplastic starch blown films using acetylated starch. J. Appl. Polym. Sci. 2021, 138, 49675. [Google Scholar] [CrossRef]
- Yusoff, N.H.; Pal, K.; Narayanan, T.; de Souza, F.G. Recent trends on bioplastics synthesis and characterizations: Polylactic acid (PLA) incorporated with tapioca starch for packaging applications. J. Mol. Struct. 2021, 1232, 129954. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, L.; Ding, N.; Liu, P.; Yao, C.; Zhang, H. Physicochemical and structural characteristics of the octenyl succinic ester of ginkgo starch. Int. J. Biol. Macromol. 2017, 94, 566–570. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Singhal, R.S. Effect of succinylation on the corn and amaranth starch pastes. Carbohydr. Polym. 2002, 48, 233–240. [Google Scholar] [CrossRef]
- Dufresne, A.; Castaño, J. Polysaccharide nanomaterial reinforced starch nanocomposites: A review. Starch-Stärke 2017, 69, 1500307. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Starch-based nano-biocomposites. Prog. Polym. Sci. 2013, 38, 1590–1628. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, H.; Chang, P.R. Transitional properties of starch colloid with particle size reduction from micro-to nanometer. J. Colloid Interface Sci. 2009, 339, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Ma, S.; Fan, L.; Tang, Z.; Zhang, R.; Na, H.; Zhu, J. Surface hydrophobic modification of starch with bio-based epoxy resins to fabricate high-performance polylactide composite materials. Compos. Sci. Technol. 2014, 94, 16–22. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, M.; Wang, L.; Zhou, J.; Tong, J. A method for improving dispersion of starch nanocrystals in water through crosslinking modification with sodium hexametaphosphate. Carbohydr. Polym. 2012, 87, 1874–1876. [Google Scholar] [CrossRef]
- Aburto, J.; Hamaili, H.; Alric, E.; Borredon, E.; Gaset, A. Advances in the Solvent-Free Synthesis of Fatty Esters of Starch; INRA: Paris, France, 1999. [Google Scholar]
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A.A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Peñaranda Contreras, O.I.; Perilla Perilla, J.E.; Algecira Enciso, N.A. Revisión de la modificación química del almidón con ácidos orgánicos. Ing. E Investig. 2008, 28, 47–52. [Google Scholar]
- López, O.V.; Zaritzky, N.E.; García, M.A. Physicochemical characterization of chemically modified corn starches related to rheological behavior, retrogradation and film forming capacity. J. Food Eng. 2010, 100, 160–168. [Google Scholar] [CrossRef]
- Rutenberg, M.W.; Solarek, D. Starch derivatives: Production and uses. In Starch: Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 311–388. [Google Scholar]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Kaur, M.; Singh, N.; Lim, S.-T. A comparison of native and oxidized normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. LWT Food Sci. Technol. 2008, 41, 1000–1010. [Google Scholar] [CrossRef]
- Kushwaha, R.; Kaur, D. Recent techniques used in modification of starches: A review. In Food Technology From Health to Wealth & Future Challenges; Bharti Publication: Delhi, India, 2018; pp. 1–15. [Google Scholar]
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films toward food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, W.; Zhang, H.; Dai, Y.; Hou, H.; Dong, H. Effects of citric acid on structures and properties of thermoplastic hydroxypropyl amylomaize starch films. Materials 2019, 12, 1565. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–Glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Berski, W.; Ptaszek, A.; Ptaszek, P.; Ziobro, R.; Kowalski, G.; Grzesik, M.; Achremowicz, B. Pasting and rheological properties of oat starch and its derivatives. Carbohydr. Polym. 2011, 83, 665–671. [Google Scholar] [CrossRef]
- Zailani, M.A.; Kamilah, H.; Husaini, A.; Seruji, A.Z.R.A.; Sarbini, S.R. Functional and digestibility properties of sago (Metroxylon sagu) starch modified by microwave heat treatment. Food Hydrocoll. 2022, 122, 107042. [Google Scholar] [CrossRef]
- Zhong, Y.; Herburger, K.; Kirkensgaard, J.J.K.; Khakimov, B.; Hansen, A.R.; Blennow, A. Sequential maltogenic α-amylase and branching enzyme treatment to modify granular corn starch. Food Hydrocoll. 2021, 120, 106904. [Google Scholar] [CrossRef]
- Rafiq, S.I.; Singh, S.; Saxena, D.C. Effect of heat-moisture and acid treatment on physicochemical, pasting, thermal and morphological properties of Horse Chestnut (Aesculus indica) starch. Food Hydrocoll. 2016, 57, 103–113. [Google Scholar] [CrossRef]
- Kurdziel, M.; Królikowska, K.; Łabanowska, M.; Pietrzyk, S.; Michalec, M. The effect of thermal and irradiation treatments on structural and physicochemical properties of octenyl succinate maize starches. Food Chem. 2020, 330, 127242. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Gao, Q. Internal structure of high degree substitution acetylated potato starch by chemical surface gelatinization. Int. J. Biol. Macromol. 2020, 145, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Ngamwonglumlert, L.; Devahastin, S.; Chindapan, N.; Chiewchan, N. Feasibility study of the use of superheated steam spray drying to produce selected food powders. Dry. Technol. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Kurdziel, M.; Łabanowska, M.; Pietrzyk, S.; Sobolewska-Zielińska, J.; Michalec, M. Changes in the physicochemical properties of barley and oat starches upon the use of environmentally friendly oxidation methods. Carbohydr. Polym. 2019, 210, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Xu, M.; Tang, W.; Wen, L.; Yang, B. Modification of structural, physicochemical and digestive properties of normal maize starch by thermal treatment. Food Chem. 2020, 309, 125733. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Ansari, S.; Estevez, L.; Hayrapetyan, S.; Giannelis, E.P.; Lai, H.-M. Preparation and properties of biodegradable starch–clay nanocomposites. Carbohydr. Polym. 2010, 79, 391–396. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, B.; Li, M.-N.; Chen, H.-Q. Effects of cross-linking with sodium trimetaphosphate on structural and adsorptive properties of porous wheat starches. Food Chem. 2019, 289, 187–194. [Google Scholar] [CrossRef]
- Punia, S. Barley starch modifications: Physical, chemical and enzymatic—A review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, F.; Song, X.; Ren, H.; Gong, H. Influence of formulation parameters on lipid oxidative stability of Pickering emulsion stabilized by hydrophobically modified starch particles. Carbohydr. Polym. 2020, 246, 116649. [Google Scholar] [CrossRef]
- Bet, C.D.; de Oliveira, C.S.; Colman, T.A.D.; Marinho, M.T.; Lacerda, L.G.; Ramos, A.P.; Schnitzler, E. Organic amaranth starch: A study of its technological properties after heat-moisture treatment. Food Chem. 2018, 264, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Pei, Y.; Qiao, M.; Ma, F.; Ren, H.; Zhao, Q. Preparation and characterizations of Pickering emulsions stabilized by hydrophobic starch particles. Food Hydrocoll. 2015, 45, 256–263. [Google Scholar] [CrossRef]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars. LWT 2019, 110, 197–206. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Tan, C.P.; Li, C.; Fu, X.; Huang, Q. Octenylsuccinate quinoa starch granule-stabilized Pickering emulsion gels: Preparation, microstructure and gelling mechanism. Food Hydrocoll. 2019, 91, 40–47. [Google Scholar] [CrossRef]
- Hu, X.-P.; Zhang, B.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Effect of high hydrostatic pressure and retrogradation treatments on structural and physicochemical properties of waxy wheat starch. Food Chem. 2017, 232, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Florido, H.; Vázquez-García, H.; Méndez-Montealvo, G.; Basilio-Cortés, U.; Navarro-Cortés, R.; Rodríguez-Marín, M.; Castro-Rosas, J.; Gómez-Aldapa, C. Effect of acid hydrolysis and OSA esterification of waxy cassava starch on emulsifying properties in Pickering-type emulsions. LWT 2018, 91, 258–264. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Schäfer, C.; Gilbert, R.G. Aggregate and emulsion properties of enzymatically-modified octenylsuccinylated waxy starches. Carbohydr. Polym. 2014, 111, 918–927. [Google Scholar] [CrossRef][Green Version]
- Ren, L.; Wang, Q.; Yan, X.; Tong, J.; Zhou, J.; Su, X. Dual modification of starch nanocrystals via crosslinking and esterification for enhancing their hydrophobicity. Food Res. Int. 2016, 87, 180–188. [Google Scholar] [CrossRef]
- Putro, J.N.; Ismadji, S.; Gunarto, C.; Soetaredjo, F.E.; Ju, Y.H. A study of anionic, cationic, and nonionic surfactants modified starch nanoparticles for hydrophobic drug loading and release. J. Mol. Liq. 2020, 298, 112034. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Double network hydrophobic starch based amphoteric hydrogel as an effective adsorbent for both cationic and anionic dyes. Carbohydr. Polym. 2020, 242, 116320. [Google Scholar] [CrossRef]
- Cova, A.; Sandoval, A.J.; Balsamo, V.; Müller, A.J. The effect of hydrophobic modifications on the adsorption isotherms of cassava starch. Carbohydr. Polym. 2010, 81, 660–667. [Google Scholar] [CrossRef]
- Barikani, M.; Mohammadi, M. Synthesis and characterization of starch-modified polyurethane. Carbohydr. Polym. 2007, 68, 773–780. [Google Scholar] [CrossRef]
- Miskeen, S.; Hong, J.S.; Choi, H.-D.; Kim, J.-Y. Fabrication of citric acid-modified starch nanoparticles to improve their thermal stability and hydrophobicity. Carbohydr. Polym. 2021, 253, 117242. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhi, C.; Jin, Z.; Miao, M. Improving properties of normal maize starch films using dual-modification: Combination treatment of debranching and hydroxypropylation. Int. J. Biol. Macromol. 2019, 130, 197–202. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, F.T.; da Silva, W.M.; El Halal, S.L.d.M.; Pinto, V.Z.; Dias, A.R.G.; da Rosa Zavareze, E. Impact of acid and oxidative modifications, single or dual, of sorghum starch on biodegradable films. Food Chem. 2017, 214, 53–60. [Google Scholar] [CrossRef]
- Biduski, B.; Evangelho, J.A.d.; Silva, F.T.d.; de Mello El Halal, S.L.; Takimi, A.S.; Carreño, N.L.V.; Dias, A.R.G.; Zavareze, E.d.R. Physicochemical properties of nanocomposite films made from sorghum-oxidized starch and nanoclay. Starch-Stärke 2017, 69, 1700079. [Google Scholar] [CrossRef]
- Bergel, B.F.; Osorio, S.D.; da Luz, L.M.; Santana, R.M.C. Effects of hydrophobized starches on thermoplastic starch foams made from potato starch. Carbohydr. Polym. 2018, 200, 106–114. [Google Scholar] [CrossRef]
- La Fuente, C.I.; de Souza, A.T.; Tadini, C.C.; Augusto, P.E.D. Ozonation of cassava starch to produce biodegradable films. Int. J. Biol. Macromol. 2019, 141, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.; Ali, T.M.; Hasnain, A. Effect of succinylation on functional and morphological properties of starches from broken kernels of Pakistani Basmati and Irri rice cultivars. Food Chem. 2016, 191, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Colussi, R.; Pinto, V.Z.; El Halal, S.L.M.; Biduski, B.; Prietto, L.; Castilhos, D.D.; da Rosa Zavareze, E.; Dias, A.R.G. Acetylated rice starches films with different levels of amylose: Mechanical, water vapor barrier, thermal, and biodegradability properties. Food Chem. 2017, 221, 1614–1620. [Google Scholar] [CrossRef]
- Sifuentes-Nieves, I.; Neira-Velázquez, G.; Hernández-Hernández, E.; Barriga-Castro, E.; Gallardo-Vega, C.; Velazquez, G.; Mendez-Montealvo, G. Influence of gelatinization process and HMDSO plasma treatment on the chemical changes and water vapor permeability of corn starch films. Int. J. Biol. Macromol. 2019, 135, 196–202. [Google Scholar] [CrossRef]
- Sifuentes-Nieves, I.; Velazquez, G.; Flores-Silva, P.C.; Hernández-Hernández, E.; Neira-Velázquez, G.; Gallardo-Vega, C.; Mendez-Montealvo, G. HMDSO plasma treatment as alternative to modify structural properties of granular starch. Int. J. Biol. Macromol. 2020, 144, 682–689. [Google Scholar] [CrossRef]
- Ding, Y.; Xiao, Y.; Ouyang, Q.; Luo, F.; Lin, Q. Modulating the in vitro digestibility of chemically modified starch ingredient by a non-thermal processing technology of ultrasonic treatment. Ultrason. Sonochem. 2021, 70, 105350. [Google Scholar] [CrossRef] [PubMed]
- Nemţanu, M.R.; Braşoveanu, M. Exposure of starch to combined physical treatments based on corona electrical discharges and ionizing radiation. Impact on physicochemical properties. Radiat. Phys. Chem. 2021, 184, 109480. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Q.-Y.; Jiang, W.; Qian, J.-Y.; Zhang, L.; Wu, M.; Rao, S.-Q.; Wu, C.-S. Effect of pulsed electric field on structural properties and digestibility of starches with different crystalline type in solid state. Carbohydr. Polym. 2019, 207, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R. Irradiation as a tool for modifying tapioca starch and development of an active food packaging film with irradiated starch. Radiat. Phys. Chem. 2020, 173, 108873. [Google Scholar] [CrossRef]
- Chung, K.M.; Moon, T.W.; Kim, H.; Chun, J.K. Physicochemical properties of sonicated mung bean, potato, and rice starches. Cereal Chem. 2002, 79, 631–633. [Google Scholar] [CrossRef]
- Iida, Y.; Tuziuti, T.; Yasui, K.; Towata, A.; Kozuka, T. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization. Innov. Food Sci. Emerg. Technol. 2008, 9, 140–146. [Google Scholar] [CrossRef]
- Goncalves, P.M.; Noreña, C.P.Z.; da Silveira, N.P.; Brandelli, A. Characterization of starch nanoparticles obtained from Araucaria angustifolia seeds by acid hydrolysis and ultrasound. LWT-Food Sci. Technol. 2014, 58, 21–27. [Google Scholar] [CrossRef]
- Herceg, Z.; Batur, V.; Jambrak, A.R.; Badanjak, M.; Brnčić, S.R.; Lalas, V. Modification of rheological, thermophysical, textural and some physical properties of corn starch by tribomechanical treatment. Carbohydr. Polym. 2010, 80, 1072–1077. [Google Scholar] [CrossRef]
- Zeng, F.; Gao, Q.-Y.; Han, Z.; Zeng, X.-A.; Yu, S.-J. Structural properties and digestibility of pulsed electric field treated waxy rice starch. Food Chem. 2016, 194, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zeng, X.-A.; Zhang, B.-S.; Yu, S.-J. Effects of pulsed electric fields (PEF) treatment on the properties of corn starch. J. Food Eng. 2009, 93, 318–323. [Google Scholar] [CrossRef]
- Han, Z.; Yu, Q.; Zeng, X.A.; Luo, D.H.; Yu, S.J.; Zhang, B.S.; Chen, X.D. Studies on the microstructure and thermal properties of pulsed electric fields (PEF)-treated maize starch. Int. J. Food Eng. 2012, 8, 1–17. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Wardman, R.H.; Abdrabbo, A. Effect of plasma treatment on the spreading of micro drops through polylactic acid (PLA) and polyester (PET) fabrics. Idea 2010, 4, 5. [Google Scholar]
- Coles, R.; Kirwan, M.J. Food and Beverage Packaging Technology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Palai, B.; Biswal, M.; Mohanty, S.; Nayak, S.K. In situ reactive compatibilization of polylactic acid (PLA) and thermoplastic starch (TPS) blends; synthesis and evaluation of extrusion blown films thereof. Ind. Crops Prod. 2019, 141, 111748. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, G.; Feng, Y.; Yin, J.; Jiang, W. Toughening polylactide with polyether-block-amide and thermoplastic starch acetate: Influence of starch esterification degree. Carbohydr. Polym. 2015, 127, 79–85. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Z.; Xiong, Z.; Zhu, J. Preparation and characterization of thermoplastic starches and their blends with poly (lactic acid). Int. J. Biol. Macromol. 2015, 77, 273–279. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Li, K.-A.; Du, A.-K. Compatibilization strategies in poly(lactic acid)-based blends. RSC Adv. 2015, 5, 32546–32565. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Gürler, N.; Paşa, S.; Temel, H. Silane doped biodegradable starch-PLA bilayer films for food packaging applications: Mechanical, thermal, barrier and biodegradability properties. J. Taiwan Inst. Chem. Eng. 2021, 123, 261–271. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H.; Lin, T.; He, C. Recent advances in stereocomplexation of enantiomeric PLA-based copolymers and applications. Prog. Polym. Sci. 2016, 62, 22–72. [Google Scholar] [CrossRef]
- Wootthikanokkhan, J.; Kasemwananimit, P.; Sombatsompop, N.; Kositchaiyong, A.; Isarankura na Ayutthaya, S.; Kaabbuathong, N. Preparation of modified starch-grafted poly (lactic acid) and a study on compatibilizing efficacy of the copolymers in poly (lactic acid)/thermoplastic starch blends. J. Appl. Polym. Sci. 2012, 126, E389–E396. [Google Scholar] [CrossRef]
- Witt, U.; Yamamoto, M.; Seeliger, U.; Müller, R.J.; Warzelhan, V. Biodegradable polymeric materials—Not the origin but the chemical structure determines biodegradability. Angew. Chem. Int. Ed. 1999, 38, 1438–1442. [Google Scholar] [CrossRef]
- Wei, D.; Wang, H.; Xiao, H.; Zheng, A.; Yang, Y. Morphology and mechanical properties of poly (butylene adipate-co-terephthalate)/potato starch blends in the presence of synthesized reactive compatibilizer or modified poly (butylene adipate-co-terephthalate). Carbohydr. Polym. 2015, 123, 275–282. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]
- Fourati, Y.; Tarrés, Q.; Mutjé, P.; Boufi, S. PBAT/thermoplastic starch blends: Effect of compatibilizers on the rheological, mechanical and morphological properties. Carbohydr. Polym. 2018, 199, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tănase, E.E.; Popa, M.E.; Râpă, M.; Popa, O. Preparation and characterization of biopolymer blends based on polyvinyl alcohol and starch. Rom. Biotechnol. Lett. 2015, 20, 10307. [Google Scholar]
- Srinivasa, P.; Ramesh, M.; Kumar, K.; Tharanathan, R. Properties and sorption studies of chitosan–Polyvinyl alcohol blend films. Carbohydr. Polym. 2003, 53, 431–438. [Google Scholar] [CrossRef]
- Follain, N.; Joly, C.; Dole, P.; Bliard, C. Properties of starch based blends. Part 2. Influence of poly vinyl alcohol addition and photocrosslinking on starch based materials mechanical properties. Carbohydr. Polym. 2005, 60, 185–192. [Google Scholar] [CrossRef]
- Ismail, H.; Zaaba, N.F. The mechanical properties, water resistance and degradation behaviour of silica-filled sago starch/PVA plastic films. J. Elastomers Plast. 2014, 46, 96–109. [Google Scholar] [CrossRef]
- Syamani, F.A.; Kusumaningrum, W.B.; Akbar, F.; Ismadi; Widyaningrum, B.A.; Pramasari, D.A. Characteristics of bioplastic made from modified cassava starch with addition of polyvinyl alcohol. IOP Conf. Ser. Earth Environ. Sci. 2020, 591, 012016. [Google Scholar] [CrossRef]
- Arboleda, G.A.; Montilla, C.E.; Villada, H.S.; Varona, G.A. Obtaining a Flexible Film Elaborated from Cassava Thermoplastic Starch and Polylactic Acid. Int. J. Polym. Sci. 2015, 2015, 627268. [Google Scholar] [CrossRef]
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous reinforcing and toughening: New nanocomposites of waterborne polyurethane filled with low loading level of starch nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Nayak, S.K. Biodegradable PBAT/Starch Nanocomposites. Polym. -Plast. Technol. Eng. 2010, 49, 1406–1418. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Ren, L.; Tong, J.; Liu, Z.; Xie, L. Preparation and characterization of surface crosslinked TPS/PVA blend films. Carbohydr. Polym. 2009, 76, 632–638. [Google Scholar] [CrossRef]
- Kahvand, F.; Fasihi, M. Plasticizing and anti-plasticizing effects of polyvinyl alcohol in blend with thermoplastic starch. Int. J. Biol. Macromol. 2019, 140, 775–781. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Bai, X.; Dong, X.; Tong, J.; Zhou, J. Effect of postcrosslinking modification with glutaraldehyde on the properties of thermoplastic starch/poly(vinyl alcohol) blend films. J. Appl. Polym. Sci. 2012, 124, 3774–3781. [Google Scholar] [CrossRef]
- Negim, E.S.M.; Rakhmetullayeva, R.K.; Yeligbayeva, G.Z.; Urkimbaeva, P.I.; Primzharova, S.T.; Kaldybekov, D.B.; Khatib, J.M.; Mun, G.A.; Craig, W. Improving biodegradability of polyvinyl alcohol/starch blend films for packaging applications. Int. J. Basic Appl. Sci. 2014, 3, 263. [Google Scholar] [CrossRef]
- Wang, D.-W.; Sun, L.-S.; Peng, X.-L.; Runt, J.; Kuo, M.-C.; Huang, K.-S.; Yeh, J.-T. Tapioca/polyvinyl alcohol thermoplastic starch materials processed with the aid of supercritical CO2. Food Packag. Shelf Life 2019, 22, 100425. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–Starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Tuhin, M.O.; Rahman, N.; Haque, M.E.; Khan, R.A.; Dafader, N.C.; Islam, R.; Nurnabi, M.; Tonny, W. Modification of mechanical and thermal property of chitosan–starch blend films. Radiat. Phys. Chem. 2012, 81, 1659–1668. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Wang, Y.; Zhang, X.; Tong, Z. Comparison of chitosan/starch composite film properties before and after cross-linking. Int. J. Biol. Macromol. 2013, 52, 275–279. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; Luchese, C.L.; Tessaro, I.C. Impact of acid type for chitosan dissolution on the characteristics and biodegradability of cornstarch/chitosan based films. Int. J. Biol. Macromol. 2019, 138, 693–703. [Google Scholar] [CrossRef]
- Hubackova, J.; Dvorackova, M.; Svoboda, P.; Mokrejs, P.; Kupec, J.; Pozarova, I.; Alexy, P.; Bugaj, P.; Machovsky, M.; Koutny, M. Influence of various starch types on PCL/starch blends anaerobic biodegradation. Polym. Test. 2013, 32, 1011–1019. [Google Scholar] [CrossRef]
- Ikeo, Y.; Aoki, K.; Kishi, H.; Matsuda, S.; Murakami, A. Nano clay reinforced biodegradable plastics of PCL starch blends. Polym. Adv. Technol. 2006, 17, 940–944. [Google Scholar] [CrossRef]
- Rosa, D.S.; Guedes, C.G.F.; Pedroso, A.G.; Calil, M.R. The influence of starch gelatinization on the rheological, thermal, and morphological properties of poly(ɛ-caprolactone) with corn starch blends. Mater. Sci. Eng. C 2004, 24, 663–670. [Google Scholar] [CrossRef]
- Cho, H.; Moon, H.; Kim, M.; Nam, K.; Kim, J. Biodegradability and biodegradation rate of poly (caprolactone)-starch blend and poly (butylene succinate) biodegradable polymer under aerobic and anaerobic environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef]
- Rosa, D.S.; Guedes, C.G.; Casarin, F. Mechanical Behavior and Biodegradation of Poly (ε-caprolactone)/Starch Blends with and without Expansor. Polym. Bull. 2005, 54, 321–333. [Google Scholar] [CrossRef]
- Wu, C.-S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stab. 2003, 80, 127–134. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Job, A.; Alves, N.; Curvelo, A.A.S.; Gandini, A. Thermoplastic starch/natural rubber blends. Carbohydr. Polym. 2003, 53, 95–99. [Google Scholar] [CrossRef]
- Trovatti, E.; Carvalho, A.J.F.; Gandini, A. A new approach to blending starch with natural rubber. Polym. Int. 2015, 64, 605–610. [Google Scholar] [CrossRef]
- Cai, Z.; Čadek, D.; Šmejkalová, P.; Kadeřábková, A.; Nová, M.; Kuta, A. The modification of properties of thermoplastic starch materials: Combining potato starch with natural rubber and epoxidized natural rubber. Mater. Today Commun. 2021, 26, 101912. [Google Scholar] [CrossRef]
- Jantanasakulwong, K.; Leksawasdi, N.; Seesuriyachan, P.; Wongsuriyasak, S.; Techapun, C.; Ougizawa, T. Reactive blending of thermoplastic starch, epoxidized natural rubber and chitosan. Eur. Polym. J. 2016, 84, 292–299. [Google Scholar] [CrossRef]
- Saetun, V.; Chiachun, C.; Riyajan, S.-A.; Kaewtatip, K. Green composites based on thermoplastic starch and rubber wood sawdust. Polym. Compos. 2017, 38, 1063–1069. [Google Scholar] [CrossRef]
- Cheong, K.S.; Balasubramaniam, J.-R.; Hung, Y.P.; Chuong, W.S.; Amartalingam, R. Development of biodegradable plastic composite blends based on sago derived starch and natural rubber. Pertanika J. Sci. Technol. 2010, 18, 411–420. [Google Scholar]
- Riyajan, S.-A.; Patisat, S. A novel packaging film from cassava starch and natural rubber. J. Polym. Environ. 2018, 26, 2845–2854. [Google Scholar] [CrossRef]
- Wang, S.; Yu, J.; Yu, J. Preparation and characterization of compatible thermoplastic starch/polyethylene blends. Polym. Degrad. Stab. 2005, 87, 395–401. [Google Scholar] [CrossRef]
- Mazerolles, T.; Heuzey, M.-C.; Soliman, M.; Martens, H.; Kleppinger, R.; Huneault, M.A. Development of multilayer barrier films of thermoplastic starch and low-density polyethylene. J. Polym. Res. 2020, 27, 44. [Google Scholar] [CrossRef]
- Arvanitoyannisa, I.; Biliaderis, C.; Ogawab, H.; Kawasakib, N. Biodegradable films made from low-density polyethylene (LDPE), rice starch and potato starch for food packaging applications: Part 1. Carbohydr. Polym. 1998, 36, 89–104. [Google Scholar] [CrossRef]
- Lisdayana, N.; Fahma, F.; Sunarti, T.C.; Iriani, E.S. Thermoplastic starch–PVA nanocomposite films reinforced with nanocellulose from oil palm empty fruit bunches (OPEFBs): Effect of starch type. J. Nat. Fibers 2020, 17, 1069–1080. [Google Scholar] [CrossRef]
- Heidarian, P.; Behzad, T.; Sadeghi, M. Investigation of cross-linked PVA/starch biocomposites reinforced by cellulose nanofibrils isolated from aspen wood sawdust. Cellulose 2017, 24, 3323–3339. [Google Scholar] [CrossRef]
- Keshk, S.M.; El-Zahhar, A.A.; Haija, M.A.; Bondock, S. Synthesis of a magnetic nanoparticles/dialdehyde starch-based composite film for food packaging. Starch-Stärke 2019, 71, 1800035. [Google Scholar] [CrossRef]
- Fahma, F.; Sunarti, T.C.; Indriyani, S.M.; Lisdayana, N. Thermoplastic cassava starch-PVA composite films with cellulose nanofibers from oil palm empty fruit bunches as reinforcement agent. Int. J. Polym. Sci. 2017, 2017, 2745721. [Google Scholar] [CrossRef]
- Fahma, F.; Lisdayana, N.; Abidin, Z.; Noviana, D.; Sari, Y.W.; Mukti, R.R.; Yunus, M.; Kusumaatmaja, A.; Kadja, G.T.M. Nanocellulose-based fibres derived from palm oil by-products and their in vitro biocompatibility analysis. J. Text. Inst. 2020, 111, 1354–1363. [Google Scholar] [CrossRef]
- Khondkar, D.; Tester, R.F.; Hudson, N.; Karkalas, J.; Morrow, J. Rheological behaviour of uncross-linked and cross-linked gelatinised waxy maize starch with pectin gels. Food Hydrocoll. 2007, 21, 1296–1301. [Google Scholar] [CrossRef]
- Guadarrama-Lezama, A.Y.; Castaño, J.; Velázquez, G.; Carrillo-Navas, H.; Alvarez-Ramírez, J. Effect of nopal mucilage addition on physical, barrier and mechanical properties of citric pectin-based films. J. Food Sci. Technol. 2018, 55, 3739–3748. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Coffin, D.R.; Fishman, M.L. Physical and mechanical properties of highly plasticized pectin/starch films. J. Appl. Polym. Sci. 1994, 54, 1311–1320. [Google Scholar] [CrossRef]
- Sabando, C.; Ide, W.; Rodríguez-Díaz, M.; Cabrera-Barjas, G.; Castaño, J.; Bouza, R.; Müller, N.; Gutiérrez, C.; Barral, L.; Rojas, J. A novel hydrocolloid film based on pectin, starch and Gunnera tinctoria and Ugni molinae plant extracts for wound dressing applications. Curr. Top. Med. Chem. 2020, 20, 280–292. [Google Scholar] [CrossRef]
- Carreño, G.; Marican, A.; Vijayakumar, S.; Valdés, O.; Cabrera-Barjas, G.; Castaño, J.; Durán-Lara, E.F. Sustained release of linezolid from prepared hydrogels with polyvinyl alcohol and aliphatic dicarboxylic acids of variable chain lengths. Pharmaceutics 2020, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.M.; Pun, M.Y.; Ishak, Z.A.M. Degradation studies during water absorption, aerobic biodegradation, and soil burial of biobased thermoplastic starch from agricultural waste/polypropylene blends. J. Appl. Polym. Sci. 2013, 129, 3656–3664. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradation of maleated linear low-density polyethylene and starch blends. Polym. Degrad. Stab. 1997, 56, 185–202. [Google Scholar] [CrossRef]
- Raee, E.; Avid, A.; Kaffashi, B. Effect of compatibilizer concentration on dynamic rheological behavior and morphology of thermoplastic starch/polypropylene blends. J. Appl. Polym. Sci. 2020, 137, 48742. [Google Scholar] [CrossRef]
- Martins, A.B.; Santana, R.M.C. Effect of carboxylic acids as compatibilizer agent on mechanical properties of thermoplastic starch and polypropylene blends. Carbohydr. Polym. 2016, 135, 79–85. [Google Scholar] [CrossRef]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A review on blending of corn starch with natural and synthetic polymers, and inorganic nanoparticles with mathematical modeling. Int. J. Biol. Macromol. 2019, 122, 969–996. [Google Scholar] [CrossRef] [PubMed]
- Ostafińska, A.; Mikešová, J.; Krejčíková, S.; Nevoralová, M.; Šturcová, A.; Zhigunov, A.; Michálková, D.; Šlouf, M. Thermoplastic starch composites with TiO2 particles: Preparation, morphology, rheology and mechanical properties. Int. J. Biol. Macromol. 2017, 101, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xu, D.; Liu, Q.; Ren, H.Q.; Zhou, M. Preparation and characterization of corn starch-nanodiamond composite films. Appl. Mech. Mater. 2014, 469, 156–161. [Google Scholar] [CrossRef]
- Hammache, Y.; Serier, A.; Chaoui, S. The effect of thermoplastic starch on the properties of polypropylene/high density polyethylene blend reinforced by nano-clay. Mater. Res. Express 2020, 7, 025308. [Google Scholar] [CrossRef]
- Toumi, N.; Guessoum, M.; Nekkaa, S. Biocomposites based on date palm flour reinforced (70/30) polypropylene/thermoplastic starch blend: Effects of flour treatment and selective dispersion. J. Adhes. Sci. Technol. 2019, 33, 2071–2092. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries− In vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Solid state photoluminescence thermoplastic starch film containing graphene quantum dots. Carbohydr. Polym. 2017, 176, 220–226. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.; Mo, X.; Yang, F.; Pang, J. Effects of nanosilica on retrogradation properties and structures of thermoplastic cassava starch. J. Appl. Polym. Sci. 2018, 135, 45687. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Lu, H.; Li, M.; Liu, J.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced dispersion stability and heavy metal ion adsorption capability of oxidized starch nanoparticles. Food Chem. 2018, 242, 256–263. [Google Scholar] [CrossRef]
- Sarkar, A.; Biswas, D.R.; Datta, S.C.; Dwivedi, B.S.; Bhattacharyya, R.; Kumar, R.; Bandyopadhyay, K.K.; Saha, M.; Chawla, G.; Saha, J.K. Preparation of novel biodegradable starch/poly (vinyl alcohol)/bentonite grafted polymeric films for fertilizer encapsulation. Carbohydr. Polym. 2021, 259, 117679. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, L.; Apostolov, A.; Karger-Kocsis, J. Characterization of layered silicate-reinforced blends of thermoplastic starch (TPS) and poly (butylene adipate-co-terephthalate). Carbohydr. Polym. 2017, 173, 566–572. [Google Scholar] [CrossRef]
- Islam, H.B.M.Z.; Susan, M.A.B.H.; Imran, A.B. High-strength potato starch/hectorite clay-based nanocomposite film: Synthesis and characterization. Iran. Polym. J. 2021, 30, 513–521. [Google Scholar] [CrossRef]
- Khodaeimehr, R.; Peighambardoust, S.J.; Peighambardoust, S.H. Preparation and characterization of corn starch/clay nanocomposite films: Effect of clay content and surface modification. Starch-Stärke 2018, 70, 1700251. [Google Scholar] [CrossRef]
- Teoh, K.H.; Ramesh, S.; Arof, A.K. Investigation on the effect of nanosilica towards corn starch–lithium perchlorate-based polymer electrolytes. J. Solid State Electrochem. 2012, 16, 3165–3170. [Google Scholar] [CrossRef]
- López, O.V.; Castillo, L.A.; Garcia, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef]
- Wu, J.; Du, X.; Yin, Z.; Xu, S.; Xu, S.; Zhang, Y. Preparation and characterization of cellulose nanofibrils from coconut coir fibers and their reinforcements in biodegradable composite films. Carbohydr. Polym. 2019, 211, 49–56. [Google Scholar] [CrossRef]
- Michel, A.; Billington, S. Characterization of poly-hydroxybutyrate films and hemp fiber reinforced composites exposed to accelerated weathering. Polym. Degrad. Stab. 2012, 97, 870–878. [Google Scholar] [CrossRef]
- Ochoa-Yepes, O.; Medina-Jaramillo, C.; Guz, L.; Famá, L. Biodegradable and edible starch composites with fiber-rich lentil flour to use as food packaging. Starch-Stärke 2018, 70, 1700222. [Google Scholar] [CrossRef]
- Glenn, G.M.; Klamczynski, A.; Holtman, K.M.; Chiou, B.-S.; Orts, W.J.; Wood, D. Cellulose fiber reinforced starch-based foam composites. J. Biobased Mater. Bioenergy 2007, 1, 360–366. [Google Scholar] [CrossRef]
- Avérous, L.; Fringant, C.; Moro, L. Plasticized starch–cellulose interactions in polysaccharide composites. Polymer 2001, 42, 6565–6572. [Google Scholar] [CrossRef]
- Versino, F.; García, M.A. Cassava (Manihot esculenta) starch films reinforced with natural fibrous filler. Ind. Crops Prod. 2014, 58, 305–314. [Google Scholar] [CrossRef]
- Liu, W.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Influence of processing methods and fiber length on physical properties of kenaf fiber reinforced soy based biocomposites. Compos. Part B Eng. 2007, 38, 352–359. [Google Scholar] [CrossRef]
- Susmitha, A.; Sasikumar, K.; Rajan, D.; Padmakumar, M.A.; Nampoothiri, K.M. Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci. 2021, 41, 100977. [Google Scholar] [CrossRef]
- Meng, L.; Xie, F.; Zhang, B.; Wang, D.K.; Yu, L. Natural biopolymer alloys with superior mechanical properties. ACS Sustain. Chem. Eng. 2018, 7, 2792–2802. [Google Scholar] [CrossRef]
- Yao, X.; Qin, Y.; Zhang, M.; Zhang, J.; Qian, C.; Liu, J. Development of active and smart packaging films based on starch, polyvinyl alcohol and betacyanins from different plant sources. Int. J. Biol. Macromol. 2021, 183, 358–368. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Marangoni Junior, L.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Raigond, P.; Sood, A.; Kalia, A.; Joshi, A.; Kaundal, B.; Raigond, B.; Dutt, S.; Singh, B.; Chakrabarti, S.K. Antimicrobial Activity of Potato Starch-Based Active Biodegradable Nanocomposite Films. Potato Res. 2019, 62, 69–83. [Google Scholar] [CrossRef]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple peel extract incorporated poly(vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-Starch Films with Natural Extracts: Physical, Chemical, Morphological and Thermal Properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Torres Vargas, O.L.; Galeano Loaiza, Y.V.; González, M.L. Effect of incorporating extracts from natural pigments in alginate/starch films. J. Mater. Res. Technol. 2021, 13, 2239–2250. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef]
- Utami, R.; Khasanah, L.U.; Manuhara, G.J.; Ayuningrum, Z.K. Effects of Cinnamon Bark Essential Oil (Cinnamomum burmannii) on Characteristics of Edible Film and Quality of Fresh Beef. Pertanika J. Trop. Agric. Sci. 2019, 42, 1173–1184. [Google Scholar]
- Aitboulahsen, M.; El Galiou, O.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of plasticizer type and essential oils on mechanical, physicochemical, and antimicrobial characteristics of gelatin, starch, and pectin-based films. J. Food Processing Preserv. 2020, 44, e14480. [Google Scholar] [CrossRef]
- De Souza, A.G.; Dos Santos, N.M.A.; da Silva Torin, R.F.; Dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Albiero, B.R.; Cipriano, L.; de Oliveira Nobre Bezerra, C.C.; Oldoni, F.C.A.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications. Cellulose 2021, 28, 6499–6511. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of starch film containing thyme essential oil microcapsules on physicochemical activity of mango. LWT 2020, 131, 109700. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antimicrobial Properties of Starch-PVA Blend Films as Affected by the Incorporation of Natural Antimicrobial Agents. Foods 2016, 5, 3. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, Z.; Han, S.; Xu, L.; Araby, S.; Cai, R.; Zhao, Y.; Lu, S.; Liu, T. A facile approach to fabricate highly sensitive, flexible strain sensor based on elastomeric/graphene platelet composite film. J. Mater. Sci. 2019, 54, 10856–10870. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Yu, M.; Ji, N.; Wang, Y.; Dai, L.; Xiong, L.; Sun, Q. Starch-based nanoparticles: Stimuli responsiveness, toxicity, and interactions with food components. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1075–1100. [Google Scholar] [CrossRef]
- Dufresne, A.; Kellerhals, M.B.; Witholt, B. Transcrystallization in Mcl-PHAs/cellulose whiskers composites. Macromolecules 1999, 32, 7396–7401. [Google Scholar] [CrossRef]
- Lin, H.; Qin, L.Z.; Hong, H.; Li, Q. Preparation of starch nanoparticles via high-energy ball milling. J. Nano Res. 2016, 40, 174–179. [Google Scholar] [CrossRef]
- Chen, C.-J.; Shen, Y.-C.; Yeh, A.-I. Physico-chemical characteristics of media-milled corn starch. J. Agric. Food Chem. 2010, 58, 9083–9091. [Google Scholar] [CrossRef]
- Lamanna, M.; Morales, N.J.; García, N.L.; Goyanes, S. Development and characterization of starch nanoparticles by gamma radiation: Potential application as starch matrix filler. Carbohydr. Polym. 2013, 97, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on supramolecular structural changes of ultrasonic treated potato starch granules. Food Hydrocoll. 2012, 29, 116–122. [Google Scholar] [CrossRef]
- Boufi, S.; Haaj, S.B.; Magnin, A.; Pignon, F.; Impéror-Clerc, M.; Mortha, G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason. Sonochem. 2018, 41, 327–336. [Google Scholar] [CrossRef]
- Grieser, F.; Ashokkumar, M.; Sostaric, J.Z. Sonochemistry and sonoluminescence in colloidal systems. In Sonochemistry and Sonoluminescence; Springer: Berlin/Heidelberg, Germany, 1999; pp. 345–362. [Google Scholar]
- Kim, J.-Y.; Lim, S.-T. Preparation of nano-sized starch particles by complex formation with n-butanol. Carbohydr. Polym. 2009, 76, 110–116. [Google Scholar] [CrossRef]
- Chin, S.F.; Azman, A.; Pang, S.C. Size controlled synthesis of starch nanoparticles by a microemulsion method. J. Nanomater. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Dong, Y.; Chang, Y.; Wang, Q.; Tong, J.; Zhou, J. Effect of operating conditions on size and morphology of amylose nanoparticles prepared by precipitation. Starch-Stärke 2015, 67, 365–372. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, C.; Jiang, S.; Xiong, L.; Sun, Q. Characterization of starch nanoparticles prepared by nanoprecipitation: Influence of amylose content and starch type. Ind. Crops Prod. 2016, 87, 182–190. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Han, J.-A.; Kweon, D.-K.; Park, J.-D.; Lim, S.-T. Effect of ultrasonic treatments on nanoparticle preparation of acid-hydrolyzed waxy maize starch. Carbohydr. Polym. 2013, 93, 582–588. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Chang, P.R.; Huneault, M.A. Comparative study on the films of poly (vinyl alcohol)/pea starch nanocrystals and poly (vinyl alcohol)/native pea starch. Carbohydr. Polym. 2008, 73, 8–17. [Google Scholar] [CrossRef]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and characterization of citric acid-modified starch nanoparticles/plasticized-starch composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Li, L.; Liu, C.; Song, C.; Wang, P. Fabrication of size-controlled starch-based nanospheres by nanoprecipitation. ACS Appl. Mater. Interfaces 2009, 1, 956–959. [Google Scholar] [CrossRef]
- Tay, S.H.; Pang, S.C.; Chin, S.F. A facile approach for controlled synthesis of hydrophilic starch-based nanoparticles from native sago starch. Starch-Stärke 2012, 64, 984–990. [Google Scholar] [CrossRef]
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the preparation of aqueous suspensions of waxy maize starch nanocrystals using a response surface methodology. Biomacromolecules 2004, 5, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Cavaille, J.-Y.; Helbert, W. New nanocomposite materials: Microcrystalline starch reinforced thermoplastic. Macromolecules 1996, 29, 7624–7626. [Google Scholar] [CrossRef]
- Wang, Y.; Khan, A.; Liu, Y.; Feng, J.; Dai, L.; Wang, G.; Alam, N.; Tong, L.; Ni, Y. Chitosan oligosaccharide-based dual pH responsive nano-micelles for targeted delivery of hydrophobic drugs. Carbohydr. Polym. 2019, 223, 115061. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ai, X.; Zhang, H.; Zhuo, W.; Mi, P. Polymeric micelles with endosome escape and redox-responsive functions for enhanced intracellular drug delivery. J. Biomed. Nanotechnol. 2019, 15, 373–381. [Google Scholar] [CrossRef]
- Liang, T.; Hou, J.; Qu, M.; Zhao, M.; Raj, I. High-viscosity α-starch nanogel particles to enhance oil recovery. RSC Adv. 2020, 10, 8275–8285. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, M.; Gao, C.; Yang, J.; Zhang, X.; Zhang, X.; Liu, Z. Ultra-small and innocuous cationic starch nanospheres: Preparation, characterization and drug delivery study. Int. J. Biol. Macromol. 2013, 58, 231–239. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, Z.; Fu, X. Preparation and characterization of starch nanoparticles in ionic liquid-in-oil microemulsions system. Ind. Crops Prod. 2014, 52, 105–110. [Google Scholar] [CrossRef]
- Zhang, F.; Pei, X.; Zhai, K.; Wang, C.; Bai, Y.; Zhang, B.; Wang, Y.; Tan, Y.; Xu, K.; Wang, P. Starch-based nanospheres modified filter paper for o/w emulsions separation and contaminants removal. Int. J. Biol. Macromol. 2020, 162, 1118–1126. [Google Scholar] [CrossRef]
- Shabana, S.; Prasansha, R.; Kalinina, I.; Potoroko, I.; Bagale, U.; Shirish, S.H. Ultrasound assisted acid hydrolyzed structure modification and loading of antioxidants on potato starch nanoparticles. Ultrason. Sonochem. 2019, 51, 444–450. [Google Scholar] [CrossRef]
- Ji, N.; Qin, Y.; Li, M.; Xiong, L.; Qiu, L.; Bian, X.; Sun, Q. Fabrication and characterization of starch nanohydrogels via reverse emulsification and internal gelation. J. Agric. Food Chem. 2018, 66, 9326–9334. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly (alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310. [Google Scholar] [CrossRef]
- Li, W.; Nie, J.; Hu, R.; Zhao, R.; Zhu, W.; Chen, X.; Li, D.; Wang, L.; Hu, L. A nanogel sensor for colorimetric fluorescence measurement of ionizing radiation doses. Chem. Commun. 2019, 55, 9614–9617. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.J.; Lim, S.A.; Lee, E.S. pH-responsive squeezing polysaccharidic nanogels for efficient docetaxel delivery. Polym. Adv. Technol. 2019, 30, 2067–2074. [Google Scholar] [CrossRef]
- Lv, H.; Cui, S.; Zhang, H.; Pei, X.; Gao, Z.; Hu, J.; Zhou, Y.; Liu, Y. Crosslinked starch nanofibers with high mechanical strength and excellent water resistance for biomedical applications. Biomed. Mater. 2020, 15, 025007. [Google Scholar] [CrossRef]
- Wang, W.; Jin, X.; Zhu, Y.; Zhu, C.; Yang, J.; Wang, H.; Lin, T. Effect of vapor-phase glutaraldehyde crosslinking on electrospun starch fibers. Carbohydr. Polym. 2016, 140, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Komur, B.; Bayrak, F.; Ekren, N.; Eroglu, M.; Oktar, F.N.; Sinirlioglu, Z.; Yucel, S.; Guler, O.; Gunduz, O. Starch/PCL composite nanofibers by co-axial electrospinning technique for biomedical applications. Biomed. Eng. Online 2017, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Starch-Derived Nanographene Oxide Paves the Way for Electrospinnable and Bioactive Starch Scaffolds for Bone Tissue Engineering. Biomacromolecules 2017, 18, 1582–1591. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Huang, L. pH and thermo dual-responsive starch-g-P(DEAEMA-co-PEGMA): Synthesis via SET-LRP, self-assembly and drug release behaviors. React. Funct. Polym. 2019, 141, 165–171. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Goff, H.D.; Nsor-Atindana, J.; Ma, J.; Zhong, F. Interfacial activity and self-assembly behavior of dissolved and granular octenyl succinate anhydride starches. Langmuir 2019, 35, 4702–4709. [Google Scholar] [CrossRef]
- Wen, N.; Lü, S.; Xu, X.; Ning, P.; Wang, Z.; Zhang, Z.; Gao, C.; Liu, Y.; Liu, M. A polysaccharide-based micelle-hydrogel synergistic therapy system for diabetes and vascular diabetes complications treatment. Mater. Sci. Eng. C 2019, 100, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Shan, C.; Jiang, K.; Chen, Z.; Du, Y. Intracellular pH-sensitive delivery CaCO3 nanoparticles templated by hydrophobic modified starch micelles. RSC Adv. 2014, 4, 10841–10844. [Google Scholar] [CrossRef]
- González-Seligra, P.; Guz, L.; Ochoa-Yepes, O.; Goyanes, S.; Famá, L. Influence of extrusion process conditions on starch film morphology. LWT 2017, 84, 520–528. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, C.; Zhai, X.; Zhang, R.; Zhang, S.; Sun, C.; Wang, W.; Hou, H. Effect of lipids with different physical state on the physicochemical properties of starch/gelatin edible films prepared by extrusion blowing. Int. J. Biol. Macromol. 2021, 185, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.D.; Merrill, E.W. Starch fragmentation during extrusion processing. Polymer 1995, 36, 1883–1886. [Google Scholar] [CrossRef]
- Colonna, P.; Doublier, J.; Melcion, J.; De Monredon, F.; Mercier, C. Extrusion cooking and drum drying of wheat starch. Cereal Chem. 1984, 61, 538–554. [Google Scholar]
- Funke, U.; Bergthaller, W.; Lindhauer, M. Processing and characterization of biodegradable products based on starch. Polym. Degrad. Stab. 1998, 59, 293–296. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, J.; Kang, X.; Wang, B.; Liu, P.; Cui, B.; Abd El-Aty, A. Development and characterization of starch films prepared by extrusion blowing: The synergistic plasticizing effect of water and glycerol. LWT 2021, 148, 111820. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Chen, L.; Yu, L.; Dean, K.; Bateman, S. Thermal Behaviour of High Amylose Cornstarch Studied by DSC. Int. J. Food Eng. 2005, 1, 1–6. [Google Scholar] [CrossRef]
- Rindlav-Westling, A.s.; Stading, M.; Hermansson, A.-M.; Gatenholm, P. Structure, mechanical and barrier properties of amylose and amylopectin films. Carbohydr. Polym. 1998, 36, 217–224. [Google Scholar] [CrossRef]
- Zhou, S.; Zhai, X.; Zhang, R.; Wang, W.; Lim, L.-T.; Hou, H. High-Throughput Fabrication of Antibacterial Starch/PBAT/AgNPs@SiO2 Films for Food Packaging. Nanomaterials 2021, 11, 3062. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Mittal, V. Polymer Nanocomposite Foams; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Martins, P.C.; Gutkoski, L.C.; Martins, V.G. Impact of acid hydrolysis and esterification process in rice and potato starch properties. Int. J. Biol. Macromol. 2018, 120, 959–965. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Poungroi, M.; Holló, B.; Mészáros Szécsényi, K. Effects of starch types on the properties of baked starch foams. J. Therm. Anal. Calorim. 2014, 115, 833–840. [Google Scholar] [CrossRef]
- Vargas-Torres, A.; Palma-Rodriguez, H.M.; Berrios, J.D.J.; Glenn, G.; Salgado-Delgado, R.; Olarte-Paredes, A.; Prieto-Mendez, J.; Hernandez-Uribe, J.P. Biodegradable baked foam made with chayotextle starch mixed with plantain flour and wood fiber. J. Appl. Polym. Sci. 2017, 134, 45565. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Baillon, F.; Fages, J. Blending and foaming thermoplastic starch with poly (lactic acid) by CO2-aided hot melt extrusion. J. Appl. Polym. Sci. 2021, 138, 50150. [Google Scholar] [CrossRef]
- Cheng, X.-H.; Wang, K.; Cheng, N.-Q.; Mi, S.-Y.; Sun, L.-S.; Yeh, J.-T. The control of expansion ratios and cellular structure of supercritical CO2-aid thermoplastic starch foams using crosslinking agents and nano-silica particles. J. Polym. Res. 2021, 28, 303. [Google Scholar] [CrossRef]
- Amirreza, Z.; Mohammad, F.; Sajad, R. Microstructural and Physical Properties of Thermoplastic Corn Starch/Polystyrene Blend Foams Affected by Different Contents and Combinations of Plasticizers. J. Polym. Environ. 2021; in press. [Google Scholar] [CrossRef]
- Viana, E.B.M.; Oliveira, N.L.; Ribeiro, J.S.; Almeida, M.F.; Souza, C.C.E.; Resende, J.V.; Santos, L.S.; Veloso, C.M. Development of starch-based bioplastics of green plantain banana (Musa paradisiaca L.) modified with heat-moisture treatment (HMT). Food Packag. Shelf Life 2022, 31, 100776. [Google Scholar] [CrossRef]
- Cheng, M.; Cui, Y.; Yan, X.; Zhang, R.; Wang, J.; Wang, X. Effect of dual-modified cassava starches on intelligent packaging films containing red cabbage extracts. Food Hydrocoll. 2022, 124, 107225. [Google Scholar] [CrossRef]
- Reneker, D.H.; Kataphinan, W.; Theron, A.; Zussman, E.; Yarin, A.L. Nanofiber garlands of polycaprolactone by electrospinning. Polymer 2002, 43, 6785–6794. [Google Scholar] [CrossRef]
- Sutjarittangtham, K.; Jaiturong, P.; Intatha, U.; Pengpat, K.; Eitssayeam, S.; Sirithunyalug, J. Fabrication of natural tapioca starch fibers by a modified electrospinning technique. Chiang Mai J. Sci. 2014, 41, 213–223. [Google Scholar]
- Kong, L.; Ziegler, G.R. Fabrication of pure starch fibers by electrospinning. Food Hydrocoll. 2014, 36, 20–25. [Google Scholar] [CrossRef]
- Fonseca, L.M.; de Oliveira, J.P.; de Oliveira, P.D.; da Rosa Zavareze, E.; Dias, A.R.G.; Lim, L.-T. Electrospinning of native and anionic corn starch fibers with different amylose contents. Food Res. Int. 2019, 116, 1318–1326. [Google Scholar] [CrossRef]
- William, A.C.; Efrén, M.P.; Yesid, G.-P.E.; Ricardo, V.-G. Comparative Study of Starch Fibers Obtained by Electro-spinning of Indigenous, Commercial and Cationic Potato Starch. J. Nat. Fibers 2020, 17, 809–819. [Google Scholar] [CrossRef]
- Raghavan, B.; Soto, H.; Lozano, K. Fabrication of Melt Spun Polypropylene Nanofibers by Forcespinning. J. Eng. Fibers Fabr. 2013, 8, 155892501300800106. [Google Scholar] [CrossRef]
- Del Ángel-Sánchez, K.; Ulloa-Castillo, N.A.; Segura-Cárdenas, E.; Martinez-Romero, O.; Elías-Zuñiga, A. Design, fabrication, and characterization of polycaprolactone (PCL)-TiO2-collagenase nanofiber mesh scaffolds by Forcespinning. MRS Commun. 2019, 9, 390–397. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Yang, B. Centrifugally spun starch-based fibers from amylopectin rich starches. Carbohydr. Polym. 2016, 137, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Hou, T.; Zhou, J.; Yang, B. Centrifugally spun ultrafine starch/PEO fibres as release formulation for poorly water-soluble drugs. Micro Nano Lett. 2018, 13, 1688–1692. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Guo, Y.; Liu, X.; Zhu, F.; Liu, Z.; Liu, X.; Yang, F. Rheological & 3D printing properties of potato starch composite gels. J. Food Eng. 2022, 313, 110756. [Google Scholar] [CrossRef]
- Leaw, Z.E.; Kong, I.; Pui, L.P. 3D printed corn starch–Gelatin film with glycerol and hawthorn berry (Crataegus pinnatifida) extract. J. Food Processing Preserv. 2021, 45, e15752. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Valencia, G.A. Reactive extrusion-processed native and phosphated starch-based food packaging films governed by the hierarchical structure. Int. J. Biol. Macromol. 2021, 172, 439–451. [Google Scholar] [CrossRef]
- Bai, J.; Pei, H.; Zhou, X.; Xie, X. Reactive compatibilization and properties of low-cost and high-performance PBAT/thermoplastic starch blends. Eur. Polym. J. 2021, 143, 110198. [Google Scholar] [CrossRef]
- Da Costa, J.C.M.; Miki, K.S.L.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef]
- Qin, Y.; Yun, D.; Xu, F.; Li, C.; Chen, D.; Liu, J. Impact of storage conditions on the structure and functionality of starch/polyvinyl alcohol films containing Lycium ruthenicum anthocyanins. Food Packag. Shelf Life 2021, 29, 100693. [Google Scholar] [CrossRef]
- Abdillah, A.A.; Charles, A.L. Characterization of a natural biodegradable edible film obtained from arrowroot starch and iota-carrageenan and application in food packaging. Int. J. Biol. Macromol. 2021, 191, 618–626. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innov. Food Sci. Emerg. Technol. 2012, 16, 211–217. [Google Scholar] [CrossRef]
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Deen, M.J. Fruit quality monitoring with smart packaging. Sensors 2021, 21, 1509. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.A.; Ilkenhans, T.; Poulston, S.; Rowsell, L.; Smith, A.W. Development of new palladium-promoted ethylene scavenger. Postharvest Biol. Technol. 2007, 45, 214–220. [Google Scholar] [CrossRef]
- Smith, A.W.; Poulston, S.; Rowsell, L.; Terry, L.A.; Anderson, J.A. A new palladium-based ethylene scavenger to control ethylene-induced ripening of climacteric fruit. Platin. Met. Rev. 2009, 53, 112–122. [Google Scholar] [CrossRef]
- Wills, R.; Warton, M. Efficacy of potassium permanganate impregnated into alumina beads to reduce atmospheric ethylene. J. Am. Soc. Hortic. Sci. 2004, 129, 433–438. [Google Scholar] [CrossRef]
- Jedermann, R.; Praeger, U.; Geyer, M.; Lang, W. Remote quality monitoring in the banana chain. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130303. [Google Scholar] [CrossRef]
- Mohan, C.; Ravishankar, C. Active and intelligent packaging systems—Application in seafood. World J. Aquac. Res. Dev. 2019, 1, 10–16. [Google Scholar]
- Panrong, T.; Karbowiak, T.; Harnkarnsujarit, N. Thermoplastic starch and green tea blends with LLDPE films for active packaging of meat and oil-based products. Food Packag. Shelf Life 2019, 21, 100331. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, L.; Yu, J.; Shao, P. Novel trends and applications of natural pH-responsive indicator film in food packaging for improved quality monitoring. Food Control 2022, 134, 108769. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT-Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2021, 126, 108039. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Basha, R.K.; Naim, M.N. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Bautista-Baños, S.; Dublan García, O.; Cabrera-Barjas, G. Corn-starch-based materials incorporated with cinnamon oil emulsion: Physico-chemical characterization and biological activity. Foods 2020, 9, 475. [Google Scholar] [CrossRef]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.; Sumi, T.; Joseph, M.; Mathew, S.; Praveen, G.; Nair, I.C.; Radhakrishnan, E. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, P.; Niazi, M.B.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2020, 40, e12725. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karim, R.; Sultan, M.T.; Paykary, M.; Johnson, S.K.; Shekarforoush, E. Comparison of starch films and effect of different rice starch-based coating formulations on physical properties of walnut during storage time at accelerated temperature. J. Food Process Eng. 2018, 41, e12607. [Google Scholar] [CrossRef]
- Oliveira, B.F.; Cruz, A.; Xe, F.; Alves, E. Cassava starch coatings for postharvest control of papaya anthracnose. Phytopathol. Mediterr. 2016, 55, 276–284. [Google Scholar]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and characterization of potato starch film with various size of nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O.V. Active films based on thermoplastic corn starch and chitosan oligomer for food packaging applications. Food Packag. Shelf Life 2017, 14, 128–136. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E. Effects of Yam Starch Films on Storability and Quality of Fresh Strawberries (Fragaria ananassa). J. Agric. Food Chem. 2003, 51, 7005–7011. [Google Scholar] [CrossRef]
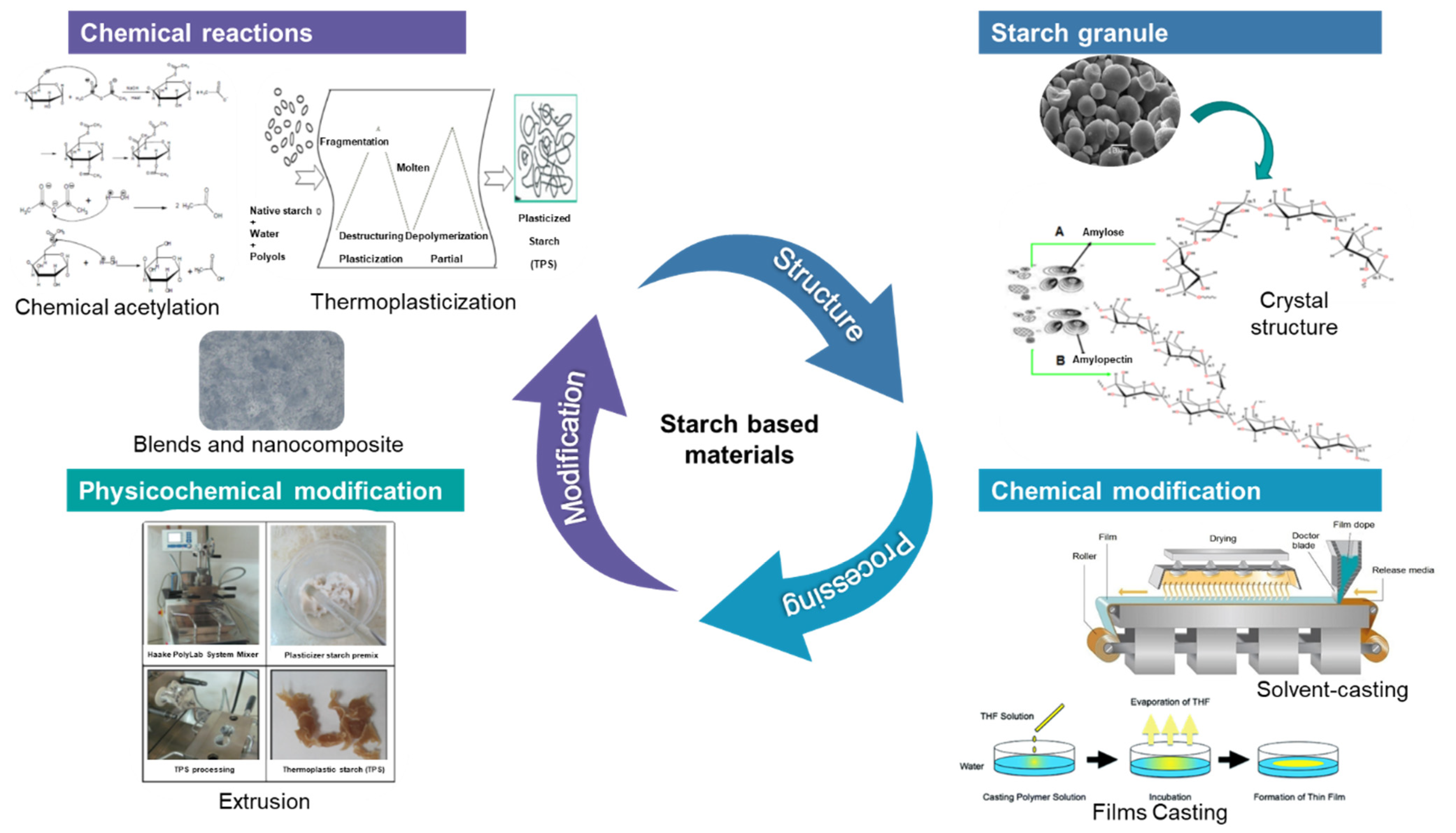
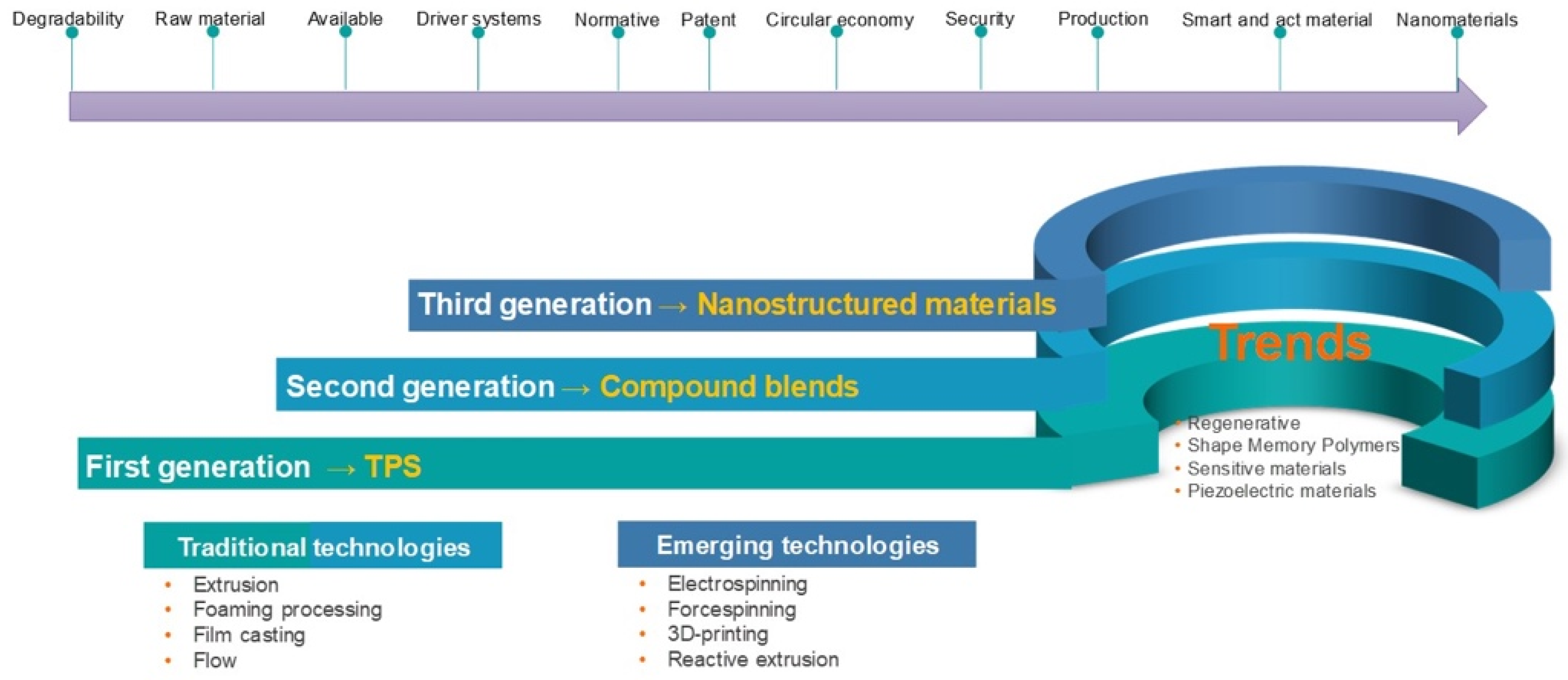

| Clasification Source | Source of Starch | Characteristic | Reference | |||
|---|---|---|---|---|---|---|
| Geometry | Size (μm) | Amylose (%) | Structure Type | |||
| Cereal | Buckwheat (Fagopyrum esculentum) | Polygonal | 5–8 | - | A | [31] |
| Amaranth | Polygonal | 1–2.5 | 4 | A | [32] | |
| Normal maize Waxy maize | Angular | 15–20 | 3–40 1–3 | A | [33] | |
| Rice White Rice red Rice black | Polygonal and angular | - | 18–20 24–26 20 | A | [34] | |
| Wild-type wheat starch High-amylose wheat starche | Spherical Deformed and heterogeneous shapes of granules | - | 32 71–84 | A B | [35] | |
| Canary seed CDC maría C05041 | Polygonal | 2–3 2.4 | 23–25 22–24 | A | [36] | |
| Tuber | Amadumbe corms purple and white | Irregular and polygonal | 1–6 | 7.5 | A | [37] |
| Arracacia xanthorrhiza Oxalis tuberosa Sweet potato | Oval and circular circular and polyhedral circular and polyhedral | 5–15 10–30 5–20 | 22 31–33 41–43 | - | [38] | |
| Yellow sweet potatoes Qin5 Qin 9 12-18-28 Shang 19 Orange sweet potatoes Su16 Qin 8 Purple sweet potatoes Qinzi 2, Quinzi 3 | The eight starch granules of sweet potato were similar, exhibiting round, polygonal, oval, semi-oval, and hemispherical shapes with different sizes. | 17–18 16–17 20–22 20–22 22–24 20–22 20–22 21–24 | 23 20 23 18–21 19 25 21 23 | C type: Qin 8, Qin 9, Qingzi 2, 12-18-28, Qingzi 3, and Su 16, and A type for Shang 19 and Qin 5. | [39] | |
| Arisaema elephas Buchet Arisaema yunnanense Buchet Arisaema erubescens (Wall.) | Three starches all had spherical, irregular and truncated shapes with central hila. | 3.0–5.0 | 29 28 32.0 | CACAA | [19] | |
| Chayote tuber starch | Oval, irregular, truncated and rounded | 7–50 | 13 | - | [40] | |
| Curcuma aeruginosa Curcuma amada Curcuma aromatica Curcuma caesia Kaempferia parviflora Zingiber montanum | Elongated, elliptical, oval, polygonal, cuboidal and spheroid | 6–25 10–30 5–28 8–30 2–15 5–20 | 22 23 25 28 24 23 | B | [41] | |
| Pulse | Pea Lentil Faba bean | Oval, kidney and irregular shapes | 10–45 | 40–42 38–40 39–40 | C | [42] |
| Chickpea | Round shape | 9–70 | 36–41 | C | [43] | |
| No conventional | Babassu mesocarp | Polygonal | - | - | C | [44] |
| Cashew nut shell | Irregular in shape and size | 26–28 | B | [45] | ||
| Jackfruit seed | Trigonal, tetragonal, round, semi-oval, and bell shape. | 3–15 | 32–34 | A | [46] | |
| Loquat (Eriobotrya japônica) seed | Oval and cylindrical | 29–45 | 15–46 | C | [47] | |
| Avocado seeds (Persea americana Mill) | Oval and ellipsoid | 10–44 | 30–31 | B | [48] | |
| Chesnut | Oval and ellipsoid | 15–22 | 15–20 | B | [49] | |
| Pehuen | Spherical | 12–21 | 38–40 | C | [13] | |
| Physical Modifications | Chemical Modifications | ||||||
|---|---|---|---|---|---|---|---|
| Source of Starch | Treatment | Conditions (Time/Temp) | Reference | Source of Starch | Treatment | Conditions (Time/Temp) | Reference |
| Metroxylon sagu | Microwave heat | 15 min at 4 °C | [85] | Corn | Branching enzyme treatment | 99 °C for 15 min | [86] |
| Horse Chestnut seeds | Heat-moisture | 110 °C for 3 h | [87] | Horse Chestnut seeds | Acid hydrolysis | 50 °C for 30 min | [87] |
| Corn | Heating in evaporating dishes | 210 °C for 30 min | [88] | Potato | Acetylation | pH 8, 30 °C stirred for 1 h | [89] |
| Wheat | Superheated steam | 130 °C, 150 °C and 170 °C for 1 and 4 min | [90] | Oat and Barley | Oxidation | 10 g Cl for 1 kg of starch using NAClO, milled for 50 min | [91] |
| Corn | Repeated dry-heat (RDHT) | Cycles of 140 °C for 4, 8, 12 and 16 h and 1 h of cooling | [92] | Corn | Octenyl succinic anhydride modification | pH 8, 35 °C, stirred for 3 h | [88] |
| Pea and lentil cultivar | Hydrothermal | 120 °C for 24 h | [93] | Wheat | Cross-linking | Stirring at 170 rpm, 40 °C for 80 min | [94] |
| Barley | Annealing (ANN) | 50 °C for 72 h | [95] | Rice starche | Esterification (octenylsuccinic anhydride (OSA) | 2 h | [96] |
| Amaranth | Heat-moisture | 150 mL min−1, 130 °C | [97] | Rice starch | Esterification (octenylsuccinic anhydride (OSA) | 35 °C, 5 h | [98] |
| Mango kernel | Heat-moisture | 3 h at 110 °C with occasional shacking | [99] | Quinoa starch | Esterification (octenylsuccinic anhydride (OSA) | 35 °C, 3 h | [100] |
| Wheat | High hydrostatic pressure | 300, 400, 500, 600 MPa; 20 °C for 30 min | [101] | Waxy cassava starch | Esterification (octenylsuccinic anhydride (OSA) | 24 h | [102] |
| Sago starch | Esterification (octenylsuccinic anhydride (OSA). | 9.65 h | [63] | ||||
| Ginkgo starch | Esterification (octenylsuccinic anhydride (OSA) | 35 °C, 8 h | [64] | ||||
| Sorghum and maize Waxy starches | Esterification octenylsuccinic anhydride (OSA)/β-amylase | - | [103] | ||||
| Waxy maize starch | Cross-linking | 40 °C, 4 h | [71] | ||||
| Waxy maize starch | Cross-linking | 40 °C, 4 h | [104] | ||||
| Native potato starch | Cross-linking | 30 °C, 20 h | [105] | ||||
| Tapioca starch | Cross-linking | 30 min | [106] | ||||
| Carboxymethyl starch | Grafting | - | [107] | ||||
| Native corn-starch | Chemically grafting | 130 °C, 24 h | [70] | ||||
| Corn starch | Grafting (DMSO) | 50 °C, 36 h | [108] | ||||
| Normal maize starch | Citric acid | room temperatura, 12 h | [109] | ||||
| Corn | Debranching by pullulanse | 55 °C for 1.5 h | [110] | ||||
| Corn | Hydroxipropilation | pH 11.5, 20 h with stirring | [110] | ||||
| Sorghum | Acid Hydrolysis | 40 °C, stirring 1h | [111] | ||||
| Sorghum | Oxidation | 40 °C, pH 9.5, 30 min | [112] | ||||
| Potato starch | Acetylation | 115°C, stirring for 5 h | [113] | ||||
| Cassava | Ozonation | gas flow of 1 L∙min−1, ozone concentration 41 mg O3∙L−1, 25 °C for 15 and 30 min | [114] | ||||
| Broken rice | Succinylation | pH 9–9.5, addition of 2%, 4% and 5% of anhydride | [115] | ||||
| Rice starch | Acetylation | 90 °C, 10, 30 and 90 min | [116] | ||||
| Non-Thermal Treatments | |||
|---|---|---|---|
| Source of Starch | Treatment | Conditions (Time/Temp) | Reference |
| Modified Starch RS4 | Ultrasound | 2 s pulses, 30 min, 25 °C | [119] |
| Corn | UV irradiation | 2.88 J/cm2 for 30 min | [88] |
| Corn | Electron beam irradiation | 6.23 MeV, 75 mA, 100 Hz, pulse duration of 3.5 µs, | [120] |
| Corn | Corona electrical discharges | 25 kV, 50 Hz, pulse duration of 100 ns, during 5 min | [120] |
| Barley | High pressure | 400, 450 and 500 MPa at 75 °C | [95] |
| Wheat and potato | Pulse electric field | Pulses at 10, 15, 20, 25 and 30 kV, 600 Hz, 6 µs pulse width | [121] |
| Tapioca | Irradiation | Gamma Cell 5000, doses of 0, 5, 10 and 20 kGy | [122] |
| Corn | Plasma | 20 rpm, 90 W during 30 min | [118] |
| Corn | HMDSO plasma | 4.5 × 10−1 mbar pressure, 30 min, 70 W | [117] |
| Blend System | Starch Source | Modifier | Processing Method | Tensile Strength (MPa) | Water Vapor Permeability (g/m Pa s) | Reference |
|---|---|---|---|---|---|---|
| PLA/Starch | Potato | Crosslinking with 3-(Aminopropyl) trime-thoxy silane (3-APTMS) | Films casting | 11 ± 2 | 15 ± 0.5 | [138] |
| PLA/Starch | Tapioca | - | Extrusion | 8 ± 2 | - | [62] |
| PLA/Starch | Corn | Maleic anhydride (MA) and epoxidized cardanol (Epicard) | Extrusion | 25 ± 12 | - | [135] |
| PLA/Starch | Corn | Poly (ether block amide) (PEBA) | Reactive blending | 22 ± 2 | - | [134] |
| PLA/Starch | Casava | maleic anhydride | Extrusion | 4 ± 1.0 | [150] | |
| PLA/Starch | Casava | PLA-g-TPS | Extrusion | 6 ± 1.0 | [140] | |
| PLA/Starch | cassava | Acetylated starch | Twin-screw extruder | 23 ± 4 | 8 ± 1.0 | [61] |
| PBAT/starch | Casava | nisin-ethylenediaminetetraacetic acid (EDTA) | Blown-film extrusion | 20 ± 10 | 2 ± 1.0 | [60] |
| PBAT/starch | Potato | Joncryl-ADR-4368,ynthesized reactive compatibilizers, SMGs | Extrusion | 20 ± 8 | - | [142] |
| PBAT/starch | Potato | Maleic anhydride (MA), citric acid (CA) | Extrusion | 10 ± 1 (MA), 5 ± 2 (CA) | [151] | |
| PBAT/starch | Cassava starch | Sodium benzoate (SB) and potassium sorbate (PS) | Blown-film extrusion | 10 ± 4 | 2 ± 1.0 | [143] |
| PBAT/starch | Tapioca | Maleic anhydride and benzoyl peroxide | Reactive extrusion | 10 ± 3 | [152] | |
| PVOH/starch | Corn | Photo crosslinking with sodium benzoate | Film casting | 16–20 MPa | NR | [153] |
| PVOH/starch | Corn | Citric acid (co-plasticizer) | melt-blending | NR | NR | [154] |
| PVOH/starch | Corn | Glutaraldehyde | Casting | 7.8–13.7 MPa | NR | [155] |
| PVOH/starch | Corn | Glacial acetic acid as a crosslinking agent | Casting | 5–240 MPa | NR | [156] |
| PVOH/OH | Cassava | Acetate solution | Casting | 1.35–13.03 MPa | NR | [149] |
| PVOH/starch | Tapioca | Supercritical CO2 | Injection molding | 20–25 MPa | NR | [157] |
| Chitosan/starch | Corn | - | Casting | 10–40 MPa | - | [158] |
| Chitosan/starch | Tapioca | - | Casting | - | 2.8–12.1 × 1010 | [159] |
| Chitosan/starch/mustard oil | Potato | 2-hydroxyethyl methacrylate (HEMA) | Casting | 1.7–13 MPA | - | [160] |
| Chitosan/starch | NR | Glutaraldehyde | Casting and solvent evaporation method | NR | 15.57–16.57 (g/m2 h) | [161] |
| Chitosan/starch | Corn | - | Casting | 3.44–19 MPa | NR | [162] |
| Chitosan/starch | corn | - | Casting | 1.6–73 MPa | 1.4–2.5 (g/m2 h kPa) | [163] |
| PCL/starch | Meritena (corn), Waxy, Amarant and GelInstant | - | Extrusion | NR | 100–1800 MPa | [164] |
| PCL/starch | NR | Maleic anhydride and clay | Extrusion | NR | 7–40 MPa | [165] |
| PCL/starch | Corn | - | Pressing and molding | NR | NR | [166] |
| PCL/starch | NR | - | ND | NR | NR | [167] |
| PCL/starch | Corn | - | Molding | NR | 5–29 MPa | [168] |
| PCL/starch | NR | Maleic anhydridegrafted-polyethylene | Mixing | NR | [169] | |
| TPS/Natural Rubber | Corn | Natural latex | Mixing | 0.7–9 MPa | NR | [170] |
| TPS/Natural Rubber | Corn | Oxidized natural rubber (ONR) | Extrusion | 0–4 MPa | NR | [171] |
| TPS/Natural Rubber | Potato | Natural rubber (NR) and epoxidized natural rubber (ENR) | Compressing and molding | 1–9 Mpa | NR | [172] |
| TPS/Natural Rubber | Cassava | Epoxidized natural rubber (ENR) | Melt blending, molding and compressing | 0–2.5 MPa | NR | [173] |
| TPS/Natural Rubber | Cassava | Rubber wood sawdust (Hevea brasiliensis) | Compression and molding | 0.33–1.23 MPa | NR | [174] |
| TPS/Natural Rubber | Sago | Natural rubber latex | Compression and molding | 4.46–25.2 MPa | NR | [175] |
| TPS/Natural Rubber | Cassava | Natural rubber latex | Casting | 4–8.5 MPa | NR | [176] |
| TPS/PE | Corn | Maleic anhydride (MAH | Bleeding and extrusion | 3.8–9.0 MPa | NR | [177] |
| TPS/PE | Potato | random ter-polymer of ethylene, acrylic and maleic anhydride | Blown film extrusion | 7–22 MPa | [178] | |
| TPS/PE | Rice and potato | - | Mixing and extrusion | 3.04–8.34 MPa | 0.1–160 (g m−1 s−1 Pa−1) × l0−13 | [179] |
| Composite System | Starch Source | Compatibilizante | Processing Techniques | Conclusions | Reference |
|---|---|---|---|---|---|
| Starch/titanium dioxide (TiO2) | Wheat | Glycerol/maleic anhydride (MA) | Solution casting-Melt mixing | Is described as a reliable, reproducible, two-step preparation of highly homogeneous TPS/mTiO2 composites, with very good dispersion of the filler. | [196] |
| Dialdehyde starch/magnetic nanoparticles | Potato | Maleic anhydride (MA) | Microwave-assisted in-situ precipitation method | The composite films manifested lower water vapor transmission, thus it can be concluded that MNPs improve the hydrophobicity and mechanical properties of MNPs/DAS composite films. | [182] |
| Starch/nanodiamond | Corn | Glycerol | Solution-blending. | The incorporation of a small amount of ND, the mechanical properties of starch were improved. To further improve the thermal stability and barrier properties of starch for food packaging applications | [197] |
| PBAT/starch/clay | Tapioca | Maleic anhydride (MA) | Reactive extrusion | Grafting with MA also improves the mechanical properties, and nanocomposite can be exploited for various commercial packaging applications. | [152] |
| TPS/PP/HDPE/nanoclaycorn | Corn | Glycerol/maleic anhydride grafted | Single-screw extruder | The addition of nano-clay to the system decreased the melt flow index, this may be due to the reaction between the modifying agent of the clay and Maleic anhydride being presented in PE-g-MA or/and the hydroxide in the glycerol. | [198] |
| TPS/PP/halloysite nanotubes | Corn | PP-g-MA | Internal mixer | The aforementioned results indicated that blending PP with TPS would successfully overcome the drawbacks of TPS such as poor mechanical properties and moisture sensitivity; in addition to improving the biodegradability of PP which is a real hazard for the environment. HNT could improve mechanical and thermal properties of the samples showing its usefulness as a promising filler. | [193] |
| TPS/PP/date palm flour | Potato | PE-g-MA, 2% w/w | Extrusion | This indicates that in addition to the interactions existing formerly between the DPF flour and the TPS phase, esterification treatment improved also the wettability of the filler by the PP phase through mediating its hydrophilic character | [199] |
| System | Starch Source | Bioactive | Results | Application | Reference |
|---|---|---|---|---|---|
| Poly (vinyl alcohol)-corn starch | Corn | Pineapple peel extract as a natural antioxidant agent | Film thickness and water vapor permeability increased slightly, antioxidant capacity increased. | Food Packaging | [225] |
| Lemon essential oil/surfactants (Span 80, Tween 80)/corn and wheat starch | Corn and wheat | Lemon essential oil | All concentrations of lemon oil were effective against selected bacteria (both Gram-negative and Gram-positive) compared with control film (without lemon oil) | Food Packaging | [226] |
| Chitosan-Starch-antioxidants | Rice | Antioxidants (from cranberry, blueberry, beetroot, pomegranate, oregano, pitaya and resveratrol, thymol and carvacrol) | The addition of natural extracts gives chitosan-starch a higher apparent density values. The addition of natural extracts provided chitosan-starch films with better thermal and physical properties | Food Packaging | [227] |
| Sodium alginate-starch | Yucca | Anthocyanin and betanin (from the exocarp of the black eggplant (Solanum melongena) and the mesocarp of beet (Beta vulgaris)) | Incorporation of natural extracts influenced the mechanical properties, however did not influence film thickness or water vapor permeability. Films with eggplant extract had higher antioxidant activity against the (DPPH) radical and were more sensitive to the exposure of gaseous amines in comparisonwith films with beet extract. | Food Packaging | [228] |
| Mung bean starch-chitosan (MSC) Water chestnut starch-chitosan (WSC) | Mung bean/Water chestnut | Hydrophobic perilla oil | The results showed that the cheese coated by WSC film containing perilla oil presented better treatment performance in terms of microbial growth delay, weight loss and shelf life length. | Food Packaging for cheese | [229] |
| Cassava starch- essential oil-sodium bentonite nanclay | Cassava | Cinnamon essential oil | The meatballs stored at ambient temperature in cassava starch film incorporated with cinnamon oil and nano-clay, significantly inhibited the microbial growth till 96 h below the FDA limits (106 CFU/g) in foods compared to control films that exceeded the limit within 48 h. | Food Packaging for meatballs | [230] |
| Starch-furcellaran-lavender essential oil-gelatin | Potato | Lavender essential oil | Antioxidant properties proved to be significantly enhanced with increasing lavender essential oil concentration. The solubility, water absorption and degree of swelling of the film decreased with increasing concentration of oils. | Food Packaging | [231] |
| Tapioca starch-cinnamon bark essential oil-glycerol | Tapioca | Cinnamon bark essential oil | Increasing cinnamon bark essential oil improves tensile strength and antibacterial activity of the film and preserved the freshness of the beef during 15 days of storage. | Food Packaging for fresh beef | [232] |
| (Gelatin-pectin-starch)-(gelatin-pectin)-(gelatin-starch)-(starch-pectin) | Potato | Mentha pulegium and Lavandula angustifolia essential oils | The incorporation of essential oils resulted in films with enhanced antibacterial properties, lower water vapor permeability, and reduced mechanical properties | Food Packaging | [233] |
| Carvacrol essential oil-corn starch-montmorillonite-tween 80/Carvacrol essential oil-glycerol-corn starch | Corn | Carvacrol essential oil | The starch-montmorillonite-carvacrol essential oil hybrid films showed antimicrobial behavior against E. coli. | Food Packaging | [234] |
| Arrowroot starch-carnauba wax nanoemulsion-cellulose nanocrystals-essential oils from Mentha spicata and Cymbopogon martinii | Arrowroot | Mentha spicata and Cymbopogon martinii | The essential oils from Mentha spicata and Cymbopogon martinii incorporation improved the thermal stability of the films and provided excellent protection against fungi Rhizopus stolonifer and Botrytis cinerea. | Food Packaging | [235] |
| Corn starch-thyme essential oil microcapsules | Corn | Thyme | The addition of thyme essential oil microcapsules to starch films increased the opacity, thickness, tensile strength and water solubility. They also showed an inhibitory effect against Botryodiplodia theobromae Pat and Colletotrichum gloeosporioides Penz and extended the shelf life of mangoes up to 10 days at 25 °C. | Food Packaging for mango | [236] |
| Corn starch-PVA- neem and oregano essential oils | Pea | Neem and oregano | Starch-PVA films with 6.7% of oregano essential oils exhibited the best physical properties, without significant differences with respect to the starch-PVA matrix, while exhibiting antibacterial activity. | Food Packaging | [237] |
| Nanoestructure | Raw Materials | Preparation Method | Size (nm) | Reference |
|---|---|---|---|---|
| Nanocrystal | Potato | Acid hydrolysis-ultrasonication | 40–70 | [151] |
| Nanocrystal | Pea | Acid hydrolysis-ultrasonication | 30–80 | [253] |
| Nanocrystal | Waxy | Acid hydrolysis-ultrasonication | 70–100 | [66] |
| Nanocrystal | High amylose maize | Acid hydrolysis | 118–130 | [18] |
| Nanospheres | Soluble starch | Micro-emulsion | 50–350 | [263] |
| Nanospheres | Native sago starch | Nanoprecipitation | 270–420 | [256] |
| Nanospheres | Corn | Microemulsion | 96–100 | [264] |
| Nanospheres | Corn | Nanoprecipitation | 90–100 | [265] |
| Nanospheres | Potato | Acid hydrolysis-ultrasonication | 40 | [266] |
| Nanogels | Corn, potato, and pea starch | Reverse emulsification | 100 | [267] |
| Nanogels | α-starch | Chemical crosslinking | 30 | [261] |
| Nanogels | Starch/poly(alginic acid-cl-acrylamide) | Chemical crosslinking | 380 | [268] |
| Nanogels | CMS | EB radiation N | 380 | [269] |
| Nanogels | Potato | Chemical crosslinking | 120–160 | [270] |
| Nanofibers | Corn | Electrospinning | 750–900 | [271] |
| Nanofibers | High amylose Maize starch | Cross-linking/Electrospinning | 300–700 | [272] |
| Nanofibers | Corn | Coaxial Electrospinning | 110–160 | [273] |
| Nanofibers | High-amylose maize starch and nGO | Electrospinning | 30–50 | [274] |
| Nanofibers | Soluble starch | Coaxial electrospinning | 90–250 | [262] |
| Micelle | Corn | Graft copolymerization/self-assemble | 20–30 | [275] |
| Micelle | Waxy Maize | Emulsion/self-assemble | 60–70 | [276] |
| Micelle | Soluble | Schiff-base bonds | [277] | |
| Micelle | Starch-octanoic | Graft copolymerization/self-assemble | 400–600 | [278] |
| Nanoparticulas | Waxy Maize | Acid hydrolysis-ultrasonication | 50–80 | [252] |
| Nanoparticulas | Waxy Maize | Enzymatically hydrolyzed-emulsion cross-linking | 80–130 | [248] |
| Nanoparticulas | Pea | Precipitation-complex formation | 50–100 | [254] |
| Nanoparticulas | Corn | Complex formation | 10–20 | [248] |
| Packaging System | Processing Techniques | Function | Food Application | Results | References |
|---|---|---|---|---|---|
| Rice starch in combination with chitosan, emulsifier (sodium caseinate), and red palm oil. | Dipping | Enhancing the shelf life of walnuts | To coat dried walnut kernels | Films with higher in elongation at break, but lower in tensile strength. Film is more flexible than the other corn and wheat starch films tested in this study. Rice starch with high flexibility produces a uniform layer on the surface of walnut. | [332] |
| Cassava starch at different concentrations (1%, 2%, 3% and 4%) | Dipping | Delay the ripening of papaya fruit (Carica papaya) | Coating papaya fruit (Carica papaya) | All cassava starch coating concentrations reduced fruit maturation and anthracnose, with the 2%, 3% and 4% coatings giving 100% disease control. | [333] |
| Nano-SiO2-potato starch | Film | Preservation the white mushroom | White mushroom | The water resistance and mechanical properties of the films were improved with the addition of nano-SiO2. Resistance to ultraviolet and thermal aging was also improved. Finally, they were more efficient against Escherichia coli (E. coli) than Staphylococcus aureus (S. aureus), improving the preservation of white fungi. | [334] |
| Corn starch (TPS) and chitosan oligomers | Film | Package perishable foods such as strawberries, ricotta, and flavored breads, | Strawberries, ricotta, and flavored breads. | Sachet type packages demonstrated to have a notable antimicrobial capability against molds and yeasts. Flavored breads were the least susceptible product to the microbial development, while strawberries and ricotta presented the highest molds and yeasts growth, respectively. | [335] |
| Yam starch-glycerol | Film | Extend storage life of strawberries stored at 4 °C and 85% RH | Strawberries | Yam Starch films significantly reduced decay of the fruits compared to control and extended the shelf life of strawberries by 21 days. | [336] |
| Material | Product | Manufacturing Company | Web Site |
|---|---|---|---|
| Granules based on corn powder/polyester + corn powder | Bio Degradable Bio One and Bio Base Rangdaneh Sirjan | RANGDANEH SIRJAN Co. Sirjan-IRAN | http://www.rangdaneh.ir (accessed on 20 November 2021) |
| BIOTEC contains 75% renewable feedstock and has a 69% biobased carbon share according to ASTM D6866 and ISO 16620-2. | BIOPLAST 105 BIOPLAST 300 BIOPLAST 400 BIOPLAST 500 BIOPLAST 900 BIOPLAST GF 106/02 BIOPLAST GS 2189 | BIOTEC GmbH and Co. KG Emmerich am Rhein-Alemania | https://es.biotec.de (accessed on 20 November 2021) |
| Starch | Mater-Bi | Novamont, S.L.U. Novara-Italia | https://www.novamontiberia.es/ (accessed on 20 November 2021) |
| Starch-PBAT | BioAgri Mulch Film | BioBag Americas, Inc. Palm Harbor-Canadian | https://www.biobagusa.com (accessed on 20 November 2021) |
| Starch from the potato processing industry and/or grain, root or seed flour based resources | Solanyl® | Rodenburg Oosterhout- The Netherlands | https://biopolymers.nl (accessed on 20 November 2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Rodríguez Llamazares, S. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 3, 136-177. https://doi.org/10.3390/polysaccharides3010007
García-Guzmán L, Cabrera-Barjas G, Soria-Hernández CG, Castaño J, Guadarrama-Lezama AY, Rodríguez Llamazares S. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides. 2022; 3(1):136-177. https://doi.org/10.3390/polysaccharides3010007
Chicago/Turabian StyleGarcía-Guzmán, Lucia, Gustavo Cabrera-Barjas, Cintya G. Soria-Hernández, Johanna Castaño, Andrea Y. Guadarrama-Lezama, and Saddys Rodríguez Llamazares. 2022. "Progress in Starch-Based Materials for Food Packaging Applications" Polysaccharides 3, no. 1: 136-177. https://doi.org/10.3390/polysaccharides3010007
APA StyleGarcía-Guzmán, L., Cabrera-Barjas, G., Soria-Hernández, C. G., Castaño, J., Guadarrama-Lezama, A. Y., & Rodríguez Llamazares, S. (2022). Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides, 3(1), 136-177. https://doi.org/10.3390/polysaccharides3010007