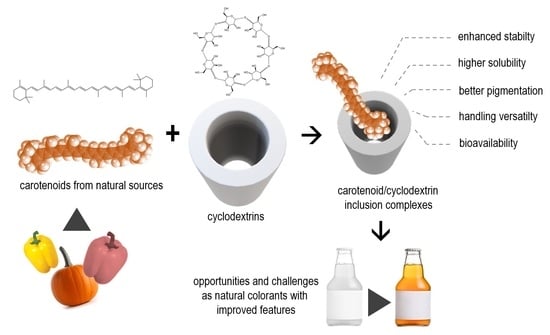
Encapsulation of Carotenoids as Food Colorants via Formation of Cyclodextrin Inclusion Complexes: A Review
Abstract
1. Introduction
2. Food Colorants: Artificial vs. Natural
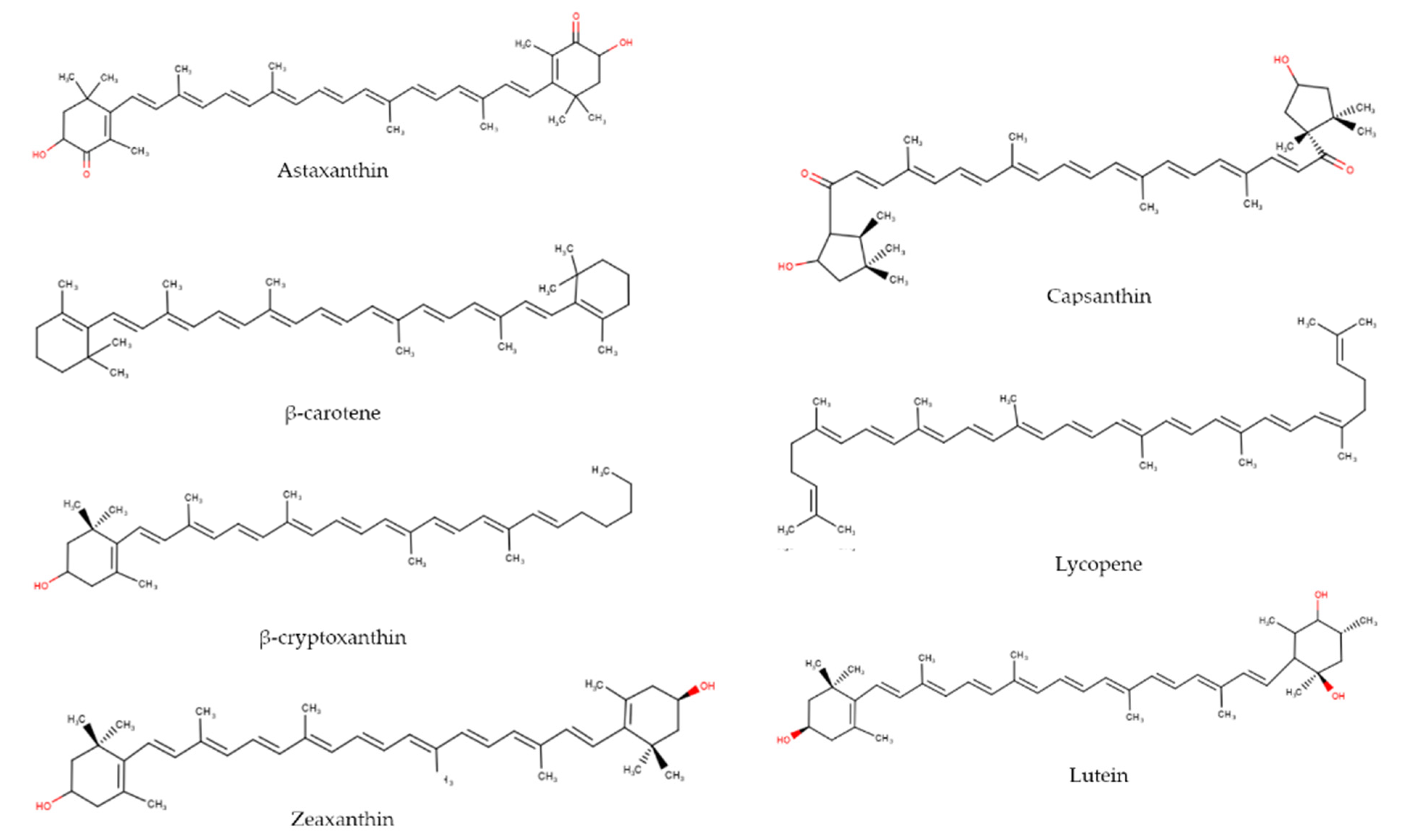
3. Carotenoids: Sources, Physicochemical Characteristics, and Stability
| Classification | Name | Sources | Associated Color |
|---|---|---|---|
| Carotene | β-carotene | Red and yellow pepper, ñame, bee pollen, carrot, tomato, squash, ahuyama, spinach, pumpkin, guayaba, apricot | Red, orange, yellow [35] |
| Lycopene | Carrot, tomato, purple cabbage | Red, orange [36] | |
| Xanthophylls | Astaxanthine | Fish, crustaceans, salmon, pomegranate | Pink-red [36] |
| β-criptoxanthin | Lemons, paprika, oranges, green pepper, papaya, squash, peach, pumpkin | Yellow-orange [35] | |
| Canthaxantin | Mushrooms, crustaceans, trout | Orange [36] | |
| Capsanthin-capsorubin | Red paprika, rice endosperm | Yellow-orange [35] | |
| Lutein | Potato, carrot, corn, tomatoes, egg yolk, papaya, pumpkin, red and yellow pepper | Yellow [36] | |
| Zeaxanthin | Papaya, egg, orange, honey, pumpkin | Yellow [36] |
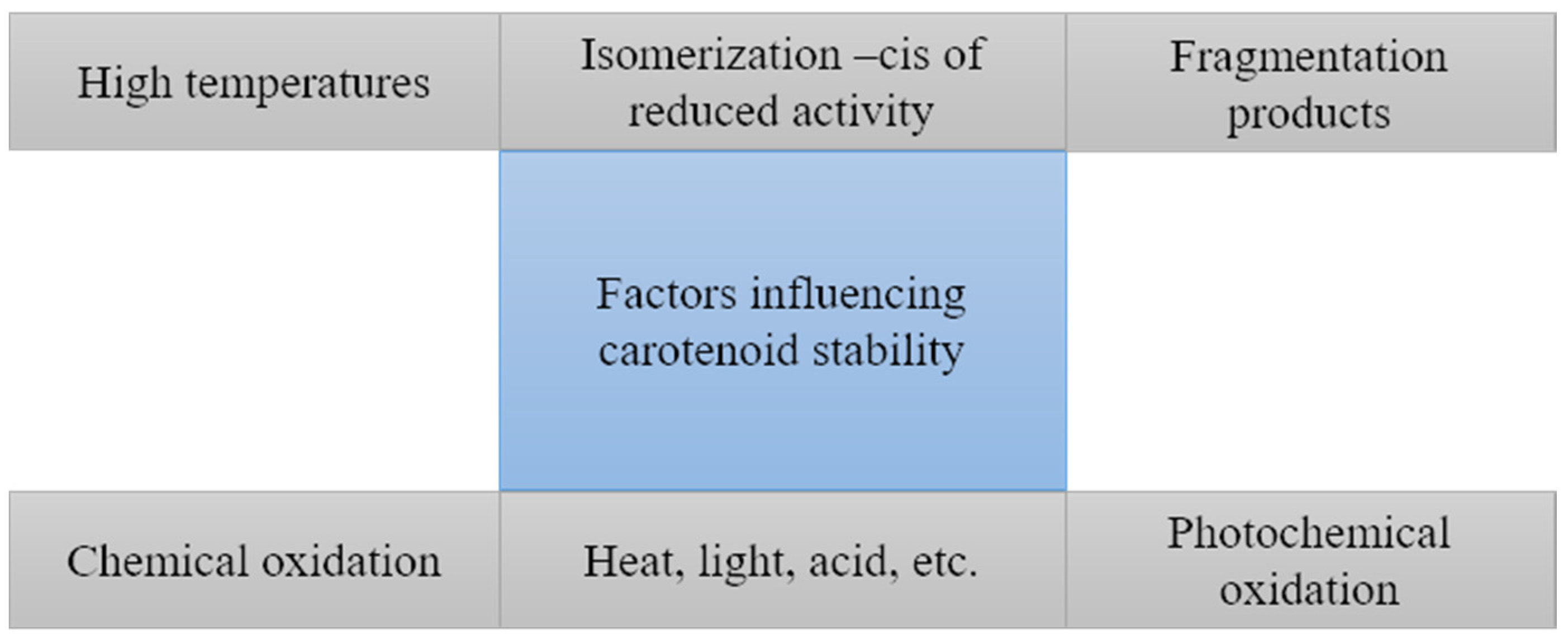
3.1. Isomerization
3.2. Oxidation
4. An Overview of Carotenoid Encapsulation Technologies
4.1. Microencapsulation
4.1.1. Coacervation
4.1.2. Spray-Drying
4.1.3. Supercritical Micronization
4.1.4. Emulsification
4.2. Nano-Encapsulation
4.2.1. Nano-Precipitation
4.2.2. Solvent Evaporation after Emulsification
4.2.3. Inclusion Complexes
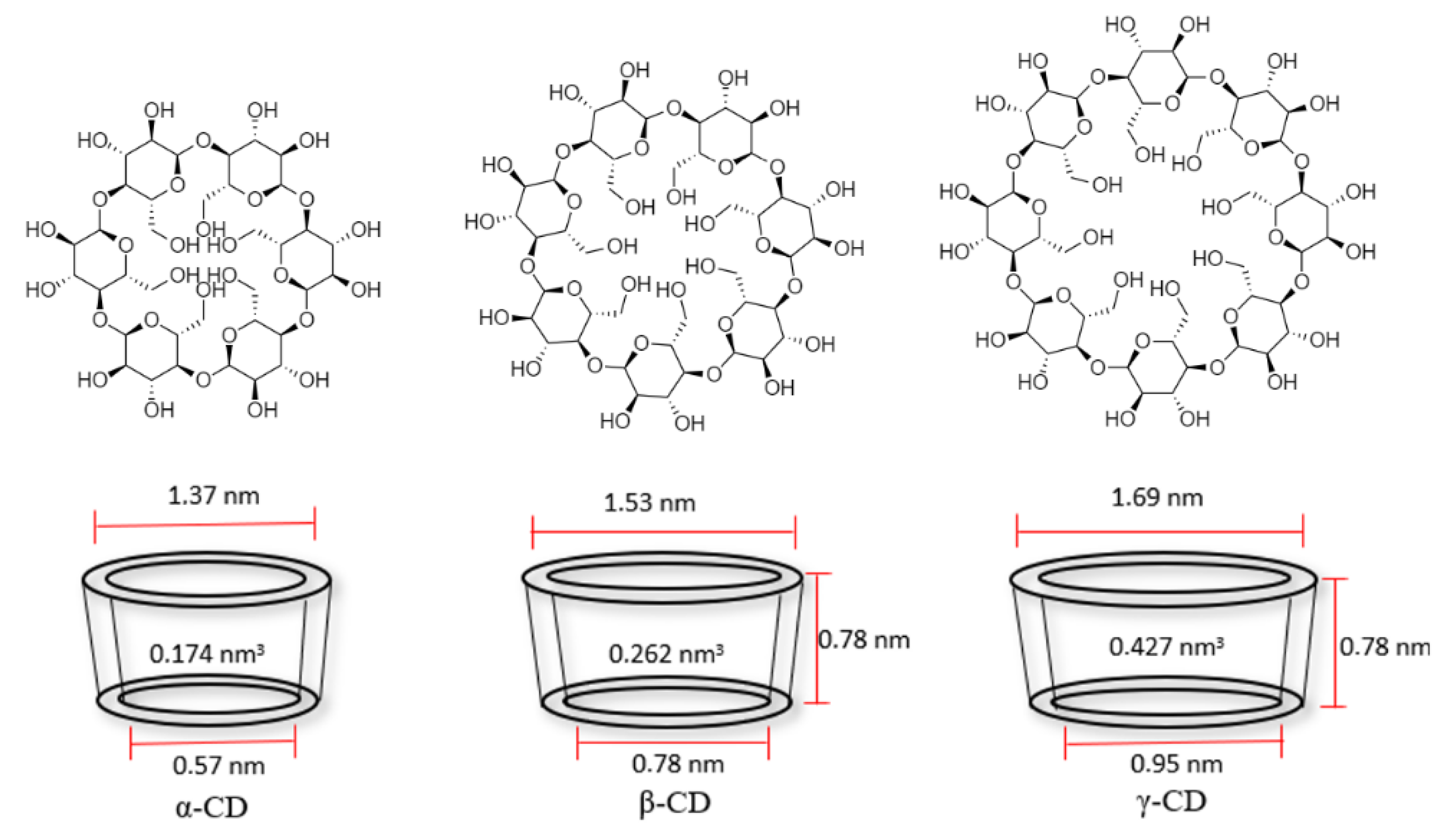
5. Cyclodextrins: Physicochemical Properties and Complex Formation Ability
| Properties | Cyclodextrin | Ref. | ||
|---|---|---|---|---|
| α-CD | β-CD | γ-CD | ||
| Glucose units | 6 | 7 | 8 | - |
| Molecular formula | C36H60O30 | C42H70O35 | C48H80O45 | - |
| Molecular weight (g/mol) | 972.8 | 1135 | 1297.1 | - |
| Melting point (°C) | 278 | 290–300 | No data | [62] |
| Boiling point (°C) | Discomposes | Discomposes | No data | [62] |
| Water solubility (25 °C) (g/100 mL) | 12.8 | 1.8 | 25.6 | [63,64] |
| Water solubility (45 °C) (g/100 mL) | 29.0 | 4.5 | 58.5 | [63,64] |
| Water solubility (60 °C) (g/100 mL) | 66.2 | 9.1 | 129.2 | [63,64] |
| Internal volume (nm3) | 0.174 | 0.262 | 0.427 | [10] |
| Internal diameter (nm) | 0.57 | 0.78 | 0.95 | [65] |
| External diameter (nm) | 1.37 | 1.53 | 1.69 | [65] |
| Ring height (nm) | No data | 0.78 | 0.78 | [65] |
6. Characterization Methods for Inclusion Complexes
6.1. Fourier Transform Infrared Spectroscopy (FTIR)
6.2. Differential Scanning Calorimetry (DSC)
6.3. Raman Spectroscopy
6.4. Proton Nuclear Magnetic Resonance (1H-NMR)
6.5. Scanning Electron Microscopy (SEM)
6.6. X-ray Diffraction (XRD)
7. Previous Studies of Encapsulation of Carotenoids by Formation of Inclusion Complexes in Cyclodextrins
8. Carotenoid/Cyclodextrin Inclusion Complex Manufacturing: A Preliminary Proposal for Industrial Scale-Up
8.1. Method 1: Co-Precipitation
8.2. Method 2: Kneading
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahra, N.; Fatima, Z.; Kalim, I.; Saeed, K. Identification of synthetic food dyes in beverages by thin layer chromatography. Pak. J. Food Sci. 2015, 25, 178–181. [Google Scholar]
- FDA. Electronic Code of Federal Regulations. Title 21, Chapter 1, Subchapter A, 2020. Available online: https://www.ecfr.gov/cgi-bin/text-idx?gp=&SID=9a60ccddf74a018d5771bc29f4c9134f&mc=true&tpl=/ecfrbrowse/Title21/21CIsubchapA.tpl (accessed on 5 April 2020).
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of Allura Red AC (E 129) in feed for cats and dogs. EFSA J. 2012, 10, 2675. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, e05714. [Google Scholar] [CrossRef]
- Schoefs, B. Plant Pigments: Properties, Analysis, Degradation. Adv. Food Nutr. Res. 2005, 49, 41–91. [Google Scholar] [CrossRef]
- Hendry, G.A.F.; Houghton, J.D. (Eds.) Natural Food Colours. In Natural Food Colorants, 2nd ed.; Blackie Academic & Professional: Glasgow, UK, 1996; pp. 40–80. [Google Scholar]
- Solymosi, K.; Latruffe, N.; Morant-Manceau, A.; Schoefs, B. Food colour additives of natural origin. In Colour Additives for Foods and Beverages; Scotter, M.J., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 3–34. [Google Scholar]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Lobo, F.A.T.; Silvana, R.; Domingues, J.; Rodrigues, S.; Costa, V.; Falcão, D.; Araújo, K.G.D.L. Inclusion complexes of yellow bell pepper pigments with β-cyclodextrin: Preparation, characterisation and application as food natural colorant. J. Sci. Food Agric. 2018, 98, 2665–2671. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- FDA. GRAS Notices. 2001. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=74&sort=GRN_No&order=DESC&startrow=1&type=basic&search=cyclodextrin (accessed on 22 May 2021).
- Gomes, L.M.M.; Petito, N.; Costa, V.G.; Falcão, D.Q.; Araújo, K.G.D.L. Inclusion complexes of red bell pepper pigments with β-cyclodextrin: Preparation, characterisation and application as natural colorant in yogurt. Food Chem. 2014, 148, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Petito, N.; Dias, D.D.S.; Costa, V.G.; Falcão, D.Q.; Araujo, K.G.D.L. Increasing solubility of red bell pepper carotenoids by complexation with 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2016, 208, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.F.; Kawaguchi, S.; Kamaya, A.; Ohshita, M.; Kabasawa, K.; Iwama, K.; Taniguchi, K.; Tsuda, S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2002, 519, 103–119. [Google Scholar] [CrossRef]
- FDA. Food Ingredient & Packaging Terms. 2018. Available online: https://www.fda.gov/food/food-ingredients-packaging/food-ingredient-packaging-terms (accessed on 5 April 2020).
- Barrow, J.; Lipman, A.; Bailey, C. Color Additives History. Reprinted from Food Safety Magazine October/November 2003 Issue, 2003. Available online: https://www.fda.gov/industry/color-additives/color-additives-history#authors (accessed on 5 March 2021).
- European Food Safety Authority (EFSA). Food Colours. 2016. Available online: https://www.efsa.europa.eu/en/topics/topic/food-colours (accessed on 5 March 2021).
- Secretaria de Salud de los Estados Unidos Mexicanos. ACUERDO por el que se determinan los aditivos y coadyuvantes en alimentos, bebidas y suplementos alimenticios, su uso y disposiciones sanitarias (Continúa en la Cuarta Sección). DOF, 2006. Available online: https://dof.gob.mx/nota_detalle_popup.php?codigo=5259470 (accessed on 5 March 2021).
- FAO/WHO. All specifications monographs from the 1st to the 65th meeting (1956–2005). In Combined Compendium of Food Additive Specifications; FAO JECFA Monogr: Rome, Italy, 2006. [Google Scholar]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A 2017, 34, 335–355. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Nascimento, K.D.O.D.; Paes, S.D.N.D.; Augusta, I.M. A Review ’Clean Labeling’: Applications of Natural Ingredients in Bakery Products. J. Food Nutr. Res. 2018, 6, 285–294. [Google Scholar] [CrossRef]
- Gallen, C.; Pla, J. Allergie et intolérance aux additifs alimentaires. Rev. Fr. d’Allergol. 2013, 53, 9–18. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the re-evaluation of vegetable carbon (E 153) as a food additive. EFSA J. 2012, 10, 2592. [Google Scholar] [CrossRef]
- Auttachoat, W.; Germolec, D.R.; Smith, M.J.; White, K.L.; Guo, T.L. Contact sensitizing potential of annatto extract and its two primary color components, cis-bixin and norbixin, in female BALB/c mice. Food Chem. Toxicol. 2011, 49, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-Microencapsulation of Anthocyanins from Cornelian Cherry Fruits and Lactic Acid Bacteria in Biopolymeric Matrices by Freeze-Drying: Evidences on Functional Properties and Applications in Food. Polymers 2020, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Milea, Ş.A.; Dima, C.V.; Enachi, E.; Dumitraşcu, L.; Barbu, V.; Bahrim, G.E.; Alexe, P.; Stănciuc, N. Combination of freeze drying and molecular inclusion techniques improves the bioaccessibility of microencapsulated anthocyanins from black rice (Oryza sativa L.) and lavender (Lavandula angustifolia L.) essential oils in a model food system. Int. J. Food Sci. Technol. 2020, 55, 3585–3594. [Google Scholar] [CrossRef]
- Nogueira, M.B.; Prestes, C.F.; Burkert, J.F.D.M. Microencapsulation by lyophilization of carotenoids produced by Phaffia rhodozyma with soy protein as the encapsulating agent. Food Sci. Technol. 2017, 37, 1–4. [Google Scholar] [CrossRef][Green Version]
- Deng, X.-X.; Chen, Z.; Huang, Q.; Fu, X.; Tang, C.-H. Spray-drying microencapsulation of β-carotene by soy protein isolate and/or OSA-modified starch. J. Appl. Polym. Sci. 2014, 131, 40399. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jimenez, A.R.; Paredes-Lopez, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Pigmentos carotenoides: Consideraciones estructurales y fisicoquímicas. Arch. Latinoam. Nutr. 2007, 57, 109–117. [Google Scholar]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef]
- Wu, L.; Lin, D. Molecular Aspects of Carotenoid Metabolizing Enzymes and Implications for Ophthalmology. In Handbook of Nutrition, Diet, and the Eye, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 415–424. [Google Scholar]
- Khoo, H.-E.; Prasad, N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.C.R.; Caramujo, M.J. Carotenoids in Aquatic Ecosystems and Aquaculture: A Colorful Business with Implications for Human Health. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A review of encapsulation of carotenoids using spray drying and freeze drying. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.L.; Khachik, F. Carotenoids in Food. Carotenoids 2009, 5, 45–66. [Google Scholar]
- Schieber, A.; Weber, F. Handbook on Natural Pigments in Food and Beverages, 1st ed.; Carle, R., Schweiggert, F., Eds.; Woodhead Publishing: Duxford, UK, 2016; pp. 101–123. [Google Scholar]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- De Boer, F.Y.; Imhof, A.; Velikov, K.P. Encapsulation of colorants by natural polymers for food applications. Color. Technol. 2019, 135, 183–194. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Techniques for Nanoencapsulation of Food Ingredients; Springer: New York, NY, USA, 2013. [Google Scholar]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.; Shivhare, U. Characterization of microcapsulated β-carotene formed by complex coacervation using casein and gum tragacanth. Int. J. Biol. Macromol. 2016, 87, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P. Microencapsulation of palm oil by complex coacervation for application in food systems. Food Chem. 2017, 220, 59–66. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids microencapsulation by spray drying method and supercritical micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef]
- Mezzomo, N.; De Paz, E.; Maraschin, M.; Martín, Á.; Cocero, M.J.; Ferreira, S.R. Supercritical anti-solvent precipitation of carotenoid fraction from pink shrimp residue: Effect of operational conditions on encapsulation efficiency. J. Supercrit. Fluids 2012, 66, 342–349. [Google Scholar] [CrossRef]
- Xia, F.; Hu, D.; Jin, H.; Zhao, Y.; Liang, J. Preparation of lutein proliposomes by supercritical anti-solvent technique. Food Hydrocoll. 2012, 26, 456–463. [Google Scholar] [CrossRef]
- Lin, K.-L.; Chng, L.-M.; Lin, J.C.-T.; Hsu, S.-L.; Young, C.-C.; Shieh, C.-J.; Chang, C.-M.J. Effect of Anti-solvent Conditions on Low Density Supercritical Fluids Precipitation of Zeaxanthin Palmitates from Lycium barbarum Fruits. J. Supercrit. Fluids 2014, 87, 104–110. [Google Scholar] [CrossRef]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, encapsulation, and health benefits of β-carotene-A review. Cogent Food Agric. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Özkan, G.; Bilek, S.E. Microencapsulation of Natural Food Colourants. Int. J. Nutr. Food Sci. 2014, 3, 145–156. [Google Scholar] [CrossRef]
- Bou, R.; Boon, C.; Kweku, A.; Hidalgo, D.; Decker, E.A. Effect of different antioxidants on lycopene degradation in oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2011, 113, 724–729. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.; Xiao, H.; McClements, D.J. Inhibition of β-carotene degradation in oil-in-water nanoemulsions: Influence of oil-soluble and water-soluble antioxidants. Food Chem. 2012, 135, 1036–1043. [Google Scholar] [CrossRef]
- Galindo-Rodriguez, S.; Allemann, E.; Fessi, H.; Doelker, E. Physicochemical Parameters Associated with Nanoparticle Formation in the Salting-Out, Emulsification-Diffusion, and Nanoprecipitation Methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Afonso, B.S.; Azevedo, A.G.; Gonçalves, C.; Amado, I.R.; Ferreira, E.C.; Pastrana, L.M.; Cerqueira, M.A. Bio-Based Nanoparticles as a Carrier of β-Carotene: Production, Characterisation and In Vitro Gastrointestinal Digestion. Molecules 2020, 25, 4497. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Souza, B.W.; Ribeiro, C.; Avides, M.C.; Quintas, M.A.; Coimbra, J.S.; Carneiro-Da-Cunha, M.G.; Vicente, A.A. Nanoemulsions of β-carotene using a high-energy emulsification–evaporation technique. J. Food Eng. 2011, 102, 130–135. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef]
- Ghosh, S.; Mishra, S.; Singh, T. Antisolvents in Perovskite Solar Cells: Importance, Issues, and Alternatives. Adv. Mater. Interfaces 2020, 7, 1–24. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar] [CrossRef]
- Horikoshi, N.K.; Nakamura, N.; Matsuzawa, N.; Yamamoto, M. Industrial Production of Cyclodextrins. In Proceedings of the First International Symposium on Cyclodextrins, Budapest, Hungary, 30 September–2 October 1981; Szejtli, J., Ed.; Springer Science and Business Media: Berlin/Heidelberg, Germany, 1982; pp. 25–39. [Google Scholar]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Caligur, V. Cyclodextrins. BioFiles 2008, 3, 32. [Google Scholar]
- Wiedenhoff, N.; Lammers, J.N.J.J. Properties of cyclodextrins. Part II. Preparation of a stable β-cyclodextrin hydrate and determination of its water content and enthalpy of solution in water from 15–30°. Carbohydr. Res. 1968, 7, 1–6. [Google Scholar] [CrossRef]
- Jozwiakowski, M.J.; Connors, K.A. Aqueous solubility behavior of three cyclodextrins. Carbohydr. Res. 1985, 143, 51–59. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- De Oliveira, V.E.; Almeida, E.W.C.; Castro, H.V.; Edwards, H.G.M.; Dos Santos, H.F.; De Oliveira, L.F.C. Carotenoids and β-Cyclodextrin Inclusion Complexes: Raman Spectroscopy and Theoretical Investigation. J. Phys. Chem. A 2011, 115, 8511–8519. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.C. Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure Appl. Chem. 1985, 57, 785–792. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Hand, E.O.; Kispert, L.D. Inclusion complexes of carotenoids with cyclodextrins: 1HNMR, EPR, and optical studies. Free. Radic. Biol. Med. 2004, 36, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, R.; Aachmann, F.L.; Larsen, K.L.; Petersen, S.B. NMR diffusion as a novel tool for measuring the association constant between cyclodextrin and guest molecules. Carbohydr. Res. 2002, 337, 841–849. [Google Scholar] [CrossRef]
- Ficarra, R.; Ficarra, P.; Di Bella, M.R.; Raneri, D.; Tommasini, S.; Calabrò, M.L.; Gamberini, M.C.; Rustichelli, C. Study of β-blockers/β-cyclodextrins inclusion complex by NMR, DSC, X-ray and SEM investigation. J. Pharm. Biomed. Anal. 2000, 23, 33–40. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Figueiras, A.; Santos, D.; Veiga, F. Preparation and Solid-State Characterization of Inclusion Complexes Formed Between Miconazole and Methyl-β-Cyclodextrin. AAPS PharmSciTech 2008, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef]
- Durante, M.; Milano, F.; De Caroli, M.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M. Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef]
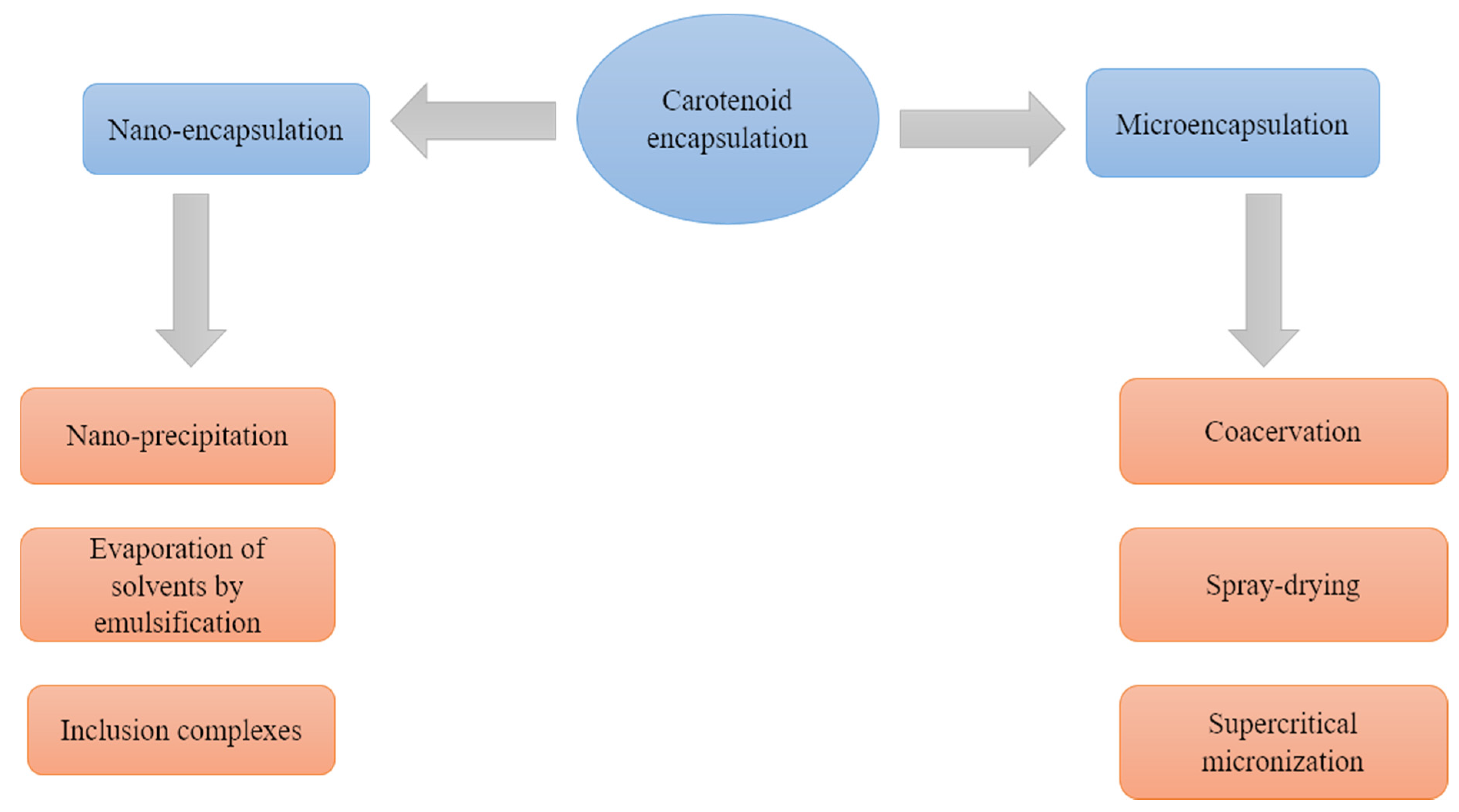


| Encapsulation Technology | Advantages | Disadvantages | Reported Applicability to Carotenoids | |
|---|---|---|---|---|
| Micro-encapsulation | Coacervation | The particle size can be engineered by stirring speed, shape of the stirrer, surface tension, and viscosity. | The required change of pH involves the use of denaturing substances, such as sulfuric or hydrochloric or organic acids. | This technique has been reported in palm oil with high carotenoid content [43], and in the encapsulation of β-carotene [44]. |
| Spray-drying | Common technology easily scalable. Particle size and key factors can be controlled by speed, flow and air temperature, as well as by the nozzle design [45]. | The use of hot air which can be deleterious, considering carotenoid sensitivity towards air and high temperatures. | The technique has been extensively used for carotenoid encapsulation, such as β-carotene and lycopene [45]. | |
| Supercritical micronization | The technique does not require high temperatures that can affect the quality of the carotenoids. In addition, using supercritical CO2 as solvent guarantees low cost and ease of downstream removal [45]. | It needs the use of more than one solvent | This method has been used to encapsulated astaxanthin [46], lutein [47] and zeaxanthin [48]. | |
| Emulsification | The oil-in-water emulsions are consider to be a low-cost, efficient method to increase the stability and bioavailability of β-carotene [49]. Multiple strategies, either high-energy and low-energy, are available. | Emulsions can be dried to form powders, but further processing is required, such as freeze-drying or spray-drying [50]. | Different types of carotenoids have improved their stability because of the use of oil water emulsions some examples are lycopene [51] and β-carotene [52]. | |
| Nano-encapsulation | Nano-precipitation | Relatively low energy requirements. It is based on the spontaneous emulsification between an internal organic phase containing the dissolved polymer, the cargo compound and the organic solvent, and an external aqueous phase [42]. | To improve the efficiency of the technique, further processing is needed, usually freeze-drying [53]. | The technique has been used to encapsulated β-carotene in polymers as ethylcellulose [54] |
| Solvent evaporation after emulsification | The size of the sphere is controlled by the stirring mode, the amount of dispersing agent, viscosity of the organic and aqueous phases, and temperature [42]. | The use of solvents can be an environmental problem. | This technique has been used to produce nano-emulsions where β-carotene has been encapsulated [55]. | |
| Inclusion complexes | The method gives the encapsulated substance high stability and provides high encapsulation yields [12]. | Only a few components can be used as a substrate for this purpose, for example cyclodextrins, and β-lactoglobulin [56]. | The technique has been used to encapsulated β-carotene, Lycopene, yellow and red pepper extract |
| Inclusion Complex | Preparation Methodologies | Mass Ratio | Recovery (%) * | Inclusion Efficiency (%) ** | Characterization Techniques | Summary | Ref. |
|---|---|---|---|---|---|---|---|
| Red pepper extract and β-cyclodextrin | Co-precipitation with ultrasound homogenization (100 W, 15 min). | (1:4) | 54.5 ± 3.6 | 62.4 ± 12.9 | FT-IR DSC Particle size Size distribution Zeta potential 1H-NMR Color stability | Greater performance and inclusion efficiency were obtained by using ultrasound over conventional (magnetic) stirring. The formation of the inclusion complex is demonstrated, with FT-IR and DSC better. High color stability in yogurt matrix was demonstrated. | [12] |
| Co-precipitation with conventional (magnetic) stirring (up to 22 ± 1 °C, ~30 min). | (1:4) | 40.8 ± 2.8 | 52.9 ± 9.4 | ||||
| Kneading | (1:4) | No data | No data | ||||
| Yellow pepper extract and β-cyclodextrin | Co-precipitation with ultrasound homogenization (50 W, 15 min) | (1:2) | 78 ± 1 | 57 ± 5 | FT-IR DSC Staining power | FT-IR and CRP techniques confirmed the formation of the inclusion complexes. Also, through the staining power it was decided that the best inclusion corresponded to 1:2. Finally, a high color stability was demonstrated in isotonic beverages. | [9] |
| (1:4) | 63 ± 4 | 73 ± 8 | |||||
| (1:6) | 51 ± 3 | 75 ± 6 | |||||
| Kneading (50 min) | (1:2) | 96 | 37 | ||||
| Red pepper extract and 2-hydroxypropyl-β-cyclodextrin | Co-precipitation with ultrasound homogenization (100 W, 5 min) | (1:4) | 91.2 ± 1.7 | 81.9 ± 2.4 | FT-IR DSC DLS 1H-NMR Solubility essay | It was observed that the amount of cyclodextrin did not affect the inclusion efficiency and high yields were obtained. The techniques of CSD, FT-IR, DLS and solubility test were the techniques that identified the formation of the inclusion complex. Finally, the use of 2-HPβCD increased water solubility by 660 times. | [13] |
| (1:6) | 92.7 ± 1.2 | 75.2 ± 7.5 | |||||
| (1:8) | 90.7 ± 1.2 | 69.0 ± 3.3 | |||||
| (1:10) | 93.0 ± 2.0 | 69.7 ± 3.7 | |||||
| Kneading (20 min) | (1:4) (1:6) (1:8) (1:10) | No data | No data | ||||
| β-carotene (BCT) and β-cyclodextrin | Co-precipitation with conventional (magnetic) stirring (5 days, N2 purge, covered with light) | (1:360) | No data | No data | FT-Raman Computational Quantum Model | The most intense band ν1 assigned to the stretching -C=C- was the most sensitive to change at the time of inclusion in Raman, and it was higher in non-bulky groups of the studied molecules, generating greater inclusion. BCT, LYC and AST presented favorable inclusion energies. The computational calculations agreed mostly with the experiments carried out. | [66] |
| Lycopene (LYC) and β-cyclodextrin | |||||||
| Astaxanthin (AST) and β-cyclodextrin | |||||||
| Tomato oil and α-cyclodextrin | Co-precipitation with stirring and nitrogen sparging (24 h) | (10:19) | No data | 61.5 | FTIR-ATR DSC SEM LCSM Antioxidant activity | The β-cyclodextrin complex showed the best dispersion in oil. α and β showed the best antioxidant activity as well as oil-dispersing agents and antioxidant carrier systems in aqueous media. A significant improvement in the stability of the lycopenes analyzed was shown by the three types of cyclodextrins. | [73] |
| Tomato oil and β-cyclodextrin | (10:23) | No data | 62.4 | ||||
| Tomato oil and γ-cyclodextrin | (10:26) | No data | 44.0 |
| Streams (kg) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled water | - | - | - | 120.0 | 60.0 | - | - | - | 19.8 | 21.8 | 60.0 | - | 60.0 | 57.0 | 57.0 | 18.8 | 38.2 | 3.0 | 3.0 | 3.0 | - | 60.0 | 60.0 | 40.2 | 19.8 | 3.0 |
| Extract | 1.0 | - | - | - | - | - | - | 0.7 | - | - | 1.0 | - | 1.0 | 0.5 | 0.5 | 0.2 | 0.3 | 0.5 | 0.5 | 0.5 | 0.5 | - | - | - | - | - |
| Ethanol | - | 1.0 | 60.0 | - | - | - | - | 1.0 | 24.9 | 14.3 | 31.0 | - | 31.0 | 23.4 | 23.4 | 7.7 | 15.7 | 7.6 | - | - | - | 7.6 | 7.6 | 5.1 | 2.5 | - |
| β-cyclodextrin | - | - | - | - | - | 2.7 | - | - | - | - | 4.0 | - | 4.0 | 1.9 | 1.9 | 0.6 | 1.3 | 0.8 | 2.1 | 2.1 | 2.1 | - | - | - | - | - |
| Refrigerant | - | - | - | - | - | - | 49.0 | - | - | - | - | 49.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Streams (kg) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled water | - | - | 60.0 | 60.0 | 4.0 | 3.3 | - | - | - | 24.0 | 19.0 | 23.0 | - | 23.0 | 78.4 | 4.6 | 60.0 | 4.6 | 4.6 | 4.6 | 60.0 | - | 24.0 | 36.0 | 78.4 | 15.7 | 62.7 |
| Extract | - | - | - | - | - | - | - | - | 1.0 | - | - | 1.0 | - | 1.0 | 0.2 | 0.9 | - | 0.9 | 0.9 | - | - | 0.9 | - | - | 0.2 | 0.0 | 0.1 |
| Carotenoids | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ethanol | - | 30.0 | - | - | - | - | - | - | - | 26.4 | - | - | - | - | 24.0 | 6.0 | 6.0 | - | - | - | 6.0 | - | 2.4 | 3.6 | 24.0 | 4.8 | 19.2 |
| β-cyclodextrin | - | - | - | - | - | - | - | 4.0 | - | - | - | 4.0 | - | 4.0 | 0.6 | 3.4 | - | 3.4 | 3.4 | - | - | 3.4 | - | - | 0.6 | 0.1 | 0.5 |
| Dextrose | 55.3 | - | - | - | - | - | 1.0 | - | - | - | 1.0 | 1.0 | 55.3 | 1.0 | 0.2 | 0.8 | 0.2 | 0.6 | 0.6 | 0.6 | 0.2 | 0.1 | - | 0.1 | 0.2 | 0.0 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of Carotenoids as Food Colorants via Formation of Cyclodextrin Inclusion Complexes: A Review. Polysaccharides 2021, 2, 454-476. https://doi.org/10.3390/polysaccharides2020028
Fuenmayor CA, Baron-Cangrejo OG, Salgado-Rivera PA. Encapsulation of Carotenoids as Food Colorants via Formation of Cyclodextrin Inclusion Complexes: A Review. Polysaccharides. 2021; 2(2):454-476. https://doi.org/10.3390/polysaccharides2020028
Chicago/Turabian StyleFuenmayor, Carlos A., Omar G. Baron-Cangrejo, and Paula A. Salgado-Rivera. 2021. "Encapsulation of Carotenoids as Food Colorants via Formation of Cyclodextrin Inclusion Complexes: A Review" Polysaccharides 2, no. 2: 454-476. https://doi.org/10.3390/polysaccharides2020028
APA StyleFuenmayor, C. A., Baron-Cangrejo, O. G., & Salgado-Rivera, P. A. (2021). Encapsulation of Carotenoids as Food Colorants via Formation of Cyclodextrin Inclusion Complexes: A Review. Polysaccharides, 2(2), 454-476. https://doi.org/10.3390/polysaccharides2020028