Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications
Abstract
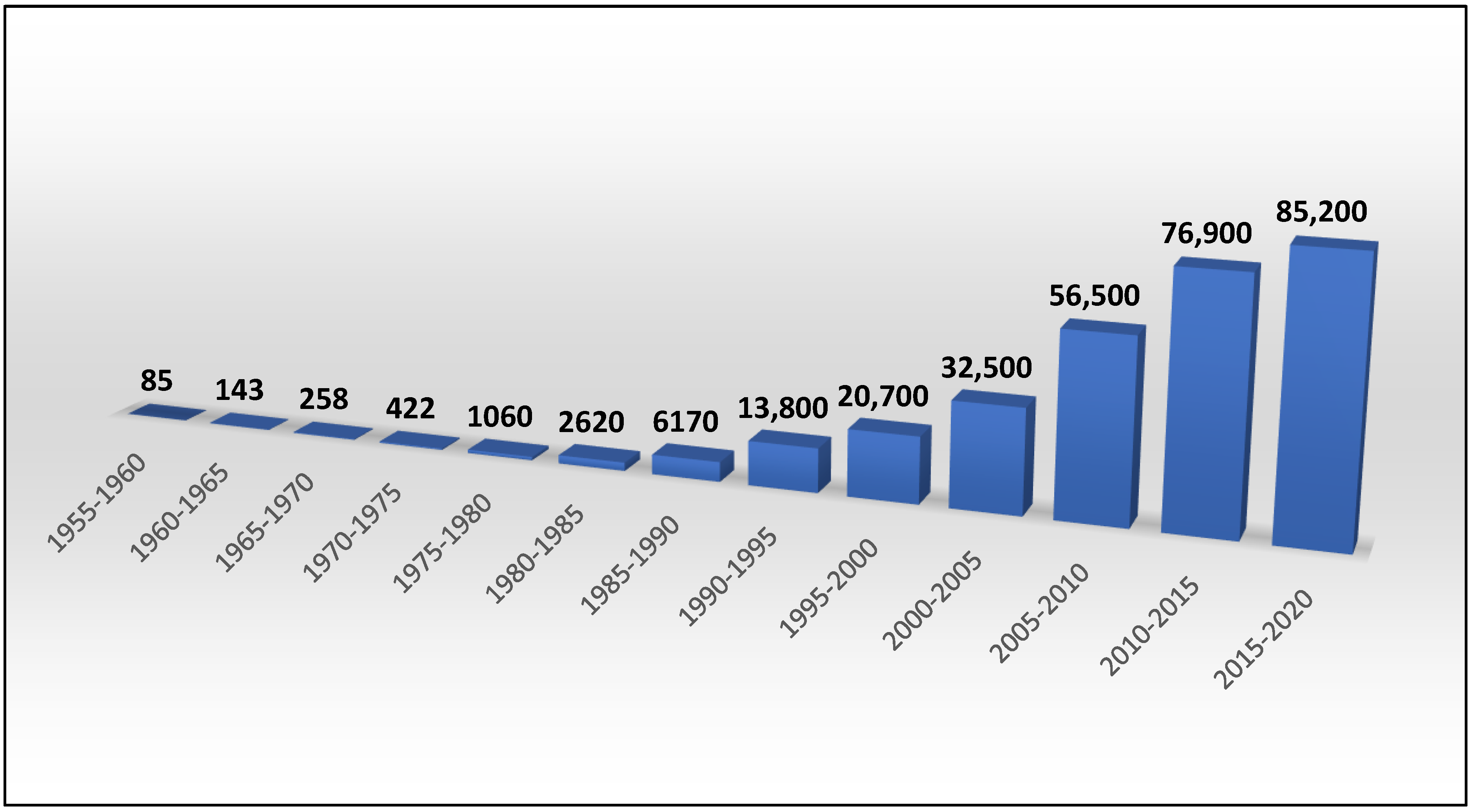
:1. Introduction—Brief History of Cyclodextrins & Their Applications
2. Structural and Chemical Properties of Cyclodextrins
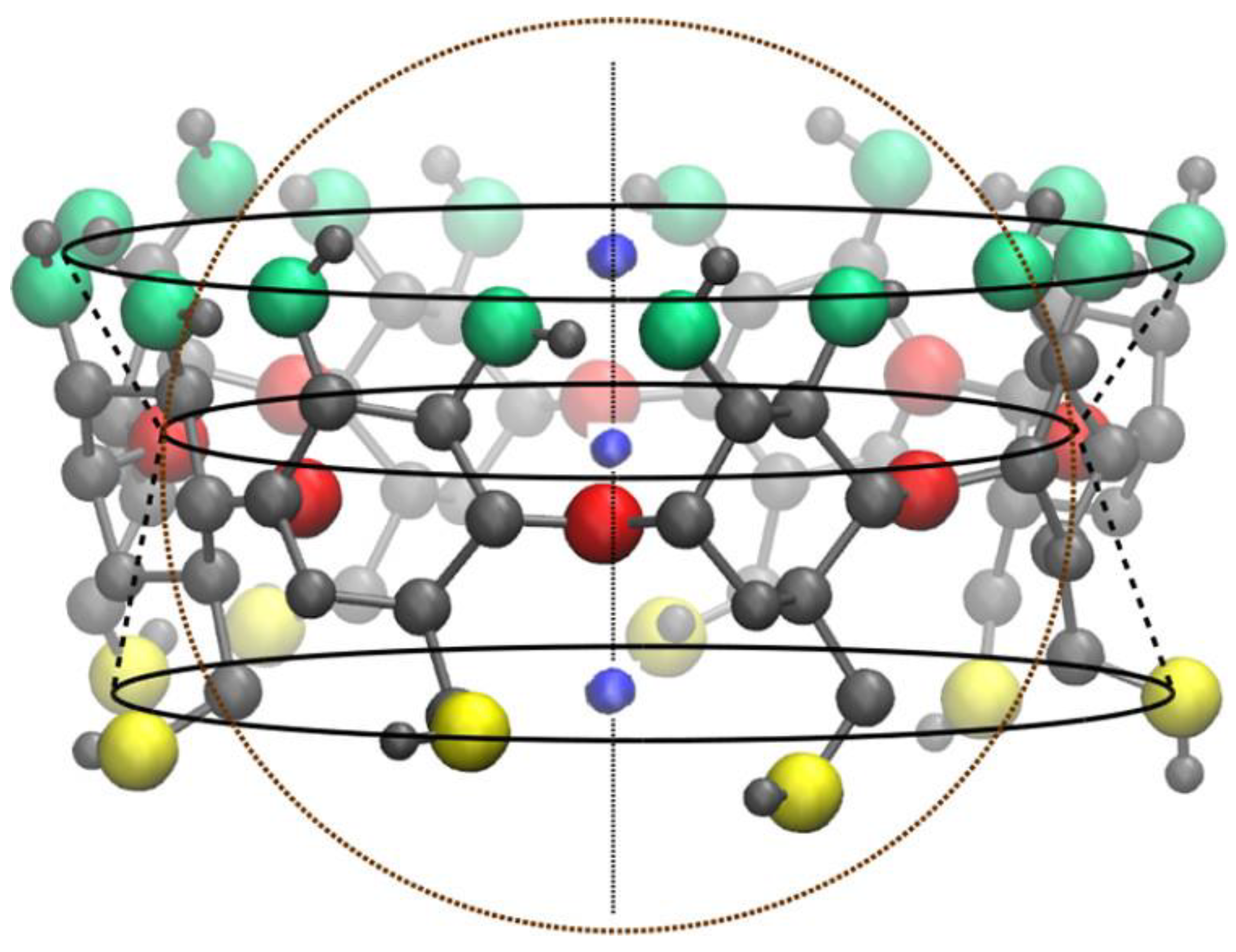
2.1. The Shape of the Native Cyclodextrins
2.2. Cyclodextrin Size Properties and Overall Flexibility
2.3. The Torsion Angle Index
2.4. Hydrogen Bonding and Cyclodextrin Aqueous Solubility
2.5. Cyclodextrin Derivatives
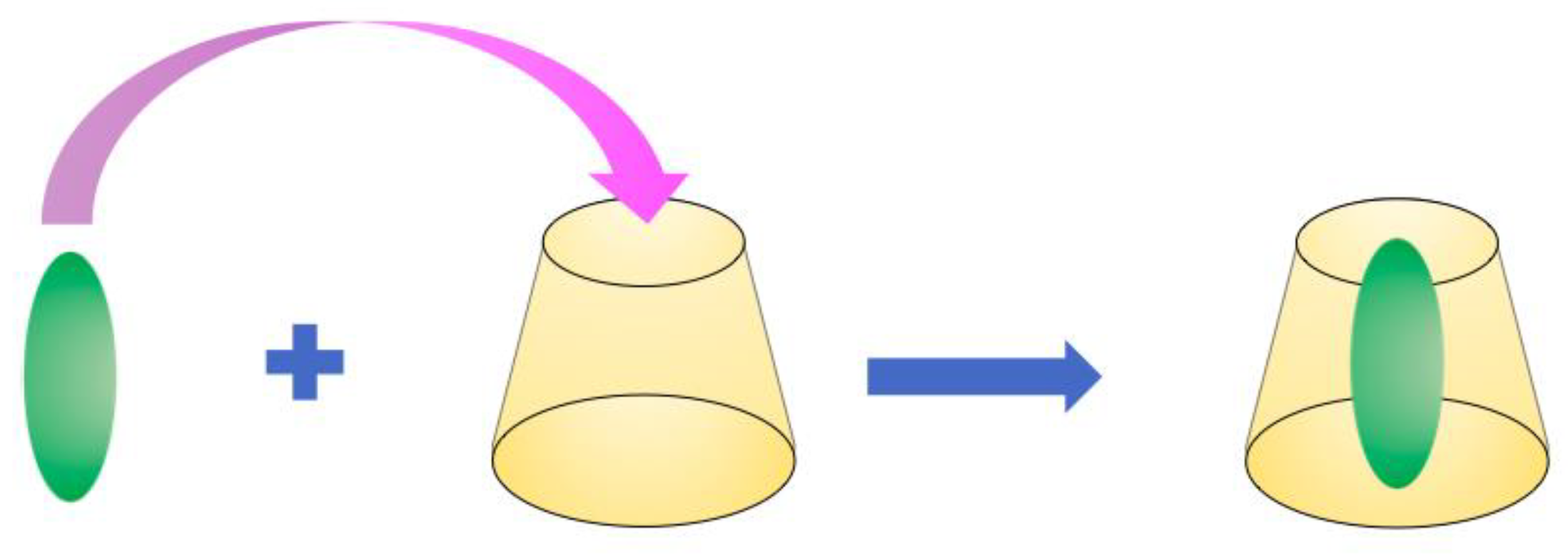
3. Complexation Properties of Cyclodextrins
3.1. Introduction
3.2. Size and Shape of the Cyclodextrin Cavity
3.3. Self-Assembly and Aggregation of Cyclodextrins and Their Complexes
Aggregation of CDs with Small Molecules
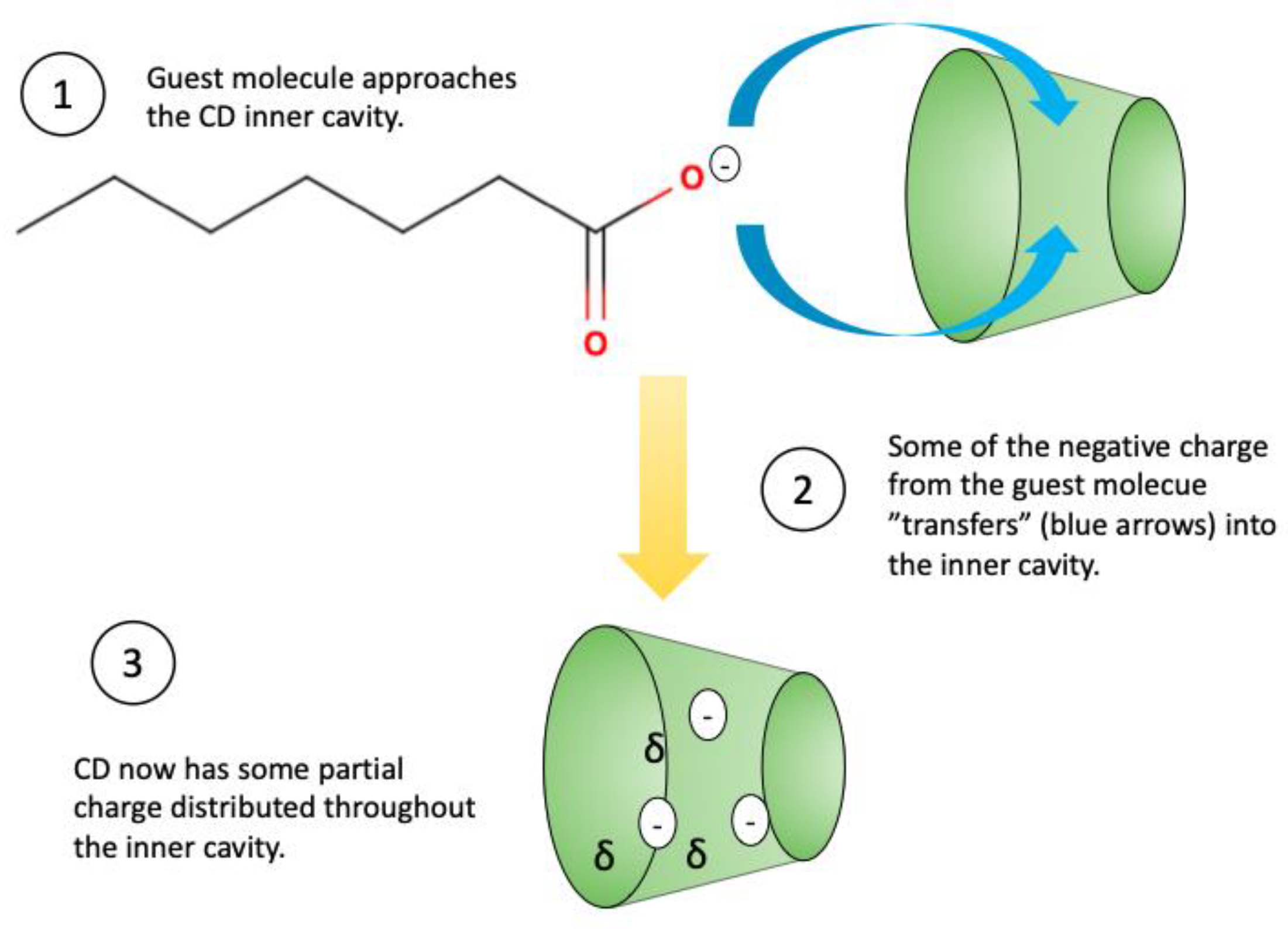
3.4. Chemical Properties of the Guest Molecule
Ionic Charge of the Guest Molecule
3.5. Intermolecular Forces between Guest and Host
3.5.1. The Dominating Van Der Waals Forces in CD Complexation
3.5.2. Hydrogen Bonding between Guest and Host
3.5.3. Release of High Energy Water Molecules and Their Role in Complexation
4. Applications of Cyclodextrins
4.1. General Applications
4.2. CDs in Solar Energy
4.3. Environmental Application of CDs
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szente, L.; Szemán, J.; Sohajda, T. Analytical Characterization of Cyclodextrins: History, Official Methods and Recommended New Techniques. J. Pharm. Biomed. Anal. 2016, 130, 347–365. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Crini, G. The Contribution of Franz Schardinger to Cyclodextrins: A Tribute on the Occasion of the Centenary of His Death. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 19–28. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- French, D. The Schardinger Dextrins. In Advances in Carbohydrate Chemistry; Wolfrom, M.L., Tipson, R.S., Eds.; Academic Press: Cambridge, MA, USA, 1957; Volume 12, pp. 189–260. [Google Scholar]
- Martin, J.; Díaz-Montaña, E.J.; Asuero, A.G. Cyclodextrins: Past and Present; IntechOpen: London, UK, 2018; ISBN 978-1-78923-069-7. [Google Scholar]
- Freudenberg, K.; Schaaf, E.; Dumpert, G.; Ploetz, T. Neue Ansichten über die Stärke. Naturwissenschaften 1939, 27, 850–853. [Google Scholar] [CrossRef]
- Cramer, F. Einschlussverbindungen; Springer: Berlin/Heidelberg, Germany, 1954. [Google Scholar]
- Sharma, N.; Baldi, A. Exploring Versatile Applications of Cyclodextrins: An Overview. Drug Deliv. 2016, 23, 729–747. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A. Thirty Years with Cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Yang, H.; Sun, W.; Gong, X.; Zhao, J.; Sun, Y.; Diao, G. A Water-Soluble Inclusion Complex of Pedunculoside with the Polymer β-Cyclodextrin: A Novel Anti-Inflammation Agent with Low Toxicity. PLoS ONE 2014, 9, e101761. [Google Scholar] [CrossRef]
- Khalid, S.H.; Bashir, M.; Asghar, S.; Mallhi, T.H.; Khan, I.U. Effect of Cyclodextrin Derivatization on Solubility and Efficacy of Drugs. In Colloid Science in Pharmaceutical Nanotechnology; IntechOpen: London, UK, 2019; ISBN 978-1-78985-596-8. [Google Scholar]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Bar, R.; Ulitzur, S. Bacterial Toxicity of Cyclodextrins: Luminuous Escherichia Coli as a Model. Appl. Microbiol. Biotechnol. 1994, 41, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Salmas, R.E.; Salvador, E.; Roewer, N.; Broscheit, J.; Förster, C. Evaluation of the Potential Toxicity of Unmodified and Modified Cyclodextrins on Murine Blood-Brain Barrier Endothelial Cells. J. Toxicol. Sci. 2016, 41, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the Cytotoxicity of β-Cyclodextrin Derivatives: Evidence for the Role of Cholesterol Extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Róka, E.; Ujhelyi, Z.; Deli, M.; Bocsik, A.; Fenyvesi, É.; Szente, L.; Fenyvesi, F.; Vecsernyés, M.; Váradi, J.; Fehér, P.; et al. Evaluation of the Cytotoxicity of α-Cyclodextrin Derivatives on the Caco-2 Cell Line and Human Erythrocytes. Molecules 2015, 20, 20269–20285. [Google Scholar] [CrossRef] [Green Version]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, e198268. [Google Scholar] [CrossRef] [Green Version]
- Irie, T.; Uekama, K. Pharmaceutical Applications of Cyclodextrins. III. Toxicological Issues and Safety Evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-Cyclodextrin (HP-β-CD): A Toxicology Review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of Water Pollution with Native Cyclodextrins and Modified Cyclodextrins: A Comparative Overview and Perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from Molecules to Applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Rincón-López, J.; Almanza-Arjona, Y.C.; Riascos, A.P.; Rojas-Aguirre, Y. Technological Evolution of Cyclodextrins in the Pharmaceutical Field. J. Drug Deliv. Sci. Technol. 2021, 61, 102156. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Cyclodextrins in Parenteral Formulations. J. Pharm. Sci. 2021, 110, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Advantages of the Combined Use of Cyclodextrins and Nanocarriers in Drug Delivery: A Review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.T.; Cagno, V.; Janeček, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vuković, L.; et al. Modified Cyclodextrins as Broad-Spectrum Antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.X.; Bai, L.; Xu, X.M.; He, J.; Pan, S.Z. Inclusion Complexation, Encapsulation Interaction and Inclusion Number in Cyclodextrin Chemistry. Coord. Chem. Rev. 2009, 253, 1276–1284. [Google Scholar] [CrossRef]
- Dos Santos, C.; Buera, P.; Mazzobre, F. Novel Trends in Cyclodextrins Encapsulation. Applications in Food Science. Curr. Opin. Food Sci. 2017, 16, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Hamoudi, M.C.; Bochot, A. Oil-Cyclodextrin Based Beads for Oral Delivery of Poorly-Soluble Drugs. Curr. Top. Med. Chem. 2014, 14, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Calleja, P.; Huarte, J.; Agüeros, M.; Ruiz-Gatón, L.; Espuelas, S.; Irache, J.M. Molecular Buckets: Cyclodextrins for Oral Cancer Therapy. Ther. Deliv. 2012, 3, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The Utility of Cyclodextrins for Enhancing Oral Bioavailability. J. Control. Release Off. J. Control. Release Soc. 2007, 123, 78–99. [Google Scholar] [CrossRef]
- Maheriya, P.M. Cyclodextrin: A Promising Candidate in Enhancing Oral Bioavailability of Poorly Water Soluble Drugs. MOJ Bioequiv. Bioavailab. 2017, 3, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Sahu, O. 4—Sustainable Cyclodextrin in Textile Applications. In The Impact and Prospects of Green Chemistry for Textile Technology; Ul-Islam, S., Butola, B.S., Eds.; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2019; pp. 83–105. ISBN 978-0-08-102491-1. [Google Scholar]
- Li, Z.; Chen, S.; Gu, Z.; Chen, J.; Wu, J. Alpha-Cyclodextrin: Enzymatic Production and Food Applications. Trends Food Sci. Technol. 2014, 35, 151–160. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as Food Ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Buschmann, H.-J.; Schollmeyer, E. Applications of Cyclodextrins in Cosmetic Products: A Review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar] [PubMed]
- Jullien, L.; Canceill, J.; Valeur, B.; Bardez, E.; Lefèvre, J.-P.; Lehn, J.-M.; Marchi-Artzner, V.; Pansu, R. Multichromophoric Cyclodextrins. 4. Light Conversion by Antenna Effect. J. Am. Chem. Soc. 1996, 118, 5432–5442. [Google Scholar] [CrossRef]
- Hajdu, C.; Gruiz, K.; Fenyvesi, É.; Nagy, Z.M. Application of Cyclodextrins in Environmental Bioassays for Soil. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 307–313. [Google Scholar] [CrossRef]
- Morillo, E.; Madrid, F.; Lara-Moreno, A.; Villaverde, J. Soil Bioremediation by Cyclodextrins: A Review. Int. J. Pharm. 2020, 591, 119943. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Gaidamauskas, E.; Norkus, E.; Butkus, E.; Crans, D.C.; Grincienė, G. Deprotonation of β-Cyclodextrin in Alkaline Solutions. Carbohydr. Res. 2009, 344, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Past, present and futute of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef] [Green Version]
- Bender, M.L.; Komiyama, M. Cyclodextrin Chemistry; Reactivity and Structure: Concepts in Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 1978; ISBN 978-3-642-66844-9. [Google Scholar]
- Tegge, G.; Szejtli, J. Cyclodextrins and Their Inclusion Complexes (Cyclodextrine und Ihre Einschlußkomplexe). Verlag Der Ungarischen Akademie Der Wissenschaften. Akadémiai Kiadó, Budapest 1982. 296 Pages, with Numerous Tables and Formulas, Cloth DM 67,50. Starch-Stärke 1982, 34, 395. [Google Scholar] [CrossRef]
- Dodziuk, H. Molecules with Holes—Cyclodextrins. In Cyclodextrins and Their Complexes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–30. ISBN 978-3-527-60898-0. [Google Scholar]
- Alvarez-Dorta, D.; León, E.I.; Kennedy, A.R.; Martín, A.; Pérez-Martín, I.; Suárez, E. Easy Access to Modified Cyclodextrins by an Intramolecular Radical Approach. Angew. Chem. Int. Ed. 2015, 54, 3674–3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harata, K. Crystallographic Study of Cyclodextrins and Their Inclusion Complexes. In Cyclodextrins and Their Complexes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 147–198. ISBN 978-3-527-60898-0. [Google Scholar]
- Plazinski, W.; Drach, M. The Dynamics of the Conformational Changes in the Hexopyranose Ring: A Transition Path Sampling Approach. RSC Adv. 2014, 4, 25028–25039. [Google Scholar] [CrossRef]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the Common Cyclodextrins and Their Larger AnaloguesBeyond the Doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Caira, M.R.; Griffith, V.J.; Nassimbeni, L.R.; van Oudtshoorn, B. Unusual 1C4 Conformation of a Methylglucose Residue in Crystalline Permethyl-β-Cyclodextrin Monohydrate. J. Chem. Soc. Perkin Trans. 2 1994, 10, 2071–2072. [Google Scholar] [CrossRef]
- Voncina, B.; Vivod, V. Cyclodextrins in Textile Finishing; IntechOpen: London, UK, 2013; ISBN 978-953-51-0892-4. [Google Scholar]
- Van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Parmar, V.; Patel, G.; Abu-Thabit, N.Y. 20—Responsive Cyclodextrins as Polymeric Carriers for Drug Delivery Applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; Volume 1, pp. 555–580. ISBN 978-0-08-101997-9. [Google Scholar]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Noreña-Caro, D.; Alvarez-Láinez, M. Experimental Design as a Tool for the Manufacturing of Filtering Media Based on Electrospun Polyacrylonitrile/β-Cyclodextrin Fibers. Int. J. Interact. Des. Manuf. IJIDeM 2016, 10, 153–164. [Google Scholar] [CrossRef]
- Cova, T.F.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M. Combining Cellulose and Cyclodextrins: Fascinating Designs for Materials and Pharmaceutics. Front. Chem. 2018, 6, 271. [Google Scholar] [CrossRef]
- Michoff, M.E.Z.; Granados, A.M.; de Rossi, R.H. Study of the Interaction of 5-(Alkylthio)-3H-1, 2-Dithiole-3-Thiones with ß-Cyclodextrin; Michigan Publishing—University of Michigan Library: Ann Arbor, MI, USA, 2005. [Google Scholar]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in Drug Delivery: An Updated Review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef]
- Atwood, J.L. Inclusion (Clathrate) Compounds. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 717–729. ISBN 978-0-12-227410-7. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Cal, K.; Centkowska, K. Use of Cyclodextrins in Topical Formulations: Practical Aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Hybl, A.; Rundle, R.E.; Williams, D.E. The Crystal and Molecular Structure of the Cyclohexaamylose-Potassium Acetate Complex1. J. Am. Chem. Soc. 1965, 87, 2779–2788. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Sabesan, M.N.; Steinrauf, L.K. Structure of Cycloheptaamylose Inclusion-Complexes: Crystal Structure of Substituted Benzoic Acid and Phenol Derivatives. Carbohydr. Res. 1981, 89, 33–53. [Google Scholar] [CrossRef]
- Maclennan, J.M.; Stezowski, J.J. The Crystal Structure of Uncomplexed-Hydrated Cyclooctaamylose. Biochem. Biophys. Res. Commun. 1980, 92, 926–932. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-Based Supramolecular Systems for Drug Delivery: Recent Progress and Future Perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as Functional Excipients: Methods to Enhance Complexation Efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.M.; Zhang, R.X.; Huang, Q.; Bai, C.C.; Wang, Z.Z. Chemical Conjugation with Cyclodextrins as a Versatile Tool for Drug Delivery. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 29–38. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic Macromolecular Material Design—The Versatility of Cyclodextrin-Based Host–Guest Chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidel, J.D.; Schluep, T. Cyclodextrin-Containing Polymers: Versatile Platforms of Drug Delivery Materials. J. Drug Deliv. 2012, 2012, e262731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, P.; Dhingra, N. Cyclodextrin—A Versatile Ingredient; BoD—Books on Demand: Norderstedt, Germany, 2018; ISBN 978-1-78923-069-7. [Google Scholar]
- Bell, A.F.; Hecht, L.; Barron, L.D. New Evidence for Conformational Flexibility in Cyclodextrins from Vibrational Raman Optical Activity. Chem.–Eur. J. 1997, 3, 1292–1298. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Inoue, Y. NMR Studies of Cyclodextrin Inclusion Complex. J. Carbohydr. Chem. 1989, 8, 29–46. [Google Scholar] [CrossRef]
- Dodziuk, H.; Koźmiński, W.; Ejchart, A. NMR Studies of Chiral Recognition by Cyclodextrins. Chirality 2004, 16, 90–105. [Google Scholar] [CrossRef]
- Dodziuk, H.; Ejchart, A.; Lukin, O.; Vysotsky, M.O. 1H and 13C NMR and Molecular Dynamics Study of Chiral Recognition of Camphor Enantiomers by α-Cyclodextrin. J. Org. Chem. 1999, 64, 1503–1507. [Google Scholar] [CrossRef]
- Ejchart, A.; Koźmiński, W. NMR of Cyclodextrins and Their Complexes. In Cyclodextrins and Their Complexes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 231–254. ISBN 978-3-527-60898-0. [Google Scholar]
- Dodziuk, H. Rigidity versus Flexibility. A Review of Experimental and Theoretical Studies Pertaining to the Cyclodextrin Nonrigidity. J. Mol. Struct. 2002, 614, 33–45. [Google Scholar] [CrossRef]
- Fujita, K.; Chen, W.-H.; Yuan, D.-Q.; Nogami, Y.; Koga, T.; Fujioka, T.; Mihashi, K.; Immel, S.; Lichtenthaler, F.W. Guest-Induced Conformational Change in a Flexible Host: Mono-Altro-β-Cyclodextrin1Part 20 of the Series Molecular Modeling of Saccharides. Tetrahedron Asymmetry 1999, 10, 1689–1696. [Google Scholar] [CrossRef]
- Kozár, T.; Venanzi, C.A. Reconsidering the Conformational Flexibility of β-Cyclodextrin. J. Mol. Struct. THEOCHEM 1997, 395–396, 451–468. [Google Scholar] [CrossRef]
- Ishizu, T.; Hirata, C.; Yamamoto, H.; Harano, K. Structure and Intramolecular Flexibility of Beta-Cyclodextrin Complex with (-)-Epigallocatechin Gallate in Aqueous Solvent. Magn. Reson. Chem. MRC 2006, 44, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G.; Ganazzoli, F. Hydration and Flexibility of α-, β-, γ- and δ-Cyclodextrin: A Molecular Dynamics Study. Chem. Phys. 2007, 333, 128–134. [Google Scholar] [CrossRef]
- Cova, T.F.; Milne, B.F.; Pais, A.A.C.C. Host Flexibility and Space Filling in Supramolecular Complexation of Cyclodextrins: A Free-Energy-Oriented Approach. Carbohydr. Polym. 2019, 205, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Pinjari, R.V.; Joshi, K.A.; Gejji, S.P. Molecular Electrostatic Potentials and Hydrogen Bonding in α-, β-, and γ-Cyclodextrins. J. Phys. Chem. A 2006, 110, 13073–13080. [Google Scholar] [CrossRef]
- Sonnendecker, C.; Thürmann, S.; Przybylski, C.; Zitzmann, F.D.; Heinke, N.; Krauke, Y.; Monks, K.; Robitzki, A.A.; Belder, D.; Zimmermann, W. Large-Ring Cyclodextrins as Chiral Selectors for Enantiomeric Pharmaceuticals. Angew. Chem. Int. Ed. 2019, 58, 6411–6414. [Google Scholar] [CrossRef]
- Ueda, H.; Endo, T. Large-Ring Cyclodextrins. In Cyclodextrins and Their Complexes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 370–380. ISBN 978-3-527-60898-0. [Google Scholar]
- Larsen, K.L. Large Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 1–13. [Google Scholar] [CrossRef]
- Ueda, H. Physicochemical Properties and Complex Formation Abilities of Large-Ring Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 53–56. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrin Inclusion Complexes. In Cyclodextrin Technology; Szejtli, J., Ed.; Topics in Inclusion Science; Springer: Dordrecht, The Netherlands, 1988; pp. 79–185. ISBN 978-94-015-7797-7. [Google Scholar]
- Saenger, W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chem. Int. Ed. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Naidoo, K.J.; Gamieldien, M.R.; Chen, J.Y.-J.; Widmalm, G.; Maliniak, A. Glucose Orientation and Dynamics in α-, β-, and γ-Cyclodextrins. J. Phys. Chem. B 2008, 112, 15151–15157. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W. Structure Aspects of Cyclodextrin Inclusion Compounds. In Proceedings of the First International Symposium on Cyclodextrins, Budapest, Hungary, 30 September–2 October 1981; Szejtli, J., Ed.; Springer: Dordrecht, The Netherlands, 1982; pp. 141–150. [Google Scholar]
- Franzini, R.; Ciogli, A.; Gasparrini, F.; Ismail, O.H.; Villani, C. Chapter 14—Recent Developments in Chiral Separations by Supercritical Fluid Chromatography. In Chiral Analysis, 2nd ed.; Polavarapu, P.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 607–629. ISBN 978-0-444-64027-7. [Google Scholar]
- Tafazzoli, M.; Ghiasi, M. Structure and Conformation of α-, β- and γ-Cyclodextrin in Solution: Theoretical Approaches and Experimental Validation. Carbohydr. Polym. 2009, 78, 10–15. [Google Scholar] [CrossRef]
- Jarvis, M.C. Relationship of Chemical Shift to Glycosidic Conformation in the Solid-State13C NMR Spectra of (1 → 4)-Linked Glucose Polymers and Oligomers: Anomeric and Related Effects. Carbohydr. Res. 1994, 259, 311–318. [Google Scholar] [CrossRef]
- Miller, M.M.; Wasik, S.P.; Huang, G.L.; Shiu, W.Y.; Mackay, D. Relationships between Octanol-Water Partition Coefficient and Aqueous Solubility. Environ. Sci. Technol. 1985, 19, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Hedges, A. Chapter 22—Cyclodextrins: Properties and Applications. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; pp. 833–851. ISBN 978-0-12-746275-2. [Google Scholar]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to Explore the Mechanism to Form Inclusion Complexes of β-Cyclodextrin with Vitamin Molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef]
- Sabadini, E.; Cosgrove, T.; do Carmo Egídio, F. Solubility of Cyclomaltooligosaccharides (Cyclodextrins) in H2O and D2O: A Comparative Study. Carbohydr. Res. 2006, 341, 270–274. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, Y.; Huang, J. Versatility of Cyclodextrins in Self-Assembly Systems of Amphiphiles. Adv. Colloid Interface Sci. 2011, 169, 13–25. [Google Scholar] [CrossRef]
- Schneiderman, E.; Stalcup, A.M. Cyclodextrins: A Versatile Tool in Separation Science. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 83–102. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; García-González, C.A.; Concheiro, A. Cyclodextrins as Versatile Building Blocks for Regenerative Medicine. J. Control. Release 2017, 268, 269–281. [Google Scholar] [CrossRef]
- Küster, T.; Stadelmann, B.; Aeschbacher, D.; Hemphill, A. Activities of Fenbendazole in Comparison with Albendazole against Echinococcus Multilocularis Metacestodes in Vitro and in a Murine Infection Model. Int. J. Antimicrob. Agents 2014, 43, 335–342. [Google Scholar] [CrossRef]
- Rodrigues, L.N.C.; Tavares, A.C.M.; Ferreira, B.T.; Reis, A.K.C.A.; Katiki, L.M. Inclusion Complexes and Self-Assembled Cyclodextrin Aggregates for Increasing the Solubility of Benzimidazoles. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Zhang, R.; Regenstein, J.M. 13—Adding Biological Function to Nonbiological Nanoparticles. In Nutrient Delivery; Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 497–534. ISBN 978-0-12-804304-2. [Google Scholar]
- Laza-Knoerr, A.L.; Gref, R.; Couvreur, P. Cyclodextrins for Drug Delivery. J. Drug Target. 2010, 18, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Otero-Espinar, F.J.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C.; Blanco-Méndez, J. Cyclodextrins in Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2010, 20, 289–301. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Eastburn, S.D.; Tao, B.Y. Applications of Modified Cyclodextrins. Biotechnol. Adv. 1994, 12, 325–339. [Google Scholar] [CrossRef]
- Seltzman, H.H.; Szulc, Z.M. Chemically Modified Cyclodextrins as Catalytic Enzyme Mimics. In Proceedings of the Eighth International Symposium on Cyclodextrins, Budapest, Hungary, 31 March–2 April 1996; Szejtli, J., Szente, L., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 267–272. [Google Scholar]
- Kataky, R.; Morgan, E. Potential of Enzyme Mimics in Biomimetic Sensors: A Modified-Cyclodextrin as a Dehydrogenase Enzyme Mimic. Biosens. Bioelectron. 2003, 18, 1407–1417. [Google Scholar] [CrossRef]
- Dagani, R. Supramolecular chemistry-polymeric nanotubes made from sugars. Chem. Eng. News 1993, 71, 4. [Google Scholar]
- Yaksh, T.L.; Jang, J.; Nishiuchi, Y.; Braun, K.P.; Ro, S.; Goodman, M. The Utility of 2-Hydroxypropyl-β-Cyclodextrin as a Vehicle for the Intracerebral and Intrathecal Administration of Drugs. Life Sci. 1991, 48, 623–633. [Google Scholar] [CrossRef]
- Adachi, H.; Irie, T.; Harayama, F.; Uekame, K. Stabilization of Prostaglandin E1 in Fatty Alcohol Propylene Glycol Ointment by Acidic Cyclodextrin Derivative, O-Carboxymethyl-O-Ethyl-β-Cyclodextrin. Chem. Pharm. Bull. 1992, 40, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Ueno, A.; Kuwabara, T.; Nakamura, A.; Toda, F.; Kuwabara, T.; Nakamura, A.; Toda, F. A Modified Cyclodextrin as a Guest Responsive Colour-Change Indicator. Nature 1992, 356, 136–137. [Google Scholar] [CrossRef]
- Schmarr, H.-G.; Mosandl, A.; Neukom, H.-P.; Grob, K. Modified Cyclodextrins as Stationary Phases for Capillary GC: Consequences of Dilution in Polysiloxanes. J. High Resolut. Chromatogr. 1991, 14, 207–210. [Google Scholar] [CrossRef]
- Otake, T.; Schols, D.; Witvrouw, M.; Naesens, L.; Nakashima, H.; Moriya, T.; Kurita, H.; Matsumoto, K.; Ueba, N.; De Clercq, E. Modified Cyclodextrin Sulphates(MCDS11) Have Potent Inhibitory Activity against HIV and High Oral Bioavailability. Antivir. Chem. Chemother. 1994, 5, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A Review on the Use of Cyclodextrins in Foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Kaatze, U. Acoustical Spectroscopy of Carbohydrate Aqueous Solutions: Saccharides; Alkyl Glycosides; Cyclodextrins. Part I. Conformer Variations. Arch. Acoust. 2010, 35, 715–738. [Google Scholar] [CrossRef] [Green Version]
- Rather, M.Y.; Nordberg Karlsson, E.; Adlercreutz, P. Complexation of Alkyl Glycosides with α-Cyclodextrin Can Have Drastically Different Effects on Their Conversion by Glycoside Hydrolases. J. Biotechnol. 2015, 200, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.Y.; Ara, K.Z.G.; Nordberg Karlsson, E.; Adlercreutz, P. Characterization of Cyclodextrin Glycosyltransferases (CGTases) and Their Application for Synthesis of Alkyl Glycosides with Oligomeric Head Group. Process Biochem. 2015, 50, 722–728. [Google Scholar] [CrossRef]
- Schurig, V. Use of Derivatized Cyclodextrins as Chiral Selectors for the Separation of Enantiomers by Gas Chromatography. Ann. Pharm. Fr. 2010, 68, 82–98. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhang, H.-Y.; Fan, Z.; Guan, X.-D. Selective Binding of Chiral Molecules of Cinchona Alkaloid by Beta- and Gamma-Cyclodextrins and Organoselenium-Bridged Bis(Beta-Cyclodextrin)s. Bioorgan. Chem. 2003, 31, 11–23. [Google Scholar] [CrossRef]
- Cai, W.; Yu, Y.; Shao, X. Chiral Recognition of Aromatic Compounds by β-Cyclodextrin Based on Bimodal Complexation. J. Mol. Model. 2005, 11, 186–193. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation and Chiral Recognition Thermodynamics of 6-Amino-6-Deoxy-β-Cyclodextrin with Anionic, Cationic, and Neutral Chiral Guests: Counterbalance between van Der Waals and Coulombic Interactions. J. Am. Chem. Soc. 2002, 124, 813–826. [Google Scholar] [CrossRef]
- Rogez-Florent, T.; Azaroual, N.; Goossens, L.; Goossens, J.-F.; Danel, C. NMR Investigation of the Complexation and Chiral Discrimination of Pyrazole Sulfonamide Derivatives with Cyclodextrins. Carbohydr. Polym. 2015, 115, 598–604. [Google Scholar] [CrossRef]
- Zerbinati, O.; Trotta, F.; Giovannoli, C.; Baggiani, C.; Giraudi, G.; Vanni, A. New Derivatives of Cyclodextrins as Chiral Selectors for the Capillary Electrophoretic Separation of Dichlorprop Enantiomers. J. Chromatogr. A 1998, 810, 193–200. [Google Scholar] [CrossRef]
- Harada, A.; Adachi, H.; Kawaguchi, Y.; Kamachi, M. Recognition of Alkyl Groups on a Polymer Chain by Cyclodextrins. Macromolecules 1997, 30, 5181–5182. [Google Scholar] [CrossRef]
- Monteil, M.; Lecouvey, M.; Landy, D.; Ruellan, S.; Mallard, I. Cyclodextrins: A Promising Drug Delivery Vehicle for Bisphosphonate. Carbohydr. Polym. 2017, 156, 285–293. [Google Scholar] [CrossRef] [PubMed]
- DiScenza, D.J.; Levine, M. Selective Detection of Non-Aromatic Pesticides via Cyclodextrin-Promoted Fluorescence Modulation. New J. Chem. 2016, 40, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Šoškić, M.; Porobić, I. Interactions of Indole Derivatives with β-Cyclodextrin: A Quantitative Structure-Property Relationship Study. PLoS ONE 2016, 11, e0154339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.S.; Boddapati, S.; Sierks, M.R. Cyclodextrins Promote Protein Aggregation Posing Risks for Therapeutic Applications. Biochem. Biophys. Res. Commun. 2009, 386, 526–531. [Google Scholar] [CrossRef]
- Łagiewka, J.; Girek, T.; Ciesielski, W. Cyclodextrins-Peptides/Proteins Conjugates: Synthesis, Properties and Applications. Polymers 2021, 13, 1759. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. Cyclodextrins as Protective Agents of Protein Aggregation: An Overview. Chem. Asian J. 2016, 11, 1648–1657. [Google Scholar] [CrossRef]
- Serno, T.; Geidobler, R.; Winter, G. Protein Stabilization by Cyclodextrins in the Liquid and Dried State. Adv. Drug Deliv. Rev. 2011, 63, 1086–1106. [Google Scholar] [CrossRef]
- Carrazana, J.; Jover, A.; Meijide, F.; Soto, V.H.; Vázquez Tato, J. Complexation of Adamantyl Compounds by β-Cyclodextrin and Monoaminoderivatives. J. Phys. Chem. B 2005, 109, 9719–9726. [Google Scholar] [CrossRef] [PubMed]
- Ngan Tran, D.; Colesnic, D.; de Beaumais, S.A.; Pembouong, G.; Portier, F.; Antelo Queijo, Á.; Tato, J.V.; Zhang, Y.; Ménand, M.; Bouteiller, L.; et al. Cyclodextrin-Adamantane Conjugates, Self-Inclusion and Aggregation versus Supramolecular Polymer Formation. Org. Chem. Front. 2014, 1, 703–706. [Google Scholar] [CrossRef]
- Díaz, D.; Escobar Llanos, C.M.; Bernad, M.J.; Mora, J.G. Binding, Molecular Mechanics, and Thermodynamics of Cyclodextrin Inclusion Complexes with Ketoprofen in Aqueous Medium. Pharm. Dev. Technol. 1998, 3, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.I.; Ural, M.S.; Pan, F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water Structure Recovery in Chaotropic Anion Recognition: High-Affinity Binding of Dodecaborate Clusters to γ-Cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6852–6856. [Google Scholar] [CrossRef] [Green Version]
- Voskuhl, J.; Waller, M.; Bandaru, S.; Tkachenko, B.A.; Fregonese, C.; Wibbeling, B.; Schreiner, P.R.; Ravoo, B.J. Nanodiamonds in Sugar Rings: An Experimental and Theoretical Investigation of Cyclodextrin-Nanodiamond Inclusion Complexes. Org. Biomol. Chem. 2012, 10, 4524–4530. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations I: Structure and Physicochemical Properties, Formation of Complexes, and Types of Complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Charumanee, S.; Titwan, A.; Sirithunyalug, J.; Weiss-Greiler, P.; Wolschann, P.; Viernstein, H.; Okonogi, S. Thermodynamics of the Encapsulation by Cyclodextrins. J. Chem. Technol. Biotechnol. 2006, 81, 523–529. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef]
- Brett, J. Goldilocks and the Three Bears; Penguin: London, UK, 2016. [Google Scholar]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef] [Green Version]
- Saad, H.Y.; Higuchi, W.I. Water Solubility of Cholesterol. J. Pharm. Sci. 1965, 54, 1205–1206. [Google Scholar] [CrossRef]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential Effects of α-, β- and γ-Cyclodextrins on Human Erythrocytes. Eur. J. Biochem. 1989, 186, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Otagiri, M.; Sunada, M.; Uekama, K.; Ohtani, Y.; Yamada, Y.; Sugiyama, Y. Cyclodextrin-Induced Hemolysis and Shape Changes of Human Erythrocytes in Vitro. J. Pharmacobiodyn. 1982, 5, 741–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Yang, L.-J.; Lin, J.; Chen, Y.; Liu, Y. Binding Behaviors of Scutellarin with α-, β-, γ-Cyclodextrins and Their Derivatives. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 149. [Google Scholar] [CrossRef]
- Wójcik, J.; Ejchart, A.; Nowakowski, M. Shape Adaptation of Quinine in Cyclodextrin Cavities: NMR Studies. Phys. Chem. Chem. Phys. 2019, 21, 6925–6934. [Google Scholar] [CrossRef] [PubMed]
- Cova, T.F.G.G.; Cruz, S.M.A.; Valente, A.J.M.; Abreu, P.E.; Marques, J.M.C.; Pais, A.A.C.C. Aggregation of Cyclodextrins: Fundamental Issues and Applications; IntechOpen: London, UK, 2018; ISBN 978-1-78923-069-7. [Google Scholar]
- Ryzhakov, A.; Do Thi, T.; Stappaerts, J.; Bertoletti, L.; Kimpe, K.; Sá Couto, A.R.; Saokham, P.; Van den Mooter, G.; Augustijns, P.; Somsen, G.W.; et al. Self-Assembly of Cyclodextrins and Their Complexes in Aqueous Solutions. J. Pharm. Sci. 2016, 105, 2556–2569. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wu, G.; Chen, D. Molecular Dynamics Simulation of Cyclodextrin Aggregation and Extraction of Anthracene from Non-Aqueous Liquid Phase. J. Hazard. Mater. 2016, 320, 169–175. [Google Scholar] [CrossRef]
- Bonnet, P.; Jaime, C.; Morin-Allory, L. Structure and Thermodynamics of α-, β-, and γ-Cyclodextrin Dimers. Molecular Dynamics Studies of the Solvent Effect and Free Binding Energies. J. Org. Chem. 2002, 67, 8602–8609. [Google Scholar] [CrossRef]
- Krois, D.; Brinker, U.H. Circular Dichroism of Cyclodextrin Complexes; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Hernández, R.; Rusa, M.; Rusa, C.C.; López, D.; Mijangos, C.; Tonelli, A.E. Controlling PVA Hydrogels with γ-Cyclodextrin. Macromolecules 2004, 37, 9620–9625. [Google Scholar] [CrossRef]
- Do, T.T.; Van Hooghten, R.; Van den Mooter, G. A Study of the Aggregation of Cyclodextrins: Determination of the Critical Aggregation Concentration, Size of Aggregates and Thermodynamics Using Isodesmic and K2–K Models. Int. J. Pharm. 2017, 521, 318–326. [Google Scholar] [CrossRef]
- Valente, A.J.M.; Carvalho, R.A.; Murtinho, D.; Söderman, O. Molecular Dynamics of Cyclodextrins in Water Solutions from NMR Deuterium Relaxation: Implications for Cyclodextrin Aggregation. Langmuir 2017, 33, 8233–8238. [Google Scholar] [CrossRef]
- Valente, A.J.M.; Carvalho, R.A.; Söderman, O. Do Cyclodextrins Aggregate in Water? Insights from NMR Experiments. Langmuir 2015, 31, 6314–6320. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fu, P.; Shen, X.; Gao, H. Cyclodextrin-Based Aggregates and Characterization by Microscopy. Micron 2008, 39, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H.; Danikiewicz, W.; Grabner, G.; Krois, D.; Brinker, U.H.; Bilewicz, R.; Chmurski, K.; Kunitake, M.; Ohira, A. Other Physicochemical Methods. In Cyclodextrins and Their Complexes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 255–332. ISBN 978-3-527-60898-0. [Google Scholar]
- Jansook, P.; Moya-Ortega, M.D.; Loftsson, T. Effect of Self-Aggregation of γ-Cyclodextrin on Drug Solubilization. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 229–236. [Google Scholar] [CrossRef]
- Muankaew, C.; Saokham, P.; Jansook, P.; Loftsson, T. Self-Assembly of Cyclodextrin Complexes: Detection, Obstacles and Benefits. Die Pharm.-Int. J. Pharm. Sci. 2020, 75, 307–312. [Google Scholar] [CrossRef]
- Bikádi, Z.; Kurdi, R.; Balogh, S.; Szemán, J.; Hazai, E. Aggregation of Cyclodextrins as an Important Factor to Determine Their Complexation Behavior. Chem. Biodivers. 2006, 3, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Johnson, E.J. The Role of Carotenoids in Human Health. Nutr. Clin. Care Off. Publ. Tufts Univ. 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, Q.; Su, Y.; Ouyang, D. Investigation of Molecular Aggregation Mechanism of Glipizide/Cyclodextrin Complexation by Combined Experimental and Molecular Modeling Approaches. Asian J. Pharm. Sci. 2019, 14, 609–620. [Google Scholar] [CrossRef]
- Chun, J.-Y.; You, S.-K.; Lee, M.-Y.; Choi, M.-J.; Min, S.-G. Characterization of β-Cyclodextrin Self-Aggregates for Eugenol Encapsulation. Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Cho, H.-S.; Choi, M.-J.; Min, S.-G.; Chun, J.-Y. Effect of Various Concentration of β-Cyclodextrin Inclusion Complexes Containing Trans-Cinnamaldehyde by Molecular Self-Assembly. Int. J. Food Eng. 2015, 11, 619–627. [Google Scholar] [CrossRef]
- Rodrigues Sá Couto, A.; Ryzhakov, A.; Larsen, K.L.; Loftsson, T. Interaction of Native Cyclodextrins and Their Hydroxypropylated Derivatives with Carbamazepine in Aqueous Solution. Evaluation of Inclusion Complexes and Aggregates Formation. ACS Omega 2019, 4, 1460–1469. [Google Scholar] [CrossRef]
- Ambrósio, A.F.; Soares-Da-Silva, P.; Carvalho, C.M.; Carvalho, A.P. Mechanisms of Action of Carbamazepine and Its Derivatives, Oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 2002, 27, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrazza, R.; Rossi, B.; Guella, G. DOSY-NMR and Raman Investigations on the Self-Aggregation and Cyclodextrin Complexation of Vanillin. J. Phys. Chem. B 2014, 118, 7147–7155. [Google Scholar] [CrossRef] [PubMed]
- Stappaerts, J.; Do Thi, T.; Dominguez-Vega, E.; Somsen, G.W.; Van den Mooter, G.; Augustijns, P. The Impact of Guest Compounds on Cyclodextrin Aggregation Behavior: A Series of Structurally Related Parabens. Int. J. Pharm. 2017, 529, 442–450. [Google Scholar] [CrossRef]
- Sayed, M.; Jha, S.; Pal, H. Complexation Induced Aggregation and Deaggregation of Acridine Orange with Sulfobutylether-β-Cyclodextrin. Phys. Chem. Chem. Phys. 2017, 19, 24166–24178. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Ukhatskaya, E.V.; Loftsson, T. Drug/Cyclodextrin: Beyond Inclusion Complexation. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 297–301. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Rakmai, J. Inclusion Complex Formation of Cyclodextrin with Its Guest and Their Applications. Biol. Eng. Med. 2017, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Street, K.W., Jr. Cyclodextrin Cavity Polarity and Chromatographic Implications. J. Liq. Chromatogr. 1987, 10, 655–662. [Google Scholar] [CrossRef]
- Heredia, A.; Requena, G.; Sánchez, F.G. An Approach for the Estimation of the Polarity of the β-Cyclodextrin Internal Cavity. J. Chem. Soc. Chem. Commun. 1985, 24, 1814–1815. [Google Scholar] [CrossRef]
- Al-Burtomani, S.K.S.; Suliman, F.O. Inclusion Complexes of Norepinephrine with β-Cyclodextrin, 18-Crown-6 and Cucurbit[7]Uril: Experimental and Molecular Dynamics Study. RSC Adv. 2017, 7, 9888–9901. [Google Scholar] [CrossRef] [Green Version]
- Jara, P.; Barrientos, L.; Herrera, B.; Sobrados, I. Inclusion compounds of α-cyclodextrin with alkylthiols. J. Chil. Chem. Soc. 2008, 53, 1474–1476. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.K.; Ghosh, N.; Mukherjee, S. Interaction of Bile Salts with β-Cyclodextrins Reveals Nonclassical Hydrophobic Effect and Enthalpy–Entropy Compensation. J. Phys. Chem. B 2016, 120, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Shimpi, S.; Chauhan, B.; Shimpi, P. Cyclodextrins: Application in Different Routes of Drug Administration. Acta Pharm. 2005, 55, 139–156. [Google Scholar]
- Schönbeck, C. Charge Determines Guest Orientation: A Combined NMR and Molecular Dynamics Study of β-Cyclodextrins and Adamantane Derivatives. J. Phys. Chem. B 2018, 122, 4821–4827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutka, A.; Gołda, B. The Effect of PH on Cyclodextrin Complexation of Trifluoperazine. Acta Pol. Pharm. 2006, 63, 3–8. [Google Scholar]
- Béni, S.; Szakács, Z.; Csernák, O.; Barcza, L.; Noszál, B. Cyclodextrin/Imatinib Complexation: Binding Mode and Charge Dependent Stabilities. Eur. J. Pharm. Sci. 2007, 30, 167–174. [Google Scholar] [CrossRef]
- Buvári, A.; Barcza, L. Complex Formation of Phenol, Aniline, and Their Nitro Derivatives with β-Cyclodextrin. J. Chem. Soc. Perkin Trans. 2 1988, 4, 543–545. [Google Scholar] [CrossRef]
- Liu, L.; Song, K.-S.; Li, X.-S.; Guo, Q.-X. Charge-Transfer Interaction: A Driving Force for Cyclodextrin Inclusion Complexation. J. Incl. Phenom. Macrocycl. Chem. 2001, 40, 35–39. [Google Scholar] [CrossRef]
- Jiménez, V.; Alderete, J.B. The Role of Charge Transfer Interactions in the Inclusion Complexation of Anionic Guests with α-Cyclodextrin. Tetrahedron 2005, 61, 5449–5456. [Google Scholar] [CrossRef]
- Yin, C.; Cui, Z.; Jiang, Y.; van der Spoel, D.; Zhang, H. Role of Host–Guest Charge Transfer in Cyclodextrin Complexation: A Computational Study. J. Phys. Chem. C 2019, 123, 17745–17756. [Google Scholar] [CrossRef]
- Fumes, B.H.; Guzzo, M.R.; Machado, A.E.H.; Okano, L.T. Study of the Mode of Inclusion for 7-Hydroxyflavone in β-Cyclodextrin Complexes. J. Braz. Chem. Soc. 2016, 27, 382–391. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A Breakthrough of Targeted Therapy in Cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, R.J.; Channing, M.A.; Gibeily, G.J.; Pillor, D.M. Disposition Requirements for Binding in Aqueous Solution of Polar Substrates in the Cyclohexaamylose Cavity. J. Am. Chem. Soc. 1977, 99, 5146–5151. [Google Scholar] [CrossRef]
- Rosanske, T.W.; Connors, K.A. Stoichiometric Model of α-Cyclodextrin Complex Formation. J. Pharm. Sci. 1980, 69, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A.; Lin, S.-F.; Wong, A.B. Potentiometric Study of Molecular Complexes of Weak Acids and Bases Applied to Complexes of α-Cyclodextrin with Para -Substituted Benzoic Acids. J. Pharm. Sci. 1982, 71, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Yu, Y.; Shao, X. Studies on the Interaction of α-Cyclodextrin with Phospholipid by a Flexible Docking Algorithm. Chemom. Intell. Lab. Syst. 2006, 82, 260–268. [Google Scholar] [CrossRef]
- Du, F.; Pan, T.; Ji, X.; Hu, J.; Ren, T. Study on the Preparation of Geranyl Acetone and β-Cyclodextrin Inclusion Complex and Its Application in Cigarette Flavoring. Sci. Rep. 2020, 10, 12375. [Google Scholar] [CrossRef]
- Bonikowski, R.; Świtakowska, P.; Kula, J. Synthesis, Odour Evaluation and Antimicrobial Activity of Some Geranyl Acetone and Nerolidol Analogues. Flavour Fragr. J. 2015, 30, 238–244. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile Components from Mango (Mangifera indica L.) Cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.-T.; Li, R. Complexation and Molecular Microcapsules of Litsea Cubeba Essential Oil with β-Cyclodextrin and Its Derivatives. Eur. Food Res. Technol. 2009, 228, 865–873. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Al-Sadeh, K.S.; Al-Bitar, M.B. Inclusion Complexes of Sunscreen Agents with β-Cyclodextrin: Spectroscopic and Molecular Modeling Studies. J. Spectrosc. 2013, 2013, e841409. [Google Scholar] [CrossRef] [Green Version]
- Steiner, T.; Saenger, W. Weak Hydrogen Bonding in Cyclodextrin Complex Stabilisation. In Current Challenges on Large Supramolecular Assemblies; Tsoucaris, G., Ed.; NATO Science Series; Springer: Dordrecht, The Netherlands, 1999; pp. 375–383. ISBN 978-94-011-5284-6. [Google Scholar]
- Ross, P.D.; Rekharsky, M.V. Thermodynamics of Hydrogen Bond and Hydrophobic Interactions in Cyclodextrin Complexes. Biophys. J. 1996, 71, 2144–2154. [Google Scholar] [CrossRef] [Green Version]
- Li, N.B.; Luo, H.Q.; Liu, S.P. Resonance Rayleigh Scattering Study of the Inclusion Complexation of Chloramphenicol with β-Cyclodextrin. Talanta 2005, 66, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Pavlova, J.A.; Lukianov, D.A.; Tereshchenkov, A.G.; Makarov, G.I.; Khairullina, Z.Z.; Tashlitsky, V.N.; Paleskava, A.; Konevega, A.L.; Bogdanov, A.A.; et al. Binding and Action of Triphenylphosphonium Analog of Chloramphenicol upon the Bacterial Ribosome. Antibiotics 2021, 10, 390. [Google Scholar] [CrossRef]
- Audino, P.G.; Masuh, H.; Zerba, E. Thermal Behaviour, Biological Activity and Conformational Study of a [Methoprene/β-Cyclodextrin] Complex in a Smoke Generating Formulation. Molecules 2005, 10, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Seidel, R.W.; Koleva, B.B. β-Cyclo dextrin 10.41-Hydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o3162–o3163. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P.; Trotta, F. Diversity of β-Cyclodextrin-Based Nanosponges for Transformation of Actives. Int. J. Pharm. 2019, 565, 333–350. [Google Scholar] [CrossRef]
- Rasheed, A.; Ashok Kumar, C.K.; Sravanthi, V.V.N.S.S. Cyclodextrins as Drug Carrier Molecule: A Review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside β-Cyclodextrin Cavity: Amount, Stability and Mechanism of Binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef]
- Angelova, S.; Nikolova, V.; Pereva, S.; Spassov, T.; Dudev, T. α-Cyclodextrin: How Effectively Can Its Hydrophobic Cavity Be Hydrated? J. Phys. Chem. B 2017, 121, 9260–9267. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety Evaluation and Risk Assessment Of D-Limonene. J. Toxicol. Environ. Health Part B 2013, 16, 17–38. [Google Scholar] [CrossRef]
- Yoshii, H.; Furuta, T.; Yasunishi, A.; Hirano, H. Minimum Number of Water Molecules Required for Inclusion of D-Limonene in the Cyclodextrin Cavity. J. Biochem. 1994, 115, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Takeshi, F.; Hidefumi, Y.; Atsuyuki, M.; Akira, Y.; Hiroshi, H. Effect of Water and Ethanol on the Formation of Inclusion Complex for D-Limonene and Cyclodextrin System. In Biochemical Engineering for 2001; Furusaki, S., Endo, I., Matsuno, R., Eds.; Springer: Tokyo, Japan, 1992; pp. 762–764. [Google Scholar]
- García-Río, L.; Mejuto, J.C.; Rodríguez-Dafonte, P.; Hall, R.W. The Role of Water Release from the Cyclodextrin Cavity in the Complexation of Benzoyl Chlorides by Dimethyl-β-Cyclodextrin. Tetrahedron 2010, 66, 2529–2537. [Google Scholar] [CrossRef]
- Rácz, C.P.; Borodi, G.; Pop, M.M.; Kacso, I.; Sánta, S.; Tomoaia-Cotisel, M. Structure of the Inclusion Complex of β-Cyclodextrin with Lipoic Acid from Laboratory Powder Diffraction Data. Acta Crystallogr. B 2012, 68, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Andreaus, J.; Dalmolin, M.C.; de Oliveira, I.B., Jr.; Barcellos, I.O. Aplicação de ciclodextrinas em processos têxteis. Quím. Nova 2010, 33, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Xu, J. Applications in the Textile Industry. In Cyclodextrins; World Scientific: Singapore, 2017; pp. 209–230. ISBN 978-981-322-965-5. [Google Scholar]
- Savarino, P.; Viscardi, G.; Quagliotto, P.; Montoneri, E.; Barni, E. Reactivity and Effects of Cyclodextrins in Textile Dyeing. Dyes Pigment. 1999, 42, 143–147. [Google Scholar] [CrossRef]
- Hu, X.; Hu, Y.; Xu, G.; Li, M.; Zhu, Y.; Jiang, L.; Tu, Y.; Zhu, X.; Xie, X.; Li, A. Green Synthesis of a Magnetic β-Cyclodextrin Polymer for Rapid Removal of Organic Micro-Pollutants and Heavy Metals from Dyeing Wastewater. Environ. Res. 2020, 180, 108796. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Jiang, H.-L.; Xie, Z.-W.; Li, Z.-T.; Xu, D.; He, F.-A. Highly Efficient Selective Adsorption of Anionic Dyes by Modified β-Cyclodextrin Polymers. J. Taiwan Inst. Chem. Eng. 2020, 108, 114–128. [Google Scholar] [CrossRef]
- Teng, M.; Li, F.; Zhang, B.; Taha, A.A. Electrospun Cyclodextrin-Functionalized Mesoporous Polyvinyl Alcohol/SiO2 Nanofiber Membranes as a Highly Efficient Adsorbent for Indigo Carmine Dye. Colloids Surf. Physicochem. Eng. Asp. 2011, 385, 229–234. [Google Scholar] [CrossRef]
- Crupi, V.; Ficarra, R.; Guardo, M.; Majolino, D.; Stancanelli, R.; Venuti, V. UV–Vis and FTIR–ATR Spectroscopic Techniques to Study the Inclusion Complexes of Genistein with β-Cyclodextrins. J. Pharm. Biomed. Anal. 2007, 44, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.S.; Abdel-Mohdy, F.A.; Fouda, M.M.G.; El-Sawy, S.M.; Hamdy, I.A.; Al-Deyab, S.S. Antimicrobial Activity of Monochlorotriazinyl-β-Cyclodextrin/Chlorohexidin Diacetate Finished Cotton Fabrics. Carbohydr. Polym. 2011, 86, 1389–1394. [Google Scholar] [CrossRef]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Lis, M.J.; García Carmona, Ó.; García Carmona, C.; Maestá Bezerra, F. Inclusion Complexes of Citronella Oil with β-Cyclodextrin for Controlled Release in Biofunctional Textiles. Polymers 2018, 10, 1324. [Google Scholar] [CrossRef] [Green Version]
- Popescu, V.; Petrea, M.; Popescu, A. Multifunctional Finishing of Cotton with Compounds Derived from MCT-β-CD and Quantification of Effects Using MLR Statistical Analysis. Polymers 2021, 13, 410. [Google Scholar] [CrossRef]
- Cireli, A.; Yurdakul, B. Application of Cyclodextrin to the Textile Dyeing and Washing Processes. J. Appl. Polym. Sci. 2006, 100, 208–218. [Google Scholar] [CrossRef]
- Kacem, I.; Laurent, T.; Blanchemain, N.; Neut, C.; Chai, F.; Haulon, S.; Hildebrand, H.F.; Martel, B. Dyeing and Antibacterial Activation with Methylene Blue of a Cyclodextrin Modified Polyester Vascular Graft. J. Biomed. Mater. Res. A 2014, 102, 2942–2951. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, I.-S. Use of β-Cyclodextrin in an Antimigration Coating for Polyester Fabric. Color. Technol. 2013, 129, 347–351. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Pu, Y.; Wang, C.; Han, J.; Jiang, M.; Liu, K. The Preparation of Thin-Walled Multi-Cavities β-Cyclodextrin Polymer and Its Static and Dynamic Properties for Dyes Removal. J. Environ. Manag. 2019, 245, 105–113. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins in Foods, Cosmetics and Toiletries. In Proceedings of the First International Symposium on Cyclodextrins, Budapest, Hungary, 30 September–2 October 1981; Szejtli, J., Ed.; Springer: Dordrecht, The Netherlands, 1982; pp. 469–480. [Google Scholar]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef]
- Maskooki, A.M.; Beheshti, S.H.R.; Valibeigi, S.; Feizi, J. Effect of Cholesterol Removal Processing Using β-Cyclodextrin on Main Components of Milk. Int. J. Food Sci. 2013, 2013, e215305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Sharma, R.; Banerjee, U.C. Biotechnological Applications of Cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Cyclodextrins in Peptide and Protein Delivery. Adv. Drug Deliv. Rev. 1999, 36, 101–123. [Google Scholar] [CrossRef]
- Zhao, Q.; Temsamani, J.; Agrawal, S. Use of Cyclodextrin and Its Derivatives as Carriers for Oligonucleotide Delivery. Antisense Res. Dev. 1995, 5, 185–192. [Google Scholar] [CrossRef]
- Bait, O.; Si–Ameur, M. Enhanced Heat and Mass Transfer in Solar Stills Using Nanofluids: A Review. Sol. Energy 2018, 170, 694–722. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zou, C. An Experimental Study on β-Cyclodextrin Modified Carbon Nanotubes Nanofluids for the Direct Absorption Solar Collector (DASC): Specific Heat Capacity and Photo-Thermal Conversion Performance. Sol. Energy Mater. Sol. Cells 2020, 204, 110240. [Google Scholar] [CrossRef]
- Fuskele, V.; Sarviya, R.M. Recent Developments in Nanoparticles Synthesis, Preparation and Stability of Nanofluids. Mater. Today Proc. 2017, 4, 4049–4060. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Walker, C.A.; Nguyen, M.; Trimble, S.; Prasher, R. Applicability of Nanofluids in High Flux Solar Collectors. J. Renew. Sustain. Energy 2011, 3, 023104. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Miedaner, A.; Ahrenkiel, P.; Himmel, M.E.; Curtis, C.; Ginley, D. Self-Assembly of Photoactive TiO2−Cyclodextrin Wires. J. Am. Chem. Soc. 2005, 127, 14968–14969. [Google Scholar] [CrossRef]
- Willner, I.; Eichen, Y. Titanium Dioxide and Cadmium Sulfide Colloids Stabilized by. Beta.-Cyclodextrins: Tailored Semiconductor-Receptor Systems as a Means to Control Interfacial Electron-Transfer Processes. J. Am. Chem. Soc. 1987, 109, 6862–6863. [Google Scholar] [CrossRef]
- Sharavath, V.; Sarkar, S.; Gandla, D.; Ghosh, S. Low Temperature Synthesis of TiO2-β-Cyclodextrin–Graphene Nanocomposite for Energy Storage and Photocatalytic Applications. Electrochim. Acta 2016, 210, 385–394. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin Functionalized Graphene Nanosheets with High Supramolecular Recognition Capability: Synthesis and Host−Guest Inclusion for Enhanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.U.; Qin, Y.; Catalano, M.; Wang, L.; Kim, M.J.; Howlader, M.M.R.; Hu, N.-X.; Deen, M.J. Tailoring MWCNTs and β-Cyclodextrin for Sensitive Detection of Acetaminophen and Estrogen. ACS Appl. Mater. Interfaces 2018, 10, 21411–21427. [Google Scholar] [CrossRef]
- Kor, K.; Zarei, K. β-Cyclodextrin Incorporated Carbon Nanotube Paste Electrode as Electrochemical Sensor for Nifedipine. Electroanalysis 2013, 25, 1497–1504. [Google Scholar] [CrossRef]
- Yin, T.; Wei, W.; Zeng, J. Selective Detection of Dopamine in the Presence of Ascorbic Acid by Use of Glassy-Carbon Electrodes Modified with Both Polyaniline Film and Multi-Walled Carbon Nanotubes with Incorporated β-Cyclodextrin. Anal. Bioanal. Chem. 2006, 386, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Gandomi, F.; Marzi Khosrowshahi, E.; Sohouli, E.; Aghaei, M.; Saleh Mohammadnia, M.; Naghian, E.; Rahimi-Nasrabadi, M. Linagliptin Electrochemical Sensor Based on Carbon Nitride-β-Cyclodextrin Nanocomposite as a Modifier. J. Electroanal. Chem. 2020, 876, 114697. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Zhang, X.; Duan, S.; Xu, S.; Zhou, C. β-Cyclodextrin Functionalized Mesoporous Silica for Electrochemical Selective Sensor: Simultaneous Determination of Nitrophenol Isomers. Electrochim. Acta 2011, 58, 142–149. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, J.; Li, S.; Guo, M.; Fan, Z. An Electrochemical Sensor for the Detection of P-Nitrophenol Based on a Cyclodextrin-Decorated Gold Nanoparticle–Mesoporous Carbon Hybrid. Analyst 2019, 144, 4400–4406. [Google Scholar] [CrossRef]
- Selvam, S.; Balamuralitharan, B.; Karthick, S.N.; Savariraj, A.D.; Hemalatha, K.V.; Kim, S.-K.; Kim, H.-J. Novel High-Temperature Supercapacitor Combined Dye Sensitized Solar Cell from a Sulfated β-Cyclodextrin/PVP/MnCO3 Composite. J. Mater. Chem. A 2015, 3, 10225–10232. [Google Scholar] [CrossRef]
- Gao, S.; Wang, L. Application of Cyclodextrin in Environmental Science. Huanjing Kexue Jinzhan 1998, 6, 80–86. [Google Scholar]
- Gibson, L.T. Mesosilica Materials and Organic Pollutant Adsorption: Part B Removal from Aqueous Solution. Chem. Soc. Rev. 2014, 43, 5173–5182. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Fourmentin, M.; Fourmentin, S.; Torri, G.; Crini, G. Silica Materials Containing Cyclodextrin for Pollutant Removal. In Cyclodextrin Applications in Medicine, Food, Environment and Liquid Crystals; Environmental Chemistry for a Sustainable World; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–182. ISBN 978-3-319-76162-6. [Google Scholar]
- Morin-Crini, N.; Fourmentin, M.; Fourmentin, S.; Torri, G.; Crini, G. Synthesis of Silica Materials Containing Cyclodextrin and Their Applications in Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 683–696. [Google Scholar] [CrossRef]
- Huang, T.; Sheng, G.; Manchanda, P.; Emwas, A.H.; Lai, Z.; Nunes, S.P.; Peinemann, K.-V. Cyclodextrin Polymer Networks Decorated with Subnanometer Metal Nanoparticles for High-Performance Low-Temperature Catalysis. Sci. Adv. 2019, 5, eaax6976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
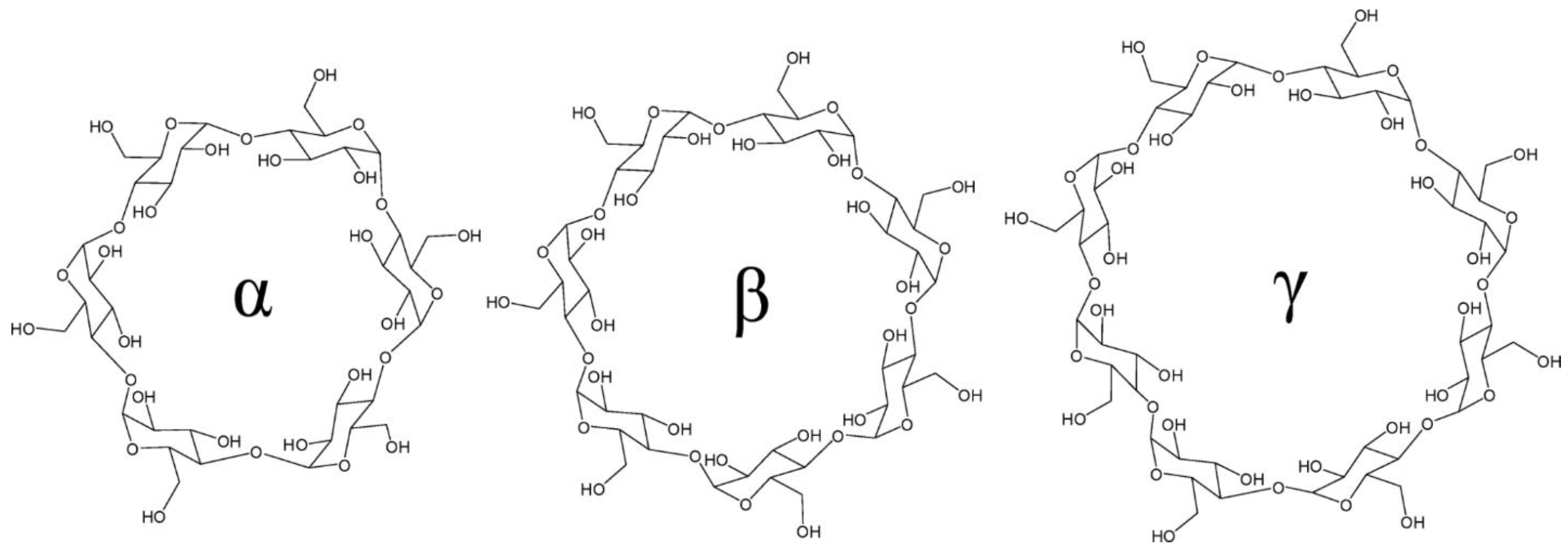


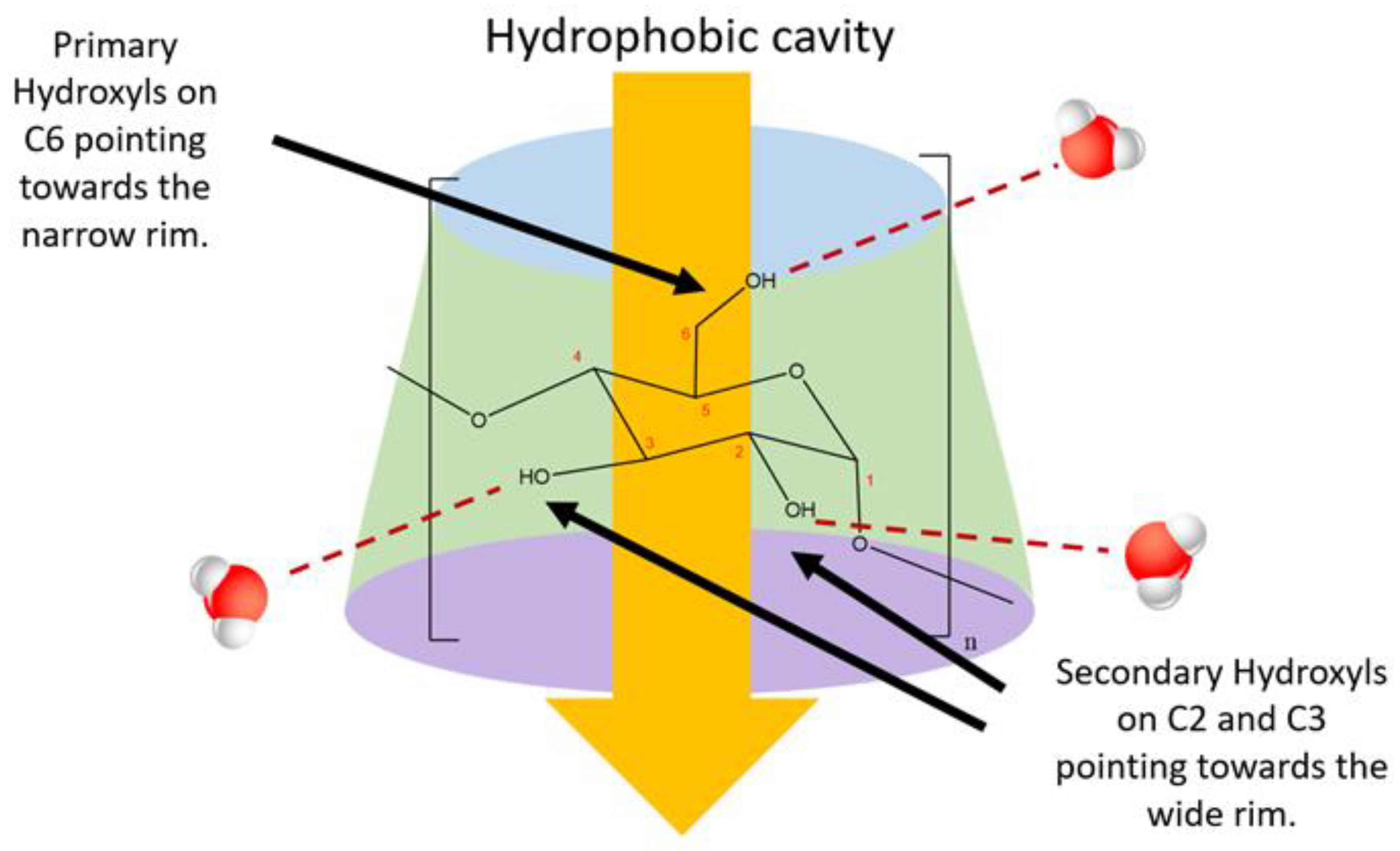
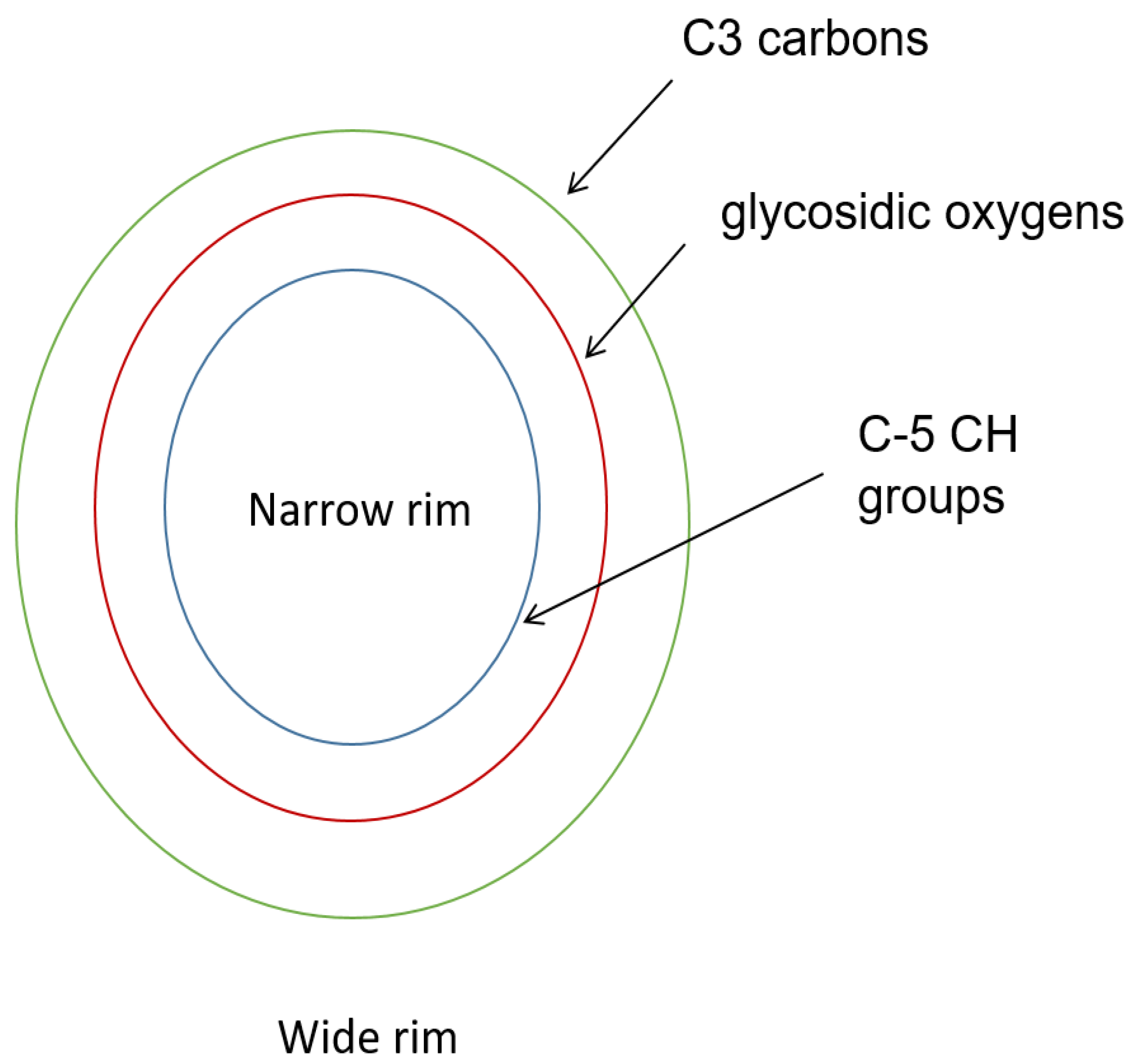
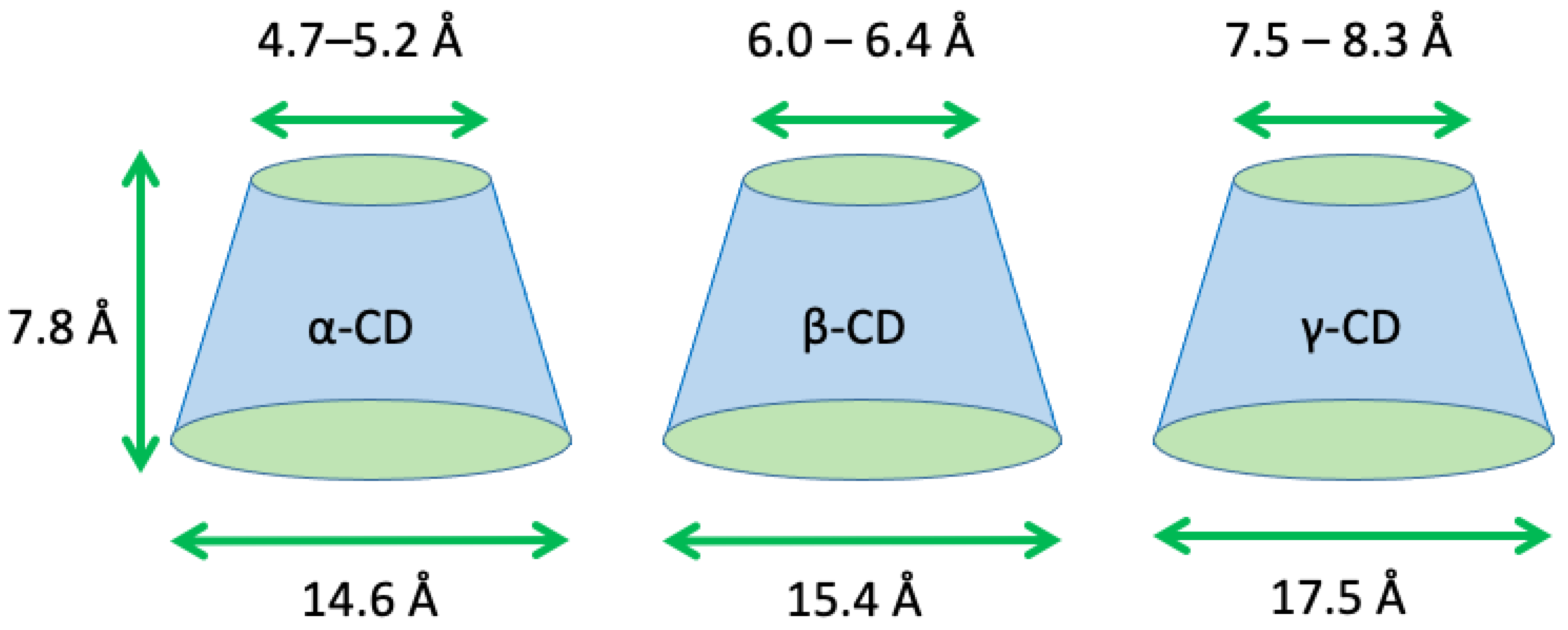



| Cyclodextrin | No. of Glucose Units | Molecular Weight | Cavity Diameter (Å) a | Outer Diameter (Å) b | Height (Å) c | Cavity Volume (Å3) d |
|---|---|---|---|---|---|---|
| α | 6 | 972 | 4.7–5.2 | 14.6 | 7.8 | 174 |
| β | 7 | 1135 | 6.0–6.4 | 15.4 | 7.8 | 262 |
| γ | 8 | 1297 | 7.5–8.3 | 17.5 | 7.8 | 427 |
| Cyclodextrin | ϕ (°) | Ψ (°) |
|---|---|---|
| α | 166 | −169 |
| β | 169 | −172 |
| γ | 165 | −169 |
| Property | α-CD | β-CD | γ-CD |
|---|---|---|---|
| LogP calculated | −12.7 | −14.82 | −16.93 |
| Acceptor count calculated | 30 | 35 | 40 |
| Donor count calculated | 18 | 21 | 24 |
| Temperature (°C) | α-CD | β-CD | γ-CD |
|---|---|---|---|
| 25 | 12.8 | 1.8 | 25.6 |
| 45 | 29.0 | 4.5 | 58.5 |
| 60 | 66.2 | 9.1 | 129.2 |
| CD Used | Albendazole Aqueous Solubility (μg/mL) | Fendendazole Aqeuous Solubility (μg/mL) |
|---|---|---|
| - | 0.4188 | 0.1054 |
| β-CD | 93.47 | 45.56 |
| HPβ-CD | 443.06 | 159.36 |
| Guest | Host | Binding Constant (M−1) | Reference |
|---|---|---|---|
| Ketoprofen | γ-CD | 2.351 × 103 | [144] |
| Ascorbic acid | β-CD | 3.655 × 103 | [104] |
| B12I122− (dodecaborate cluster) | γ-CD | 6.7 × 105 | [145] |
| 9-triamantane carboxylic acid | γ-CD | 5.0 × 105 | [146] |
| Size and shape of the CD cavity CD/CD-complex self-assembly or aggregation Chemical properties of guest molecule(s) Expulsion of “high-energy” water molecules (applies to aqueous solutions only) |
| Guest | Host (s) | Type of Charge (i.e., Positive and/or Negative) | Effect on CD Binding | Ref. |
|---|---|---|---|---|
| Adamantane derivatives | β-CD, DM-β-CD, TM-β-CD | Positive, Negative | Positively charged end of guests pointed out the wide rim, negative charge had no effect | [190] |
| Trifluoperazine | β-CD, DM-β-CD, HP-β-CD | Less positive (i.e., more negative) | Stronger binding | [191] |
| Imatinib | β-CD | 0, +1, +2, and +3 | Weakest binding at +1, strongest binding at +3 | [192] |
| p-nitrophenol, p-nitrophenolate | α-CD, β-CD | 0 (p-nitrophenol) −1 (p-nitrophenolate) | Anionic guest bound more strongly | [193,194] |
| Some carboxylic acids and their conjugate bases | α-CD | 0 (carboxylic acids) −1 (conjugate bases) | Negligible effect | [195] |
| nitrobenzene, carboxybenzene, benzoate, 4-nitrophenol, 4-nitrophenolate | α-CD | 0 (nitrobenzene) 0 (carboxybenzene) −1 (benzoate) 0 (4-nitrophenol) −1 (4-nitrophenolate) | Negligible effect | [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1-31. https://doi.org/10.3390/polysaccharides3010001
Poulson BG, Alsulami QA, Sharfalddin A, El Agammy EF, Mouffouk F, Emwas A-H, Jaremko L, Jaremko M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides. 2022; 3(1):1-31. https://doi.org/10.3390/polysaccharides3010001
Chicago/Turabian StylePoulson, Benjamin Gabriel, Qana A. Alsulami, Abeer Sharfalddin, Emam. F. El Agammy, Fouzi Mouffouk, Abdul-Hamid Emwas, Lukasz Jaremko, and Mariusz Jaremko. 2022. "Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications" Polysaccharides 3, no. 1: 1-31. https://doi.org/10.3390/polysaccharides3010001
APA StylePoulson, B. G., Alsulami, Q. A., Sharfalddin, A., El Agammy, E. F., Mouffouk, F., Emwas, A.-H., Jaremko, L., & Jaremko, M. (2022). Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides, 3(1), 1-31. https://doi.org/10.3390/polysaccharides3010001






