Abstract
Polysaccharide biomaterials have gained significant importance in the manufacture of nanoparticles used in colon-targeted drug delivery systems. These systems are a form of non-invasive oral therapy used in the treatment of various diseases. To achieve successful colonic delivery, the chemical, enzymatic and mucoadhesive barriers within the gastrointestinal (GI) tract must be analyzed. This will allow for the nanomaterials to cross these barriers and reach the colon. This review provides information on the development of nanoparticles made from various polysaccharides, which can overcome multiple barriers along the GI tract and affect encapsulation efficiency, drug protection, and release mechanisms upon arrival in the colon. Also, there is information disclosed about the size of the nanoparticles that are usually involved in the mechanisms of diffusion through the barriers in the GI tract, which may influence early drug degradation and release in the digestive tract.
1. Introduction
Polysaccharides are biopolymers constituted by simple sugar monomers. They are usually isolated from plant material, marine plants, and exogenous metabolites of some bacteria. The polysaccharides chain comprises monosaccharide units linked by glycosidic bonds with hydroxyl, carboxyl, and amino groups. Carbohydrates are stable, non-toxic, hydrophilic, and biodegradable biomolecules in the human body [1,2]. Polysaccharides can be used as matrices for encapsulation, immobilization, and controlled release for many active compounds. This matrix are also used in the food, pharmaceutical, biomedical, and chemical industries. Polysaccharides are the most abundant biomolecules, followed by proteins, lipids, and nucleic acids. Polysaccharides studied as a matrix for encapsulation include chitosan, pectin, alginate, starch, and dextran [3,4].
Encapsulation occurs when liquid and solid particles are trapped in a matrix or coated by a polymer material. The preparation of a proper encapsulating agent is an essential factor in enhancing encapsulation efficiency. Encapsulating agents must have the ability to preserve bioactive compounds under different processes and storage conditions [5]. A controlled release system will increase drug retention time and enhance paracellular and transcellular absorption. The release mechanisms occur by diffusion, biodegradation, and osmosis [6,7]. The application of nanotechnology to encapsulate nutrients and drugs has aroused significant interest and demand due to its great benefits. In addition, nanotechnology has been associated with safety, environmental, ethical, and regulatory problems related to human health and environmental impacts. Information related to the security of nanoparticles is limited. In general, nanoparticles have unique characteristics, such as their small size. This property allows them to cross different biological barriers (e.g., intestinal and mucosal epithelial cells) [8,9].
Natural biopolymers are considered biodegradable and safe for human consumption because they are not toxic and do not affect cell viability, making them ideal for drug delivery systems [10,11]. Other kinds of metallic, solid, and polymeric nanoparticles can be toxic for humans. Researchers have used strategies of coating toxic nanoparticles with biopolymers to reduce their toxicity [12,13]. At the moment, no legislation regulates the use of nanoparticles in the food and pharmaceutical industries. Therefore, most countries have regulations for risk assessment when using nanotechnology. Relevant regulatory agencies include the U.S. Food and Drug Administration (FDA), the European Union, the Australian Government Department of Health, and Health Canada [14].
It is necessary to develop and appropriately evaluate nanomaterials to determine their risk to human health. When manufacturing these nanoparticles, the elements that must be considered are particle and distribution size, shape, state of aggregation/disaggregation, solubility, surface charge, and surface morphology [15,16]. The size and morphology of nanoparticles enhance their functionality, and are dependent on the manufacturing technique for each nanomaterial. These properties allow a high loading capacity, high encapsulation efficiency, stability, sustained release profile, bioavailability of bioactive compounds, and biocompatibility [17,18].
The nanoparticle technology based on polysaccharides plays a vital role in controlling drugs, bioactive agents, and genes for oral administrations. These release systems can occur by diffusion (barrier/matrix), degradation (chemical or physical matrix), or changes in the environment (e.g., pH, ionic strength, and pressure) [19]. Most drugs or bioactive agents administered orally are absorbed in the upper GI tract, but their delivery in the colon is necessary for additional results in specific therapies. Some areas of interest are colonic delivery of peptides and proteins, probiotic bacteria, and microbiota replacement therapies [20,21]. The main release mechanisms in the colon are degradation by colonic microbiota, time and pH-controlled release. Colon release formulations are generally designed to prevent degradation in the stomach and upper GI tract. The colon’s main function is the absorption of water, ions, and the storage of feces. The large intestine is colonized by many bacteria—approximately 1012 per gram of intestinal content. The microorganisms in the colon are beneficial for human health because they are responsible for fermenting indigestible dietary fiber [22].
This review focuses on polysaccharide-based nanoparticles used for oral administration in colon-directed therapies, such as drug administration, peptides, and proteins. This contribution aims to present important information from the literature that shows various polysaccharide-based nanomaterials. Therefore, these nanomaterials can reach the colon through physiological barriers due to their morphological characteristics, such as size, shape, and surface charge.
2. Polysaccharides
Various polymers with complex structures and specific functions have developed naturally. These include amino acids, nucleobases, and mono- and disaccharides. According to the nature of their heteroatom present in the main chain natural polymers can be classified into four types, as shown in Table 1 [23,24]. Polysaccharides are biopolymers isolated from plant, animal, microbial, and algae sources. These are made up of more than ten monosaccharide units linked by O-glycosidic bonds. Due to their great abundance in hydroxyl groups polysaccharides can be modified by carboxylation, esterification, and amination, thereby improving their functional properties [25]. According to their nature, polysaccharides have excellent biocompatibility, biodegradability, non-toxicity, and cell specificity. Therefore, they are considered ideal for various biomedical applications, such as drug, gene delivery, and wound dressing [26,27].

Table 1.
Natural polymers and their types.
2.1. Chitosan
Chitosan is a unique linear poly-cationic polysaccharide derived from chitin by deacetylation. It is found primarily in the exoskeleton of crustaceans, such as shrimp and crabs. Its chemical structure consists of a chain of β-(1-4)-linked glucosamine and N-acetyl d-glucosamine units. It has functional groups such as polyhydroxy and amino, and these groups can lead to hydrogen bonds. Chitosan is the second most abundant polysaccharide after cellulose. It is used for pharmaceutical applications due to its reactive functional groups, biocompatibility, biodegradability, gel-forming ability, non-toxicity, high charge density, and low pH solubility. This polysaccharide can interact electrostatically with mucus or negatively charged mucosal surfaces, giving it an excellent mucoadhesive property for developing systems for oral administration. The presence of its positively charged amino group (–NH3+) can interact with negatively charged proteoglycans on the cell surface, enabling better intestinal absorption of the drug by opening the tight junctions between the epithelial cells [28].
2.2. Hyaluronic Acid
Hyaluronic acid is a non-sulfated, negatively charged glycosaminoglycan composed of D-glucuronic acid and N-acetyl-d-glucosamine linked by β-(1-3) and β-(1-4) bonds. It is a macromolecule produced and secreted by cells as a linear polymer that is not bound to a polypeptide. It is a biocompatible biopolymer of natural origin present in the skin, connective tissues, synovial fluid of the joints, neural tissues, vitreous humor, and has the ability to regulate lubrication. This polysaccharide can be chemically modified due to its groups, such as the carboxylic acid of glucuronic acid, primary and secondary hydroxyl groups, and N-acetyl groups, which can alter its properties such as hydrophobicity, biological activity, viscoelasticity, water retention, biocompatibility, cell proliferation, wound regeneration, and specific signal transduction and cell interactions through cell surface receptors [29].
2.3. Pectin
Pectin is a linear heteropolysaccharide that constitutes the cell wall of plants and consists of a linear unbranched chain of α-(1-4) linked d-galacturonic acid units (homogalacturonan) with uronic acids. This polysaccharide contains hydroxyl and carboxyl groups, residues esterified with methyl ether distributed in its linear chain, and a certain quantity of neutral sugars present in the side chains. It is principally composed of galacturonic acid, methyl ester, and sugar units such as arabinose, galactose, and rhamnose. Pectin can form hydrogels in the presence of its ionized carboxyl groups (–COO−) that interact with its positively charged anions. For this reason, it is an anionic, biodegradable, biocompatible, and non-toxic polysaccharide used for its mucoadhesive properties in the oral delivery of drugs to the colon. Pectin can remain intact in the upper gastrointestinal tract and be degraded in the colon by pectinases [30].
2.4. Guar Gum
Guar gum is a non-ionic polysaccharide. It is extracted from the seeds of Cyamopsis tetragonolobus and consists of a linear chain of (1-4)-β-d-mannopyranosil units with α-d-galactopyranosyl units attached by (1–6) ramifications. This polysaccharide presents an extraordinarily viscous property due to its intermolecular chain entanglement of galactose side chains. It is a biocompatible and biodegradable polysaccharide with the ability to form gels in aqueous solutions, and is used to formulate hydrophilic matrices for drug delivery due to its enzymatic degradation in the colon [31].
2.5. Dextran
Dextran is a complex branched glucan consisting of α-d-(1-6) glycosidic links and branched at α-(1-3). It is obtained naturally from the lactic acid bacterium. It is a hydrophilic, biodegradable, and biocompatible polysaccharide with abundant hydroxyl groups. This polysaccharide is biocompatible, highly hydrophilic, and shows low protein adsorption. Like other polysaccharides, dextran has many hydroxyl groups, allowing it to be easily conjugated with drugs and proteins to prevent drug absorption in the small intestine [32].
2.6. Alginate
Alginate is an anionic polysaccharide consisting of β-(1-4)-d-mannuronic acid and α-(1-4) l-guluronic acid residues. The α-(1-4) l-guluronic bonds can be cross-linked with divalent ions by sodium ion exchange, forming a gel matrix and the retention of encapsulated charges. Due to their anionic nature, they can interact with cationic compounds. It is extracted mainly from brown marine algae (Laminaria hyperborean) and soil bacteria (Azobacter vinelandii). This polysaccharide has physicochemical properties such as biodegradability, biocompatibility, low immunogenicity, good mucoadhesion, and non-toxicity. Due to its composition, sequence of arrangement, and molecular weight, the alginate also has functional groups such as polyhydroxy and carboxyl distributed throughout its chain. It is highly reactive and with the possibility of chemical modification (oxidation, amidation, esterification, and sulphation) [33].
2.7. Arabinoxylans
Arabinoxylans (AX) are non-starch polysaccharides found mainly in the cell wall, outer layer, and endosperm of cereals. The AX are composed of a linear chain of β-(1-4) xylose units branched to arabinose units in positions C(O)-3 and C(O)-2. Arabinose can be esterified with monomeric or dimeric ferulic acid (AF). AX are desirable polysaccharides for application in the food, pharmaceutical, and biomedical industries. Similarly, they have become an attractive alternative due to their biodegradability, biocompatibility, non-toxicity, hydrophilic, and gelling properties. Due to their functional properties, AX can act as prebiotics, antioxidants, emulsifiers, and immunomodulators. The gels made from this polysaccharide have been studied for their potential as a drug delivery system directed to the colon because they are biocompatible and have the ability to retain water. These covalent gels can cross the conditions of the upper gastrointestinal tract and be fermented in the colon by the colonic microbiota to release the drug [34,35].
3. Polysaccharide Based-Nanoparticles
Drugs can be loaded into a polysaccharide matrix or be bound to the external surface, improving their aqueous solubility and stability (e.g., proteins). They are used to enhance slow enzymatic degradation, targeting mucosal recognition, receptor binding, adhesion, and transport systems [36,37]. Polysaccharides have presented advantages and benefits when they are used as biomaterials for the manufacture of nanoparticles. Compared to nanoparticles synthesized from other materials, such as metals or synthetic polymers, polysaccharide nanoparticles do not present toxicity problems due to their biocompatibility and biodegradability, thus providing safety, better storage, and physiological stability [38,39].
Nanotechnology-based drug delivery systems aim to target different routes of administration. When designing polysaccharide-based nanoparticles for use in biomedicine and other industries, it is important to consider the route of administration, retention time, and therapy [40]. These factors may be dependent on the polysaccharide used, its size, and its structure. One possible disadvantage when using polysaccharides for manufacturing nanoparticles is their high molecular weights. In addition, in many cases, the low solubility of some polysaccharides limits their potential in chemical modification [27]. Due to the polysaccharide properties, the size of polysaccharide-based nanoparticles has been considered between 10 and 1000 nm [41,42].
These nanoparticles can carry drugs, proteins, peptides, or DNA material to target a specific organ or cell. Depending on the technique, the drug or other active compound can adhere, dissolve, encapsulate, or entrap into the nanoparticle matrix [43]. The significant interest in this technology is due to the fact that the nanoparticles can target specific organs or Diana cells, and reduce side effects. Nanoparticles have a larger surface area, and their smaller size allows them to enter smaller capillaries and cells. Nanoparticle size and surface charge are highly important because many secondary properties, such as surface area, toxicity, degradation, targeting, and absorption mechanism, are related [18]. Polysaccharide-based nanoparticles have shown significant potential in biological and pharmaceutical applications. Natural polysaccharides used to fabricate nanoparticles include chitosan, alginate, pectin, guar gum, dextran, hyaluronic acid, and arabinoxylan (Table 2).

Table 2.
Natural polysaccharides, properties, and their degradation mechanism.
Previously studied polysaccharides used to fabricate nanoparticles include the manufacture of acrylate chitosan-based nanoparticles cross-linked with tripolyphosphate anions (TPPs). The authors obtained chitosan nanoparticles with an average diameter of 356 nm and a Z potential of 13.7 mV [44]. In another study, nanoparticles were synthesized by β-lactoglobulin-pectin to encapsulate an anticancer drug (bipyridine ethyl dithiocarbamate Pt (II) nitrate), and they obtained nanoparticles with sizes of 200–250 nm [45]. The fabricated nanoparticles based on bovine serum albumin (BSA)-dextran conjugate to encapsulate curcumin showed nanoparticles with a size of 115 nm [46]. Similarly, arabinoxylan cross-linked with laccase was developed and showed a hydrodynamic diameter of 328 nm [47].
3.1. Physicochemical Characteristics of Polysaccharide-Based Nanoparticles
3.1.1. Size and Shape
The development of polysaccharide-based nanoparticles has progressed significantly in biomedicine in applications of drug, protein, and gene control delivery [55] and tissue engineering [56]. These nanomaterials have contributed to therapies for the treatment of various diseases such as cancer [57], diabetes [58], wound treatment [59], and inflammation [60]. These nanoparticles present a rapid absorption and release behavior, which allows a high diffusion capacity and volume change. Furthermore, their size and shape can be easily adapted and controlled to reduce desirable side effects [61]. The characterization of nanoparticles (size, surface charge, shape, and agglomeration) is highly important during their fabrication, because they have to overcome barriers in the human body, such as cell internalization, tissue penetration, localization directed at tumors, and circulation through blood vessels (Figure 1). The properties of nanoparticles related to their size and shape are their colloidal stability, specific surface, optical properties, in vivo behavior, and cellular absorption [62,63]. Boverhof et al. [14] analyzed 14 different regulatory authorities for the use of nanomaterials with a particular emphasis on safety and impact on human health and the environment. These authorities agreed to define the word “nanomaterial”, which is a representative characteristic of its size. As a definition, a nanomaterial has at least one external dimension or surface structure in the nanometric range (~1–100 nm). Furthermore, these regulatory authorities indicated that the upper field of the nanoscale can be more than 100 nm and must present physical or chemical properties, and their biological effects are attributable to its size (e.g., bioavailability, lower toxicity, and lower dose) [64,65,66,67].
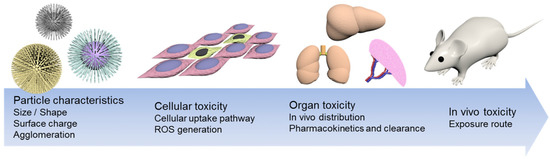
Figure 1.
Illustration of the main properties of nanoparticles. Adapted from [68]. Open access.
The nanoparticle’s size/shape are important parameters because they contribute to the biological processes. Small-sized particles present a higher surface-to-volume ratio, leading to the faster release of the drug. This size allows the particles to cross cell membranes, organs, and tissues. These include transport through the vasculature, phagocytosis, endocytosis, and intracellular transport. However, the size should not be too small because it has been established that the size threshold in renal excretion is usually 6 to 8 nm. In addition, sizes smaller than 6 nm can be coated with serum proteins circulating through the bloodstream, which favors an increase in the hydrodynamic diameter, blocking renal excretion [69]. Conversely, nanoparticles larger than 100 nm are more likely to accumulate at the injection site or be trapped by macrophages in the spleen, lung, and liver [70].
3.1.2. Superficial Charge and Aggregation
The surface charge measures the Z potential and represents a simple measurement of the particles electrical characteristics. This property determines the stability of colloidal dispersions and is affected by the ionic strength and pH of the dispersion. The Z potential indicates the degree of repulsion between adjacent particles, charged in a dispersion [19]. Aggregation occurs when several particles are joined together strongly, resulting in the sum of surface areas of the individual particles. The size and shape of the aggregation have a significant influence on the properties of the nanomaterial. These aggregates are indivisible structures and cannot be separated by external forces [14]. The surface charge determines the dispersion, aggregation, or flocculation of the particles. It can influence stability, and a neutral charge leads to instability of the nanoparticles, causing precipitation. A high Z potential confers stability to the particles, thus preventing their aggregation. When this value is low, the particles tend to attract each other and form flocs. Negatively charged nanoparticles have low phagocytic absorption, increasing their time in the bloodstream. Additionally, positively charged particles cause increased phagocytosis due to the interaction of the anionic cell membrane with the nanoparticles [71]. As the particle passes through the systemic circulation, a positive surface charge facilitates interaction with plasma proteins, resulting in aggregation. Therefore, the charge density in the nanoparticles when interacting with body fluids can be altered [18,69,72]. The use of nanotechnology in drug delivery enables better targeting of delivery to cells and tissues [55].
3.2. Cellular Uptake Mechanisms
The cellular uptake mechanisms to deliver biological substances and therapeutic agents require internalization to specific compartments or organelles to affect a cellular level. First, nutrient uptake, communication between cells, their microenvironment, and cell adhesion occur through the plasma membrane by different mechanisms. Due to of their concentration gradients, hydrophobic or nonpolar compounds can easily diffuse through the plasma membrane. Then, ions and amino acids are transported across the plasma membrane by active transport mechanisms, such as integral membrane protein pumps or ion channels. Finally, nanoscale hydrophilic biomacromolecules are conventionally transported into the cell by endocytosis [73].
Endocytosis is an active transport process in which a cell membrane wraps itself around an object and encloses it in vesicles or vacuoles pinched off from its cytoplasmic membrane. Endocytosis is divided into two main mechanisms: phagocytosis and pinocytosis (Figure 2). Phagocytosis is the primary uptake for larger particles by the cell membrane forming an internal phagosome, and occurs principally in certain types of phagocytes (macrophages and neutrophils). Phagocytes can take up relatively large particles (1 micron). This mechanism occurs when a particle, cells, and pathogens are recognized by phagocytes circulating through the blood and tissues. They attach to the phagocytes’ surface receptors, allowing further internalization. Once recognized, the plasma membrane of phagocytes encloses the particles within a cup-shaped protrusion and fuses to produce a phagosome that is pinched off inward [74].
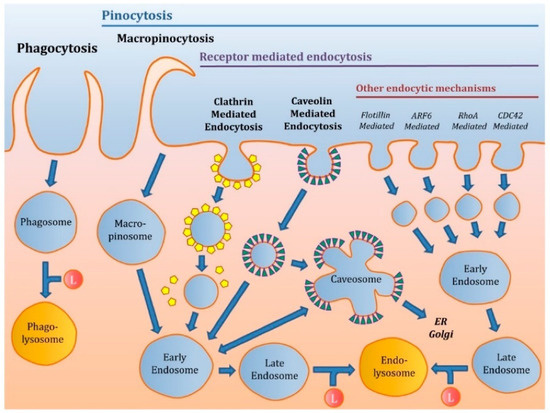
Figure 2.
Different mechanisms of endocytosis. Adapted from [76]. Open access.
Pinocytosis highlights the uptake of smaller particles. Pinocytosis is subdivided into clathrin-dependent and clathrin-independent pathways, divided into caveolae-mediated endocytosis, micropinocytosis, and other endocytic mechanisms. The clathrin-mediated endocytosis mechanism is the main endocytosis pathway, through which cells absorb necessary materials. This is known as receptor-mediated uptake, which involves the capture of particular macromolecules (ligands) or ligand-coated particles after attaching to receptors on the cell membrane’s surface. This binding (receptor-ligand) initiates a process in the plasma membrane. It results in the formation of “coated pits”, where the receptor concentration is greater than that in the remaining area of the cell membrane. Non-specific adsorptive uptake is a receptor-independent mechanism, and the uptake occurs through hydrophobic or electrostatic interactions, eventually allowing the internalization process. The macropinocytosis mechanism is a process that allows the uptake of larger particles that cannot easily cross other endocytosis pathways. In this mechanism, the plasma membrane generates ruffles of diverse shapes that absorb extracellular fluids via closing the membrane ruffles and forming large vesicles (macropinosomes) [75].
3.3. Polysaccharide Based-Nanoparticles and their Impact on Cellular Uptake
The biological response of the nanoparticles is measured in animal cell culture studies before starting in vivo administration [77]. In a biological system, nanoparticles can easily pass through the membranes and most biological barriers, and easily penetrate the body, cells, organs, and tissues, presenting problems such as potential toxicity [8]. The physicochemical characteristics of the nanoparticles contribute to the cellular uptake mechanisms. The smaller sizes of the nanoparticles allow them to internalize capillaries and thus easily target the cell, providing greater therapeutic efficiency and less toxicity. Materials internalized by endocytosis are delivered to lysosomes and are subject to degradation after the cleavage of the budding vesicles. Delivery of nanoparticles into lysosomes via clathrin-mediated endocytosis has been a widely used route for drug delivery [78]. The intracellular disposition of chitosan nanoparticles in macrophages has been previously studied. Chitosan nanoparticles with a size of 250 nm were prepared and collocated in a murine macrophage cell line (RAW 264.7) as a model macrophage. The results showed that the nanoparticles follow two mechanisms of macrophage (clathrin-mediated endocytosis pathway and phagocytosis) internalization, indicating cellular digestion of the nanoparticles [79]. In another study, alginate-based nanoparticles were fabricated. The in vitro study was performed in Caco-2 cells. The results showed that nanoparticles with a size of 50/120 nm internalized via clathrin-mediated endocytosis, and the mechanisms for nanoparticles with sizes of 420 and 730 nm were caveolae-mediated endocytosis and macropinocytosis, respectively [80]. In addition, in a study using chitosan-based nanoparticles (CS-NPs), the CS-NPs showed an average diameter of 281 nm and a negative surface charge (−0.3 mV). The cell experiments indicated that CS-NPs presented higher cell internalization efficiency in Raw 264.7 macrophages. These results show excellent potential for the use of these CS-NPs as a macrophage-targeting drug delivery system in further studies [81].
Nanoparticle behavior and motion in blood flow, membrane adhesion strength, and cell uptake pathways and effectiveness are influenced by particle shape. The surface charge of nanoparticles can also influence the uptake mechanism. Positively charged nanoparticles can be successfully absorbed by cells through electrostatic interactions with the negatively charged cell plasma membrane (clathrin-mediated endocytosis). In contrast, negatively charged nanoparticles are more likely to use caveolae-mediated routes [82].
The cytotoxicity of chitosan nanoparticles in Caco-2 cells was evaluated previously [83]. The nanoparticles had sizes of 25 and 333 nm and a Z potential of 5.3 mV. The results showed that in vitro cytotoxicity was mainly influenced by the particle size and not by its surface charge. The Caco-2 cells were more sensitive to cytotoxicity with 25 nm nanoparticles because these were able to internalize the cells. Similarly, silica nanoparticles (SiNPs) coated with chitosan (CH) and alginate (A) for the encapsulation of insulin have been previously reported [13]. The SiNP-CHs were synthesized with sizes of ~600 nm and with a Z potential of −13 mV, and the SiNP-A presented sizes of ~390 nm and a Z potential of +19 mV. Cytotoxicity was evaluated by the viability of HepG2 and Caco-2 cells. At concentrations of 100 µg/mL, nanoparticles coated with chitosan and alginate did not show toxicity. In the same manner, insulin-loaded chitosan-pectin nanoparticles were developed [84]. Particles with a size of 240–420 nm were obtained, and their cell viability was evaluated using Caco-2 cells. The particles loaded with insulin showed a moderate decrease in cell viability, which could be attributed to the degree of acetylation of chitosan and the presence of insulin; similarly, this did not represent a toxicity problem. In addition, chitosan-g-poly(methyl methacrylate) nanoparticles thiolate by conjugating N-acetyl cysteine were synthesized [85]. The authors obtained nanoparticle sizes ranging from 100 to 330 nm and a positive surface charge due to the presence of free amide groups protonated to chitosan. Cell viability results using Caco-2 and HT29-MTX cells showed that, after 4 h, nanoparticles had viability > 90%, making it acceptable for a non-cytotoxic material. The permeability studies showed that the chitosan-based nanoparticles penetrated the cell monolayer in which mucus is present.
4. Colon
The colon, or large intestine, extends from the ileum to the rectum, with a length of approximately 1.5 m and an average diameter of 6 cm. It is composed primarily of the cecum, ascending, transverse, descending, and sigmoid colons, the rectum, and anus. A complex layer of mucosa covers the colon. The mucus secreted by the large intestine mucosa is responsible for protecting the intestinal wall from excoriation and bacterial activity. It provides the medium for agglutination of feces [86,87]. The colon’s main function is to absorb the remainder of the water and salts secreted from the small intestine. It is responsible for bacterial fermentation, synthesis of small amounts of vitamins, storage, and excretion of feces. The microbiota that resides in the colon ferments the dietary fiber from the carbohydrates in the diet. The products of its digestion are gases (e.g., hydrogen, carbon dioxide, nitrogen, and sometimes methane) and short-chain fatty acids (e.g., acetic, propionic, butyric, and lactic acid) [88,89]. The pH from the cecum to the rectum varies from 5.5 to 7; the change in pH is influenced by the diet and people’s health status [22]. The colon is inhabited by abundant microbiota (400 different species) of aerobic and anaerobic microorganisms. The microbiota has a significant impact on the host physiology, energy homeostasis, immune system, digestion, and the synthesis of vitamins. The colonic microbiota is mostly anaerobic, and the main species belong to the genera Bacteroides, Bifidobacterium, Eubacterium, and Lactobacillus. The colon harbors a population of 1011 bacteria per gram of intestinal content. These microorganisms produce a large panel of enzymes responsible for breaking dietary fibers that are not digested in the small intestine [88,90,91].
The colon does not have villi as observed in the small intestine; instead, it has a crescentic mucosal fold called the plica semilunaris which increases the internal surface area of the colon by about 1300 cm2. The colon is composed of four prominent layers: (1) Serous (squamous epithelium) is found in regions of the colon with a peritoneal surface. (2) Muscular regions are groups of autonomous muscles that control movement and mixing within the colon. (3) Submucosa comprises connective tissue containing blood and lymphatic vessels, fat cells, and nerve ganglia. The ganglia communicate with the innermost layer of the colon: the mucosa. (4) The mucosa is subdivided into three layers: the muscularis mucosa, lamina propria, and epithelium (Figure 3) [86]. The epithelium secretes the mucosa from the stomach to the colon. This secretion forms an adherent gel (mucus) on the surface of the mucous membranes. Its function is to act as a protective barrier between the underlying epithelium and the lumen due to harmful agents, destructive hydrolases, and microorganisms. It also acts as a lubricant to facilitate the passage of digestive material, protecting the underlying epithelium from mechanical stress and providing an essential environment for the enteric microbiota. The colon mucus is a very sticky translucent gel with a thickness of 830 µm [92].
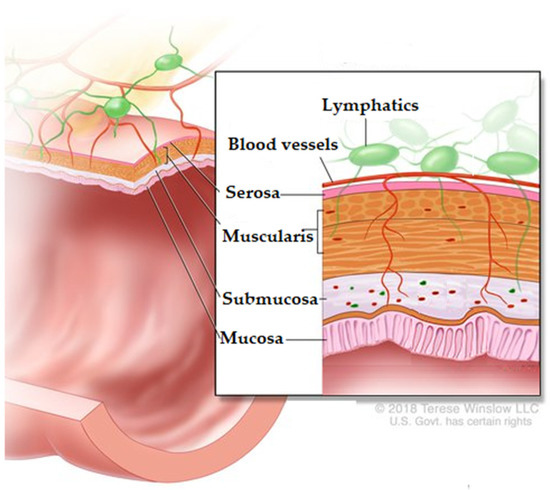
Figure 3.
Histological layers of the colon. Adapted from [93]. Rights holder: © (2021) Terese Winslow LLC, U.S. Govt. has certain rights.
5. Colon-Targeted Drug Delivery
Colon-targeted drug delivery is widely studied for the local treatment of various diseases, such as ulcerative colitis, colonic pathologies [60] colon cancer [94], and in the systemic administration of drugs, including proteins and peptides [95]. The administration of drugs and peptides for colon-targeted delivery is a challenge because they have to pass through the upper GI tract and not be absorbed. Thus, it is imperative to protect the drug during its journey through the GI tract [96]. The colon has been studied as a suitable site for the absorption of drugs, peptides, and proteins because it has a neutral pH and less diversity of digestive enzymes. In addition, the mucosa of the colon facilitates the absorption of several drugs and the colon’s proteolytic activity is less than that in the small intestine. The colon also has a long residence time of 5 days [90].
Polysaccharide-based nanoparticles protect drugs, peptides, and proteins from hydrolysis and enzymatic degradation in the duodenum and jejunum, thus enabling proper release of the drug in the ileum or colon, improving its systemic bioavailability [97]. The administration of a drug to the colon can be oral or rectal [98]. Oral administration is the most convenient and preferred route for people because it is painless, non-invasive, and easy to handle [94]. The best candidates for colon-targeted delivery are drugs that show poor stomach absorption to the intestine (peptides and proteins) [99]. The development of formulations and new technologies, such as nanoparticles, for the delivery of drugs, peptides, and proteins depends on the physicochemical nature of the drug, the biomaterial, and the therapy. Carriers such as biopolymer nanoparticles can be used as matrices, hydrogels, or coating agents. This type of carrier must protect the drug, peptides, and proteins from the challenging conditions of the stomach and intestine, increase intestinal absorption in the bloodstream, allow specific cells in the human body to be reached, and ensure controlled release [100,101].
6. Factors That Influence Colon-Targeted Drug Delivery
The colon as a drug delivery site offers excellent advantages due to its pH, residence time, and low enzyme activity (Figure 4). The administration of drugs to the colon depends on its physiological factors to ensure optimal efficiency after oral administration. The nanoparticles must be manufactured considering their residence time in the GI tract, the environment (pH, microorganism, food, etc.), and the intestinal fluid volume [102]. For oral administration of drugs, peptides, and proteins, it is necessary to cross multiple barriers present in the GI tract, which are designed to break down nutrients and prevent the entry of pathogens. The barriers that influence the arrival and absorption of drugs, peptides, and proteins in the colon are classified into chemical, enzymatic, and mucus barriers [15]. Each is described below, and nanoparticle studies are shown in Table 3.
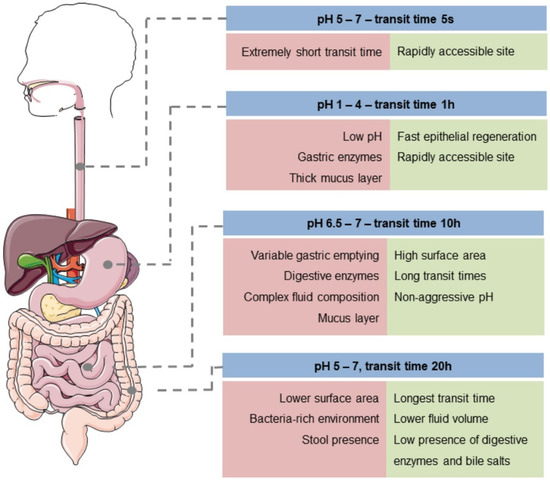
Figure 4.
Conditions in the GI tract. Reprinted by permission from John Wiley and Sons [103] Copyright (2021).

Table 3.
Polysaccharide-based nanoparticle colon-specific drug delivery.
6.1. Chemical Barrier
In colon-targeted oral drug delivery systems such as drugs, peptides, and proteins are exposed to aggressive environments when crossing through the stomach and small intestine until they reach the colon. The first barrier they must cross is chemical. The pH along the GI tract destroys the administered molecules, reducing their effectiveness. As previously mentioned, the colon has a neutral pH (5.5–7), unlike the stomach and small intestine. The GI tract is divided into the upper and lower tract. The upper tract includes the oral cavity, pharynx, esophagus, stomach (pH 1 and 3.5), and the small intestine’s initial section (duodenum, pH 6). The lower GI tract includes the remainder of the small intestine (jejunum, pH 6 and ileum, pH 7), and the segments of the large intestine (cecum, colon, and rectum) [22,127]. Polysaccharide-based nanoparticles can be degraded in the stomach by electrostatic interactions, degrading the polymer network and destroying the nanoparticle. Therefore, for these conditions, the approach is to design pH-dependent nanoparticle systems using resistant matrices and enteric coatings to prevent drug release in the upper GI tract [49].
The fabrication of chitosan and guar gum-based nanoparticles to encapsulate curcumin was previously reported [128]. Highly charged, monodisperse, hydrophilic colloidal nanoparticles were formed with an average diameter range of 250–290 nm. Under gastric stimulation conditions using a simulated gastric fluid (SGF) (2 g/L sodium chloride; 3.2 g/L pepsin; pH 1.2), the nanoparticles released 16% of the load after 3 h and remained stable, preserving their structural integrity. The release of nanoparticles in this system was controlled by the diffusion and swelling of the particles, which was presented in an acidic environment due to the possible protonation of the chitosan amide group. In the simulated intestinal fluid (SIF) (6 g/L potassium dihydrogen phosphate; 3.2 g/L pancreatin; 5 g/L bile salts; pH 7.0), the release of curcumin of the nanoparticles was 43% after 2 h of incubation. This could be attributable to the decrease in the solubility of chitosan at an intestinal pH close to its pKa (6.5) and the swelling of guar gum at pH ~7, resulting in greater porosity in the nanoparticles which resulted in the release of curcumin. In another study, polylactic-co-glycolic acid (PLGA)-modified nanoparticles using chitosan were manufactured [129]. The size of the nanoparticles increased from 132.8 nm to 172.7 nm due to the use of chitosan. The increase in size helped to increase the encapsulation efficiency from 65.8 to 87.1%. In vitro studies showed that modified nanoparticles responded at pH 5.5 by accelerating drug release. In addition, the development of modified nanogels based on soy protein and dextran to encapsulate riboflavin has been studied [130]. Morphological characterization analyses showed spherical core-layer structures, with sizes within the range of 32 to 40 nm. The size allowed an encapsulation efficiency of 65.9%. In vitro studies simulating GI tract conditions showed that these nanogels are stable in SGF and presented greater release in SIF.
6.2. Enzymatic Barrier
Enzymatic degradation is a challenge for polysaccharide-based nanoparticles. Some polysaccharides are usually susceptible to various digestive enzymes found in the GI tract. The breakdown of lipids and proteins occurs in the stomach by gastric lipases and pepsins, and continuous degradation in the small intestine by pancreatic enzymes (lipase, trypsin, and elastase) [127]. The polysaccharides that are not degraded by gastric and intestinal enzymes are degraded and fermented in the colon by the colonic microbiota. This degradation reduces polysaccharides to oligosaccharides, and their fermentation results in the production of metabolites and short-chain fatty acids [131]. Polysaccharides are often used to deliver drugs by film coating and matrix formation. Matrices based on non-starch polysaccharides are not decomposed in the stomach because anaerobic bacteria ferment them in the colon [54]. These microbiota-activated systems have shown promise in colon-targeted drug delivery due to anaerobic microbiota and their specific enzymatic activity. Polysaccharides fermentable by these microorganisms and used to manufacture drug delivery systems are pectin, guar gum, inulin, chitosan, and arabinoxylan. These polysaccharides can resist being passed through the upper GI tract and be metabolized in the colon to release the drug, peptides, or proteins [97].
In a previous study, citrus pectin-based nanoparticles were coated using Eudragit S100 [132]. These nanoparticles were designed to release 5-fluorouracil (5-FU) in the colon to attack colorectal cancer and presented a spherical shape, an average size of 218.12 nm, and a Z potential of 27.5 mV. In vitro matrix degradation studies showed that nanoparticles were degraded by pectinase, and the drug release started after 4 h in SIF (pH 6.8). In vivo studies to evaluate site specificity showed that a smaller quantity of the drug was lost in the upper GI tract. An increase in the concentration of 5-FU was observed in the colon due to degradation by its microbiota.
6.3. Mucus Barrier
The mucus present in the small and large intestine is a sticky, elastic, and viscous layer. This layer is produced by goblet cells and is responsible for protecting epithelial cells by capturing hydrophobic molecules; it also protects them from physical damage caused by eating food. Also, the mucus layer is water and mucins, which contains salts, bacteria, carbohydrates, enzymes, and immunoglobulins. These components, which are coated with proteoglycans, give the layer a negative charge [133]. Although the pore sizes of mucus gels are around 100 to 200 nm, it has been confirmed the mucus layer can entrap molecules with high molecular weight and low permeability (e.g., insulin) and positively charged nanoparticles with a hydrophobic nature. Hence, this is one of the obstacles that nanoparticles must cross when drugs are administered orally. Many nanomaterials tend to be immobilized by the mucus layer and cannot reach the intestinal epithelial cells of the colon [133,134]. Polysaccharides are mucoadhesive biomaterials due to their hydrophilic groups (hydroxyl, carboxyl, amide, and sulfate), which bind to mucus by hydrogen bonding and hydrophobic or electrostatic interactions [44,98]. The negatively charged mucosa attracts positively charged nanoparticles, such as cationic polymers (chitosan and derivatives). Nanoparticles coated with cationic groups or thiol groups have a longer residence time in the GI tract. The mucoadhesive and mucopenetration properties of nanoparticles are important for oral administration because mucoadherent particles are removed from mucosal tissue [15].
Mucoadhesion is defined as the adhesion between materials (mucosa-material). Formulations designed for mucoadhesive dosing allow prolonged retention at the site, maintaining the controlled release rate of the drug, therefore increasing its bioavailability. Mucoadhesion can be affected by various factors, such as hydrophobicity, molecular weight, cross-linking, swelling, spatial conformation, pH, and polymer concentration. The transit time of these systems depends on the physiological renewal time of the mucus layer. The intestinal mucin turnover time is between 50 and 270 min, so the particles adhere to the mucus for between 4 and 5 h [92]. In the mucus-penetrating mechanism, nanoparticles must avoid adhering to the mucosa. In the design of mucus-penetrating nanoparticles, the particle easily penetrates the luminal mucus layer to break through the underlying adherent mucus layer. Once the nanoparticles penetrate, they are closer to the cells, and a higher dose of the drug is exposed to later bind to the underlying epithelium, improving drug delivery [134].
The manufacture of a chitosan-based nanoparticle system (NPCS) to encapsulate insulin has been previously reported [135]. The nanoparticles had a size of 253.2 nm and a Z potential of 28.2 mV. In this study, the effect of nanoparticles to adhere to and infiltrate the mucus layer was evaluated. The results showed that the NPCS could penetrate through the epithelial mucus layer and approach the epithelial cells. In another study, insulin-loaded polyurethane-alginate (PU-AG)-based nanoparticles were evaluated as a model for oral administration [136]. The nanoparticles reached sizes of 73 nm and a Z potential of −27.3 mV. Ex vivo mucoadhesion studies report that these nanoparticles presented high mucoadhesiveness and were strongly attached to the intestinal lumen. This resulted from the mucoadhesive property of alginate due to its carboxyl groups and the negative charge of the nanoparticle.
6.4. Absorption Colon-Specific Drug Delivery
The transport of drugs across the colonic mucosa depends on different factors, including colonic residence time, susceptibility to destruction during uptake, diffusional characteristics, colonic motility, and integrity of the epithelium. Alterations on the mucus layer develop various pathological conditions affecting colonic delivery; thus, the colon requires an intact mucous layer (Figure 5) [102]. The absorption mechanism for oral drugs is complex because they must be soluble in gastric fluid and be absorbed by the stomach, small intestine, or colon [137]. There are two main routes for oral drug absorption in the colon: the transcellular and paracellular routes. The transcellular route is the method by which most drugs are successfully delivered in the colon. Small molecules readily diffuse across colonic epithelial cells by a series of partitions between membrane lipids and aqueous environments. The greatest passive absorption of the drug occurs in the proximal colon due to the lipid fluidity of the membrane in the proximal colonocytes. The active transcellular route initiates drug uptake at the luminal surface through carrier-mediated uptake, receptor-mediated endocytosis, or pinocytotic endocytosis. Paracellular transport is controlled by the sieving action of the intercellular occluding junctions found at the apical neck of cells in the GI tract. This barrier is cation selective, limits the transit of larger compounds, and provides a barrier for the uptake of pathogens found in the lumen of the GI tract. Transcellular ion gradients drive the movement of water and ions through the paracellular pathway. Some drugs have local effects on the GI tract, whereas most are delivered to the bloodstream via systemic circulation to act elsewhere in the human body [96,131].
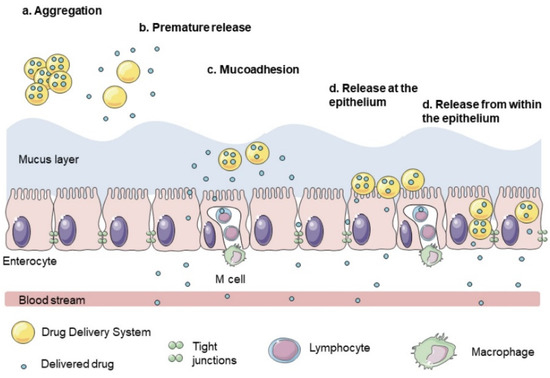
Figure 5.
Barriers to the colonic transport of drugs. Reprinted by permission from John Wiley and Sons [103]. Copyright (2021).
The size of the nanoparticles is an important factor because it affects or benefits their absorption. Studies to assess the specificity of the drug release site can be affected by factors such as the pH of biological fluids, the enzyme system, the interaction between the drug, and the biological environment. Previous studies that discuss this factor (size) include the manufacture of chitosan-based nanoparticles by nano-emulsion, modifying their surface with a penetrating peptide (CPP CS NP) to encapsulate and release insulin [138]. The formed monodisperse spherical particles had ultra-small sizes (12 nm). The nanoparticles were able to penetrate Caco-2 cells in the in vitro assay through paracellular and intracellular pathways.
Similarly, the manufacture of calcium phosphate nanoparticles coated with vitamin B12-chitosan and alginate (ViTB12-Chi-CPNPs) to encapsulate and release insulin was previously reported [139]. The nanoparticles were synthesized with particle sizes of <250 nm and Z potential of +32.56 mV. Studies in the monolayer of Caco-2 cells showed greater uptake of ViTB12-Chi-CPNPs. A reduction in transepithelial electrical resistance measurements (TEER) was observed, resulting in the paracellular transport of insulin due to the opening of tight epithelial junctions. In another study, the author’s manufactured alginate/dextran-based nanoparticles were loaded with insulin and coated with chitosan and albumin to improve the permeability of insulin through the intestinal epithelium [140]. The nanoparticle sizes obtained were 300 nm. To study the permeability of the nanoparticles, they used Caco-2/HT29-MTX-/Raji B cells. This model mimics the intestinal cellular monolayer due to its content of erythrocytes, goblet cells, and M cells in the Peyer’s patches. The results showed that these nanoparticles increased interactions with cell monolayers.
7. Conclusions and Perspectives
The use of polysaccharide-based nanoparticles for oral drug delivery to the colon has become an essential strategy for treating several diseases. The use of polysaccharides to fabricate these nanomaterials improves their biocompatibility and biodegradability properties. Targeted drug delivery offers attractive properties when materials are developed at a nanometric scale because their size is related to the particle’s stability, surface area, toxicity, degradation, and absorption mechanisms. Similarly, its size depends mainly on the encapsulated drugs, the type of polysaccharide, and the manufacturing technique. In this review, various studies are discussed, focusing on the physicochemical properties of nanoparticles and their potential to cross the barriers present in the GI, especially in the colon region.
Polysaccharide-based nanoparticles have significant advantages over other nanoparticles (metallic, ceramic, synthetic polymers, etc.). Currently, polysaccharide-based nanoparticles are generally investigated in terms of their physicochemical properties, such as size, surface charge, and encapsulation efficiency, via in vitro and in vivo tests. However, once nanoparticles are administered as drug delivery systems or as part of medical interventions, they tend to present specific interactions with elements of the human body, such as organs, tissues, cells, or hormonal systems. Therefore, it is crucial to investigate the possible side effects of these materials to a greater extent; however, this may significantly impact their use, application, and safety. Also, due to the extensive use and manufacture of polysaccharide-based nanoparticles in the nanomedicine and pharmaceutical areas, more scrutiny is needed of regulatory safety as they continue developing this field. Currently, it is known that polysaccharide-based nanoparticles are considered to be non-toxic, biocompatible, and biodegradable; nonetheless, more in-depth research should be undertaken on the long-term toxicity risks they may pose. Nanoparticle safety is critical because this technology is increasingly playing an essential role in medicine and pharmaceuticals. Although regulatory organizations exist in each country, and each has established its criteria for using nanoparticles, it is necessary to develop international legislation for the applications, safety, and restrictions of nanoparticles. This regulation would open the door to more clinical trials and regulatory standards to set future precedents for the use and benefits of polysaccharide-based nanoparticles.
Author Contributions
Y.D.A.-F. writing—original draft preparation, review and editing; E.C.-M. supervision, project administration, resources, validation, writing—review and editing; A.C.-M., A.R.-C., J.L.-M., J.T.-C. and A.L.M.-L. supervision, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the National Council of Science and Technology (CONACyT) and Research Center for Food and Development (CIAD, A.C.) in Mexico for the financial support during the Ph.D. studies of Yubia De Anda-Flores.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Pawar, V.; Bavya, M.C.; Rohan, K.V.; Srivastava, R. Advances in Polysaccharide-Based Antimicrobial Delivery Vehicles. In Racing for the Surface; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 267–295. [Google Scholar]
- Krishnamurthy, R. Giving Rise to Life: Transition from Prebiotic Chemistry to Protobiology. Acc. Chem. Res. 2017, 50, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Datta, L.P.; Manchineella, S.; Govindaraju, T. Biomolecules-derived biomaterials. Biomaterials 2020, 230, 119633. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Reinholz, J.; Landfester, K.; Mailänder, V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F. Application of Biopolymers in Microencapsulation Processes. In Biopolymers for Food Design; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 191–222. [Google Scholar]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT 2017, 85, 37–44. [Google Scholar] [CrossRef]
- Prabhu, S.; Chenreddy, S.; Thio, A.; Khamas, W.; Wang, J.; Thakkar, A. Preclinical systemic toxicity evaluation of chitosan-solid–lipid nanoparticle-encapsulated aspirin and curcumin in combination with free sulforaphane in BALB/c mice. Int. J. Nanomed. 2016, 11, 3265–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.K.; Roy, P.; Mondal, M.K.; Roy, D.; Gayen, P.; Chowdhury, P.; Babu, S.P. Development of chitosan based gold nanomaterial as an efficient antifilarial agent: A mechanistic approach. Carbohydr. Polym. 2017, 157, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; Fangueiro, J.; Severino, P.; De Souza, A.L.R.; Martins-Gomes, C.; Fernandes, P.M.V.; Calpena, A.C.; Gremião, M.P.; Souto, E.B.; Silva, A.M. The Influence of Polysaccharide Coating on the Physicochemical Parameters and Cytotoxicity of Silica Nanoparticles for Hydrophilic Biomolecules Delivery. Nanomaterials 2019, 9, 1081. [Google Scholar] [CrossRef] [Green Version]
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascon-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Vazquez, N.A.R.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
- Carvalho, P.; Felício, M.R.; Santos, N.; Gonçalves, S.; Domingues, M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef]
- Tiwari, A.; Verma, A.; Panda, P.K.; Saraf, S.; Jain, A.; Jain, S.K. Stimuli-responsive polysaccharides for colon-targeted drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 1, pp. 547–566. [Google Scholar]
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Im, E.; Jung, Y.; Yoo, J.-W. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharmacal Res. 2020, 43, 153–169. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.M.; Meneguin, A.B.; Akhter, D.T.; Fletcher, N.; Houston, Z.H.; Bell, C.; Thurecht, K.J.; Gremião, M.P.D. Understanding the role of colon-specific microparticles based on retrograded starch/pectin in the delivery of chitosan nanoparticles along the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2021, 158, 371–378. [Google Scholar] [CrossRef]
- Wahlgren, M.; Axenstrand, M.; Håkansson, Å.; Marefati, A.; Pedersen, B.L. In Vitro Methods to Study Colon Release: State of the Art and An Outlook on New Strategies for Better In-Vitro Biorelevant Release Media. Pharmaceutics 2019, 11, 95. [Google Scholar] [CrossRef] [Green Version]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Borchmann, D.E.; Carberry, T.P.; Weck, M. “Bio”-Macromolecules: Polymer-Protein Conjugates as Emerging Scaffolds for Therapeutics. Macromol. Rapid Commun. 2013, 35, 27–43. [Google Scholar] [CrossRef]
- Ding, W.; Wu, Y. Sustainable dialdehyde polysaccharides as versatile building blocks for fabricating functional materials: An overview. Carbohydr. Polym. 2020, 248, 116801. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Ahmed, T.; Aljaeid, B. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Khotimchenko, M. Pectin polymers for colon-targeted antitumor drug delivery. Int. J. Biol. Macromol. 2020, 158, 1110–1124. [Google Scholar] [CrossRef]
- BeMiller, J.N. Guar, Locust Bean, Tara, and Cassia Gums. In Carbohydrate Chemistry for Food Scientists; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 241–252. [Google Scholar]
- Chen, F.; Huang, G.; Huang, H. Preparation and application of dextran and its derivatives as carriers. Int. J. Biol. Macromol. 2020, 145, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Da Silva, C.F.; Andrade, L.N.; Oliveira, D.D.L.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Rivera, D.E.V. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Oxidative Med. Cell. Longev. 2018, 2018, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-López, A.L.; Carvajal-Millan, E.; Sotelo-Cruz, N.; Micard, V.; Rascón-Chu, A.; López-Franco, Y.; Lizardi-Mendoza, J.; Canett-Romero, R. Enzymatically cross-linked arabinoxylan microspheres as oral insulin delivery system. Int. J. Biol. Macromol. 2019, 126, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Fan, Y.; Hu, Y.; Cheng, G.; Xu, F. Polysaccharide–Peptide Conjugates: A Versatile Material Platform for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005978. [Google Scholar] [CrossRef]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive polymers: Novel tool for drug delivery. Artif. Cells Nanomed. Biotechnol. 2013, 42, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Jenjob, R.; Phakkeeree, T.; Crespy, D. Saccharides, oligosaccharides, and polysaccharides nanoparticles for biomedical applications. J. Control. Release 2018, 284, 188–212. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano Struct. Nano Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Pereira, M.A.; Rebouças, J.D.S.; Ferraz-Carvalho, R.; de Redín, I.L.; Guerra, P.V.; Gamazo, C.; Brodskyn, C.I.; Irache, J.M.; Santos-Magalhaes, N. Poly(anhydride) nanoparticles containing cashew nut proteins can induce a strong Th1 and Treg immune response after oral administration. Eur. J. Pharm. Biopharm. 2018, 127, 51–60. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 33–93. [Google Scholar]
- Ramos, A.P.; Cruz, M.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Eliyahu, S.; Aharon, A.; Bianco-Peled, H. Acrylated Chitosan Nanoparticles with Enhanced Mucoadhesion. Polymers 2018, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Izadi, Z.; Divsalar, A.; Saboury, A.A.; Sawyer, L. β-lactoglobulin-pectin Nanoparticle-based Oral Drug Delivery System for Potential Treatment of Colon Cancer. Chem. Biol. Drug Des. 2016, 88, 209–216. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- De Anda-Flores, Y.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Martínez-López, A.L.; Marquez-Escalante, J.; Brown-Bojorquez, F.; Tanori-Cordova, J. Covalently Cross-Linked Nanoparticles Based on Ferulated Arabinoxylans Recovered from a Distiller’s Dried Grains Byproduct. Processes 2020, 8, 691. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Bianchera, A.; Bettini, R. Polysaccharide nanoparticles for oral controlled drug delivery: The role of drug–polymer and interpolymer interactions. Expert Opin. Drug Deliv. 2020, 17, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascón-Chu, A.; Díaz-Baca, J.A.; Carvajal-Millan, E.; Pérez-López, E.; Hotchkiss, A.T.; González-Ríos, H.; Balandrán-Quintana, R.; Campa-Mada, A.C. Electrosprayed Core–Shell Composite Microbeads Based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control. Polymers 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Dheer, D.; Arora, D.; Jaglan, S.; Rawal, R.; Shankar, R. Polysaccharides based nanomaterials for targeted anti-cancer drug delivery. J. Drug Target. 2016, 25, 1–16. [Google Scholar] [CrossRef]
- Aduba, J.D.C.; Yang, H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering 2017, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Martínez-López, A.L.; Carvajal-Millan, E.; Micard, V.; Rascón-Chu, A.; Brown-Bojorquez, F.; Sotelo-Cruz, N.; López-Franco, Y.L.; Lizardi-Mendoza, J. In vitro degradation of covalently cross-linked arabinoxylan hydrogels by bifidobacteria. Carbohydr. Polym. 2016, 144, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2019, 27, 19151–19168. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Qi, F.; Cheng, Y.; Lu, X.; Wang, L.; Zhao, J.; Zhao, B. Enhanced bone regeneration using an insulin-loaded nano-hydroxyapatite/collagen/PLGA composite scaffold. Int. J. Nanomed. 2017, 13, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Malhaire, H.; Gimel, J.-C.; Roger, E.; Benoît, J.-P.; Lagarce, F. How to design the surface of peptide-loaded nanoparticles for efficient oral bioavailability? Adv. Drug Deliv. Rev. 2016, 106, 320–336. [Google Scholar] [CrossRef]
- Beloqui, A.; Rieux, A.D.; Préat, V. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv. Drug Deliv. Rev. 2016, 106, 242–255. [Google Scholar] [CrossRef]
- Tosi, G.; Duskey, J.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2019, 17, 23–32. [Google Scholar] [CrossRef]
- Health Canada. 2011 Policy Statement on Health Canada’s Working Definition for Nanomaterial—Canada.ca. Available online: https://www.canada.ca/en/health-canada/services/science-research/reports-publications/nanomaterial/policy-statement-health-canada-working-definition.html (accessed on 1 May 2021).
- ISO 8968-1:2014. Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. Available online: https://www.iso.org/obp/ui/#iso:std:iso:8968:-1:ed-2:v1:en (accessed on 1 May 2021).
- SCENIHR Communication on the Second Regulatory Review on Nanomaterials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52012DC0572&from=EN (accessed on 1 May 2021).
- SCENIHR Opinion on the Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_045.pdf (accessed on 1 May 2021).
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [Green Version]
- de Crozals, G.; Bonnet, R.; Farre, C.; Chaix, C. Nanoparticles with multiple properties for biomedical applications: A strategic guide. Nano Today 2016, 11, 435–463. [Google Scholar] [CrossRef]
- Fernández, E.F.; Santos-Carballal, B.; De Santi, C.; Ramsey, J.M.; MacLoughlin, R.; Cryan, S.-A.; Greene, C.M. Biopolymer-Based Nanoparticles for Cystic Fibrosis Lung Gene Therapy Studies. Materials 2018, 11, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.M.; Esfanjani, A.F. Instrumental analysis and characterization of nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 524–544. [Google Scholar]
- Griffin, B.; Guo, J.; Presas, E.; Donovan, M.; Alonso, M.J.; O’Driscoll, C.M. Pharmacokinetic, pharmacodynamic and biodistribution following oral administration of nanocarriers containing peptide and protein drugs. Adv. Drug Deliv. Rev. 2016, 106, 367–380. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinelli, F.; Ortolà; Óscar, F.; Makvandi, P.; Perale, G.; Rossi, F. In vivo drug delivery applications of nanogels: A review. Nanomedicine 2020, 15, 2707–2727. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.-J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.-X.; Zheng, C.L. Intracellular disposition of chitosan nanoparticles in macrophages: Intracellular uptake, exocytosis, and intercellular transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Liu, C.-G.; Yu, Y. Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydr. Polym. 2015, 124, 274–279. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Ma, L.; Zu, M.; Song, H.; Xiao, B. Oral administration of chondroitin sulfate-functionalized nanoparticles for colonic macrophage-targeted drug delivery. Carbohydr. Polym. 2019, 223, 115126. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, J.W.; Saunders, M.; Lim, L.-Y. Cytotoxicity of monodispersed chitosan nanoparticles against the Caco-2 cells. Toxicol. Appl. Pharmacol. 2012, 262, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.V.; Yoshida, C.M.P.; Pereira, S.M.S.S.; Goycoolea, F.M.; Franco, T.T. Electrostatic Self-Assembled Chitosan-Pectin Nano- and Microparticles for Insulin Delivery. Molecules 2017, 22, 1707. [Google Scholar] [CrossRef]
- Noi, I.; Schlachet, I.; Kumarasamy, M.; Sosnik, A. Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers 2018, 10, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharucha, A.E.; Camilleri, M. Physiology of the Colon and Its Measurement. In Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 1676–1688. [Google Scholar]
- Arévalo-Pérez, R.; Maderuelo, C.; Lanao, J.M. Recent advances in colon drug delivery systems. J. Control Release 2020, 327, 703–724. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Valencia-Rivera, D.E.; Carvajal-Millan, E.; Astiazaran-Garcia, H.; Micard, V.; Rascón-Chu, A. Fermentation of Ferulated Arabinoxylan Recovered from the Maize Bioethanol Industry. Processes 2021, 9, 165. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- Winslow, T. Colon or Rectal Cancer Stage 0. Available online: https://visualsonline.cancer.gov/details.cfm?imageid=9147 (accessed on 7 May 2021).
- Song, Q.; Jia, J.; Niu, X.; Zheng, C.; Zhao, H.; Sun, L.; Zhang, H.; Wang, L.; Zhang, Z.; Zhang, Y. An oral drug delivery system with programmed drug release and imaging properties for orthotopic colon cancer therapy. Nanoscale 2019, 11, 15958–15970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.; Zhao, A. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.-W.; Park, B.J.; Han, H.-K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tam, M.; Samaei, S.; Lerouge, S.; Barralet, J.; Stevenson, M.M.; Cerruti, M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017, 48, 247–257. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Rajput, R.; Narkhede, J.; Naik, J.B. Nanogels as nanocarriers for drug delivery: A review. ADMET DMPK 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghomi, M.; Ashrafizadeh, M.; Tafazoli, A.; Agarwal, T.; Delfi, M.; Akhtari, J.; Zare, E.N.; Padil, V.V.; Zarrabi, A.; et al. A review on advances in graphene-derivative/polysaccharide bionanocomposites: Therapeutics, pharmacogenomics and toxicity. Carbohydr. Polym. 2020, 250, 116952. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020, 32, e1901935. [Google Scholar] [CrossRef]
- Wu, J.-L.; Tian, G.-X.; Yu, W.-J.; Jia, G.-T.; Sun, T.-Y.; Gao, Z.-Q. pH-Responsive Hyaluronic Acid-Based Mixed Micelles for the Hepatoma-Targeting Delivery of Doxorubicin. Int. J. Mol. Sci. 2016, 17, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilipenko, I.; Korzhikov-Vlakh, V.; Sharoyko, V.; Zhang, N.; Schäfer-Korting, M.; Rühl, E.; Zoschke, C.; Tennikova, T. pH-Sensitive Chitosan–Heparin Nanoparticles for Effective Delivery of Genetic Drugs into Epithelial Cells. Pharmaceutics 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, A.I.; Lima, S.; Reis, S. Application of pH-Responsive Fucoidan/Chitosan Nanoparticles to Improve Oral Quercetin Delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, A.; Ziora, Z.; Tamer, T.; Khalifa, R.; Hassan, M.; Mohy-Eldin, M.; Blaskovich, M. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef]
- Elgegren, M.; Kim, S.; Cordova, D.; Silva, C.; Noro, J.; Cavaco-Paulo, A.; Nakamatsu, J. Ultrasound-Assisted Encapsulation of Sacha Inchi (Plukenetia volubilis Linneo.) Oil in Alginate-Chitosan Nanoparticles. Polymers 2019, 11, 1245. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; Lucas, R.; Benito, E.; De-Paz, M.-V. Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release. Int. J. Mol. Sci. 2019, 20, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrarathna, H.; Liyanage, T.; Edirisinghe, S.; Dananjaya, S.; Thulshan, E.; Nikapitiya, C.; Oh, C.; Kang, D.-H.; De Zoysa, M. Marine Microalgae, Spirulina maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef] [Green Version]
- Dyawanapelly, S.; Koli, U.; Dharamdasani, V.; Jain, R.; Dandekar, P. Improved mucoadhesion and cell uptake of chitosan and chitosan oligosaccharide surface-modified polymer nanoparticles for mucosal delivery of proteins. Drug Deliv. Transl. Res. 2016, 6, 365–379. [Google Scholar] [CrossRef]
- Sabra, R.; Roberts, C.J.; Billa, N. Courier properties of modified citrus pectinate-chitosan nanoparticles in colon delivery of curcumin. Colloid Interface Sci. Commun. 2019, 32, 100192. [Google Scholar] [CrossRef]
- Prabahar, K.; Udhumansha, U.; Qushawy, M. Optimization of Thiolated Chitosan Nanoparticles for the Enhancement of in Vivo Hypoglycemic Efficacy of Sitagliptin in Streptozotocin-Induced Diabetic Rats. Pharmaceutics 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhakar, S.; Chandran, S.V.; Selvamurugan, N.; Nazeer, R.A. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Biol. Macromol. 2020, 150, 281–288. [Google Scholar] [CrossRef]
- Shailender, J.; Ravi, P.R.; Sirukuri, M.R.; Dalvi, A.; Priya, O.K. Chitosan nanoparticles for the oral delivery of tenofovir disoproxil fumarate: Formulation optimization, characterization and ex vivo and in vivo evaluation for uptake mechanism in rats. Drug Dev. Ind. Pharm. 2018, 44, 1109–1119. [Google Scholar] [CrossRef]
- He, Z.; Santos, J.L.; Tian, H.; Huang, H.; Hu, Y.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.-Q. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials 2017, 130, 28–41. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Kenechukwu, F.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Hirpara, M.R.; Manikkath, J.; Sivakumar, K.; Managuli, R.S.; Gourishetti, K.; Krishnadas, N.; Shenoy, R.; Jayaprakash, B.; Rao, C.M.; Mutalik, S. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: Development and in vitro and in vivo evaluations. Int. J. Biol. Macromol. 2018, 107, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Singh, S.; Renukuntla, J.; Govender, T. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surfaces B Biointerfaces 2017, 158, 650–657. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Chakraborty, M.; Bhattacharya, S.; Mishra, R.; Kundu, P. Oral insulin delivery by self-assembled chitosan nanoparticles: In vitro and in vivo studies in diabetic animal model. Mater. Sci. Eng. C 2013, 33, 376–382. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Van Can, M.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909–915. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Tan, C.P.; Basri, M.; Saari, N. Improved In Vivo Efficacy of Anti-Hypertensive Biopeptides Encapsulated in Chitosan Nanoparticles Fabricated by Ionotropic Gelation on Spontaneously Hypertensive Rats. Nanomaterials 2017, 7, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; He, Z.; Sun, C.; Yang, C.; Zhao, P.; Liu, L.; Leong, K.W.; Mao, H.-Q.; Liu, Z.; Chen, Y. Uniform Core-Shell Nanoparticles with Thiolated Hyaluronic Acid Coating to Enhance Oral Delivery of Insulin. Adv. Health Mater. 2018, 7, e1800285. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Sun, X.; Bai, J.; Dong, J.; Zhang, B.; Gao, Z.; Wu, J. Doxorubicin-loaded dual-functional hyaluronic acid nanoparticles: Preparation, characterization and antitumor efficacy in vitro and in vivo. Mol. Med. Rep. 2018, 19, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, B.; Zhou, X.; Li, X.; Lin, W.; Chen, G.; Qiu, R. Self-Assembled Modified Soy Protein/Dextran Nanogel Induced by Ultrasonication as a Delivery Vehicle for Riboflavin. Molecules 2016, 21, 282. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 Coated Citrus Pectin Nanoparticles for Colon Targeting of 5-Fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- McGuckin, M.; Thornton, D.; Whitsett, J.A. Mucins and Mucus. In Mucosal Immunology; Elsevier BV: Amsterdam, The Netherlands, 2015. [Google Scholar]
- García-Díaz, M.; Birch, D.; Wan, F.; Nielsen, H.M. The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv. Drug Deliv. Rev. 2018, 124, 107–124. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, K.-J.; Tseng, M.T.; Wey, S.-P.; Su, F.-Y.; Chuang, E.-Y.; Hsu, C.-W.; Chen, C.-T.; Sung, H.-W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Mukherjee, D.; Mishra, R.; Kundu, P.P. Development of pH sensitive polyurethane–alginate nanoparticles for safe and efficient oral insulin delivery in animal models. RSC Adv. 2016, 6, 41835–41846. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Barbari, G.R.; Dorkoosh, F.A.; Amini, M.; Sharifzadeh, M.; Atyabi, F.; Balalaie, S.; Tehrani, N.R.; Tehrani, M.R. A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides. Int. J. Nanomed. 2017, 12, 3471–3483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef]
- Lopes, M.; Shrestha, N.; Correia, A.; Shahbazi, M.-A.; Sarmento, B.; Hirvonen, J.T.; Veiga, F.; Seiça, R.; Ribeiro, A.; Santos, H.A. Dual chitosan/albumin-coated alginate/dextran sulfate nanoparticles for enhanced oral delivery of insulin. J. Control. Release 2016, 232, 29–41. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).