Characterization of Cassava Starch and Its Structural Changes Resulting of Thermal Stress by Functionally-Enhanced Derivative Spectroscopy (FEDS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Thermal Treatment of Cassava
2.2. Analysis by Infrared Spectroscopy with Fourier Transform Coupled to the Total Attenuated Reflectance Technique (FTIR-ATR)
2.3. FEDS Analysis
2.4. Computational Analysis
3. Results and Discussion
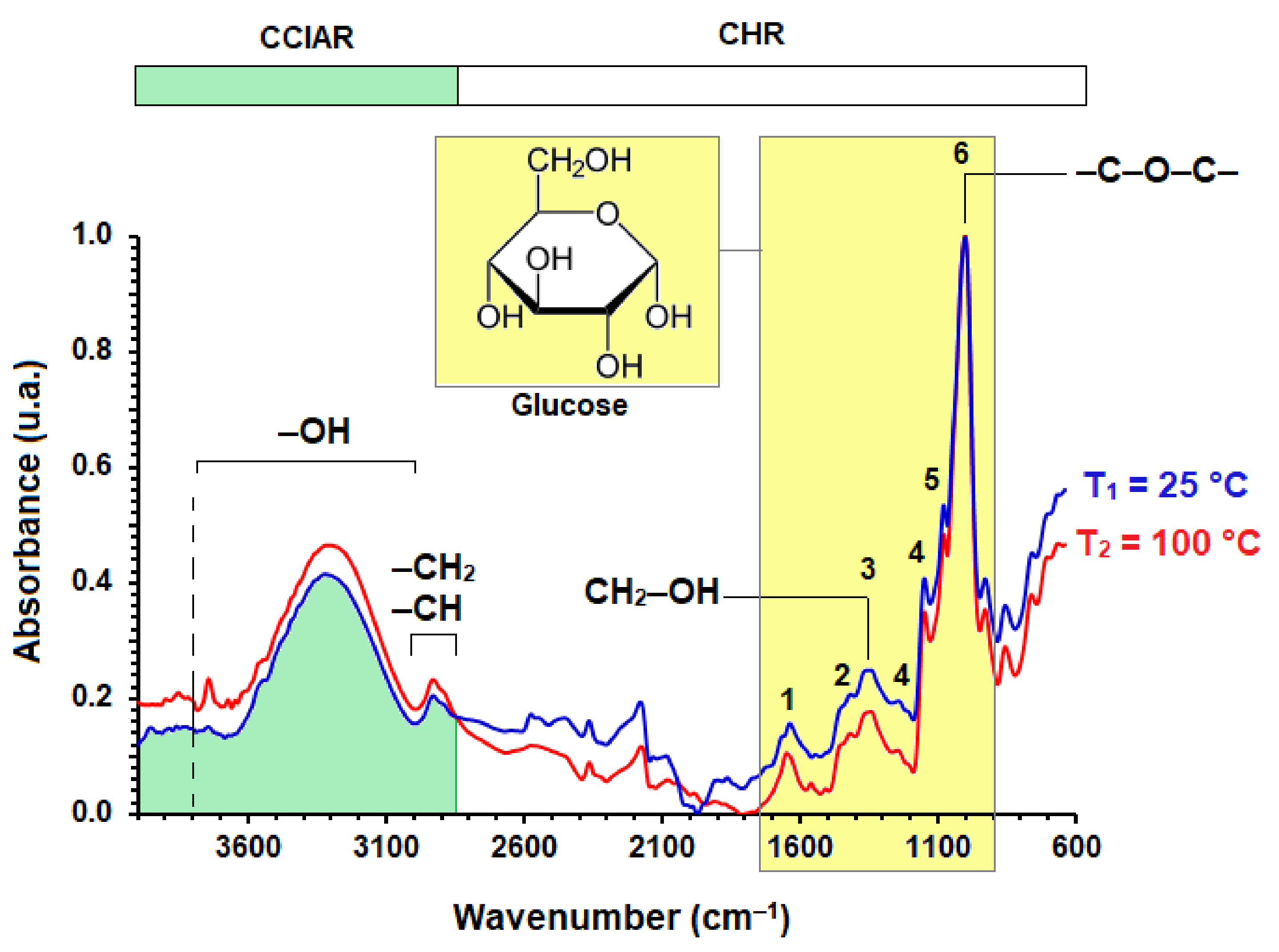
3.1. Cassava Starch Characterization at 25 °C and Starch Dried at 100 °C
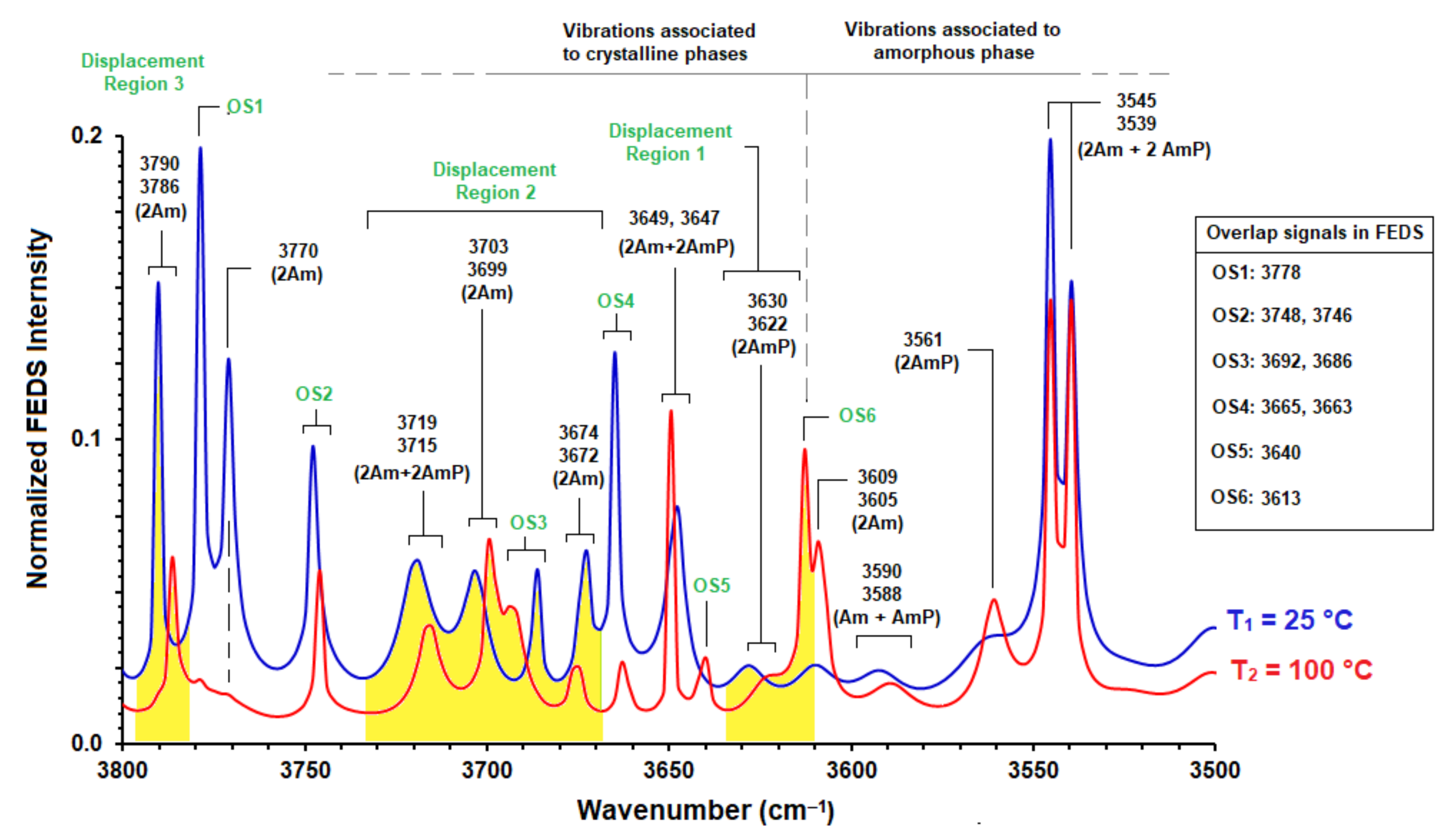
3.2. Study by FEDS of Chain-Chain Intermolecular Association Region Associated to the Hydroxyl Band
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teleky, B.E.; Dan, C.V. Biomass-derived production of itaconic acid as a building block in specialty polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [Green Version]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of Acetylation on the Properties of Corn Starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Li, H.L.; Xie, B.J.; Shi, J. Impact of the Catalytic Activity of Iodine on the Granule Morphology, Crystalline Structure, Thermal Properties and Water Solubility of Acetylated Corn (Zea Mays) Starch Synthesized under Microwave Assistance. Ind. Crop. Prod. 2011, 33, 302–309. [Google Scholar] [CrossRef]
- Garces, V.; Palencia, M.; Combatt, E.M. Development of Bacterial Inoculums Based on Biodegradable Hydrogels for Agricultural Applications. J. Sci. Technol. Appl. 2017, 2, 13–23. [Google Scholar] [CrossRef]
- Palencia, M.; Mora, M.; Palencia, S. Biodegradable Polymer Hydrogels Based in Sorbitol and Citric Acid for Controlled Release of Bioactive Substances from Plants (Polyphenols). Curr. Chem. Biol. 2017, 11, 36–43. [Google Scholar] [CrossRef]
- Rodríguez-Pineda, L.M.; Muñoz-Prieto, E.D.J.; Rius-Alonso, C.A.; Palacios-Alquisira, J. Preparation and Characterization of Potato Starch Microparticles with Acrylamide by Microwave Radiation. Cienc. Desarro. 2018, 9, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Arrieta-Almario, A.A.; Mendoza-Fandiño, J.M.; Palencia-Luna, M.S. Composite Material Elaborated from Conducting Biopolymer Cassava Starch and Polyaniline. Rev. Mex. Ing. Química 2019, 19, 707–715. [Google Scholar] [CrossRef]
- Palencia, M.; Lerma, T.; Garcés, V.; Mora, M.; Martínez, J.; Palencia, S.L. Principles of polymer synthetic green chemistry. In Eco-Friendly Functional Polymers: An Approach from Application-Targeted Green Chemistry, 1st ed.; Elsevier Science Publishing Co Inc: New York, NY, USA, 2021; pp. 5–22. [Google Scholar]
- Arrieta, A.; Palencia, M. Estudio Electroquímico de Un Biopolímero Compuesto PPy/Almidón de Cassava. Rev. Latinoam. Metal. Mater. 2016, 36, 26–35. [Google Scholar]
- Arrieta, A.; Combatt, E.; Palencia, M. Electrochemical Study of Cassava Starch Conductive Biopolymers Synthesized at Different PH. Adv. J. Food Sci. Technol. 2018, 15, 148–151. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Zhao, Y.; Tan, L.; Zhang, J.; Du, Z.; Zhang, H. Lipophilization and Molecular Encapsulation of p-Coumaric Acid by Amylose Inclusion Complex. Food Hydrocoll. 2019, 93, 270–275. [Google Scholar] [CrossRef]
- Maraphum, K.; Saengprachatanarug, K.; Wongpichet, S.; Phuphaphud, A.; Posom, J. In-Field Measurement of Starch Content of Cassava Tubers Using Handheld Vis-near Infrared Spectroscopy Implemented for Breeding Programmes. Comput. Electron. Agric. 2020, 175, 105607. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Wiens, R.A.; Gough, K.M.; Aluko, R.E. Influence of Acetylation on Physicochemical and Morphological Characteristics of Pigeon Pea Starch. Food Hydrocoll. 2020, 100, 105424. [Google Scholar] [CrossRef]
- Omoregie, H. Chemical Properties of Starch and Its Application in the Food Industry. In Chemical Properties of Starch, 1st ed.; Emeje, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Schnupf, U.; Willett, J.L.; Bosma, W.; Momany, F.A. DFT Conformation and Energies of Amylose Fragments at Atomic Resolution. Part 1: Syn Forms of α-Maltotetraose. Carboh. Res. 2009, 344, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Afanasjeva, N.; Castillo, L.; Sinisterra, J. Biomasa Lignocelulósica. Parte I: Transformación de biomasa. J. Sci. Technol. Appl. 2017, 3, 27–43. [Google Scholar] [CrossRef]
- Fu, H.Y.; Li, H.D.; Xu, L.; Yin, Q.B.; Yang, T.M.; Ni, C.; Cai, C.B.; Yang, J.; She, Y.B. Detection of Unexpected Frauds: Screening and Quantification of Maleic Acid in Cassava Starch by Fourier Transform Near-Infrared Spectroscopy. Food Chem. 2017, 227, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Paixão e Silva, G.D.L.; Bento, J.A.C.; Soares Júnior, M.S.; Caliari, M. Trend of Modification by Autoclave at Low Pressure and by Natural Fermentation in Sweet Potato and Cassava Starches. Polysaccharides 2021, 2, 354–372. [Google Scholar] [CrossRef]
- Santha, N.; Sudha, K.G.; Vijayakumari, K.P.; Nayar, V.U.; Moorthy, S.N. Raman and Infrared Spectra of Starch Samples of Sweet Potato and Cassava. J. Chem. Sci. 1990, 102, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared Spectroscopy as a Tool to Characterise Starch Ordered Structure—a Joint FTIR–ATR, NMR, XRD and DSC Study. Carb. Pol. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garces, V.; Palencia, M. Biosynthesis and Biotransformations of Biopolymers: Starch and Poly(Hydroxyalkanoates). J. Sci. Technol. Appl. 2020, 9, 4–9. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kurc, B.; Rymaniak, Ł.; Lijewski, P.; Fuć, P. Kinetics and Thermodynamics of Thermal Degradation of Different Starches and Estimation the OH Group and H2O Content on the Surface by TG/DTG-DTA. Polymers 2020, 12, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulik, S.; Jekle, M.; Becker, T. Mechanically and Thermally Induced Degradation and Modification of Cereal Biopolymers during Grinding. Polymers 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kačíková, D.; Kubovský, I.; Ulbriková, N.; Kačík, F. The Impact of Thermal Treatment on Structural Changes of Teak and Iroko Wood Lignins. Appl. Sci. 2020, 10, 5021. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nakamura, Y.; Tamada, Y.; Kurosu, H.; Kameda, T. The influence of thermal treatments on the secondary structure of silk fibroin scaffolds and their interaction with fibroblasts. PeerJ Mater. Sci. 2020, 2, e8. [Google Scholar] [CrossRef]
- Faroongsarng, D.; Wongpoowarak, W.; Mitrevej, A. Starch gelatinization under thermal stress. Pharm. Dev. Technol. 1999, 4, 531–538. [Google Scholar] [CrossRef]
- Lu, D.L.; Yang, H.; Shen, X.; Lu, W.P. Effects of high temperature during grain filling on physicochemical properties of waxy maize starch. J. Integrat. Agr. 2016, 15, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Palencia, M.; Lerma, T.A.; Combatt, E.M. Hydrogels Based in Cassava Starch with Antibacterial Activity for Controlled Release of Cysteamine-Silver Nanostructured Agents. Curr. Chem. Biol. 2017, 11, 28–35. [Google Scholar] [CrossRef]
- Palencia, M.; Lerma, T.; Garcés, V.; Mora, M.; Martínez, J.; Palencia, S.L. Polymer biosynthesis and biotransformations. In Eco-Friendly Functional Polymers: An Approach from Application-Targeted Green Chemistry, 1st ed.; Elsevier Science Publishing Co Inc: Cambridge, MA, USA, 2021; pp. 89–104. [Google Scholar]
- Otálora, A.; Palencia, M. Application of Functionally-Enhanced Derivative Spectroscopy (FEDS) to the Problem of the Overlap of Spectral Signals in Binary Mixtures: Triethylamine-Acetone. J. Sci. Technol. Appl. 2019, 6, 96–107. [Google Scholar] [CrossRef]
- Bantadjan, Y.; Rittiron, R.; Malithong, K.; Narongwongwattana, S. Establishment of an Accurate Starch Content Analysis System for Fresh Cassava Roots Using Short-Wavelength Near Infrared Spectroscopy. ACS Omega 2020, 5, 11210–11216. [Google Scholar] [CrossRef]
- Ferreira-Villadiego, J.; Garcia-Echeverri, J.; Vidal Mejia, M.V.; Pasqualino, J.; Meza-Catellar, P.; Lambis, H. Chemical Modification and Characterization of Starch Derived from Plantain (Musa Paradisiaca) Peel Waste, as a Source of Biodegradable Material. Chem. Eng. Trans. 2018, 65, 763–768. [Google Scholar] [CrossRef]
- Awolu, O.O.; Odoro, J.W.; Adeloye, J.B.; Lawal, O.M. Physicochemical Evaluation and Fourier Transform Infrared Spectroscopy Characterization of Quality Protein Maize Starch Subjected to Different Modifications. J. Food Sci. 2020, 85, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Palencia, M. Functionally-Enhanced Derivative Spectroscopy (FEDS): A Methodological Approach. J. Sci. Technol. Appl. 2020, 9, 29–34. [Google Scholar] [CrossRef]
- Palencia, M. Functional transformation of Fourier-transform mid-infrared spectrum for improving spectral specificity by simple algorithm based on wavelet-like functions. J. Adv. Res. 2018, 14, 53–62. [Google Scholar] [CrossRef]
- Restrepo, D.F.; Palencia, M.; Palencia, V.J. Study by Attenuated Total Reflectance Spectroscopy of Structural Changes of Humified Organic Matter by Chemical Perturbations via Alkaline Dissolution. J. Sci. Technol. Appl. 2018, 4, 49–59. [Google Scholar] [CrossRef]
- García-Quintero, A.; Combatt, E.M.; Palencia, M. Structural Study of Humin and Its Interaction with Humic Acids by Fourier-Transform Mid-Infrared Spectroscopy. J. Sci. Technol. Appl. 2018, 4, 28–39. [Google Scholar] [CrossRef]
- Lerma, T.A.; Garcés, V.; Palencia, M. Novel Multi- and Bio-Functional Hybrid Polymer Hydrogels Based on Bentonite-Poly(Acrylic Acid) Composites and Sorbitol Polyesters: Structural and Functional Characterization. Eur. Polym. J. 2020, 128, 109627. [Google Scholar] [CrossRef]
- Palencia, S.L.; García, A.; Palencia, M. Mid-Infrared Vibrational Spectrum Characterization of the Outer Surface of Candida Albicans by Functionally Enhanced Derivative Spectroscopy. J. Appl. Spectrosc. 2021, 88, 166–180. [Google Scholar] [CrossRef]
- Palencia, S.L.; García, A.; Palencia, M. Multiple Surface Interaction Mechanisms Direct the Anchoring, Co-Aggregation and Formation of Dual-Species Biofilm between Candida Albicans and Helicobacter Pylori. J. Adv. Res. 2021. [Google Scholar] [CrossRef]
- Anaya-Tatis, L.R.; Libreros, K.H.; Palencia, V.J.; Atencio, V.J.; Palencia, M. Mid-Infrared Spectral Characterization of Fish Scales: “Bocachico” (Prochilodus Magdalenae) by Functionally-Enhanced Derivative Spectroscopy (FEDS)—A Methodological Approach. J. Sci. Technol. Appl. 2019, 6, 28–39. [Google Scholar] [CrossRef]
- Palencia, M. Deconvolution of IR Spectra by Functionally-EnhancednDerivative Spectroscopy (FEDS): Why Does It Work? J. Sci. Technol. Appl. 2020, 9, 18–28. [Google Scholar] [CrossRef]
- Gao, X.; Liu, W.; Liu, H.; He, S.; Tang, M.; Zhu, C. Reaction Mechanism of Chitosan/Acrylamides Dimer: A DFT Study. J. Phys. Org. Chem. 2017, 31, e3775. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tomilin, F.N.; Pozdnyakova, A.A.; Vasilyeva, N. Yu.; Malyar, Y.N.; Kuznetsova, S.A.; Avramov, P.V. Theoretical DFT Interpretation of Infrared Spectra of Biologically Active Arabinogalactan Sulphated Derivatives. Chem. Pap. 2020, 74, 4103–4113. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushiko, N.I.; Zhbankov, R.G. Investigation of Stretching Vibrations of Glycosidic Linkages in Disaccharides and Polysaccarides with Use of IR Spectra Deconvolution. Biopolymers 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Mina, J.; Valadez-González, A.; Herrera-Franco, P.; Zuluaga, F.; Delvasto, S. Physicochemical characterization of natural and acetylated thermoplastic cassava starch. Dyna 2011, 78, 166–173. [Google Scholar]
- Lomeli, M.G.; Barrios-Guzman, A.J.; García, S.; Rivera-Prado, J.; Manríquez-González, R. Chemical and Mechanical Evaluation of Bio-composites Based on Thermoplastic Starch and Wood Particles Prepared by Thermal Compression. Bioresources 2014, 9, 2960–2974. [Google Scholar] [CrossRef]
- Nybacka, L. FTIR Spectroscopy of Glucose. Degree of Bachelor, Master Programme in Electrical Engineering, Uppsala Universitet, Uppsala, Sweden, 2016. [Google Scholar]
- Xie, F.; Zhang, B.; Wang, D.K. Starch Thermal Processing. In Starch-Based Materials in Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 187–227. [Google Scholar]
- Morley, M.J.; Miles, C.A. Modelling the thermal conductivity of starch-water gels. J. Food Eng. 1997, 33, 1–14. [Google Scholar] [CrossRef]
- Sopa, C.; Cumnueng, W.; Thavachai, T.; Juntanee, U.; Jatuphong, V. Effects of temperature and concentration on thermal properties of cassava starch solutions. Songklanakarin J. Sci. Technol. 2008, 30, 405–411. [Google Scholar]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR spectral collection of conservation materials in the extended region of 4000-80 cm–1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; de Leon, A. Analysis of the structure and vibrational spectra of glucose and fructose. Eclect. Química 2006, 31, 15–21. [Google Scholar] [CrossRef]


| Theoretical Wavenumber (cm−1) | Signal Assignation | ||
|---|---|---|---|
| 2Am | 2AmP | Total | (From Theoretical Data) |
| 3784 | 3784 | 6-C of 2Am-m1 | |
| 3757 | 3757 | 3757 | 6-C of 2AmP-m1 or 2AmP-m2 |
| 3739 | 3739 | 1-C of 2AmP-m2 | |
| 3730 | 3730 | 3-C of 2Am-m2 and 2AmP-m1 | |
| 3685 | 3685 | 2-C of 2Am-m2 | |
| 3658 | 3658 | 4-C of 2Am-m2 | |
| 3631 | 3631 | 3631 | 6-C 2Am-m2 and 2-C 2AmP-m1 |
| 3577 | 3577 | 2-C of 2Am-m2 | |
| v (cm−1) | v − k (cm−1) | v (cm−1) | Observations |
|---|---|---|---|
| by DFT | by FEDS | ||
| 3784 | 3600 | 3609 | 6-C of 2Am-m1 |
| 3757 | 3573 | 3590 | 1-C of Am-m2; 6-C or 3-C of 2AmP-m1 or 2AmP-m2 |
| 3739 | 3555 | 3561 | 1-C of 2AmP-m2 |
| 3730 | 3546 | 3545 | Signal splitting in 3-C of 2Am-m2 and 2AmP-m1 (no specific assignment) |
| 3539 | |||
| 3685 | 3501 | 3502 | 2-C of 2Am-m2 |
| 3658 | 3474 | 3482 | Signal splitting in both cases related to 4-C of 2Am-m2 (no specific assignment) |
| 3460 | |||
| 3631 | 3447 | 3443 | Signal splitting in 6-C of 2Am-m2 and 2-C of 2AmP-m1 (no specific assignment) |
| 3418 | |||
| 3577 | 3393 | 3392 | 2-C of 2Am-m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garces, V.; García-Quintero, A.; Lerma, T.A.; Palencia, M.; Combatt, E.M.; Arrieta, Á.A. Characterization of Cassava Starch and Its Structural Changes Resulting of Thermal Stress by Functionally-Enhanced Derivative Spectroscopy (FEDS). Polysaccharides 2021, 2, 866-877. https://doi.org/10.3390/polysaccharides2040052
Garces V, García-Quintero A, Lerma TA, Palencia M, Combatt EM, Arrieta ÁA. Characterization of Cassava Starch and Its Structural Changes Resulting of Thermal Stress by Functionally-Enhanced Derivative Spectroscopy (FEDS). Polysaccharides. 2021; 2(4):866-877. https://doi.org/10.3390/polysaccharides2040052
Chicago/Turabian StyleGarces, Viviana, Angélica García-Quintero, Tulio A. Lerma, Manuel Palencia, Enrique M. Combatt, and Álvaro A. Arrieta. 2021. "Characterization of Cassava Starch and Its Structural Changes Resulting of Thermal Stress by Functionally-Enhanced Derivative Spectroscopy (FEDS)" Polysaccharides 2, no. 4: 866-877. https://doi.org/10.3390/polysaccharides2040052
APA StyleGarces, V., García-Quintero, A., Lerma, T. A., Palencia, M., Combatt, E. M., & Arrieta, Á. A. (2021). Characterization of Cassava Starch and Its Structural Changes Resulting of Thermal Stress by Functionally-Enhanced Derivative Spectroscopy (FEDS). Polysaccharides, 2(4), 866-877. https://doi.org/10.3390/polysaccharides2040052









