Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems
Abstract
:1. Introduction
2. Polysaccharides
2.1. Chitosan
2.2. Hyaluronic Acid
2.3. Pectin
2.4. Guar Gum
2.5. Dextran
2.6. Alginate
2.7. Arabinoxylans
3. Polysaccharide Based-Nanoparticles
3.1. Physicochemical Characteristics of Polysaccharide-Based Nanoparticles
3.1.1. Size and Shape
3.1.2. Superficial Charge and Aggregation
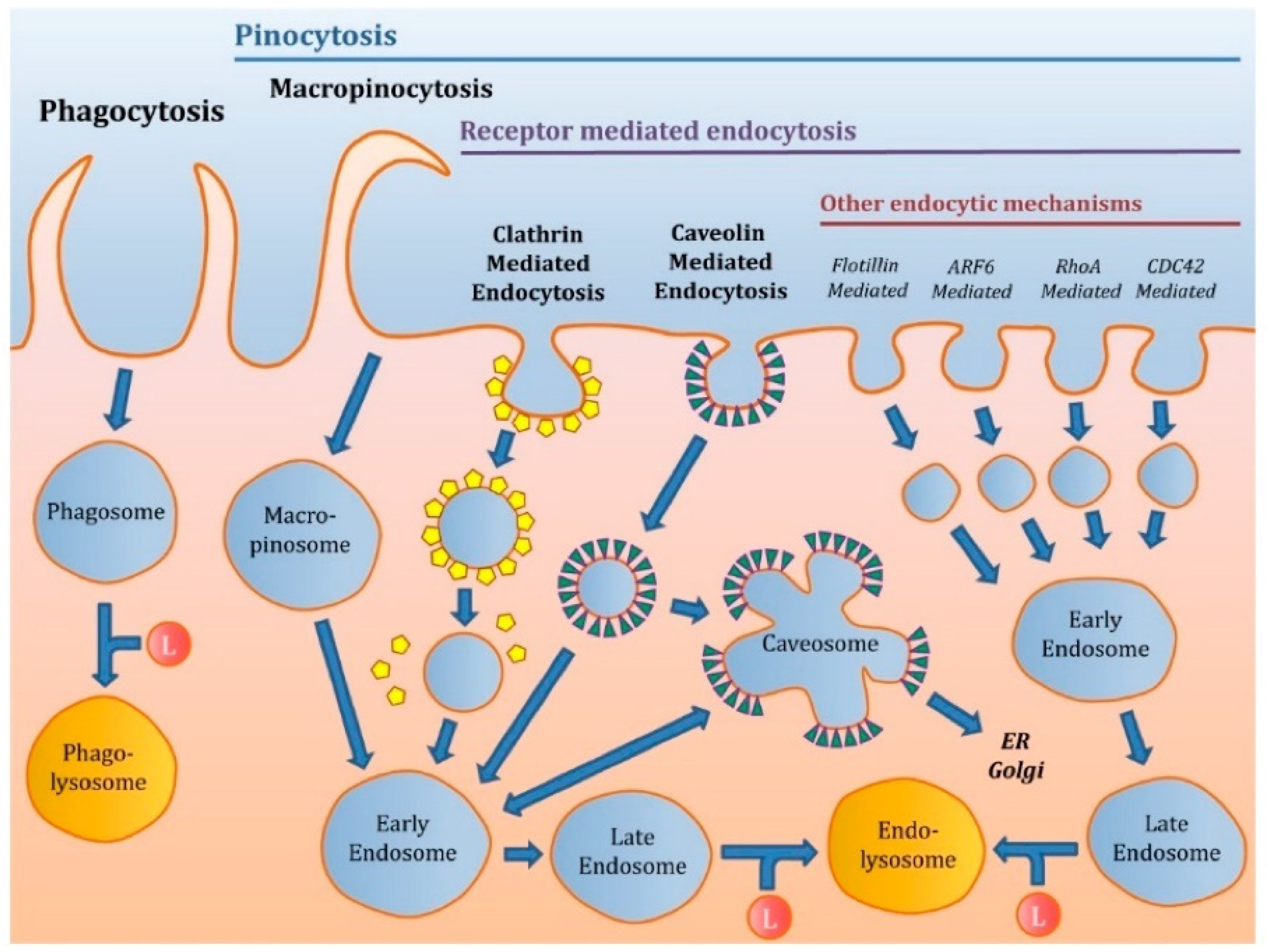
3.2. Cellular Uptake Mechanisms
3.3. Polysaccharide Based-Nanoparticles and their Impact on Cellular Uptake
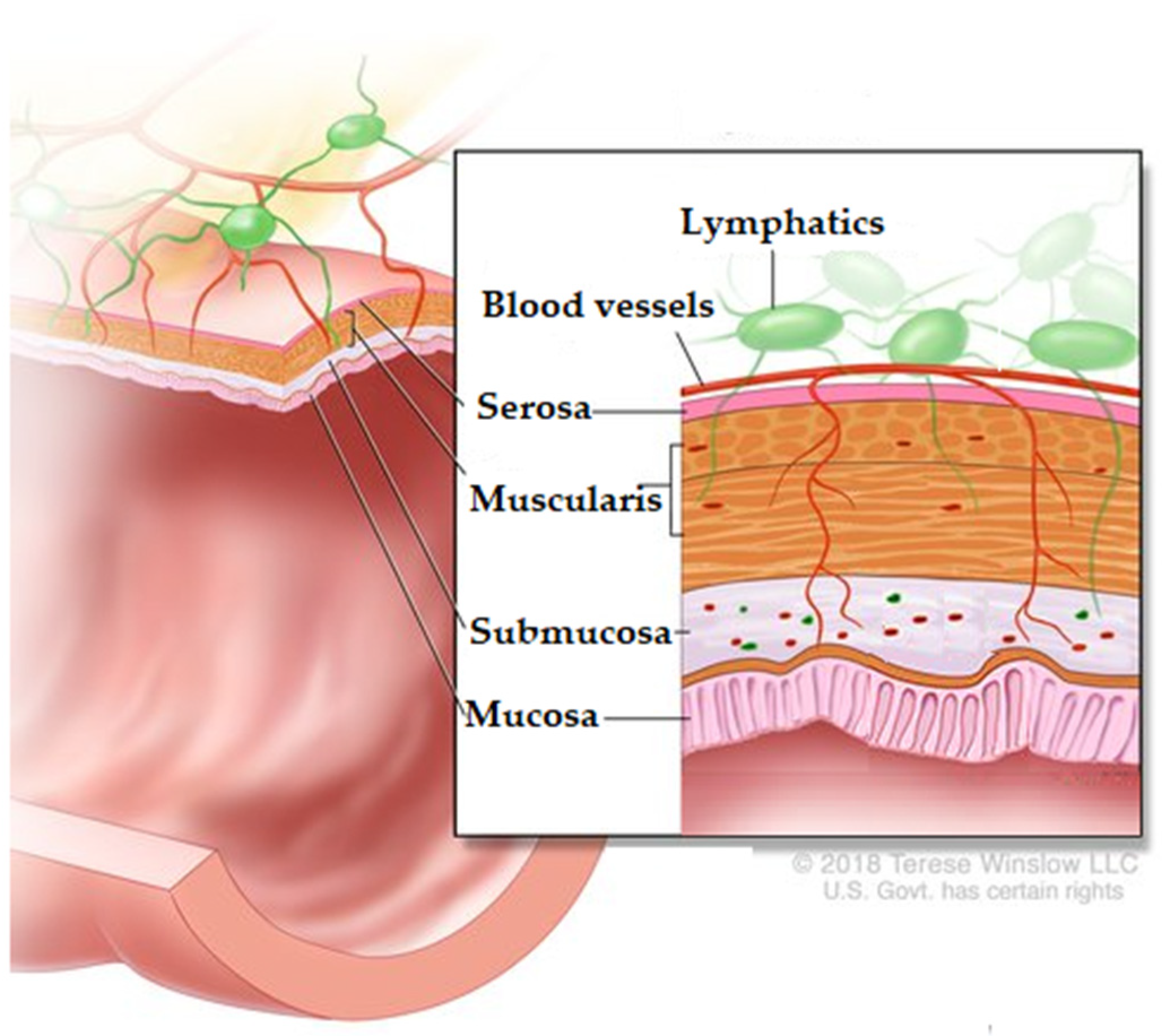
4. Colon
5. Colon-Targeted Drug Delivery
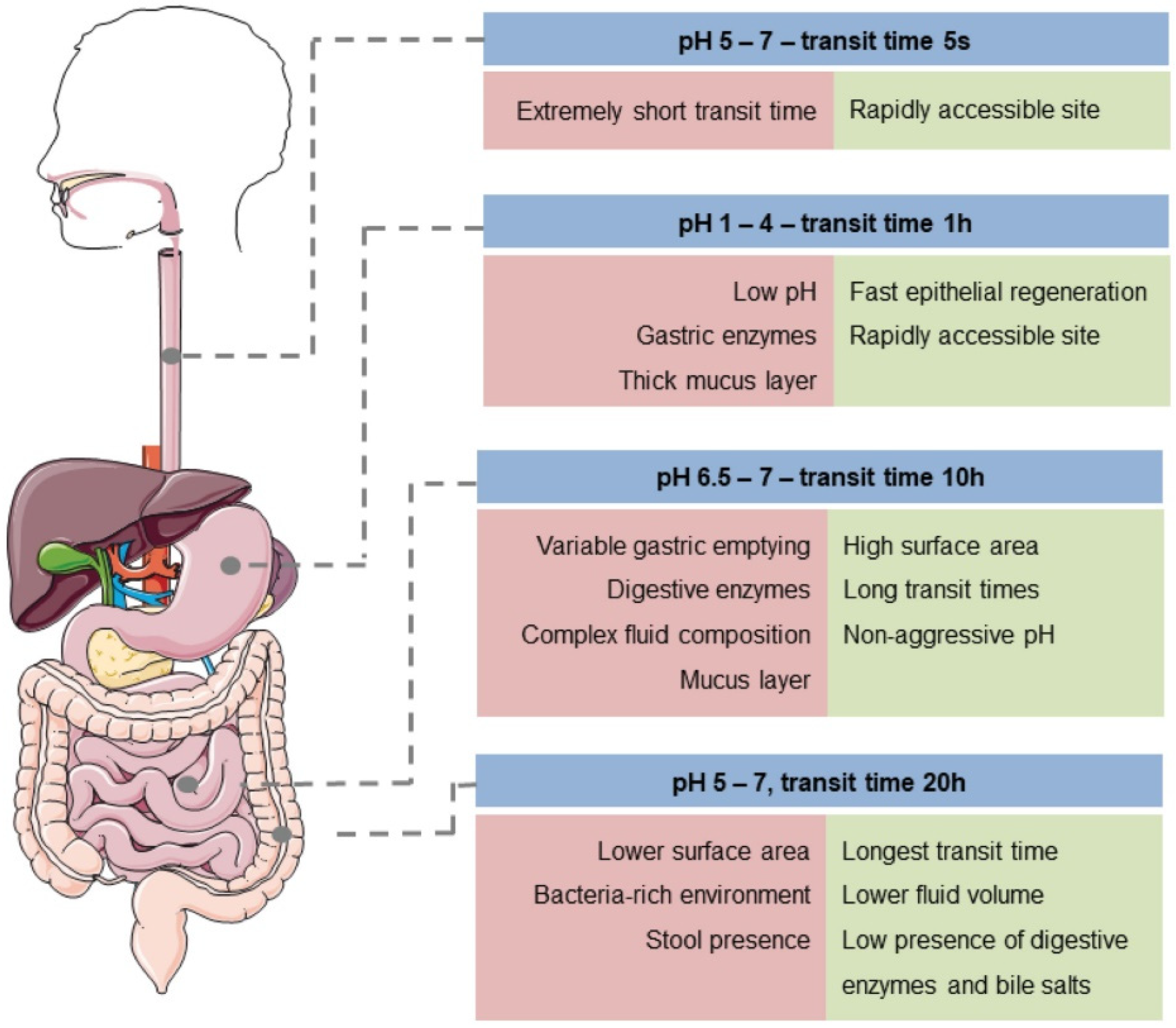
6. Factors That Influence Colon-Targeted Drug Delivery
6.1. Chemical Barrier
6.2. Enzymatic Barrier
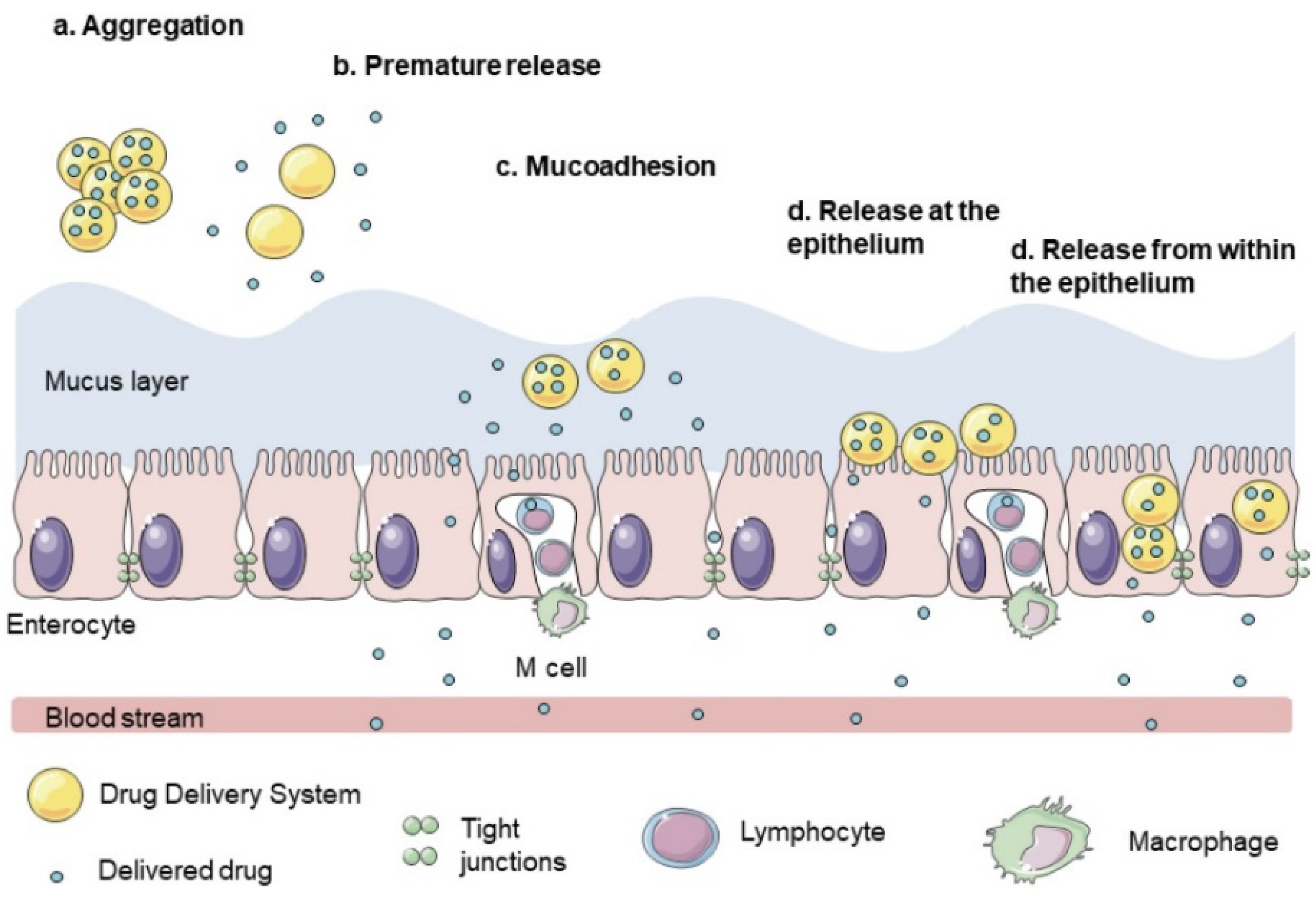
6.3. Mucus Barrier
6.4. Absorption Colon-Specific Drug Delivery
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Pawar, V.; Bavya, M.C.; Rohan, K.V.; Srivastava, R. Advances in Polysaccharide-Based Antimicrobial Delivery Vehicles. In Racing for the Surface; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 267–295. [Google Scholar]
- Krishnamurthy, R. Giving Rise to Life: Transition from Prebiotic Chemistry to Protobiology. Acc. Chem. Res. 2017, 50, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Datta, L.P.; Manchineella, S.; Govindaraju, T. Biomolecules-derived biomaterials. Biomaterials 2020, 230, 119633. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Reinholz, J.; Landfester, K.; Mailänder, V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F. Application of Biopolymers in Microencapsulation Processes. In Biopolymers for Food Design; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 191–222. [Google Scholar]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT 2017, 85, 37–44. [Google Scholar] [CrossRef]
- Prabhu, S.; Chenreddy, S.; Thio, A.; Khamas, W.; Wang, J.; Thakkar, A. Preclinical systemic toxicity evaluation of chitosan-solid–lipid nanoparticle-encapsulated aspirin and curcumin in combination with free sulforaphane in BALB/c mice. Int. J. Nanomed. 2016, 11, 3265–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.K.; Roy, P.; Mondal, M.K.; Roy, D.; Gayen, P.; Chowdhury, P.; Babu, S.P. Development of chitosan based gold nanomaterial as an efficient antifilarial agent: A mechanistic approach. Carbohydr. Polym. 2017, 157, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; Fangueiro, J.; Severino, P.; De Souza, A.L.R.; Martins-Gomes, C.; Fernandes, P.M.V.; Calpena, A.C.; Gremião, M.P.; Souto, E.B.; Silva, A.M. The Influence of Polysaccharide Coating on the Physicochemical Parameters and Cytotoxicity of Silica Nanoparticles for Hydrophilic Biomolecules Delivery. Nanomaterials 2019, 9, 1081. [Google Scholar] [CrossRef] [Green Version]
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascon-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Vazquez, N.A.R.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
- Carvalho, P.; Felício, M.R.; Santos, N.; Gonçalves, S.; Domingues, M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef]
- Tiwari, A.; Verma, A.; Panda, P.K.; Saraf, S.; Jain, A.; Jain, S.K. Stimuli-responsive polysaccharides for colon-targeted drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 1, pp. 547–566. [Google Scholar]
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Im, E.; Jung, Y.; Yoo, J.-W. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharmacal Res. 2020, 43, 153–169. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.M.; Meneguin, A.B.; Akhter, D.T.; Fletcher, N.; Houston, Z.H.; Bell, C.; Thurecht, K.J.; Gremião, M.P.D. Understanding the role of colon-specific microparticles based on retrograded starch/pectin in the delivery of chitosan nanoparticles along the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2021, 158, 371–378. [Google Scholar] [CrossRef]
- Wahlgren, M.; Axenstrand, M.; Håkansson, Å.; Marefati, A.; Pedersen, B.L. In Vitro Methods to Study Colon Release: State of the Art and An Outlook on New Strategies for Better In-Vitro Biorelevant Release Media. Pharmaceutics 2019, 11, 95. [Google Scholar] [CrossRef] [Green Version]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Borchmann, D.E.; Carberry, T.P.; Weck, M. “Bio”-Macromolecules: Polymer-Protein Conjugates as Emerging Scaffolds for Therapeutics. Macromol. Rapid Commun. 2013, 35, 27–43. [Google Scholar] [CrossRef]
- Ding, W.; Wu, Y. Sustainable dialdehyde polysaccharides as versatile building blocks for fabricating functional materials: An overview. Carbohydr. Polym. 2020, 248, 116801. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Ahmed, T.; Aljaeid, B. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Khotimchenko, M. Pectin polymers for colon-targeted antitumor drug delivery. Int. J. Biol. Macromol. 2020, 158, 1110–1124. [Google Scholar] [CrossRef]
- BeMiller, J.N. Guar, Locust Bean, Tara, and Cassia Gums. In Carbohydrate Chemistry for Food Scientists; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 241–252. [Google Scholar]
- Chen, F.; Huang, G.; Huang, H. Preparation and application of dextran and its derivatives as carriers. Int. J. Biol. Macromol. 2020, 145, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Da Silva, C.F.; Andrade, L.N.; Oliveira, D.D.L.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Rivera, D.E.V. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Oxidative Med. Cell. Longev. 2018, 2018, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-López, A.L.; Carvajal-Millan, E.; Sotelo-Cruz, N.; Micard, V.; Rascón-Chu, A.; López-Franco, Y.; Lizardi-Mendoza, J.; Canett-Romero, R. Enzymatically cross-linked arabinoxylan microspheres as oral insulin delivery system. Int. J. Biol. Macromol. 2019, 126, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Fan, Y.; Hu, Y.; Cheng, G.; Xu, F. Polysaccharide–Peptide Conjugates: A Versatile Material Platform for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005978. [Google Scholar] [CrossRef]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive polymers: Novel tool for drug delivery. Artif. Cells Nanomed. Biotechnol. 2013, 42, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Jenjob, R.; Phakkeeree, T.; Crespy, D. Saccharides, oligosaccharides, and polysaccharides nanoparticles for biomedical applications. J. Control. Release 2018, 284, 188–212. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano Struct. Nano Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Pereira, M.A.; Rebouças, J.D.S.; Ferraz-Carvalho, R.; de Redín, I.L.; Guerra, P.V.; Gamazo, C.; Brodskyn, C.I.; Irache, J.M.; Santos-Magalhaes, N. Poly(anhydride) nanoparticles containing cashew nut proteins can induce a strong Th1 and Treg immune response after oral administration. Eur. J. Pharm. Biopharm. 2018, 127, 51–60. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 33–93. [Google Scholar]
- Ramos, A.P.; Cruz, M.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Eliyahu, S.; Aharon, A.; Bianco-Peled, H. Acrylated Chitosan Nanoparticles with Enhanced Mucoadhesion. Polymers 2018, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Izadi, Z.; Divsalar, A.; Saboury, A.A.; Sawyer, L. β-lactoglobulin-pectin Nanoparticle-based Oral Drug Delivery System for Potential Treatment of Colon Cancer. Chem. Biol. Drug Des. 2016, 88, 209–216. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- De Anda-Flores, Y.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Martínez-López, A.L.; Marquez-Escalante, J.; Brown-Bojorquez, F.; Tanori-Cordova, J. Covalently Cross-Linked Nanoparticles Based on Ferulated Arabinoxylans Recovered from a Distiller’s Dried Grains Byproduct. Processes 2020, 8, 691. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Bianchera, A.; Bettini, R. Polysaccharide nanoparticles for oral controlled drug delivery: The role of drug–polymer and interpolymer interactions. Expert Opin. Drug Deliv. 2020, 17, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascón-Chu, A.; Díaz-Baca, J.A.; Carvajal-Millan, E.; Pérez-López, E.; Hotchkiss, A.T.; González-Ríos, H.; Balandrán-Quintana, R.; Campa-Mada, A.C. Electrosprayed Core–Shell Composite Microbeads Based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control. Polymers 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Dheer, D.; Arora, D.; Jaglan, S.; Rawal, R.; Shankar, R. Polysaccharides based nanomaterials for targeted anti-cancer drug delivery. J. Drug Target. 2016, 25, 1–16. [Google Scholar] [CrossRef]
- Aduba, J.D.C.; Yang, H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering 2017, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Martínez-López, A.L.; Carvajal-Millan, E.; Micard, V.; Rascón-Chu, A.; Brown-Bojorquez, F.; Sotelo-Cruz, N.; López-Franco, Y.L.; Lizardi-Mendoza, J. In vitro degradation of covalently cross-linked arabinoxylan hydrogels by bifidobacteria. Carbohydr. Polym. 2016, 144, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2019, 27, 19151–19168. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Qi, F.; Cheng, Y.; Lu, X.; Wang, L.; Zhao, J.; Zhao, B. Enhanced bone regeneration using an insulin-loaded nano-hydroxyapatite/collagen/PLGA composite scaffold. Int. J. Nanomed. 2017, 13, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Malhaire, H.; Gimel, J.-C.; Roger, E.; Benoît, J.-P.; Lagarce, F. How to design the surface of peptide-loaded nanoparticles for efficient oral bioavailability? Adv. Drug Deliv. Rev. 2016, 106, 320–336. [Google Scholar] [CrossRef]
- Beloqui, A.; Rieux, A.D.; Préat, V. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv. Drug Deliv. Rev. 2016, 106, 242–255. [Google Scholar] [CrossRef]
- Tosi, G.; Duskey, J.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2019, 17, 23–32. [Google Scholar] [CrossRef]
- Health Canada. 2011 Policy Statement on Health Canada’s Working Definition for Nanomaterial—Canada.ca. Available online: https://www.canada.ca/en/health-canada/services/science-research/reports-publications/nanomaterial/policy-statement-health-canada-working-definition.html (accessed on 1 May 2021).
- ISO 8968-1:2014. Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. Available online: https://www.iso.org/obp/ui/#iso:std:iso:8968:-1:ed-2:v1:en (accessed on 1 May 2021).
- SCENIHR Communication on the Second Regulatory Review on Nanomaterials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52012DC0572&from=EN (accessed on 1 May 2021).
- SCENIHR Opinion on the Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_045.pdf (accessed on 1 May 2021).
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [Green Version]
- de Crozals, G.; Bonnet, R.; Farre, C.; Chaix, C. Nanoparticles with multiple properties for biomedical applications: A strategic guide. Nano Today 2016, 11, 435–463. [Google Scholar] [CrossRef]
- Fernández, E.F.; Santos-Carballal, B.; De Santi, C.; Ramsey, J.M.; MacLoughlin, R.; Cryan, S.-A.; Greene, C.M. Biopolymer-Based Nanoparticles for Cystic Fibrosis Lung Gene Therapy Studies. Materials 2018, 11, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.M.; Esfanjani, A.F. Instrumental analysis and characterization of nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 524–544. [Google Scholar]
- Griffin, B.; Guo, J.; Presas, E.; Donovan, M.; Alonso, M.J.; O’Driscoll, C.M. Pharmacokinetic, pharmacodynamic and biodistribution following oral administration of nanocarriers containing peptide and protein drugs. Adv. Drug Deliv. Rev. 2016, 106, 367–380. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinelli, F.; Ortolà; Óscar, F.; Makvandi, P.; Perale, G.; Rossi, F. In vivo drug delivery applications of nanogels: A review. Nanomedicine 2020, 15, 2707–2727. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.-J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.-X.; Zheng, C.L. Intracellular disposition of chitosan nanoparticles in macrophages: Intracellular uptake, exocytosis, and intercellular transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Liu, C.-G.; Yu, Y. Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydr. Polym. 2015, 124, 274–279. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Ma, L.; Zu, M.; Song, H.; Xiao, B. Oral administration of chondroitin sulfate-functionalized nanoparticles for colonic macrophage-targeted drug delivery. Carbohydr. Polym. 2019, 223, 115126. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, J.W.; Saunders, M.; Lim, L.-Y. Cytotoxicity of monodispersed chitosan nanoparticles against the Caco-2 cells. Toxicol. Appl. Pharmacol. 2012, 262, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.V.; Yoshida, C.M.P.; Pereira, S.M.S.S.; Goycoolea, F.M.; Franco, T.T. Electrostatic Self-Assembled Chitosan-Pectin Nano- and Microparticles for Insulin Delivery. Molecules 2017, 22, 1707. [Google Scholar] [CrossRef]
- Noi, I.; Schlachet, I.; Kumarasamy, M.; Sosnik, A. Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers 2018, 10, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharucha, A.E.; Camilleri, M. Physiology of the Colon and Its Measurement. In Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 1676–1688. [Google Scholar]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent advances in colon drug delivery systems. J. Control Release 2020, 327, 703–724. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Valencia-Rivera, D.E.; Carvajal-Millan, E.; Astiazaran-Garcia, H.; Micard, V.; Rascón-Chu, A. Fermentation of Ferulated Arabinoxylan Recovered from the Maize Bioethanol Industry. Processes 2021, 9, 165. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- Winslow, T. Colon or Rectal Cancer Stage 0. Available online: https://visualsonline.cancer.gov/details.cfm?imageid=9147 (accessed on 7 May 2021).
- Song, Q.; Jia, J.; Niu, X.; Zheng, C.; Zhao, H.; Sun, L.; Zhang, H.; Wang, L.; Zhang, Z.; Zhang, Y. An oral drug delivery system with programmed drug release and imaging properties for orthotopic colon cancer therapy. Nanoscale 2019, 11, 15958–15970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.; Zhao, A. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.-W.; Park, B.J.; Han, H.-K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tam, M.; Samaei, S.; Lerouge, S.; Barralet, J.; Stevenson, M.M.; Cerruti, M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017, 48, 247–257. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Rajput, R.; Narkhede, J.; Naik, J.B. Nanogels as nanocarriers for drug delivery: A review. ADMET DMPK 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghomi, M.; Ashrafizadeh, M.; Tafazoli, A.; Agarwal, T.; Delfi, M.; Akhtari, J.; Zare, E.N.; Padil, V.V.; Zarrabi, A.; et al. A review on advances in graphene-derivative/polysaccharide bionanocomposites: Therapeutics, pharmacogenomics and toxicity. Carbohydr. Polym. 2020, 250, 116952. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020, 32, e1901935. [Google Scholar] [CrossRef]
- Wu, J.-L.; Tian, G.-X.; Yu, W.-J.; Jia, G.-T.; Sun, T.-Y.; Gao, Z.-Q. pH-Responsive Hyaluronic Acid-Based Mixed Micelles for the Hepatoma-Targeting Delivery of Doxorubicin. Int. J. Mol. Sci. 2016, 17, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilipenko, I.; Korzhikov-Vlakh, V.; Sharoyko, V.; Zhang, N.; Schäfer-Korting, M.; Rühl, E.; Zoschke, C.; Tennikova, T. pH-Sensitive Chitosan–Heparin Nanoparticles for Effective Delivery of Genetic Drugs into Epithelial Cells. Pharmaceutics 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, A.I.; Lima, S.; Reis, S. Application of pH-Responsive Fucoidan/Chitosan Nanoparticles to Improve Oral Quercetin Delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, A.; Ziora, Z.; Tamer, T.; Khalifa, R.; Hassan, M.; Mohy-Eldin, M.; Blaskovich, M. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef]
- Elgegren, M.; Kim, S.; Cordova, D.; Silva, C.; Noro, J.; Cavaco-Paulo, A.; Nakamatsu, J. Ultrasound-Assisted Encapsulation of Sacha Inchi (Plukenetia volubilis Linneo.) Oil in Alginate-Chitosan Nanoparticles. Polymers 2019, 11, 1245. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; Lucas, R.; Benito, E.; De-Paz, M.-V. Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release. Int. J. Mol. Sci. 2019, 20, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrarathna, H.; Liyanage, T.; Edirisinghe, S.; Dananjaya, S.; Thulshan, E.; Nikapitiya, C.; Oh, C.; Kang, D.-H.; De Zoysa, M. Marine Microalgae, Spirulina maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef] [Green Version]
- Dyawanapelly, S.; Koli, U.; Dharamdasani, V.; Jain, R.; Dandekar, P. Improved mucoadhesion and cell uptake of chitosan and chitosan oligosaccharide surface-modified polymer nanoparticles for mucosal delivery of proteins. Drug Deliv. Transl. Res. 2016, 6, 365–379. [Google Scholar] [CrossRef]
- Sabra, R.; Roberts, C.J.; Billa, N. Courier properties of modified citrus pectinate-chitosan nanoparticles in colon delivery of curcumin. Colloid Interface Sci. Commun. 2019, 32, 100192. [Google Scholar] [CrossRef]
- Prabahar, K.; Udhumansha, U.; Qushawy, M. Optimization of Thiolated Chitosan Nanoparticles for the Enhancement of in Vivo Hypoglycemic Efficacy of Sitagliptin in Streptozotocin-Induced Diabetic Rats. Pharmaceutics 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhakar, S.; Chandran, S.V.; Selvamurugan, N.; Nazeer, R.A. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Biol. Macromol. 2020, 150, 281–288. [Google Scholar] [CrossRef]
- Shailender, J.; Ravi, P.R.; Sirukuri, M.R.; Dalvi, A.; Priya, O.K. Chitosan nanoparticles for the oral delivery of tenofovir disoproxil fumarate: Formulation optimization, characterization and ex vivo and in vivo evaluation for uptake mechanism in rats. Drug Dev. Ind. Pharm. 2018, 44, 1109–1119. [Google Scholar] [CrossRef]
- He, Z.; Santos, J.L.; Tian, H.; Huang, H.; Hu, Y.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.-Q. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials 2017, 130, 28–41. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Kenechukwu, F.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Hirpara, M.R.; Manikkath, J.; Sivakumar, K.; Managuli, R.S.; Gourishetti, K.; Krishnadas, N.; Shenoy, R.; Jayaprakash, B.; Rao, C.M.; Mutalik, S. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: Development and in vitro and in vivo evaluations. Int. J. Biol. Macromol. 2018, 107, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Singh, S.; Renukuntla, J.; Govender, T. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surfaces B Biointerfaces 2017, 158, 650–657. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Chakraborty, M.; Bhattacharya, S.; Mishra, R.; Kundu, P. Oral insulin delivery by self-assembled chitosan nanoparticles: In vitro and in vivo studies in diabetic animal model. Mater. Sci. Eng. C 2013, 33, 376–382. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Van Can, M.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909–915. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Tan, C.P.; Basri, M.; Saari, N. Improved In Vivo Efficacy of Anti-Hypertensive Biopeptides Encapsulated in Chitosan Nanoparticles Fabricated by Ionotropic Gelation on Spontaneously Hypertensive Rats. Nanomaterials 2017, 7, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; He, Z.; Sun, C.; Yang, C.; Zhao, P.; Liu, L.; Leong, K.W.; Mao, H.-Q.; Liu, Z.; Chen, Y. Uniform Core-Shell Nanoparticles with Thiolated Hyaluronic Acid Coating to Enhance Oral Delivery of Insulin. Adv. Health Mater. 2018, 7, e1800285. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Sun, X.; Bai, J.; Dong, J.; Zhang, B.; Gao, Z.; Wu, J. Doxorubicin-loaded dual-functional hyaluronic acid nanoparticles: Preparation, characterization and antitumor efficacy in vitro and in vivo. Mol. Med. Rep. 2018, 19, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.; Zhou, X.; Li, X.; Lin, W.; Chen, G.; Qiu, R. Self-Assembled Modified Soy Protein/Dextran Nanogel Induced by Ultrasonication as a Delivery Vehicle for Riboflavin. Molecules 2016, 21, 282. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- McGuckin, M.; Thornton, D.; Whitsett, J.A. Mucins and Mucus. In Mucosal Immunology; Elsevier BV: Amsterdam, The Netherlands, 2015. [Google Scholar]
- García-Díaz, M.; Birch, D.; Wan, F.; Nielsen, H.M. The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv. Drug Deliv. Rev. 2018, 124, 107–124. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, K.-J.; Tseng, M.T.; Wey, S.-P.; Su, F.-Y.; Chuang, E.-Y.; Hsu, C.-W.; Chen, C.-T.; Sung, H.-W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Mukherjee, D.; Mishra, R.; Kundu, P.P. Development of pH sensitive polyurethane–alginate nanoparticles for safe and efficient oral insulin delivery in animal models. RSC Adv. 2016, 6, 41835–41846. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Barbari, G.R.; Dorkoosh, F.A.; Amini, M.; Sharifzadeh, M.; Atyabi, F.; Balalaie, S.; Tehrani, N.R.; Tehrani, M.R. A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides. Int. J. Nanomed. 2017, 12, 3471–3483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef]
- Lopes, M.; Shrestha, N.; Correia, A.; Shahbazi, M.-A.; Sarmento, B.; Hirvonen, J.T.; Veiga, F.; Seiça, R.; Ribeiro, A.; Santos, H.A. Dual chitosan/albumin-coated alginate/dextran sulfate nanoparticles for enhanced oral delivery of insulin. J. Control. Release 2016, 232, 29–41. [Google Scholar] [CrossRef] [PubMed]

| Natural Polymers | Types | Examples |
|---|---|---|
| Hydrocarbon polymers | Natural rubber | |
| Carbon-oxygen | Carbohydrates | Cellulose; starch; chitin; chitosan; pullulan |
| Carbon-oxygen-nitrogen/Sulphur | Proteins | Soya protein; gelatin; casein |
| Carbon-oxygen-nitrogen-phosphorus | Nucleic acids | DNA, RNA |
| Polysaccharide | Chain Characteristics | Properties | Crosslinker | Degradation Mechanism | Comments | Reference |
|---|---|---|---|---|---|---|
| Chitosan | β-(1-4)-linked N-acetyl d-glucosamine | -Positive Z potential -pKa 6.5 -Protonated at acidic and neutral pH -Mucoadhesive -Cationic | Calcium TPP | Enzymatic | Degradation by enzymes (β-glucosidase) | [48] |
| Alginate | β-(1-4)-d-mannuronic acid and α-(1-4) l-guluronic acid residues | -Water-soluble -Anionic -Hydrophobicity | Divalent cations Ca2+, Cu2+, Zn2+ or Mn2+ | pH-responsive | Degradation by enzymes (glucuronidases etc.) | [33,49] |
| Pectin | (1-4)-linked α-d-galacturonic acid residues | -Water-soluble -High methoxy -Low methoxy | Ca2+ ions Lacasse | Enzymatic | Degradation by Bacteroides species | [50,51] |
| Guar Gum | (1-4)- β-d-mannopyranose units with α-d-galactopyranosyl units attached by (1-6) linkages | -Water-soluble | Epichlorohydrin | pH-responsive and enzymatic | Degradation by bacteria (Bacteroides, ruminococci, bifidobacteria) | [19,31] |
| Dextran | α-(1-6) glycosidic links and branched at α-(1-3) position | -Mucoadhesive -Hydrophilic | Diamine | Enzymatic | Degradation by esterases and endodextranases | [52] |
| Hyaluronic Acid | D-glucuronic acid and N-acetyl-d-glucosamine linked by β-(1-3) and β-(1-4) bond | -Anionic -Water-absorption -Water soluble | pH temperature ionic | pH-responsive and enzymatic | Degradation by hyaluronidases | [53] |
| Arabinoxylan | Xylose β-1-4 linkages and α-l-arabinose substitutions (α-1-3 and α-1-2) | -Highly ferulated -Water-soluble -Neutral | Laccase Peroxidase | Enzymatic | Degradation by bacteria (Bifidobacterium and Bacteroides) | [34,54] |
| Polysaccharide | Drug | Barrier | Size (nm) | Mechanism | Study | Fabrication Technique | Reference |
|---|---|---|---|---|---|---|---|
| HA–GA/HA–His | Doxorubicin | Chemical | 147.5–607.6 | pH responsive | In vitro | Ultrasonic dispersion | [104] |
| Chitosan/Pectin | Insulin | Chemical | 240–420 | pH responsive | In vitro | Electrostatic self-assembly | [84] |
| Chitosan-Heparin | Oligonucleotides | Chemical | 145 | pH responsive | In vitro | Spontaneous polyelectrolyte complexation | [105] |
| Fucoidan/Chitosan | Quercetin | Chemical | 300–400 | pH responsive | In vitro | Polyelectrolyte self-assembly | [106] |
| Q-AmCs | Curcumin | Chemical | 162 | pH responsive | In vitro | Ionic gelation | [107] |
| Alginate-Chitosan | BSA | Enzymatic | 320–340 | Enzymatic | In vitro | Nano-emulsion | [108] |
| Chitosan | Resveratrol | Enzymatic | 115 | Enzymatic | In vitro | Synthesized block-copolymer | [109] |
| Modified Pectin | Enzymatic | 64.11 | Enzymatic | In vitro | Sonication | [110] | |
| COS-PLGA | BSA | Mucoadhesive | 170.7 | Muchoadhesiveness | Mucoadhesive strength | Double emulsion solvent evaporation by homogenization | [111] |
| ACS | Mucoadhesive | 356 | Muchoadhesiveness | Mucoadhesive strength | Ionic gelation | [44] | |
| Pectinate-Chitosan | Curcumin | Mucoadhesive | 218.1 | Muchoadhesiveness | Mucoadhesive strength | Ionic gelation | [112] |
| Thiolated Chitosan | Sitagliptin | Mucoadhesive | 160.3 | Muchoadhesiveness | Mucoadhesive strength | Ionic gelation | [113] |
| Chitosan | Insulin | Enzymatic | 220 | Enzymatic | In vivo | Ionic gelation | [114] |
| Chitosan | TDF | Enzymatic | 156 | Enzymatic | In vivo | Ionic gelation | [115] |
| Chitosan | Insulin | Chemical | 45 and 115 | pH-responsive | In vivo | Ionic gelation | [116] |
| Chitosan+Mucin | Insulin | Mucoadhesive | 504.1 | Muchoadhesiveness | In vivo | Self-gelation | [117] |
| Chitosan/Alginate | Naringenin | Chemical | 150–300 | pH responsive | In vivo | Ionic gelation | [118] |
| Chitosan | IFNα | Chemical | 36 | Epithelium | In vivo | Ionic gelation | [119] |
| PEGylated Chitosan | Rosuvastatin | Chemical | <200 | Epithelium | In vivo | Mediated reaction | [120] |
| Chitosan | Vancomycin | Chemical | 220 | pH-responsive | In vivo | Ionic gelation | [121] |
| Succinyl Chitosan/Alginate | Quercetin | Chemical | 90 | pH-responsive | In vivo | Ionic crosslinking | [122] |
| Chitosan/Alginate | Lovastatin | Chemical | 50–100 | pH-responsive | In vivo | Ionic gelation | [123] |
| Chitosan-AntBiop | Enzymatic | 162.7 | Enzymatic | In vivo | Ionotropic gelation | [124] | |
| Thiolated Hyaluronic Acid | Insulin | Mucoadhesive | 75 | Muchoadhesiveness | In vivo | Ring-opening reaction | [125] |
| Hyaluronic Acid | DOX | Chemical | 238.1 to 156.7 | pH-responsive | In vivo | Two-step reaction | [126] |
| Chitosan-modified | Curcumin | Chemical | 281 | Epithelium | In vivo | Ionic gelation | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Anda-Flores, Y.; Carvajal-Millan, E.; Campa-Mada, A.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Tanori-Cordova, J.; Martínez-López, A.L. Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides 2021, 2, 626-647. https://doi.org/10.3390/polysaccharides2030038
De Anda-Flores Y, Carvajal-Millan E, Campa-Mada A, Lizardi-Mendoza J, Rascon-Chu A, Tanori-Cordova J, Martínez-López AL. Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides. 2021; 2(3):626-647. https://doi.org/10.3390/polysaccharides2030038
Chicago/Turabian StyleDe Anda-Flores, Yubia, Elizabeth Carvajal-Millan, Alma Campa-Mada, Jaime Lizardi-Mendoza, Agustin Rascon-Chu, Judith Tanori-Cordova, and Ana Luisa Martínez-López. 2021. "Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems" Polysaccharides 2, no. 3: 626-647. https://doi.org/10.3390/polysaccharides2030038
APA StyleDe Anda-Flores, Y., Carvajal-Millan, E., Campa-Mada, A., Lizardi-Mendoza, J., Rascon-Chu, A., Tanori-Cordova, J., & Martínez-López, A. L. (2021). Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides, 2(3), 626-647. https://doi.org/10.3390/polysaccharides2030038








